Abstract
As the continuation of a previous study, synthetic peptides corresponding to the extracellular domains of human gonadotropin-releasing hormone (GnRH) receptor were used to generate additional monoclonal antibodies which were further characterized biochemically and immunologically. Among those identified to recognize GnRH receptor, monoclonal antibodies designated as GHR-103, GHR-106 and GHR-114 were found to exhibit high affinity (Kd ≤ 1 × 10−8 M) and specificity to GnRH receptor as judged by the whole cell binding immunoassay and Western blot assay. Both anti-GnRH receptor monoclonal antibodies and GnRH were shown to compete for the same binding site of GnRH receptor on the surface of cultured cancer cells. Growth inhibitions of cancer cells cultured in vitro were demonstrated by cellular apoptosis experiments (TUNEL and MTT assays) under different conditions of treatment with GHR-106 monoclonal antibody or GnRH analogs. It was generally observed that both GnRH I and GHR-106 effectively induce the apoptosis of cultured cancer cells as determined by TUNEL and MTT assays. Consistently, suppressions of gene expressions at mRNA levels were demonstrated with several ribosomal proteins (P0, P1, P2 and L37), when cancer cells were incubated with GnRH or GHR-106. The widespread expressions of GnRH receptor in almost all of the studied human cancer cell lines were also demonstrated by RT-PCR and Western blot assay, as well as indirect immunofluorescence assay with either of these monoclonal antibodies as the primary antibody. In view of the longer half life of antibodies as compared to that of GnRH or its analogs, anti-GnRH receptor monoclonal antibodies in humanized forms could function as GnRH analogs and serve as an ideal candidate of anti-cancer drugs for therapeutic treatments of various cancers in humans as well as for fertility regulations.
Keywords: Gonadotropin-releasing hormone receptor (GnRH receptor), Anti-GnRH receptor monoclonal antibodies, Growth inhibition of cancer cells, Anti-cancer drugs, Apoptosis, Anti-proliferation
Introduction
Gonadotropin-releasing hormone (GnRH) (both types I and II hormone) was initially identified as a decapeptide hormone [1]. Its function is to stimulate the release of gonadotropin, LH and FSH from anterior pituitary through specific binding to the GnRH receptor located on the external membrane of selected cell types [1, 2]. Subsequent studies revealed that GnRH and its receptor also play extra pituitary roles in numerous normal and malignant cells or tissues, the mechanisms of which are still being actively explored [2–7]. Anti-proliferative effects of GnRH or its analogs on cancer cells of different human tissue origins have been reported [8–11].
Numerous clinical studies have also been reported regarding the use of cytotoxic GnRH analogs or GnRH analogs alone as anti-cancer drugs [12–19]. These drugs are formulated by cross-linking cytotoxic anti-cancer agents to GnRH analogs such as AN-152 and AN-207 [12–15]. Numerous GnRH agonists or antagonists were also employed to suppress cancer cell (especially prostate and ovarian cancer cell) growth [15–19]. However, limited and variable efficacy was observed in most cases [6]. Nevertheless, this approach has been shown to be effective in cancer treatments with lower toxicity and improved efficacy when compared to non-targeted systemic chemotherapy [12].
Development of antibody-based anti-cancer drugs has been an alternative approach to human cancer treatments in recent years [20, 21]. However, it is important that the drug target on the cancer cell surface is clearly identified, expressed with high abundance and its mechanisms of action well elucidated. The advantages of this approach include reduced side effects and a relatively long half life (5–20 days) of circulating antibody drugs upon treatments. In view of these considerations, monoclonal antibodies (Mabs) against GnRH receptor could be an ideal choice to neutralize cancer cells if the target specificity and the mechanisms to inhibit cancer cell growth can be established. Although a Mab designed as F1G4, raised against synthetic peptide of N1-29 amino acid residues of human GnRH receptor has been reported to bind T47D human breast cancer cell line and OVCAR-3 ovarian cancer cell line, not all of the cells in either cell line were reactive with the antibody [22, 23]. It has also been reported that polyclonal antibodies, which were raised against N5-17 amino acid residues of mouse pituitary GnRH receptor, could cross-react with human ovarian cancer cell line, suggesting the feasibility of using such antibodies for the treatment of proliferative disease of ovary or uterus [24, 25]. However, the antigen specificity of these antibodies has never been well characterized.
