Abstract
Polyamines are essential cell constituents whose depletion results in growth cessation. Here we have investigated potential mechanisms of action of polyamines in supporting mammalian cell proliferation. We demonstrate that polyamines regulate translation both at the initiation and at the elongation steps. l-α-Difluoromethylornithine treatment resulting in polyamine depletion reduces protein synthesis via inhibition of translation initiation. N1-Guanyl-diaminoheptane (GC7), a spermidine analogue that inhibits eukaryotic initiation factor 5A (eIF5A) hypusination, also caused inhibition of translation initiation. In contrast, depletion of eIF5A by short hairpin RNA inhibits translation elongation as was recently demonstrated in yeast and Drosophila. These results suggest that in addition to competing with spermidine in the hypusination reaction, GC7 also competes with spermidine at yet undefined sites required for translation initiation. Finally, we show that either polyamine depletion or GC7 treatment induced eIF2α phosphorylation and reduced phosphorylation of 4E-BP, thus setting the molecular basis for the observed inhibition of translation initiation.
Keywords: Cell Metabolism, Polyamines, Translation Elongation Factors, Translation Initiation Factors, Translation Regulation, 4E-BP, eIF2 Alpha
Introduction
The polyamines are small organic polycations that are essential for the process of cellular proliferation as their depletion leads to growth inhibition (1, 2). Although their exact mode of action is far from being resolved, they are suggested to play important roles in regulating fundamental cellular processes such as signaling, replication, transcription, and translation (2). Polyamines regulate translation in a specific and global manner. They regulate the expression of antizyme, a central regulator of the polyamine biosynthesis pathway, by promoting ribosomal frameshifting (3, 4) and suppress the expression of S-adenosylmethionine decarboxylase, a key enzyme in the polyamine biosynthesis pathway, via interaction with specific sequences of upstream open reading frames (5). At the global level, polyamines may regulate translation via hypusination of the putative translation factor eIF5A.2 eIF5A, a highly conserved and essential protein (6–11), is the only protein that contains the unusual spermidine-derived amino acid residue hypusine (8, 12, 13), which is essential for its biological activity (13, 14). The requirement of eIF5A hypusination for cell growth raised the possibility that eIF5A hypusination may represent the major or even the entire requirement of polyamines for cellular proliferation (7, 15, 16). eIF5A, which was originally suggested to function as a translation initiation factor (17), was recently demonstrated to function in regulating translation elongation in yeast and Drosophila (18–20).
Here we show that also in mammalian cells, eIF5A regulates translation elongation. However, we show that in addition to regulating translation elongation through eIF5A hypusination, polyamines regulate translation initiation through an eIF5A-independent mechanism by modulating phosphorylation of eIF2α and 4E-BP1, two key regulators of the process of translation initiation.
MATERIALS AND METHODS
Cell Culture Conditions, Transfection, and Infection
NIH3T3 mouse fibroblasts and HEK-293T cell lines were grown in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% (v/v) fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin (Biological Industries). NIH3T3 cells were transfected using JetPEI (Source BioScience AUTOGEN) following the manufacturer's instructions. HEK-293T cells were transfected using the calcium phosphate method (21).
Mouse GIPZ plasmids bearing lentiviral shRNAmir against eIF5A and a non-silencing shRNAmir (Open Biosystems) were co-transfected with a second generation lentiviral packaging system plasmid (22) into HEK-293T cells. The medium was replaced after 10 h, and following an additional 60 h of incubation, the medium was collected, filtrated through a 0.45-μm cellulose acetate filter, and used to infect NIH3T3 cells in the presence of 8 μg/ml Polybrene (Sigma). Sixteen hours after infection, the cells were washed five times with phosphate-buffered saline and incubated in fresh medium containing 1 μg/ml puromycin.
Polyamine Analysis
Cells grown in 10-cm dishes were harvested, sedimented, and resuspended in 100 μl of phosphate-buffered saline. The cells were lysed in 3% perchloric acid, and precipitated material was removed by centrifugation (5 min at 13,000 rpm). The supernatant was collected for polyamine analysis, whereas the pellet was used for normalization by DNA quantification (DNA was quantified by resuspending the pellet in 400 μl of 4% diphenylamine (Sigma) in acetic acid, 400 μl of 10% perchloric acid, and 20 μl of 1:500 acetaldehyde (Sigma) and by incubation for 16 h at 30 °C followed by optical density determination at 595 and 700 nm). For polyamine analysis, 100 μl of the perchloric acid supernatant were mixed with 200 μl of 6 mg/ml dansyl chloride (in acetone). After the addition of 10 mg of sodium carbonate, the mixture was incubated for 16 h in the dark. To neutralize residual dansyl chloride, 50 μl of 100 mg/ml l-proline solution were added and incubated for 1 h at room temperature. Dansylated derivatives were extracted into 250 μl of toluene. Portions of 50–100 μl were spotted on a silica 60 F254 (Merck) TLC plate, and the dansylated derivatives were resolved by thin layer chromatography using ethyl acetate/cyclohexane (1:1.5) as a solvent and visualized by UV illumination. Dansylated derivatives of known polyamines served as markers.
