Abstract
Phosphate is critical for the maintenance of skeletal integrity, is a necessary component of important biomolecules, and is central to signal transduction and cell metabolism. It is becoming clear that endocrine communication occurs between the skeleton, kidney, and the intestine to maintain proper serum phosphate concentrations, with the kidney being the primary site for minute-to-minute regulation. Identification of genetic alterations in Mendelian disorders of hypo- and hyperphosphatemia has led to the isolation of novel genes and new roles for existing proteins in the control of renal phosphate handling, such as Fibroblast growth factor-23 (FGF23) and its processing systems, the co-receptor α-Klotho (KL), and phosphate transporters. Recent findings also indicate that FGF23 has feedback mechanisms intertwined with parathyroid hormone (PTH) and vitamin D that control phosphate homeostasis. This review will highlight genetic, and in vitro and in vivo findings, and discuss how these clinical and experimental discoveries have uncovered novel aspects of renal phosphate handling, as well as opened new research and therapeutic avenues.
Keywords: hypophosphatemia, tumoral calcinosis, Klotho, tumor induced osteomalacia, ADHR, XLH, ARHR, hyperphosphatemia, Fibroblast growth factor-23, FGF23, PTH, vitamin D
Introduction
Serum phosphate levels are regulated by complex processes involving the intestine, skeleton, and the kidneys. Maintaining serum phosphate levels is critical for proper bone development and for skeletal integrity. Additionally, phosphorus is a necessary component of DNA and RNA, and is essential for cellular metabolism as an energy source in the form of ATP1. Phosphate is abundant in the diet, and intestinal absorption is efficiently regulated1. However, the mechanisms of renal phosphate regulation, the most critical organ system for maintaining short-term serum phosphate concentrations, are incompletely understood. Investigating the molecular etiology of Mendelian disorders characterized by disturbed renal ion homeostasis has been instrumental in identifying new regulators which guide kidney phosphate handling.
This review will address major factors in renal phosphate metabolism, including Fibroblast growth factor-23 (FGF23), parathyroid hormone (PTH), and vitamin D, as well as disorders of hypo- and hyperphosphatemia that have identified novel systems important for phosphate balance. Recent advances in a novel intra-renal signaling axis upstream of phosphate reabsorption will also be discussed.
Phosphate Regulation
Phosphate, which composes approximately 1% of total body weight, is widely distributed in the soft tissues of the body, both in inorganic form and as a component of organic molecules, including nucleic acids and membrane phospholipids, as well as other phosphoproteins. However, these non-osseous phosphates comprise less than 20% of the total body content. The remainder is stored in the bone matrix. In humans, the average phosphate intake in an adult is 800–1600 mg/day2, and this large range is primarily due to the high phosphate levels in Western diets. The normal range for serum phosphate in adults is 2.5–4.5 mg/dl3, and in children is higher and varies with age (the younger the child, the higher the phosphate)4.
Phosphate taken in through the diet is initially absorbed by the small intestine through an active, sodium-dependent process and a passive, diffusional process that is load-dependent5. The Type II sodium-phosphate co-transporters are responsible for the majority of physiological phosphate transport. There are three members of this family, NPT2a (SLC34A1), NPT2b (SLC34A2), and NPT2c (SLC34A3). NPT2a is primarily expressed in the apical brush border membrane of the kidney proximal tubule and is central to renal phosphate reabsorption. NPT2b is primarily expressed in the small intestine and is regulated by vitamin D. NPT2b has a low affinity for phosphate, and humans with NPT2b inactivating mutations do not have a phosphate phenotype6. However, a conditional-null Npt2b mouse model demonstrated this transporter has a primary role in active intestinal phosphate absorption7. Interestingly, deletion of Npt2b resulted in an increase in renal proximal tubule Npt2a expression, and the animals showed normal serum phosphate concentrations, most likely due to compensatory renal reabsorption. Thus changes in intestinal phosphate absorption may affect renal phosphate handling, perhaps indirectly through alterations in serum phosphate concentrations, or potentially through the production of intestinally-derived circulating peptides8. These findings indicate that intestinal phosphate absorption may have important dynamics that are yet to be uncovered.
The skeleton represents the largest reservoir of phosphate, primarily complexed with calcium in the form of hydroxyapatite crystals, which constitute the main inorganic component of the mineralized bone matrix. The majority of phosphate retained in the body is deposited in bone as both crystalline hydroxyapatite, which constitutes 85% of bone, and amorphous calcium phosphate that makes up the remaining 15%9. As serum phosphate decreases, it is resorbed from bone through the activity of PTH and vitamin D10.
The major organ regulating minute-to-minute phosphate homeostasis is the kidney, and approximately 70% of filtered phosphate is reabsorbed within the proximal tubule where NPT2a and NPT2c are localized2. Phosphate transport across the renal proximal tubular cell is largely unidirectional and involves uptake across the brush border membrane, translocation across the cell, and efflux at the basolateral membrane. The rate-limiting step in the overall reabsorptive process is phosphate uptake at the cell surface, and consequently is the major site of its regulation1. Npt2a, and to a lesser extent, Npt2c, actively transport phosphate from the phosphate-rich nephron lumen into proximal tubule cells. In mice, Npt2a is responsible for approximately 70% of active phosphate transport11 with Npt2c likely compromising the remaining 30%. Npt2c is expressed in the renal proximal tubule brush border membrane, with a more restricted expressional pattern than Npt2a, and this transporter was originally thought to primarily play a role in neonatal phosphate balance in mice12. In humans, NPT2c may play a larger role in kidney phosphate reabsorption as inactivating mutations in this gene lead to a hypophosphatemic syndrome13, 14 (see below).
Control of Renal Phosphate Reabsorption: PTH
The primary function of PTH is to regulate serum calcium concentrations. In this regard, hypocalcemia stimulates the parathyroid glands to produce and release PTH. PTH increases the expression of the proximal tubule 25(OH) vitamin D 1-αhydroxylase15, the enzyme that produces the active form of vitamin D, 1,25(OH)2 vitamin D (1,25(OH)2D), and increases calcium reabsorption in the renal distal convoluted tubule (DCT). PTH also stimulates the release of calcium from bones into the extracellular fluid by increasing osteoclastic bone resorption16. In addition to its effects on calcium, PTH is one of the best characterized hormonal regulators of serum phosphate concentrations. Following PTH delivery in vitro or in vivo, NPT2a protein expression in the proximal tubule apical membrane is reduced (Figure 1)17. This effect results from relatively rapid internalization of Npt2a and Npt2c proteins and to subsequent degradation18, 19, independent of transcriptional control of the co-transporters20. These effects are mirrored in patients with defects in PTH regulation, as patients with hyperparathyroidism develop renal phosphate wasting, and those with hypoparathyroidism have increased renal phosphate reabsorption. To regulate sodium-phosphate co-transporter expression in the apical membrane of proximal tubule cells, PTH signals through the type 1 PTH receptor (PTHR1) via PKA and PKC, as well as through the MAPK pathway21, 22 to control internalization and degradation.