In our previous study [2], preliminary data were reported for the generations and partial characterizations of several Mabs which were generated against synthetic peptides corresponding to the extracellular domains of human GnRH receptor. These included immunohistochemical studies of human pituitary tissues, selected cancer cell lines and human sperm as well as RT-PCR to demonstrate the expressions of GnRH receptor in these tissues or cancer cells. In this study, efforts were made to further characterize additional Mabs against extracellular domains of human GnRH receptor. These antibodies were analyzed biochemically and immunologically with respect to their specificity and affinity to this receptor. Anti-proliferative effects of some of these Mabs were demonstrated through cellular apoptosis experiments with cancer cells cultured in vitro as well as mRNA expressions of selected ribosomal proteins by RT-PCR.
Materials and methods
Chemicals
All the chemicals were purchased from Sigma Chemical Company (St. Louis, MO) unless otherwise mentioned.
Cell lines
The ovarian cancer cell line of serous origin, OC-3-VGH was established by Department of Obstetrics and Gynecology at Veterans General Hospital, Taipei, Taiwan [26]. The cancer cells were cultured in RPMI medium containing 10% fetal calf serum. For comparative purposes, as many as 33 human cancer cell lines were obtained from American Type Culture Collection (ATCC) Co. (Rockville, MD). They were cultured and harvested separately according to the supplier’s instructions. The origins of these human cancer cell lines are listed in Table 1 of this report.
Table 1.
Expressions of GnRH receptor 1 in cancer cell lines as detected by RT-PCR, indirect immunofluorescence staining and Western blot assays
| Origins | Cell line designation | ATCC No. | Relative GnRHR1 signala |
|---|---|---|---|
| Breast | MCF7 | HTB-22 | ±, +c |
| MDA-MB-231 | HTB-26 | +, +d | |
| MDA-435 | ±, ±c | ||
| T-47D | HTB-133 | ±, +c | |
| Cervix | C-33A | HTB-31 | +, +c, +d |
| SiHa | HTB-35 | + | |
| ME-180 | HTB-33 | +, ++c | |
| Colon | HCT 115 | + | |
| HCT 116 | CCL-247 | +, +c | |
| HT-29 | HTB-38 | + | |
| SW-48 | CCL-231 | ±, +c | |
| Glioblastoma | HTB-14 | U-87 MG | + |
| Hepatocellular | Hep3B | HB-8064 | + |
| HepG2 | HB-8065 | +, +c | |
| Hep-2 | +, ±c | ||
| Lung | A549 | CCL-185 | +, ±c, +d |
| Calu-6 | HTB-56 | + | |
| H441 | HTB-174 | +, ±d | |
| MRC-5b | CCL-171 | +, +c | |
| WI-38b | CCL-75 | + | |
| Lymphoma | HEL 1 | ±, +d | |
| Lekemia | K-562 | CCL-243 | +, ±c, +d |
| Melanoma | MMAN | ± | |
| MMRU | + | ||
| SK-MEL-3 | HTB-69 | ± | |
| Neuroblastoma | SH-SY5Y | CRL-2266 | + |
| Human Ovarian | SK-OV-3 | HTB-77 | ±, +d |
| OC-3-VGH | +, +d | ||
| OVCAR-3 | HTB-161 | ±, +d | |
| Placenta | JEG-3 | HTB-36 | + |
| Prostate | DU 145 | HTB-81 | + |
| PC-3 | CRL-1435 | ± | |
| T-cell Leukemia | Jurkat | TIB-152 | − |
aThe strength of the signal in the order of ++ (very strong), + (strong), ± (weak), − (invisible)
bNormal hyperplastic and proliferated fibroblast cell lines derived from fetal lung tissue
cPositive staining confirmed with indirect immunofluorescence staining by GHR-106 Mab
dPositive staining confirmed with Western blot assay (probe GHR-103 or GHR-106 Mab)
Generation and selection of monoclonal antibodies against human GnRH receptor
The method for the generations of hybridomas secreting Mabs against the extracellular domains of human GnRH receptor has been published previously [2, 26]. Briefly, oligopeptides corresponding to the N-terminal amino acid residues 1–29, 182–193, 195–206 and 293–306 in the extracellular domains of human GnRH receptor were custom synthesized by Gemed Lab, South San Francisco, CA and Genescript, Piscataway NJ. They were conjugated separately to Keyhole Limpet hemocyanin (KLH) by standard methods and used as immunogen to generate Mabs as previously described [2]. Detailed procedures for production of Mabs through immunization, cell fusion and screening steps have been published elsewhere [2, 27]. Several cell fusion experiments were performed with mice immunized with KLH-conjugated peptide (N1-29). However, none of the Mabs could be generated by using other three synthetic peptides as immunogens due to poor immune response [2]. Three of the Mabs, designated as GHR-103, GHR-106 and GHR-114, respectively, were selected for further characterizations and presented in this study. Preliminary characterizations of these Mabs were preformed similarly to those published previously including immunohistochemical staining with pituitary tissue sections and selected cancer cell lines [2]. ELISA with microwells coated with synthetic peptides or with cancer cell extract was employed for the binding or binding inhibition assay presented in this study.
