Abstract
Activation of Ca2+ signaling induced by receptor stimulation and mechanical stress plays a critical role in the development of cardiac hypertrophy. A canonical transient receptor potential protein subfamily member, TRPC6, which is activated by diacylglycerol and mechanical stretch, works as an upstream regulator of the Ca2+ signaling pathway. Although activation of protein kinase G (PKG) inhibits TRPC6 channel activity and cardiac hypertrophy, respectively, it is unclear whether PKG suppresses cardiac hypertrophy through inhibition of TRPC6. Here, we show that inhibition of cGMP-selective PDE5 (phosphodiesterase 5) suppresses endothelin-1-, diacylglycerol analog-, and mechanical stretch-induced hypertrophy through inhibition of Ca2+ influx in rat neonatal cardiomyocytes. Inhibition of PDE5 suppressed the increase in frequency of Ca2+ spikes induced by agonists or mechanical stretch. However, PDE5 inhibition did not suppress the hypertrophic responses induced by high KCl or the activation of protein kinase C, suggesting that PDE5 inhibition suppresses Ca2+ influx itself or molecule(s) upstream of Ca2+ influx. PKG activated by PDE5 inhibition phosphorylated TRPC6 proteins at Thr69 and prevented TRPC6-mediated Ca2+ influx. Substitution of Ala for Thr69 in TRPC6 abolished the anti-hypertrophic effects of PDE5 inhibition. In addition, chronic PDE5 inhibition by oral sildenafil treatment actually induced TRPC6 phosphorylation in mouse hearts. Knockdown of RGS2 (regulator of G protein signaling 2) and RGS4, both of which are activated by PKG to reduce Gαq-mediated signaling, did not affect the suppression of receptor-activated Ca2+ influx by PDE5 inhibition. These results suggest that phosphorylation and functional suppression of TRPC6 underlie prevention of pathological hypertrophy by PDE5 inhibition.
Keywords: Channels/Calcium, Membrane/Channels, Phosphorylation, Signal Transduction/Cyclic Nucleotides/Cyclic GMP, Signal Transduction/Phosphodiesterases, Signal Transduction/Protein Kinases/Cyclic Nucleotide, Cardiac Hypertrophy, TRPC Channel
Introduction
Pathological hypertrophy of the heart, induced by pressure overload, such as chronic hypertension and aortic stenosis, is a major risk factor for heart failure and cardiovascular mortality (1). Neurohumoral factors, such as norepinephrine, angiotensin II (Ang II),2 and endothelin-1 (ET-1), and mechanical stress are believed to be prominent contributors for pressure overload-induced cardiac hypertrophy (2, 3). Neurohumoral factors stimulate Gq protein-coupled receptors, leading to a sustained increase in [Ca2+]i through activation of phospholipase C. Mechanical stress also increases [Ca2+]i through Ca2+ influx-dependent pathways (4). The increase in [Ca2+]i induces activation of Ca2+-sensitive effectors, such as Ca2+/calmodulin-dependent serine/threonine phosphatase calcineurin (3, 5), Ca2+/calmodulin-dependent kinase II (6, 7), and calmodulin-binding transcription factor (8), which in turn induces hypertrophic gene expressions. Although the mechanism of Ca2+-mediated hypertrophy is extensively analyzed, it is not fully understood how these Ca2+ targets specifically decode the alteration of [Ca2+]i under the conditions of the rhythmic Ca2+ increases required for contraction.
In excitable cardiomyocytes, increases in the frequency or amplitude of Ca2+ transients evoked by Ca2+ influx-induced Ca2+ release have been suggested to encode signals for induction of hypertrophy (9). A partial depolarization of plasma membrane by receptor stimulation is reported to increase the frequency of Ca2+ oscillations, leading to activation of nuclear factor of activated T cells (NFAT), a transcription factor that is predominantly regulated by calcineurin (10). Recent reports have indicated that transient receptor potential canonical (TRPC) subfamily proteins play an essential role in agonist-induced membrane depolarization (11, 12). The relevance of TRPC channels to pathological hypertrophy is underscored by the observations that heart-targeted transgenic mice expressing TRPC channels caused hypertrophy (13, 14) and that TRPC proteins were up-regulated in hypertrophied and failing hearts (14–17). Among seven TRPC subfamilies, increased channel activities of TRPC1, TRPC3, and TRPC6 have been implicated in cardiac hypertrophy in vivo. TRPC1 is known to function not only as a Ca2+-permeable channel-forming subunit but also as an accessory protein to form the Ca2+ signaling complex (18). Endogenous TRPC1 and TRPC3 proteins are associated with each other to form native store-operated channels in HEK293 cells (19). In addition, diacylglycerol (DAG)-sensitive TRPC3, TRPC6, and TRPC7 proteins assemble to homotetramers or heterotetramers that function as DAG-activated cation channels (20). We have previously reported that TRPC3 and TRPC6 mediate Ang II-induced membrane depolarization, followed by Ca2+ influx through voltage-dependent Ca2+ channels in rat neonatal cardiomyocytes (21). Either knockdown of TRPC3 or TRPC6 channels completely suppressed Ang II-induced hypertrophy. Thus, TRPC1, TRPC3, and TRPC6 may form multimers in cardiomyocytes, which function as DAG-activated cation channels. Furthermore, we have recently demonstrated that treatment with a TRPC3 channel-selective blocker suppresses mechanical stretch-induced NFAT activation and pressure overload-induced cardiac hypertrophy in mice (22). Thus, inhibition of TRPC3-containing multimeric channels may represent a novel therapeutic strategy for preventing cardiac hypertrophy.
Phosphorylation of TRPC channels has been reported to modulate channel activity (23–25). For example, Fyn, an Src family Tyr kinase, physically interacts with the N-terminal region of TRPC6 proteins, and Tyr phosphorylation of TRPC6 enhances its channel activity (23). It has also been demonstrated that Src-dependent Tyr phosphorylation of TRPC3 is essential for DAG-activated cation influx (24). In contrast, Ser/Thr phosphorylation of TRPC3 channel attenuates its channel activity (25). Activation of PKG is known to regulate [Ca2+]i at multiple levels (26). PKG activation by a NO donor or cGMP analog has been reported to inhibit voltage-dependent L-type Ca2+ channels by α1-adrenergic receptor stimulation in cardiomyocytes (27). Several reports have shown that TRPC3 and TRPC6 channel activities are greatly attenuated by PKG-catalyzed phosphorylation of TRPC6 at threonine 69 (Thr69) and TRPC3 at Thr11 and Ser263 (25, 28). The physiological importance of negative regulation of TRPC6 channels by the NO-cGMP-protein kinase G (PKG) signaling pathway has been reported in vascular smooth muscle cells (28). However, the role of PKG-dependent negative regulation of TRPC6 channels in the heart is still unknown.
