Abstract
The facial somatosensory map in the cortex is derived from facial representations that are first established at the brainstem level and then serially ‘copied’ at each stage of the somatosensory pathway. Recent studies have provided insights into the molecular mechanisms involved in the development of somatotopic maps of the face and whiskers in the trigeminal nuclei of the mouse brainstem. This work has revealed that early molecular regionalization and positional patterning of trigeminal ganglion and brainstem target neurons are established by homeodomain transcription factors, the expression of which is induced and maintained by signals from the brain and face. Such position-dependent information is fundamental in transforming the early spatial layout of sensory receptors into a topographic connectivity map that is conferred to higher brain levels.
Somatosensory pathways are characterized by a high degree of order. The relay of somatosensory stimuli including touch, pain and temperature from the body surface to the cortex involves the generation of point-to-point connectivity maps that enable an individual to constantly be aware of the nature and the positional origin of the stimulus. At all levels of the pathway, the spatial arrangement of neurons and their afferent fibres provides a somatotopic representation — that is, it faithfully reiterates the physical distribution of sensory receptors on the body surface. Such spatial organization was exemplified in the concept of the homunculus by Penfield and Boldrey1. However, body maps are not simply linear transformations of the body surface. Distinct body parts are mapped at different scales depending on their sensory importance, which is also directly reflected by the density of their surface receptors.
Mammals display species-specific facial specializations (such as the human lips, elephant proboscis, tactile nasal appendages of the star-nosed mole and rodent whiskers) that have prominent roles in fine tactile discrimination. As a result, in mammals, and rodents in particular, facial cerebral representations are prominent — their mapping taking up more cortical space than the mapping of other body parts. Facial somatosensory input is relayed through the trigeminal circuit, through which receptor distribution and input from distinct face regions is topographically and serially wired to the brainstem, thalamus and neocortex2–7 (FIG. 1).
Figure 1. Trigeminal circuit and face maps in the mouse brain.
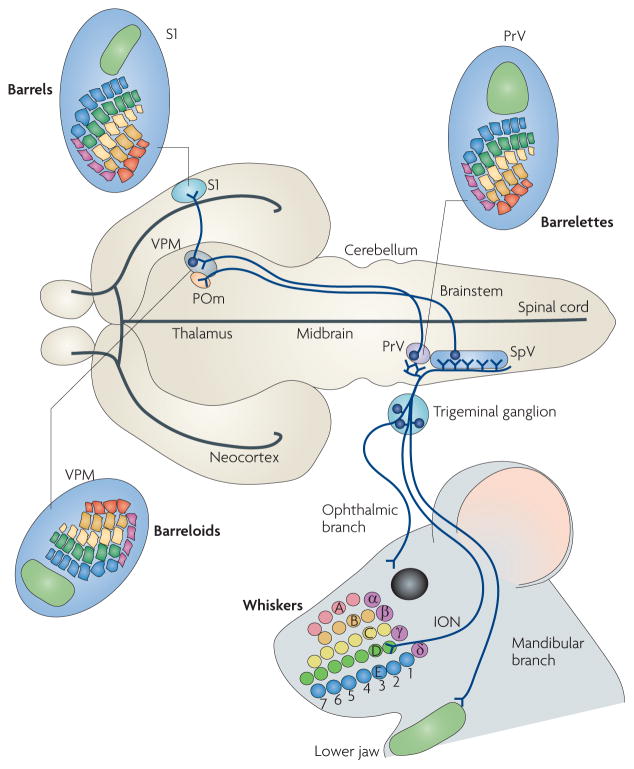
The ophthalmic (supplying the skin above the eye and forehead), maxillary (supplying the whiskers, upper jaw and lip) and mandibular (supplying the lower jaw and lip) branches of the trigeminal ganglion convey an inverted face map to the brainstem trigeminal nuclei — the rostral principal nucleus (PrV) and the caudal spinal nucleus (SpV). The whiskers and sinus hairs on the snout are innervated by the infraorbital branch of the maxillary nerve (ION). Here, five rows of whiskers (A–E) and the straddle whiskers (α –δ are indicated and colour coded. In the brainstem, radial collaterals emerge from the central trigeminal axons and innervate the PrV and SpV, where they form whisker-specific patterns (barrelettes). In the PrV, the facial map is inverted, with the mandibular fields represented dorsally and maxillary and ophthalmic fields represented ventrally. Similarly, the whisker rows A–E are represented in an inverted fashion. Trigeminothalamic axons from the PrV (lemniscal pathway17,18) project to the contralateral dorsomedial part of the ventral posteromedial nucleus (VPM) in the thalamus, where the whisker-related neural modules (barreloids) and face map again shift their orientation. SpV neurons project instead to the posteromedial (POm) nucleus (paralemniscal pathway19,20) and to the ventrolateral VPM (extralemniscal pathway20; not shown here for simplicity). Finally, thalamocortical axons from the VPM convey the facial map and whisker patterning to the somatosensory cortex (S1), where barrels form. Figure is modified, with permission, from REF. 72 © (2006) American Association for the Advancement of Science.
A longstanding question is to what extent the central pattern is influenced by signals from peripheral inputs versus intrinsic genetic mechanisms. This intensely debated issue has focused on the establishment of a cortical pattern in the rodent whisker-to-barrel pathway8–12 (FIG. 1). The current view is that cortical maps develop through an interplay between mechanisms that are intrinsic to cortical progenitors and neurons, which establish and position cortical areas, and extrinsic mechanisms imposed by thalamocortical input relaying information from the periphery8–10,13,14. However, a complete understanding of the relative importance of such mechanisms in generating somatotopic patterning in cortical areas has been complicated by the fact that the facial map is not directly wired to the cortex, but is first processed through intermediate stations. In addition, the postnatal appearance of the cortical pattern coincides with a critical period of plasticity, during which wiring can be influenced by whisker-dependent neural activity. During this period, sensory loss or deprivation alters the development of the cortical pattern15,16, further complicating the ability to distinguish between genetic and epigenetic influences in building facial maps.
Recently, genetic, molecular and cellular studies in the rodent have begun to uncover the mechanisms underlying the establishment of facial somatotopic organization at peripheral and brainstem levels and the topographic connectivity between these two components of the circuit. Here, we bring a critical synthesis of the results from various studies on the development of topographic and patterned projections from the rodent face to the trigeminal brainstem, the formation of the face map and whisker-specific patterning in the principal sensory trigeminal nucleus. We also discuss how such a map is conveyed to the somatosensory thalamus. The emerging picture is one of early spatial segregation and molecular regionalization of progenitors and neurons in the brainstem and peripheral sensory ganglia by sets of homeodomain transcription factors. This provides an underlying genetic framework that has been adapted in different species to enable the mapping of diverse mammalian facial morphologies and sensory specializations to the brain.
The rodent trigeminal pathway
FIGURE 1 summarizes the somatotopic organization of the trigeminal circuit in the rodent. Distinct regions of the face are innervated by first-order neurons, the cell bodies of which reside in the trigeminal ganglion and which project primary central afferents, forming the trigeminal tract, to the brainstem trigeminal sensory nuclei. Upon entering the brainstem, the primary afferent axons bifurcate into ascending and descending branches. Collaterals from these branches innervate second-order neurons in the rostral principal nucleus (PrV) and the caudal spinal nucleus (SpV). Both nuclei project to the contralateral thalamic ventral postero-medial (VPM) nucleus17–19, and the SpV also projects to the posteromedial (POm) nucleus19,20. Finally, thalamic neurons send topographic projections to the specific layers of the S1 somatosensory cortex that are devoted to the representation of the orofacial structures.
In nocturnal rodents, a large portion of the somato-sensory representation in the brain is devoted to the mystacial vibrissae (whiskers) (see Supplementary information S1 (box)). Sensory input from individual whiskers is relayed and somatotopically mapped at each level of the pathway as spatially ordered sets of neuronal modules. These modules are called barrelettes in the brainstem, barreloids in the VPM and barrels in the neocortex5,21,22. The arrangement of these modules faithfully copies the spatial layout of vibrissae follicles on the snout (FIG. 1).
Patterning trigeminal ganglion neurons
First-order trigeminal ganglion sensory neurons bridge the facial sensory periphery and the brainstem, where facial maps and whisker representations are first formed. A large body of studies, which have focused on the neurons of the trunk dorsal root ganglia (DRG) and in part on trigeminal ganglion neurons, have identified genetic programmes that regulate the differentiation of somatosensory progenitors into specific somatosen-sory modalities and the innervation of distinct types of skin receptors23,24. By contrast, information about how trigeminal ganglion progenitors and neurons acquire specific positional identities with respect to their facial targets has been sparse. However, recent work has begun to shed light on these processes and has identified some of the intrinsic transcriptional programmes and extrinsic inducing signals that are involved (FIG. 2).
Figure 2. Positional molecular patterning of trigeminal ganglion divisions.
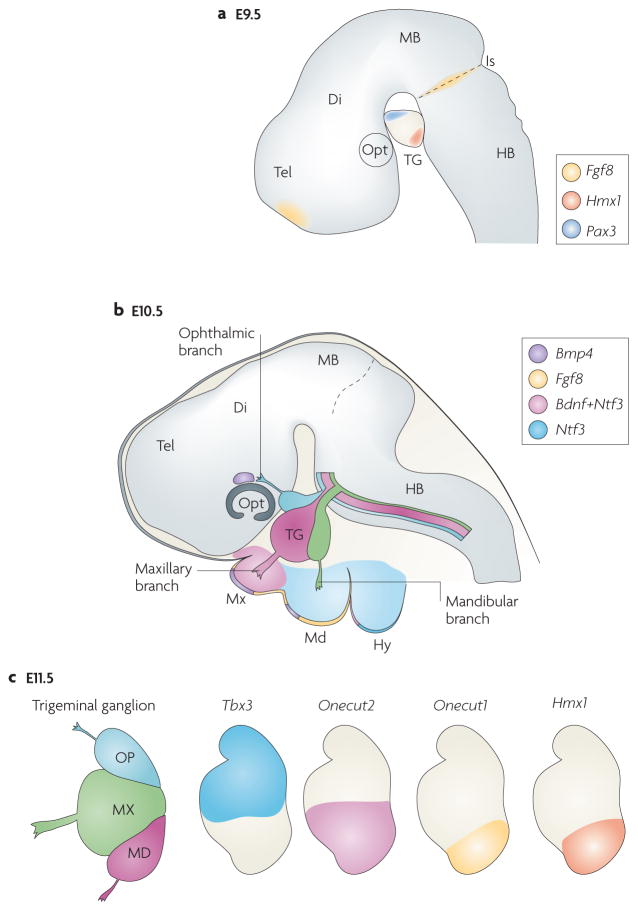
a | Diagram of the brain at embryonic day (E) 9.5 showing the trigeminal ganglion (TG), its position relative to the hindbrain (HB) and isthmic organizer (Is). Wnt (not shown) and fibroblast growth factor 8 (FGF8) signalling molecules originating at the isthmus cooperate for the spatially restricted induction of the homeodomain transcription factor paired box protein PAX3 in the ophthalmic placode and in progenitors that will become ophthalmic neurons38,39. Moreover, expression of the Hmx1 homeodomain transcription factor is restricted to the ventral, mandibular trigeminal ganglion division44. b | Expression patterns of signalling molecules in the developing trigeminal peripheral targets at E10.5. Neurotrophin 3 (Ntf3) and brain-derived neurotrophic factor (Bdnf) are expressed by both the target epithelium and the mesenchyme through which the TG axons extend53. Bone morphogenetic protein 4 (Bmp4) is expressed in regions adjacent to ophthalmic and maxillary regions, although not in mandibular axons, and differentially regulates positionally restricted expression of homeodomain transcription factors in TG neurons44. c | Differential homeodomain gene expression in TG divisions at E11.5. T box family transcription factor Tbx3 is restricted to dorsal (ophthalmic and maxillary) TG neurons, whereas Onecut1 (also known as Oc1 and Hnf6) and Hmx1 are expressed by ventral (mandibular) TG neurons, and Onecut2 (also known as Oc2 and Hnf6b) is expressed in the mandibular and ventral half of the maxillary divisions. BMP4 is required to maintain expression of Tbx3 in dorsal TG neurons while suppressing the transcription of Onecut1, Onecut2 and Hmx1 (REF. 44). Di, diencephalon; Hy, hyoid arch; MB, midbrain; Md, mandibular region of the first branchial arch; Mx, maxillary region of the first branchial arch; Opt, optic cup; Tel, telencephalon.
