Abstract
An oral sustained release dosage form of cinnarizine HCl (CNZ) based on gastric floating matrix tablets was studied. The release of CNZ from different floating matrix formulations containing four viscosity grades of hydroxypropyl methylcellulose, sodium alginate or polyethylene oxide, and gas-forming agent (sodium bicarbonate or calcium carbonate) was studied in simulated gastric fluid (pH 1.2). CNZ release data from the matrix tablets were analyzed kinetically using Higuchi, Peppas, Weibull, and Vergnaud models. From water uptake, matrix erosion studies, and drug release data, the overall release mechanism can be explained as a result of rapid hydration of polymer on the surface of the floating tablet and formation of a gel layer surrounding the matrix that controls water penetration into its center. On the basis of in vitro release data, batch HP1 (CNZ, HPMC-K100LV, SBC, LTS, and MgS) was subjected to bioavailability studies in rabbits and was compared with CNZ suspension. It was concluded that the greater bioavailability of HP1 was due to its longer retention in the gastric environment of the test animal. Batch no. HP1 of floating tablet in rabbits demonstrated that the floating tablet CNZ could be a 24-h sustained release formulation.
Key words: bioavailability, cinnarizine HCl, floating tablet, HPMC, kinetic models
INTRODUCTION
Cinnarizine HCl (CNZ) is an effective vestibular sedative and acts by depressing the labyrinth. It is a weak base with poor aqueous and pH-dependent solubility (0.29 mg/ml, in 0.01 N HCL (pH 2.0), 0.002 mg/ml in pH 6.5 phosphate buffer). The dissolution rate of CNZ differs among commercially available pharmaceutical preparations, and this difference affects its bioavailability after oral administration.
Gastric floating drug delivery (GFDD) offers a number of benefits for drugs with poor bioavailability because of narrow absorption window in the upper part of the gastrointestinal tract such as riboflavin (1), ranitidine (2), nitrofurantoin, furosemide (3), and theophylline (4). Floating dosage forms are retained at the site of absorption, and the longer retention enhances the bioavailability.
GFDD is also useful for drugs that act locally in the proximal part of gastrointestinal tract such as antibiotics for eradication of Helicobacter pylori in the treatment of peptic ulcer (5), for drugs that are unstable in the intestinal fluid such as captopril (6), and for drugs that exhibit poor solubility in the intestinal tract such as diazepam (7) and verapamil HCl (8).
Many approaches have been reported for controlling the residence time of a drug delivery system in a particular region of the gastrointestinal tract, such as intragastric floating systems (FDDS), high-density systems, mucoadhesive systems, magnetic systems, unfoldable, extendable, or expandable systems, and superporous, biodegradable hydrogel systems.
Excipients which generate CO2 in the stomach produce effective buoyancy for more than 24 h. Fukuda et al. (9) investigated floating hot-melt extruded tablets for gastroretentive controlled drug release system. Wei et al. (10) and Xiaoqiang et al. (11) reported that floating tablets containing HPMC and sodium bicarbonate generated CO2 gas in simulated gastric fluid and rendered the tablets buoyant. Ichigawa et al. (12) developed floating systems by coating the sustained release granules with a tartaric acid layer, a sodium bicarbonate layer, and a polymeric film consisting of polyvinyl acetate and shellac.
Hydrocolloids of natural and semisynthetic origin are commonly used for the development of FDDS. Floating matrix systems containing HPMC as the matrix forming excipient swell and form a gel layer with entrapped air around the tablet core after contact with gastric fluid, and this gel layer controls the drug release (13,14).
According to pH partition theory, a weakly basic drug like CNZ should theoretically be absorbed more at the intestinal pH. However, CNZ solubility in alkaline pH is very low; therefore, its absorption from the intestine would be low and is the reason of selection of FDDS for continuous delivery of CNZ in the stomach.
It is reported that for weakly basic drugs with poor solubility in the basic environment, the floating systems is an attractive option to increase its oral bioavailability (15). The objective of the present study was to fabricate controlled release formulations of CNZ and to study the in vitro and in vivo performance (bioavailability) of the controlled release floating formulations.
MATERIALS AND METHODS
Materials
Cinnarizine HCl was generously gifted by Wallace Pharmaceutical, Goa. HPMC (K100LV, K4M, K15M, K100MCR) were purchased from Dow Chemicals, USA. All other chemicals were of analytical grade.
Fabrication of Matrix Floating System
Accurately weighed quantities of CNZ, HPMC (different grades) and other excipients sufficient for a batch of 200 tablets (Table I) were mixed by geometric method of dilution and tumbled for 30 min. The blend was then lubricated with magnesium stearate for 5 min and directly compressed into tablets of average weight 300 mg using single punch tabletting machine (Manesty E2) with 10 mm standard concave punches. The hardness of the tablets was kept in the vicinity of 4–6 kg/cm2.
Table I.
Formulation Composition of CNZ Gastrofloating Tablets
| Batch code | CNZ (mg) | K100 LV | K4M | K15M | K100 MCR | SA | PEO | CAG | SBC | CA | TA | CC | LTS | MgS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HP1 | 90 | 135 | – | – | – | 27 | – | – | – | 45 | 3 | |||
| HP2 | 90 | 0 | 135 | – | – | 27 | – | – | – | 45 | 3 | |||
| HP3 | 90 | 0 | 135 | 27 | – | – | – | 45 | 3 | |||||
| HP4 | 90 | 0 | 135 | 27 | – | 45 | 3 | |||||||
| HP5 | 90 | 135 | – | – | – | – | – | – | – | – | – | 27 | 45 | 3 |
| HP6 | 90 | 135 | 27 | 5 | 40 | 3 | ||||||||
| HP7 | 90 | 135 | 27 | 5 | 40 | 3 | ||||||||
| HP8 | 90 | 135 | 5 | 27 | 40 | 3 | ||||||||
| HP9 | 90 | 135 | 5 | 27 | 40 | 3 | ||||||||
| HP10 | 90 | 135 | 27 | 45 | 3 | |||||||||
| HP11 | 90 | 135 | 27 | 45 | 3 | |||||||||
| HP12 | 90 | 135 | 27 | 45 | 3 |
Amounts are milligrams per tablet
CNZ cinnarizine, SBC sodium bicarbonate, CA citric acid, TA tartaric acid, CC calcium carbonate, LTS lactose, MgS magnesium stearate, SA sodium alginate, PEO polyethylene oxide, CAG λ-carrageenan
Uniformity of Weight and Hardness
According to Indian pharmacopoeia, 20 tablets were selected at random, weight together and individually for the determination of weight of tablets. The mean and standard deviations were calculated. Five tablets were selected at random and the hardness of each tablet was measured on Monsanto hardness tester.