Inhibition of cGMP-dependent phosphodiesterase 5 (PDE5) enhances basal PKG activity through an increase in intracellular cGMP concentration. In fact, chronic treatment with sildenafil, a PDE5 inhibitor, exhibits the anti-hypertrophic effects in mice (29, 30) and in patients with systolic heart failure (31). It has been reported that RGS2 mediates cardiac compensation to pressure overload and anti-hypertrophic effects of PDE5 inhibition in mice (32). Because PKG-dependent phosphorylation of RGS2 enhances GTPase activity of the α-subunit of Gq protein (Gαq), this may explain the cGMP-dependent disruption of intracellular Ca2+ signaling induced by Gq-coupled receptor stimulation. However, we here found that inhibition of PDE5 also suppresses Ca2+ responses induced by the DAG analog and mechanical stretch (which may not require the activation of Gαq signaling) in rat neonatal cardiomyocytes. We also demonstrate that inhibition of PDE5 actually induces phosphorylation of TRPC6 proteins at Thr69, leading to inhibition of TRPC6-mediated Ca2+ signaling. These results suggest that PKG-dependent inhibition of TRPC6 channel activity is required for the anti-hypertrophic effects of PDE5 inhibition.
EXPERIMENTAL PROCEDURES
Materials and Cell Cultures
A PDE5-selective inhibitor (PDE5-I; 4-{[3′,4′-(methylenedioxy)benzyl]amino}-6-methoxyquinazoline), 4-methyl-4′-[3,5-bis(trifluoromethyl)-1H-pyrazol-1-yl]-1,2,3-thiadiazole-5-carboxanilide (BTP2), and KT5823 were purchased from Calbiochem. 8-Bromo-cGMP (8-Br-cGMP), phorbol 12-myristrate 13-acetate, S-nitroso-N-acetyl-dl-penicillamine, 1-oleoyl-2-acetyl-sn-glycerol (OAG), and ET-1 were from Sigma. Ang II was from Peptide Lab. Fura2/AM was from Dojindo. Bis(1,3-dibutylbarbituric acid)trimethine oxonol (DiBAC4(3)) was from Molecular Probes. Collagenase and Fugene 6 were from Roche Applied Science. Stealth small interfering RNA (siRNA) oligonucleotides, Alexa Fluor 568 phalloidin, and Lipofectamine 2000 were purchased from Invitrogen. Revatio (sildenafil citrate) was from Pfizer. The cDNAs coding a dominant negative mutant of TRPC6 (DN-TRPC6) and the TRPC6 (T69A) mutant were constructed as described (21, 28). Anti-TRPC6 was from Alomone. Phospho-Thr69 TRPC6 antiserum was generated against phospho-TRPC6 peptide (CHRRQ(P)TILREK). The phospho-TRPC6 antibody was purified by an antigen column. Isolation of rat neonatal cardiomyocytes and adenoviral infection of LacZ, green fluorescent protein, wild type (WT) TRPC6, or DN-TRPC6 were described (33). For knockdown of rat RGS proteins, cells were transfected with siRNAs (100 nm each) for RGS2 (AGAAAUAGCUCAAACGGGUCUUCCA) and RGS4 (UUUGAAAGCUGCCAGUCCACAUUCA) or control scrambled siRNAs for RGS2 (UUCACGGAACCGACCUUAAAUA) and RGS4 (AAAUAGCGUCUGACCACCCUUAGGU), using Lipofectamine 2000 for 72 h.
Reporter Activity
Measurement of NFAT-dependent luciferase activity and brain natriuretic peptide (BNP) promoter activity was performed as described previously (21). Briefly, cardiomyocytes (5 × 105 cells) plated on 24-well dishes were transiently co-transfected with 0.45 μg of pNFAT-Luc and 0.05 μg of pRL-SV40 control plasmid or with 0.3 μg of pBNP-Luc and 0.2 μg of pRL-SV40 using Fugene 6. Expression of the constitutively active mutant of green fluorescent protein-fused NFAT proteins (CA-NFAT) was performed as described (34). Forty-eight h after transfection, cells were stimulated with Ang II (1 μm), ET-1 (100 nm), or mechanical stretch (21) for 6 h (for NFAT) or 24 h (for BNP).
Measurement of [Ca2+]i and Membrane Potential
The intracellular Ca2+ concentration ([Ca2+]i) of cardiomyocytes or HEK293 cells was determined as described (35, 36). Briefly, HEK293 cells were transfected for 48 h with vector (pCI-neo), WT TRPC6, or TRPC6 (T69A) mutant using Fugene 6. Cardiomyocytes (1 × 106 cells) were plated on gelatin-coated glass bottom 35-mm dishes or on laminin-coated silicone rubber culture dishes (4 cm2; STREX) and were loaded with 1 μm fura-2/AM at 37 °C for 30 min (37). As we measured the changes in [Ca2+]i of the same cells before and after mechanical stretch, we treated cells with 20% of transient stretch for 3 s using automatic stretch systems (STB-150; STREX). Measurement and analysis of membrane potential were performed using DiBAC4(3) as described (21). The fluorescence intensity was measured with a video image analysis system (Aquacosmos, Hamamatsu Photonics).
Animal Models and Drug Treatment
All experiments on male C57BL6/J mice (C57BL6/J) were performed in accordance with the Guide for the Care and Use of Laboratory Animals prepared by Kyushu University. Sildenafil (100 mg/kg/day) was orally administered once a day for 1 week, and then hearts were removed and homogenized with radioimmune precipitation buffer.
Western Blot Analysis
TRPC6-expressing HEK293 cells (3 × 105 cells) or cardiomyocytes (1 × 106 cells) plated on 6-well dishes were directly harvested with 2× SDS sample buffer (200 μl). After centrifugation, supernatants (20–40 μl) were fractionated by 8% SDS-polyacrylamide gel and then transferred onto polyvinylidene difluoride membrane. For measurement of TRPC6 phosphorylation in mouse hearts, supernatants (100 μg of proteins) without boiling treatment were applied onto SDS-polyacrylamide gel. The expression and phosphorylation of endogenous TRPC6 proteins were detected by anti-TRPC6 (dilution rate, 1:1000) and anti-phospho-TRPC6 (1:1000) antibodies. We visualized the reactive bands using Supersignal® West Pico Luminol/Enhancer solution (Pierce). The optical density of the film was scanned and measured with Scion Image software.
Measurement of Hypertrophic Responses of Cardiomyocytes
Measurement of cardiomyocyte hypertrophy was performed as described (21, 34). Cardiomyocytes were fixed by paraformaldehyde and then stained with Alexa Fluor 548 phalloidin to visualize actin filaments. Digital photographs were taken at ×600 magnification with confocal microscopy (FV-10i, Olympus) or a Biozero microscope (BZ-8000, Keyence), and the average values of the cardiomyocyte area (n > 100 cells) were calculated using a BZ-II analyzer (Keyence). Protein synthesis was measured by [3H]leucine incorporation. After cells were stimulated with Ang II or ET-1 for 2 h, [3H]leucine (1 μCi/ml) was added to the culture medium and further incubated for 6 h. The incorporated [3H]leucine was measured using a liquid scintillation counter.
Statistical Analysis
The results are shown as means ± S.E. All experiments were repeated at least three times. Statistical comparisons were made with a two-tailed Student's t test or analysis of variance followed by the Student-Newman-Keuls procedure with significance imparted at p values of <0.05.