Position-dependent patterning of trigeminal ganglion progenitors: brain-derived signals
The forming trigeminal ganglion initially adjoins the neural tube. Trigeminal ganglion progenitors are generated at early developmental stages (between embryonic day (E) 8.25 and E9 in the mouse) from both neural crest cells migrating from the level of rhombomere (r)1–r2 and the ectodermal placodes that contribute to the ophthalmic and maxillomandibular components of the trigeminal ganglion25–40. In zebrafish, chemokine attraction mediated by CXC-chemokine receptor 4B plays a part in guiding trigeminal primary sensory neurons to the site of ganglion assembly41. Furthermore, SLIT1–ROBO2 -mediated cooperative interactions between neural crest and placodal cells as well as adhesion mediated by E- and N-cadherin contribute to the proper positioning of sensory neurons and trigeminal ganglion condensation in both zebrafish and chick37,41–43.
One of the earliest signs of trigeminal ganglion molecular regionalization is the induction of the homeodomain transcription factor paired box protein PAX3 in the ophthalmic placode and in progenitors that are programmed to become ophthalmic neurons38,39 (FIG. 2a). In the chick, the basic helix-loop-helix transcription factors neurogenin 1 (NGN1) and NGN2 have also been identified as early molecular markers of max-illomandibular and ophthalmic placodal neurogenesis, respectively32,38,40. Moreover, in the developing mouse trigeminal ganglion, the HMX1 homeodomain transcription factor is restricted to the mandibular portion as early as E9.5 (REF. 44) (FIG. 2a).
This early somatotopic arrangement is laid out before the onset of trigeminal ganglion axon outgrowth, prompting the question of the origin of the signals involved in induction and maintenance of this early pattern. Diffusible signals from the neuroectoderm of prospective midbrain and rostral hindbrain regions are required to induce and maintain Pax3 expression in the ophthalmic placode38,39,45. Recently, Wnt and fibroblast growth factor 8 (FGF8) signalling molecules originating at the isthmus (the boundary region between midbrain and hindbrain46) have been shown to induce Pax3 expression and the subsequent differentiation of trigeminal placodes47. This is intriguing given that the isthmic source of FGF8 is also involved in concomitant regional patterning of the r1–r2 hindbrain and derived neural crest48,49. Thus, the same set of brain-derived signals from the isthmus may simultaneously establish early positional differences in both the rostral hindbrain and in trigeminal ganglion neural progenitors. Interestingly, an FGF8 source at the rostral margin of the telencephalon is also involved in the early spatial patterning and positioning of the progenitor area, which generates the cortical facial map50.
Position-dependent patterning of trigeminal ganglion neurons: face-derived signals
The regional pre-patterning of the early trigeminal ganglion described above must be subsequently refined to allow the topographic innervation of distinct facial regions and sensory receptors. Target-derived signals may feed back and influence the positional identities of early trigeminal ganglion neurons and guide their axons along specific pathways.
In the past two decades, the identities of such periphery-derived signals have been actively sought. In vitro collagen gel-embedded co-culture assays indicated that the developing maxillary epithelium in mice secretes a chemotropic factor (termed maxillary factor) that attracts the trigeminal ganglion axons earliest51,52. This factor was later identified as a combination of neuro-trophin 3 (NTF3) and brain-derived neurotrophic factor (BDNF)53. NTF3 and BDNF are produced by both the target epithelium and the mesenchyme through which the trigeminal ganglion axons extend53 (FIG. 2b). However, the initial trajectories of trigeminal axons were largely unaffected in mice deficient in both BDNF and NTF3, indicating that these molecules have a neurotrophic rather than a tropic role in axon guidance53. Members of the nerve growth factor and glia-derived neurotrophin family, the collapsin–semaphorin family and their receptor neuropilin, as well as the Slit ligands and their Robo receptors have also been shown to be involved in regulating peripheral and central pathfinding, branching and arborization of trigeminal ganglion axons54–60 (FIGS 3,4).
Figure 3. Late expression patterns of homeodomain transcription factors and guidance molecules in the developing TG and PrV.
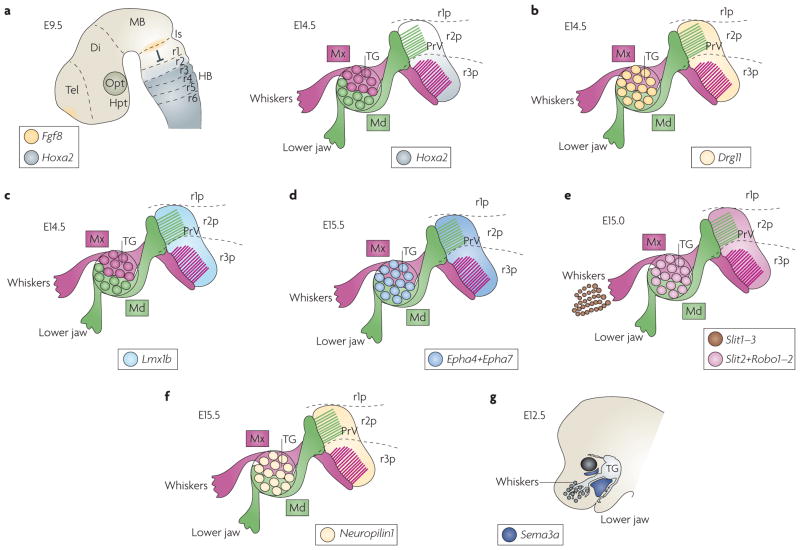
a | In the rostral principal nucleus (PrV) at embryonic day (E) 14.5 (right panel), homeobox A2 (Hoxa2) is differentially expressed in rhombomere 2 (r2)- or r3-derived postmitotic progenies. Hoxa2 is expressed at high levels in the ventral r3-derived portion (r3p), but is barely expressed in the dorsal r2-derived portion (r2p) of the PrV. By contrast, in the hindbrain at E9.5 (left panel), the anterior-most expression domain of Hoxa2 is in r2. Fibroblast growth factor 8 (FGF8)-mediated repression from the isthmus (Is) prevents Hox gene expression in r1 (and perhaps allows only low Hoxa2 expression levels in r2)48. b | At E14.5, the paired homeodomain factor dorsal root ganglion 11 (Drg11; also known as Drgx) is expressed throughout the PrV and in trigeminal ganglion cells90. c | At E14.5, the LIM homeodomain transcription factor Lmx1b is expressed in PrV but not in trigeminal ganglia (TG)54. d | Ephrin receptor A4 (Epha4) and Epha7 are expressed in the PrV and TG at E15.5 (REF. 72). e | Expression of the Slit ligands and Robo receptors. Slit1, Slit2 and Slit3 are expressed in whisker follicles and Slit2, Robo1 and Robo2 are expressed in both PrV and TG59,60. f | Expression of the receptor Neuropilin1 at E14.5 occurs in both the PrV and TG. g | The drawing represents X-gal staining at E12.5 of transgenic mice with heterozygous Lacz-knock-in into the semaphorin 3A gene (Sema3a)139. The trigeminal nerve seldom invades the Sema3a-expressing area. Di, diencephalon; HB, hindbrain; Hpt, hypothalamus; MB, midbrain; Md, mandibular branch of the trigeminal nerve; Mx, maxillary branch of the trigeminal nerve; Opt, optic cup; Tel, telencephalon.
Figure 4. Relationship between rhombomere progenies and PrV somatotopy.
