Abstract
Background
The successful treatment of patients with type 1 diabetes by islet transplantation is affected by a multitude of factors, of which infusion of the highest quality tissue is essential. The current standard pre-transplant quality assessments lack sensitivity, accuracy, and objectivity in the determination of islet viability and potency. We hypothesized that a multi-parametric approach focused on islet cell metabolic state, mitochondrial integrity, and in vitro glucose stimulated insulin secretion could provide data predictive of in vivo function. The objective of this study was to validate a novel set of islet quality assays and develop a simplified islet quality scoring system for both basic research and clinical applications.
Methods
A series of 42 human islet preparations were screened using standard and novel methods, which included determination of yield, viability by fluorescent microscopy, glucose stimulated insulin secretion (GSIS), percentage of islet loss in culture, quantification of adenine nucleotides, flow cytometric measurement of viability, apoptosis, and mitochondrial membrane potential (MMP). In vivo functional potency was tested by minimal model transplant in streptozotocin-induced diabetic NOD.scid mice.
Results
Functionally potent islet preparations showed significantly greater numbers of cells with polarized MMP, higher ATP/ADP ratios and increased glucose induced insulin secretion. The MMP, ATP/ADP, and GSIS data were combined into a single islet scoring formula that showed >86% accuracy in predicting in vivo functional potency.
Conclusions
Our study demonstrates that a multi-parametric approach using objective assessments focused on islet cell mitochondrial integrity and in vitro function can provide data predictive of in vivo function.
Keywords: Islet, Diabetes, Mitochondria, Transplantation, Flow Cytometry
INTRODUCTION
Isolated pancreatic islet transplantation has become an effective treatment for restoration of blood glucose control in patients with type 1 diabetes and hypoglycemia unawareness. However, major challenges remain in extending the function of islet grafts post transplant and reducing the quantity of islets required to “cure” individual patients. Despite significant advances in islet isolation and culture methodologies, as reported in the “Collaborative Islet Transplant Registry, 2009 Annual Report”, primary graft non-function (PNF) or early graft loss (EGL) still occurs in as many as 10% of islet transplant recipients post-infusion (1). Also documented in the CITR 2009 Annual report is the steady decline in islet graft function over time, with 42% of patients showing complete loss of islet function at four years post-infusion.
Islet cell viability and function are negatively impacted by the physical, chemical and temperature stresses that occur prior to and during pancreas recovery, preservation, transport, and isolation (2). Specifically, these events lead to low islet yields, diminished insulin content, accumulation of reactive oxygen species (ROS), ATP depletion, induction of apoptosis and necrosis. The mitochondria play a critical role in the cells response to both extrinsic and intrinsic stresses. Exposure of islets to pro-inflammatory cytokines such as IL-1β, excess reactive oxygen species, hypoxia, and electron chain inhibitors have all been shown to initiate a series of events that lead to mitochondrial dysfunction and apoptosis (3-5). Specific markers of mitochondrial dysfunction include: ATP depletion (an indicator of reduced oxidative phosphorylation), depolarization of the mitochondrial transmembrane potential (an indicator of permeability transition pore opening), and activation of pro-apoptotic caspase enzymes (an indicator of the release into the cytosol of proteins, such as cytochrome c, Smac/DIABLO and AIF) (6, 7).
We hypothesized that quantitative analysis of surrogate markers of islet cell health, with a focus on indicators of mitochondrial integrity and function, would provide sensitive and predictive data as to the ability of human islet preparations to restore normoglycemia in diabetic NOD.scid mice using a minimal islet mass model. The novel assays evaluated in this study allowed for the objective quantification of islet adenine nucleotides as indicators of metabolic state, phosphatidyl serine translocation and active caspase enzymes as indicators of apoptosis, and mitochondrial membrane potential (MMP). We performed a retrospective analysis of these assays in comparison to standard practice assessments for a series of 42 human islet preparations. In vivo islet functional potency was determined by graft performance following transplant into streptozotocin (STZ)-induced diabetic immunodeficient NOD.scid mice or a subset of human patients with type I diabetes. The primary endpoint of the study was to characterize human islet quality with greater precision than the current minimal standards requested by the United States Food and Drug Aministration for product release prior to transplantation.
MATERIALS AND METHODS
Islet isolation and culture
Human pancreata were obtained through the University of Wisconsin Hospital and Clinics Organ Procurement Organization with consent obtained for research from next of kin and authorization by the Health Sciences Institutional Review Board. Islets were isolated using a modification of the automated Ricordi method (8). Collagenase NB1, neutral protease (Serva Electrophoresis, Heidelberg, Germany), and DNase (Roche, Indianapolis, IN) were infused into the main pancreatic duct. Islets were separated from exocrine tissue by centrifugation on a continuous Biocoll gradient (Biochrom AG, Berlin, Germany) in a COBE 2991 cell processor (Lakewood, CO). For islet equivalent (IEQ) determination, islet samples were stained with dithizone (Sigma-Aldrich Corp., St. Louis, MO) and sized using an eyepiece reticle and inverted microscope. Islets were cultured in M1A media (Mediatech, Herndon, VA) at 25°C in a 5% CO2 incubator for 24 to 36h.
INS-1 Cell Culture
INS-1 cells (clone 823/13) were obtained as the kind gift of Dr. Christopher Newgard (Duke University) and were cultured at 37°C in a 5% CO2/95% air incubator in RPMI 1640 media supplemented with 10% FBS, 2 mM L-glutamine, 1 mM sodium pyruvate, 50 mM 2-mercaptoethanol, 25 mM Hepes and 100 U/ml penicillin/100 mg/ml streptomycin (9).