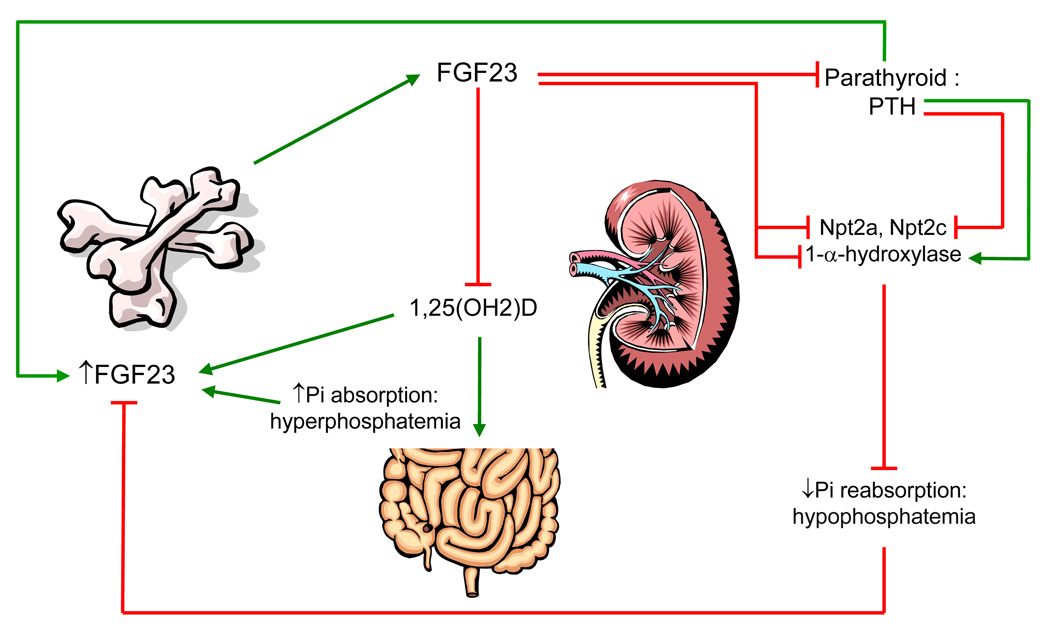
Figure 1. FGF23 regulatory systems in phosphate metabolism.
FGF23 is produced in bone and secreted into the circulation, potentially in response to increased phosphate, 1,25(OH)2D, and PTH. FGF23 acts in the kidney to decrease Npt2a and Npt2c expression and decrease 1,25(OH)2D production, resulting in hypophosphatemia. Potentially, in a novel feedback loop FGF23 may reduce PTH mRNA and protein. (Red arrows indicate suppressive effects; green arrows indicate stimulatory effects).
1,25(OH)2D regulates serum phosphate concentrations by increasing intestinal calcium and phosphate absorption, and at high concentrations, increasing phosphate mobilization from bone through increased osteoclast activity23. The opposing effects of PTH and vitamin D on the kidney and the intestine, respectively, keep phosphate levels in balance while preserving calcium ion homeostasis24. Recent findings indicate that both PTH and vitamin D production could be influenced by FGF23 in negative feedback loops (Figure 1), adding further complexity to this regulatory system (see below).
Fibroblast growth factor-23
FGF23, identified as the causative gene in autosomal dominant hypophosphatemic rickets (ADHR)25, plays a central role in phosphate regulation26. The FGF23 gene, composed of three exons encoding a 251 residue polypeptide, is located on the human chromosome 12p1325. Although FGF23 mRNA can be detected at low levels in many tissues including heart, liver, thyroid/parathyroid, and small intestine, it is predominantly expressed in bone25 by osteoblasts, osteocytes, flattened bone lining cells, and osteoprogenitor cells27.
Full-length FGF23 (32 kD) is the biologically active form of the protein which can be cleaved into 20 and 12 kDa fragments. The N-terminal region of FGF23 contains a conserved FGF-homology domain, whereas the C-terminus comprises a unique 72-amino acid tail28. Intracellular proteolysis and inactivation of FGF23 occurs at the subtilisin-like proprotein convertase (SPC) site R176HTR179/S180, (RXXR/S motif) that separates the FGF-like domain from the C-terminal tail25, 28. The FGF23 ADHR mutations, R176Q, R179Q, and R179W, destroy this site25, 29 and stabilize the full-length active form of the protein. In support of these genetic observations, when full-length FGF23, the N-terminal fragment, or the C-terminal fragment is delivered in vivo, only the intact hormone causes a reduction in serum phosphate concentrations28. Interestingly, recent evidence supports that the 72-residue C-terminal fragment of FGF23 is able to bind to an FGFR1-KL signaling complex in vitro, and competitively decrease FGF23 bioactivity in vitro and in vivo30.
Regulation of FGF23 and in vivo renal bioactivity
In mice, increased dietary phosphate increases serum phosphate concentrations and circulating FGF23, and the reciprocal relationship is present for low phosphate diets31. These findings have also been reported in some human studies, but the effect of phosphate on FGF23 appears to be less robust than in mice32–34. Whether phosphate has direct effects on the skeleton to control FGF23 production and secretion is currently unknown.
Similar to PTH, FGF23 functions to reduce phosphate reabsorption in the proximal tubule. However, in the converse manner to PTH, FGF23 reduces expression of the renal vitamin D 1-α-hydroxylase and increases expression of the catabolic 25(OH)D-24-hydroxylase, thus decreasing circulating 1,25(OH)2D concentrations (Figure 1)35. The transgenic over-expression of FGF23 results in marked hypophosphatemia36, 37 due to renal phosphate wasting through the down-regulation of Npt2a and Npt2c36, 37. As expected, the FGF23 transgenic mice have rickets/osteomalacia36, 37, similar to patients with ADHR, X-linked hypophosphatemia (XLH), and tumor-induced osteomalacia (TIO). In disorders of elevated FGF23 such as ADHR and XLH, the serum vitamin D concentrations are referred to as ‘inappropriately low or normal,’ as the physiological response to the prevailing hypophosphatemia should be an increase in 1,25(OH)2D. Collectively, these observations are consistent with a negative feedback process between kidney and bone as 1,25(OH)2D stimulates FGF23 promoter activity in vitro38, 39 and production in vivo40 (Figure 1).
The Fgf23-null mouse has the reciprocal phosphate phenotype to the FGF23 transgenic mice, with severe hyperphosphatemia and elevated 1,25(OH)2D resulting in soft tissue calcifications, growth retardation, and abnormal bone mineralization41, 42. A reduction in FGF23 bioactivity leads to increased circulating 1,25(OH)2D (Figure 1) most likely through release of 1-α-hydroxylase suppression43 (Figure 1), as observed in both Fgf23-null mice, mice null for the FGF23 co-receptor α-Klotho (KL)41, 42, 44, and patients with hyperphosphatemic tumoral calcinosis (TC)45–47. Hyperphosphatemia is also observed in the Galnt3-null mouse, a model of familial TC48, which arises due to altered glycosylation and increased degradation of FGF2349. Confirming the relationship between FGF23 and vitamin D, mating the Fgf23-null or KL-null to the 1-α-hydroxylase (Cyp27B1)-null or VDR-null mice results in a reversal of the hyperphosphatemia associated with genomic loss of FGF23 and KL43, 50. Thus, the pathogenesis observed in the Fgf23- and KL-null mice may largely be due to the elevated 1,25(OH)2D concentrations.
Recently, other minerals, such as iron, have been implicated to alter FGF23 expression in humans, which then results in osteomalacia51. It was reported that some patients receiving parenteral iron infusions became hypophosphatemic with decreased 1,25(OH)2D52, 53. Case reports have since demonstrated that these patients significantly increased serum FGF23 in response to this treatment54, 55. Although a striking relationship between iron infusion and elevated FGF23 is present, the mechanisms underlying these observations are currently unknown.
Box 1. Mediators of FGF23 expression.
High serum/dietary phosphate: increase
1,25(OH)2D: increase
PTH: increase
Iron infusion: increase
FGFs/Wnt activity: increase and/or decrease?