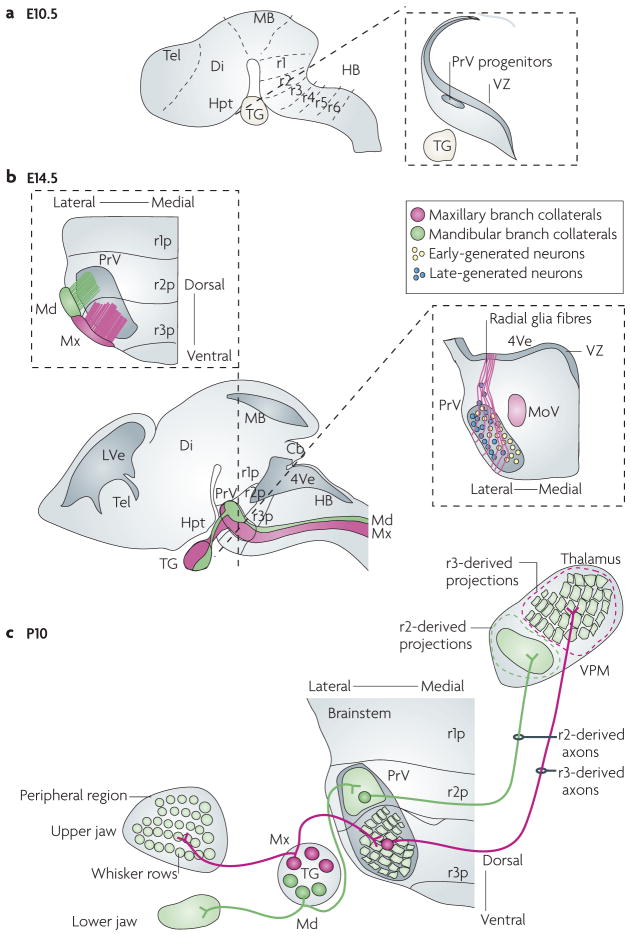
a | The developing mouse brain at embryonic day (E) 10.5. The left drawing shows rhombomere compartments of the hindbrain. The right illustration depicts a representative coronal section through the rhombomere 2 (r2) region of the hindbrain. At this stage, rostral principal nucleus (PrV) progenitors first emerge from the ventricular zone (VZ). b | Lateral view of the mouse brain at E14.5 (bottom left panel). Central axons of the trigeminal nerve — the mandibular branch (shown in green) and the maxillary branch (shown in pink) — enter the hindbrain, form the trigeminal tract and innervate the PrV. The upper section shows the somatotopic relationship between the r2- and r3-derived progenies contributing to the PrV and their targeting by distinct trigeminal nerve branches. Specifically, mandibular-branch axons mainly arborize into the r2-derived portion of PrV, but not the r3-derived component72. Conversely, r3-derived neurons receive selective collateral input from whisker-related, but not mandibular, maxillary afferents72. The right section shows the migration of PrV neurons from the VZ along radial fibres and their ‘inside-out’ distribution in the PrV, with neurons that are formed early settling more medially than neurons that are formed late86. c | Topography of the trigeminal circuit at postnatal day 10. The illustration shows the topography of afferent and efferent axonal connections of the PrV nucleus in relation to rhombomere-derived neuronal progenies (r1p–r3p). The rhombomere-specific spatial segregation of neurons in the mature PrV underlies the parcelling of mandibular and maxillary (whisker-related) segments of the face map. Cb, cerebellum; Di, diencephalon; HB, hindbrain; Hpt, hypothalamus; LVe, lateral ventricle; MB, midbrain; MoV, motor trigeminal nucleus; Tel, telencephalon; 4Ve, fourth ventricle; VPM, ventral posterior medial nucleus .
Recently, bone morphogenetic protein 4 (BMP4) has been identified as a peripheral target-derived signalling molecule. In the mouse trunk, BMP4 signalling has been shown to control the final neuron number in DRGs and to regulate the extent of peripheral innervation of skin targets61. Notably, in the head, BMP4 is a putative face-derived retrograde signalling molecule that differentially regulates the positionally restricted expression of homeodomain transcription factors in trigeminal ganglion neurons during peripheral axonal targeting44. In mice, at E10.5, BMP4 is expressed in regions adjacent to ophthalmic and maxillary, but not mandibular, axons44 (FIG. 2b). At this stage, Hmx1 expression is already restricted to the ventral (mandibular) trigeminal ganglion portion (see above and FIG. 2a). As the trigeminal ganglion axons reach their peripheral targets between E10.5 and E11.5, the expression of T box family transcription factor TBX3 becomes restricted to dorsal (ophthalmic and maxillary) trigeminal ganglion neurons. By contrast, the homeodomain transcription factors Onecut1 (also known as OC1 and HNF6) and HMX1 are expressed by ventral (mandibular) trigeminal ganglion neurons, and OC2 becomes expressed in the ventral half of the maxillary and mandibular trigeminal ganglion components44 (FIG. 2c).
BMP4 is required to maintain expression of Tbx3 in dorsal trigeminal ganglion neurons while suppressing Oc1, Oc2 and Hmx1 transcription44. BMP4-mediated signal transduction is achieved through phosphory-lation of Smad family transcription factors. Conditional Smad4-knockout mice have smaller trigeminal ganglia than wild-type mice, and ventral maxillary and mandibular axons tangle without reaching their peripheral targets44. Finally, BMP4 also has a prominent role in regulating the morphology of facial elements, and BMPs (including BMP4) and downstream signalling molecules are expressed in the developing hindbrain as well33,62–65. These findings therefore suggest that BMP signalling has several effects during face morphogenesis and map formation, including positional regulation of transcription in the trigeminal ganglion.
Face map patterning in the brainstem
The PrV and SpV both contain inverted face map representations (BOX 1). Barrelettes — each of which corresponds to a single whisker and sinus hair on the ipsilateral side of the face — are present in the PrV and in two of the three subnuclei of the SpV, interpolaris and caudalis. Of these nuclei, it is the PrV that transmits the patterned face map template to the contralateral thalamus and subsequently the somatosensory barrel cortex66,67. The ascending branches of the trigeminal tract axons convey the face map to the PrV and the descending branches to the SpV subnuclei. Virtually nothing is known about the molecular determinants of face map patterning in the SpV. Here, we review recent insights into the molecular and cellular mechanisms of PrV development.
Box 1. Wiring facial pattern.
Embryonic trigeminal ganglion cells are spindle shaped with two axonal processes emerging from the opposite ends of the cell body. One axon grows towards peripheral targets, navigating through non-neuronal cells, and the other enters the adjacent hindbrain. In rats and mice, the peripheral and central processes of trigeminal ganglion cells show a distinct, target-directed growth as soon as they emit axonal processes68,85,130,131. The development and differentiation of the trigeminal pathway of the rat and mouse are strikingly similar, although there is a slight shift in the timing of events by a couple of days in rats, corresponding to the longer duration of the gestation in these species85,130–132.
From the beginning of the process of peripheral trigeminal neuron projection, the trigeminal nerve is topographically organized44,68,72. The three components of the peripheral trigeminal projections — the ophthalmic, maxillary and mandibular nerves — follow specific routes that are separated from each other (see the figure and FIG. 1). In rodents, the infraorbital component of the maxillary division is the largest nerve, and it enters the developing whisker pad from a caudal to rostral direction. Non-overlapping multicoloured carbocyanine dye or dextran injections along the dorsoventral axis of the developing whisker field, or the ophthalmic, maxillary and mandibular zones clearly show this topographic organization of trigeminal projections in the rat and mouse embryos44,68,72. Thus, the dorsoventral axis of the face map is preserved in the peripheral nerves.
Within the embryonic trigeminal ganglion, there is also a general somatotopic segregation of trigeminal ganglion neuron cell bodies, contributing to the three subdivisions44,72,85. The dorsoventral axis of the face map is, however, shifted medially by approximately 450 and by almost 900 in the trigeminal tract as it enters the hindbrain, as a consequence of the differential growth of the face and brain. Thus, as the trigeminal tract is being laid down in the brainstem, the dorsal trigeminal fields are represented medially and ventral fields are represented laterally. As the brainstem develops further, there is a final rotation of 900 in the dorsoventral axis of facial topography so that the lateral (mandibular) branch becomes dorsal and the medial (ophthalmic) branch becomes ventral. So, although the somatotopic relationships between distinct facial trigeminal fields are preserved, this spatial arrangement during development accounts for the inverted representation of the face map in the brainstem trigeminal nuclei. The rostrocaudal axis of the face map is represented along the mediolateral axis of the adult brainstem trigeminal nuclei. This axis of the face map has been related to progressive termination of peripheral trigeminal axons along the caudal to rostral (oculonasal) axis of the face and their central counterparts along the lateral to medial axis in the brainstem85.

Position-dependent patterning of PrV neuron subsets
In the mature brainstem, the dorsal PrV contains representations of the lower jaw and lips, whereas the barrelettes, which represent the array of whiskers, are located ventrally (FIG. 1). Several lines of evidence indicate that point-to-point somatosensory periphery-related neural maps and patterns are conveyed by the whisker-related afferents to their target cells at each synaptic relay station68–71. However, much less is known about the intrinsic mechanisms of PrV patterning that underlie the parcelling of facial map subdivisions, and how they contribute to the development of somatotopic organization in the PrV. Just as the wiring of an electrical appliance requires the plug to fit into a size- and shape-matched socket, there is emerging evidence that intrinsic patterning of the PrV might contribute to the wiring of facial map formation at the brainstem level72.
The PrV originates from the rostral hindbrain72,73. Starting at about E8.5 in the mouse, the hindbrain neuroepithelium becomes partitioned along the rostro-caudal axis into spatially segregated compartments — the rhombomeres25,74. Such a metameric cellular organization has been highly conserved in vertebrate evolution and has a fundamental role in the segmental organization of nuclei and columns of the mature brainstem72–83. Rostral rhombomere identity and early patterning is under the influence of FGF8 signals from the isthmic organizer46,48 (see below and FIG. 3a), like the neural crest and placode-derived progenitors of trigeminal ganglion cells (see above and FIG. 2a). An intriguing possibility is therefore that a shared set of rostrocaudal positional coordinates may organize trigeminal ganglion and hindbrain neural progenitors. This could set out an early basic programme that allows for the subsequent somatotopic organization of neurons and matching of face-to-brainstem topographic connectivity.
Indeed, there is strong evidence that the gross topographic organization of the face map in the brainstem is related to the positional (rhombomeric) origin of PrV neuron subsets along the rostrocaudal axis72. Long-term genetic fate mapping revealed that the mouse PrV is mainly composed of r2- and r3-derived postmitotic progenies that remain physically segregated from each other in specific subsets in the mature nucleus72 (FIG. 4). Moreover, the map of the lower jaw and lips contained in the ‘dorsal’ portion of the PrV (which corresponds to the rostral part at early stages) is comprised only of neurons derived from r2 (REF. 72), whereas the barrelette map contained in the ‘ventral’ (early caudal) portion of PrV is entirely contributed by the progeny of r3 (REF. 72). The persistence of cohesion properties84 that maintain physical segregation of postmitotic progenies of r2 and r3 suggests that the differential expression of cell adhesion molecules provides a cellular pattern on which to build precise neuronal connectivity and a facial somatotopic map. Thus, the spatial segregation of PrV neurons derived from distinct rhombomeres underlies the parcelling of mandibular and maxillary (whisker-related) segments of the face map in the PrV. The hindbrain segmentation and spatial segregation of PrV neuron subsets may also provide a cellular framework for ordered connectivity of incoming facial afferents and trigeminothalamic projections.
Establishing topography of afferent PrV connectivity
The pioneering central trigeminal ganglion afferents enter the brainstem at the level of r2 (REF. 81) around E12 in the rat (E9.5–E10 in the mouse), before the peripheral processes of trigeminal ganglion neurons have reached their peripheral targets85. Ascending tract axons travel a short distance in the rostral hindbrain and stop at the r1–r2 boundary72, whereas the descending axons travel over a longer course and abruptly stop at upper cervical levels of the spinal cord (FIG. 4b). Recent evidence indicates that early signals from the r2 neuroepithelium are required to restrict the rostral path of the trigeminal tract and to prevent the ascending axons from entering r1 (REF. 72) (see below). Whether the caudal boundary of the descending trigeminal projections is also determined by rhombomere-derived signals remains to be determined.