Fluorescent microscopy assessment of islet viability
Islets were suspended in PBS pH 7.4 and stained with fluorescein diacetate (FDA) [10 μg/ml] (Sigma Aldrich, St. Louis, MO) and propidium iodide (PI) [10̣μg/ml] (Invitrogen, Carlsbad, CA) with fluorescence observed using a Zeiss Axiovert 200 MAT/TV inverted microscope (Carl Zeiss Microimaging Inc., Thornwood, NY). Approximately 100 individual islets were digitally photographed and the percentage of live (green) versus dead (red) cells estimated manually by two separate observers.
Glucose stimulated insulin secretion assays
Islets were handpicked into oxygen (95%O2/5%CO2) saturated basal Krebs-Ringer Bicarbonate Buffer (KRB, 137 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4-7H2O, 2.5 mM CaCl2-2H2O, 25 mM NaHCO3, 0.25% BSA, 3.3 mM glucose) followed by incubation at 37°C for 30 min. in a 5%CO2/95% air incubator. Groups of 8 – 10 islets from the equilibration cultures were then transferred to fresh oxygen saturated KRB containing either 3.3 mM or 16.7 mM glucose and incubated an additional 60 min. in a 37°C water bath with gentle shaking. Secreted insulin in the media was measured by ELISA (Millipore, Billerica, MA) and values normalized to extracted islet DNA (Quant-iT PicoGreen, Invitrogen). Data are shown as a stimulation index (mean of secreted insulin from quintuplicate samples incubated with 16.7 mM/3.3 mM).
Adenine nucleotide quantification by HPLC
Total islet cellular extracts were prepared by phenol:chloroform:isoamyl alcohol extraction (10) followed by a two step ether extraction of residual phenol (11). Islet extracts were run on a Discovery C-18 column (Sigma) on a HP1100 series quad pump reverse phase HPLC with a variable wavelength UV detector set to 254nm. Analysis of chromatograms was performed using Chemstation software (Agilent Technologies,Waldbronn, Germany) with peak identification by co-elution with known ATP, ADP, AMP, GTP, and GDP (Sigma-Aldrich) standards.
Multi-parametric flow cytometry assessments
Islets were dispersed into single cell suspensions by incubation with trypsin [0.05%] and EDTA [0.53 mM] for 5 minutes at 37°C followed by gentle passage through a narrow gauge pipet tip. Dispersed cells were suspended in KRB containing 3.3 mM glucose and stained separately with probes for apoptosis (Annexin V PE, Invitrogen and Caspace (VADFMK) FITC, Promega, Madison, WI), viability (FDA, Sigma-Aldrich), and MMP (JC-1, Invitrogen) for 30 minutes in a 37°C incubator with 5% CO2. A beta cell specific probe was not included and data reflect the entire heterogeneous population of cells in the purified (on average >80%) islet preparation. After staining, cells were washed twice in KRB and analyzed for fluorescence using a BD LSR II™ flow cytometer (Becton Dickenson, Franklin Lakes, NJ). ToPro3 (Invitrogen) was added to samples just prior to data acquisition to identify necrotic cells. A minimum of 10,000 single cell gated events were collected and analyzed using Flo Jo (Treestar Inc. Ashland, OR) and GraphPad Prism software (GraphPad, San Diego, CA). Cellular fragments and free nuclei were excluded from intact cells based upon forward (FSC) and side scatter (SSC) light parameters. Data were further restricted to single cells by gating out aggregates based upon forward scatter area (FSC-A) and forward scatter width (FSC-W) parameters. Viable cells were defined as FDA bright and ToPro3−, apoptotic cells as Annexin V+ and/or VADFMK+, and cells with polarized MMP as JC-1 redbright and JC-1 greendull. Treatment of islet cells with the mitochondria depolarizing agent CCCP prior to JC-1 staining was used as a control to allow for objective establishment of the MMP+ gate.
In vivo islet functional assessment
NOD.scid male mice (>6 wks of age), purchased from The Jackson Laboratory (Bar Harbor, MA), were used as islet recipients following guidelines established by the University of Wisconsin-Madison Institutional Animal Care and Use Committee. Diabetes was induced by single intraperitoneal injection of 150 mg/kg streptozotocin (STZ, Sigma-Aldrich Corp.). Glucose was measured daily using an Ascensia Elite glucometer (Bayer, Burr Ridge, IL). Mice were considered diabetic if non-fasting blood glucose was > 300 mg/dL for 3 consecutive days. Mice (at least n=2/assay) were anesthetized by 2% isoflurane and islets were implanted underneath the kidney capsule. Blood glucose was measured for a total of 30 days after transplantation. Potent islet grafts produced sustained lowering of blood glucose to < 200 mg/dL within 14 days of transplant in a minimum of one recipient at a dose of 1000 IEQ/mouse. Those that failed to reverse diabetes were considered as preparations with poor function. The restoration and maintenance of normoglycemia due to islet graft function was proven by removal of the graft-bearing kidney with return to the hyperglycemic state within 24h. Four individual human subjects were transplanted intraportally with human islets following a US FDA and UW-Madison IRB approved clinical trial (Clinicaltrials.gov NCT00214253). Satisfactory graft performance was indicated by a reduction in insulin requirements of 20 units/day, lowering of HbA1c% and measureable c-peptide at 3 months post infusion.