Determination of Protein Synthesis Activity
For determination of global protein synthesis activity, cells were plated at a density of 6 × 104 cells in 6-cm plates. At the indicated times, the cells were washed twice with phosphate-buffered saline and incubated for 20 min in methionine-free Dulbecco's modified Eagle's medium supplemented with 10% dialyzed serum (HyClone) and 50 μCi/ml l-[35S]methionine. Cells were washed four times with phosphate-buffered saline, scraped, and collected by centrifugation. The pellets were lysed, and aliquots containing 30 μg of protein were loaded onto GF/C glass microfiber filters and subjected to trichloroacetic acid precipitation. For determination of [35S]methionine incorporation into nascent chains across the polysomal profile, 100 μl from each fraction were loaded onto the glass microfiber filters, and radioactivity was determined using the BAS-2500 bio-imaging analyzer (Fujifilm).
Polysomal Extraction and Sucrose Gradient Preparation
Cells were harvested 2 min after the addition of cycloheximide (100 μg/ml) to the growth medium. The cells were transferred to ice, washed twice with ice-cold buffer A (20 mm Tris-HCl pH 8, 140 mm KCl, 5 mm MgCl2, and 100 μg/ml cycloheximide), and collected by scraping and centrifugation. Following resuspension of cells in 450 μl of buffer A, 50 μl of lysis buffer (0.2% Triton X-100, 1% deoxycholate, 10 mm dithiothreitol, protease inhibitor mixture (Sigma), and 150 units of RNasin (EURx)) were added and incubated for 20 min on ice with frequent vortexing. The lysed cells were centrifuged, and the polysome-containing supernatant was collected. Two hundred micrograms of RNA from these polysomal fractions were loaded on 10–50% sucrose gradients (prepared in 20 mm Tris-HCl, pH 8, 140 mm KCl, 5 mm MgCl2, 500 μm dithiothreitol, 100 μg/ml cycloheximide, and 150 units of RNasin). The gradients were centrifuged for 90 min at 41,000 rpm at 4 °C, 250-μl fractions were collected, and the RNA concentrations were determined (at 260 nm) using the NanoDrop ND-1000 spectrophotometer (Thermo Scientific).
Isoelectric Focusing
Cells were lysed in low salt hypotonic buffer (10 mm HEPES, pH 7.3, 1.5 mm MgCl2, 20 mm KCl, 500 μm dithiothreitol, 1% Triton X-100) supplemented with protease inhibitor mixture for 20 min followed by five brief rounds of sonication. These samples were fractionated by electrophoresis in slab isoelectric focusing gels, pH 3–7 (Invitrogen). The resolved proteins were electroblotted onto a nitrocellulose membrane for immunoblot analysis.
Immunoblot Analysis
Cellular extracts were prepared by lysing cells in radioimmune precipitation buffer (50 mm Tris-HCl, pH 8, 150 mm KCl, 1.0% Nonidet P-40 (IGEPAL), 0.5% sodium deoxycholate, 0.1% SDS) supplemented with a protease inhibitor mixture. Equal portions of protein were resolved by electrophoresis in SDS-polyacrylamide gel, electroblotted onto a nitrocellulose membrane, and incubated with the indicated antibodies followed by horseradish peroxidase-conjugated anti-IgG antibodies. The antibodies used were: mouse monoclonal antibody against antizyme (Az1), rabbit polyclonal antibody against eIF2α (Santa Cruz Biotechnology), rabbit polyclonal antibody against eIF2α-[pS52] (BIOSOURCE), rabbit monoclonal antibody against 4E-BP1 (Cell Signaling Technology), and mouse anti-β-actin antibody (Sigma). Signals were developed using EZ-ECL (Biological Industries), and the membranes were exposed to x-ray films and developed.