The trigeminal tract elongates along the lateral margin of the entire rostrocaudal axis of the hindbrain, growing for several days without branching. In the meantime, the PrV nucleus is formed86,87. In the rat, most PrV neurons are generated between E12 and E17 (REFS 88,89). Similarly, in the mouse PrV, progenitors first emerge from the r2–r3 ventricular zone around E10.5 (FIG. 4a), and by E15.5 the nucleus is mostly formed90. Newly formed PrV neurons migrate along radial fibres and seem to settle in the ventrolateral hindbrain in an ‘inside-out’ sequence, with neurons that are formed early being located more medially than neurons that are formed late in the mature nucleus86 (FIG. 4b). Remarkably, the medio-lateral axis of the mature PrV encodes the rostrocaudal axis of the face map (see above). It is unknown whether the timing of PrV neuron formation might instruct trigeminal ganglion afferent topography along this axis, although this is an intriguing possibility.
At around E17 in the rat (E14.5–E15 in the mouse), central trigeminal ganglion axons start emitting radially oriented interstitial collaterals into the brainstem trigeminal nuclei, and begin forming synaptic terminals that will eventually replicate the facial pattern68, 85. At the level of the PrV, spatially restricted patterns of collateralization–arborization occur in individual trigeminal nerve branches, correlating with the rhombomeric origin of PrV neurons72 (FIG. 4b). Specifically, mandibular-branch axons mainly arborize into the r2-derived portion of PrV, but not the r3-derived component. Conversely, r3-derived neurons receive selective collateral input from whisker-related, but not mandibular, afferents. Although PrV neuron segmental differences may be instructive in achieving this spatially restricted collateral targeting72, these early patterns of connectivity of trigeminal ganglion afferents reciprocally establish basic facial somatotopy in the PrV nucleus.
Establishing topography of PrV efferent connectivity
Although the barreloids were identified 30 years ago22,91, few studies have focused on the development of the lemniscal pathway, which connects the PrV to the thalamus. A combined anterograde tracing and electrophysiological study in the rat showed that trigeminothalamic fibres reach the thalamus by E17, begin branching in the VPM by E18 and start elaborating arbours shortly before birth92. Thus, by the time incoming trigeminal ganglion afferents establish first contact with PrV brainstem neurons, their axonal projections have made substantial progress towards the contralateral VPM, suggesting that PrV axonal pathfinding is controlled independently of peripheral inputs. At postnatal stages, genetic labelling of r2- or r3-derived axon fibres in the trigeminal lemniscus revealed rhombomere-specific topographic mapping of axonal arbours of PrV neurons to specific areas of the VPM thalamus; specifically, r3-derived PrV projections precisely map to the barreloid area, whereas r2-derived axons map to an area including the representation of the lower jaw and lips72 (FIG. 4c).
An extensive account of the developmental mechanisms intrinsic to the thalamus is outside the scope of this Review and has been dealt with elsewhere14,25,93. Briefly, VPM neurons are generated from E13 in the rat and migrate and aggregate to form the VPM94,95 shortly before the arrival of trigeminothalamic fibres. Interestingly, these fibres are synaptically active as soon as they enter the VPM92. In the developing rat VPM, excitatory responses are mediated solely by NMDARs (N-methyl 3-aspartate receptors) until postnatal day (P)1, and influences of GABA (γ-aminobutyric acid) begin around E18 (REF. 92). Whisker-specific neural patterning in the VPM, and subsequently in the primary somatosensory cortex, depends on the inputs from the barrelette cells67 (BOX 2). In the mature thalamus, VPM cells orient their dendrites and somata in relation to PrV afferent terminal patches, forming the barreloids. The rodent VPM contains primarily barreloid cells, which project to the barrel cortex, and inhibitory inputs to the VPM come from the zona incerta and reticular nucleus96–98.
Box 2. Barrelette neurons and NMDA receptors.
Whisker-specific patterning (barrelettes) in the brainstem occurs between embryonic day (E) 19 and E20 in the rat132,133 and at birth in mice4. Whisker afferents of different modalities interdigitate, overlap and form the sausage-shaped cores of the barrelettes134,135.
Both the rat and the mouse rostral principal nucleus (PrV) contain three classes of neurons: barrelette neurons, interbarrelette neurons and GABA (γ-aminobutyric acid)-ergic interneurons. Barrelette neurons are characterized by polarized dendritic trees that make contact with the whisker-specific bands of afferent terminals. They display a transient K+ current and receive monosynaptic excitatory and disynaptic inhibitory inputs upon stimulation of the trigeminal tract136. Interbarrelette neurons have dendritic trees that span multiple barrelettes. They are distinguished by a low-threshold T-type Ca2+ current and receive excitatory inputs from many sources136. GABAergic interneurons provide disynaptic inhibition to barrelette neurons136. Class-specific membrane properties and synaptic responses are present in the PrV at birth.
Postsynaptic responses in the late embryonic and early postnatal PrV are mediated predominantly by NMDARs (N-methyl 3-aspartate receptors)132. Genetic studies showed that, in the absence of two NMDAR subunits (NR1 or NR2B), barrelettes do not form, even though trigeminal afferents target properly and establish a gross topographic order126,127 (FIG. 5). In transgenic mice with a 70–80% reduction of Nr1 expression in the PrV, whisker-specific refinement in the PrV or downstream centres does not occur128. Morphological analyses of afferent arbours and dendritic orientation in the PrV of NR1-deficient mice have led to the interpretation that NMDARs act as stop-stabilization signals for afferent arbours and dendritic trees129. Ca2+ signalling through the NMDARs is essential in the patterning of the PrV. Point mutations in the NR1 subunit that abolish the Mg2+ block and Ca2+ permeability impair the coincidence detection properties of NMDARs. The phenotypes of these mutant mice137,138 are similar to those reported for the Nr1-deficient and Nr1-knockdown mice.
Molecular mechanisms of PrV patterning and connectivity
The homeodomain transcription factors of the homeobox (Hox) gene family (comprising 39 genes in mammals) are well known for their conserved role in providing positional identity and patterning information to cells along the main rostrocaudal axis of the embryo99. In the developing hindbrain, Hox genes have a fundamental role in conferring rostrocaudal identity and patterning information to rhombomere neuroepithelial compartments100,101. Moreover, the early segmental Hox expression patterns are often maintained through later stages in subsets of rhombomere-derived postmitotic progenitors and projection neurons that contribute to developing brainstem columns and nuclei72,102–105. It is becoming increasingly clear that such an intrinsic molecular regionalization of developing nuclei by late Hox expression programmes may be involved in regulating late aspects of neural circuit formation, such as stereotypical neuronal migration and the control of topographic patterns of afferent and efferent connectivity, both in sensory and motor systems72,101,105–108.
Recently, Hoxa2 has been shown to have an important role in the developing trigeminal system72. In the mouse embryo at E9.5, the expression domain of Hoxa2 encompasses the entire hindbrain up to a sharp anterior border at the r1–r2 boundary109–112 (FIG. 3a). Hoxa2 is the only Hox gene expressed in r2 (albeit at low levels), whereas in r3 it is co-expressed (at high levels) only with its paralogue Hoxb2 (REF. 109) (FIG. 3a). The Hoxa2 anterior expression limit in r2 (and perhaps its low expression level) is regulated by repressive isthmic FGF8 signalling that prevents Hox gene expression in r1 (REF. 48) (FIG. 3a) and, probably, in the trigeminal ganglion placode and r1–r2-derived neural crest47,49. The expression of Hoxa2 is then maintained through later stages with a spatially restricted pattern in the developing PrV nucleus72 (FIG. 3a). Unlike other markers such as the paired homeodomain factor dorsal root ganglion 11 (Drg11; also known as Drgx) (see below) that are more homogenously expressed90 (FIG. 3b), high Hoxa2 expression levels are only observed in the ventral r3-derived portion of PrV (the future barrelette area), and almost no transcripts are present in the dorsal r2-derived portion (the mandibular area).
Conditional targeted inactivation revealed distinct spatiotemporal roles of Hoxa2 (REF. 72) (FIG. 5). Lack of Hoxa2 in r2 results in pathfinding errors within the trigeminal tract — axons make aberrant projections into the cerebellum72. Thus, early Hoxa2 expression in the r2 neuroepithelium might control a putative ‘stop’ signal for incoming trigeminal tract afferents. Selective inactivation of Hoxa2 in r3 does not overtly affect r3 identity, probably owing to partial compensation by Hoxb2 (REF. 102). However, it specifically impairs collateral formation from incoming trigeminal ganglion whisker-related afferents and arborization onto PrV neurons, resulting in a lack of barrelette patterning72 (FIG. 5a). Similar effects are seen when Hoxa2 inactivation is induced just before collateral formation72 (FIG. 5a). Thus, late Hoxa2 expression in PrV neurons may be an important regulator of trigeminal ganglion central afferent arborization, supporting the idea that this process requires maturation of target cells113. Hoxa2 function in PrV neurons may indeed regulate the expression of molecules involved in trigeminal afferent arborization, such as neurotrophins and their receptors54, Slit proteins and Robo receptors59,60 and/or semaphorins and neuropilin receptors57. Interestingly, Hoxa2 directly regulates ROBO2 levels in response to Slit signalling in migrating precerebellar neurons105. It is not known whether Hoxa2 also regulates Slit–Robo signalling in the trigeminal system, although it seems likely.
Figure 5. Phenotypes of the trigeminal system in mutant mice.
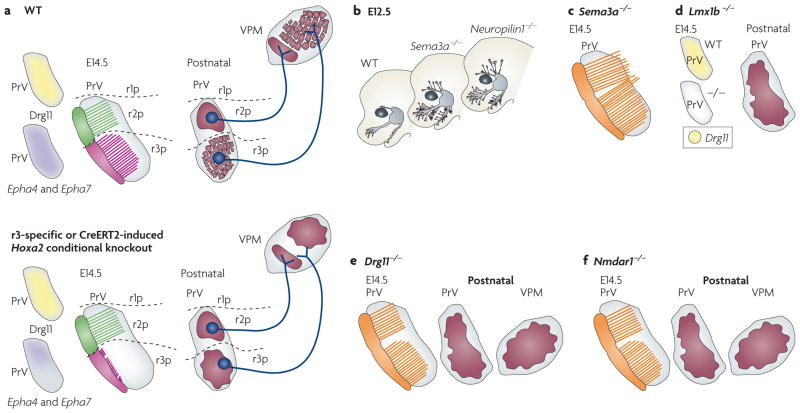
a The trigeminal system at embryonic day (E) 14.5. The upper panel represents the wild type (WT) and the lower panel represents the similar phenotypes of rhombomere 3 (r3)-specific or temporally induced homeobox A2 (Hoxa2) conditional mutants. The left drawings show the expression of dorsal root ganglion 11 (Drg11; also known as Drgx), ephrin receptor A4 (Epha4) and Epha7 in the rostral principal nucleus (PrV). The middle drawings show the collaterals of trigeminal mandibular branches (shown in green) and whisker-related maxillary branches (shown in pink) in the PrV in relation to the spatial restriction of rhombomere progenies. The right drawings show the projection of the r2- and r3- derived axons and the somatotopic map of the PrV and ventral posteromedial nucleus (VPM) at postnatal stages. Inactivation of Hoxa2 in r3 or before collateral formation specifically impairs collateral formation from incoming trigeminal ganglion (TG) whisker-related afferents and arborization onto PrV neurons, resulting in lack of barrelette pattern72. Mutant PrV neuron projections can normally be traced to the contralateral VPM, but display topographic mapping defects that correlate with reduced Epha4 and Epha7 expression in prenatal PrV and result in lack of barreloids72. b | Schematic representation of the peripheral distribution of the trigeminal branches in semaphorin 3A (Sema3a)- and Neuropilin1-knockout mice. SEMA3A and its receptor neuropilin have a role in restricting peripheral (shown in grey) and central (shown in orange) trigeminal axons to specific routes but are not involved in whisker-related patterning in the PrV57. c | Phenotypes of the central branch of the trigeminal nerve in Sema3a-knockout mice. Note that the central trigeminal ganglion axon collaterals (shown in orange) pass beyond the PrV. d | The trigeminal system in a LIM homeobox transcription factor 1β (Lmx1b) mutant. The left drawings show loss of Drg11 expression in the mutant PrV at E14.5. The right drawing shows the loss of somatotopic map in the postnatal mutant PrV. e,f | The trigeminal system in Drg11- and Nmdar1 (N-methyl 3-aspartate receptor 1) knockout mice, respectively. The PrV phenotypes of Lmx1B, Drg11 and Nmdar1 knockouts are similar in that they all lack barrelettes.