Data analysis and statistics
A retrospective data analysis was performed based on the score of each individual assay and the capacity of the islet preparation to reverse diabetes in diabetic NOD.scid mice at a dose of 1000 IEQ transplanted under the kidney capsule. Descriptive statistics, Student’s T test, Fisher’s Exact test and Mann-Whitney U test and Receiver Operator Characteristic curve analysis were performed using GraphPad Prism Software (GraphPad Software, Inc., San Diego, CA). ROC curves illustrate the trade-off between sensitivity and specificity of a test (see Figure 4). Tests which cannot discriminate between true and false positives show a sensitivity plot that is not significantly different from the line of identity and a p value >0.05 when the Area Under the Curve (AUC) is calculated. Test cut-off values were selected based upon a user defined balance between the highest level of sensitivity and specificity, which produces the highest Likelihood Ratio (LR) of accurate discrimination. The LR is the ratio of the probabilities of the test discriminating True Positives from False Positives (LR = Sensitivity/1-Specificity). Multivariate discriminant analysis was performed with Statistica software (StatSoft, Tulsa, OK) to evaluate the significance of assays data in combination. Statistical significance was set at a p value <0.05.
Figure 4. Assessment of the predictive value of islet quality assays by Receiver Operator Characteristic analysis.
A subset of the standard and extended islet quality assessment data shown in Figure 3 was further analyzed by ROC analysis to evaluate the ability of each test to accurately discriminate potent (“Sensitivity”) from poor functioning (“Specificity”) preparations. ROC curve graphs which illustrate the trade-off between sensitivity and specificity are shown for islet yield (A), flow cytometric quantification of islet cell MMP (B), fluorescence microscopy-based islet viability (C), HPLC ATP/ADP measurement (D), islet loss during culture (E), and glucose-induced insulin secretion (F). Tests which cannot discriminate between true and false positives (A, C, and D) show a Sensitivity plot that is not significantly different from the line of Identity and a p value >0.05 when the Area Under the Curve (AUC) is calculated.
RESULTS
Islet adenine nucleotides reveal cellular energy state
Shown in Figure 1 are HPLC chromatograms of extracts prepared from the rat insulinoma cell line INS-1 (A and B) and two separate human islet preparations (C and D). The INS-1 extract shown in Fig. 1B was spiked with known quantities of ATP, ADP, and AMP standards to allow for chromatogram peak identification. The adenine nucleotide HPLC profile shown in Fig. 1C is a representative poor performing human islet preparations that had a low ATP/ADP ratio (3.8) and did not restore normoglycemia in STZ-diabetic NOD.scid mice when transplanted under the kidney capsule at a dose of 1000 IEQ/mouse. In contrast, the adenine nucleotide profile of the human islet preparation shown in Fig.1D showed a greater ATP/ADP ratio (12.6) and the islets cured diabetic NOD.scid mice when transplanted at a dose of 1000 IEQ/mouse. Guanosine triphosphate (GTP) is also identified by this HPLC method, however in our study its quantity did not correlate with islet cell viability or in vivo function (data not shown).
Figure 1. HPLC quantification of total islet adenine nucleotides.
Representative chromatograms of HPLC separation of adenine nucleotides from total cellular extracts of INS-1 cells (A), INS-1 cell extracts spiked with ATP, ADP, and AMP standards (B), non-curing (Poor) human islets (C) and curing (Good) human islets(D) when transplanted under the kidney capsule of streptozotocin-induced diabetic NOD.scid mice at a dose of 1000 IEQ/mouse. The insets show the calculated ATP/ADP nucleotide ratios after quantification of the identified peak areas. Data shown are representative of 5 independent INS-1 samples and 14 good and 25 poor human islet preparations.
Flow cytometry analysis of islet cells provides quantification of viability, apoptosis, and mitochondrial transmembrane potential
Human islets were dispersed into single cells and stained separately with probes for viability, apoptosis, and mitochondrial transmembrane potential (MMP). Representative dot plots are shown in Figure 2 demonstrating the flow cytometric method of gating and quantification of probe labeled cells. Intact islet cells were selected based upon forward (FSC-A) and side scatter (SSC) light parameters and further restricted to single cells by exclusion of aggregates based upon FSC-A and forward scatter width (FSC-W) parameters. Viable islet cells were distinguished as FDAbright ToPro3− , apoptotic cells as Annexin V+ and/or VADFMK+, and MMP+ as JC-1 Red>Green. The mitochondrial membrane depolarizing agent CCCP was added to a duplicate JC-1 stained sample to allow for objective establishment of the gate defining MMP+ from MMP− islet cells (data not shown). The data shown in the left panels of Figure 2 are from a representative islet preparation that restored normoglycemia in STZ-diabetic NOD.scid mice when transplanted under the kidney capsule at a dose of 1000 IEQ/mouse. The data shown in the right panels of Figure 2 are from a representative islet preparation that did not cure diabetic NOD.scid mice when transplanted at a dose of 1000 IEQ/mouse.
Figure 2. Flow cytometry assessment of human islet cells.
Representative dot plots from single-cell flow cytometry analysis of dispersed human islets. Cellular fragments and free nuclei were excluded from intact cells based upon forward (FSC-A) and side scatter (SSC-A) light parameters (A and B). Data were further restricted to single cells by gating out aggregates based upon FSC-A and forward scatter width (FSC-W) parameters (C and D). Quantification of viability (FDA and Topro3, E and F), apoptosis (Annexin V and VADFMK, G and H), and mitochondrial membrane potential (JC-1, I and J). The left-hand panels show representative staining for an islet preparation that cured STZ-induced diabetic NOD.scid mice at a dose of 1000 IEQ/mouse, while the right-hand panels show islet cell staining from a non-curing preparation.