Induction of apoptosis of cancer cells by GnRH and GHR-106
In order to investigate the anti-proliferative or apoptosis effects of GHR-106 on cancer cells, In Situ Cell Death Detection Kit, POD (Roche, Canada) was employed for detection and quantitation of apoptosis at a single cell level. Briefly, OC-3-VGH ovarian cancer cells were cultured in RPMI-1640 medium at 37°C in a CO2 (5%) incubator for 2–3 days until all cancer cells are attached to microwells. Following removal of cell culture medium, fresh serum-free medium was added for an additional 3 h incubation in a CO2 incubator. After the serum-free starving period, the cells were incubated in fresh medium containing 10% fetal calf serum, and GnRH or GHR-106 of known concentrations was added for co-incubation of 24–72 h. As the negative control, normal mouse IgG of the same concentration was used for the same incubation period.
At the end of incubation, the attached cells were removed from tissue culture wells by appropriate cell detachment solution (Accutase, Sigma). Apoptosis of treated cancer cells was determined quantitatively by TUNEL assay with the instructions provided by Cell Death Detection Kit, POD (Roche, Canada). Percent increases of apoptosis cells after treatments with either GnRH or GHR-106 were obtained by subtracting the spontaneous apoptosis of the negative control.
MTT assay for anti-proliferation of cancer cells by GnRH and GHR-106
MTT assay [16, 17] was performed on cancer cells on a 24-well plate. A total of 1 × 104 cells were dispensed into each well and cultured for 48 h. Following removal of cultured media, fresh medium containing GnRH (0.1 μg/ml) or GHR-106 (10–20 μg/ml) was added to each well for an additional 48 h incubation. Subsequently, the culture medium was removed, and 200 μl of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide) (2.5 mg/ml in PBS, Sigma) was added to each well. After 5 h of incubation with MTT at 37°C in a 5% CO2 incubator, the supernatant was removed, and 200 μl of dimethylsulfoxide (DMSO) was added to each well followed by shaking at 150 rpm for 5 min. Absorbance at 560 nm was determined spectrophotometrically. The cells without any antibody treatment served as the negative control.
Western blot and indirect immunofluorescence assay
Western blot assay was performed according to the previously reported procedure with minor modifications [2]. Briefly, the cancer cells (104–105 cells/ml) from OC-3-VGH or other cell lines were solubilized in SDS sample buffer containing 1% SDS and 5% β-mercaptoethanol followed by boiling for 3 min. The samples were loaded on 12% acrylamide gels with BioRad molecular size markers. The rest of Western blot procedure was the same as previously described [2]. For negative control, normal mouse IgG of the same concentration was used instead.
For indirect immunofluorescence (IIF) assay, cancer cells (trypsinized) from the selected cell lines were fixed on slides with absolute methanol. GHR-106 Mab of known dilutions (0.1–5 μg/ml) was used as the primary antibody in RPMI-1640 medium containing 10% bovine calf serum. Following 1 h incubation, the cell-coated slides were washed five times with PBS containing 1% BSA and 0.1% Tween-20. Goat anti-mouse IgG labeled with FITC (Sigma) of suitable dilutions (1:200–1,000) were added for an additional hour of incubation at room temperature. Following wash, immunofluorescence staining was observed under the Zeiss microscope with fluorescent attachment. For the negative control, normal mouse IgG of the same dilution was used for comparison.
Total RNA extraction and cDNA synthesis
Total RNA was extracted from different cell lines (105–107 cells) using QIAGEN RNeasy mini kit. RNase-free DNase set was included to avoid genomic gene interference. Reverse transcription (RT) of total RNA (0.5–5 μg/20 μl) to cDNA was performed by using oligo (dT)15 primers and EasyScript™ First Strand cDNA Synthesis Kit from Applied Biological Materials (Abm) Inc. (Richmond, BC, Canada) following the manufacturer’s protocol. Reaction mixtures with RNA template but without reverse-transcriptase or with reverse-transcriptase but without RNA template were used as the negative controls for cDNA synthesis.