RESULTS
Polyamine Depletion and Inhibition of eIF5A Hypusination Inhibit Cellular Proliferation
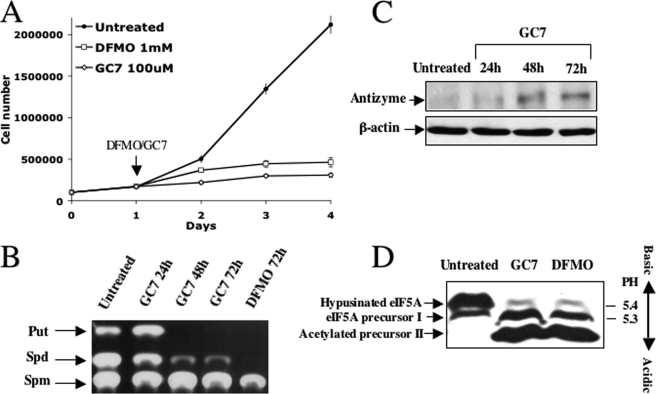
To compare the effect of inhibition of eIF5A hypusination to that of polyamine depletion on the process of cell proliferation, NIH3T3 cells were treated either with DFMO, a mechanism-based suicide inhibitor of ornithine decarboxylase (ODC), the first enzyme in the biosynthesis of polyamines (23), or with N1-guanyl-diaminoheptane (GC7), a spermidine analogue that competitively and reversibly inhibits deoxyhypusine synthase, the first enzyme in the hypusination of eIF5A (24, 25). As shown in Fig. 1A, both treatments inhibited cellular proliferation with similar efficiency. GC7 was used at a concentration (100 μm) shown previously to inhibit growth of NIH3T3 cells (26). As GC7 may also affect cellular polyamine content in addition to its interference with eIF5A hypusination(16), we set out to determine polyamine levels in GC7- and DFMO-treated cells. Although DFMO was more effective in reducing cellular polyamines, GC7 also caused reduction in putrescine and spermidine (Fig. 1B). The ability of GC7 to reduce cellular polyamines raised the possibility that as a spermidine analogue, it may induce antizyme (Az), a polyamine-induced protein that inhibits cellular proliferation due to its ability to stimulate ODC degradation and inhibit polyamine uptake (27, 28). Indeed, Western blot analysis demonstrated efficient induction of Az (Fig. 1C), suggesting that in addition to its proposed ability to inhibit growth by blocking eIF5A hypusination, GC7 also inhibits growth by inducing Az and reducing cellular polyamines. We have also compared the effect of both treatments on eIF5A hypusination by fractionating extracts of control and treated cells in a slab isoelectric focusing gel. As shown in Fig. 1D, both treatments inhibited eIF5A hypusination, resulting in accumulation of unhypusinated eIF5A precursors.
FIGURE 1.
Polyamine depletion arrests growth of mammalian cells and inhibits eIF5A hypusination. A, NIH3T3 cells were treated with DFMO (1 mm) and with GC7 (100 μm). Cells were counted at the indicated times (the presented results represent triplicate experiments). Error bars indicate S.D. B, NIH3T3 were treated with GC7 and DFMO for the indicated times, and cellular extracts were prepared and subjected to polyamine analysis as described under “Materials and Methods.” The position of the polyamine markers is indicated. Put, putrescine; Spd, spermidine; Spm, spermine. C, NIH3T3 cells were treated with GC7. At the indicated time points, cellular extracts were prepared and subjected to Western blot analysis using anti-Az antibodies. D, NIH3T3 cells were treated with GC7 for 3 days and with DFMO for 4 days. Cellular extracts were resolved in a slab isoelectric focusing gel (pH 3–7), electroblotted to nitrocellulose, and probed with anti-eIF5A antibodies.