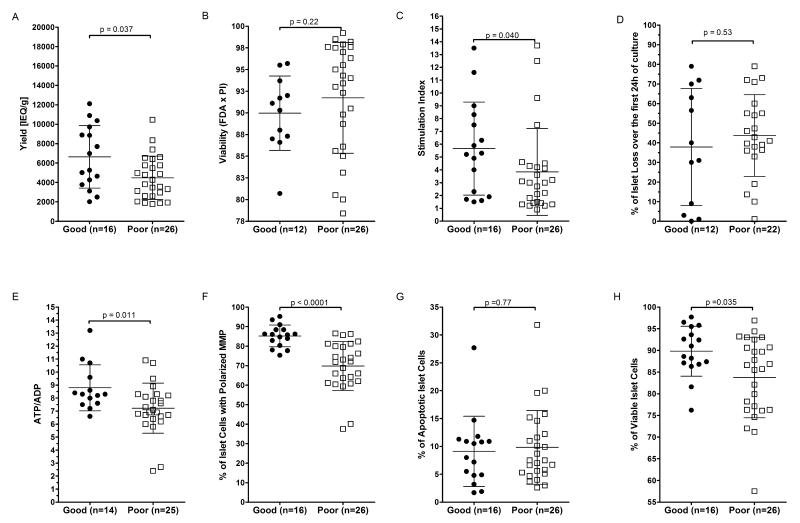
Comparison of quality control assessment methods
We retrospectively analyzed a series of 42 human islet preparations assessed by both standard practice and the novel adenine nucleotide and flow cytometry assays to determine if significant differences could be found between islets with good vs poor in vivo function (Figure 3). Each preparation was designated as either “Good” or “Poor” based upon in vivo function and all demographic and islet quality data were segregated accordingly. None of the pancreas donor demographic parameters were statistically different between the two groups (Table 1). Among the panel of standard islet quality assessments evaluated (viability by fluorescent microscopy, glucose induced insulin secretion, and islet loss during culture), only glucose-induced insulin secretion index (p=0.040) was significantly different between islet preparations showing good vs poor in vivo function. Islet yield in IEQ/g did differ significantly (p=0.037) between the two groups, but these data do not represent a true quality assessment. Flow cytometric quantification of islet cell viability (p=0.035), MMP (p<0.0001), and HPLC quantification of ATP/ADP ratio (p=0.011) were all significantly different between islet preparations showing good vs poor in vivo function.
Figure 3. Comparison of multiple islet quality assessments.
Human islet preparation quality was assessed using standard methods including yield (A), viability by fluorescence microscopy of islets stained with FDA and PI (B), GSIS SI (C), and percentage of islet loss during the first 24h of culture (D)) and novel methods including (ATP/ADP ratio (E), flow cytometry quantification of islet cell MMP (F), apoptosis (G), and viability (H). Data shown are the individual values for each sample and sample size is indicated on each graph. Islet quality data was segregated into groups based upon good (closed circles) or poor (open squares) in vivo function (see Methods). Bars indicate the mean and standard deviation for each group. P value calculated by non-parametric Mann-Whitney U test.
Table 1.
Comparison of pancreas donor demographics
| In Vivo Islet Function |
||||
|---|---|---|---|---|
| Donor Characteristics | Unit of Measure | Good (n=16) a | Poor (n=26) a | p-valueb |
| Age | years | 40.8 ± 10.4 | 47.2 ± 10.5 | 0.060 |
| Gender | male/female | 10/6 | 17/9 | 1.000 |
| Weight | kg | 101.1 ± 18.8 | 98.2 ± 26.4 | 0.705 |
| Height | m | 1.76 ± 0.09 | 1.72 ± 0.10 | 0.191 |
| BMI | weight [kg]/height [m]2 | 32.7 ± 4.3 | 32.9 ± 7.8 | 0.926 |
| Cause of Death | traumatic/atraumatic | 7/9 | 8/18 | 0.511c |
| Intensive Care time | days | 2.9 ± 1.9 | 3.0 ± 2.2 | 0.922c |
| Cardiac/Respiratory Arrest | Y/N | 4/12 | 3/23 | 0.397c |
| Chest compression | Y/N | 4/12 | 2/24 | 0.180c |
| OR Procedures | Y/N | 3/13 | 8/18 | 0.485c |
| Defibrillation | Y/N | 2/14 | 1/25 | 0.547c |
| Donor Management | ||||
| Vasopressors | Y/N | 11/5 | 17/5 | 0.713c |
| Antibiotics | Y/N | 11/4 | 19/5 | 0.711c |
| Steroids | Y/N | 10/6 | 13/13 | 0.530c |
| Insulin | Y/N | 9/7 | 12/13 | 0.751c |
| Lab Profile | ||||
| SGOT | U/l | 178.4 ± 453.7 | 86.3 ± 212.5 | 0.377 |
| SGPT | U/l | 146.9 ± 324.2 | 78.1 ± 208.9 | 0.406 |
| Amylase | U/l | 108.2 ± 141.5 | 52.4 ± 35.5 | 0.077 |
| Lipase | U/l | 46.8 ± 52.4 | 62.9 ± 71.1 | 0.441 |
| Glucose | mg/dL | 182.3 ± 72.3 | 166.0 ± 76.9 | 0.508 |
| Pancreas Recovery | ||||
| Cold ischemia | H:m | 6:38± 2:15 | 7.17 ± 2.55 | 0.450 |
Human islet preparations were segregated into “Good” vs “Poor” groups based upon in vivo function (see Methods). Data shown are the mean ± SD for continuous data.
P valued calculated by two tailed Student’s t-test, except where indicated (c) by Fisher’s Exact test.