Friability and Bulk Density
The friability test was carried out in Roch Friabilator. Ten tablets were weighted (Wo) initially and put in a rotating drum. Then the tablets were subjected to 100 falls of 6 in. height. After completion of rotation, the tablets were again weighted (W).
 |
1 |
The average density of tablets was determined using gas multipycnometer. The weight of tablet was divided by the volume of tablet determined from the multipycnometer. Three measurements were carried out and the average bulk density was calculated.
Buoyancy Lag Time Determination
The buoyancy of the tablets was studied at 37 ± 0.5°C in 100 ml of simulated gastric fluid at pH 1.2 (without pepsin, USP). The duration of tablet floatation was observed visually. The evaluation was conducted in triplicate for each batch of tablets.
High-Performance Liquid Chromatography Procedure
The drug concentration for in vitro study was analyzed by validated high-performance liquid chromatography (HPLC) method using a reverse-phase microparticulate C18 (Bondapak C18, particle size 5 µm, 30 cm × 4 mm column Waters Associate, Milford, MA, USA) (16). The mobile phase was prepared by mixing acetate buffer (20 ml glacial acetic acid, adjusted to pH 4) and methanol (30:70) at a flow rate of 1.5 ml/min. The UV detector was a fixed at 253 nm wavelength. Sensitivity of assay was good at 1 ng/ml with a linear relationship between peak areas and CNZ concentrations of 10–250 ng/ml (r2 = 0.999).
In Vitro Dissolution Test
The in vitro release rates of CNZ matrix tablets were determined using the USP XXIII basket apparatus at 150 rpm, and a temperature of 37 ± 0.5°C was maintained. Release testing was carried out in 900 ml simulated gastric fluid (pH 1.2). Samples (5 ml) were withdrawn at different time intervals, filtered through a 0.8-µm filter, and assayed by HPLC method. The withdrawn volume was replaced with an equal volume of prewarmed (37°C) media. Six tablets of each formulation were used in the dissolution test.
Water Uptake Studies
Water uptake studies were performed by equilibrium weight gain method (17) using USP XXVII basket dissolution test apparatus. The tablets were accurately weighed and placed in a dissolution basket. The basket was immersed in a dissolution vessel containing 900 ml of 0.1 N HCl (pH 1.2) maintained at 37 ± 0.5°C; speed of rotation was 100 rpm. At regular intervals, the basket-matrix system was removed from the dissolution vessel, blotted with tissue paper to remove excess water, and reweighed.
The percentage water uptake (i.e., the degree of swelling due to absorbed medium) was calculated using the following equation:
 |
2 |
where Wo and Wt are weights of dry and swelled tablet at time t, respectively.
Matrix Erosion Studies
Matrix erosion studies were performed by a method similar to Ebube et al. (18). USP XXVII type I dissolution test apparatus was used for this purpose. The dry matrices were weighed, placed in dissolution basket containing 900 ml of 0.1 N HCl (pH 1.2) maintained at 37 ± 0.5°C, and the basket was rotated at 100 rpm. At regular intervals, the whole basket-matrix assembly was removed from the dissolution vessels and dried to a constant weight in a hot air oven at 50°C.The matrix erosion (E) at time, t, was estimated from Eq. 2.
 |
3 |
Wdt and Wo are weights of dried tablet and initial weight of dry tablet at time t, respectively.
Animal Study Design and Method
The experiments were conducted under approval of the ethical committee of the institution. For the comparative in vivo pharmacokinetic study, healthy rabbits (New Zealand albino) of either sex weighing 2.5–3.0 kg were acclimatized in the animal room for 15 days and fasted for 12 h before dose administration with free access to drinking water. The test formulation of batch HP1 was compared with CNZ suspension in 2% sodium carboxymethyl cellulose (CMC; reference) with the following study design: Single dose, open label, two period, two treatment, randomized, and complete crossover design under fasted condition. CNZ (10 ml of 9 mg/ml) suspension in 2% sodium CMC was used as reference. Floating tablet formulation (batch HP1) containing 90 mg of CNZ was used as test. Washout period of 1 week was allowed between successive runs.
Dosing and Blood Sample Collections
For oral administration, the rabbits were placed in a restraining device specially designed to protect the rabbits from spinal injury. The reference suspension was administered orally to the rabbits using a syringe and flexible tubing. A wooden biting block with a central opening was placed between the upper and lower teeth. The tube was passed through the opening and lowered carefully in the stomach. Once the tube was properly inserted into the stomach, the syringe was attached and the dose was administered. To assure the complete delivery of dose, a small volume (2 ml) of water was used to flush the tube. For the test formulation, the same procedure without tube was followed.
Blood samples (2 ml) were withdrawn from ear vein of each animal with a 24-G, 1-in. needle and collected directly in tubes containing 300 µl of sodium citrate solution (2%, w/v). Blood samples were collected at 0 (pretreatment), 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 h (ten time points).
The blood samples were centrifuged at 4,000 rpm (for 10 min at 4°C, Remi cooling centrifuge, Mumbai), and the separated plasma samples were stored in clean screw capped 5-ml polypropylene plasma tubes (Laxbro, Mumbai) at −22°C in a deep freezer, until further analysis.
Extraction of Drug from Plasma
One-milliliter plasma sample was taken in a glass centrifuge tube; 0.15 ml of 0.5 M HCl was added and gently mixed. Extraction was done with 1 ml of chloroform. After shaking for 1 min and centrifugation for 15 min at 2,000 rpm, the organic layer was aspirated off and transferred to a second tube by means of disposable Pasteur pipette. The collected organic layer was evaporated to dryness under nitrogen gas flow on a water bath at 50°C. The residue was dissolved in 100 µl of methanol, and 0.20-µl aliquot was applied for HPLC (Bondpack C18 column, 1.5 ml/min, ambient 50°C, retention time 8.76 ± 0.03 min, Cecil UK). The method is a slight modification of Hasan et al. (16) with a detection limit of 10 ng/ml. For the calibration curve, blood collected from unmediated rabbits was spiked with CNZ solution (10–100 in 0.5 N HCl) and carried through the procedure described above.
Determination of Pharmacokinetic Parameters and Their Statistical Evaluation
The highest observed plasma CNZ concentration Cmax and the time to reach Cmax relative to the time of dosing (Tmax) were noted from plasma concentration vs time profile. The biological half-life (t1/2) was calculated using the formula t1/2 = 0.6931/Kel. Total area under the concentration vs. time curve (AUC0 − t) was obtained by linear trapezoidal method (Table II).
