Abstract
Purpose
To introduce a novel approach for the time-dependent quantification of risk factors for prostate cancer (PCa) detection after an initial negative biopsy.
Patients and Methods
Data for 1,871 men with initial negative biopsies and at least one follow-up biopsy were available. Piecewise exponential regression models were developed to quantify hazard ratios (HRs) and define cumulative incidence curves for PCa detection for subgroups with specific patterns of risk factors over time. Factors evaluated included age, race, serum prostate-specific antigen (PSA) concentration, PSA slope, digital rectal examination, dysplastic glands or prostatitis on biopsy, ultrasound gland volume, urinary symptoms, and number of negative biopsies.
Results
Four hundred sixty-five men had PCa detected, after a mean follow-up time of 2.8 years. All of the factors were independent predictors of PCa detection except for PSA slope, as a result of its correlation with time-dependent PSA level, and race. PSA (HR = 3.90 for > 10 v 2.5 to 3.9 ng/mL), high-grade prostatic intraepithelial neoplasia/atypical glands (HR = 2.97), gland volume (HR = 0.39 for > 50 v < 25 mL), and number of repeat biopsies (HR = 0.36 for two v zero repeat biopsies) were the strongest predictors. Men with high-risk versus low-risk event histories had a 20-fold difference in PCa detection over 5 years.
Conclusion
Piecewise exponential models provide an approach to longitudinal analysis of PCa risk that allows clinicians to see the interplay of risk factors as they unfold over time for individual patients. With these models, it is possible to identify distinct subpopulations with dramatically different needs for monitoring and repeat biopsy.
INTRODUCTION
Because of an early detection marker, prostate-specific antigen (PSA), that is measured in serum and the lack of any practical means for imaging lesions, prostate cancer (PCa) is the only solid tumor that is routinely detected by indirect tissue sampling (ie, without visualization of a clear-cut suspicious lesion). Seventy to 80% of the approximately 1.2 million patients who undergo prostate biopsy each year in the United States receive negative results (ie, no cancer) but cannot be completely reassured because a cancer may have been missed by sampling error. Twelve biopsy cores from an 18-gauge needle allow histologic examination of only approximately 0.04% of a prostate with a fairly typical volume.1 Many patients undergo repeat biopsies because clinicians do not have a clear basis for stratifying individuals who need intensive follow-up and those who do not.
Previous studies have investigated how specific clinical or histologic variables are associated with detection of PCa on subsequent biopsies.1–8 Here, we use longitudinal data from a large cohort to present a new approach to modeling risk of PCa after a negative biopsy. This method uses the piecewise exponential model, which is similar to the Cox regression model for survival analysis in that it enables consideration of time-dependent covariates (ie, risk factors that vary over time) and it can be modified to allow the hazard ratio (HR) for a risk factor to vary with time. However, unlike the standard Cox model, the piecewise model can provide estimates of the actual probability of cancer detection over time for subsets of patients with specific patterns of change in the risk factors. This ability to estimate the probability of cancer detection with time-varying risk profiles inherently resembles how clinicians make decisions as a patient's event history unfolds over time.
Herein, we present estimates of the independent association of routinely available data elements to risk of PCa detection and illustrate how this approach identifies subsets of patients with widely divergent needs for follow-up. The results show that many men with common event histories have a low cumulative risk of PCa detection up to 5 years after a negative biopsy, and thus, clinicians can help these patients by avoiding the cost and psychological distress of unnecessary biopsies.
PATIENTS AND METHODS
All participants were volunteers in a community-based PCa screening study described previously.9–11 We obtained serum from 24,893 men older than age 40 years who were enrolled and observed between May 1991 and November 2001. Transrectal ultrasound–guided biopsy was recommended for elevated PSA values or suspicious digital rectal examination (DRE); men with abnormal PSA or DRE were re-examined in 6 months, whereas men with normal results returned in 1 year. In May 1995, the threshold PSA level for biopsy changed from 4.0 to 2.5 ng/mL, and the minimum number of cores per biopsy increased from four to six cores (mean cores in a representative subset, 8.9 cores). Data on the exact number of cores were not available. Study protocols were approved by the Human Subjects Committee at Washington University, St Louis, MO, and all participants provided informed consent.
The study population for the current analysis includes 1,871 men (5,421 repeat biopsies) whose first biopsy was negative and who had at least one repeat biopsy. This article focuses on models for time to biopsy-confirmed PCa, which are right censored on date of the last negative biopsy for patients without PCa. A piecewise exponential model with time-dependent covariates was fit to the data with the following covariates: age, race, urinary symptoms, ultrasound prostate volume, PSA, PSA slope, an indicator variable for the change in 1995 in PSA threshold for biopsy and number of needle cores per biopsy, time from study entry to first repeat biopsy, number of repeat biopsies, atypical glands or high-grade prostatic intraepithelial neoplasia (HGPIN), chronic prostatitis reported on biopsy, and DRE result. To avoid problems with nonlinearity, most models broke continuous variables, such as serum PSA and gland volume, into ordinal categories. We used PSA cutoff values conventionally used in clinical practice and gland volume cutoff values that roughly divided the population by quartiles. To evaluate possible selection bias, we fit additional models that included participants who did not undergo a repeat biopsy after an initial negative one. These models, in which participants were censored on the date of their last study visit, gave similar results (Appendix, online only).
For analyses presented here, PSA slope was calculated as a time-dependent covariate based on a linear regression of PSA values over a period extending 36 months before a participant's current visit. Atypical glands were defined as cytomorphologic abnormalities in the prostatic epithelium that did not meet strict criteria for HGPIN or PCa. Atypical glands and HGPIN were grouped in final analyses because agreement between pathologists regarding these entities was questionable during the study period and thus misclassification could be substantial. Patients considered positive for urinary symptoms reported at least one of the following: frequency, urgency, or reduced stream. In the final models, some variables (urinary symptoms, suspicious DRE, prostatitis, or HGPIN/atypical glands) are treated as absorbing features, meaning their value remains constant throughout follow-up once they occur. These variables reflect conditions that are presumably long lasting or permanent; however, they may not be detected on subsequent visits and thus may be misclassified if treated as time dependent.
