Abstract
IFN-regulatory factor 5 (IRF-5), a member of the IRF family, is a transcription factor that has a key role in the induction of the antiviral and inflammatory response. When compared with C57BL/6 mice, Irf5−/− mice show higher susceptibility to viral infection and decreased serum levels of type I IFN and the inflammatory cytokines IL-6 and TNF-α. Here, we demonstrate that IRF-5 is involved in B-cell maturation and the stimulation of Blimp-1 expression. The Irf5−/− mice develop an age-related splenomegaly, associated with a dramatic accumulation of CD19+B220− B cells and a disruption of normal splenic architecture. Splenic B cells from Irf5−/− mice also exhibited a decreased level of plasma cells. The CD19+ Irf5−/− B cells show a defect in Toll-like receptor (TLR) 7- and TLR9-induced IL-6 production, and the aged Irf5−/− mice have decreased serum levels of natural antibodies; however, the antigen-specific IgG1 primary response was already dependent in IRF-5 in young mice, although the IgM response was not. Analysis of the profile of transcription factors associated with plasma cell differentiation shows down-regulation of Blimp-1 expression, a master regulator of plasma cell differentiation, which can be reconstituted with ectopic IRF-5. IRF-5 stimulates transcription of the Prdm1 gene encoding Blimp-1 and binds to the IRF site in the Prdm1 promoter. Collectively, these results reveal that the age-related splenomegaly in Irf5−/− mice is associated with an accumulation of CD19+B220− B cells with impaired functions and show the role of IRF-5 in the direct regulation of the plasma cell commitment factor Blimp-1 and in B-cell terminal differentiation.
Keywords: IRF-5, antigenic response, B-cell development, Blimp-1, Toll-like receptors
The roles of IFN-regulatory factor (IRF) 3 and IRF-7 in the antiviral response have been well established, and their function in induction of type I Ifn genes has been extensively characterized (1, 2). The in vitro studies indicated that IRF-5 may also be involved in the antiviral response, but it was only recently that Irf5−/− mice became available and the importance of IRF-5 in the antiviral and inflammatory response in vivo was clearly demonstrated (3, 4).
Irf5−/− mice exhibit high susceptibility to viral infection and show reductions in serum levels of type I IFN as well as inflammatory cytokines (4). Irf5−/− mice also show resistance to lethal shock induced by unmethylated CpG DNA and LPS (3). IRF-5 expression is induced by type I IFN and viral infection. In humans, IRF-5 is expressed in multiple spliced variants (5), and a distinct polymorphism in the IRF-5 gene is associated with autoimmune diseases such as systemic lupus erythematosus (6) and rheumatoid arthritis (7).
The aim of this study was to examine the role of IRF-5 in B-cell development and differentiation, because we had observed that Irf5−/− mice exhibit age-related splenomegaly associated with a large increase in CD19+ cells. Here, we demonstrate that Irf5−/− mice show a dramatic increase in CD19+B220− cells and attenuation of plasma cell development. Addressing the molecular mechanism responsible for this impairment, we show that IRF-5 regulates expression of the plasma cell maturation protein Blimp-1. Blimp-1 encoded by Prdm1 is required for the formation of Ig-secreted plasma cells (8). In mice, B cells specific for Blimp-1 deficiency result in attenuation of plasma cell development and a reduction in the humoral antigenic response (9). Thus, our results reveal the importance of IRF-5 in B-cell development and the commitment to terminal B-cell differentiation by stimulating expression of Blimp-1, the master regulator of plasma cell differentiation.
Results
Irf5−/− Mice Displayed Age-Related Splenomegaly.