Table II.
Drug Release Kinetics of CNZ Floating Matrix Tablets
| Batch code | First order | Zero order | Higuchi | Peppas | Weibull model | Hixson–Crowell model | Vergnaud | Erosion (%/min) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n value | R 2 value | β | α | R 2 | K | R 2 | n value | |||||
| HP1 | 0.840 | 0.944 | 0.977 | 0.895 | 0.996 | 1.35 | 6.21 | 0.996 | 0.271 | 0.946 | 0.599 | 0.21 |
| HP2 | 0.936 | 0.978 | 0.912 | 0.724 | 0.955 | 0.886 | 15.07 | 0.955 | 0.069 | 0.985 | 0.468 | 0.173 |
| HP3 | 0.984 | 0.993 | 0.969 | 0.794 | 0.992 | 0.924 | 17.38 | 0.992 | 0.086 | 0.987 | 0.464 | 0.124 |
| HP4 | 0.984 | 0.984 | 0.955 | 0.727 | 0.989 | 0.832 | 22.60 | 0.989 | 0.109 | 0.945 | 0.286 | 0.102 |
| HP5 | 0.963 | 0.973 | 0.984 | 0.832 | 0.989 | 1.09 | 8.07 | 0.989 | 0.162 | 0.979 | 0.507 | 0.20 |
| HP6 | 0.795 | 0.987 | 0.961 | 0.737 | 0.962 | 1.05 | 7.87 | 0.962 | 0.204 | 0.886 | – | – |
| HP7 | 0.732 | 0.995 | 0.978 | 0.841 | 0.995 | 1.30 | 6.14 | 0.995 | 0.278 | 0.898 | – | – |
| HP8 | 0.890 | 0.988 | 0.971 | 0.809 | 0.993 | 1.20 | 6.31 | 0.993 | 0.241 | 0.959 | – | – |
| HP9 | 0.895 | 0.988 | 0.981 | 0.680 | 0.986 | 1.02 | 6.50 | 0.989 | 0.214 | 0.955 | – | – |
| HP10 | 0.960 | 0.966 | 0.943 | 0.612 | 0.969 | 0.742 | 17.13 | 0.989 | 0.088 | 0.961 | 0.180 | 0.17 |
| HP11 | 0.810 | 0.981 | 0.864 | 0.905 | 0.940 | 1.41 | 6.28 | 0.940 | 0.369 | 0.817 | 0.261 | 0.186 |
| HP12 | 0.972 | 0.983 | 0.966 | 0.804 | 0.977 | 0.945 | 15.36 | 0.977 | 0.091 | 0.975 | 0.772 | 0.158 |
Data Analysis
The differences in average of data were compared by simple analysis of variance (one-way analysis of variance) or independent sample t test (Origin 6.1 USA). The significance of the difference was determined at 95% confident limit (α = 0.05).
RESULTS AND DISCUSSION
Evaluation of Tablets
The hardness of formulated tablets was found in the vicinity of 4–6 kg/cm2 with the friability values between 0.11 and 0.45. CNZ content was found within 100 ± 2.5% of labeled amount. The formulated floating tablets complied with the USP requirements for weight variation and friability.
In Vitro Buoyancy
The gastric floating system employed sodium bicarbonate as a gas-forming agent dispersed in the matrix tablet. Sodium bicarbonate reacted with hydrochloric acid and evolved carbon dioxide that was entrapped throughout the tablets (19).
The in vitro buoyancy studies showed that formulations containing HPMC K100LV (Batch nos. HP1, HP6, and HP7) showed good buoyancy with a lag time of 40–45 s and floatation time of more than 12 h in the test medium (Table III). HPMC tablets hydrated immediately after contact with the test medium. The generated gas gets entrapped and protected by the gel formed by hydration of the polymer and keeps the whole tablet buoyant on the surface of the test medium for as long as 12 h. It was also observed that increase in viscosity of the HPMC grade results in increased floating lag time for batches HP2, HP3, and HP4 (Table III). Calcium carbonate was equally effective as gas generating agent as compared to sodium bicarbonate.
Table III.
Physical Parameters of Fabricated Tablets of CNZ
| Batch no. | Weight mg ± SD (n=20) | Hardness Kg/cm2 ± SD (n = 3) | Friability (%) | Bulk density ± SD (n = 3) | Drug content (%) ± SD (n = 3) | Floating lag time ± SD (n = 3) |
|---|---|---|---|---|---|---|
| HP1 | 300 (1.36) | 4.5 (0.50) | 0.14 | 0.90 (0.007) | 99.30 (0.89) | 45 (9.01) s |
| HP2 | 302 (1.17) | 4.45 (0.39) | 0.16 | 0.91 (0.02) | 100.26 (1.12) | 1.2 (0.5) min |
| HP3 | 303 (1.16) | 4.55 (0.40) | 0.11 | 0.91 (0.012) | 99.89 (1.69) | 1.56 (0.67) min |
| HP4 | 302 (1.37) | 4.96 (0.59) | 0.18 | 0.90 (0.011) | 100.06 (1.61) | 2.6 (0.80) min |
| HP5 | 299 (1.50) | 5.33 (0.35) | 0.34 | 0.90 (0.006) | 99.40 (1.73) | 7.93 (0.95) min |
| HP6 | 305 (1.73) | 4.86 (0.24) | 0.13 | 0.90 (0.007) | 99.96 (0.67) | 40 (3.05) s |
| HP7 | 305 (2.18) | 4.89 (0.4) | 0.34 | 0.89 (0.015) | 99.62 (2.10) | 44.2 (7.76) s |
| HP8 | 305 (1.81) | 4.99 (0.10) | 0.41 | 0.88 (0.013) | 99.60 (1.53) | 6.56 (1.46) min |
| HP9 | 306 (1.45) | 4.9 (0.10) | 0.27 | 0.90 (0.005) | 99.75 (1.7) | 8.90 (0.87) min |
| HP10 | 301 (1.47) | 4.67 (0.29) | 0.21 | 0.93 (0.011) | 100.06 (1.45) | 4.6 (0.40) min |
| HP11 | 301 (1.54) | 4.69 (0.29) | 0.24 | 0.90 (0.021) | 100.51 (1.95) | 3.76 (0.48) min |
| HP12 | 302 (2.00) | 4.5 (0.50) | 0.37 | 0.93 (0.009) | 99.36 (0.59) | 6.76 (0.96) min |
In vitro buoyancy studies revealed that formulations HP10, HP11, and HP12 containing sodium alginate, polyethylene oxide (PEO), and carrageenan showed higher floating lag time. Apparently, the buoyancy of the tablet is governed by both the swelling of the hydrocolloid upon contact with the dissolution fluid and the presence of voids in the center of the tablet, which vary from polymer to polymer.