RESULTS
Suppression of Diacylglycerol-mediated Ca2+ Responses by Inhibition of PDE5
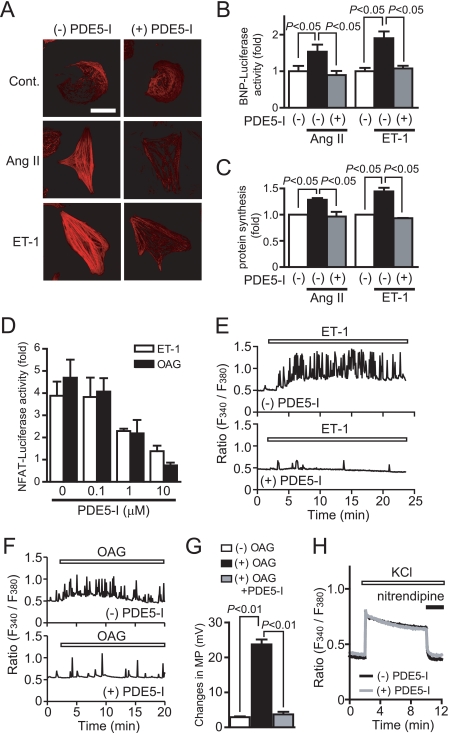
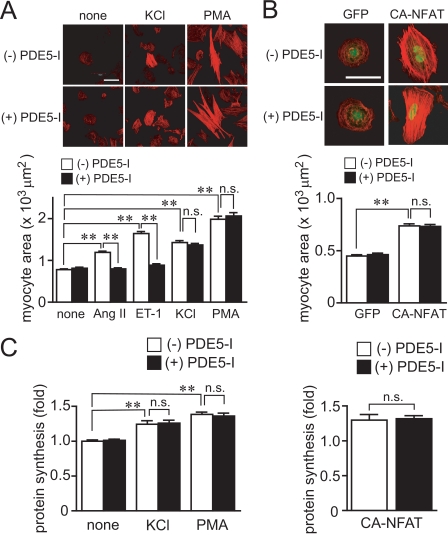
We first investigated whether inhibition of PDE5 suppresses agonist-induced Ca2+ responses and hypertrophic responses in rat cardiomyocytes. Treatment with PDE5-I completely suppressed Ang II- or ET-1-induced hypertrophic responses, such as an increase in cell size, actin reorganization, hypertrophic gene (BNP) expression, and protein synthesis (Fig. 1, A–C). Stimulation of cardiomyocytes with ET-1 increases the frequency of Ca2+ oscillations through voltage-dependent Ca2+ channels (38). PDE5-I also suppressed ET-1-induced NFAT activation and oscillatory and sustained increase in [Ca2+]i (Fig. 1, D and E). Although expression of CA-NFAT increased NFAT activity about 2.5-fold, this NFAT activation was not suppressed by PDE5-I (data not shown). These results suggest that PDE5-I inhibits NFAT activity through inhibition of Ca2+ responses. We have previously shown that DAG-sensitive TRPC channels (TRPC3 and TRPC6) mediate Ang II-induced activation of voltage-dependent Ca2+ influx through membrane depolarization (21). Treatment with OAG increased NFAT activity and the frequency of Ca2+ spikes, which were suppressed by PDE5-I (Fig. 1, D and F). In addition, the OAG-induced membrane depolarization, as determined by DiBAC4(3) imaging, was completely suppressed by PDE5-I pretreatment (Fig. 1G). Furthermore, nitrendipine-sensitive voltage-dependent Ca2+ influx-mediated increase in [Ca2+]i induced by high KCl was not affected by PDE5-I pretreatment (Fig. 1H). In addition, the hypertrophic responses induced by high KCl (Fig. 2, A and C) or the expression of CA-NFAT (Fig. 2B) were not suppressed by PDE5 inhibition. Although DAG also activates protein kinase C-dependent hypertrophic signaling pathway (3), PDE5-I did not suppress the phorbol 12-myristrate 13-acetate-induced hypertrophic responses (Fig. 2, A and C). These results suggest that PDE5-I suppresses agonist-induced Ca2+ responses and cardiomyocyte hypertrophy through inhibition of DAG-mediated membrane depolarization.
FIGURE 1.
Inhibition of PDE5 suppresses agonist-induced cardiomyocyte hypertrophic responses through inhibition of DAG-mediated Ca2+ signaling. A–C, effects of PDE5-I on agonist-induced hypertrophic responses (actin reorganization (A), BNP expression (B), and protein synthesis (C)). Cardiomyocytes were treated with PDE5-I (10 μm) for 20 min before the addition of Ang II (1 μm) or ET-1 (100 nm). Scale bar, 50 μm. D, effects of PDE5-I on NFAT activation induced by ET-1 and OAG (30 μm). E–H, average time courses of Ca2+ responses induced by ET-1 (E), OAG (F), and KCl (H) in the absence or presence of PDE5-I. G, effects of PDE5-I on OAG-induced increase in membrane potential (MP). Cardiomyocytes were treated with OAG for 20 min, and maximal increase in MP was calculated from peak changes in DiBAC4(3) fluorescence intensity (21). H, voltage-dependent Ca2+ influx was evoked by KCl (8 mm) for 8 min, and nitrendipine (10 μm) was added to inhibit the activities of voltage-dependent Ca2+ channels.
FIGURE 2.
Inhibition of PDE5 does not suppress the agonist-independent cardiomyocyte hypertrophic responses. A and C, effects of PDE5-I on hypertrophic responses (actin reorganization, protein synthesis, and increases in area of cardiomyocytes) induced by KCl and phorbol 12-myristrate 13-acetate (PMA). Cardiomyocytes were stimulated with Ang II (1 μm), ET-1 (100 nm), KCl (5 mm), or phorbol 12-myristrate 13-acetate (1 μm) for 48 h. B and C, effects of PDE5-I on hypertrophic growth (B) and protein synthesis (C) in green fluorescent protein- and CA-NFAT-expressing cardiomyocytes. Scale bar, 50 μm. **, p < 0.01; n.s., no significance.
Inhibition of TRPC6 Channel Activity by PDE5 Inhibition
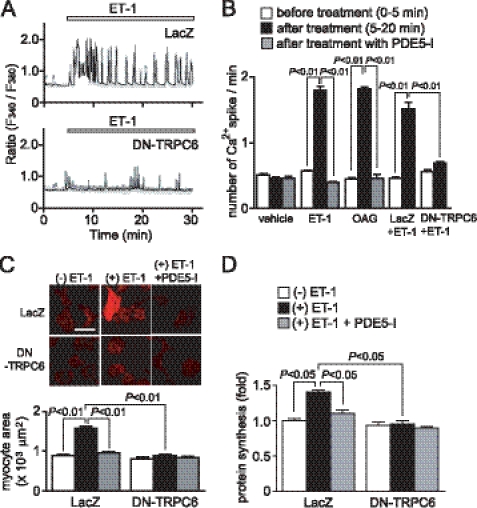
To investigate the involvement of TRPC6 in agonist-induced cardiomyocyte hypertrophy, we used DN-TRPC6 (21). As shown in Fig. 3A, treatment of cardiomyocytes with ET-1 or OAG significantly increased the frequency of Ca2+ spikes, which were completely suppressed by PDE5 inhibition (Fig. 3, A and B). Expression of DN-TRPC6 also completely suppressed ET-1-induced increases in the frequency of Ca2+ spikes (Fig. 3, A and B) and hypertrophic responses (Fig. 3, C and D). The anti-hypertrophic effect of PDE5-I was completely abolished in DN-TRPC6-expressing myocytes, suggesting that PDE5-I suppresses the TRPC6-mediated hypertrophic signaling pathway.