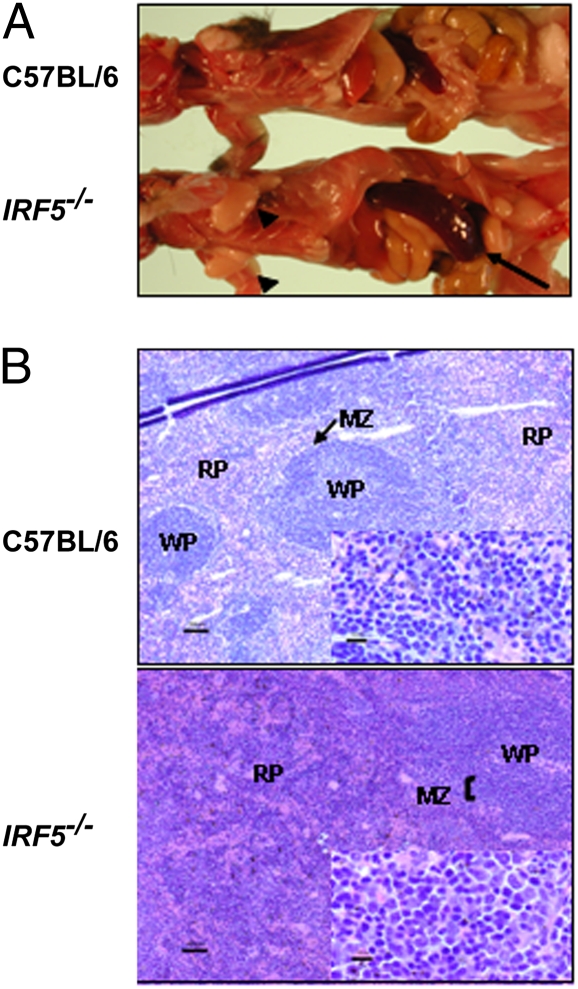
The young Irf5−/− mice did not show any characteristic phenotype and had normal-sized littermates. However, with increasing age, these mice developed splenomegaly when compared with age-matched C57BL/6 mice (Fig. 1). Analysis of splenic cellularity showed no significant difference in spleen weight and total cell number in spleens of young Irf5−/− mice (8 weeks old) compared with age-matched C57BL/6 mice (Table S1); however, with increasing age, splenomegaly developed in 70–80% of Irf5−/− mice. Thus, spleen weight of 10-month-old Irf5−/− mice increased about 2-fold when compared with age-matched C57BL/6 mice. The enlargement of lymph nodes was less frequent and was seen only in 30–40% of mice.
Fig. 1.
Splenomegaly and alteration of splenic architecture in aging Irf5−/− mice. (A) Spleens (arrows) and lymph nodes (arrowheads) of C57BL/6 mice and Irf5−/− mice (10 months old). (B) Spleens of C57BL/6 mice and Irf5−/− mice (10 months old) were fixed in 3.7% formaldehyde, sectioned, and stained with H&E. White pulp (WP), red pulp (RP), and marginal zones (MZ) are marked.
Analysis of the spleen architecture in H&E-stained paraffin-embedded sections of spleens from Irf5−/− mice and age-matched C57BL/6 mice showed that Irf5−/− mice exhibit an expansion of marginal zones surrounding the white pulp by mononuclear cells (Fig. 1). The red pulp was also expanded diffusely by mononuclear cells, erythrocytes, and myeloid precursors (extramedullary hematopoiesis). This phenomenon was observed in 6-month-old Irf5−/− mice and continued to develop in 8- and 12-month-old mice (Fig. S1). Expansion of marginal zones in Irf5−/− mice suggested that IRF-5 may affect the development of B cells.
Characterization of Splenic B Cells of Irf5−/− Mice.
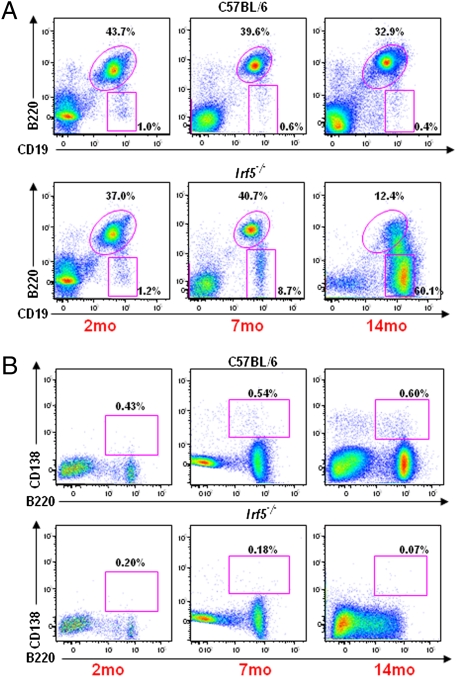
To determine the profile of splenic cells in the large spleens of old Irf5−/− mice, we analyzed distinct populations of splenocytes by five-color flow cytometry, comparing the splenocytes of old Irf5−/− and age-matched C57BL/6 mice (Fig. 2 and Fig. S2). In the old (14 months old) Irf5−/− mice, the CD19+ B-cell population represented ≈90% of the splenocytes, whereas in the age-matched C57BL/6 mice, only about 35% of splenocytes were B cells. The number of splenic CD4+ T cells in the old Irf5−/− mice was decreased by 3-fold, and there was a slight reduction in the levels of CD8+ T cells when compared with the age-matched C57BL/6 mice (Table S2). However, the decline of CD4+ T cells over age resulted an inversion of the CD4/CD8 T-cell ratio in Irf5−/− mice compared with age-matched C57BL/6 animals. The CD19+ B cells from Irf5−/− mice were heterogeneous for B220 and had a larger size than normal resting B cells. The majority of B cells were C19+B220−, indicating possible accumulation of plasmablasts (Table S2). The increase in the number of CD19+B220+ cells in Irf5−/− mice was only marginal (1.5-fold), and there was no difference in the number of IgM+IgD+ mature B cells and an increase (6-fold) of the immature B cells (IgMhigh, IgDlow) (Fig. S2B and Table S2). Finally, the spleens of Irf5−/− mice showed fewer CD138high, B220high plasma cells throughout their age (37% of the controls at 14 months of age) (Fig. 2B and Table S2). The large cell size, uniform IgM staining, and high B-cell count (∼400–600 × 106 in a total spleen) suggest the presence of B-cell lymphoma (BCL).