In Vitro Drug Release
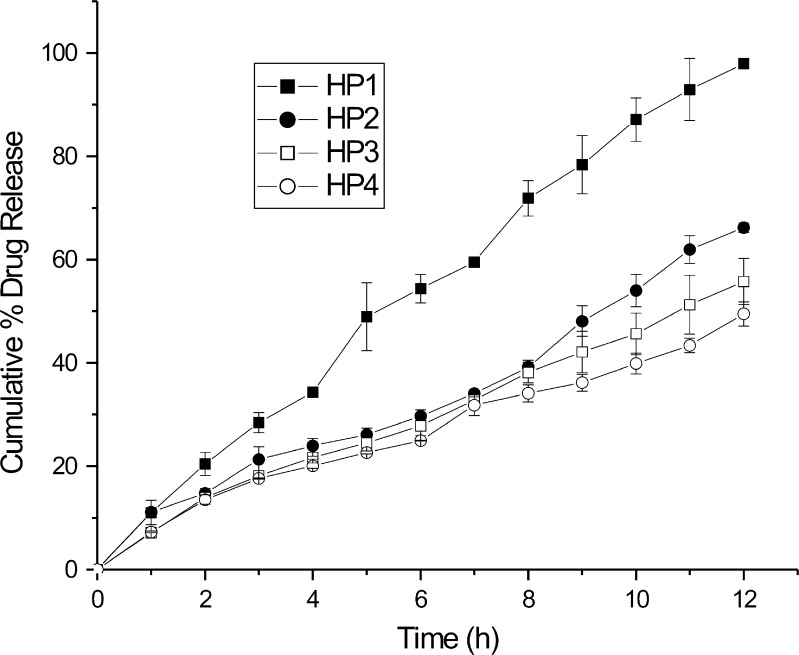
The dissolution of all formulated CNZ floating tablet was performed in 0.1 N HCl to keep them in the acidic environment of stomach for prolonged periods. The four grades of HPMC (K100 LV, K4M, K15M, and K100 MCR) differ in their molecular weight and viscosity. It has been reported that polymers of different viscosity grades can affect drug absorption. In addition to the effect on the floating lag time, molecular weight or viscosity of the polymer also affect the drug release from the matrix tablets. Batches HP1 to HP4 were studied to compare the effect of viscosity of polymer (HPMC) on drug release. There was an apparent difference in CNZ release pattern between the HPMC of different viscosity grades as the fraction of CNZ decreases with increase in the viscosity of the polymer. From the dissolution profiles shown in Fig. 1, it was observed that formulation HP4 containing HPMC K100 MCR showed the slowest release rate of the drug when compared with others, due to its higher viscosity.
Fig. 1.
Effect of viscosity grade of HPMC on drug release from floating matrix tablet
As can be seen from Fig. 1, a low viscosity polymer (HPMC K100 LV) yielded a faster drug release. It has been reported that increased viscosity results in a corresponding decrease in the drug release rate. Wan et al. (21) reported that HPMC of higher viscosity grades result in thicker gel formation with decrease in drug release. As soon as the matrix tablet comes in contact with the dissolution media, imbibition of the dissolution medium by the floating tablet takes place, initiating the formation of a gel layer of the polymer around the tablet. The diffusion of dissolved drug through this gel layer is the determining factor in the improvement of dissolution rate. From the Stokes–Einstein equation, the diffusion coefficient is inversely proportional to the viscosity. Hence, it can be inferred that increasing the viscosity of polymer decreases the drug release rate of the drug. The drug release rate decreased in the rank order HP4 > HP3 > HP2 > HP1.
Although the drug release rate mainly depends on the concentration of the hydrophilic polymer, the entrapped gas within the hydrogel also influences the release of the drug from the matrix. The study of the effect of the gas-forming agent on drug release suggested that drug release was higher with sodium bicarbonate as compared to calcium carbonate (Fig. 2). This may be due to different gas-forming capacity and the solubility of the particular gas-forming agent because of the high aqueous solubility of sodium bicarbonate than calcium carbonate. It was observed that drug release rate was increased with incorporation of tartaric acid and citric acid (Fig. 3), which apparently is due to the quicker formation of CO2 with these acids.
Fig. 2.
Effect of CO2 forming agent on drug release from floating matrix tablet
Fig. 3.
Effect of CA and TA combination with SBC and CC on drug release from floating matrix tablet
This was expected because constant weight tablets with higher porosity had greater thickness, and thicker tablets possess larger surface area. Analysis of the effect of calcium carbonate, citric acid, tartaric acid, and sodium bicarbonate concentration indicated that swelling and erosion were faster in the tablets containing higher amounts of gas-forming agents due to higher porosity of tablets.
Among the polymers used in this study, carrageenan retarded the drug dissolution rate up to 60.13% as compared to sodium alginate, polyethylene oxide, and HPMC-K100 LV (Fig. 4). The dissolution rate decreased in the rank order for the four polymers: carrageenan > sodium alginate > HPMC K100 LV > polyethylene oxide. For a dissolvable polymer matrix, polymer dissolution is another important mechanism that can modulate the drug delivery rate. While either swelling or dissolution can be the predominant factor for a specific type of polymer, in most cases, drug release kinetics is a result of a combination of these two mechanisms.
Fig. 4.
Effect of different polymers on drug release from floating matrix tablet
Initially, a rapid release was observed in the batches prepared with polyethylene oxide and HPMC matrices as compared to carrageenan and sodium alginate (Fig. 4). The slow rate of polymer hydration of sodium alginate and carrageenan might be the reason for this, as it is well known that the rate of hydration of polymer in a hydrophilic matrix system during dissolution has profound influence on drug release (21–23).
Generally, in hydrophilic matrix tablets, the release rate is influenced by two factors. Firstly, as the surface area of the polymer is not large enough to cover the drug particle at the surface of the matrix, therefore there is a greater chance of higher drug release. Secondly, if the polymer does not hydrate quickly, the surface barrier cannot be formed immediately, which may cause a larger portion of drug release during the initial phase of the drug release process. Thus, the surface area as well as the hydration of polymer can play an important role in drug releases from matrix tablets.