The piecewise exponential model, easily implemented using existing software, incorporates covariates into an actuarial life-table approach to survival analysis in the same way that the Cox model incorporates covariates into a Kaplan-Meier approach. Both methods have been shown to yield nearly equivalent results.12 Under a piecewise model, follow-up time is partitioned into discrete nonoverlapping intervals within which the event rate is assumed to be constant but allowed to vary across intervals. To achieve optimal efficiency, we partitioned time into nine nonoverlapping intervals having roughly equal numbers of events per interval.13 Estimation was carried out using maximum likelihood as implemented in the NLMIXED procedure of SAS (SAS Institute, Cary NC). Time-dependent variables were updated on the actual date of change rather than the beginning or end date of the relevant time interval. To depict curves comparing the cumulative incidence of PCa among patient subgroups, we created hypothetical event histories and used parameter estimates from the model to compute cumulative incidence curves over time.
RESULTS
Selected features of the study population are listed in Table 1. The mean number of biopsies (sessions, not cores) performed per patient, including the initial biopsy, was 2.9. Analysis of bivariate relationships between cancer detection and key predictors at successive biopsies (Appendix Table A1, online only) revealed that the percentage of positive biopsies decreased only slightly from the second to the fifth biopsy and beyond, illustrating how strongly bivariate results are confounded by differences in the risk profile of the population as the biopsy number increases. In a previous analysis, we reported that the Gleason grade did not vary significantly according to the number of biopsies performed.14
Table 1.
Selected Clinical Characteristics of Patients Who Underwent Repeat Biopsies
| Characteristic | No. of Patients (N = 1,871) | % |
|---|---|---|
| Prostate cancer detected | 465 | 24.9 |
| < 2 years of follow-up | 253 | 54.4 |
| 2-5 years of follow-up | 148 | 31.8 |
| > 5 years of follow-up | 64 | 13.8 |
| Race/ethnicity | ||
| White | 1,745 | 93.3 |
| Black | 107 | 5.7 |
| Other | 9 | 0.5 |
| PSA at baseline, ng/mL* | ||
| < 2.5 | 437 | 23.4 |
| 2.5-3.9 | 537 | 28.7 |
| 4.0-6.9 | 689 | 36.8 |
| 7.0-9.9 | 131 | 7.0 |
| ≥ 10.0 | 72 | 3.8 |
| Digital rectal examination at baseline | ||
| Suspicious for cancer | 650 | 34.7 |
| Not suspicious | 1,217 | 65.3 |
| Baseline biopsy histology | ||
| HGPIN or atypical glands† | 252 | 13.5 |
| Prostatitis | 221 | 11.8 |
| Urinary symptoms at baseline‡ | 746 | 39.9 |
| Age at baseline, years | ||
| Mean | 62.2 | |
| SD | 7.4 | |
| Median | 62.0 | |
| Range | 41.0-87.0 | |
| Follow-up time, years | ||
| Mean | 2.8 | |
| SD | 2.4 | |
| Median | 2.0 | |
| Range | 0.4-10.4 | |
| Total No. of biopsies§ | ||
| Mean | 2.9 | |
| SD | 1.4 | |
| Median | 2.0 | |
| Range | 2.0-11.0 | |
| Time between initial and repeat biopsy, months | ||
| Mean | 18.3 | |
| SD | 19.6 | |
| Median | 12.2 | |
| Range | 0.5-121.3 | |
| No. of PSA tests before baseline biopsy | ||
| Mean | 3.1 | |
| SD | 3.0 | |
| Median | 2.0 | |
| Range | 0-16.0 | |
| Gland volume by TRUS, mL | ||
| Mean | 44.6 | |
| SD | 24.4 | |
| Median | 39.0 | |
| Range | 9.0-187.0 | |
Abbreviations: PSA, prostate-specific antigen; HGPIN, high-grade prostatic intraepithelial neoplasia; SD, standard deviation; TRUS, transrectal ultrasound.
Baseline was defined as the date of the initial negative prostate biopsy.
Atypical glands refer to glands with an intact basement membrane that are variable in size or shape with little or no interposed stroma between them. The lining of these glands, unlike the normal two-cell layered epithelium containing basal and luminal cells, may contain either a single layer of cells with prominent, eosinophilic nuclei or a proliferative glandular epithelium with papillae, sometimes progressing to a cribriform pattern. Often, they contain many but not all the features characteristic of carcinoma or are too small to justify a definitive diagnosis of cancer.
Urinary symptoms were defined as frequency, urgency, or reduced stream.
Note that the analyses were restricted to patients who had at least one repeat biopsy. Eight hundred thirty-five patients (44.6%) had more than one repeat biopsy.
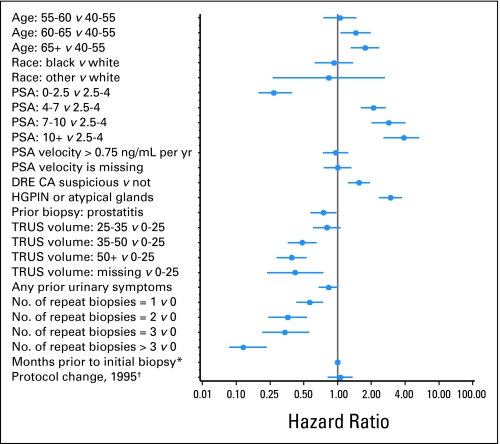
HRs and 95% CIs from a multivariable piecewise exponential model are listed in Table 2 and shown in Figure 1. In the first 3 months after an initial negative biopsy, the cancer detection rate among baseline control participants (whose covariates have HR = 1.00) was 1.08 events per 100 person-years at risk. This baseline rate increased by two-fold over the next 4 years and then increased significantly again to approximately four events per 100 person-years during the fifth year (HR = 3.78). Age, suspicious DRE, HGPIN/atypical glands, and time-dependent serum PSA were positively and significantly associated with risk. PSA slope was not associated with risk unless time-dependent PSA was removed from the model; PSA slope was also significant when baseline PSA was included in the model. Prostatitis on biopsy, the number of negative biopsies, and gland volume more than 35 mL all were inversely associated with PCa detection. There were strong linear trends for decreasing risk at successively larger gland volumes. An inverse association for the presence of urinary symptoms approached statistical significance (P = .07). Note that changes in the study protocol in 1995 had no independent effect on risk of PCa detection.
Table 2.