Fig. 2.
Analysis of B-cell subtypes in spleens of C57BL/6 and Irf5−/− mice at 2, 7, and 14 months (mo) of age. Total splenocytes from C57BL/6 (Top) and Irf5−/− (Bottom) mice aged 2, 7, and 14 mo were stained with CD19 and B220 (A) or B220 and CD138 (B), respectively, and analyzed by flow cytometry. CD19+ B cells and CD138+ plasma cells were gated on live lymphocytes by forward and side scatter.
In contrast, analysis of the splenic lymphocyte population in young (8 weeks old) Irf5−/− mice did not show significant differences in the levels of T cells and B-cell subtypes from the C57BL/6 mice (Fig. 2, Fig. S2, and Table S2). Many of the phenotypic changes arise gradually, with significant alterations apparent by 6–7 months of age, at a time when overall splenocyte numbers have not yet changed (Fig. 2 and Fig. S2). However, the increase in the level of CD19+B220− cells was already detected in 7-month-old mice. Further characterization of the B-cell profile as a consequence of aging of the Irf5−/− mice, as well as determination of the clonality of B-cell expansion, is under investigation, and hence not included in the present study.
IRF-5 expression in distinct stages of B-cell lineage was determined by flow cytometry. As shown in Fig. S3, IRF-5 is expressed in B220+IgM+IgD+ mature B cells and its levels decline in CD138+ plasma cells. As expected, expression of IRF-5 was not detected in B cells of Irf5−/− mice.
Functional Characterization of Irf5−/− B Cells.
Serum levels of inflammatory cytokines in infected Irf5−/− mice were decreased when compared with the C57BL/6 mice, and the Irf5−/− CD11c+ cells showed a decrease in TNF-α and IL-6 production on MyD88 activation (4). We therefore analyzed IL-6 production in Irf5−/− B cells from young (8 weeks old) and old (14 months old) Irf5−/− mice and age-matched C57BL/6 controls stimulated in vitro with Toll-like receptor (TLR) 4, TLR7, and TLR9 ligands (Fig. S4A). There was no significant difference in IL-6 production in response to LPS stimulation between C57BL/6 and Irf5−/− mice (old and young). However, on stimulation with R848 or CpG DNA, B cells from both young and old Irf5−/− mice produced lower levels of IL-6 than the B cells from C57BL/6 mice. There was no down-modulation of TLR7 expression in Irf5−/− B cells, indicating that the attenuation of IL-6 expression is not attributable to the lower expression of TLR7 in these cells (Fig. S4B). The expression of TLR9 was very low both in Irf5−/− B cells and B cells from C57BL/6 mice, and the levels did not differ. These data show that IRF-5 has a critical role in the TLR7- and TLR9-mediated activation of IL-6 in splenic B cells.
Because the analysis of B-cell differentiation in Irf5−/− mice suggests a decrease in plasma cells, which are the major source of Igs, we measured and compared the level of natural antibodies in serum of naive Irf5−/− mice and C57BL/6 mice. No significant differences in the serum levels of IgG subtypes were observed between naive young (8 weeks old) Irf5−/− mice and C57BL/6 mice (Fig. S5). However, in aged (10 months old) Irf5−/− mice, the levels of IgG1, IgG2a, and IgG2b were decreased, whereas the levels of IgM were increased compared with the serum levels of the C57BL/6 mice. The high levels of IgM and low levels of IgG suggest that the Irf5−/− B cells have either defects in isotype switching or attenuation of the terminal differentiation to IgG-secreting plasma cells.