Renal FGF23 bioactivity and associations with α-Klotho
FGF23 binds to a signaling complex composed of the co-receptor α-Klotho (KL) and a fibroblast growth factor receptor (FGFR) for bioactivity56. KL is expressed in limited tissues including choroid plexus, parathyroid glands, and the kidney44, which provides for tissue-specific effects of circulating FGF23. In vitro evidence supports associations between FGFR1c and KL as part of a receptor complex to elicit FGF23 signaling through the mitogen activated protein kinase (MAPK) cascade and phospho-ERK1/2 (p-ERK1/2)56. Interactions between multiple FGFRs and KL have also been identified in vitro57. Underscoring the importance of the formation of a KL-FGFR receptor complex, high levels of FGF23 signaling in vitro occur when KL and FGFR1c are co-expressed56, and this activity can be blocked by anti-FGF23 antibodies that disrupt FGFR-FGF23-KL associations58. In further support of direct FGF23-KL interactions, the Fgf23- and KL-null animals have identical hyperphosphatemic phenotypes41, 42, 44, 59. Additionally, a recessive, loss of function mutation in the human KL gene resulted in impaired KL expression and activity in vitro, and to a severe tumoral calcinosis (TC) phenotype, most likely due to end-organ resistance to FGF2345. Interestingly, in the converse situation, a case has been reported with a novel balanced translocation that increases the expression of α-KL in the circulation. This genetic rearrangement was associated with elevated FGF23 and PTH, and hypophosphatemic rickets60, however the molecular mechanisms underlying this phenotype are currently unknown.
In studies towards identifying the renal Fgfr that mediates FGF23 bioactivity, the Fgfr3- and Fgfr4-null mice were mated to the Hyp mouse model of XLH. Genetic removal of these receptors did not reverse the hypophosphatemic phenotype observed in the Hyp mouse61, indicating that these receptors individually may not be the primary FGFRs that transduce FGF23 signaling with KL in vivo. Thus, whether FGFR1 and/or FGFR2 are responsible for FGF23-dependent signaling, or whether KL can partner with multiple FGFRs in vivo is currently unknown.
KL is produced as at least two isoforms due to alternative splicing of the same gene. Membrane bound KL (mKL) is a 130 kD single-pass transmembrane protein characterized by a large extracellular region (KL1 and KL2 domains) and a very short (10 residue) intracellular domain that does not possess signaling capabilities62. The secreted form of KL (sKL) is approximately 80 kD and is spliced within exon 3 to result in a KL protein that does not contain the transmembrane region, and is thus secreted into the circulation62. A third isoform of KL (‘cut KL’, or cKL), also found in the circulation, can be derived by the proteolytic processing of KL near the extracellular membrane surface63.
Although KL permits FGF23 signaling when co-expressed with FGFRs in vitro, the mechanisms underlying FGF23 bioactivity in the kidney in vivo are unclear. In this regard, KL protein and mRNA have been mapped to the distal convoluted tubule (DCT)44, 64, whereas FGF23 mediates effects on Npt2a, Npt2c, and the vitamin D metabolizing enzymes within the proximal tubule35, 36 (Figure 2). Further, in vitro experiments support that the mKL isoform is capable of initiating FGF23-dependent MAPK bioactivity64. In contrast to PTH, which has direct effects on the proximal tubule to regulate Npt2a expression17, it was recently demonstrated that following FGF23 injection, initial FGF23 activity (5–10 min) as tested through detection of phospho-ERK1/2, occurs in the renal DCT, and was not localized with Npt2a in the proximal tubule64. Therefore, there is likely a spatial separation of initial FGF23 bioactivity in the DCT from NPT2a, however at this time it cannot be ruled out that a novel, direct signaling process occurs in the proximal tubule following FGF23 delivery. It has also been proposed that soluble KL, produced in the DCT, could have direct effects in the proximal tubule as a mediator of FGF23 activity65. Additional mechanistic studies are needed to clarify the role of the soluble KL isoforms in phosphate handling, but increased activity of circulating soluble KL could potentially explain the phenotype of the hypophosphatemic patient with the balanced translocation described above60. KL is an attractive therapeutic target for disorders involving increased FGF23 bioactivity, although it will be necessary to first determine which KL isoforms are responsible for FGF23-mediated phosphate handling, and whether soluble forms of KL regulate FGF23 activity within, as well as outside of the kidney.
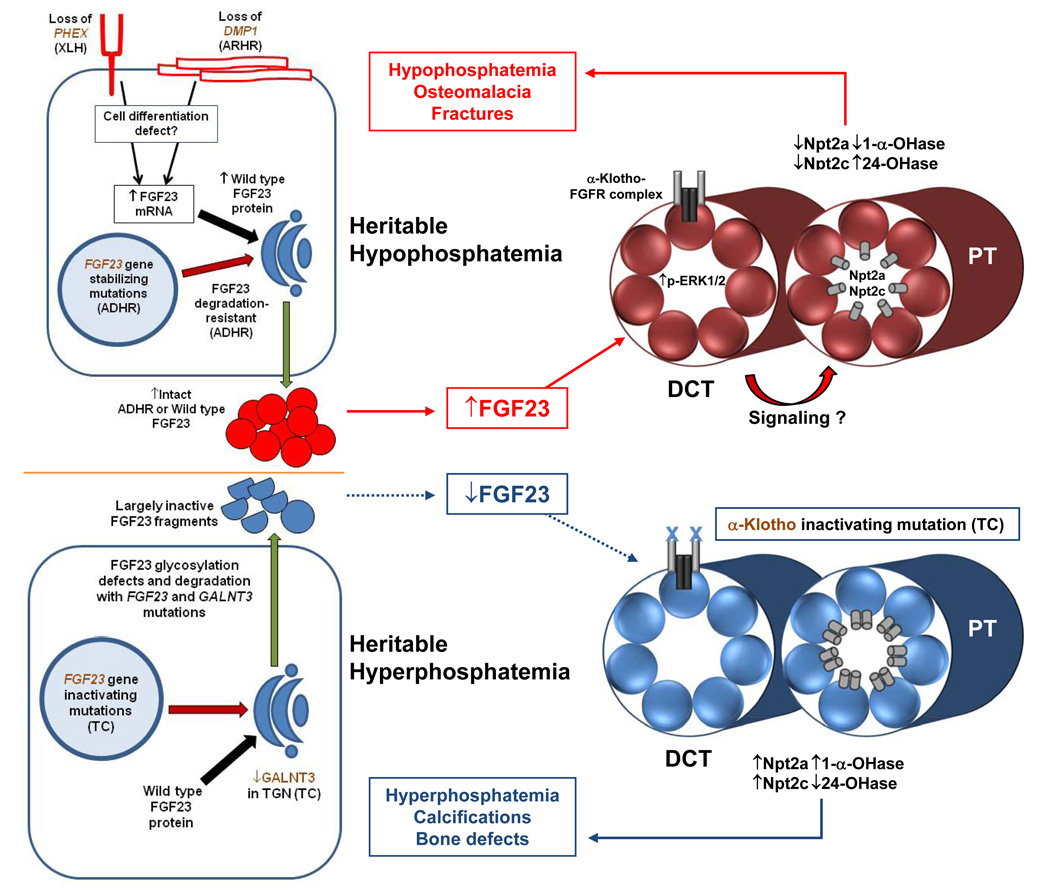
Figure 2. The molecular and physiological consequences of genetic alterations in heritable hypo- and hyperphsophatemia.
FGF23 is produced in osteoblasts and osteocytes (left). In hypophosphatemic disorders (outlined in red and red arrows), loss of PHEX and DMP1, in XLH and ARHR, respectively, are associated with a cell differentiation defect that causes elevated FGF23 by unknown mechanisms. The ADHR gain of function alterations in FGF23 result in a more stable full length protein. Circulating FGF23 (wild type or ADHR-mutant) signals through p-ERK1/2 in the renal DCT and down-regulates Npt2a, Npt2c, and the 1-α-hydroxylase, and increases the catabolic 24-hydroxylase in the proximal tubule through unknown mechanisms. This process leads to hypophosphatemia, osteomalacia, and fracture. In the hyperphosphatemic disorder TC (outlined in blue and blue arrows), loss of function mutations in FGF23 and GALNT3 result in incompletely glycosylated FGF23, which increases susceptibility to proteolysis and results in the secretion of inactive FGF23 fragments. Loss of FGF23 bioactivity results in the converse expression of the sodium-phosphate co-transporters and vitamin D metabolizing enzymes. The effect on serum biochemistries is hyperphosphatemia with elevated 1,25(OH)2D, which leads to ectopic and vascular calcifications.