Treatment with Either DFMO or GC7 Inhibits Translation Initiation
Because treatment with DFMO and with GC7 both inhibited eIF5A hypusination, a modification required for its putative function in regulating translation, we next characterized their effects on protein synthesis activity in the treated cells. As shown in Fig. 2A, both treatments caused significant inhibition of protein synthesis activity in the treated cells. To investigate the mechanism that accounts for this inhibition, polysomal profiles of whole cell extracts from control and from GC7- and DFMO-treated cells were analyzed by velocity sedimentation in sucrose gradients. The fractionation was performed in the presence of cycloheximide to inhibit ribosomal elongation during the analysis process. GC7 and DFMO both reduced the amount of polysomes while increasing the 80 S peak, a pattern compatible with inhibition of translation initiation (Fig. 2B). Indeed, the polysomal profiles of the GC7- and DFMO-treated cells were similar to that obtained following arsenite treatment, which is known to impair translation initiation by stimulating eIF2α phosphorylation (29) (Fig. 2C). Moreover, they were profoundly different from that obtained following treatment with a low concentration of cycloheximide, an inhibitor of the elongation process (Fig. 2D). To further confirm the notion that treatment with GC7, an inhibitor of eIF5A hypusination, actually impairs translation initiation, control and GC7-treated cells were pulse-labeled with [35S]methionine prior to their lysis and fractionated on sucrose gradient. Because unincorporated [35S]methionine sediments at the light fractions of the gradient, association of the radioactivity with the heavy fractions represents incorporation into initiation complexes and nascent polypeptide chains. As shown in Fig. 2E, despite the increase in the 80 S peak of the gradient representing the GC7-treated cells, there was a decrease in the radioactivity in this peak, strongly supporting the notion that translation initiation was inhibited. The 80 S peak of the polysomal profile may contain a mixture of empty initiation complexes lacking mRNA or real initiation complexes containing mRNA. Empty complexes are distinguished from mRNA-containing complexes by their increased sensitivity to high salt concentration (30, 31). As shown in Fig. 2F, the 80 S peak of the GC7-treated cells was not sensitive to the addition of 200 mm KCl to the sucrose gradient, indicating that this peak represents mRNA-containing translation initiation complexes. Interestingly, spermine was recently demonstrated to affect the initiation step of mitochondrial protein synthesis (32).
FIGURE 2.
DFMO and GC7 inhibit translation at the initiation step. A, NIH3T3 cells were treated with DFMO or GC7. At the indicated time points, the cells were labeled with [35S]methionine ([35]S-Met), and radioactivity incorporated into proteins was determined by trichloroacetic acid precipitation (the presented results represent two experiments). Error bars indicate S.D.; PSL, photostimulus luminescence. B, NIH3T3 cells were treated with DFMO (for 4 days) or GC7 (for 3 days). Cycloheximide was added to the growth medium shortly before harvesting. Whole cell extracts were prepared and resolved on sucrose gradients to visualize the indicated ribosomal species. The ratio between polysomes and monosomes (P/M) was calculated by dividing the area under the polysomes by that under the 40 S, 60 S, and 80 S peaks. C, NIH3T3 cells were treated with arsenite (250 μm) for 40 min. Whole cell extracts were prepared and fractionated as in A. D, cells were either untreated or treated with cycloheximide (5 μg/ml) 30 min prior to their harvest. Whole cell extracts were prepared and fractionated as in A. E, control NIH3T3 cells and cells treated with GC7 (100 μm for 3 days) were labeled with 100 μCi/ml [35S]methionine for 10 min before harvesting. Whole cell extracts were prepared and fractionated to obtain the polysomal profiles. Aliquots from the individual fractions were spotted on a filter paper, and the radioactivity incorporated into proteins was determined using a PhosphorImager. F, control and GC7-treated cells were harvested as described and fractionated in sucrose gradients containing 45 mm or 200 mm KCl.
GC7 Inhibits Translation Initiation Even in the Presence of Polyamines
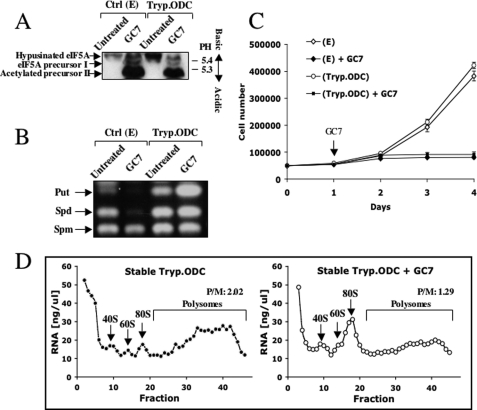
As shown above, treatment with either DFMO or GC7 resulted in polyamine depletion and inhibition of eIF5A hypusination. To test whether inhibition of eIF5A hypusination by GC7 without depletion of polyamines suffices to inhibit translation and cellular proliferation, NIH3T3 cells that were stably transfected with trypanosome ODC were treated with GC7. Because trypanosome ODC is refractory to the effect of Az (33, 34), it is expected that in these cells, induction of Az by GC7 will not result in polyamine depletion. Indeed, while inhibiting eIF5A hypusination (Fig. 3A), GC7 failed to reduce polyamine levels in these cells (Fig. 3B). Interestingly, although not depleting polyamines in the trypanosome ODC-expressing cells, GC7 inhibited their growth (Fig. 3C) and inhibited translation initiation (Fig. 3D). These findings suggest that GC7 inhibits translation initiation without depleting cellular polyamines, either by inhibiting eIF5A hypusination or through an independent mechanism.