Hazard Ratio Estimates From a Multivariable Piecewise Exponential Survival Model: Right-Censored Model With End Point of Time to Detection of Prostate Cancer
| Covariate | Hazard Ratio | 95% CI | Probabilities of Remaining Cancer Free at Each Time Interval |
|---|---|---|---|
| Follow-up time, months | |||
| 0-3 | 1.00 | — | 0.990 |
| 3-6 | 2.31 | 1.27 to 3.34 | 0.968 |
| 6-9 | 2.09 | 1.10 to 3.09 | 0.948 |
| 9-12 | 1.43 | 0.65 to 2.21 | 0.934 |
| 12-24 | 2.10 | 1.20 to 3.00 | 0.859 |
| 24-36 | 2.04 | 1.07 to 3.01 | 0.792 |
| 36-48 | 1.67 | 0.79 to 2.54 | 0.741 |
| 48-60 | 3.78 | 1.92 to 5.64 | 0.637 |
| > 60 | 3.33 | 1.70 to 4.95 | — |
| Age, years | |||
| 40-54 | 1.00 | — | |
| 55-59 | 1.06 | 0.76 to 1.48 | |
| 60-64 | 1.46 | 1.07 to 1.98 | |
| ≥ 65 | 1.76 | 1.32 to 2.35 | |
| Race | |||
| White | 1.00 | — | |
| Black | 0.93 | 0.63 to 1.37 | |
| Other | 0.84 | 0.27 to 2.63 | |
| PSA, ng/mL | |||
| < 2.5 | 0.27 | 0.19 to 0.39 | |
| 2.5-3.9 | 1.00 | — | |
| 4.0-6.9 | 2.10 | 1.63 to 2.70 | |
| 7.0-9.9 | 2.87 | 2.02 to 4.06 | |
| ≥ 10.0 | 3.90 | 2.59 to 5.87 | |
| PSA slope, ng/mL/yr | |||
| < 0.75 | 1.00 | — | |
| ≥ 0.75 | 0.97 | 0.75 to 1.25 | |
| Missing | 1.01 | 0.76 to 1.33 | |
| DRE | |||
| Not suspicious | 1.00 | — | |
| PCa suspicious* | 1.56 | 1.25 to 1.95 | |
| HGPIN or atypical glands* | |||
| No | 1.00 | — | |
| Yes | 2.97 | 2.35 to 3.77 | |
| Prostatitis present* | |||
| No | 1.00 | — | |
| Yes | 0.75 | 0.58 to 0.97 | |
| TRUS volume, mL | |||
| < 25 | 1.00 | — | |
| 25-34 | 0.81 | 0.61 to 1.07 | |
| 35-49 | 0.49 | 0.36 to 0.65 | |
| ≥ 50 | 0.39 | 0.29 to 0.53 | |
| Missing | 0.42 | 0.23 to 0.75 | |
| Prior urinary symptoms* | |||
| No | 1.00 | — | |
| Yes | 0.83 | 0.68 to 1.02 | |
| No. of repeat negative biopsies | |||
| 0 | 1.00 | — | |
| 1 | 0.57 | 0.43 to 0.74 | |
| 2 | 0.36 | 0.24 to 0.54 | |
| 3 | 0.34 | 0.21 to 0.56 | |
| > 3 | 0.12 | 0.06 to 0.23 | |
| Months prior to initial biopsy | 1.00 | 1.00 to 1.01 | |
| Protocol changes in 1995† | |||
| No | 1.00 | — | |
| Yes | 1.06 | 0.83 to 1.37 |
Abbreviations: PSA, prostate-specific antigen; DRE, digital rectal examination; PCa, prostate cancer; HGPIN, high-grade prostatic intraepithelial neoplasia; TRUS, transrectal ultrasound.
These variables are absorbing; once they occur, the patient remains positive for that factor throughout the remainder of follow-up.
In May 1995, the minimum number of biopsy cores increased from four to six cores, and PSA threshold for initial biopsy decreased from 4.0 to 2.5 ng/mL.
Fig 1.
Hazard ratios and 95% CIs for risk of prostate cancer after an initial negative biopsy—right-censored model (n = 1,871). (*) Number of months from first study visit to initial biopsy (baseline date for this analysis). (†) Binary variable to indicate change in May 1995 in minimum number of biopsy cores (four to six cores) and prostate-specific antigen (PSA) threshold for biopsy (4.0 to 2.5 ng/mL). DRE, digital rectal examination; CA, cancer; HGPIN, high-grade prostatic intraepithelial neoplasia; TRUS, transrectal ultrasound.
Controlling for all other risk factors, there was a sharp decline in risk after the first repeat biopsy and a strong downward trend with successive negative biopsies. The rate of cancer detection was significantly lower (by 37%) among men who had a second repeat negative biopsy compared with men who had only one biopsy over the same course of follow-up. After a third repeat biopsy, the relative hazard was reduced by 88% compared with men with no repeat negative biopsies and by 79% compared with men with one repeat biopsy. These results suggest that additional biopsies after a second negative biopsy are usually not beneficial, assuming that other time-varying risk factors remain constant.
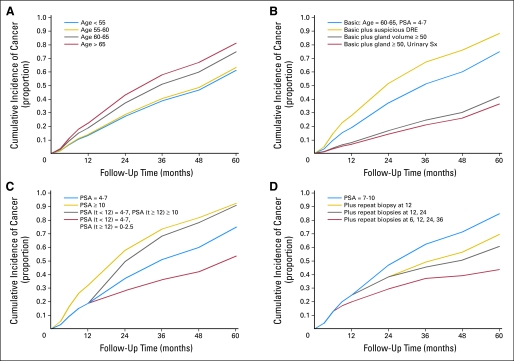
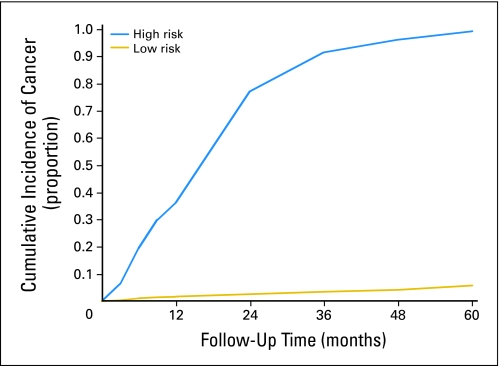
Figures 2 to 4 depict cumulative incidence curves illustrating the effects of various event histories on PCa detection. Certain combinations of risk factors produce dramatic contrasts in cancer risk. We compiled features into arbitrary high- and low-risk histories based on initial PSA, PSA increase, HGPIN, gland volume, and prostatitis. The high- and low-risk groups in Figure 4 have 5-year cumulative risks of greater than 95% and less than 5%, respectively.