FIGURE 3.
TRPC6 mediates ET-1-induced cardiomyocyte hypertrophic responses. A, Ca2+ responses induced by ET-1 (100 nm) in LacZ- and DN-TRPC6-overexpressing cardiomyocytes. B, results of the frequency of Ca2+ oscillations induced by ET-1 or OAG (30 μm). Cardiomyocytes were pretreated with PDE5-I (10 μm) 35 min before agonist stimulation. C and D, effects of DN-TRPC6 on ET-1-induced actin reorganization and increase in cell size (C) and protein synthesis (D). Scale bar, 50 μm.
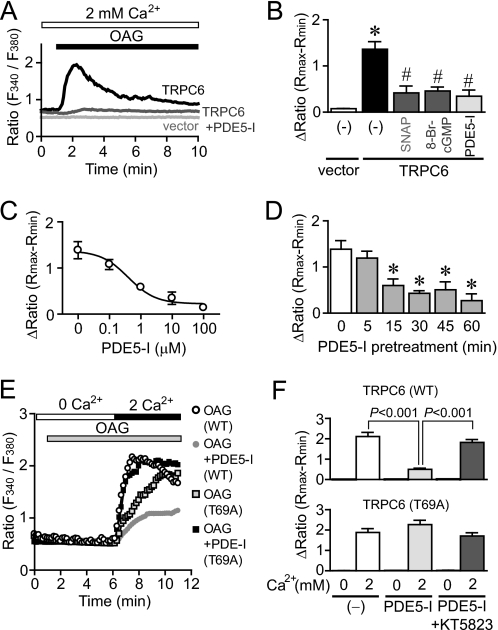
Takahashi et al. (28) have recently reported that activation of the NO-cGMP-PKG pathway by extracellular treatment with a NO donor or cGMP analog inhibits TRPC6 channel activity through phosphorylation of TRPC6 at Thr69. Thus, we next examined whether PDE5 inhibition attenuates TRPC6 channel activity. Compared with vector-expressing HEK293 cells, treatment with OAG induced a marked increase in [Ca2+]i of TRPC6-expressing cells (Fig. 4A). The TRPC6-mediated increase in [Ca2+]i was significantly suppressed by pretreatment with PDE5-I as well as S-nitroso-N-acetyl-dl-penicillamine and 8-Br-cGMP (Fig. 4B). The IC50 value of inhibition of the TRPC6-mediated increase in [Ca2+]i by PDE5-I was 0.41 ± 0.08 μm (Fig. 4C). More than 15 min of pretreatment with PDE5-I was required for the suppression of the TRPC6-mediated increase in [Ca2+]i induced by OAG (Fig. 4D). PDE5-I suppressed the Ca2+ influx-mediated increase in [Ca2+]i induced by OAG in TRPC6 (WT)-expressing cells, which was completely abolished by co-treatment with a PKG-selective inhibitor, KT5823 (Fig. 4, E and F). These results suggest that activation of PKG is required for the inhibition of TRPC6 channel activity. In fact, the suppression of OAG-induced Ca2+ influx by PDE5-I treatment was abolished in TRPC6 (T69A)-expressing cells. Thus, PKG-dependent phosphorylation of TRPC6 at Thr69 may be essential for inhibition of TRPC6 channel activity by PDE5 inhibition.
FIGURE 4.
PKG-dependent suppression of TRPC6-mediated Ca2+ influx by PDE5 inhibition. A, average time courses of Ca2+ responses induced by OAG (30 μm) in vector and TRPC6-expressing HEK293 cells with or without PDE5-I. B, peak increases in [Ca2+]i induced by OAG in vector- and TRPC6-expressing cells. HEK293 cells were treated with S-nitroso-N-acetyl-dl-penicillamine (SNAP) (100 μm), 8-Br-cGMP (100 μm), or PDE5-I (10 μm) for 30 min before the addition of OAG (30 μm). C and D, concentration-dependent (C) and time-dependent (D) suppression of OAG-induced [Ca2+]i increases by PDE5-I. E and F, effects of PDE5-I on OAG-induced Ca2+ influx-mediated [Ca2+]i increases in TRPC6 (WT)- and TRPC6 (T69A)-expressing cells. HEK293 cells were treated with KT5823 (1 μm) for 30 min before the addition of OAG. *, p < 0.05 versus vector (white bar); #, p < 0.05 versus TRPC6 (−) control (black bar).
Phosphorylation of TRPC6 Proteins at Thr69 by PDE5 Inhibition
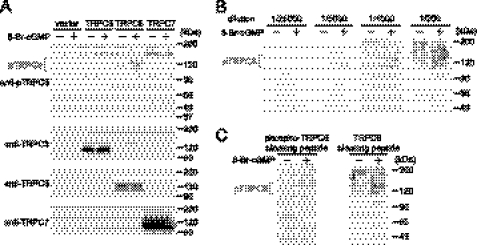
In order to examine whether inhibition of PDE5 actually induces phosphorylation of TRPC6 proteins in cardiomyocytes, we generated a phospho-specific TRPC6 (Thr69) antibody. Because TRPC6 proteins have two glycosylation sites (39), a single 100 kDa band and smear 110–120 kDa bands due to several patterns of glycosylation were observed in TRPC6 wild type (WT)-overexpressing HEK293 cells (Fig. 5A). Phosphorylation of TRPC6 proteins was observed only when HEK293 cells were stimulated with 8-Br-cGMP. In contrast, this phosphorylation was not observed in vector-, TRPC3-, or TRPC7-expressing HEK293 cells, even when cells were stimulated with 8-Br-cGMP. The TRPC6 phosphorylation could be detectable when the antibody was diluted from 1,000- to 5,000-fold (Fig. 5B). Furthermore, the TRPC6 phosphorylation bands were completely abolished by the treatment with phospho-TRPC6-blocking peptide but not by control blocking peptide (Fig. 5C). These results clearly suggest that our phospho-specific TRPC6 antibody specifically recognized the phosphorylation of rodent TRPC6 at Thr69.
FIGURE 5.
Specific recognition of TRPC6 phosphorylation at Thr69 by a phospho-specific antibody. A, phosphorylation of TRPC6 at Thr69 induced by PKG activation in vector-, TRPC3-, TRPC6-, and TRPC7-expressing HEK293 cells. HEK293 cells were treated with 8-Br-cGMP (100 μm) for 2 h. B, optimization of dilution of anti-phospho-TRPC6 antibody. Antibody (0.56 mg/ml) was diluted with Tris-buffered saline plus 0.1% Tween 20 (TBS-T) and incubated with blots for 1 h at room temperature. C, effect of treatment with a TRPC6-blocking peptide on the recognition of TRPC6 phosphorylation by this antibody. Blots were incubated with phospho-TRPC6 antibody diluted 1:1000 in TBS-T with phospho-TRPC6-blocking peptide or non-phosphorylated TRPC6-blocking peptide (10 μg/ml) for 1 h at room temperature.