FGF23 and PTH
Recent evidence points to a role for FGF23 in directly regulating PTH production, as the FGF23 co-receptor KL is highly expressed in the parathyroid glands44. Independent, parallel studies demonstrated that FGF23 delivery to bovine parathyroid cells in vitro, or to rats in vivo, increased p-ERK1/2 activity and reduced PTH mRNA and circulating concentrations, respectively66, 67 (Figure 1). In further support of a PTH-FGF23 axis, a patient with Jansen’s metaphyseal chondrodysplasia, caused by activating mutations in the Type 1 PTH/PTHrP receptor (PTHR1), had significantly elevated serum FGF23 levels, despite persistent hypophosphatemia68, suggesting that PTH could potentially increase FGF23 production in bone. In support of this hypothesis, in vitro experiments have shown a direct relationship between PTH and FGF23. In a study reported in preliminary form, treatment of primary cultures of differentiated calvarial osteoblasts/osteocytes with PTH, PTHrP, and cAMP analogs resulted in dose- and time-dependent increases in Fgf23 mRNA69. Moreover, a transgenic mouse model carrying the same Jansen’s PTHR1 mutation (H223R) specifically in osteocytes showed elevated serum Fgf2368, 70. These studies support the idea that PTH may increase FGF23 in bone, and subsequently FGF23 may activate KL and an FGFR in the parathyroids to reduce PTH production (Figure 1). Certainly, these systems are more complex than realized, as patients with primary hyperparathyroidism have been reported to have variable serum FGF2371, and the fact that PTH could potentially regulate FGF23 indirectly through 1,25(OH)2D. These findings also indicate that additional regulatory loops may be involved in FGF23 production, and speculatively, that these loops may be physiologically ‘prioritized’. In this regard, the hypophosphatemia in primary hyperparathyroidism may strongly suppress FGF23 production at the genomic level and not permit PTH-dependent increases in FGF23. Collectively, these findings underscore the complexity of phosphate homeostasis, and further study is needed to resolve potentially new regulatory pathways between FGF23, PTH, and vitamin D.
Disorders of reduced renal phosphate reabsorption
As described above, ADHR is caused by gain of function mutations in FGF23 (Figure 2; Table 1). ADHR is a rare renal phosphate wasting disorder characterized by hypophosphatemia, elevated FGF23 serum levels, inappropriately normal 1,25(OH)2D levels, normal PTH, and normocalcemia72 (Table 1). In general, patients with ADHR present with bone pain, osteomalacia, fractures, and tooth abscesses, similar to XLH patients. However, ADHR is unique among the hypophosphatemic syndromes in that patients manifest the disease in two distinct groups either as children, with bone pain, weakness, and insufficiency fractures; or as adults, with low TmP/GFR and no extremity deformities, but potentially with pseudofractures. Additionally, unaffected carriers of ADHR have also been observed72. Importantly, the degree of disease manifestation in ADHR is correlated with serum FGF23 concentrations73.
Table 1.
Molecular and physiological manifestations in disorders involving FGF23
| Syndrome | OMIM Ref. nos. |
Gene | Mutation type |
Effect on FGF23-related systems |
Effect on serum Pi |
Effect on serum 1,25D |
Intact FGF23 ELISA |
C-terminal FGF23 ELISA |
|---|---|---|---|---|---|---|---|---|
| ADHR | 193100 (FGF23: 605380) |
FGF23 | Gain of function | Stabilize full-length FGF23 | ↓ | *↔ | ↔ or ↑ | ↔ or ↑ |
| XLH | 307800 (PHEX: 300550) |
PHEX | Loss of function | Increased FGF23 production in osteocytes | ↓ | *↔ or ↓ | ↔ or ↑ | ↔ or ↑ |
| ARHR | 241520 (DMP1: 600980) |
DMP1 | Loss of function | Increased FGF23 production in osteocytes | ↓ | *↔ | ↔ or ↑ | ↔ or ↑ |
| MAS/FD | 174800 | GNAS1 | Gain of function | Increased FGF23 production in lesions | ↓ | *↔ | ↔ or ↑ | ↔ or ↑ |
| TIO | 605380 (FGF23) |
-- | -- | FGF23 produced by tumor | ↓ | *↔ or ↓ | ↔ or ↑ | ↔ or ↑ |
| TC | 211900 | FGF23 | Loss of function | Destabilize full-length, active FGF23 | ↑ | ↔ or ↑ | ↓ | ↑ |
| TC | 211900 (GALNT3: 601756) |
GALNT3 | Loss of function | Destabilize full-length, active FGF23 | ↑ | ↔ or ↑ | ↓ | ↑ |
| HHS | 610233 | GALNT3 | Loss of function | Destabilize full-length, active FGF23 | ↑ | ↔ or ↑ | ↓ | ↑ |
| TC | 211900 (KL: 604824) |
KL | Loss of function | Decreased FGF23-dependent signaling | ↑ | ↔ or ↑ | ↑ | ↑ |
Inappropriately normal 1,25D
X-linked hypophosphatemic rickets (XLH) is the most common heritable phosphate wasting disorder, occurring in approximately 1/20,000 births. In contrast to ADHR, XLH is a fully penetrant dominant disorder with variable severity74. Patients with XLH present with defects similar to ADHR patients (Table 1), but also manifest enthesopathy (calcification of the tendons and ligaments). XLH is caused by inactivating mutations in PHEX (Phosphate-regulating gene with homologies to endopeptidases on the X chromosome)75, a member of the M13 family of membrane-bound metalloproteases. Studies show that there is no predominant PHEX mutation that leads to XLH76.
PHEX is expressed in osteoblasts and osteocytes, and odontoblasts in teeth77. Serum FGF23 is elevated in most XLH patients (Table 1)78–80, and is also elevated in Hyp mice, which display approximately 10-fold higher concentrations than wild type controls81. Quantitative real-time RT-PCR (qPCR) analysis examining bone revealed that Fgf23 mRNA is also elevated82. Additionally, renal Npt2a expression is reduced by ~50% in this model83. These findings provide support for a physiological connection between PHEX activity and FGF23 expression.
A relatively new disorder, autosomal recessive hypophosphatemic rickets (ARHR) in which patients have Dentin matrix protein-1 (DMP1) inactivating mutations, has been identified 84, 85. ARHR is characterized by a similar biochemical phenotype to that of ADHR and XLH (Table 1), including elevated serum FGF23 concentrations in some patients. Of significance, ARHR patients manifest peri-osteocytic lesions upon bone biopsy, a hallmark of XLH84.
DMP1 is a member of the ‘short integrin-binding ligand interacting glycoprotein’ (SIBLING) family of skeletal matrix proteins86, and is highly expressed in osteocytes and in the canaliculi within the bone matrix. DMP1 has been proposed to have roles in canaliculi function and structural support87 and regulating hydroxyapatite formation88. The Dmp1-null mouse has a severe hypophosphatemic rickets phenotype and marked elevation of Fgf2384. Histomorphometric and EM analyses of Dmp1-null bone and ARHR patient iliac crest biopsies demonstrated malformed osteocytes and disorganization of the cannicular system84. Fgf23 was shown to be elevated in Dmp1-null osteocytes by in situ hybridization, consistent with the elevated serum levels. These findings revealed an important role for skeletal matrix protein in bone cell formation and in the downstream regulation of phosphate handling. Mechanistic studies using the Dmp1-null mouse demonstrated that loss of Dmp1 impairs osteocyte maturation and gene expression, leading not only to elevated Fgf23 (Figure 2), but also to the inappropriate expression of Type I collagen and alkaline phosphatase in osteocytes, which may indicate a general cell defect84. The prevailing hypophosphatemia results in pathological changes in bone mineralization in this model84, which can be largely, but not completely abrogated by administering a high phosphate diet. Importantly, Dmp1-null mice parallel the phenotypes of Hyp mice (and patients with ARHR and XLH), which all share a distinctive bone histology characterized by periosteocytic lesions of non-mineralized bone84, abnormal osteocyte morphology89, and an ‘intrinsic’ bone defect that cannot be completely resolved by increased delivery of phosphate. Thus, these findings suggest that PHEX may also have a role in osteocyte maturation that leads to over expression of FGF23 (Figure 2). More recently, gene array analyses of bone RNA from Hyp mice shows alterations in components of Fgf and Wnt signaling39. Both of these pathways are known to be central to cell differentiation and skeletal development, therefore these autocrine systems may either underlie, or be a response to, the potential differentiation defects observed in XLH/Hyp and ARHR/Dmp1-null mice.