Statistical evaluation of islet quality assessments
The islet quality tests showing the most statistically significant difference between groups by univariate analysis, were further analyzed by Receiver Operator Characteristic (ROC) curve analysis (12) to evaluate the ability of each test to accurately discriminate good from poor functioning islet preparations (Figure 4). Data for islet yield, viability by FDA × PI, and islet loss in culture were included in the ROC analysis for comparison. ROC calculations and optimized cut-off values are shown for each of the selected assays (Table 2). Among standard islet assessment methods, yield (p=0.036) and GSIS (p=0.038) provided significant predictive value although the likelihood ratio (LR) of both was <3.0. Flow cytometric quantification of MMP+ islet cells (p <0.0001) and HPLC-based detection of ATP/ADP ratio (p=0.011) both showed significant predictive value with LR’s >3.0. Multivariate discriminant statistical analysis was performed to evaluate the predictive significance of combinations of MMP, GSIS, and ATP assays. As shown in Table 2, the highest level of sensitivity and specificity was observed when the MMP data was combined with the ATP/ADP and GSIS data (85.7% overall success rate of classification, p<0.0001). From the multivariate discriminant analysis, coefficients were derived that could be used with the raw MMP, GSIS SI, and ATP/ADP data to calculate a single Islet Quality Score (IQS) (Table 2). Good preparations showed an average IQS of 1.22 ± 0.73 while poor performing preparations had an average IQS of −0.75 ± 1.13 (p<0.0001, two-tailed Student’s t test).
Table 2.
Evaluation of the Predictive Success of Each Islet Quality Test
| Receiver Operator Characteristic Analysis | ||||||
|---|---|---|---|---|---|---|
| Test (n=42) | AUCa (>0.8) |
p value | Test Cut-Off Value |
Sensitivity (%Detect +) |
Specificity (% Detect −) |
Likelihood Ratio (>3) |
| Yield [IEQ/g] | 0.695 | 0.036 | 3671 | 46.2 | 81.3 | 2.5 |
| GSIS (S.I.) | 0.692 | 0.038 | 3.8 | 65.4 | 68.8 | 2.1 |
| MMP+ % (Flow Cytometry) |
0.887 | <0.0001 | 80.1 | 76.9 | 81.3 | 4.1 |
| ATP/ADP (HPLC) | 0.749 | 0.011 | 7.2 | 52.0 | 92.9 | 7.3 |
| Multivariate Discriminant Analysis | |||
|---|---|---|---|
| Match Between Observed and Predicted Classifications of in vivo Islet Functionb |
|||
| Test (n=42) | Sensitivity (%) | Specificity (%) | Overall Rate of Successful Classification |
| MMP + ATP/ADP | 75.0 | 84.6 | 81.0 |
| MMP + GSIS | 81.3 | 84.6 | 83.3 |
| ATP/ADP + GSIS | 43.8 | 80.8 | 66.7 |
| MMP + ATP/ADP + GSIS | 87.5 | 84.6 | 85.7 |
|
cIslet Quality Score = % of MMP+ islet cells × (0.087) + GSIS SI × (0.15) + ATP/ADP × (0.25) − 9.20 | |||
|---|---|---|---|
| Good (n=16) | Poor (n=26) | P valued | |
|
Islet Quality Scores
(mean±SD) |
1.22 ± 0.73 | −0.75 ± 1.13 | < 0.0001 |
Receiver Operator Characteristic analysis (Graphpad Prism Software).
Data shown are the rates of successful matching between observed and predicted isolation classifications based upon multivariate discriminant analysis (Statistica software) of each islet quality test either alone or in combination (n=42).
Formula determined by multivariate discriminate analysis of all 42 human islet preparations. Data shown are the mean ± SD.
p value calculated by two-tailed Student’s t test.
Relationship between Islet Quality Score and In Vivo Function
Figure 5 shows representative kinetic curves of glycemic control in diabetic NOD.scid mice after transplantation of 1000 or 2000 IEQ doses of human islets under the kidney capsule. Representative islet preparations with an Islet Quality Score less than 0 (Fig. 5, left panels) were unable to reverse diabetes at 1000 IEQ/mouse, but in some cases could restore normoglycemia at a dose of 2000 IEQ/mouse. In contrast, representative islet preparations with an Islet Quality Score above 0 (Fig. 5, right panels) were able to reverse diabetes at both the 1000 and 2000 IEQ doses.
Figure 5. Glycemic control of NOD.scid mice transplanted with human islet preparations with varying islet quality scores.
Streptozotocin-induced diabetic NOD.scid mice were transplanted under the kidney capsule with 1000 or 2000 IEQ doses of human islet preparations with Islet Quality Scores (IQS) <0 (left panels) or >0 (right panels). Mice were considered cured if blood glucose levels fell below < 200 mg/dL within 14 days of transplant and were maintained at or below that level until return to the hyperglycemic state following nephrectomy of the graft bearing kidney.
DISCUSSION
Multiple studies of isolated human islet quality argue that the current standard practice assays of viability and potency lack the sensitivity and objectivity needed to accurately predict islet graft transplant suitability (13-15). The financial costs of islet isolation and transplantation combined with the high risk of recipient sensitization after failure, demand that all efforts be made to minimize PNF and EGL (16). We and others have sought to develop methods of predicting islet transplant outcome based upon the detection of markers of oxidative stress, cellular phenotype, mitochondrial membrane integrity, insulin secretory function, and oxygen consumption rate (4, 17-20). The seminal work of Ichii et al. (17) and Papas et al. (19) demonstrated by two separate methods that assessing islet mitochondrial integrity could provide objective data that correlated with in vivo function after kidney capsule transplantation in diabetic immunodeficient mice. Changes in mitochondrial integrity and function are well established as early indicators of apoptosis and cell death (21, 22). The objective of this study was to validate a simplified method of islet quality assessment that is inexpensive, requires standard laboratory equipment found in the majority of academic research institutions, and can be utilized for both basic research and clinical islet applications.