In postnatal conditional Hoxa2-deficient mice, many de-afferented PrV neurons eventually die postnatally, probably owing to a lack of neurotrophic support and/or support mediated by neural activity. Surviving mutant PrV neuron projections can normally be traced to the contralateral VPM. However, they have topographic mapping defects and aberrantly target the ventromedial area — which normally hosts the lower-jaw representation — and not the barreloid area72 (FIG. 5a). Such topographic mapping alterations correlate with, and may be in part mediated by, reduced ephrin receptor A4 (EphA4) and EphA7 expression in the prenatal PrV (FIGS 3d,5a), as these receptors are important for topographic mapping in sensory systems (for example, see REF. 114). These results further support the idea that basic facial somatotopy at the brainstem level is established early in development and can be traced to early and late patterns of Hox gene expression in specific rhombomeres and brainstem trigeminal sensory neurons.
Only a few other homeodomain transcription factors have so far been shown to be functionally important for PrV development, including DRG11, LIM homeobox transcription factor 1β (LMX1β) and T cell leukaemia homeobox 3 (TLX3; also known as RNX). Drg11 is expressed in the PrV and in trigeminal ganglion cells, but not in the barrelette-forming components of SpV interpolaris and caudalis90,115,116. Pathfinding errors for central trigeminal ganglion afferents are observed in Drg11-deficient foetuses from E16.5, whereas peripheral trigeminal ganglion projections are largely normal90. By E18.5, the PrV in mutant mice is smaller than in control mice owing to increased cell death. Such abnormalities lead to a failure to develop whisker-related patterns in the PrV, VPM and S1 cortex (FIG. 5e). Interestingly, the whisker-related pattern is not recovered even when the cell death defect is rescued in trigeminal ganglion and PrV neurons, indicating that DRG11 is involved in patterning these structures115. TLX3 (REFS 117,118) and LMX1β54,119 act upstream and positively regulate Drg11 expression in the PrV (FIG. 5d). However, both of these homeodomain factors seem to also function in several processes that are independent of DRG11. Tlx proteins, in particular, are involved in determining glutamatergic neurotransmitter cell phenotype120. Finally, Drg11 expression is not affected in Hoxa2-deficient mice72, indicating that the transcription factors encoded by these genes might function in parallel, independent pathways and/or as cofactors in the same pathway.
Conclusions and future directions
The results discussed here underscore the importance of the brainstem in organizing the representation of facial pattern in the brain and open several exciting research directions and questions for the future.
These findings have important implications for our understanding of the basic principles that allow almost any type of facial morphology and sensory specialization to be mapped in the brain throughout vertebrate evolution. Work in recent years strongly supported the view that heterochronic (time-varying), heterotopic (place-varying) or quantitative changes in the expression of key signalling molecules (such as BMP4, FGF8, Wnt and Sonic hedgehog protein) in the epithelium of the face might underlie the evolution and variation of facial morphology and sensory receptors in different vertebrates33,64,65,121–123. We now provide a conceptual framework to begin to investigate how distinct facial regions are mapped at different scales — not only within species (such as maps of the whiskers versus maps of the lower jaw and lips in rodents) but also between different vertebrates (for example, by comparing maxillary or ophthalmic facial map components in the mouse and chick, respectively).
The conservation of the segmental organization of the hindbrain in all vertebrates could mean that a similar cellular ‘scaffold’ is available on which to build an ordered neural circuit. Conversely, differences in the qualitative or quantitative distributions of Hox gene products in the progenies of distinct rhombomeres during hindbrain maturation might allow for changes in the amount of space that is allocated to the wiring of each peripheral trigeminal component (maxillary, mandibular or ophthalmic) on hindbrain target neurons in different vertebrates. This would result in maps at different scales and might direct the development of topographic equivalence at thalamic and cortical levels. Comparative studies of the trigeminal circuit in other vertebrate models are required to support such a speculative model.
Several other fascinating questions remain to be investigated. Although the coarse segregation of trigeminal nerve afferents is well documented, the fine point-to-point map formation is as yet unexplained. The mechanism that reproduces the high spatial order of the facial whisker array in the r3-derived barrelettes is a challenge for future studies. The current evidence suggests that, during prenatal development, trigeminal ganglion cell bodies and peripheral axons that innervate whisker rows have an ordered spatial pattern44,68,85. Such information is at a coarser level than the single whisker follicle, and detailed prenatal mapping of the central pattern of terminal arbours of identified whisker afferents is currently lacking. Furthermore, although the trigeminal ganglion is molecularly regionalized (see above) this does not seem to be at a finer level than ophthalmic–maxillary–mandibular subdivisions, with little suggestion of a molecular pre-pattern that could underlie a whisker-specific somatotopy.
The whisker follicle pattern is first established in the facial skin independently of innervation124. However, reciprocal interactions between the developing target tissues and trigeminal ganglion innervation play a major part in establishing an approximate soma-totopy between the dorsoventral axes of the face and the brainstem. What we still do not know is how the peripheral process of a trigeminal ganglion neuron communicates its location in space to the central process, and the nature of the molecular mechanisms that are involved in mapping the caudal to rostral axis of the face along the mediolateral axis of the PrV. One possibility is that near-neighbour relationships between trigeminal ganglion axons, the gradients of caudal to rostral innervation in the whisker pad, and its mapping along the lateral to medial direction in the brainstem enable the fine-tuning of the face map68,85. In zebrafish, repulsive interactions between individual trigeminal primary sensory axons control the shape and size of terminal arbours125. The molecular mechanisms involved in such axonal repulsion, and whether it is also at work in the developing mammalian trigeminal system, remains to be determined.
As for the point-to-point mapping of the whiskers and sinus hairs at the brainstem level, it is well established that postsynaptic NMDARs contribute to the refinement of single barrelette patterns. In NMDAR-knockout mice, dendrite remodelling in barrelette neurons is impaired and trigeminal primary terminal arbours undergo sustained expansion and occupy abnormally large territories in the PrV, resulting in severe impairment of whisker-specific patterning126–129. However, the topographic organization of the trigeminal nerve onto the brainstem is maintained in the PrV of the mutant mice129. It is not known whether there are molecular identity markers for individual cell types in the PrV that distinguish barrelette cells from interneurons and GABAergic neurons. Moreover, whether there is a single barrelette-specific molecular patterning code that operates during prenatal development and is further refined by activity-dependent mechanisms is yet to be determined. The signals that induce aggregation of barrelette neurons and orientation of their dendrites towards the patterned distribution of trigeminal afferent terminals are presently unknown. Are there similarities or molecular recognition cues between barrelette cells, barreloid cells and the barrel cells in the cortex? Single-cell gene profiling, detailed neuronal tracing and investigation of single-whisker microcircuits during prenatal and postnatal development, spatiotemporal modulation of gene expression in conditional mouse knockouts and three-dimensional reconstruction of barrelette development will undoubtedly provide answers to these exciting questions.
Supplementary Material
Glossary
- Homunculus
Literally ‘little man’, it refers to the somatosensory and motor body maps in the human brain
- Critical period
A finite but modifiable developmental time window during which sensory experience-mediated input provides information that is essential for normal maturation of sensory circuits
- Epigenetics
Changes in phenotype or gene expression caused by mechanisms other than genetic factors
- Neural crest
Groups of cells that migrate from the neural tube to the periphery, where they give rise to a wide range of cell types
- Rhombomeres
Neuroepithelial segments found transiently in the embryonic hindbrain that adopt distinct molecular and cellular properties, restrictions in cell mixing and ordered domains of gene expression
- Neuroectoderm
Part of the ectoderm that gives rise to the neural crest and neural tube
- Ventricular zone
The proliferative region of the mammalian brain adjacent to the brain ventricles that gives rise to neurons and glia
Footnotes
Competing interests statement
The authors declare no competing financial interests.