PCR
All primers required for PCR amplifications were obtained from Integrated DNA Technologies (San Diego, CA) and listed as follows: GnRHR1 (486 bp): 5′-CAGAAGAAAGAGAAAGGGAAAAAGC-3′ (sense) and 5′-GATGAAAAGAGGGATGATGAAGAGG-3′ (antisense); P0 (431 bp): 5′-TTGTGTTCACCAAGGAGG-3′ (sense) and 5′-GTAGCCAATCTGCAGACAG-3′ (antisense), respectively; P1 (476 bp): 5′-CAAGGTGCTCGGTCCTTC-3′ (sense) and 5′-GAACATGTTATAAAAGAGG-3′ (antisense); P2 (386 bp): 5′-TCCGCCGCAGACGCCGC-3′ (sense) and 5′-TGCAGGGGAGCAGGAATT-3′ (antisense); L37 (340 bp): 5′-CAGAAGCGAGATGACGAAGG-3′ (sense) and 5′-CCAGAACATTTATTGCATGAC-3′ (antisense) [7]. A housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified to check the functional integrity of cDNA and used as internal control by using 5′-GAAATCCCATCACCATCTTCC-3′ (sense) and 5′-CCAGGGGTCTTACTCCTTGG-3′ (antisense) as primers (805 bp) [28].
In this study, the nucleotide sequence of the sense primer and that of the antisense primer were selected with the expected size of PCR product of 486 bp, extending from position 1944 of Exon 1 at 5′ end to position 2429 of Exon 2 at the 3″ end of GnRH receptor as reported previously [2, 29].
PCR was performed by using 2× PCR Plus MasterMix kit (ABM, Richmond, BC, Canada) according to manufacturer’s protocols. After denaturing at 94°C for 4 min, 20–35 cycles were performed under the following conditions: denaturing at 94°C for 40 s, annealing at 50°C for 40 s, polymerizing at 72°C for 1 min, and at last complete extension at 72°C for 7 min. At the end, the PCR product was checked by 1.5% agarose gel electrophoresis. The relative signal intensities of different PCR products on the agarose gel were semi-quantitatively analyzed by ImageQuant image analysis software. The intensity of GAPDH control was adjusted to 100% in each case for comparative purposes. The negative control from cDNA synthesis was further used in PCR reaction and served as the negative control.
Results
Characterizations of anti-GnRH receptor monoclonal antibodies