Among the limited set of donor demographic parameters evaluated in this study, none were found to differ significantly, although donor age was increased in islet preparations with poor in vivo function. This observation is concerning as the availability of pancreata from younger donors is decreasing due to the changing demographics of the US population. Studies by others (23, 24) have shown a correlation between donor BMI and islet isolation yield, but this finding was not replicated in our study. Those preparations that showed good in vivo function did however on average come from higher yielding isolations. This observation suggests that technical aspects of the islet isolation procedure itself may play a significant role in post-isolation viability and functional potency.
Islet viability measurement by FDA × PI staining and quantification by fluorescent microscopy did not predict post transplant outcomes. It is important to emphasize the lack of sensitivity of this method as the majority of islet preparations assessed in this study had passing viability scores of ≥ 80%. Islet GSIS measured in vitro was significantly greater for preparations that subsequently demonstrated in vivo functional potency. The inconsistent linkage between in vitro insulin secretory capacity and in vivo potency published by others, suggests that this assay is prone to error due to experimentor and protocol variability (18, 25).
Essential to the viability and function of cells are their energy content and redox state, which can be diagnosed in part by quantification of total cellular adenine nucleotides (ATP, ADP, and AMP) (26-28). Islet ATP/ADP ratios were found to be significantly greater in preparations that demonstrated satisfactory functional potency in vivo. The islet ATP/ADP ratios reported in this study were higher than those published previously from our laboratory (4). The differences reflect a modification to the islet extract preparation methodology that increases the recovery of total adenine nucleotides, producing values consistent with publications by others (28). Islet cellular guanosine nucleotides can also be identified by our HPLC method and have been reported to be linked to apoptosis and insulin secretory function (29). We did not however find a correlation between human islet GTP/GDP ratios and either the percentage of apoptotic islet cells measured by flow cytometry or in vivo function following transplant into diabetic mice (data not shown).
Among the flow cytometry assessments, the percentage of islet cells with polarized mitochondria (MMP+) showed the most statistically significant difference (p<0.0001) between good and poor functioning preparations (85.2% ± 6.0 vs 69.6% ± 12.8, mean ± SD, respectively). The percentage of apoptotic islet cells, as measured by flow cytometry analysis of extracellular phosphatidyl serine expression and activation of caspase enzymes, was not found to differ significantly between the two groups. This may in part be due to the partial loss of apoptotic cells during the enzymatic and mechanical dispersion of the islets into single cells. In some rare cases, islet preparations with high levels of intra-islet necrosis can yield low numbers of intact cells for analysis (data not shown). We have established an assay threshold value of 70% for the percentage of gated intact cells required to ensure that the data are not compromised by enrichment for viable cells.
While it has been reported that the number of viable beta cells is an important factor in determining the potential of an islet preparation to reverse diabetes, it is also true that an equivalent or greater percentage of endocrine and non-endocrine cell types comprise islet preparations (17, 30, 31). The islet preparations analyzed in this study had purities on average ≥ 80% (range, 70 to 90%) and the flow cytometry data analysis method eliminates the majority of non-islet cells (data not shown). Our data demonstrate that the assessment of all cells within the heterogeneous islet preparation is valid and sufficient for the prediction of post-transplant outcomes. However, as beta cell content has been shown to vary between islet preparations, it remains possible that differences in functional potency may in part be related to differences in the quantity of viable beta cells.
An equally important aspect of this study was that the islet quality assays selected (HPLC-based ATP/ADP, GSIS, and flow cytometry) can be completed pre-transplant. This multi-assay approach minimizes the risk of inaccurate islet quality assessment as a result of dependency on any one assay. The simplicity of the approach derives from the focus on key objective markers of islet cell health and in vitro function without the need for customized analytical equipment or specialized personnel. Moreover, alternative methodologies for obtaining ojective and quantitative data on islet ATP and ADP content, MMP, and glucose-induced insulin secretion can be utilized (e.g. chemiluminescence and fluorescence assays using a microplate reader, and dynamic perfusion, respectively).
To further simplify our model, we completed a multivariate discriminant statistical analysis to evaluate the assays in combination and to derive an islet quality scoring formula using the raw data. Calculation of the IQS for the complete set of 42 isolations showed that functionally potent islets had an average score of 1.22 ± 0.73 and their in vivo potency was classified correctly in over 85% of cases. Based upon a minimal IQS of ≥ 0, cut-off values can be proposed (MMP+% of ≥ 77%, ATP/ADP of ≥ 7.2 and a GSIS SI of ≥ 3.4) in which an islet preparation has a statistically significant likelihood of good function after transplantation under the kidney capsule of diabetic NOD.scid mice at a dose of 1000 IEQ/mouse. In the present study, four human subjects were transplanted with islet preparations released for transplant based upon the proposed MMP, ATP/ADP, and GSIS SI cut-off values. The average IQS of these islet preparations was 1.7 ± 0.91 and at 3 months post transplant the function of these preparations was demonstrated by an average decrease in daily insulin requirements from 0.36 ± 0.26 to 0.06 ± 0.08 units/day, increased fasting C-peptide from 0.33±0.29 to 1.21±0.50 ng/mL, and a reduction in HbA1c from 6.8% ±1.2 to 5.6% ± 0.28.