Parikh et al. (20) prepared a floating tablet of CNZ using HPMC K100 and sodium bicarbonate and tested in a multicompartment dissolution apparatus. Complete release was reported within 24 h with nearly zero-order release rate and no precipitation observed in the intestinal compartment, giving 100% of CNZ transferred to absorption compartment. In our case, we developed a floating tablet with different polymers and tested the effect of excipients on in vitro release of CNZ. The main emphasis of our study was on different kinetic parameters of formulation. In vivo study was conducted of one batch (HP1) and result compared with test formulation. In vivo data was promising with in vitro release of developed formulation. We used the USP XXIII basket apparatus for in vitro release study. In spite of the different in experimental protocol, some of our formulations also showed near zero-order release as that of the authors under reference.
Swelling Kinetics and Drug Release
Swelling is a very important characteristic of polymers that controls the drug release from the matrix via diffusion mechanism that depends on the rate of penetrant entry into the matrix. The effect of swelling behavior of different polymers is recorded in Fig. 5.
Fig. 5.
Percentage swelling profile of CNZ floating matrix tablet containing different polymers
In case of sodium alginate tablets (k = 229.09), rapid swelling in axial direction was observed as compared to carrageenan (k = 2.68), HPMC (k = 25.04), and polyethylene oxide (k = 86.09) matrices. As per Vergnaud, analysis of the swelling exponent, n, was found to be 0.599 (R = 0.869) and 0.180 (R = 0.987) for HPMC- and sodium alginate-based tablets, respectively (Fig. 5), which confirmed anomalous kinetics and diffusion controlled swelling (22). Diffusional coefficient values obtained from least squares linear regression analysis were 0.768 and 0.89 for sodium alginate and HPMC, respectively. This indicates that drug release from sodium alginate and HPMC tablets was controlled primarily by non-Fickian diffusion through pores and channels in the structure. HPMC tablets upon contact with the dissolution medium swell due to the disruption of hydrogen bondings among the polymeric chains and form a thick gel layer at the tablet surface, which gets eroded simultaneously. These parameters are responsible for controlling drug release rate from HPMC tablets (25). Water inserts itself into the hydrogen bonds between adjacent polymer chains. The macromolecular chains initially get rotational freedom and begin to occupy more space which results in polymer swelling. The penetrating medium fills the voids between the polymer chains and diffuses into denser regions of the polymer, and drug dissolution takes place at the boundary between the infiltrated region and the gel layer. Therefore, dissolved drug release depends upon the diffusion toward the outer most boundary between the swollen matrix and as well as erosion/dissolution of the polymer upon prolonged contact with dissolution medium. Since the hydration character of the polymer and subsequent physical properties of then hydrated gel layer may critically influence drug release, any change in the properties of the hydrated surface layer influences the performance of sodium alginate, carrageenan, and polyethylene oxide matrices.
Erosion Kinetics and Drug Release
In hydrophilic matrix systems, the carrier on the surface of the matrix initially hydrates during dissolution to generate an outer viscous gel layer. This phase is then sequentially followed by matrix bulk hydration, swelling, and erosion. The overall dissolution rate is controlled by the rate of matrix swelling, drug diffusion through the gel layer (26), and/ or matrix erosion (27). In the present study, it was observed that the erosion rate of the matrices decreased with increase in polymer viscosity. This indicates that increasing the viscosity forms a strong viscous gel layer, which is durable and resistant to erosion.
The rate of erosion of HPMC and sodium alginate was 0.21% and 0.176%/min, respectively (Fig. 6), i.e., HPMC matrices exhibited relatively higher erosion (≈1.2 times) than to sodium alginate matrices resulting in higher drug release (97.95% and 61.23%, respectively) at the end of 12 h (Fig. 6). Although the degree of water uptake and swelling of sodium alginate was higher than that of HPMC-K100LV, the rate of erosion of sodium alginate tablets was less significant than that of HPMC tablets.
Fig. 6.
Percentage erosion profile of CNZ floating matrix tablet containing different polymers
In case of carrageenan and polyethylene oxide, erosion data were fitted to erosion kinetics, and regression parameters were calculated (Table II). Carrageenan matrices (0.158%/min) showed less erosion rate as compared to PEO (0.186%/min) and other polymers used, overall resulting into lower drug release.
Water-soluble drugs are released primarily by diffusion of dissolved drugs molecules across the gel layer, while poorly soluble drugs are released predominantly by erosion mechanism. The contribution of each mechanism in the overall drug release process is influenced by both, the drug solubility, and also by the physical and mechanical properties of the gel barrier formed (28). At pH 1.2, carboxylate anions on the component uronic acid residues (sodium alginate) are rapidly converted to free carboxyl group (alginic acid) which has the ability to swell on hydration but which is usually insoluble (29). These result in a tough and rubbery outer layer in contrast to the viscous gelatinous layer formed around the HPMC tablets. The differences in the susceptibility of the hydrated layers to erosion of sodium alginate with respect to HPMC is accountable for lower drug release, as release rate of poorly soluble drugs like CNZ depends predominantly on surface erosion.
The relative contribution of drug diffusion was further confirmed by subjecting the dissolution data to Heuristic model. There are two competing mechanisms of drug release: swelling and erosion of the material occurring simultaneously resulting into moving boundary conditions which continuously modify the effective diffusivity of the drug. Erosion increases the drug dissolution rate thus compensating to some extent of high swelling and consequent slowing of drug diffusion by increasing diffusional path length.
It is observed that in the case of HPMC, PEO, and sodium alginate matrices (Fig. 6), Fickian diffusion predominates in the initial period of the dissolution, gradually decreasing until polymer erosion becomes predominant. In the later stage during the dominating diffusion period, the thickness of the viscous gel layer around the matrix will increase with time creating a longer path length for the drug to diffuse into the dissolution medium. Thereafter, the polymer chains increasingly relax, disentangle, and erode. It is noted that drug release due to erosion takes over the declining diffusional release mechanism about the half way through the dissolution time period.
On the other hand with carrageenan, the diffusional contribution is relatively higher. Carrageenan shows moderate swelling and solvent penetration rate but has a very slow rate of erosion. These factors combine to result in a low drug release.
Kinetic Analysis of Drug Release
A tablet composed of a polymeric matrix on contact with water builds a gel layer around the tablet, which governs the drug release. In order to establish the mechanism of drug release and swelling kinetics, the experimental data were fitted to zero-order, Higuchi, Korsmeyer–Peppas, Weibull, and Hixon–Crowell models (30).
The coefficients of regression were in a range between 0.966–0.995 (zero order), 0.964–0.984 (Higuchi), 0.940–0.996 (Peppas), and 0.732–0.984 (first order). However, the exponential equation is recommended to be used only for data corresponding up to 70% of drug release.