FIGURE 3.
GC7 inhibits translation initiation without depleting cellular polyamines. A, control NIH3T3 cells stably transfected with empty vector (E) and NIH3T3 cells stably expressing trypanosome ODC (Tryp.ODC) were treated with GC7 (100 μm) for 3 days. Cellular extracts were prepared and fractionated in a slab isoelectric focusing gel as described in the legend for Fig. 1. B, control NIH3T3 and trypanosome ODC-expressing NIH3T3 cells were treated with GC7 (100 μm) for 3 days and subjected to polyamine analysis as in Fig. 1B. Put, putrescine; Spd, spermidine; Spm, spermine. C, control NIH3T3 and trypanosome ODC-expressing NIH3T3 cells were treated with GC7, and their growth rate was determined as in Fig. 1A (the presented results represent triplicate experiments). Error bars indicate S.D. D, wild type and trypanosome ODC-expressing NIH3T3 cells were treated with GC7 (100 μm for 3 days) and polysomal profiles were prepared as in Fig. 2A. P/M, the ratio between polysomes and monosomes.
Depletion of eIF5A Inhibits Translation Elongation
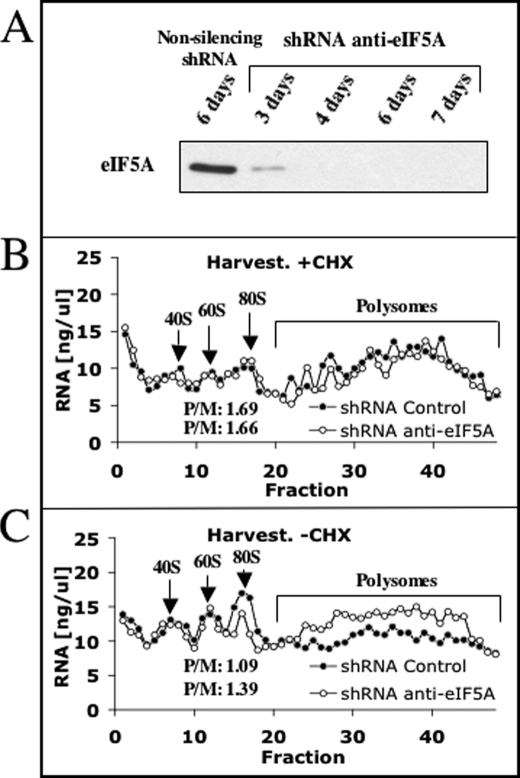
Our findings that treatment with GC7, an inhibitor of eIF5A hypusination, leads to inhibition of translation initiation contradict recent reports supporting the involvement of hypusinated eIF5A in the process of translation elongation in yeast and Drosophila (18–20), perhaps suggesting a different role for this factor in mammalian cells. Because these studies used genetic manipulation to regulate eIF5A levels and modification, whereas we employ inhibitors of hypusine synthesis that may have pleiotropic effects, we have utilized shRNA as an independent approach to determine the role of mammalian eIF5A in regulating the translation process. Infection of NIH3T3 cells with lentivirus expressing shRNA to eIF5A resulted in effective depletion of this protein (Fig. 4A), and as expected, in substantial growth inhibition (not shown). Sucrose gradient analysis performed on extracts of these cells and cells infected with a non-silencing virus in the presence of cycloheximide showed that there is practically no difference in the polysomal profiles (Fig. 4B). To facilitate the detection of possible inhibition of translation elongation, the polysomal analysis was repeated in the absence of cycloheximide. Under these conditions, polysomal analysis demonstrated that the level of polysomes in the cells treated with shRNA to eIF5A was preserved, whereas the level of polysomes in control cells was diminished (Fig. 4C). These results suggest that as in yeast and Drosophila, eIF5A also regulates translation elongation in mammalian cells and suggest that GC7 inhibits translation initiation via a mechanism that is independent of its ability to inhibit eIF5A hypusination. However, because shRNA-mediated eIF5A depletion is not likely to be complete, we cannot rule out the possibility that in addition to its role in the elongation process, eIF5A may also be required for the initiation process.
FIGURE 4.
Depletion of eIF5A results in inhibition of translation elongation. A, NIH3T3 cells were infected with a lentivirus encoding shRNA to eIF5A. Cellular extracts were prepared at the indicated times, and the level of eIF5A was determined by Western blot analysis. B and C, cells were harvested 6 days after infection either with (B, +CHX) or without (C, −CHX) cycloheximide and fractionated on a sucrose gradient as in Fig. 2.