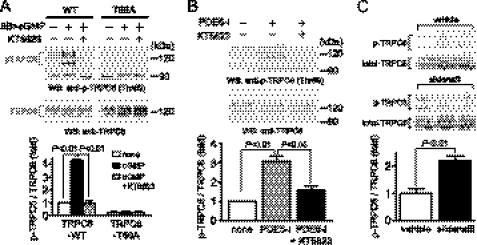
Activation of PKG by 8-Br-cGMP and PDE5-I stimulated the phosphorylation of TRPC6 proteins, which was completely suppressed by the treatment with KT5823 in TRPC6-WT-expressing HEK293 cells (Fig. 6, A and B). The PKG-mediated TRPC6 phosphorylation was not observed in TRPC6 (T69A)-expressing cells, indicating the specificity of this antibody. Treatment with PDE5-I significantly increased the phosphorylation of native TRPC6 proteins in rat cardiomyocytes, which was completely suppressed by KT5823 (Fig. 6B). We further examined whether inhibition of PDE5 actually phosphorylates TRPC6 proteins in vivo. Takimoto et al. (29) have previously reported that chronic treatment with sildenafil (100 mg/kg/day) prevents and reverses cardiac hypertrophy induced by pressure overload in mice. We found that oral treatment with sildenafil (100 mg/kg/day) for 1 week, under the same conditions as in their report, actually increases TRPC6 phosphorylation levels in mouse hearts (Fig. 6C).
FIGURE 6.
PKG-dependent phosphorylation of Thr69 in TRPC6 by PDE5 inhibition. A, PKG-dependent phosphorylation of TRPC6 proteins at Thr69 in TRPC6 (WT)- and TRPC6 (T69A)-expressing HEK293 cells. HEK293 cells were treated with KT5823 (1 μm) for 20 min before the addition of 8-Br-cGMP (100 μm) and PDE5-I (10 μm) for 30 min. B, PKG-dependent TRPC6 phosphorylation by PDE5-I. Cardiomyocytes were treated with KT5823 for 20 min before the addition of PDE5-I (10 μm) for 1 h. C, effects of sildenafil on the phosphorylation of TRPC6 in mice. One week after oral administration with sildenafil (100 mg/kg/day), hearts were lysed with radioimmune precipitation buffer, and 100 μg of proteins were applied to SDS-PAGE.
Inhibition of TRPC6 Phosphorylation at Thr69 Diminishes the Anti-hypertrophic Effects of PDE5 Inhibition
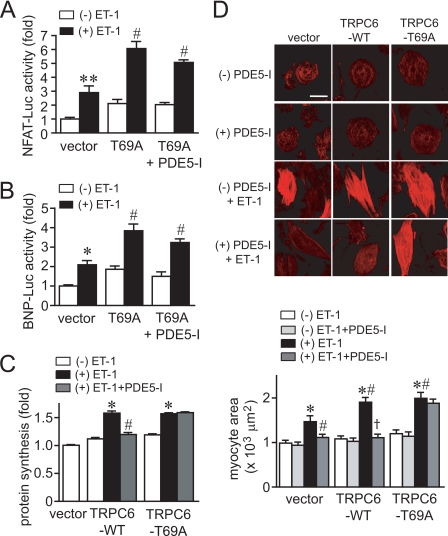
Overexpression of TRPC6 (T69A) enhanced the increase in NFAT activity and BNP expression induced by ET-1, which was not suppressed by PDE5-I (Fig. 7, A and B). In addition, expression of TRPC6 (WT) or TRPC6 (T69A) enhanced the ET-1-induced hypertrophic responses of cardiomyocytes (Fig. 7, C and D). Although PDE5-I completely suppressed the ET-1-induced hypertrophic responses in control (vector)- or TRPC6 (WT)-expressing cardiomyocytes, PDE5-I did not suppress hypertrophic responses in TRPC6 (T69A)-expressing cardiomyocytes. These results suggest that inhibition of TRPC6 channel activity via its PKG-dependent phosphorylation at Thr69 participates in anti-cardiomyocyte hypertrophic effects of PDE5 inhibition.
FIGURE 7.
Phosphorylation of Thr69 is essential for the anti-hypertrophic effects of PDE5 inhibition. A and B, effects of PDE5-I on ET-1-induced NFAT activation (A) and BNP gene expression (B) in TRPC6 (T69A)-expressing cardiomyocytes. C and D, effects of PDE5-I on the ET-1-induced protein synthesis (C) and increase in the size of TRPC6 (T69A)-overexpressing cardiomyocytes (D). Cardiomyocytes were treated with PDE5-I (10 μm) for 20 min before the addition of ET-1 (100 nm). Scale bar, 50 μm. *, p < 0.05; **, p < 0.01 versus without (−) ET-1 within vector. #, p < 0.05 versus vector with (+) ET-1. †, p < 0.01 versus TRPC6-WT with ET-1.
Suppression of Mechanical Stretch-induced Ca2+ Responses by PDE5 Inhibition
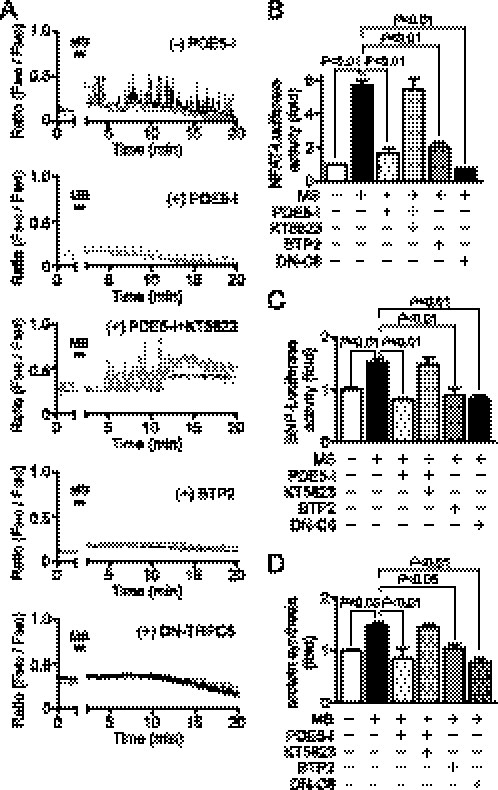
Because mechanical stress is also involved in the development of cardiac hypertrophy, we next examined whether PDE5-I inhibits Ca2+ responses induced by mechanical stretch. In control cardiomyocytes, a periodic increase in [Ca2+]i was observed after cells were stretched by 20% for 3 s with a speed of 20 mm/s (Fig. 8A). A bis(triluoromethyl)pyrazole derivative, BTP2, is recently used as a selective inhibitor of the TRPC1 to -7 channels. We previously reported that BTP2 at 3 μm suppresses TRPC6-mediated Ca2+ influx by 80% in HEK293 cells (22). Treatment with PDE5-I or BTP2 abolished mechanical stretch-induced increase in [Ca2+]i (Fig. 8A). Mechanical stretch-induced increases in NFAT-dependent luciferase activity, BNP-luciferase activity, and protein synthesis were suppressed by PDE5-I, which was canceled by co-treatment with KT5823 (Fig. 8, B–D). We also found that mechanical stretch-induced increases in [Ca2+]i, NFAT activation, and hypertrophic responses were completely suppressed by the expression of DN-TRPC6 (Fig. 8, B–D). These results suggest that PDE5-I suppresses mechanical stretch-induced Ca2+ responses linked to cardiomyocyte hypertrophic responses through inhibition of TRPC6 channels.
FIGURE 8.