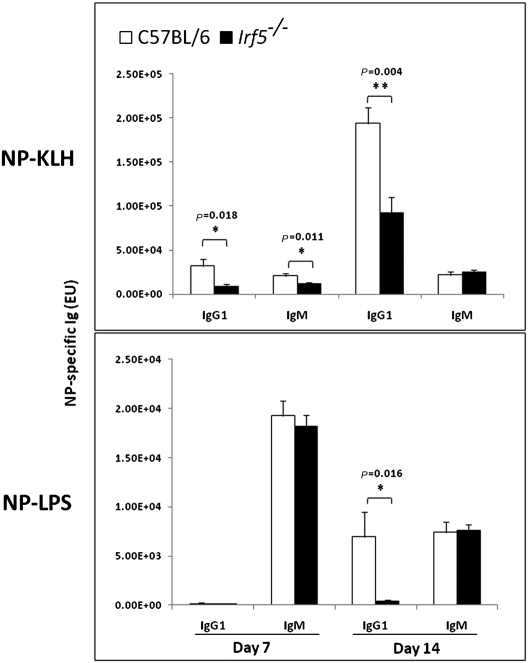
Although the young Irf5−/− mice (8 weeks old) did not show major changes in the B-cell repertoire (Fig. 2), the antibody response to T-cell-dependent antigen, following primary immunization of these mice, showed a significant reduction in IgG1 (P = 0.018) and a transient reduction in IgM (P = 0.011). The early antigenic response to T-cell-independent antigen was very low, but there was a significant decrease in the antigen-specific IgG1 (P = 0.016), whereas there was no difference in IgM levels between Irf5−/− mice and age-matched controls (Fig. 3). The antigen-specific responses of the other IgG subclasses, as well as the secondary responses, are being evaluated. These data suggest that IRF-5 expression in B cells drives the secretion of the antigen-specific IgG1 both in T-cell-dependent and -independent primary immunization, although the impairment in the serum levels of natural antibodies could be detected primarily in aged mice.
Fig. 3.
Antibody response to T-cell-dependent and -independent antigens in Irf5−/− and C57BL/6 mice. Groups of five Irf5−/− and C57BL/6 female mice (8 weeks old) were injected i.p. with 4-Hydroxy-3-nitrophenyl (NP); Keyhole Limpet Hemocyanin (KLH) NP-KLH/alum (1:1, 100 μg) or NP-LPS (20 μg) and bled 7 and 14 days later. Antigen-specific IgG1 and IgM were determined by ELISA, as described in Materials and Methods. Values indicate mean titers for groups of five animals, and the error bars indicate the SEM. The P values are given at the top of the respective figures. *p ≤ 0.05 **p ≤ 0.005
IRF-5 Regulates Expression of Blimp-1, a Master of Plasma Cell Differentiation.
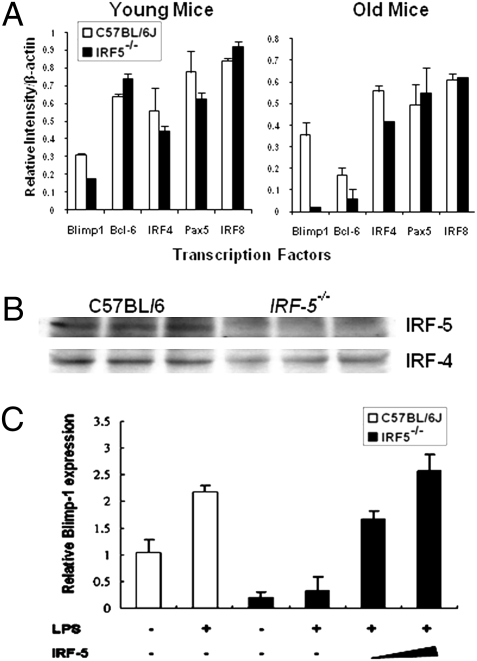
Because the Irf5−/− mice showed decreased levels of splenic plasma cells and IgG production, we analyzed expression of the transcription factors required for plasma cell differentiation in Irf5−/− B cells (10). The paired box protein 5 (PAX-5) (11) is expressed throughout the B-cell lineages, and inhibition of PAX-5 expression is the first step in plasma cell differentiation (12). The down-regulation of nuclear phosphoprotein BCL-6 terminates the germinal cell (GC) transcription cascade, and the expression of the B-lymphocyte-induced maturation protein Blimp-1 is critical for differentiation to plasma cells and IgG production (13, 14). Blimp-1 expression is regulated on a transcriptional level and can be induced both by TLR ligands (15) and IRF-4 (16). Analysis of the expression of these factors by semiquantitative RT-PCR shows that purified B cells from both young and old Irf5−/− mice exhibit significantly lower levels of Blimp-1 mRNA than B cells from the age-matched C57BL/6 mice (Fig. 4A). The expression of Blimp-1 on the protein level could not be evaluated because it is very low even in unstimulated C57BL/6 B cells. There was also a decrease in IRF-4 expression in Irf5−/− B cells, seen both on RNA and protein levels (Fig. 4 A and B), whereas the expression of PAX-5 and IRF-8 was not modulated. BCL-6 expression was not greatly modulated in Irf5−/− mice, and the observed differences may be not significant. Collectively, these data indicate that the expression of Blimp-1 and IRF-4 is down-regulated in B cells of Irf5−/− mice.
Fig. 4.