Fig 2.
Cumulative incidence of prostate cancer with varying (A) age; (B) digital rectal examination (DRE), gland volume, and urinary symptoms (Sx); (C) prostate-specific antigen (PSA); and (D) number of negative biopsies. Each graph includes a basic event history curve shown with a blue line and alternative histories are shown with gold, gray, and red lines. All covariates not mentioned are set to reference levels as in Table 2. Note the small effect of age and DRE relative to PSA and gland volume and that for men with persistent PSA of 7 to 10 ng/mL, risk remains elevated after several negative biopsies.
Fig 4.
Cumulative incidence curves for prostate cancer detection among men age 55 to 60 years with high- or low-risk event histories. High risk is defined as gland volume less than 25 mL, prostate-specific antigen (PSA) increasing from 4 to 7 ng/mL to more than 10 ng/mL at 12 months, and high-grade prostatic intraepithelial neoplasia/atypical glands at baseline. Low risk is defined as gland volume more than 50 mL, PSA of 2.5 to 4 ng/mL and steady, prostatitis on initial biopsy, and negative biopsies at 6 and 12 months.
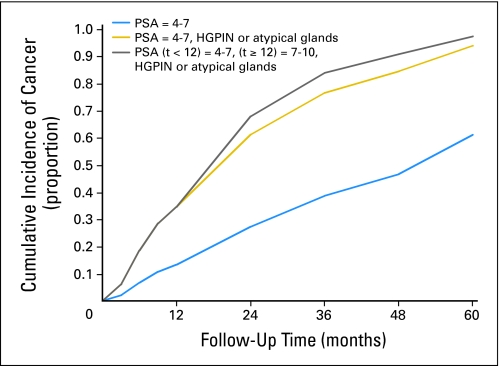
Fig 3.
Cumulative incidence curves for prostate cancer detection among men less than age 55 years with prostate-specific antigen (PSA) of 4 to 7 ng/mL at baseline. Each graph includes a basic event history curve shown with a blue line and alternative histories shown with gold and gray lines. All covariates not mentioned are set to reference levels as in Table 2. High-grade prostatic intraepithelial neoplasia (HGPIN)/atypical glands on initial biopsy increases cumulative risk by approximately 40% at 4 years. A PSA increase at 12 months adds a small amount of additional risk; however, absolute risk is already asymptotically approaching 100%.
DISCUSSION
False-positive results are an inevitable consequence of current practices for screening and early detection of PCa. For men who have a negative prostate biopsy, the identification of risk factors for subsequent detection of cancer and the quantification of actual risk remain significant concerns. In this report, we demonstrated that piecewise exponential modeling, a statistical approach not previously applied to this problem, can estimate the probability of cancer detection for subgroups of men whose risk factor patterns vary in specific ways as time elapses after a negative biopsy. Several factors considered in previous studies, including age, elevated total PSA, suspicious DRE, smaller gland volume, presence of HGPIN or atypical features on biopsy, and number of previous biopsies, were all independently associated with risk. Moreover, two simple variables rarely considered in earlier analyses—presence of urinary symptoms and histologic evidence of prostatitis—were independent, negative risk factors.
These models can identify patient subgroups with unusually high or low risk after a negative biopsy. To illustrate, a 55-year-old man with a large gland, stable PSA less than 4 ng/mL, evidence of prostatitis, and two additional negative biopsies has a predicted 5-year risk of approximately 4%, more than 20 times lower than a similar-age man with a small gland, increasing PSA, and atypical biopsy findings. In fact, the former patient's absolute risk is comparable to that of a man randomly selected from the US general population; based on Surveillance, Epidemiology, and End Results data for 2001 to 2005, the 5-year cumulative incidence for a man (white) age 55 years is approximately 2%. Therefore, common event histories can have sufficiently high negative predictive value to discourage close patient follow-up or additional biopsies. Although PCa detection decreased substantially after the initial negative biopsy, there were participants whose event histories indicated an uncomfortably high risk after two negative biopsies. The crude probability of detecting PCa on successive biopsies is a function of opposing forces—the culling out of larger tumors on earlier biopsies, which decreases subsequent yield, and the tendency for patients who receive more biopsies to have stronger risk factors and a longer time for tumors to grow, which pushes yield upward. Although this report focuses on identification of risk factors and presentation of the method, ongoing analyses are quantifying the accuracy of these models in predicting outcomes for individual patients.
Serum PSA level, updated over time, was one of the strongest predictors of PCa on repeat biopsy, with a 14-fold risk difference between patients with PSA less than 2.5 ng/mL versus more than 10 ng/mL. In agreement with others, we found that the association of PSA velocity with risk virtually disappeared when updated PSA was included in the model.7 HGPIN was another strong predictor, in contrast to recent reports that cancer prevalence on repeat biopsy was only marginally greater among men with HGPIN versus men with completely benign findings.15,16 The prognostic significance of isolated HGPIN may have declined as a result of more intensive biopsy sampling schemes and the tendency for repeated PSA testing to cull out larger tumors in screened populations. However, other recent studies using multivariable adjustment techniques identified HGPIN as a risk factor for PCa on repeat biopsy, leading us to speculate that this association may be obscured by confounding in some cohorts.7,8,17 Indeed, our unadjusted results show only a slightly increased cancer prevalence at the second biopsy among men with HGPIN/atypical glands at initial biopsy (Appendix Table A1). The term atypical glands encompasses a range of cytomorphologic lesions that lie below the threshold for a cancer diagnosis as a result of their small size or lack of a basement membrane. This categorization preceded the designation of atypical small acinar proliferation, which may be more strongly associated with cancer on subsequent biopsy.18 In fact, the HRs for HGPIN and atypical glands were similar in our data, and thus, we combined them. Carver et al17 reported a lower HR for atypical small acinar proliferation compared with HGPIN, suggesting that the former category as used by most pathologists is probably itself rather heterogeneous.