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/gene
Hoxa2 | Pax3
UniProtKB: http://www.uniprot.org
BDNF | BMP4 | Drg11 | FGF8 | PAX3 | NTF3 | ROBO2 | SLIT1
FURTHER INFORMATION
Filippo M. Rijli’s homepage: http://www.fmi.ch/rijli.f/
See online article: S1 (box)
References
- 1.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- 2.Belford GR, Killackey HP. The development of vibrissae representation in subcortical trigeminal centers of the neonatal rat. J Comp Neurol. 1979;188:63–74. doi: 10.1002/cne.901880106. [DOI] [PubMed] [Google Scholar]
- 3.Ma PM. The barrelettes — architectonic vibrissal representations in the brainstem trigeminal complex of the mouse. I Normal structural organization. J Comp Neurol. 1991;309:161–199. doi: 10.1002/cne.903090202. [DOI] [PubMed] [Google Scholar]
- 4.Ma PM. Barrelettes — architectonic vibrissal representations in the brainstem trigeminal complex of the mouse. II Normal post-natal development. J Comp Neurol. 1993;327:376–397. doi: 10.1002/cne.903270306. [DOI] [PubMed] [Google Scholar]
- 5.Ma PM, Woolsey TA. Cytoarchitectonic correlates of the vibrissae in the medullary trigeminal complex of the mouse. Brain Res. 1984;306:349–374. doi: 10.1016/0006-8993(84)90390-1. This report coined the term ‘barrelettes’ for the whisker-specific neural modules. [DOI] [PubMed] [Google Scholar]
- 6.Rebsam A, Seif I, Gaspar P. Refinement of thalamocortical arbors and emergence of barrel domains in the primary somatosensory cortex: a study of normal and monoamine oxidase A knock-out mice. J Neurosci. 2002;22:8541–8552. doi: 10.1523/JNEUROSCI.22-19-08541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox K. Barrel Cortex. Cambridge University Press; New York: 2008. [Google Scholar]
- 8.Grove EA, Fukuchi-Shimogori T. Generating the cerebral cortical area map. Annu Rev Neurosci. 2003;26:355–380. doi: 10.1146/annurev.neuro.26.041002.131137. [DOI] [PubMed] [Google Scholar]
- 9.O’Leary DD, Chou SJ, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- 11.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 12.Cohen-Tannoudji M, Babinet C, Wassef M. Early determination of a mouse somatosensory cortex marker. Nature. 1994;368:460–463. doi: 10.1038/368460a0. [DOI] [PubMed] [Google Scholar]
- 13.Erzurumlu RS, Kind PC. Neural activity: sculptor of ‘barrels’ in the neocortex. Trends Neurosci. 2001;24:589–595. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Bendito G, Molnar Z. Thalamocortical development: how are we going to get there? Nature Rev Neurosci. 2003;4:276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- 15.Belford GR, Killackey HP. The sensitive period in the development of the trigeminal system of the neonatal rat. J Comp Neurol. 1980;193:335–350. doi: 10.1002/cne.901930203. [DOI] [PubMed] [Google Scholar]
- 16.Durham D, Woolsey TA. Effects of neonatal whisker lesions on mouse central trigeminal pathways. J Comp Neurol. 1984;223:424–447. doi: 10.1002/cne.902230308. [DOI] [PubMed] [Google Scholar]
- 17.Arsenault D, Zhang ZW. Developmental remodelling of the lemniscal synapse in the ventral basal thalamus of the mouse. J Physiol. 2006;573:121–132. doi: 10.1113/jphysiol.2006.106542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deschenes M, Timofeeva E, Lavallee P. The relay of high-frequency sensory signals in the whisker-to-barreloid pathway. J Neurosci. 2003;23:6778–6787. doi: 10.1523/JNEUROSCI.23-17-06778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veinante P, Jacquin MF, Deschenes M. Thalamic projections from the whisker-sensitive regions of the spinal trigeminal complex in the rat. J Comp Neurol. 2000;420:233–243. doi: 10.1002/(sici)1096-9861(20000501)420:2<233::aid-cne6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 20.Pierret T, Lavallee P, Deschenes M. Parallel streams for the relay of vibrissal information through thalamic barreloids. J Neurosci. 2000;20:7455–7462. doi: 10.1523/JNEUROSCI.20-19-07455.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. This study coined the term ‘barrels’ for cytoarchitectonic cortical units that correspond to whiskers on the face in a one-to-one fashion. [DOI] [PubMed] [Google Scholar]
- 22.Van der Loos H. Barreloids in mouse somatosensory thalamus. Neurosci Lett. 1976;2:1–6. doi: 10.1016/0304-3940(76)90036-7. This report describes the cellular organization of the VPM in relation to whisker representation and introduced the term ‘barreloids’. [DOI] [PubMed] [Google Scholar]
- 23.Anderson DJ. Lineages and transcription factors in the specification of vertebrate primary sensory neurons. Curr Opin Neurobiol. 1999;9:517–524. doi: 10.1016/S0959-4388(99)00015-X. [DOI] [PubMed] [Google Scholar]
- 24.Marmigere F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nature Rev Neurosci. 2007;8:114–127. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- 25.Kiecker C, Lumsden A. Compartments and their boundaries in vertebrate brain development. Nature Rev Neurosci. 2005;6:553–564. doi: 10.1038/nrn1702. [DOI] [PubMed] [Google Scholar]
- 26.Ayer-Le Lievre CS, Le Douarin NM. The early development of cranial sensory ganglia and the potentialities of their component cells studied in quail-chick chimeras. Dev Biol. 1982;94:291–310. doi: 10.1016/0012-1606(82)90349-9. [DOI] [PubMed] [Google Scholar]
- 27.Noden DM. Somatotopic organization of the embryonic chick trigeminal ganglion. J Comp Neurol. 1980;190:429–444. doi: 10.1002/cne.901900303. [DOI] [PubMed] [Google Scholar]
- 28.D’Amico-Martel A, Noden DM. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am J Anat. 1983;166:445–468. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- 29.Couly G, Le Douarin NM. Head morphogenesis in embryonic avian chimeras: evidence for a segmental pattern in the ectoderm corresponding to the neuromeres. Development. 1990;108:543–558. doi: 10.1242/dev.108.4.543. [DOI] [PubMed] [Google Scholar]
- 30.Lumsden A, Sprawson N, Graham A. Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development. 1991;113:1281–1291. doi: 10.1242/dev.113.4.1281. [DOI] [PubMed] [Google Scholar]
- 31.Serbedzija GN, Bronner-Fraser M, Fraser SE. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development. 1992;116:297–307. doi: 10.1242/dev.116.2.297. [DOI] [PubMed] [Google Scholar]
- 32.Begbie J, Ballivet M, Graham A. Early steps in the production of sensory neurons by the neurogenic placodes. Mol Cell Neurosci. 2002;21:502–511. doi: 10.1006/mcne.2002.1197. [DOI] [PubMed] [Google Scholar]
- 33.Santagati F, Rijli FM. Cranial neural crest and the building of the vertebrate head. Nature Rev Neurosci. 2003;4:806–818. doi: 10.1038/nrn1221. [DOI] [PubMed] [Google Scholar]
- 34.Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- 35.Streit A. Early development of the cranial sensory nervous system: from a common field to individual placodes. Dev Biol. 2004;276:1–15. doi: 10.1016/j.ydbio.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 36.Schlosser G. Induction and specification of cranial placodes. Dev Biol. 2006;294:303–351. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Shiau CE, Lwigale PY, Das RM, Wilson SA, Bronner-Fraser M. Robo2-Slit1 dependent cell-cell interactions mediate assembly of the trigeminal ganglion. Nature Neurosci. 2008;11:269–276. doi: 10.1038/nn2051. [DOI] [PubMed] [Google Scholar]
- 38.Stark MR, Sechrist J, Bronner-Fraser M, Marcelle C. Neural tube-ectoderm interactions are required for trigeminal placode formation. Development. 1997;124:4287–4295. doi: 10.1242/dev.124.21.4287. [DOI] [PubMed] [Google Scholar]
- 39.Baker CV, Stark MR, Marcelle C, Bronner-Fraser M. Competence, specification and induction of Pax-3 in the trigeminal placode. Development. 1999;126:147–156. doi: 10.1242/dev.126.1.147. [DOI] [PubMed] [Google Scholar]
- 40.Xu H, Dude CM, Baker CV. Fine-grained fate maps for the ophthalmic and maxillomandibular trigeminal placodes in the chick embryo. Dev Biol. 2008;317:174–186. doi: 10.1016/j.ydbio.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Knaut H, Blader P, Strähle U, Schier AF. Assembly of trigeminal sensory ganglia by chemokine signaling. Neuron. 2005;47:653–666. doi: 10.1016/j.neuron.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Kerstetter AE, Azodi E, Marss JA, Liu Q. Cadherin-2 function in the cranial ganglia and lateral line system of developing zebrafish. Dev Dyn. 2004;230:137–143. doi: 10.1002/dvdy.20021. [DOI] [PubMed] [Google Scholar]
- 43.Shiau CE, Bronner-Fraser M. N-cadherin acts in concert with Slit1-Robo2 signaling in regulating aggregation of placode-derived cranial sensory neurons. Development. 2009;136:4155–4164. doi: 10.1242/dev.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodge LK, et al. Retrograde BMP signaling regulates trigeminal sensory neuron identities and the formation of precise face maps. Neuron. 2007;55:572–586. doi: 10.1016/j.neuron.2007.07.010. The authors identify BMP4 as a face-derived retrograde signaling molecule that differentially regulates positionally restricted expression of homeodomain transcription factors in TG neurons. [DOI] [PubMed] [Google Scholar]
- 45.McCabe KL, Bronner-Fraser M. Essential role for PDGF signaling in ophthalmic trigeminal placode induction. Development. 2008;135:1863–1874. doi: 10.1242/dev.017954. [DOI] [PubMed] [Google Scholar]
- 46.Wurst W, Bally-Cuif L. Neural plate patterning: upstream and downstream of the isthmic organizer. Nature Rev Neurosci. 2001;2:99–108. doi: 10.1038/35053516. [DOI] [PubMed] [Google Scholar]
- 47.Canning CA, Lee L, Luo SX, Graham A, Jones CM. Neural tube derived Wnt signals cooperate with FGF signaling in the formation and differentiation of the trigeminal placodes. Neural Dev. 2008;3:35. doi: 10.1186/1749-8104-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Irving C, Mason I. Signalling by FGF8 from the isthmus patterns anterior hindbrain and establishes the anterior limit of Hox gene expression. Development. 2000;127:177–186. doi: 10.1242/dev.127.1.177. [DOI] [PubMed] [Google Scholar]
- 49.Trainor PA, Ariza-McNaughton L, Krumlauf R. Role of the isthmus and FGFs in resolving the paradox of neural crest plasticity and prepatterning. Science. 2002;295:1288–1291. doi: 10.1126/science.1064540. [DOI] [PubMed] [Google Scholar]
- 50.Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- 51.Lumsden AG, Davies AM. Earliest sensory nerve fibres are guided to peripheral targets by attractants other than nerve growth factor. Nature. 1983;306:786–788. doi: 10.1038/306786a0. [DOI] [PubMed] [Google Scholar]