Inhibition of PDE5 suppresses Ca2+ responses and cardiomyocyte hypertrophic responses induced by mechanical stretch. A, typical traces of Ca2+ responses induced by mechanical stretch (MS) in the absence or presence of PDE5-I, KT5823, or BTP2. Cardiomyocytes were treated with PDE5-I (10 μm) or BTP2 (5 μm) for 35 min before MS. DN-TRPC6 proteins were expressed using adenoviral infection. B–D, effects of PDE5-I, BTP2, and DN-TRPC6 on the MS-induced NFAT activation (B) and hypertrophic responses (BNP gene expression (C) and protein synthesis (D)).
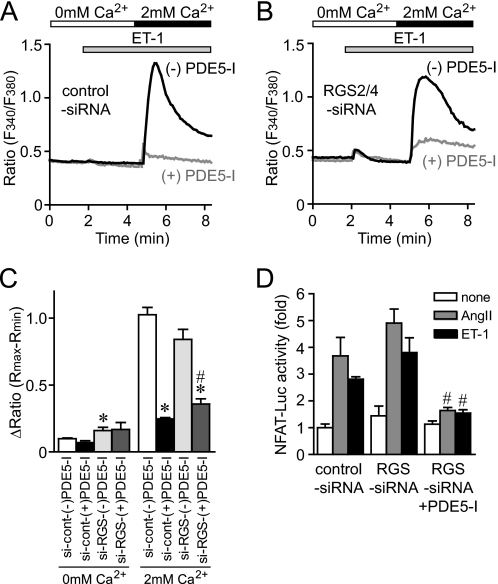
Knockdown of RGS2 and RGS4 Does Not Affect the Effect of PDE5-I
It has been recently reported that PKG-dependent phosphorylation of RGS2 and RGS4 mediates the anti-hypertrophic effects in mouse hearts (32, 40, 41). Thus, we next examined the involvement of RGS proteins in the inhibition of agonist-induced Ca2+ responses by PDE5 inhibition, using siRNAs for RGS2 and RGS4. We confirmed that the treatment of cardiomyocytes with siRNAs for RGS2/4 reduced the expression levels of RGS2 and RGS4 mRNAs to 18.5 ± 5.2 and 26.7 ± 7.8%, respectively. Knockdown of RGS2/4 proteins did not affect Ca2+ responses and NFAT activation induced by ET-1 (Fig. 9). The addition of extracellular Ca2+ induced a Ca2+ influx-mediated increase in [Ca2+]i by ET-1 stimulation, which was significantly suppressed by PDE5-I treatment in control myocytes (Fig. 9, A–C). The Ca2+ influx-mediated [Ca2+]i increases and increase in NFAT activity by agonist stimulation were slightly enhanced in RGS2/4-deficient myocytes and were also significantly suppressed by PDE5-I treatment (Fig. 9, B–D). These results suggest that RGS2 and RGS4 proteins are not mainly involved in the inhibition of agonist-induced Ca2+ responses by PDE5 inhibition in cardiomyocytes.
FIGURE 9.
PDE5-I suppresses ET-1-induced Ca2+ responses in RGS2/4-down-regulated cardiomyocytes. A and B, average time courses of Ca2+ responses induced by ET-1 in the absence or presence of PDE5-I in control siRNA-treated cardiomyocytes (A) and RGS2/4 siRNAs-treated cardiomyocytes (B). C, peak Ca2+ releases (0 mm Ca2+) and Ca2+ influx-mediated increases in [Ca2+]i (2 mm Ca2+) induced by ET-1 (100 nm). D, effects of knockdown of RGS2/4 proteins on PKG-dependent inhibition of ET-1-induced NFAT activation by PDE5-I. *, p < 0.05 versus control siRNA-treated cardiomyocytes without PDE5-I; #, p < 0.05 versus RGS2/4 siRNA-treated cardiomyocytes without PDE5-I.
DISCUSSION
In this study, we have demonstrated that inhibition of PDE5 suppresses agonist-induced and mechanical stretch-induced hypertrophic responses in rat cardiomyocytes. The increases in the frequency of Ca2+ oscillations induced by OAG or mechanical stretch are greatly attenuated by PDE5 inhibition. PDE5-I suppresses OAG-induced membrane depolarization, suggesting the inhibition of DAG-sensitive TRPC channels by PDE5 inhibitor. Treatment with PDE5-I actually induces PKG-dependent phosphorylation of TRPC6 proteins at Thr69 and inhibits TRPC6-mediated Ca2+ responses. Because the inhibition of ET-1-induced hypertrophic responses by PDE5-I was abolished in TRPC6 (T69A)-expressing cardiomyocytes, we suggest that phosphorylation of TRPC6 is required for the anti-hypertrophic effects of PDE5 inhibition. In addition, PDE5-I-induced suppression of Ca2+ influx induced by ET-1 was not abolished by the knockdown of RGS2 and RGS4, both of which are reported to be activated by PKG. This result emphasizes the physiological importance of TRPC6 phosphorylation by PDE5-I in suppressing Ca2+ influx induced by agonist stimulation and mechanical stretch.
Higazi et al. (38) has recently reported that inositol-1,4,5-trisphosphate-induced Ca2+ release from perinuclear inositol-1,4,5-trisphosphate receptors by ET-1 stimulation or membrane depolarization preferentially couples to the calcineurin/NFAT pathway to induce hypertrophy. They have also demonstrated that the potentiation of Ca2+ influx activity induced by isoproterenol or BayK8644 induces activation of calcineurin/NFAT signaling pathway via Ca2+ release from perinuclear inositol-1,4,5-trisphosphate receptors. This mechanism may be involved in the process of voltage-dependent Ca2+ influx-mediated NFAT activation evoked by TRPC6 activation. Although we did not measure nuclear Ca2+ concentrations, PDE5-I may inhibit the increase in nuclear Ca2+ concentrations because PDE5-I suppresses NFAT activation induced by ET-1.
Because PDE5-I did not suppress the high KCl-induced increase in [Ca2+]i (Fig. 1), we suggest that PKG activation by PDE5-I does not inhibit L-type Ca2+ channels. In contrast, Fiedler et al. (27) have reported that overexpression of PKG type I suppresses single L-type Ca2+ channel open probability and [Ca2+]i transient amplitude. This discrepancy can be explained by the intensity of PKG activation. Although we show that PDE5-I increased the TRPC6 phosphorylation level about 3-fold, the treatment with 8-Br-cGMP induced a more than 5-fold increase in TRPC6 phosphorylation level (data not shown). This suggests that PDE5-I moderately activates PKG, and this activation is insufficient to inhibit L-type Ca2+ channel activity.
Inhibition of PDE5 suppresses mechanical stretch-induced Ca2+ responses in cardiomyocytes. Because the pattern of Ca2+ spikes is similar to those induced by ET-1 or OAG stimulation, membrane depolarization may be also involved in this mechanism. This idea is supported by the reports that DAG-sensitive TRPC channels work as stretch-activated depolarizing channels in the vascular system (42–44). It has recently been reported that TRPC6 is activated by mechanical stretch through two pathways: G protein-mediated indirect activation and direct activation of TRPC6 (42). Furthermore, TRPC6 can be synergistically activated by mechanical force in the presence of Gq-coupled receptor stimulation (43). We have previously reported that mechanical stretch increases the concentration of extracellular nucleotides, which stimulate Gq protein-coupled P2Y receptors (37). Thus, extracellular nucleotides released by mechanical stretch and mechanical force may synergistically increase TRPC6 channel activity in rat neonatal cardiomyocytes.