The n values for the Peppas model ranged from 0.724 to 0.794 for batches HP2, HP3, and HP4 indicating that the release of the drug from the floating tablets followed non-Fickian diffusion from the batches prepared from various grade of HPMC. Varying the HPMC grade increased the n value that is indicative of the release mechanism from diffusion toward a relaxation and erosion controlled process. The presence of CO2 bubbles produced after reaction of sodium bicarbonate with the acidic dissolution medium decreases the drug release rate. The matrix containing sodium bicarbonate indicates a little more contribution of relaxation and erosion to release mechanism. The n values were observed to be 0.895 and .905 for batch HP1 (HPMC K 100LV) and HP10 (PEO), respectively, indicating zero-order (case II) transport. The drug release from both batches involves two mechanisms, diffusion through swelling and release via polymer dissolution, thus indicating that swelling and erosion of polymer governs the release kinetics.
As expected, an increase in the polymer content brought about a corresponding decrease in drug release rate. The effect of polymer content is attributed to an increasing tortuosity and length of the diffusion path through the matrix as the polymer content increases.
HPMC hydrogels have several important characteristics that play an essential role in drug diffusion including swelling ratio and specific mesh or pore size. Swelling ratio is a function of the network structure, hydrophilicity, and ionization of the functional groups. The pore size is the space available for drug transport. The drug characteristics are as important as those of the gel. The size, shape, and ionization of the drug affect its diffusion through the gel layer (30). The drug diffusion through most types of polymeric systems is often best described by Fickian diffusion, but other processes in addition to diffusion are also important.
The n values for HP10, HP11, and HP12 matrices are 0.612, 0.905, and 0.804 indicating a non-Fickian (anomalous) behavior, and the drug diffuses partly through the swollen matrix and partly through the gradually expanding hydrated matrix with increasing diffusion path length.
The dissolution data for carrageenan (HP12) and sodium alginate (HP10) tablets showed linearity over the entire dissolution period with correlation coefficient 0.966 and 0.943; however, the data for PEO (HP11) tablets showed curvilinear behavior with correlation coefficient of 0.864 which indicates a relatively poor fit in Higuchi’s release model.
The fitted equation and correlation coefficient of each model is shown in Table II. The Hixson–Crowell equation was best fitted to HPMC tablet (batches HP2 to HP3) and indicates an erosion-dependent release mechanism (24). Although the Weibull function also presents good fits for batches HP1, HP3, HP7, and HP8, the best regression functions rely more on the description of shape, having not a direct connection with mechanism. It can describe the dissolution curve in terms of applicable parameters. This model gives information about shape parameter. The shape parameter characterizes the curves either as exponential (b = 1; case 1), sigmoid, S-shaped, with upward curvature followed by a turning point (b > 1; case 2), or parabolic, with a higher slop and after that consistent with the exponential (b > 1; case 3).
Overall, the release mechanisms from these polymers can be explained as a result of rapid hydration of the polymers on the surface of the tablets, which results in a gel or a highly viscous solution surrounding the matrix that restrict water penetration in to the center. The net result is a reduction of the rate of drug release as a function of time. From this study, it can be concluded that the drug release predominantly follows non-Fickian diffusion for all the four polymers used.
Pharmacokinetic Evaluation
The plasma concentrations of CNZ from oral CNZ suspension in 2% sodium CMC and controlled release tablet (HP1) are shown in Fig. 7. The study was aimed to evaluate the controlled release floating tablet over 12 h. The batch HP1 was selected for in vivo study on the basis of its in vitro results. It was concluded that CNZ was released from the HP1 tablets in a controlled manner and subsequently got absorbed in vivo. The mean pharmacokinetic parameters of the tested tablet and suspension are shown in Table IV. The difference of bioavailability between the HP1 tablet and reference oral suspension was very significant. CNZ concentration of controlled release tablet of CNZ was detected till the end of 24 h postadministration, while the plasma concentration of cinnarizine HCl suspension in 2% sodium CMC was detected till only the end of 8 h (Fig. 7). This indicated prolonged release and its subsequent in vivo absorption of HP1 tablet. In case of CNZ suspension, there was a quick absorption and a sharp elimination phase, while in case of controlled release tablet (HP1), the absorption phase was slow and prolonged. The post absorption phase after Cmax for the controlled release tablet could be attributed to a combination of elimination of already absorbed drug and continued absorption from the slow release of CNZ from the tablets in vivo.
Fig. 7.
Plasma concentration profiles of CNZ from floating controlled release tablets (HP1) and reference (oral suspension) in healthy rabbits
Table IV.
Summary of Mean Pharmacokinetic Parameters for CNZ from Floating Tablet (HP1) and 2% Sodium CMC in Healthy Rabbits
| S. no | Pharmacokinetic parameters | Controlled release (gastric floating) tablet (HP1; mean ± SD) | Oral suspension of CNZ in 2% sodium CMC (mean ± SD) |
|---|---|---|---|
| 1 | C max (ng/ml) | 121.46 ± 15.84 | 158.59 ± 24.07 |
| 2 | T max (h) | 4.00 ± 1.41 | 2.00 |
| 3 |

|
2,545.07 ± 579.56 | 892.705 ± 181.9 |
| 4 | Slope | 0.03 ± 0.004 | 0.104 ± 0.02 |
| 5 | K el (h−1) | 0.08 ± 0.01 | 0.24 ± 0.04 |
| 6 | t 1/2 (h) | 8.89 ± 1.15 | 2.95 ± 0.43 |
SD standard deviation,  mean area under plasma time curve, CNZ cinnarizine, CMC carboxymethyl cellulose
mean area under plasma time curve, CNZ cinnarizine, CMC carboxymethyl cellulose
The absorption of CNZ from suspension was rapid; the mean Tmax was 2 h, while in the controlled release tablet (HP1), the mean Tmax was 4.0 h. This showed that the controlled release tablet was effective in delaying the peak plasma concentration, thus indicating prolonged plasma concentration of CNZ from the controlled release tablets in vivo.
The mean peak plasma concentration (Cmax) of controlled release tablet (HP1) was 121.46 ± 15.84 ng/ml, while that of CNZ suspension at the same dose was 158.59 ± 24.07 ng/ml. This showed that the controlled release formulation effectively reduced the amount of drug released and consequently absorbed in vivo in the initial phase.