Irf5−/− B cells show decreased expression of Blimp-1, which can be rescued by ectopic IRF-5. (A) RNA was isolated from purified splenic B cells of C57BL/6 and Irf5−/− mice, and the relative levels of Blimp-1,BCL6,IRF-4,PAX5, and IRF-8 expression were determined by semiquantitative RT-PCR. The intensity of analyzed bands was normalized to β-actin levels. (B) Expression of IRF-5 and IRF-4 in purified splenic B cells of C57BL/6 and Irf5−/− mice was determined by Western blot analysis. (C) Purified splenic B cells from C57BL/6 and Irf5−/− mice were stimulated with LPS (100 μg/mL) for 3 days and then infected with retrovirus expressing the IRF-5 gene. RNA was isolated from infected cells 3 days postinfection, and the relative levels of Blimp-1 transcripts in transduced and untransduced Irf5−/− cells and controls (C57BL/6 B cells) were detected by real-time RT-PCR.
Blimp-1 is encoded by the Prdm1 gene. To demonstrate that the low levels of Prdm1 transcripts in Irf5−/− B cells are attributable to the absence of IRF-5 and not to decreased levels of IRF-4, we reconstituted Irf5−/− B cells with full-length IRF-5 cDNA by retroviral transduction (Fig. 4C). LPS stimulated Blimp-1 expression in splenic B cells (15) of C57BL/6 mice, although it did not enhance levels of Prdm1 transcripts in Irf5−/− B cells (Fig. 4C). However, reconstitution of IRF-5 expression in LPS-stimulated Irf5−/− B cells significantly increased the relative levels of Prdm1 transcripts (Fig. 4C). These data show unambiguously that the attenuation of Blimp-1 levels in Irf5−/− B cells is attributable to the absence of Irf5−/− expression and imply that IRF-5 is a transcriptional activator of Prdm1.
Sequence analysis of the Prdm1 promoter showed the presence of interferon regulatory factor (IRF) and interferon stimulated response element (ISRE)-like domains (Fig. S6A). We used the DNA pull-down assay to examine whether IRF-5 can bind to this sequence. We have shown previously (4) that murine IRF-5 is activated by viral infection and by the TLR7 or TLR9 mediated MyD88 signaling pathway. MyD88 activated IRF-5 bound effectively to the deoxyoligonucleotide corresponding to the Prdm1 IRF binding site, although it did not bind to a mutated IRF site or to an unrelated oligonucleotide (Fig. S6A). Under the same conditions, there was no significant binding of IRF-5 in the absence of MyD88 activation.
To determine whether the binding of IRF-5 to the IRF domain is functional, we examined whether IRF-5 can stimulate the transcriptional activity of the Prdm1 promoter, using a transient transfection assay with a Prdm1 promoter-regulated firefly luciferase expression vector. The Prdm1 promoter was shown to be constitutively active in a transient transfection assay (17); however, as shown in Fig. S6B, MyD88-activated ectopic IRF-5 stimulated activity of the Prdm1 promoter in a dose-dependent manner. In the absence of MyD88, IRF-5 was unable to activate the Prdm1 promoter above the basal levels. MyD88 alone did not stimulate the activity of the Prdm1 promoter at the concentration levels used. We have shown previously that MyD88 activation of IRF-5 includes tumor necrosis factors (TNF) receptor associated factor-6 (TRAF-6)-mediated K63 ubiquitination and phosphorylation by a yet unknown kinase (18). It remains to be determined what activates IRF-5 in B cells of naive mice, and we are presently addressing this question.
Discussion
In the present study, we have demonstrated that IRF-5 is required for B-cell maturation and expression of Blimp-1 a hallmark of plasma cells. Two other members of the IRF family, IRF-4 and IRF-8, have been shown to have critical functions in different stages of B-cell development. Although in pre-B cells, some of their functions are redundant (18, 19), in later stages of B-cell development, IRF-4 suppresses BCL-6 transcription (20) and regulates receptor editing, Ig class switching, and plasma cell differentiation (21, 22). IRF-8 functions in the GC B-cell transcription program and directly stimulates expression of the BCL-6 gene (23). Altogether, these data indicate that several members of the IRF family can affect B-cell development, although at steps distinct from IRF-5.
IRF-5 is activated by the MyD88 signaling pathway, and alteration of B-cell function was observed in MyD88−/− mice (24), which failed to develop long-lived plasma cells and a long-term antibody-mediated antiviral response (25). MyD88 activation of B cells was required to generate a primary response to T-cell-dependent antigen, and the impairment was predominantly in the IgG2a/c class, whereas the impairment in the antigen-specific IgG1 response showed some variation (24, 26). Our data indicate that IRF-5 is required for the primary antigen responses in the IgG1 class, whereas the IgM response was generally independent of IRF-5. The impairment in the antigen-specific IgG1 was already detected in young mice that had not yet developed splenomegaly or accumulation of CD19+B220− cells. These data suggest that IRF-5 is required in T helper (Th)-2 responses; however, it still remains to be determined whether there is also impairment in the IgG2a/c subclasses, which are associated with the Th-1 response, and this question is being examined.