Polyamine Depletion or Treatment with GC7 Stimulates eIF2α Phosphorylation and Reduces 4E-BP Phosphorylation
Two factors regulate translation initiation through interaction with other components of the translation initiation machinery. The first is 4E-BP, which competes with eIF4G on binding to the cap-binding factor eIF4E. Phosphorylation of 4E-BP weakens its binding to eIF4E, enabling assembly of the cap-binding complex and thus stimulating initiation of cap-dependent translation (35–37). The second is eIF2α, whose phosphorylation on Ser-52 converts eIF2 from a substrate to a competitive inhibitor of the guanine nucleotide exchange factor eIF2B, thus inhibiting translation initiation (35–37). To determine whether treatment with DFMO or GC7 affects the phosphorylation state of these two factors, cellular extracts from control and GC7- and DFMO-treated cells were fractionated by SDS-PAGE and subjected to Western blot analysis using anti-4E-BP and anti-phosphorylated eIF2α antibodies. Treatment of wild-type NIH3T3 cells with GC7 or DFMO (Fig. 5A) and trypanosome ODC-expressing NIH3T3 cells with GC7 (Fig. 5B) stimulated eIF2α phosphorylation and inhibited 4E-BP phosphorylation, changes that are fully compatible with inhibition of translation initiation.
FIGURE 5.
Treatment with either GC7 or DFMO affects the phosphorylation state of 4E-BP and eIF2α. Control NIH3T3 cells, stably transfected with empty vector or trypanosome ODC-expressing (Tryp.ODC) NIH3T3 cells, were treated with GC7 (3 days) or DFMO (4 days). Cellular extracts were prepared and subjected to Western blot analysis using anti-eIF2α, anti-eIF2α-P (recognizing only its phosphorylated form), and anti-4E-BP. P indicates phosphorylation.
The Initiation Block Provoked by DFMO or GC7 Treatments Is Developed Gradually and Prevails over the Elongation Block
Because modulation of cellular polyamines affected both translation initiation and elongation, we next examined the kinetics of these two processes. For this purpose, NIH3T3 cells were treated with DFMO or GC7, cellular extracts were prepared at the indicated time points, and eIF2α and 4E-BP phosphorylation and eIF5A hypusination were determined. As shown in Fig. 6A, in DFMO-treated cells, the increase in eIF2α phosphorylation and decrease in 4E-BP phosphorylation were developed gradually and paralleled the disappearance of hypusinated eIF5A and appearance of the unhypusinated forms. The effect of GC7 treatment on eIF2α phosphorylation was the most rapid, noted already at 3 h after treatment, followed by a decrease in 4E-BP phosphorylation observed after 6 h. Hypusinated eIF5A started to disappear only 24 h after treatment, an observation compatible with its stable nature. Interestingly, newly synthesized unhypusinated precursor already appeared 3 h after the addition of GC7. More importantly, both treatments resulted in gradual development of a translation initiation block starting already at 24 and 48 h in GC7- and DFMO-treated cells, respectively (Fig. 6, C and D). The polysomal profiles demonstrated higher increase in the 80 S peak in the GC7-treated cells than in the DFMO-treated cells, perhaps because the initiation block is developed simultaneously with the elongation block in the DFMO-treated cells.
FIGURE 6.
The translation initiation block induced by DFMO or GC7 is developed from early time points. NIH3T3 cells were treated with DFMO (A and C) or GC7 (B and D). Cellular extracts were prepared at the indicated time points and used for determination of the phosphorylation (P) state of eIF2α and 4E-BP and the hypusination state of eIF5A (A and B) or for the preparation of polysomal profiles (C and D). Ctrl, control. P/M, the ratio between polysomes and monosomes.