Tumor induced osteomalacia (TIO) is an acquired disorder of renal phosphate wasting associated with tumors, typically arising from a mesenchymal origin90. Patients with TIO share similar biochemical and skeletal phenotypes with patients with ADHR, XLH, and ARHR, and may also report weakness. The study of this disorder introduced the idea for circulating factors produced by the tumor, referred to as “phosphatonins,” that act upon the kidney to reduce phosphate reabsorption, due to the fact that surgical removal of the tumor leads to a reversal of the hypophosphatemia and metabolic bone disease91, 92. FGF23 mRNA is highly expressed in TIO tumors35, 93, 94 (Figure 2), and patients can have markedly elevated serum FGF23, that rapidly returns to control levels after tumor resection78, 95. As support for the phosphatonin theory, transplant of tumor tissue into mice resulted in mirroring the original hypophosphatemic syndrome in these animals96.
Hereditary hypophosphatemic rickets with hypercalciuria (HHRH) was first described in a consanguineous Bedouin tribe97. Similar to the disorders described above, HHRH is characterized by hypophosphatemia with rickets and short stature, however, the distinguishing biochemical characteristic of this disorder when compared to the FGF23-related diseases such as ADHR and XLH is the notable increase in serum 1,25(OH)2D as a compensatory response to the prevailing hypophosphatemia97. Affected individuals also have markedly elevated urine calcium excretion with increased intestinal absorption of phosphorus and calcium, which can lead to nephrolithiasis.
To identify the genetic defect responsible for HHRH, homozygosity mapping was undertaken. With this approach, the disease locus was narrowed to the portion of chromosome 9q34 containing the SLC34A3 gene, which encodes NPT2c (Table 1)13, 14. Following direct DNA sequencing of NPT2c in unrelated HHRH kindreds, recessive, single-nucleotide deletions, compound heterozygous deletions and missense mutations have been identified. These changes are predicted to be loss of function, and most likely reduce renal phopshate absorption through decreasing apical membrane expression of NPT2c or the uncoupling of sodium-phosphate co-transport in the proximal tubule98, 99. An important implication of identifying the HHRH changes in NPT2c is that this transporter appears to have a significant role in renal Pi reabsorption throughout life, and is not limited to a role in phosphate homeostasis early in life, as hypothesized. The fact that NPT2c mutations lead to disease in humans, but not when genetically deleted in mice100, may also indicate that this transporter plays a more significant role in humans than other species. There have also been reports of mutations in NPT2a and NHERF, which regulates Npt2a expression and activity, leading to hypophosphatemia101, 102. Further in vitro and in vivo studies will be useful in determining the molecular mechanisms underlying these syndromes.
Approximately half of all patients affected by polyostotic fibrous dysplasia associated with McCune-Albright syndrome (MAS/FD) will develop hypophosphatemic rickets/osteomalacia27 (Table 1). MAS/FD is caused by somatic mutations in GNAS1, and characterized by benign fibrous tissue that replaces normal bone. Individuals with MAS/FD who develop hypophosphatemia and isolated renal phosphate wasting have elevated serum FGF23, which is produced by osteogenic cells in the fibrous lesions27.
Box 2. Animal models of human disorders involving FGF23.
-
Hypophosphatemia:
XLH: Hyp mouse
ARHR: Dmp1-null mouse
TIO: FGF23 transgenic mice
-
Hyperphosphatemia:
TC/HHS: Galnt3-null mouse
TC: Fgf23-null mouse
TC: KL-null mouse
Disorders of increased renal phosphate reabsorption
Tumoral calcinosis (TC) is an autosomal recessive syndrome characterized by hyperphosphatemia, and normal or elevated 1,25(OH)2D concentrations, often with the development of severe ectopic and vascular calcifications103; a phenotype that closely resembles that of the Fgf23- and KL-null mice37, 44. TC was first determined to be caused by inactivating mutations in the GalNAc transferase 3 (GALNT3) gene, which initiates O-linked glycosylation (Table 1)47. Mature active FGF23 protein is O-glycosylated in three regions, with the most critical being within the 176RH177T178R179/S180 SPC-like domain, at position T178104. This key glycosylation site likely prevents intracellular degradation of FGF23 at the RXXR motif by SPC-like enzymes in the trans-Golgi network104 (Figure 2). When inactivating GALNT3 mutations are present in TC patients, only minute amounts of intact FGF23 are produced, leading to hyperphosphatemia49. In support of this, subsequent analysis of the Galnt3-null mouse revealed that this model has reduced circulating intact Fgf23 and increased Fgf23 circulating fragments, but has increased bone Fgf23 mRNA. These studies indicate that the mice display the expected physiological response to increased circulating phosphate and 1,25(OH)2D by increasing Fgf23 transcription, but the defective O-glycosylation reduces intact, bioactive Fgf23 concentrations48.
The TC phenotype is also caused by recessive inactivating mutations in the conserved FGF-like domain of FGF23 (Figure 2; Table 1), which destabilize the full-length form of the hormone105–107. Strikingly, patients with mutations in GALNT3 and FGF23, both have low intact serum FGF23 levels, but significantly elevated C-terminal fragments of FGF2347, 49, which likely represents an attempt to increase FGF23 production. The biochemical profile of elevated C-terminal serum FGF23 in conjunction with low intact serum FGF23 is specific for GALNT3- and FGF23-TC. Importantly, it has recently been shown in vitro, that recombinant FGF23 carrying FGF23-TC mutations has defective O-glycosylation, thus making this species of FGF23 more prone to intracellular degradation. These observations likely provide clarification for the identical serum FGF23 profile of patients with FGF23- and GALNT3-mutant TC108. Hyperostosis-hyperphosphatemia syndrome (HHS) was first described as a separate disorder from TC due to radiological findings of abnormalities involving cortical bone hyperostosis109. However mutational analysis of patients with HHS revealed identical mutations to those of patients diagnosed with GALNT3-TC and the same inappropriate intact/C-terminal FGF23 concentrations106, 109, thus the two syndromes are allelic. The molecular mechanisms underlying the large range of phenotypes between TC and in HHS are currently unknown.
A homozygous mutation in KL, H193R, was shown to be a third cause of TC in a single case45 (Figure 2). This patient presented with hyperphosphatemia, hypercalcemia, elevated PTH, and ectopic calcifications in the heel and brain45. The missense mutation is localized within a highly conserved region in exon 1, present in all isoforms of KL. In vitro analysis of the mutant protein illustrated decreased mature, glycosylated KL expression, as well as reduced FGF23-dependent signaling through MAPK, thus this mutation is assumed to prevent FGF23 bioactivity in vivo. In contrast to TC patients with mutations in GALNT3 and FGF23, the KL-TC patient had significantly elevated serum intact and C-terminal FGF23 (>150-fold control). This increase is likely a response to the significant hyperphosphatemia and elevated 1,25(OH)2D, as the FGF23 gene itself is not altered in this patient. These findings provide important support for direct interactions between FGF23 and KL.