A reduction in cancer detection is expected when randomly sampling a larger volume gland; however, given the imprecision of the ultrasound measurements, the influence of gland volume (a 61% difference in risk between extreme quartiles) was surprisingly strong. Previous investigators reported higher PSA density (PSA divided by gland volume) as a risk factor on repeat biopsy; however, considering PSA and volume separately allows ascertainment of their independent effects.6,19 Although PSA density based on transition zone volume might better reflect benign prostatic hyperplasia as a cause for PSA elevation,4,20 transition zone and total volume are highly correlated, and there are problems with accurately measuring transition zone volume in everyday practice. However, the presence of urinary voiding symptoms has rarely been considered as having predictive value. A recent cross-sectional study on initial biopsy results observed a strong inverse association between the American Urological Association Symptom Score and odds of PCa detection.21 Our results indicate that even a crude measure of symptoms can identify men whose PSA elevations are more likely a result of benign prostatic hyperplasia than cancer.
Few studies have considered prostatitis, another alternate explanation for PSA elevation, although a recent small study reported that inflammation approached statistical significance as a negative predictor.22 This does not contradict the hypothesis that inflammation is important in the early development of PCa.23 Chronic prostatic inflammation is often diffuse, heterogeneously distributed, or quite subtle when viewed with conventional stains; hence, biopsies recorded as showing inflammation may have been especially pronounced. Moreover, the etiologic effect of inflammation on cancer development may precede the initial biopsy by decades. A PSA decrease after antibiotic therapy has been observed, although PSA was not associated with inflammatory findings on negative biopsies in one study.24,25 Nevertheless, more accurate methods for reporting inflammation on prostate biopsies could yield important information regarding both short-term and long-term cancer risk.
To our knowledge, this is the largest prospective analysis of repeat prostate biopsy to date, and the number of factors found to be independently associated with PCa detection (nine factors) is noteworthy. This suggests how complex it is for physicians to estimate risk during patient counseling, particularly as the risk profile changes over time. Logistic regression, the most common modeling approach in previous studies, is limited because it ignores variation in the time to cancer detection and is unable to account for changes in risk factors over time. Logistic regression can also lead to confusing results if it is not clear whether the outcome refers to cancer on a particular (eg, first repeat) biopsy or over a series of biopsies. For example, a previous study from our data set reported that the number of biopsies was positively associated with ultimate PCa status, which makes sense because each biopsy increases the likelihood of finding cancer.26 Machine learning techniques (eg, artificial neural networks) are free of modeling assumptions and are more capable of uncovering complex interactions between variables; however, they have other disadvantages and do not provide better results for this particular problem than equation-based methods.6,27,28 The approach most similar to ours is Cox regression with time-dependent covariates, which, to our knowledge, has been used in only two previous studies.7,17
Apart from its substantial statistical power, this analysis benefited from a relatively uniform evaluation protocol in an academic setting and a high degree of continuity and compliance. In this sense, the study represents a proof-of-concept setting for discovering relationships between risk factors and PCa detection; generalizability of our results to community practice populations remains to be determined. We were unable to include some potentially interesting factors such as serum free PSA and family history because of excessive missing data. However, family history was a relatively weak risk factor in studies of either initial or repeat biopsies.7,21 We were also unable to include some biopsy details, such as the exact number of cores or number of cores containing HGPIN.29 The great majority of study participants were white, and thus, we were unable to determine how results might differ in nonwhite men. Finally, our results focus on detection of all PCa; however, it is also important to focus on cancers that are most likely to be aggressive. Ongoing work addresses this, as well as the validation of clinical prediction models and the incorporation of molecular markers as risk indicators in histologically benign tissue.30–32
Welch et al33 reported that 44% of men age 65 to 70 years with an initial negative prostate biopsy undergo a repeat biopsy within 5 years, a percentage that is undoubtedly higher for younger men. Thus, the number of repeat biopsies performed in the United States and the associated costs, in both financial and emotional terms, are formidable. Approaches based on one or two risk factors may reduce biopsies at the expense of failure to detect some patients with cancer whose outcomes could have been altered by early intervention. A more detailed understanding of PCa risk after a negative biopsy, based on the preceding history of each individual patient, holds the most promise for reducing negative biopsies while maintaining adequate sensitivity. At least part of the consistent decline in PCa mortality over the last dozen years is attributable to earlier detection and treatment; however, sustaining or expanding that benefit at a lower cost remains a major priority for research.34 See the Appendix for additional discussion.
Acknowledgment
We gratefully acknowledge Kimberly Roehl, Alfred Rademaker, and Xiaoying Yu for their contributions to this work.
Appendix
Exclusion of Patients Who Did Not Receive Any Repeat Biopsies
The analysis featured in this article included only those patients with an initial negative biopsy who underwent at least one additional biopsy. This means there was at least one opportunity to determine their prostate cancer (PCa) status during follow-up. After a negative biopsy, patients were allowed to remain under follow-up even if they waived a recommended repeat biopsy or had a recommendation rescinded as a result of a change in indication. In Roehl et al,14 we reported that men who underwent at least one repeat biopsy were more likely to have a prostate-specific antigen (PSA) increase or a suspicious digital rectal examination than men who waived all repeat biopsies. However, this fact alone would not introduce selection bias unless the actual degree of association between a predictor and PCa detection was different between men who were included versus excluded in our analyses. To test for selection bias, we fit separate models that included all 3,544 patients who had at least one negative biopsy, right-censoring at the date of their last study visit. The results were similar to those presented here; for example, the hazard ratios for PSA categories of 4.0 to 6.9, 7.0 to 9.9, and ≥ 10 ng/mL (relative to 2.5 to 3.9 ng/mL) were 2.24, 2.82, and 4.24, respectively, close to the results listed in Table 2. Although the relative hazard estimates were similar, the survival time distribution for the expanded data set was obviously different because after their last biopsy, patients contributed additional time at risk with no end point. The continued follow-up of men who did not undergo a repeat biopsy after an initial negative one is a strength of our study because it provides important additional data for modeling the effect of the covariates on PCa detection. However, we are primarily interested in modeling the likelihood of PCa detection assuming that additional biopsies will be performed; thus, we chose the restricted data set for the main analysis.