- 52.Lumsden AG, Davies AM. Chemotropic effect of specific target epithelium in the developing mammalian nervous system. Nature. 1986;323:538–539. doi: 10.1038/323538a0. [DOI] [PubMed] [Google Scholar]
- 53.O’Connor R, Tessier-Lavigne M. Identification of maxillary factor, a maxillary process-derived chemoattractant for developing trigeminal sensory axons. Neuron. 1999;24:165–178. doi: 10.1016/s0896-6273(00)80830-2. This study identified the putative maxillary factor that attracts the earliest trigeminal peripheral axons as a combination of NTF3 and BDNF, for which a neurotrophic role was shown. [DOI] [PubMed] [Google Scholar]
- 54.Erzurumlu RS, Chen ZF, Jacquin MF. Molecular determinants of the face map development in the trigeminal brainstem. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:121–134. doi: 10.1002/ar.a.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobayashi H, Koppel AM, Luo Y, Raper JA. A role for collapsin-1 in olfactory and cranial sensory axon guidance. J Neurosci. 1997;17:8339–8352. doi: 10.1523/JNEUROSCI.17-21-08339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitsukawa T, et al. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron. 1997;19:995–1005. doi: 10.1016/s0896-6273(00)80392-x. [DOI] [PubMed] [Google Scholar]
- 57.Ulupinar E, Datwani A, Behar O, Fujisawa H, Erzurumlu R. Role of semaphorin III in the developing rodent trigeminal system. Mol Cell Neurosci. 1999;13:281–292. doi: 10.1006/mcne.1999.0747. Pathfinding defects in mice lacking SEM3A illustrate the role of this repulsive axon guidance molecule in restricing peripheral and central projections of trigeminal ganglion axons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White FA, Behar O. The development and subsequent elimination of aberrant peripheral axon projections in Semaphorin3A null mutant mice. Dev Biol. 2000;225:79–86. doi: 10.1006/dbio.2000.9822. [DOI] [PubMed] [Google Scholar]
- 59.Ozdinler PH, Erzurumlu RS. Slit2, a branching-arborization factor for sensory axons in the mammalian CNS. J Neurosci. 2002;22:4540–4549. doi: 10.1523/JNEUROSCI.22-11-04540.2002. Axon branching effects of SLIT2 are demonstrated in organotypic wholemount cultures of the trigeminal ganglion brainstem preparations from rat embryos. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma L, Tessier-Lavigne M. Dual branch-promoting and branch-repelling actions of Slit/Robo signaling on peripheral and central branches of developing sensory axons. J Neurosci. 2007;27:6843–6851. doi: 10.1523/JNEUROSCI.1479-07.2007. Defects in peripheral projections of the ophthalmic branch of the trigeminal nerve are shown in multiple Slit-deficient mouse embryos. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guha U, et al. Target-derived BMP signaling limits sensory neuron number and the extent of peripheral innervation in vivo. Development. 2004;131:1175–1186. doi: 10.1242/dev.01013. [DOI] [PubMed] [Google Scholar]
- 62.Graham A, Francis-West P, Brickell P, Lumsden A. The signalling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest. Nature. 1994;372:684–686. doi: 10.1038/372684a0. [DOI] [PubMed] [Google Scholar]
- 63.Nie X, Luukko K, Kettunen P. BMP signalling in craniofacial development. Int J Dev Biol. 2006;50:511–521. doi: 10.1387/ijdb.052101xn. [DOI] [PubMed] [Google Scholar]
- 64.Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ. Bmp4 and morphological variation of beaks in Darwin’s finches. Science. 2004;305:1462–1465. doi: 10.1126/science.1098095. [DOI] [PubMed] [Google Scholar]
- 65.Wu P, Jiang TX, Suksaweang S, Widelitz RB, Chuong CM. Molecular shaping of the beak. Science. 2004;305:1465–1466. doi: 10.1126/science.1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Erzurumlu RS, Ebner FF. Maintenance of discrete somatosensory maps in subcortical relay nuclei is dependent on an intact sensory cortex. Brain Res Dev Brain Res. 1988;44:302–308. doi: 10.1016/0165-3806(88)90229-5. [DOI] [PubMed] [Google Scholar]
- 67.Killackey HP, Fleming K. The role of the principal sensory nucleus in central trigeminal pattern formation. Brain Res. 1985;354:141–145. doi: 10.1016/0165-3806(85)90077-x. [DOI] [PubMed] [Google Scholar]
- 68.Erzurumlu RS, Jhaveri S. Trigeminal ganglion cell processes are spatially ordered prior to the differentiation of the vibrissa pad. J Neurosci. 1992;12:3946–3955. doi: 10.1523/JNEUROSCI.12-10-03946.1992. The first application of carbocyanine labelling to developing trigeminal axons confirmed the topographic order of the trigeminal projections from the periphery to the brainstem and showed that trigeminothalamic projections from the PrV alone are responsible for whisker-specific pattern formation in the thalamus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Erzurumlu RS, Jhaveri S. Thalamic axons confer a blueprint of the sensory periphery onto the developing rat somatosensory cortex. Brain Res Dev Brain Res. 1990;56:229–234. doi: 10.1016/0165-3806(90)90087-f. [DOI] [PubMed] [Google Scholar]
- 70.Erzurumlu RS, Jhaveri S. Emergence of connectivity in the embryonic rat parietal cortex. Cereb Cortex. 1992;2:336–352. doi: 10.1093/cercor/2.4.336. [DOI] [PubMed] [Google Scholar]
- 71.Senft SL, Woolsey TA. Computer-aided analyses of thalamocortical afferent ingrowth. Cereb Cortex. 1991;1:336–347. doi: 10.1093/cercor/1.4.336. [DOI] [PubMed] [Google Scholar]
- 72.Oury F, et al. Hoxa2- and rhombomere-dependent development of the mouse facial somatosensory map. Science. 2006;313:1408–1413. doi: 10.1126/science.1130042. This study mapped the rhombomeric origin and Hoxa2-dependent patterning of the mouse PrV and its whisker and lower-jaw representations. [DOI] [PubMed] [Google Scholar]
- 73.Marin F, Puelles L. Morphological fate of rhombomeres in quail/chick chimeras: a segmental analysis of hindbrain nuclei. Eur J Neurosci. 1995;7:1714–1738. doi: 10.1111/j.1460-9568.1995.tb00693.x. [DOI] [PubMed] [Google Scholar]
- 74.Lumsden A. The cellular basis of segmentation in the developing hindbrain. Trends Neurosci. 1990;13:329–335. doi: 10.1016/0166-2236(90)90144-y. [DOI] [PubMed] [Google Scholar]
- 75.Carpenter EM, Goddard JM, Chisaka O, Manley NR, Capecchi MR. Loss of Hox-A1 (Hox-1.6) function results in the reorganization of the murine hindbrain. Development. 1993;118:1063–1075. doi: 10.1242/dev.118.4.1063. [DOI] [PubMed] [Google Scholar]
- 76.Clarke JD, Lumsden A. Segmental repetition of neuronal phenotype sets in the chick embryo hindbrain. Development. 1993;118:151–162. doi: 10.1242/dev.118.1.151. [DOI] [PubMed] [Google Scholar]
- 77.Cordes SP. Molecular genetics of cranial nerve development in mouse. Nature Rev Neurosci. 2001;2:611–623. doi: 10.1038/35090039. [DOI] [PubMed] [Google Scholar]
- 78.Farago AF, Awatramani RB, Dymecki SM. Assembly of the brainstem cochlear nuclear complex is revealed by intersectional and subtractive genetic fate maps. Neuron. 2006;50:205–218. doi: 10.1016/j.neuron.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 79.Fraser S, Keynes R, Lumsden A. Segmentation in the chick embryo hindbrain is defined by cell lineage restrictions. Nature. 1990;344:431–435. doi: 10.1038/344431a0. [DOI] [PubMed] [Google Scholar]
- 80.Lumsden A, Keynes R. Segmental patterns of neuronal development in the chick hindbrain. Nature. 1989;337:424–428. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- 81.Metcalfe WK, Mendelson B, Kimmel CB. Segmental homologies among reticulospinal neurons in the hindbrain of the zebrafish larva. J Comp Neurol. 1986;251:147–159. doi: 10.1002/cne.902510202. [DOI] [PubMed] [Google Scholar]
- 82.Pasqualetti M, Diaz C, Renaud JS, Rijli FM, Glover JC. Fate-mapping the mammalian hindbrain: segmental origins of vestibular projection neurons assessed using rhombomere-specific Hoxa2 enhancer elements in the mouse embryo. J Neurosci. 2007;27:9670–9681. doi: 10.1523/JNEUROSCI.2189-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wingate RJ, Lumsden A. Persistence of rhombomeric organisation in the postsegmental hindbrain. Development. 1996;122:2143–2152. doi: 10.1242/dev.122.7.2143. [DOI] [PubMed] [Google Scholar]
- 84.Wizenmann A, Lumsden A. Segregation of rhombomeres by differential chemoaffinity. Mol Cell Neurosci. 1997;9:448–459. doi: 10.1006/mcne.1997.0642. [DOI] [PubMed] [Google Scholar]
- 85.Erzurumlu RS, Killackey HP. Development of order in the rat trigeminal system. J Comp Neurol. 1983;213:365–380. doi: 10.1002/cne.902130402. The first study depicting topographic order in the infraorbital nerve, the trigeminal ganglion and brainstem projections in rat embryos. [DOI] [PubMed] [Google Scholar]
- 86.al-Ghoul WM, Miller MW. Orderly migration of neurons to the principal sensory nucleus of the trigeminal nerve of the rat. J Comp Neurol. 1993;330:464–475. doi: 10.1002/cne.903300403. [DOI] [PubMed] [Google Scholar]
- 87.Altman J, Bayer SA. Development of the brain stem in the rat. IV Thymidine-radiographic study of the time of origin of neurons in the pontine region. J Comp Neurol. 1980;194:905–929. doi: 10.1002/cne.901940411. [DOI] [PubMed] [Google Scholar]
- 88.Nornes HO, Morita M. Time of origin of the neuronsin the caudal brain stem of the rat. An autoradiographic study. Dev Neurosci. 1979;2:101–114. [Google Scholar]
- 89.Miller MW, Muller SJ. Structure and histogenesis of the principal sensory nucleus of the trigeminal nerve: effect of prenatal exposure to ethanol. J Comp Neurol. 1989;282:570–580. doi: 10.1002/cne.902820408. [DOI] [PubMed] [Google Scholar]
- 90.Ding YQ, Yin J, Xu HM, Jacquin MF, Chen ZF. Formation of whisker-related principal sensory nucleus-based lemniscal pathway requires a paired homeodomain transcription factor, Drg11. J Neurosci. 2003;23:7246–7254. doi: 10.1523/JNEUROSCI.23-19-07246.2003. The first study to show the involvement of a homeodomain transcription factor, DRG11, in PrV development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Belford GR, Killackey HP. Vibrissae representation in subcortical trigeminal centers of the neonatal rat. J Comp Neurol. 1979;183:305–321. doi: 10.1002/cne.901830207. The first study depicting the development of pattern formation along the subcortical trigeminal centers of postnatal rats. [DOI] [PubMed] [Google Scholar]