Because Kwan et al. (25) reported that the activation of PKG by NO donor and 8-Br-cGMP increases phosphorylation at Thr11 and Ser263 of human TRPC3 proteins, it is possible that inhibition of PDE5 also results in phosphorylation of TRPC3 proteins and reduction of TRPC3 channel activity. Although Ser263 of human TRPC3 protein is conserved among humans, rats, and mice, Thr11 is not present in mouse and rat TRPC3 proteins. We have tried to generate an antibody to recognize the phosphorylated form of Ser263 in TRPC3 but failed to obtain the useful antibody. However, we confirmed that PDE5-I inhibits TRPC3-mediated Ca2+ influx induced by OAG in TRPC3-expressing HEK293 cells, which was abolished in TRPC3 (S325A)-expressing HEK293 cells (n = 2; data not shown). Because the Ser325 in mouse TRPC3 protein is identical to Ser263 in human TRPC3 protein, this result implies that the inhibition of TRPC3 channel activity by PDE-I may also be involved in the anti-hypertrophic effects of PDE5 inhibition.
We have not yet been able to identify the individual roles of TRPC6 and TRPC3 channels in cardiomyocytes. Despite their high degree of structural and functional similarity, TRPC3, TRPC6, and TRPC7 are substantially different in their basal channel activities (45). The basal channel activity of TRPC6 is tightly regulated (39). In contrast, TRPC3 and TRPC7 have considerable constitutive activity when expressed in various cell lines (46). Despite the low basal activity of TRPC6 channels, results from transgenic mice with cardiomyocyte-specific expression of TRPC3 or TRPC6 channels show that the up-regulation of TRPC6 channel proteins is essential for the development of cardiac hypertrophy (13, 14). In pathological conditions, hearts are exposed to mechanical stress and neurohumoral factors. Therefore, a characteristic of TRPC6 channels that is synergistically activated by mechanical stretch in the presence of a low concentration of agonist (43) may explain the mechanism of induction of pathological hypertrophy. Because TRPC3 has high constitutive activity among the DAG-activated TRPC3/6/7 family and is up-regulated in smooth muscle cells from TRPC6-deficient mice (45), the TRPC6-deficient mouse heart may cause excessive hypertrophy by pressure overload like a TRPC3-transgenic mouse heart (13). In addition, there is no pharmacological tool that selectively inhibits TRPC6 channel activity. Generation of transgenic mice with heart-specific expression of the dominant negative TRPC6 mutant, which moderately inhibits the function of TRPC6 channels without any compensation, will be necessary for understanding the pathophysiological role of TRPC6 in the heart.
In conclusion, we demonstrated that inhibition of DAG-sensitive TRPC channel activities through PKG-dependent phosphorylation is required for the anti-hypertrophic effects of PDE5 inhibition in rat cardiomyocytes. Our finding will provide a new insight for the creation of therapeutic strategies for heart failure.
Acknowledgments
We thank Marina Ariyoshi and Shinji Oda for measurement of Ca2+ imaging during the early stage of this study. We also thank Dr. Koichiro Kuwahara (Kyoto University) for helpful comments.
This study was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to M. Nishida, M. Nakaya, and H. Kurose), a grant-in-aid for scientific research on Innovative Areas (to M. Nishida), a grant-in-aid for scientific research on Priority Areas (H. Kurose), and grants from the Naito Foundation, the Nakatomi Foundation, the Sapporo Bioscience Foundation (M. Nishida), and the Mochida Memorial Foundation for Medical and Pharmaceutical Research (to M. Nakaya).
- Ang II
- angiotensin II
- BNP
- brain natriuretic peptide
- CA-NFAT
- constitutively active NFAT
- DAG
- diacylglycerol
- DiBAC4(3)
- bis(1,3-dibutylbarbituric acid)trimethine oxonol
- DN-TRPC6
- dominant negative TRPC6
- ET-1
- endothelin-1
- BTP2
- 4-methyl-4′-[3,5-bis(trifluoromethyl)-1H-pyrazol-1-yl]-1,2,3-thiadiazole-5-carboxanilide
- NFAT
- nuclear factor of activated T cells
- OAG
- a DAG derivative, 1-oleoyl-2-acetyl-sn-glycerol
- TRPC
- transient receptor potential canonical
- PDE5-I
- phosphodiesterase 5-selective inhibitor: 4-{[3′,4′-(methylenedioxy)benzyl]amino}-6-methoxyquinazoline
- RGS
- regulator of G protein signaling
- PKG
- protein kinase G
- WT
- wild type
- 8-Br-cGMP
- 8-bromo-cyclic GMP
- siRNA
- small interfering RNA.
REFERENCES
- 1.Mann D. L. (1999) Circulation 100, 999–1008 [DOI] [PubMed] [Google Scholar]
- 2.Dorn G. W., 2nd, Force T. (2005) J. Clin. Invest. 115, 527–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heineke J., Molkentin J. D. (2006) Nat. Rev. Mol. Cell Biol. 7, 589–600 [DOI] [PubMed] [Google Scholar]
- 4.Liao X. D., Tang A. H., Chen Q., Jin H. J., Wu C. H., Chen L. Y., Wang S. Q. (2003) Biochem. Biophys. Res. Commun. 310, 405–411 [DOI] [PubMed] [Google Scholar]
- 5.Molkentin J. D., Lu J. R., Antos C. L., Markham B., Richardson J., Robbins J., Grant S. R., Olson E. N. (1998) Cell 93, 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frey N., McKinsey T. A., Olson E. N. (2000) Nat. Med. 6, 1221–1227 [DOI] [PubMed] [Google Scholar]
- 7.Ramirez M. T., Zhao X. L., Schulman H., Brown J. H. (1997) J. Biol. Chem. 272, 31203–31208 [DOI] [PubMed] [Google Scholar]
- 8.Song K., Backs J., McAnally J., Qi X., Gerard R. D., Richardson J. A., Hill J. A., Bassel-Duby R., Olson E. N. (2006) Cell 125, 453–466 [DOI] [PubMed] [Google Scholar]
- 9.Berridge M. J., Bootman M. D., Roderick H. L. (2003) Nat. Rev. Mol. Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- 10.Colella M., Grisan F., Robert V., Turner J. D., Thomas A. P., Pozzan T. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 2859–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Large W. A. (2002) J. Cardiovasc. Electrophysiol. 13, 493–501 [DOI] [PubMed] [Google Scholar]
- 12.Yao X., Garland C. J. (2005) Circ. Res. 97, 853–863 [DOI] [PubMed] [Google Scholar]
- 13.Nakayama H., Wilkin B. J., Bodi I., Molkentin J. D. (2006) FASEB J. 20, 1660–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuwahara K., Wang Y., McAnally J., Richardson J. A., Bassel-Duby R., Hill J. A., Olson E. N. (2006) J. Clin. Invest. 116, 3114–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishida M., Kurose H. (2008) Naunyn Schmiedebergs Arch. Pharmacology 378, 395–406 [DOI] [PubMed] [Google Scholar]