DISCUSSION
We show here that polyamines are required for the translation process in mammalian cells both at the initiation and at the elongation steps and that as suggested before (16, 38, 39), the requirement of polyamines in these translational processes is likely to account for their essentiality in the process of cellular proliferation. Translation elongation is affected via the use of spermidine as a precursor in the hypusination of eIF5A, a factor originally thought to function in translation initiation (17). As shown here for mammalian cells, and as recently shown in yeast and Drosophila (18–20), eIF5A actually functions as a translation elongation factor. We further show that polyamines affect the initiation process by modulating the phosphorylation of eIF2α and 4E-BP, a modification critical for the ability of these factors to regulate translation initiation. Depletion of cellular polyamines using DFMO, an irreversible mechanism-based inhibitor of ODC, inhibited both translation processes. GC7, a spermidine analogue that competitively and reversibly inhibits the hypusination reaction, also depletes cellular polyamines by inducing Az. Nevertheless, it inhibits translation initiation even in the presence of a normal level of polyamines, suggesting that as in the case of the hypusination reaction, GC7 may compete with spermidine at enzymatic or regulatory sites required for modulation of eIF2α and 4E-BP phosphorylation. However, because the magnitude (level) of the initiation block induced by GC7 is lower in this case, as compared with the effect in control cells, it is possible that the two blocks are developed in parallel, whereas the initiation block develops faster and prevails the elongation block. In all experiments, GC7 acted more rapidly and more profoundly than DFMO, probably because its competition with cellular polyamines starts immediately, whereas the depletion of polyamines by DFMO develops gradually.
It was suggested that polyamine analogues inhibit growth by inducing Az, thus depleting cellular polyamines (40). As we show here, polyamine analogues may also exert their toxicity by competing with the natural polyamines on sites that regulate translation either at the initiation or at the elongation steps.
eIF5A and its hypusination are essential for cellular proliferation and viability (6, 7, 9, 41). It was argued that the spermidine-dependent modification of eIF5A might represent the main or even the only requirement of polyamine for the process of cellular proliferation (7). As we show here, this possibility is rather unlikely as polyamine depletion leads also to a translation initiation block via an eIF5A-independent mechanism. Kinetic studies demonstrate that the initiation block established after DFMO and GC7 treatment precedes and predominates the elongation block provoked by inhibition of eIF5A hypusination, which was revealed only when cell growth was inhibited by shRNA to eIF5A. Our demonstration that a translation initiation block precedes the eIF5A-dependent elongation block is in agreement with two studies suggesting that polyamines may affect growth independently of eIF5A hypusination and that this independent effect precedes the eIF5A-dependent effect (15, 16). Further studies will be required to elucidate the molecular mechanism that underlines the requirement of polyamines for translation initiation and how the polyamine-dependent hypusination of eIF5A affects translation elongation.
Supplementary Material
Acknowledgments
We thank O. Elroy-Stein and O. Shifman for helpful comments and discussions.
This work was authored, in whole or in part, by National Institutes of Health staff. This work was supported by a grant from the M. D. Moross Institute for Cancer Research at the Weizmann Institute and by the Israel Science Foundation.
This article was selected as a Paper of the Week.
- eIF
- eukaryotic initiation factor
- DFMO
- l-α-difluoromethylornithine
- GC7
- N1-guanyl-diaminoheptane
- shRNA
- short hairpin RNA
- ODC
- ornithine decarboxylase
- Az
- antizyme.
REFERENCES
- 1.Igarashi K., Kashiwagi K. (2010) Int. J. Biochem. Cell Biol. 42, 39–51 [DOI] [PubMed] [Google Scholar]
- 2.Pegg A. E. (2009) IUBMB Life 61, 880–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsufuji S., Matsufuji T., Miyazaki Y., Murakami Y., Atkins J. F., Gesteland R. F., Hayashi S. (1995) Cell 80, 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rom E., Kahana C. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 3959–3963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mize G. J., Morris D. R. (2001) RNA 7, 374–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee I., Gross S. R., Kinzy T. G., Chen K. Y. (2006) Mol. Genet. Genomics 275, 264–276 [DOI] [PubMed] [Google Scholar]