Old Irf5−/− mice have shown a decrease in the levels of CD138+B220+ B cells and a marked accumulation of large CD19+B220− cells that may represent preblast or plasmablast B cells, indicating that aged Irf5−/− mice have defects in plasma cell development. Alternatively, these cells could represent expansion of B1 cells, which are the major producers of the circulating IgM present at high levels in the serum of old Irf5−/− mice (8). We are presently examining this possibility. There was no significant accumulation of CD19+B220− B cells in young mice; however, increased levels were already detected in 7-month-old mice (Fig. 2A). There was also a decrease in the expression of the transcription factor Blimp-1, a hallmark of plasma cells in both young and old mice. Blimp-1 is zinc finger-containing transcriptional repressor encoded by the Prdm1 gene, which has a critical role in the development and maintenance of antibody-secreting plasma cells by down-regulating expression of PAX-5 and BCL-6 (27) as well as c-myc (28). Mice with B-lineage-specific Blimp-1 deletion do not generate plasma cells (29), and ectopic expression of Blimp-1 in mature B cells drives plasma cell differentiation (27). In mice, Blimp-1 is expressed in plasma cells and at low levels in B-1 cells, although it is not expressed in memory B cells (30). Recent studies indicate that Blimp-1 is also important for T-cell differentiation, where it represses expression of IL-2 and IFN γ genes (14) and regulates differentiation of follicular T cells (31). Thus, Blimp-1 function is required both in B cells and T cells; however, its major impact in B cells is on terminal differentiation of plasma cells, whereas in T cells it seems to be important in the regulation of immune response.
The role of IRF-4 in plasma cell differentiation has been well established, and Irf4−/− mice do not generate plasma cells (21). IRF-4 also up-regulates Blimp-1 expression in B cells (16) and interferes with IRF-5 activation in the TLR-MyD88 signaling pathway (32). Thus, the roles of IRF-4 and IRF-5 in the activation of Blimp-1 and plasma cell differentiation are distinct, suggesting that the function of IRF-5 may be to enhance plasma cell differentiation in infected cells when IRF-5 is stimulated by IFN or activated by TLR signaling. Interestingly, both IRF-5 and Blimp-1 can be induced by the type I IFN signaling pathway, which is required for plasma cell differentiation and antibody responses to influenza virus infection in vivo (33). The Blimp-1 DNA binding motif is very similar to the IRF-3 and IRF-5 DNA binding motifs, which have been shown to function as positive regulators of IFNB gene transcription in the antiviral response (34). However, in contrast to these two factors, Blimp-1 functions as a repressor of IFNB gene transcription (35).
It was shown that BCL-6 and Blimp-1 developed a mutual suppression loop, and although Blimp-1 suppressed BCL-6 expression in plasma cells, BCL-6 repressed Blimp-1 expression in GC B cells (16). Expression of BCL-6 in B cells of Irf5−/− mice was a little higher in young mice and a little lower in old mice than in C57BL/6 mice and needs to be evaluated further in larger groups of mice. Down-regulation of Prdm1 expression by BCL-6 results in attenuation of plasmacytic differentiation (36). Dysregulated expression of BCL-6 in B cells also drives B cells into tumor precursors, because BCL-6 suppresses transcription of the p53 tumor suppressor gene (37). Enhanced expression of BCL-6 and inactivation of Blimp-1 occur in a large fraction of diffuse large-cell lymphomas (38).
In conclusion, we have identified IRF-5 as a critical component of the B-cell transcriptional differentiation program and have shown that IRF-5 directly regulates transcription of the Prdm1 gene encoding Blimp-1, an essential regulator of plasma cell development. To our knowledge, the role of IRF-5 in the transcriptional activation of Prdm1 and plasma cell development is a previously undescribed finding. There are several indications that the impairment of IRF-5 expression may also be associated with leukemogenesis. Human IRF-5 is constitutively expressed in lymphoid tissue and peripheral lymphocytes in multiple spliced variants, but it is undetectable in the majority of leukemia cells from patients with hematological malignancies (39). A dominant negative mutant of IRF-5 that down-regulates transcriptional activity of IRF5 in vitro was detected in the blood of patients with Chronic lymphocytic leukemia (CLL) (40). Further, Kaposi sarcoma herpes virus (KSHV)-encoded nuclear protein viral IRF-3, expressed in human B cell lymphoma-Primary effusion lymphoma (PEL) lines, interacts with IRF-5 and inhibits its transcriptional activity (41). Distinct polymorphisms in the IRF-5 gene are associated with a predisposition to autoimmune diseases (6). It still remains to be determined whether genetic or epigenetic modulation of IRF-5 expression or function is associated with the occurrence of some B-cell malignancies.
Materials and Methods
Detailed methods are described in SI Text.
Mice.