Right-Censored Versus Interval-Censored Models
Although this report deals with right-censored models of time to PCa detection, we also fit similar models that used interval-censored time to PCa detection. This latter approach assumes that PCa becomes detectable at some point during the interval between a positive and negative biopsy. Models for interval-censored event times are commonly used when the event of interest is determined at scheduled visits. Although not shown, the interval-censored results were similar to those presented here; a separate methodologic report will focus on a comparison of these two approaches. It should be noted that both the right-censored and interval-censored analyses assume that the underlying censoring mechanism is noninformative (discussed further later in the Appendix).
Timing of Repeat Biopsies: Informative Versus Noninformative Censoring
Similar to the concern about bias as a result of patients failing to undergo a repeat biopsy, there also may be concern about biased risk estimates for factors that influence the urologist's decision about timing of a repeat biopsy. Under certain circumstances, the association between a time-dependent risk factor and an end point can be affected by the timing of examinations performed to ascertain the end point. In our main analysis, patients were right censored at the time of their last biopsy. If timing of a future biopsy is dependent on a patient's actual end point status at that future time, then the examination timing scheme (and the related censoring mechanism) is said to be informative; in this case, survival and hazard functions cannot be estimated without taking additional data (which may not be available) into account. For example, if a patient requests a biopsy because he is experiencing symptoms as a result of PCa, then the examination timing is informative (ie, not independent of end point status at the time of censoring). However, if the timing of the examinations (and the last biopsy in particular) is determined at random or at fixed, preassigned intervals, then the examination scheme is obviously noninformative. Similarly, as discussed in Gruger et al (Gruger J, Kay R, Schumacher M. Biometrics 47:595-605, 1991), decisions about timing of examinations made under a doctor's care would represent a noninformative censoring mechanism. In the screening cohort and in community practice, a urologist may decide to schedule an early repeat biopsy in a patient with elevated PSA or a high-grade prostatic intraepithelial neoplasia on a prior biopsy. This decision, in and of itself, would not lead to biased estimates of association between PSA or high-grade prostatic intraepithelial neoplasia and PCa detection because the timing of that next biopsy in this case is not determined by other unmeasured covariates that are related to the patient's true underlying disease state.
For example, if a urologist believed—implausibly—that left-handedness was a risk factor for having a detectable PCa, then scheduling earlier repeat biopsies for left-handed men would not mean that the probability of PCa detection at that future time would be any greater for left-handed versus right-handed men, unless handedness was a true risk indicator. Although biopsy timing decisions in our analysis did take covariate histories into account, patients did not influence the timing of biopsies as a result of symptoms; PCa was asymptomatic at presentation in this screening cohort. Therefore, we conclude that it is reasonable to assume that the censoring mechanism used in our analyses was noninformative and hence unbiased.
Additional Notes on Age and Follow-Up Time As Predictors
Some previous reports,5,8 but not all,6,7 identified increasing age as a positive predictor of PCa on repeat biopsy. The moderate trend we observed for age is plausible because, once confounding variables are controlled, the probability of harboring undetected cancer obviously increases with age. Our results also indicate, for the first time to our knowledge, that risk varies over follow-up time when all predictors are held constant, notably with a significant increase in risk after 4 years, which is logically consistent with the emergence of newly detectable lesions.
Additional Notes on PSA Velocity
Our finding that PSA velocity does not predict PCa detection when time-dependent PSA is included simultaneously in models is also consistent with a large Swedish study that reported that PSA velocity and most recent PSA were highly correlated and that velocity did not improve long-term prediction of PCa once recent PSA was considered (Ulmert D, Serio AM, O'Brien MF, et al. J Clin Oncol 26:835-841, 2008). PSA velocity, time-dependent PSA, and most recent PSA are not identical, however, and the relative performance of these indicators for predicting an outcome may depend on the context. Recent data suggest that velocity is a particularly useful indicator of PCa aggressiveness (Carter HB, Ferrucci L, Kettermann A, et al. J Natl Cancer Inst 98:1521-1527, 2006; D'Amico AV, Chen MH, Roehl KA, et al. N Engl J Med 351:125-135, 2004), and in the context of cancer prediction after a negative biopsy, we found that PSA velocity was a significant predictor when combined in a model with baseline rather than time-dependent PSA. Alternative formulations for PSA velocity gave similar results.
Table A1.
Diagnosis of Prostate Cancer According to Clinical Characteristics of Patients Before Successive Prostate Biopsies
| Characteristic | Biopsy 2 |
Biopsy 3 |
Biopsy 4 |
Biopsy 5+* |
||||
|---|---|---|---|---|---|---|---|---|