The mean biological half-life (t1/2) of CNZ from controlled release tablet and suspension was 8.89 ± 1.15 and 2.95 ± 0.43 h, respectively, that is nearly three times higher. This indicates that the declining phase of the plasma concentration–time curve involves an input function in addition to the elimination function, i.e., it is not a true elimination phase (31). The plasma half-life values are the same for the same drug substance, regardless of the dosage form. The difference observed here is due to prolonged absorption of the controlled release tablets (HP1); there is prolonged continuous introduction of CNZ into the blood stream. Therefore, the controlled release tablets (HP1) shows to have a longer plasma half-life, i.e., the drug stays in the plasma for a longer time than the suspension of CNZ (32).
The rate of elimination (Kel) of controlled release tablet (0.08 h−1) was less than that of suspension of CNZ suspension (0.24 h−1), which implies that the drug was slowly released and absorbed from the controlled release tablets than from the suspension of CNZ.
The mean area under plasma time curve  of controlled release tablet was 2545.07 ± 579.56 ng h/ml, while the mean
of controlled release tablet was 2545.07 ± 579.56 ng h/ml, while the mean  of oral reference suspension was 892.705 ± 181.9 ng h/ml. Thus, the overall absorption of CNZ from the controlled release tablet was 2.85 times more than its suspension which shows enhancement of bioavailability of test formulation with respect to reference product at the same dose. Based on these results, it can be concluded that the greater bioavailability obtained from the FDDS is due to its prolonged gastric residence time. The pharmacokinetic parameters of a drug suspension is commensurate with literature cited values (10–70 ng/ml in 10 h) of cinnarizine HCl commercial tablets, and the higher bioavailability and prolonged plasma drug concentration (17.5–137.5 ng/ml in 24 h) indicated that the objective of this study are met successfully.
of oral reference suspension was 892.705 ± 181.9 ng h/ml. Thus, the overall absorption of CNZ from the controlled release tablet was 2.85 times more than its suspension which shows enhancement of bioavailability of test formulation with respect to reference product at the same dose. Based on these results, it can be concluded that the greater bioavailability obtained from the FDDS is due to its prolonged gastric residence time. The pharmacokinetic parameters of a drug suspension is commensurate with literature cited values (10–70 ng/ml in 10 h) of cinnarizine HCl commercial tablets, and the higher bioavailability and prolonged plasma drug concentration (17.5–137.5 ng/ml in 24 h) indicated that the objective of this study are met successfully.
CONCLUSION
Gastrofloating tablets of CNZ were fabricated by direct compression. Tablet containing HPMC K4100LV (HP1) showed good buoyancy with very short lag time (45 s) and long floatation time of more than 12 h in simulated gastric fluid. In vitro release of tablets was obtained for more than 8 h. In vitro release data were fitted to various kinetic models and drug release predominantly follows non-Fickian diffusion for all the four polymers.
Overall, this study concludes that viscosity is a major factor affecting the drug release and floating properties of FDDS. It was concluded from the results of the pharmacokinetic study that the overall bioavailability of the floating controlled release formulation (HP1) was 2.85 times greater in comparison to oral suspension of CNZ as indicated by greater  .The t1/2 and Tmax of the test tablets was more than that of reference which indicates slow release and absorption of cinnarizine HCl from controlled release formulation. The pharmacokinetic parameters of CNZ suspension is commensurate with literature cited values (10–70 ng/ml in 10 h) of CNZ commercial tablet, and the higher bioavailability and prolonged plasma drug concentration (17.5–137.5 ng/ml in 24 h) indicate that the objective of this study are met successfully.
.The t1/2 and Tmax of the test tablets was more than that of reference which indicates slow release and absorption of cinnarizine HCl from controlled release formulation. The pharmacokinetic parameters of CNZ suspension is commensurate with literature cited values (10–70 ng/ml in 10 h) of CNZ commercial tablet, and the higher bioavailability and prolonged plasma drug concentration (17.5–137.5 ng/ml in 24 h) indicate that the objective of this study are met successfully.
Acknowledgments
The authors are grateful to University Grant Commission (UGC), India for Senior Research Fellowship to Ramesh C. Nagarwal and D. N. Ridhurkar for supporting this work.
Contributor Information
Ramesh C. Nagarwal, Email: rcnagarwal.rs.phe@itbhu.ac.in
Devendra N. Ridhurkar, Email: devendraridhurkar@gmail.com
J. K. Pandit, Email: dr.jkpandit@gmail.com
References
- 1.Ahmed IS, Ayres JW. Bioavailability of riboflavin from a gastric retention formulation. Int J Pharm. 2007;330:146–154. doi: 10.1016/j.ijpharm.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 2.Williams MF, Dukes GE, Heizer W, Han Y, Herman DJ, Lampin T, et al. Influence of gastrointestinal site of drug delivery on the absorption characteristics of ranitidine. Pharm Res. 1992;9:1190–1194. doi: 10.1023/A:1015860007380. [DOI] [PubMed] [Google Scholar]
- 3.Hwang SJ, Park H, Park K. Gastric retentive drug-delivery systems. Crit Rev Ther Drug Carrier Syst. 1998;15:243–284. [PubMed] [Google Scholar]
- 4.Desai S, Bolton S. A floating controlled-release drug delivery systems: in vitro–in vivo evaluation. Pharm Res. 1993;10:1321–1325. doi: 10.1023/A:1018921830385. [DOI] [PubMed] [Google Scholar]
- 5.Hilton AK, Deasy PB. In vitro and in vivo evaluation of an oral sustained-release floating dosage form of amoxycillin trihydrate. Int J Pharm. 1992;86:79–88. doi: 10.1016/0378-5173(92)90033-X. [DOI] [Google Scholar]
- 6.Matharu RS, Singhavi NM. Novel drug delivery system for captopril. Drug Dev Ind Pharm. 1992;18:1567–1574. doi: 10.3109/03639049209040859. [DOI] [Google Scholar]