CKD and emerging therapeutics
As described herein, a large body of evidence supports that FGF23 and its signaling complexes are central to renal phosphate homeostasis. In clinical practice, the most common form of pathological change involving serum phosphate is hyperphosphatemia from chronic kidney disease (CKD), which is now estimated to affect 1 in 8 in the US alone110. The increase in serum phosphate balance in these patients results from several different mechanisms, however the most important factor is reduced renal phosphate excretion. A further aggravating factor is secondary hyperparathyroidism, which is manifested by a large proportion of patients with CKD, and may result in increased bone resorption and further elevations in phosphate arising from skeletal stores111.
Due to the increase in serum phosphate in CKD patients and the known physiological response of FGF23, it was hypothesized that patients with advanced renal disease may have elevated FGF23112. This has now been demonstrated using multiple FGF23 serum assays78, 95, and importantly, in CKD patients assessed as quartiles with similar serum phosphate concentrations, the groups with elevated FGF23 demonstrated a 5–6 fold increase in the odds for mortality113. Further, the circulating FGF23 may be in part responsible for the suppressed 1,25(OH)2D concentrations observed in these patients, as FGF23 is known to reduce the renal 1-α-hydroxylase and stimulate the catabolic 24-hydroxylase40. Importantly, this may occur under situations of hyperparathyroidism in CKD, which should have the opposite effects on vitamin D metabolism.
Progress towards potential treatments for the heritable hypophosphatemias was initially tested in model systems, and is underway in clinical trials through the use of inactivating monoclonal antibodies targeting FGF23. In the Hyp mouse, the anti-FGF23 completely reversed the hypophosphatemia and associated bone disease, including the osteomalacia and reduced bone length, after four weeks of treatment58. Humanized anti-FGF23 therapy (‘KRN23’) is now undergoing Phase I clinical trial in patients with XLH. It is tempting to speculate that similar classes of therapies could be applied to FGF23 or its mediators of activity to modulate renal phosphate and vitamin D metabolism, or potentially PTH production, in patients with early stage CKD. In patients with partial renal function, suppression of FGF23 activity may elevate 1,25(OH)2D, however this approach could also increase renal phosphate reabsorption, and further raise serum phosphate concentrations. Certainly, additional studies examining the role of FGF23 in animal models will need to be performed to relate these observations to potential future therapeutics targeting FGF23 in this disorder.
Conclusions
In sum, the regulation of renal phosphate handling is complex and controlled by overlapping endocrine processes. PTH is primarily responsible for calcium balance, but plays significant roles in kidney phosphate homeostasis by directly acting upon the proximal tubule. The molecular discoveries associated with the heritable hypophosphatemias and TC demonstrate that FGF23 is central to phosphate and vitamin D metabolism in humans. Increased FGF23 bioactivity is associated with renal phosphate loss and inappropriately normal 1,25(OH)2D concentrations, whereas decreased FGF23 bioactivity results in increased renal phosphate reabsorption and elevated 1,25(OH)2D. Further, the identification of NPT2c inactivating mutations has revealed novel mechanisms guiding the direct control of proximal tubule phosphate handling. Finally, the recently-discovered relationships between the kidney, skeleton, and intestine will provide new and important insight into phosphate homeostasis under normal circumstances and in disease.
Acknowledgments
Financial disclosures/Acknowledgements
KEW receives royalties for licensing the FGF23 gene to Kyowa Hakko Kirin, Ltd. The authors would like to acknowledge support by NIH grant DK063934 (KEW), the Showalter Foundation, Genzyme Corporation, and the Indiana Genomics Initiative (INGEN) of Indiana University, supported in part by the Lilly Endowment, Inc. We also acknowledge the editorial and scientific contributions of Ms. Lelia J. Summers.
References
- 1.Takeda E, Taketani Y, Sawada N, Sato T, Yamamoto H. The regulation and function of phosphate in the human body. Biofactors. 2004;21:345–355. doi: 10.1002/biof.552210167. [DOI] [PubMed] [Google Scholar]
- 2.Tenenhouse HS. Regulation of phosphorus homeostasis by the type iia na/phosphate cotransporter. Annu Rev Nutr. 2005;25:197–214. doi: 10.1146/annurev.nutr.25.050304.092642. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg BG, Winters RW, Graham JB. The normal range of serum inorganic phosphorus and its utility as a discriminant in the diagnosis of congenital hypophosphatemia. J Clin Endocrinol Metab. 1960;20:364–379. doi: 10.1210/jcem-20-3-364. [DOI] [PubMed] [Google Scholar]
- 4.Burritt MF, et al. Pediatric reference intervals for 19 biologic variables in healthy children. Mayo Clin Proc. 1990;65:329–336. doi: 10.1016/s0025-6196(12)62533-6. [DOI] [PubMed] [Google Scholar]
- 5.Walton J, Gray TK. Absorption of inorganic phosphate in the human small intestine. Clin Sci (Lond) 1979;56:407–412. doi: 10.1042/cs0560407. [DOI] [PubMed] [Google Scholar]
- 6.Corut A, et al. Mutations in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly associated with testicular microlithiasis. Am J Hum Genet. 2006;79:650–656. doi: 10.1086/508263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabbagh Y, et al. Intestinal Npt2b Plays a Major Role in Phosphate Absorption and Homeostasis. J Am Soc Nephrol. 2009 doi: 10.1681/ASN.2009050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berndt T, et al. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc Natl Acad Sci U S A. 2007;104:11085–11090. doi: 10.1073/pnas.0704446104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silve C, Friedlander G. In: The Kidney: Physiology & Pathophysiology. Seldin DW, Giebisch G, editors. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 1885–1904. [Google Scholar]
- 10.Baron R, editor. Anatomy and Biology of Bone Matrix and Cellular Elements. Washington, D.C.: American Society for Bone and Mineral Research; 2003. [Google Scholar]
- 11.Beck L, et al. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci U S A. 1998;95:5372–5377. doi: 10.1073/pnas.95.9.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohkido I, Segawa H, Yanagida R, Nakamura M, Miyamoto K. Cloning, gene structure and dietary regulation of the type-IIc Na/Pi cotransporter in the mouse kidney. Pflugers Arch. 2003;446:106–115. doi: 10.1007/s00424-003-1010-6. [DOI] [PubMed] [Google Scholar]
- 13.Lorenz-Depiereux B, et al. Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet. 2006;78:193–201. doi: 10.1086/499410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergwitz C, et al. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet. 2006;78:179–192. doi: 10.1086/499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omdahl JL, Gray RW, Boyle IT, Knutson J, DeLuca HF. Regulation of metabolism of 25-hydroxycholecalciferol by kidney tissue in vitro by dietary calcium. Nat New Biol. 1972;237:63–64. doi: 10.1038/newbio237063a0. [DOI] [PubMed] [Google Scholar]
- 16.Parfitt AM. The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone disease. Part IV of IV parts: The state of the bones in uremic hyperaparathyroidism--the mechanisms of skeletal resistance to PTH in renal failure and pseudohypoparathyroidism and the role of PTH in osteoporosis, osteopetrosis, and osteofluorosis. Metabolism. 1976;25:1157–1188. doi: 10.1016/0026-0495(76)90024-x. [DOI] [PubMed] [Google Scholar]
- 17.Bacic D, et al. The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int. 2006;69:495–503. doi: 10.1038/sj.ki.5000148. [DOI] [PubMed] [Google Scholar]
- 18.Pfister MF, et al. Parathyroid hormone-dependent degradation of type II Na+/Pi cotransporters. J Biol Chem. 1997;272:20125–20130. doi: 10.1074/jbc.272.32.20125. [DOI] [PubMed] [Google Scholar]