| No. of Patients With Cancer/Total No. of Patients | % | No. of Patients With Cancer/Total No. of Patients | % | No. of Patients With Cancer/Total No. of Patients | % | No. of Patients With Cancer/Total No. of Patients | % | |
| All patients | 260/1,871 | 14 | 117/835 | 14 | 47/410 | 11 | 25/208 | 12 |
| Age, years | ||||||||
| 40-54 | 38/340 | 11 | 23/141 | 16 | 3/62 | 5 | 2/30 | 7 |
| 55-59 | 46/358 | 13 | 14/164 | 9 | 10/88 | 11 | 5/44 | 11 |
| 60-64 | 65/447 | 15 | 27/201 | 13 | 10/93 | 11 | 7/41 | 17 |
| ≥ 65 | 111/726 | 15 | 53/329 | 16 | 24/167 | 14 | 11/93 | 12 |
| PSA, ng/mL | ||||||||
| < 2.5 | 30/360 | 8 | 11/77 | 14 | 1/25 | 4 | 1/4 | 25 |
| 2.5-3.9 | 68/522 | 13 | 22/222 | 10 | 11/93 | 12 | 8/44 | 18 |
| 4.0-6.9 | 115/740 | 16 | 60/388 | 15 | 23/189 | 12 | 10/95 | 11 |
| 7.0-9.9 | 27/163 | 17 | 18/93 | 19 | 8/65 | 12 | 4/37 | 11 |
| ≥ 10.0 | 19/83 | 23 | 6/54 | 11 | 4/38 | 11 | 2/28 | 7 |
| PSA slope, ng/mL/yr | ||||||||
| < 0.75 | 125/1,129 | 11 | 77/600 | 13 | 31/293 | 11 | 19/156 | 12 |
| ≥ 0.75 | 59/409 | 14 | 34/216 | 16 | 15/111 | 14 | 6/49 | 12 |
| Missing | 76/333 | 23 | 6/19 | 32 | 1/6 | 17 | 0/3 | 0 |
| DRE | ||||||||
| Not suspicious | 166/1,140 | 15 | 72/526 | 14 | 29/256 | 11 | 16/124 | 13 |
| Suspicious | 94/731 | 13 | 45/309 | 15 | 18/154 | 12 | 9/84 | 11 |
| HGPIN/atypical glands | ||||||||
| No | 205/1,448 | 14 | 58/491 | 12 | 13/197 | 7 | 7/93 | 8 |
| Yes | 55/423 | 13 | 59/344 | 17 | 34/213 | 16 | 18/115 | 16 |
| Prostatitis present | ||||||||
| No | 224/1,497 | 15 | 89/614 | 14 | 33/275 | 12 | 18/128 | 14 |
| Yes | 36/374 | 10 | 28/221 | 13 | 14/135 | 10 | 7/80 | 9 |
| TRUS volume, mL | ||||||||
| < 25 | 56/330 | 17 | 30/117 | 26 | 7/49 | 14 | 3/17 | 18 |
| 25-34 | 80/471 | 17 | 34/206 | 17 | 11/68 | 16 | 6/37 | 16 |
| 35-49 | 71/533 | 13 | 27/249 | 11 | 19/152 | 13 | 9/65 | 14 |
| ≥ 50 | 49/517 | 9 | 24/261 | 9 | 10/141 | 7 | 7/89 | 8 |
| Urinary symptoms | ||||||||
| No | 186/1,120 | 17 | 63/474 | 13 | 24/226 | 11 | 16/113 | 14 |
| Yes | 74/751 | 10 | 54/361 | 15 | 23/184 | 13 | 9/95 | 9 |
Abbreviations: PSA, prostate-specific antigen; DRE, digital rectal examination; HGPIN, high-grade prostatic intraepithelial neoplasia; TRUS, transrectal ultrasound.
For patients with five or more biopsies, risk factor information at biopsy 5 is used.
Footnotes
Supported in part by the Urological Research Foundation and by Prostate Specialized Program of Research Excellence Grant No. P50 CA90386-05S2 and Robert H. Lurie Comprehensive Cancer Center Grant No. P30 CA60553 from the National Institutes of Health/National Cancer Institute.
Presented in part at the 103rd Annual Meeting of the American Urological Association, Orlando, FL, May 17-22, 2008.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: William J. Catalona, Beckman Coulter (C), deCODE Genetics (U), Nanosphere (U), Ohmx Corporation (U) Stock Ownership: None Honoraria: William J. Catalona, Beckman Coulter, Imodex, GlaxoSmithKline Research Funding: William J. Catalona, Beckman Coulter, deCODE Genetics, Ohmx Corporation Expert Testimony: None Other Remuneration: William J. Catalona, Astellas Pharma
AUTHOR CONTRIBUTIONS
Conception and design: Peter H. Gann, Edward Vonesh
Administrative support: Peter H. Gann
Provision of study materials or patients: William J. Catalona
Collection and assembly of data: Peter H. Gann, Angela Fought, Ryan Deaton, Edward Vonesh
Data analysis and interpretation: Peter H. Gann, Angela Fought, Ryan Deaton, William J. Catalona, Edward Vonesh
Manuscript writing: Peter H. Gann, Angela Fought, William J. Catalona, Edward Vonesh
Final approval of manuscript: Peter H. Gann, Angela Fought, Ryan Deaton, William J. Catalona, Edward Vonesh
REFERENCES
- 1.Fleshner NE, O'Sullivan M, Fair WR. Prevalence and predictors of a positive repeat transrectal ultrasound guided needle biopsy of the prostate. J Urol. 1997;158:505–508. [PubMed] [Google Scholar]
- 2.Catalona WJ, Beiser JA, Smith DS. Serum free prostate specific antigen and prostate specific antigen density measurements for predicting cancer in men with prior negative prostatic biopsies. J Urol. 1997;158:2162–2167. doi: 10.1016/s0022-5347(01)68187-4. [DOI] [PubMed] [Google Scholar]
- 3.Chun FK, Briganti A, Graefen M, et al. Development and external validation of an extended repeat biopsy nomogram. J Urol. 2007;177:510–515. doi: 10.1016/j.juro.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 4.Djavan B, Zlotta A, Remzi M, et al. Optimal predictors of prostate cancer on repeat prostate biopsy: A prospective study of 1,051 men. J Urol. 2000;163:1144–1148. [PubMed] [Google Scholar]
- 5.Fowler JE, Jr, Bigler SA, Miles D, et al. Predictors of first repeat biopsy cancer detection with suspected local stage prostate cancer. J Urol. 2000;163:813–818. [PubMed] [Google Scholar]
- 6.Garzotto M, Park Y, Mongoue-Tchokote S, et al. Recursive partitioning for risk stratification in men undergoing repeat prostate biopsies. Cancer. 2005;104:1911–1917. doi: 10.1002/cncr.21420. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Corona E, Ohori M, Scardino PT, et al. A nomogram for predicting a positive repeat prostate biopsy in patients with a previous negative biopsy session. J Urol. 2003;170:1184–1188. doi: 10.1097/01.ju.0000087451.64657.fa. [DOI] [PubMed] [Google Scholar]
- 8.O'Dowd GJ, Miller MC, Orozco R, et al. Analysis of repeated biopsy results within 1 year after a noncancer diagnosis. Urology. 2000;55:553–559. doi: 10.1016/s0090-4295(00)00447-7. [DOI] [PubMed] [Google Scholar]
- 9.Catalona WJ, Smith DS, Ornstein DK. Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/mL and benign prostate examination: Enhancement of specificity with free PSA measurements. JAMA. 1997;277:1452–1455. [PubMed] [Google Scholar]