- 7.Sheth PR, Tossounian J. The hydrodynamically balanced system (HBSE): a novel drug delivery system for oral use. Drug Dev Ind Pharm. 1984;10:313–339. doi: 10.3109/03639048409064653. [DOI] [Google Scholar]
- 8.Chen GL, Hao WH. In vitro performance of floating sustained-release capsule of verapamil. Drug Dev Ind Pharm. 1998;24:1067–1072. doi: 10.3109/03639049809089950. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda M, Peppas NA, McGinity JW. Floating hot-melt extruded tablets for gastroretentive controlled drug release system. J Control Release. 2006;115:121–129. doi: 10.1016/j.jconrel.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Wei Z, Yu Z, Bi D. Design and evaluation of a two-layer floating tablet for gastric retention using cisapride as a model drug. Drug Dev Ind Pharm. 2001;27:469–474. doi: 10.1081/DDC-100104323. [DOI] [PubMed] [Google Scholar]
- 11.Xiaoqiang X, Minjie S, Feng Z, Yiqiao H. Floating matrix dosage form for phenoporlamine hydrochloride based on gas forming agent: in vitro and in vivo evaluation in healthy volunteers. Int J Pharm. 2006;310:139–145. doi: 10.1016/j.ijpharm.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Ichigawa M, Watanabe S, Miyake Y. A new multiple-unit oral floating dosage system. I: preparation and in vitro evaluation of floating and sustained-release characteristics. J Pharm Sci. 1991;80:1062–1106. doi: 10.1002/jps.2600801113. [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner S, Tivadar A, Vrečer F, Kristl J. Development of floating tablets as a new approach to the treatment of Helicobacter pylori infections. Acta Pharm. 2001;51:21–33. [Google Scholar]
- 14.Cedillo-Ramirez E, Villafuerte-Robles L, Hernandez-Leon A. Effect of added pharmatose DCL11 on the sustained-release of metronidazole from methocel K4M and carbopol 971 NF floating matrices. Drug Dev Ind Pharm. 2006;32:955–965. doi: 10.1080/03639040500534127. [DOI] [PubMed] [Google Scholar]
- 15.Sopimath SK, Kulkarni AR, Rudzinski WE, Aminabhavi TM. Microspheres as floating drug delivery systems to increase gastric retention of drugs. Drug Metab Rev. 2001;33:149–160. doi: 10.1081/DMR-100104401. [DOI] [PubMed] [Google Scholar]
- 16.Hasan SM, Elmosallamy AF, Abbas AB. LC and TLC determination of cinnarizine in pharmaceutical preparations and serum. J Pharm Biomed Anal. 2002;28:711–719. doi: 10.1016/S0731-7085(01)00662-8. [DOI] [PubMed] [Google Scholar]
- 17.Roy DS, Rohera BD. Comparative evaluation of rate of hydration and matrix erosion of HEC and HPC and study of drug release from their matrices. Eur J Pharm Sci. 2000;16:193–199. doi: 10.1016/s0928-0987(02)00103-3. [DOI] [PubMed] [Google Scholar]
- 18.Ebube NK, Hikal AH, Jones AB. Sustained release of acetaminophen from heterogeneous matrix tablets: influence of polymer ratio, polymer loading, and co-active on drug release. Pharm Dev Technol. 1997;2:161–170. doi: 10.3109/10837459709022621. [DOI] [PubMed] [Google Scholar]
- 19.Choi BY, Park HJ, Hwang SJ, Park JB. Preparation of alginate beads for floating drug delivery system: effects of CO2 gas-forming agents. Int J Pharm. 2002;239:81–91. doi: 10.1016/S0378-5173(02)00054-6. [DOI] [PubMed] [Google Scholar]
- 20.Parikh RK, Parikh CP, Delvadia RR, Patel SM. A novel multicompartment dissolution apparatus for evaluation of floating dosage form containing poorly soluble weakly basic drug. Dissolution Technologies. 2006;13:14–19. [Google Scholar]
- 21.Wan LS, Heng PW, Wong LF. Relationship between swelling and drug release in a hydrophilic matrix drug. Dev Ind Pharm. 1993;19:1201. doi: 10.3109/03639049309063012. [DOI] [Google Scholar]
- 22.Colombo P, Bettini R, Santi P, Ascentiis A, Peppas NA. Analysis of the swelling and release mechanisms from drug delivery systems with emphasis on drug solubility and water transport. J Control Release. 1996;39:231. doi: 10.1016/0168-3659(95)00158-1. [DOI] [Google Scholar]
- 23.Mitchell K, Ford JL, Armstrong DJ, Elliot PN, Hogan JE, Rostron C. The influence of drugs on the properties of gels and swelling characteristics of matrices containing methylcellulose or hydroxypropylmethylcellulose. Int J Pharm. 1993;100:165–173. doi: 10.1016/0378-5173(93)90087-V. [DOI] [Google Scholar]
- 24.Yan G, Li H, Zhang R, Ding D. Preparation and evaluation of a sustained-release formulation of nifedipine HPMC tablets. Drug Dev Ind Pharm. 2000;26:681–686. doi: 10.1081/DDC-100101284. [DOI] [PubMed] [Google Scholar]
- 25.Conti S, Maggi L, Segale L, Ochoa Machiste E, Conte U, Grenier P, et al. Matrices containing NaCMC and HPMC: 1. Dissolution performance characterization. Int J Pharm. 2007;333:136–142. doi: 10.1016/j.ijpharm.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 26.Harland R, Gazzaninga A, Sangalli M, Colomb P, Peppas NA. Drug/polymer matrix swelling and dissolution. Pharm Res. 1988;5:488. doi: 10.1023/A:1015913207052. [DOI] [PubMed] [Google Scholar]
- 27.Bain J, Tan S, Gandarton D, Solomon M. Comparison of the in vitro release characteristics of a wax matrix and a hydrogel sustained release diclofenac sodium tablet. Drug Dev Ind Pharm. 1991;17:215. doi: 10.3109/03639049109043821. [DOI] [Google Scholar]
- 28.Alderman DA. A review of cellulose ethers in hydrophilic matrices for oral controlled-release dosage forms. Int J Pharm. 1984;5:1–9. [Google Scholar]
- 29.Hodson AC, Mitchell JR, Davies MC, Melia CD. Structure and behavior in hydrophilic matrix sustained release dosage forms: 3. The influence of pH on the sustained-release performance and internal gel structure of sodium alginate matrices. J Control Release. 1995;33:143–152. doi: 10.1016/0168-3659(94)00076-7. [DOI] [Google Scholar]
- 30.Costa C, Lobo CJM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–133. doi: 10.1016/S0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 31.Hasan EI, Amro BI, Arafat T, Badwan AA. Assessment of a controlled release hydrophilic matrix formulation for metoclopramide HCl. Eur J Pharm Biopharm. 2003;55:339–344. doi: 10.1016/S0939-6411(03)00022-5. [DOI] [PubMed] [Google Scholar]
- 32.Paulson SK, Vaughn MB, Jessen SM, Lawal Y, Gresk CJ, Yan B, et al. Pharmacokinetics of celecoxib after oral administration in dogs and humans: effect of food and site of absorption. J Pharmacol Exp Ther. 2001;297(2):638–645. [PubMed] [Google Scholar]