- 19.Lotscher M, et al. New aspects of adaptation of rat renal Na-Pi cotransporter to alterations in dietary phosphate. Kidney Int. 1996;49:1012–1018. doi: 10.1038/ki.1996.146. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi F, et al. Effects of dietary Pi on the renal Na+-dependent Pi transporter NaPi-2 in thyroparathyroidectomized rats. Biochem J. 1998;333(Pt 1):175–181. doi: 10.1042/bj3330175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Traebert M, Volkl H, Biber J, Murer H, Kaissling B. Luminal and contraluminal action of 1–34 and 3–34 PTH peptides on renal type IIa Na-P(i) cotransporter. Am J Physiol Renal Physiol. 2000;278:F792–F798. doi: 10.1152/ajprenal.2000.278.5.F792. [DOI] [PubMed] [Google Scholar]
- 22.Bacic D, et al. Involvement of the MAPK-kinase pathway in the PTH-mediated regulation of the proximal tubule type IIa Na+/Pi cotransporter in mouse kidney. Pflugers Arch. 2003;446:52–60. doi: 10.1007/s00424-002-0969-8. [DOI] [PubMed] [Google Scholar]
- 23.Portale AA, Halloran BP, Morris RC., Jr Physiologic regulation of the serum concentration of 1,25-dihydroxyvitamin D by phosphorus in normal men. J Clin Invest. 1989;83:1494–1499. doi: 10.1172/JCI114043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ADHR-Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 26.White KE, Larsson TE, Econs MJ. The roles of specific genes implicated as circulating factors involved in normal and disordered phosphate homeostasis: frizzled related protein-4, matrix extracellular phosphoglycoprotein, and fibroblast growth factor 23. Endocr Rev. 2006;27:221–241. doi: 10.1210/er.2005-0019. [DOI] [PubMed] [Google Scholar]
- 27.Riminucci M, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112:683–692. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimada T, et al. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. 2002;143:3179–3182. doi: 10.1210/endo.143.8.8795. [DOI] [PubMed] [Google Scholar]
- 29.White KE, et al. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–2086. doi: 10.1046/j.1523-1755.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 30.Goetz R, et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0902006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perwad F, et al. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146:5358–5364. doi: 10.1210/en.2005-0777. [DOI] [PubMed] [Google Scholar]
- 32.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91:3144–3149. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 33.Nishida Y, et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 2006;70:2141–2147. doi: 10.1038/sj.ki.5002000. [DOI] [PubMed] [Google Scholar]
- 34.Ito N, et al. Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J Bone Miner Metab. 2007;25:419–422. doi: 10.1007/s00774-007-0779-3. [DOI] [PubMed] [Google Scholar]
- 35.Shimada T, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsson T, et al. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–3094. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- 37.Shimada T, et al. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun. 2004;314:409–414. doi: 10.1016/j.bbrc.2003.12.102. [DOI] [PubMed] [Google Scholar]
- 38.Kolek OI, et al. 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1036–G1042. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- 39.Liu S, et al. Novel regulators of Fgf23 expression and mineralization in Hyp bone. Mol Endocrinol. 2009;23:1505–1508. doi: 10.1210/me.2009-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimada T, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 41.Shimada T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sitara D, et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–432. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hesse M, Frohlich LF, Zeitz U, Lanske B, Erben RG. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. 2007;26:75–84. doi: 10.1016/j.matbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Kuro-o M, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 45.Ichikawa S, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14:385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- 47.Topaz O, et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36:579–581. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- 48.Ichikawa S, et al. Ablation of the Galnt3 gene leads to low-circulating intact fibroblast growth factor 23 (Fgf23) concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology. 2009;150:2543–2550. doi: 10.1210/en.2008-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garringer HJ, et al. The role of mutant UDP-N-acetyl-alpha-D-galactosamine-polypeptide N-acetylgalactosaminyltransferase 3 in regulating serum intact fibroblast growth factor 23 and matrix extracellular phosphoglycoprotein in heritable tumoral calcinosis. J Clin Endocrinol Metab. 2006;91:4037–4042. doi: 10.1210/jc.2006-0305. [DOI] [PubMed] [Google Scholar]
- 50.Ohnishi M, Nakatani T, Lanske B, Razzaque MS. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int. 2009;75:1166–1172. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schouten BJ, Hunt PJ, Livesey JH, Frampton CM, Soule SG. FGF23 elevation and hypophosphataemia following intravenous iron polymaltose - a prospective study. J Clin Endocrinol Metab. 2009 doi: 10.1210/jc.2008-2396. [DOI] [PubMed] [Google Scholar]
- 52.Sato K, et al. Saccharated ferric oxide (SFO)-induced osteomalacia: in vitro inhibition by SFO of bone formation and 1,25-dihydroxy-vitamin D production in renal tubules. Bone. 1997;21:57–64. doi: 10.1016/s8756-3282(97)00084-7. [DOI] [PubMed] [Google Scholar]
- 53.Sato K, Shiraki M. Saccharated ferric oxide-induced osteomalacia in Japan: ironinduced osteopathy due to nephropathy. Endocr J. 1998;45:431–439. doi: 10.1507/endocrj.45.431. [DOI] [PubMed] [Google Scholar]
- 54.Schouten BJ, Doogue MP, Soule SG, Hunt PJ. Iron polymaltose-induced FGF23 elevation complicated by hypophosphataemic osteomalacia. Ann Clin Biochem. 2009;46:167–169. doi: 10.1258/acb.2008.008151. [DOI] [PubMed] [Google Scholar]
- 55.Shimizu Y, et al. Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: another form of FGF23-related hypophosphatemia. Bone. 2009;45:814–816. doi: 10.1016/j.bone.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 56.Urakawa I, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 57.Kurosu H, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aono Y, et al. Therapeutic Effects of Anti-FGF23 Antibodies in Hypophosphatemic Rickets/Osteomalacia. J Bone Miner Res. 2009 doi: 10.1359/jbmr.090509. [DOI] [PubMed] [Google Scholar]
- 59.Segawa H, et al. Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am J Physiol Renal Physiol. 2007;292:F769–F779. doi: 10.1152/ajprenal.00248.2006. [DOI] [PubMed] [Google Scholar]
- 60.Brownstein CA, et al. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci U S A. 2008;105:3455–3460. doi: 10.1073/pnas.0712361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu S, Vierthaler L, Tang W, Zhou J, Quarles LD. FGFR3 and FGFR4 do not mediate renal effects of FGF23. J Am Soc Nephrol. 2008;19:2342–2350. doi: 10.1681/ASN.2007121301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsumura Y, et al. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 63.Imura A, et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 64.Farrow EG, Davis SI, Summers LJ, White KE. Initial FGF23-mediated signaling occurs in the distal convoluted tubule. J Am Soc Nephrol. 2009;20:955–960. doi: 10.1681/ASN.2008070783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kurosu H, Kuro OM. The Klotho gene family as a regulator of endocrine fibroblast growth factors. Mol Cell Endocrinol. 2009;299:72–78. doi: 10.1016/j.mce.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 66.Krajisnik T, et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195:125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 67.Ben-Dov IZ, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown WW, et al. Hypophosphatemia with elevations in serum fibroblast growth factor 23 in a child with Jansen's metaphyseal chondrodysplasia. J Clin Endocrinol Metab. 2009;94:17–20. doi: 10.1210/jc.2008-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rhee Y, R. Lee EF, Bivi N, Lezcano V, Plotkin L, White K, Bellido T. FGF23 Gene Expression Is Upregulated by PTH Receptor Activation In Osteocytes In Vitro and In Vivo: A Parathyroid-Bone Link Influencing the Endocrine Function of Osteocytes. J Bone Miner Res. 2009;24 [Google Scholar]