- 10.Catalona WJ, Smith DS, Ratliff TL, et al. Detection of organ-confined prostate cancer is increased through prostate-specific antigen-based screening. JAMA. 1993;270:948–954. [PubMed] [Google Scholar]
- 11.Catalona WJ, Smith DS, Wolfert RL, et al. Evaluation of percentage of free serum prostate-specific antigen to improve specificity of prostate cancer screening. JAMA. 1995;274:1214–1220. [PubMed] [Google Scholar]
- 12.Vonesh EF, Schaubel DE, Hao W, et al. Statistical methods for comparing mortality among ESRD patients: Examples of regional/international variations. Kidney Int. 2000;57(suppl):S19–S27. [Google Scholar]
- 13.Allison PD. Cary, NC: SAS Institute; 1995. Survival Analysis Using the SAS System: A Practical Guide. [Google Scholar]
- 14.Roehl KA, Antenor JA, Catalona WJ. Serial biopsy results in prostate cancer screening study. J Urol. 2002;167:2435–2439. [PubMed] [Google Scholar]
- 15.Gokden N, Roehl KA, Catalona WJ, et al. High-grade prostatic intraepithelial neoplasia in needle biopsy as risk factor for detection of adenocarcinoma: Current level of risk in screening population. Urology. 2005;65:538–542. doi: 10.1016/j.urology.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Vis AN, Hoedemaeker RF, Roobol M, et al. The predictive value for prostate cancer of lesions that raise suspicion of concomitant carcinoma: An evaluation from a randomized, population-based study of screening for prostate cancer. Cancer. 2001;92:524–534. doi: 10.1002/1097-0142(20010801)92:3<524::aid-cncr1351>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 17.Carver BS, Bozeman CB, Simoneaux WJ, et al. Race is not a predictor of prostate cancer detection on repeat prostate biopsy. J Urol. 2004;172:1853–1855. doi: 10.1097/01.ju.0000141248.28904.fd. [DOI] [PubMed] [Google Scholar]
- 18.Bostwick DG, Meiers I. Atypical small acinar proliferation in the prostate: Clinical significance in 2006. Arch Pathol Lab Med. 2006;130:952–957. doi: 10.5858/2006-130-952-ASAPIT. [DOI] [PubMed] [Google Scholar]
- 19.Eggener SE, Roehl KA, Catalona WJ. Predictors of subsequent prostate cancer in men with a prostate specific antigen of 2.6 to 4.0 ng/ml and an initially negative biopsy. J Urol. 2005;174:500–504. doi: 10.1097/01.ju.0000165203.40973.0f. [DOI] [PubMed] [Google Scholar]
- 20.Singh H, Canto EI, Shariat SF, et al. Predictors of prostate cancer after initial negative systematic 12 core biopsy. J Urol. 2004;171:1850–1854. doi: 10.1097/01.ju.0000119667.86071.e7. [DOI] [PubMed] [Google Scholar]
- 21.Nam RK, Toi A, Klotz LH, et al. Assessing individual risk for prostate cancer. J Clin Oncol. 2007;25:3582–3588. doi: 10.1200/JCO.2007.10.6450. [DOI] [PubMed] [Google Scholar]
- 22.Sajadi KP, Kim T, Terris MK, et al. High yield of saturation prostate biopsy for patients with previous negative biopsies and small prostates. Urology. 2007;70:691–695. doi: 10.1016/j.urology.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Nelson WG, DeWeese TL, DeMarzo AM. The diet, prostate inflammation, and the development of prostate cancer. Cancer Metastasis Rev. 2002;21:3–16. doi: 10.1023/a:1020110718701. [DOI] [PubMed] [Google Scholar]
- 24.Chang SG, Kim CS, Jeon SH, et al. Is chronic inflammatory change in the prostate the major cause of rising serum prostate-specific antigen in patients with clinical suspicion of prostate cancer? Int J Urol. 2006;13:122–126. doi: 10.1111/j.1442-2042.2006.01244.x. [DOI] [PubMed] [Google Scholar]
- 25.Sindhwani P, Wilson CM. Prostatitis and serum prostate-specific antigen. Curr Urol Rep. 2005;6:307–312. doi: 10.1007/s11934-005-0029-y. [DOI] [PubMed] [Google Scholar]
- 26.Loeb S, Roehl KA, Yu X, et al. Use of prostate-specific antigen velocity to follow up patients with isolated high-grade prostatic intraepithelial neoplasia on prostate biopsy. Urology. 2007;69:108–112. doi: 10.1016/j.urology.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 27.Kattan MW. Comparison of Cox regression with other methods for determining prediction models and nomograms. J Urol. 2003;170(suppl):S6–S9. doi: 10.1097/01.ju.0000094764.56269.2d. [DOI] [PubMed] [Google Scholar]
- 28.Remzi M, Anagnostou T, Ravery V, et al. An artificial neural network to predict the outcome of repeat prostate biopsies. Urology. 2003;62:456–460. doi: 10.1016/s0090-4295(03)00409-6. [DOI] [PubMed] [Google Scholar]
- 29.Kronz JD, Allan CH, Shaikh AA, et al. Predicting cancer following a diagnosis of high-grade prostatic intraepithelial neoplasia on needle biopsy: Data on men with more than one follow-up biopsy. Am J Surg Pathol. 2001;25:1079–1085. doi: 10.1097/00000478-200108000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Ananthanarayanan V, Deaton RJ, Yang XJ, et al. Alpha-methylacyl-CoA racemase (AMACR) expression in normal prostatic glands and high-grade prostatic intraepithelial neoplasia (HGPIN): Association with diagnosis of prostate cancer. Prostate. 2005;63:341–346. doi: 10.1002/pros.20196. [DOI] [PubMed] [Google Scholar]
- 31.Dhir R, Vietmeier B, Arlotti J, et al. Early identification of individuals with prostate cancer in negative biopsies. J Urol. 2004;171:1419–1423. doi: 10.1097/01.ju.0000116545.94813.27. [DOI] [PubMed] [Google Scholar]
- 32.Nonn L, Ananthanarayanan V, Gann PH. Evidence for field cancerization of the prostate. Prostate. 2009;69:1470–1479. doi: 10.1002/pros.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welch HG, Fisher ES, Gottlieb DJ, et al. Detection of prostate cancer via biopsy in the Medicare-SEER population during the PSA era. J Natl Cancer Inst. 2007;99:1395–1400. doi: 10.1093/jnci/djm119. [DOI] [PubMed] [Google Scholar]
- 34.Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control. 2008;19:175–181. doi: 10.1007/s10552-007-9083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]