It is however important to point out that not all islet preparations with low IQS showed a complete lack of in vivo function in mice. Many islet preparations with IQS<0 were capable of curing STZ-diabetic NOD.scid mice when transplanted at a dose of 2000 IEQ, but not a sustained cure at the minimal dose of 1000 IEQ. This observation demonstrates that islet mass must also be considered when evaluating overall preparation quality and suitability for transplant. In some cases, a very large islet mass may compensate for a low IQS and support its clinical utilization. It is also important to state that with the current data set a statistically significant correlation between IQS and quantitative in vivo function variables, such as time to reversal of diabetes, could not be shown. Additional studies are needed to understand the relationship between islet mass and islet quality and how these factors affect long term graft survival and function.
From our data we propose that the release criteria for clinical islet transplantation should be based upon objective assessments of; 1) mitochondrial integrity, 2) insulin secretion, 3) mass, 4) identity/morphology, and 5) sterility. We acknowledge that implementation of an extended islet quality assessment program prior to islet transplant does not guarantee better long term function, but should decrease or eliminate cases of PNF and EGL. We hypothesize that transplantation of islets that pass an extended quality assessment will lead to better long term results, however further follow up is needed. Correlation between the Islet Quality Score and post-infusion potency will need continued study in humans as one method of meeting this objective. Studies are underway to understand the predictive value of the Islet Quality Score as a release criterion prior to clinical islet transplantation.
In summary, our findings demonstrate that multi-parametric quantification of markers of islet cellular health provides a highly sensitive method to predict post transplant functional potency in diabetic NOD.scid mice.
ACKNOWLEDGEMENTS
This study was supported in part by grants from the NIH (DK071218-01, U42 RR023240) and Roche Organ Transplantation Research Foundation (636383041). Special thanks to Kathy Schell for expert assistance with flow cytometry analysis, the Organ Procurement Organization at the University of Wisconsin for providing human pancreata for research, and Dr. Jamie Sperger for careful review of this manuscript.
Abbreviations
- ADP
adenine diposphate
- AMP
adenine monophosphate
- ATP
adenine triphosphate
- EGL
Early graft loss
- FDA
fluorescein diacetate
- GSIS
glucose stimulated insulin secretion
- HPLC
high pressure liquid chromatography
- IEQ
islet equivalent
- IP
islet particle
- MMP
mitochondria membrane potential
- NOD
nonobese diabetic
- PI
propidium iodide
- PNF
primary graft non-function
- SCID
severe combined immunodeficient
- SI
stimulation index
- STZ
streptozotocin
Footnotes
Matthew S. Hanson: Participated in the research design, writing of the paper, performance of the research, data analysis. This author’s work was supported by grants from the NIH (DK071218-01, U42 RR023240) and Roche Organ Transplantation Research Foundation (636383041). No conflicts of interest.
Elisa Park: Participated in the performance of the research and data analysis. This author’s work was supported by grants from the NIH (U42 RR023240).
No conflicts of interest.
Mallory Sears: Participated in the performance of the research and data analysis. This author’s work was supported by grants from the NIH (U42 RR023240).
No conflicts of interest.
Krista K. Greenwood: Participated in the performance of the research and data analysis. This author’s work was supported by grants from the NIH (U42 RR023240).
No conflicts of interest.
Juan Sebastian Danobeitia: Participated in the performance of the research and data analysis. This author’s work was supported by grants from the NIH (U42 RR023240).
Debra A. Hullett: Participated in the performance of the research and data analysis. This author’s work was supported by internal funds of the UW Department of Surgery.
Luis A. Fernandez: Participated in the research design, writing of the paper, and data analysis. This author’s work was supported by grants from the NIH (DK071218-01, U42 RR023240) and Roche Organ Transplantation Research Foundation (636383041).No conflicts of interest.
Clinical Trial: ClinicalTrials.gov (NCT00214253)
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Collaborative Islet Transplant Registry . Sixth Annual Report. Collaborative Islet Transplant Registry; Rockville, MD: 2009. p. 240. [Google Scholar]
- 2.Abdelli S, Ansite J, Roduit R, et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes. 2004;53(11):2815. doi: 10.2337/diabetes.53.11.2815. [DOI] [PubMed] [Google Scholar]
- 3.Schwarznau A, Hanson MS, Sperger JM, et al. IL-1beta receptor blockade protects islets against pro-inflammatory cytokine induced necrosis and apoptosis. J Cell Physiol. 2009 doi: 10.1002/jcp.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armann B, Hanson MS, Hatch E, Steffen A, Fernandez LA. Quantification of basal and stimulated ROS levels as predictors of islet potency and function. Am J Transplant. 2007;7(1):38. doi: 10.1111/j.1600-6143.2006.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas DA, Stauffer C, Zhao K, et al. Mitochondrial targeting with antioxidant peptide SS-31 prevents mitochondrial depolarization, reduces islet cell apoptosis, increases islet cell yield, and improves posttransplantation function. J Am Soc Nephrol. 2007;18(1):213. doi: 10.1681/ASN.2006080825. [DOI] [PubMed] [Google Scholar]