- 7.Chattopadhyay M. K., Park M. H., Tabor H. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 6554–6559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen K. Y., Liu A. Y. (1997) Biol. Signals 6, 105–109 [DOI] [PubMed] [Google Scholar]
- 9.Childs A. C., Mehta D. J., Gerner E. W. (2003) Cell Mol. Life Sci. 60, 1394–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park M. H., Lee Y. B., Joe Y. A. (1997) Biol. Signals 6, 115–123 [DOI] [PubMed] [Google Scholar]
- 11.Schnier J., Schwelberger H. G., Smit-McBride Z., Kang H. A., Hershey J. W. (1991) Mol. Cell. Biol. 11, 3105–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper H. L., Park M. H., Folk J. E., Safer B., Braverman R. (1983) Proc. Natl. Acad. Sci. U.S.A. 80, 1854–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolff E. C., Kang K. R., Kim Y. S., Park M. H. (2007) Amino Acids 33, 341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanelli C. F., Valentini S. R. (2007) Amino Acids 33, 351–358 [DOI] [PubMed] [Google Scholar]
- 15.Hyvönen M. T., Keinänen T. A., Cerrada-Gimenez M., Sinervirta R., Grigorenko N., Khomutov A. R., Vepsäläinen J., Alhonen L., Jänne J. (2007) J. Biol. Chem. 282, 34700–34706 [DOI] [PubMed] [Google Scholar]
- 16.Nishimura K., Murozumi K., Shirahata A., Park M. H., Kashiwagi K., Igarashi K. (2005) Biochem. J. 385, 779–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benne R., Hershey J. W. (1978) J. Biol. Chem. 253, 3078–3087 [PubMed] [Google Scholar]
- 18.Gregio A. P., Cano V. P., Avaca J. S., Valentini S. R., Zanelli C. F. (2009) Biochem. Biophys. Res. Commun. 380, 785–790 [DOI] [PubMed] [Google Scholar]
- 19.Patel P. H., Costa-Mattioli M., Schulze K. L., Bellen H. J. (2009) J. Cell Biol. 185, 1181–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saini P., Eyler D. E., Green R., Dever T. E. (2009) Nature 459, 118–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham F. L., van der Eb A. J. (1973) Virology 54, 536–539 [DOI] [PubMed] [Google Scholar]
- 22.Naldini L., Blömer U., Gallay P., Ory D., Mulligan R., Gage F. H., Verma I. M., Trono D. (1996) Science 272, 263–267 [DOI] [PubMed] [Google Scholar]
- 23.Bitonti A. J., Bacchi C. J., McCann P. P., Sjoerdsma A. (1985) Biochem. Pharmacol 34, 1773–1777 [DOI] [PubMed] [Google Scholar]
- 24.Jakus J., Wolff E. C., Park M. H., Folk J. E. (1993) J. Biol. Chem. 268, 13151–13159 [PubMed] [Google Scholar]
- 25.Park M. H., Wolff E. C., Lee Y. B., Folk J. E. (1994) J. Biol. Chem. 269, 27827–27832 [PubMed] [Google Scholar]
- 26.Shi X. P., Yin K. C., Ahern J., Davis L. J., Stern A. M., Waxman L. (1996) Biochim. Biophys. Acta 1310, 119–126 [DOI] [PubMed] [Google Scholar]
- 27.Iwata S., Sato Y., Asada M., Takagi M., Tsujimoto A., Inaba T., Yamada T., Sakamoto S., Yata J., Shimogori T., Igarashi K., Mizutani S. (1999) Oncogene 18, 165–172 [DOI] [PubMed] [Google Scholar]
- 28.Kahana C. (2009) Cell Mol. Life Sci. 66, 2479–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brostrom C. O., Prostko C. R., Kaufman R. J., Brostrom M. A. (1996) J. Biol. Chem. 271, 24995–25002 [DOI] [PubMed] [Google Scholar]
- 30.Martin T. E., Hartwell L. H. (1970) J. Biol. Chem. 245, 1504–1506 [PubMed] [Google Scholar]
- 31.Sivan G., Kedersha N., Elroy-Stein O. (2007) Mol. Cell. Biol. 27, 6639–6646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christian B. E., Haque M. E., Spremulli L. L. (2010) Biochem. Biophys. Res. Commun. 391, 942–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghoda L., Phillips M. A., Bass K. E., Wang C. C., Coffino P. (1990) J. Biol. Chem. 265, 11823–11826 [PubMed] [Google Scholar]
- 34.Li X., Coffino P. (1992) Mol. Cell. Biol. 12, 3556–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dever T. E. (2002) Cell 108, 545–556 [DOI] [PubMed] [Google Scholar]
- 36.Gingras A. C., Raught B., Sonenberg N. (1999) Annu. Rev. Biochem. 68, 913–963 [DOI] [PubMed] [Google Scholar]
- 37.Sonenberg N., Hinnebusch A. G. (2009) Cell 136, 731–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogasawara T., Ito K., Igarashi K. (1989) J. Biochem. 105, 164–167 [DOI] [PubMed] [Google Scholar]
- 39.Shimogori T., Kashiwagi K., Igarashi K. (1996) Biochem. Biophys. Res. Commun. 223, 544–548 [DOI] [PubMed] [Google Scholar]
- 40.Mitchell J. L., Leyser A., Holtorff M. S., Bates J. S., Frydman B., Valasinas A. L., Reddy V. K., Marton L. J. (2002) Biochem. J. 366, 663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee Y., Kim H. K., Park H. E., Park M. H., Joe Y. A. (2002) Mol. Cell Biochem. 237, 69–76 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.