- 16.Bush E. W., Hood D. B., Papst P. J., Chapo J. A., Minobe W., Bristow M. R., Olson E. N., McKinsey T. A. (2006) J. Biol. Chem. 281, 33487–33496 [DOI] [PubMed] [Google Scholar]
- 17.Seth M., Zhang Z. S., Mao L., Graham V., Burch J., Stiber J., Tsiokas L., Winn M., Abramowitz J., Rockman H. A., Birnbaumer L., Rosenberg P. (2009) Circ. Res. 105, 1023–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori Y., Wakamori M., Miyakawa T., Hermosura M., Hara Y., Nishida M., Hirose K., Mizushima A., Kurosaki M., Mori E., Gotoh K., Okada T., Fleig A., Penner R., Iino M., Kurosaki T. (2002) J. Exp. Med. 195, 673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zagranichnaya T. K., Wu X., Villereal M. L. (2005) J. Biol. Chem. 280, 29559–29569 [DOI] [PubMed] [Google Scholar]
- 20.Hofmann T., Schaefer M., Schultz G., Gudermann T. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 7461–7466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onohara N., Nishida M., Inoue R., Kobayashi H., Sumimoto H., Sato Y., Mori Y., Nagao T., Kurose H. (2006) EMBO J. 25, 5305–5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiyonaka S., Kato K., Nishida M., Mio K., Numaga T., Sawaguchi Y., Yoshida T., Wakamori M., Mori E., Numata T., Ishii M., Takemoto H., Ojida A., Watanabe K., Uemura A., Kurose H., Morii T., Kobayashi T., Sato Y., Sato C., Hamachi I., Mori Y. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 5400–5405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hisatsune C., Kuroda Y., Nakamura K., Inoue T., Nakamura T., Michikawa T., Mizutani A., Mikoshiba K. (2004) J. Biol. Chem. 279, 18887–18894 [DOI] [PubMed] [Google Scholar]
- 24.Vazquez G., Wedel B. J., Kawasaki B. T., Bird G. S., Putney J. W., Jr. (2004) J. Biol. Chem. 279, 40521–40528 [DOI] [PubMed] [Google Scholar]
- 25.Kwan H. Y., Huang Y., Yao X. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 2625–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kass D. A., Champion H. C., Beavo J. A. (2007) Circ. Res. 101, 1084–1095 [DOI] [PubMed] [Google Scholar]
- 27.Fiedler B., Lohmann S. M., Smolenski A., Linnemuller S., Pieske B., Schroder F., Molkentin J. D., Drexler H., Wollert K. C. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 11363–11368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi S., Lin H., Geshi N., Mori Y., Kawarabayashi Y., Takami N., Mori M. X., Honda A., Inoue R. (2008) J. Physiol. 586, 4209–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takimoto E., Champion H. C., Li M., Belardi D., Ren S., Rodriguez E. R., Bedja D., Gabrielson K. L., Wang Y., Kass D. A. (2005) Nat. Med. 11, 214–222 [DOI] [PubMed] [Google Scholar]
- 30.Hsu S., Nagayama T., Koitabashi N., Zhang M., Zhou L., Bedja D., Gabrielson K. L., Molkentin J. D., Kass D. A., Takimoto E. (2009) Cardiovasc. Res. 81, 301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis G. D., Lachmann J., Camuso J., Lepore J. J., Shin J., Martinovic M. E., Systrom D. M., Bloch K. D., Semigran M. J. (2007) Circulation 115, 59–66 [DOI] [PubMed] [Google Scholar]
- 32.Takimoto E., Koitabashi N., Hsu S., Ketner E. A., Zhang M., Nagayama T., Bedja D., Gabrielson K. L., Blanton R., Siderovski D. P., Mendelsohn M. E., Kass D. A. (2009) J. Clin. Invest. 119, 408–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishida M., Maruyama Y., Tanaka R., Kontani K., Nagao T., Kurose H. (2000) Nature 408, 492–495 [DOI] [PubMed] [Google Scholar]
- 34.Nishida M., Onohara N., Sato Y., Suda R., Ogushi M., Tanabe S., Inoue R., Mori Y., Kurose H. (2007) J. Biol. Chem. 282, 23117–23128 [DOI] [PubMed] [Google Scholar]
- 35.Nishida M., Sugimoto K., Hara Y., Mori E., Morii T., Kurosaki T., Mori Y. (2003) EMBO J. 22, 4677–4688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishida M., Tanabe S., Maruyama Y., Mangmool S., Urayama K., Nagamatsu Y., Takagahara S., Turner J. H., Kozasa T., Kobayashi H., Sato Y., Kawanishi T., Inoue R., Nagao T., Kurose H. (2005) J. Biol. Chem. 280, 18434–18441 [DOI] [PubMed] [Google Scholar]
- 37.Nishida M., Sato Y., Uemura A., Narita Y., Tozaki-Saitoh H., Nakaya M., Ide T., Suzuki K., Inoue K., Nagao T., Kurose H. (2008) EMBO J. 27, 3104–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higazi D. R., Fearnley C. J., Drawnel F. M., Talasila A., Corps E. M., Ritter O., McDonald F., Mikoshiba K., Bootman M. D., Roderick H. L. (2009) Mol. Cell 33, 472–482 [DOI] [PubMed] [Google Scholar]
- 39.Dietrich A., Mederos y Schnitzler M., Emmel J., Kalwa H., Hofmann T., Gudermann T. (2003) J. Biol. Chem. 278, 47842–47852 [DOI] [PubMed] [Google Scholar]
- 40.Huang J., Zhou H., Mahavadi S., Sriwai W., Murthy K. S. (2007) Am. J. Physiol. Cell Physiol. 292, C200–C208 [DOI] [PubMed] [Google Scholar]
- 41.Tokudome T., Kishimoto I., Horio T., Arai Y., Schwenke D. O., Hino J., Okano I., Kawano Y., Kohno M., Miyazato M., Nakao K., Kangawa K. (2008) Circulation 117, 2329–2339 [DOI] [PubMed] [Google Scholar]
- 42.Mederos y Schnitzler M., Storch U., Meibers S., Nurwakagari P., Breit A., Essin K., Gollasch M., Gudermann T. (2008) EMBO J. 27, 3092–3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inoue R., Jensen L. J., Jian Z., Shi J., Hai L., Lurie A. I., Henriksen F. H., Salomonsson M., Morita H., Kawarabayashi Y., Mori M., Mori Y., Ito Y. (2009) Circ. Res. 104, 1399–1409 [DOI] [PubMed] [Google Scholar]
- 44.Gottlieb P., Folgering J., Maroto R., Raso A., Wood T. G., Kurosky A., Bowman C., Bichet D., Patel A., Sachs F., Martinac B., Hamill O. P., Honoré E. (2008) Pflugers Arch. 455, 1097–1103 [DOI] [PubMed] [Google Scholar]
- 45.Dietrich A., Mederos Y Schnitzler M., Gollasch M., Gross V., Storch U., Dubrovska G., Obst M., Yildirim E., Salanova B., Kalwa H., Essin K., Pinkenburg O., Luft F. C., Gudermann T., Birnbaumer L. (2005) Mol. Cell. Biol. 25, 6980–6989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trebak M., Vazquez G., Bird G. S., Putney J. W., Jr. (2003) Cell Calcium 33, 451–461 [DOI] [PubMed] [Google Scholar]