- 6.van Loo G, Saelens X, van Gurp M, MacFarlane M, Martin SJ, Vandenabeele P. The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ. 2002;9(10):1031. doi: 10.1038/sj.cdd.4401088. [DOI] [PubMed] [Google Scholar]
- 7.Fariss MW, Chan CB, Patel M, Van Houten B, Orrenius S. Role of mitochondria in toxic oxidative stress. Mol Interv. 2005;5(2):94. doi: 10.1124/mi.5.2.7. [DOI] [PubMed] [Google Scholar]
- 8.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 9.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49(3):424. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 10.Gellerich FN, Kunz W. Cause and consequences of dynamic compartmentation of adenine nucleotides in the mitochondrial intermembrane space in respect to exchange of energy rich phosphates between cytosol and mitochondria. Biomed Biochim Acta. 1987;46(8-9):S545. [PubMed] [Google Scholar]
- 11.Noack H, Kunz WS, Augustin W. Evaluation of a procedure for the simultaneous determination of oxidized and reduced pyridine nucleotides and adenylates in organic phenol extracts from mitochondria. Anal Biochem. 1992;202(1):162. doi: 10.1016/0003-2697(92)90222-s. [DOI] [PubMed] [Google Scholar]
- 12.Linden A. Measuring diagnostic and predictive accuracy in disease management: an introduction to receiver operating characteristic (ROC) analysis. J Eval Clin Pract. 2006;12(2):132. doi: 10.1111/j.1365-2753.2005.00598.x. [DOI] [PubMed] [Google Scholar]
- 13.Ricordi C, Gray DW, Hering BJ, et al. Islet isolation assessment in man and large animals. Acta Diabetol Lat. 1990;27(3):185. doi: 10.1007/BF02581331. [DOI] [PubMed] [Google Scholar]
- 14.Eckhard M, Brandhorst D, Winter D, et al. The role of current product release criteria for identification of human islet preparations suitable for clinical transplantation. Transplant Proc. 2004;36(5):1528. doi: 10.1016/j.transproceed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Barnett MJ, McGhee-Wilson D, Shapiro AM, Lakey JR. Variation in human islet viability based on different membrane integrity stains. Cell Transplant. 2004;13(5):481. doi: 10.3727/000000004783983701. [DOI] [PubMed] [Google Scholar]
- 16.Campbell PM, Senior PA, Salam A, et al. High risk of sensitization after failed islet transplantation. Am J Transplant. 2007;7(10):2311. doi: 10.1111/j.1600-6143.2007.01923.x. [DOI] [PubMed] [Google Scholar]
- 17.Ichii H, Inverardi L, Pileggi A, et al. A novel method for the assessment of cellular composition and beta-cell viability in human islet preparations. Am J Transplant. 2005;5(7):1635. doi: 10.1111/j.1600-6143.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- 18.Street CN, Lakey JR, Shapiro AM, et al. Islet graft assessment in the Edmonton Protocol: implications for predicting long-term clinical outcome. Diabetes. 2004;53(12):3107. doi: 10.2337/diabetes.53.12.3107. [DOI] [PubMed] [Google Scholar]
- 19.Papas KK, Colton CK, Nelson RA, et al. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. Am J Transplant. 2007;7(3):707. doi: 10.1111/j.1600-6143.2006.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraker C, Timmins MR, Guarino RD, et al. The use of the BD oxygen biosensor system to assess isolated human islets of langerhans: oxygen consumption as a potential measure of islet potency. Cell Transplant. 2006;15(8-9):745. doi: 10.3727/000000006783981440. [DOI] [PubMed] [Google Scholar]
- 21.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 22.Duchen MR. Roles of mitochondria in health and disease. Diabetes. 2004;53(Suppl 1):S96. doi: 10.2337/diabetes.53.2007.s96. [DOI] [PubMed] [Google Scholar]
- 23.Lakey JR, Warnock GL, Rajotte RV, et al. Variables in organ donors that affect the recovery of human islets of Langerhans. Transplantation. 1996;61(7):1047. doi: 10.1097/00007890-199604150-00010. [DOI] [PubMed] [Google Scholar]
- 24.Nano R, Clissi B, Melzi R, et al. Islet isolation for allotransplantation: variables associated with successful islet yield and graft function. Diabetologia. 2005;48(5):906. doi: 10.1007/s00125-005-1725-3. [DOI] [PubMed] [Google Scholar]
- 25.Sweet IR, Gilbert M, Jensen R, et al. Glucose stimulation of cytochrome C reduction and oxygen consumption as assessment of human islet quality. Transplantation. 2005;80(8):1003. doi: 10.1097/01.tp.0000178381.35014.37. [DOI] [PubMed] [Google Scholar]
- 26.Matsui Y, Kitade H, Kamiya T, et al. Adenylate energy charge of rat and human cultured hepatocytes. In Vitro Cell Dev Biol Anim. 1994;30A(9):609. doi: 10.1007/BF02631260. [DOI] [PubMed] [Google Scholar]
- 27.Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185(8):1481. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradbury DA, Simmons TD, Slater KJ, Crouch SP. Measurement of the ADP:ATP ratio in human leukaemic cell lines can be used as an indicator of cell viability, necrosis and apoptosis. J Immunol Methods. 2000;240(1-2):79. doi: 10.1016/s0022-1759(00)00178-2. [DOI] [PubMed] [Google Scholar]
- 29.Li G, Segu VB, Rabaglia ME, Luo RH, Kowluru A, Metz SA. Prolonged depletion of guanosine triphosphate induces death of insulin-secreting cells by apoptosis. Endocrinology. 1998;139(9):3752. doi: 10.1210/endo.139.9.6207. [DOI] [PubMed] [Google Scholar]
- 30.Pipeleers D, Keymeulen B, Chatenoud L, et al. A view on beta cell transplantation in diabetes. Ann N Y Acad Sci. 2002;958:69. doi: 10.1111/j.1749-6632.2002.tb02948.x. [DOI] [PubMed] [Google Scholar]
- 31.Jayaraman S. A novel method for the detection of viable human pancreatic beta cells by flow cytometry using fluorophores that selectively detect labile zinc, mitochondrial membrane potential and protein thiols. Cytometry A. 2008;73(7):615. doi: 10.1002/cyto.a.20560. [DOI] [PubMed] [Google Scholar]