- 92.Leamey CA, Ho SM. Afferent arrival and onset of functional activity in the trigeminothalamic pathway of the rat. Brain Res Dev Brain Res. 1998;105:195–207. doi: 10.1016/s0165-3806(97)00170-3. These authors show that rat trigeminothalamic fibres are synaptically active as soon as they reach the thalamus. [DOI] [PubMed] [Google Scholar]
- 93.Lim Y, Golden JA. Patterning the developing diencephalon. Brain Res Rev. 2007;53:17–26. doi: 10.1016/j.brainresrev.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 94.Altman J, Bayer SA. Development of the rat thalamus: I. Mosaic organization of the thalamic neuroepithelium. J Comp Neurol. 1988;275:346–377. doi: 10.1002/cne.902750304. [DOI] [PubMed] [Google Scholar]
- 95.Altman J, Bayer SA. Development of the rat thalamus: IV. The intermediate lobule of the thalamic neuroepithelium, and the time and site of origin and settling pattern of neurons of the ventral nuclear complex. J Comp Neurol. 1989;284:534–566. doi: 10.1002/cne.902840405. [DOI] [PubMed] [Google Scholar]
- 96.Deschenes M, Timofeeva E, Lavallee P, Dufresne C. The vibrissal system as a model of thalamic operations. Prog Brain Res. 2005;149:31–40. doi: 10.1016/S0079-6123(05)49003-2. [DOI] [PubMed] [Google Scholar]
- 97.Sherman SM, Guillery RW. Functional organization of thalamocortical relays. J Neurophysiol. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- 98.Trageser JC, et al. State-dependent gating of sensory inputs by zona incerta. J Neurophysiol. 2006;96:1456–1463. doi: 10.1152/jn.00423.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Krumlauf R. Hox Genes and patterning in mammals. Annu Rev Cell Dev Biol. 2009;45:431–456. doi: 10.1146/annurev.cellbio.042308.113423. [DOI] [PubMed] [Google Scholar]
- 100.Lumsden A, Krumlauf R. Patterning the vertebrate neuraxis. Science. 1996;274:1109–1115. doi: 10.1126/science.274.5290.1109. [DOI] [PubMed] [Google Scholar]
- 101.Narita Y, Rijli FM. Hox genes in neural patterning and circuit formation in the mouse hindbrain. Curr Top Dev Biol. 2009;88:139–167. doi: 10.1016/S0070-2153(09)88005-8. [DOI] [PubMed] [Google Scholar]
- 102.Davenne M, et al. Hoxa2 and Hoxb2 control dorsoventral patterns of neuronal development in the rostral hindbrain. Neuron. 1999;22:677–691. doi: 10.1016/s0896-6273(00)80728-x. [DOI] [PubMed] [Google Scholar]
- 103.Gaufo GO, Flodby P, Capecchi MR. Hoxb1 controls effectors of sonic hedgehog and Mash1 signaling pathways. Development. 2000;127:5343–5354. doi: 10.1242/dev.127.24.5343. [DOI] [PubMed] [Google Scholar]
- 104.Gaufo GO, Wu S, Capecchi MR. Contribution of Hox genes to the diversity of the hindbrain sensory system. Development. 2004;131:1259–1266. doi: 10.1242/dev.01029. [DOI] [PubMed] [Google Scholar]
- 105.Geisen MJ, et al. Hox paralog group 2 genes control the migration of mouse pontine neurons through slit-robo signaling. PLoS Biol. 2008;6:e142. doi: 10.1371/journal.pbio.0060142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dasen JS, Jessell TM. Hox networks and the origins of motor neuron diversity. Curr Top Dev Biol. 2009;88:169–200. doi: 10.1016/S0070-2153(09)88006-X. [DOI] [PubMed] [Google Scholar]
- 107.Dasen JS, Liu JP, Jessell TM. Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature. 2003;425:926–933. doi: 10.1038/nature02051. [DOI] [PubMed] [Google Scholar]
- 108.Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 109.Hunt P, et al. A distinct Hox code for the branchial region of the vertebrate head. Nature. 1991;353:861–864. doi: 10.1038/353861a0. [DOI] [PubMed] [Google Scholar]
- 110.Krumlauf R. Hox genes and pattern formation in the branchial region of the vertebrate head. Trends Genet. 1993;9:106–112. doi: 10.1016/0168-9525(93)90203-t. [DOI] [PubMed] [Google Scholar]
- 111.Prince V, Lumsden A. Hoxa-2 expression in normal and transposed rhombomeres: independent regulation in the neural tube and neural crest. Development. 1994;120:911–923. doi: 10.1242/dev.120.4.911. [DOI] [PubMed] [Google Scholar]
- 112.Tan DP, et al. Murine Hox-1.11 homeobox gene structure and expression. Proc Natl Acad Sci USA. 1992;89:6280–6284. doi: 10.1073/pnas.89.14.6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Erzurumlu RS, Jhaveri S, Takahashi H, McKay RD. Target-derived influences on axon growth modes in cultures of trigeminal neurons. Proc Natl Acad Sci USA. 1993;90:7235–7239. doi: 10.1073/pnas.90.15.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vanderhaeghen P, et al. A mapping label required for normal scale of body representation in the cortex. Nature Neurosci. 2000;3:358–365. doi: 10.1038/73929. [DOI] [PubMed] [Google Scholar]
- 115.Jacquin MF, et al. In DRG11 knock-out mice, trigeminal cell death is extensive and does not account for failed brainstem patterning. J Neurosci. 2008;28:3577–3585. doi: 10.1523/JNEUROSCI.4203-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rebelo S, et al. DRG11 immunohistochemical expression during embryonic development in the mouse. Dev Dyn. 2007;236:2653–2660. doi: 10.1002/dvdy.21271. [DOI] [PubMed] [Google Scholar]
- 117.Logan C, Wingate RJ, McKay IJ, Lumsden A. Tlx-1 and Tlx-3 homeobox gene expression in cranial sensory ganglia and hindbrain of the chick embryo: markers of patterned connectivity. J Neurosci. 1998;18:5389–5402. doi: 10.1523/JNEUROSCI.18-14-05389.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qian Y, Shirasawa S, Chen CL, Cheng L, Ma Q. Proper development of relay somatic sensory neurons and D2/D4 interneurons requires homeobox genes Rnx/Tlx-3 and Tlx-1. Genes Dev. 2002;16:1220–1233. doi: 10.1101/gad.982802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dai JX, Hu ZL, Shi M, Guo C, Ding YQ. Postnatal ontogeny of the transcription factor Lmx1b in the mouse central nervous system. J Comp Neurol. 2008;509:341–355. doi: 10.1002/cne.21759. [DOI] [PubMed] [Google Scholar]
- 120.Cheng L, et al. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nature Neurosci. 2004;7:510–517. doi: 10.1038/nn1221. [DOI] [PubMed] [Google Scholar]
- 121.Shigetani Y, Nobusada Y, Kuratani S. Ectodermally derived FGF8 defines the maxillomandibular region in the early chick embryo: epithelial-mesenchymal interactions in the specification of the craniofacial ectomesenchyme. Dev Biol. 2000;228:73–85. doi: 10.1006/dbio.2000.9932. [DOI] [PubMed] [Google Scholar]
- 122.Shigetani Y, et al. Heterotopic shift of epithelial-mesenchymal interactions in vertebrate jaw evolution. Science. 2002;296:1316–1319. doi: 10.1126/science.1068310. [DOI] [PubMed] [Google Scholar]
- 123.Shigetani Y, Sugahara F, Kuratani S. A new evolutionary scenario for the vertebrate jaw. Bioessays. 2005;27:331–338. doi: 10.1002/bies.20182. [DOI] [PubMed] [Google Scholar]
- 124.Andres FL, Van der Loos H. Whisker patterns form in cultured non-innervated muzzle skin from mouse embryos. Neurosci Lett. 1982;30:37–41. doi: 10.1016/0304-3940(82)90008-8. [DOI] [PubMed] [Google Scholar]
- 125.Sagasti A, Guido MR, Raible DW, Schier AF. Repulsive interactions shape the morphologies and functional arrangement of zebrafish peripheral sensory arbors. Curr Biol. 2005;15:804–814. doi: 10.1016/j.cub.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 126.Kutsuwada T, et al. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron. 1996;16:333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 127.Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell. 1994;76:427–437. doi: 10.1016/0092-8674(94)90108-2. This study is the first demonstration that NMDAR function is essential in patterning of the trigeminal afferents in the PrV but not in their topographic ordering. [DOI] [PubMed] [Google Scholar]
- 128.Iwasato T, et al. NMDA receptor-dependent refinement of somatotopic maps. Neuron. 1997;19:1201–1210. doi: 10.1016/s0896-6273(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 129.Lee LJ, Lo FS, Erzurumlu RS. NMDA receptor-dependent regulation of axonal and dendritic branching. J Neurosci. 2005;25:2304–2311. doi: 10.1523/JNEUROSCI.4902-04.2005. This study describes the role of NMDARs in trigeminal axonal branching and dendritic orientation of PrV barrelette cells in Nmdar mutant mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stainier DY, Gilbert W. Pioneer neurons in the mouse trigeminal sensory system. Proc Natl Acad Sci USA. 1990;87:923–927. doi: 10.1073/pnas.87.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stainier DY, Gilbert W. Neuronal differentiation and maturation in the mouse trigeminal sensory system, in vivo and in vitro. J Comp Neurol. 1991;311:300–312. doi: 10.1002/cne.903110210. [DOI] [PubMed] [Google Scholar]
- 132.Waite PM, Ho SM, Henderson TA. Afferent ingrowth and onset of activity in the rat trigeminal nucleus. Eur J Neurosci. 2000;12:2781–2792. doi: 10.1046/j.1460-9568.2000.00161.x. This study showed that embryonic trigeminal axons are synaptically active as they enter the brainstem trigeminal nuclei. [DOI] [PubMed] [Google Scholar]
- 133.Chiaia NL, et al. Evidence for prenatal competition among the central arbors of trigeminal primary afferent neurons. J Neurosci. 1992;12:62–76. doi: 10.1523/JNEUROSCI.12-01-00062.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hayashi H. Distributions of vibrissae afferent fiber collaterals in the trigeminal nuclei as revealed by intra-axonal injection of horseradish peroxidase. Brain Res. 1980;183:442–446. doi: 10.1016/0006-8993(80)90478-3. [DOI] [PubMed] [Google Scholar]
- 135.Henderson TA, Jacquin MF. What Makes Subcortical Barrels? Plenum; New York: 1995. pp. 123–187. [Google Scholar]
- 136.Lo FS, Guido W, Erzurumlu RS. Electrophysiological properties and synaptic responses of cells in the trigeminal principal sensory nucleus of postnatal rats. J Neurophysiol. 1999;82:2765–2775. doi: 10.1152/jn.1999.82.5.2765. Membrane properties and synaptic responses of PrV cells in neonatal rats are described. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rudhard Y, et al. Absence of whisker-related pattern formation in mice with NMDA receptors lacking coincidence detection properties and calcium signaling. J Neurosci. 2003;23:2323–2332. doi: 10.1523/JNEUROSCI.23-06-02323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Single FN, et al. Dysfunctions in mice by NMDA receptor point mutations NR1 (N598Q) and NR1 (N598R) J Neurosci. 2000;20:2558–2566. doi: 10.1523/JNEUROSCI.20-07-02558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Taniguchi M, et al. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron. 1997;19:519–530. doi: 10.1016/s0896-6273(00)80368-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