- 70.O'Brien CA, et al. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One. 2008;3:e2942. doi: 10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kobayashi K, et al. Regulation of plasma fibroblast growth factor 23 by calcium in primary hyperparathyroidism. Eur J Endocrinol. 2006;154:93–99. doi: 10.1530/eje.1.02053. [DOI] [PubMed] [Google Scholar]
- 72.Econs MJ, McEnery PT. Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Metab. 1997;82:674–681. doi: 10.1210/jcem.82.2.3765. [DOI] [PubMed] [Google Scholar]
- 73.Imel EA, Hui SL, Econs MJ. FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J Bone Miner Res. 2007;22:520–526. doi: 10.1359/jbmr.070107. [DOI] [PubMed] [Google Scholar]
- 74.Tenenhouse HS, Roy S, Martel J, Gauthier C. Differential expression, abundance, and regulation of Na+-phosphate cotransporter genes in murine kidney. Am J Physiol. 1998;275:F527–F534. doi: 10.1152/ajprenal.1998.275.4.F527. [DOI] [PubMed] [Google Scholar]
- 75.HypConsortium. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 76.Ichikawa S, et al. Mutational survey of the PHEX gene in patients with X-linked hypophosphatemic rickets. Bone. 2008;43:663–666. doi: 10.1016/j.bone.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beck L, et al. Pex/PEX tissue distribution and evidence for a deletion in the 3' region of the Pex gene in X-linked hypophosphatemic mice. J Clin Invest. 1997;99:1200–1209. doi: 10.1172/JCI119276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jonsson KB, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 79.Weber TJ, Liu S, Indridason OS, Quarles LD. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res. 2003;18:1227–1234. doi: 10.1359/jbmr.2003.18.7.1227. [DOI] [PubMed] [Google Scholar]
- 80.Yamazaki Y, et al. Elevated circulatory and expression level of fibroblast growth factor (FGF)-23 in hypophosphatemic mice. Bone. 2003;32 [Google Scholar]
- 81.Aono Y, et al. The neutralization of FGF-23 ameliorates hypophosphatemia and rickets in Hyp mice. J Bone Miner Metab. 2003;18 [Google Scholar]
- 82.Liu S, et al. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278:37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- 83.Tenenhouse HS, Beck L. Renal Na(+)-phosphate cotransporter gene expression in X-linked Hyp and Gy mice. Kidney Int. 1996;49:1027–1032. doi: 10.1038/ki.1996.149. [DOI] [PubMed] [Google Scholar]
- 84.Feng JQ, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lorenz-Depiereux B, et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38:1248–1250. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fisher LW, Fedarko NS. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res. 2003;44 Suppl 1:33–40. [PubMed] [Google Scholar]
- 87.Feng JQ, et al. The Dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. J Dent Res. 2003;82:776–780. doi: 10.1177/154405910308201003. [DOI] [PubMed] [Google Scholar]
- 88.Ling Y, et al. DMP1 depletion decreases bone mineralization in vivo: an FTIR imaging analysis. J Bone Miner Res. 2005;20:2169–2177. doi: 10.1359/JBMR.050815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yuan B, et al. Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia. J Clin Invest. 2008;118:722–734. doi: 10.1172/JCI32702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Folpe AL, et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004;28:1–30. doi: 10.1097/00000478-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 91.Ryan EA, Reiss E. Oncogenous osteomalacia. Review of the world literature of 42 cases and report of two new cases. Am J Med. 1984;77:501–512. doi: 10.1016/0002-9343(84)90112-8. [DOI] [PubMed] [Google Scholar]
- 92.Econs MJ, Drezner MK. Tumor-induced osteomalacia--unveiling a new hormone. N Engl J Med. 1994;330:1679–1681. doi: 10.1056/NEJM199406093302310. [DOI] [PubMed] [Google Scholar]
- 93.White KE, et al. The autosomal dominant hypophosphatemic rickets (ADHR) gene is a secreted polypeptide overexpressed by tumors that cause phosphate wasting. J Clin Endocrinol Metab. 2001;86:497–500. doi: 10.1210/jcem.86.2.7408. [DOI] [PubMed] [Google Scholar]
- 94.Jan De Beur SM, et al. Tumors associated with oncogenic osteomalacia express genes important in bone and mineral metabolism. J Bone Miner Res. 2002;17:1102–1110. doi: 10.1359/jbmr.2002.17.6.1102. [DOI] [PubMed] [Google Scholar]
- 95.Yamazaki Y, et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 96.Chalew SA, Lovchik JC, Brown CM, Sun CC. Hypophosphatemia induced in mice by transplantation of a tumor-derived cell line from a patient with oncogenic rickets. J Pediatr Endocrinol Metab. 1996;9:593–597. doi: 10.1515/jpem.1996.9.6.593. [DOI] [PubMed] [Google Scholar]
- 97.Tieder M, et al. Hereditary hypophosphatemic rickets with hypercalciuria. N Engl J Med. 1985;312:611–617. doi: 10.1056/NEJM198503073121003. [DOI] [PubMed] [Google Scholar]
- 98.Jaureguiberry G, Carpenter TO, Forman S, Juppner H, Bergwitz C. A novel missense mutation in SLC34A3 that causes hereditary hypophosphatemic rickets with hypercalciuria in humans identifies threonine 137 as an important determinant of sodium-phosphate cotransport in NaPi-IIc. Am J Physiol Renal Physiol. 2008;295:F371–F379. doi: 10.1152/ajprenal.00090.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Levi M. Novel NaPi-2c mutations that cause mistargeting of NaPi-2c protein and uncoupling of Na-Pi cotransport cause HHRH. Am J Physiol Renal Physiol. 2008;295:F369–F370. doi: 10.1152/ajprenal.90327.2008. [DOI] [PubMed] [Google Scholar]
- 100.Segawa H, et al. Type IIc Sodium-Dependent Phosphate Transporter Regulates Calcium Metabolism. J Am Soc Nephrol. 2008 doi: 10.1681/ASN.2008020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prie D, et al. Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N Engl J Med. 2002;347:983–991. doi: 10.1056/NEJMoa020028. [DOI] [PubMed] [Google Scholar]
- 102.Khundmiri SJ, et al. Novel regulatory function for NHERF-1 in Npt2a transcription. Am J Physiol Renal Physiol. 2008;294:F840–F849. doi: 10.1152/ajprenal.00180.2007. [DOI] [PubMed] [Google Scholar]
- 103.Inclan ALP, Schaeffer PC, Goldsmith RS, Chausmer AB. Tumoral Calcinosis. JAMA. 1943;121:5. [Google Scholar]
- 104.Kato K, et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281:18370–18377. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- 105.Benet-Pages A, et al. FGF23 is processed by proprotein convertases but not by PHEX. Bone. 2004;35:455–462. doi: 10.1016/j.bone.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 106.Garringer HJ, et al. Two novel GALNT3 mutations in familial tumoral calcinosis. Am J Med Genet A. 2007;143:2390–2396. doi: 10.1002/ajmg.a.31947. [DOI] [PubMed] [Google Scholar]
- 107.Larsson T, et al. A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J Clin Endocrinol Metab. 2005;90:2424–2427. doi: 10.1210/jc.2004-2238. [DOI] [PubMed] [Google Scholar]
- 108.Bergwitz C, et al. Defective O-Glycosylation due to a Novel Homozygous S129P Mutation Is Associated with Lack of Fibroblast Growth Factor 23 Secretion and Tumoral Calcinosis. J Clin Endocrinol Metab. 2009 doi: 10.1210/jc.2009-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Frishberg Y, et al. Identification of a recurrent mutation in GALNT3 demonstrates that hyperostosis-hyperphosphatemia syndrome and familial tumoral calcinosis are allelic disorders. J Mol Med. 2005;83:33–38. doi: 10.1007/s00109-004-0610-8. [DOI] [PubMed] [Google Scholar]
- 110.Coresh J, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 111.Silver J, Kilav R, Sela-Brown A, Naveh-Many T. Molecular mechanisms of secondary hyperparathyroidism. Pediatr Nephrol. 2000;14:626–628. doi: 10.1007/s004670000355. [DOI] [PubMed] [Google Scholar]
- 112.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 113.Gutierrez OM, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]