Irf5−/− mice (generously provided by T. Mak, University of Toronto, Toronto, ON, Canada) were back-crossed to C57BL/6 mice for at least six generations (4). C57BL/6 mice were obtained from the Jackson Laboratory and were bred in animal housing. Animal housing and all experiments were carried out in compliance with protocols approved by The Johns Hopkins University Animal Use and Care Committee. Data presented represent analysis of this Irf5−/− mice colony. Whether the B cell phenotype is determined by the genotype and retained in the Irf5−/− cre+/+ mice is being analyzed.
B-Cell Purification and Flow Cytometry.
Resting B lymphocytes were isolated from the spleen with CD43 microbeads (Miltenyi Biotech), cultured in RPMI supplemented with L-glutamine, sodium pyruvate, 50 μM 2-mercaptoethanol, and 10% (vol/vol) FBS (GIBCO-BRL). Single-cell suspensions from spleen were stained with phycoerythrin (PE)-Cy5-conjugated CD4; FITC-conjugated CD8 or IgD; PE-Cy7-conjugated B220; PE-conjugated IgM, CD19, or CD21; Allophycocyanin (APC) APC-conjugated CD23; biotin-conjugated CD138; and streptavidin-APC (BD Biosciences-Pharmingen) and analyzed by using flow cytometry. FACS analysis was performed as described in SI Materials and Methods.
RT-PCR and Retrovirus Transduction of B Cells.
Total RNA was isolated from purified splenic B cells and reverse-transcribed to cDNA using a polyA primer (Fermentas Life Sciences; details and primers are provided in SI Text). Retroviral plasmid carrying the murine IRF-5 cDNA and GFP gene was prepared as described (42). Virus preparation and transduction are fully described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. T. Mak for the Irf5−/− mice; Dr. K. Fitzgerald for the Blimp-1 reporter plasmid; Dr. Mumtaz Balkhi for performing the pull-down assay; Drs. J. Cerny, E. Szomolanyi-Tsuda, N. Baumgarth and A. Paun for their advice during the course of this study; and M. Paul and H. Lee for their help with mouse colonies. The study was supported by National Institute of Allergy and Infectious Diseases Grants R01 AI067632-02A1 and CA 19737-24A1 and by the pilot grant from the Alliance for Lupus Foundation (to P.M.P.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911193107/DCSupplemental.
References
- 1.Paun A, Pitha PM. The IRF family, revisited. Biochimie. 2007;89:744–753. doi: 10.1016/j.biochi.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001;19:623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- 3.Takaoka A, et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 4.Paun A, et al. Functional characterization of murine interferon regulatory factor 5 (IRF-5) and its role in the innate antiviral response. J Biol Chem. 2008;283:14295–14308. doi: 10.1074/jbc.M800501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mancl ME, et al. Two discrete promoters regulate the alternatively spliced human interferon regulatory factor-5 isoforms. Multiple isoforms with distinct cell type-specific expression, localization, regulation, and function. J Biol Chem. 2005;280:21078–21090. doi: 10.1074/jbc.M500543200. [DOI] [PubMed] [Google Scholar]
- 6.Graham RR, et al. Three functional variants of IFN regulatory factor 5 (IRF5) define risk and protective haplotypes for human lupus. Proc Natl Acad Sci USA. 2007;104:6758–6763. doi: 10.1073/pnas.0701266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sigurdsson S, et al. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am J Hum Genet. 2005;76:528–537. doi: 10.1086/428480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumgarth N, et al. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savitsky D, Calame K. B-1 B lymphocytes require Blimp-1 for immunoglobulin secretion. J Exp Med. 2006;203:2305–2314. doi: 10.1084/jem.20060411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuxa M, Skok JA. Transcriptional regulation in early B cell development. Curr Opin Immunol. 2007;19:129–136. doi: 10.1016/j.coi.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Delogu A, et al. Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells. Immunity. 2006;24:269–281. doi: 10.1016/j.immuni.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Kallies A, et al. Initiation of plasma-cell differentiation is independent of the transcription factor Blimp-1. Immunity. 2007;26:555–566. doi: 10.1016/j.immuni.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Shaffer AL, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 14.Martins GA, Cimmino L, Liao J, Magnusdottir E, Calame K. Blimp-1 directly represses Il2 and the Il2 activator Fos, attenuating T cell proliferation and survival. J Exp Med. 2008;205:1959–1965. doi: 10.1084/jem.20080526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schliephake DE, Schimpl A. Blimp-1 overcomes the block in IgM secretion in lipopolysaccharide/anti-mu F(ab′)2-co-stimulated B lymphocytes. Eur J Immunol. 1996;26:268–271. doi: 10.1002/eji.1830260142. [DOI] [PubMed] [Google Scholar]
- 16.Sciammas R, et al. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity. 2006;25:225–236. doi: 10.1016/j.immuni.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Tunyaplin C, Shapiro MA, Calame KL. Characterization of the B lymphocyte-induced maturation protein-1 (Blimp-1) gene, mRNA isoforms and basal promoter. Nucleic Acids Res. 2000;28:4846–4855. doi: 10.1093/nar/28.24.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu R, Medina KL, Lancki DW, Singh H. IRF-4,8 orchestrate the pre-B-to-B transition in lymphocyte development. Genes Dev. 2003;17:1703–1708. doi: 10.1101/gad.1104803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, et al. IRF8 regulates B-cell lineage specification, commitment, and differentiation. Blood. 2008;112:4028–4038. doi: 10.1182/blood-2008-01-129049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saito M, et al. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer Cell. 2007;12:280–292. doi: 10.1016/j.ccr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Klein U, et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat Immunol. 2006;7:773–782. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- 22.Pathak S, Ma S, Trinh L, Lu R. A role for interferon regulatory factor 4 in receptor editing. Mol Cell Biol. 2008;28:2815–2824. doi: 10.1128/MCB.01946-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CH, et al. Regulation of the germinal center gene program by interferon (IFN) regulatory factor 8/IFN consensus sequence-binding protein. J Exp Med. 2006;203:63–72. doi: 10.1084/jem.20051450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 25.Guay HM, Andreyeva TA, Garcea RL, Welsh RM, Szomolanyi-Tsuda E. MyD88 is required for the formation of long-term humoral immunity to virus infection. J Immunol. 2007;178:5124–5131. doi: 10.4049/jimmunol.178.8.5124. [DOI] [PubMed] [Google Scholar]
- 26.Barr TA, Brown S, Mastroeni P, Gray D. B cell intrinsic MyD88 signals drive IFN-gamma production from T cells and control switching to IgG2c. J Immunol. 2009;183:1005–1012. doi: 10.4049/jimmunol.0803706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner CA, Jr, Mack DH, Davis MM. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 28.Lin KI, Lin Y, Calame K. Repression of c-myc is necessary but not sufficient for terminal differentiation of B lymphocytes in vitro. Mol Cell Biol. 2000;20:8684–8695. doi: 10.1128/mcb.20.23.8684-8695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein U, Dalla-Favera R. Germinal centres: Role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 30.Blink EJ, et al. Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J Exp Med. 2005;201:545–554. doi: 10.1084/jem.20042060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston RJ, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Negishi H, et al. Negative regulation of Toll-like-receptor signaling by IRF-4. Proc Natl Acad Sci USA. 2005;102:15989–15994. doi: 10.1073/pnas.0508327102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coro ES, Chang WL, Baumgarth N. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J Immunol. 2006;176:4343–4351. doi: 10.4049/jimmunol.176.7.4343. [DOI] [PubMed] [Google Scholar]
- 34.Kuo TC, Calame KL. B lymphocyte-induced maturation protein (Blimp)-1, IFN regulatory factor (IRF)-1, and IRF-2 can bind to the same regulatory sites. J Immunol. 2004;173:5556–5563. doi: 10.4049/jimmunol.173.9.5556. [DOI] [PubMed] [Google Scholar]
- 35.Keller AD, Maniatis T. Identification and characterization of a novel repressor of beta-interferon gene expression. Genes Dev. 1991;5:868–879. doi: 10.1101/gad.5.5.868. [DOI] [PubMed] [Google Scholar]
- 36.Tunyaplin C, et al. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J Immunol. 2004;173:1158–1165. doi: 10.4049/jimmunol.173.2.1158. [DOI] [PubMed] [Google Scholar]
- 37.Phan RT, Dalla-Favera R. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature. 2004;432:635–639. doi: 10.1038/nature03147. [DOI] [PubMed] [Google Scholar]
- 38.Lenz G, et al. Aberrant immunoglobulin class switch recombination and switch translocations in activated B cell-like diffuse large B cell lymphoma. J Exp Med. 2007;204:633–643. doi: 10.1084/jem.20062041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnes BJ, Kellum MJ, Pinder KE, Frisancho JA, Pitha PM. Interferon regulatory factor 5, a novel mediator of cell cycle arrest and cell death. Cancer Res. 2003;63:6424–6431. [PubMed] [Google Scholar]
- 40.Yang L, et al. Functional analysis of a dominant negative mutation of interferon regulatory factor 5. PLoS One. 2009;4:e5500. doi: 10.1371/journal.pone.0005500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wies E, et al. The Kaposi's sarcoma-associated herpesvirus-encoded vIRF-3 inhibits cellular IRF-5. J Biol Chem. 2009;284:8525–8538. doi: 10.1074/jbc.M809252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma S, Turetsky A, Trinh L, Lu R. IFN regulatory factor 4 and 8 promote Ig light chain kappa locus activation in pre-B cell development. J Immunol. 2006;177:7898–7904. doi: 10.4049/jimmunol.177.11.7898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.