Besides the human GnRH receptor-specific Mabs that were reported in the previous study [2], three additional high affinity ones were selected and characterized in the present study. They were designated as GHR-103, GHR-106 and GHR-114, respectively. All were generated with immunogens consisting of a synthetic peptide corresponding to that of N1-29 amino acid residues in the extracellular domains of human GnRH receptor. Typical immunohistochemical staining with pituitary tissue sections and selected cancer cells were performed to establish their relative specificity to GnRH receptor [2]. Details of this analysis were presented in the previous study [2] and will not be elaborated here.
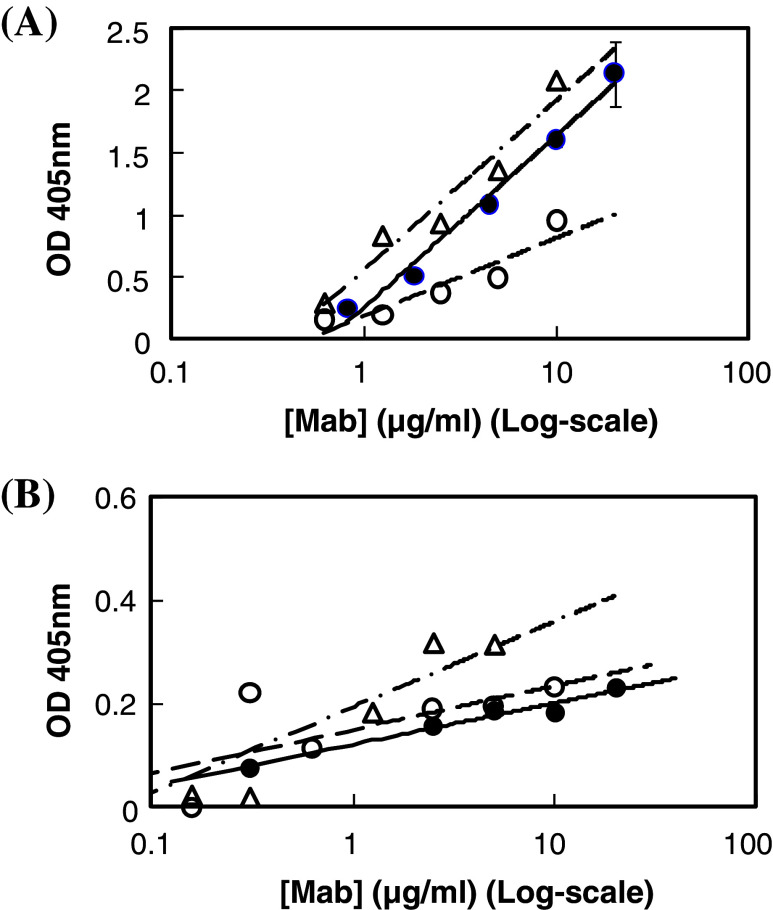
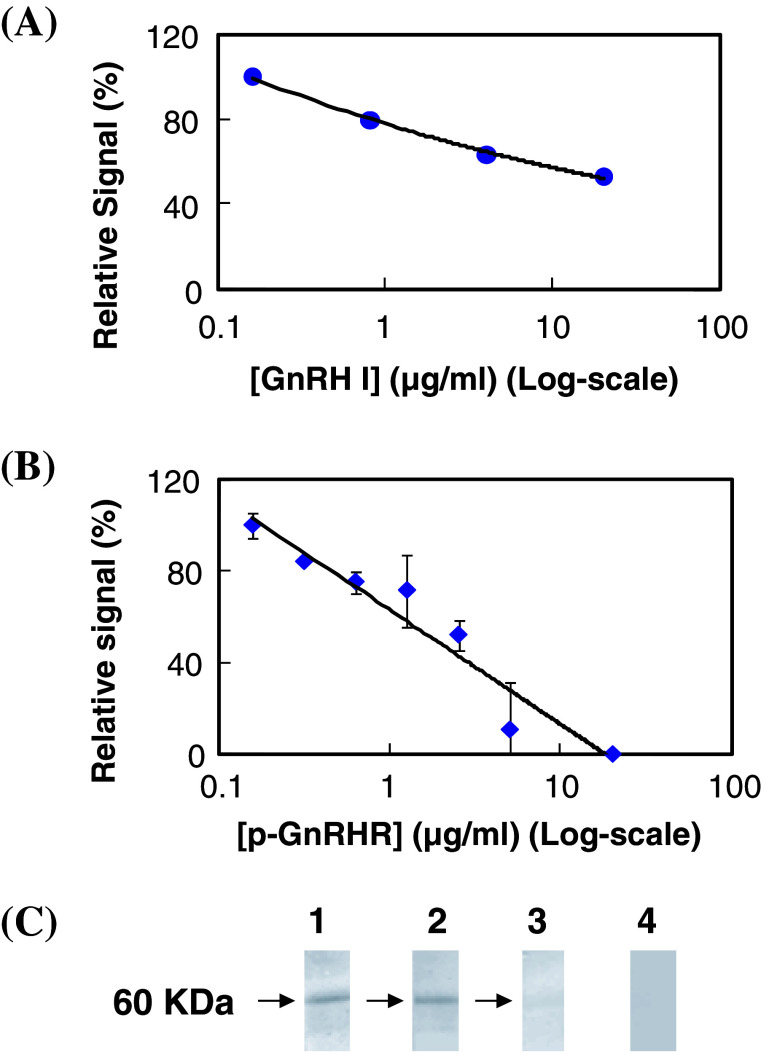
ELISA with microwells coated with synthetic peptide or cancer cells was employed to estimate the affinity constant between anti-GnRH receptor Mabs and GnRH receptor in the present study. As shown in Fig. 1a, GHR-103, GHR-106 and GHR-114 were shown to reveal dose-dependent binding to N1-29 synthetic peptide coated on microwells. A similar dose-dependent binding was observed between GHR-103, GHR-106 or GHR-114 and OC-3-VGH cancer cells coated on microwells as shown in Fig. 1b. Based on the results of these binding assays, comparable dissociation constant (Kd) was estimated to be 2 × 10−9 M for GHR-103 and GHR-106 and slightly higher for GHR-114. Furthermore, GnRH I was shown to inhibit the binding between GHR-106 and OC-3-VGH cancer cells coated on microwells. This was clearly demonstrated by the decrease in relative signal intensity of antibody binding to cancer cells with increasing GnRH concentrations ranging from 0.2 to 20 μg/ml (Fig. 2a). Similarly, N1-29 synthetic peptide was also shown to inhibit GHR-106 binding to immobilized cancer cells as shown in Fig. 2b.
Fig. 1.
a ELISA to reveal the dose-dependent binding of GHR-103, GHR-106 and GHR-114 monoclonal antibodies to well-coated synthetic peptide corresponding to the N1-29 amino acid residues of human GnRH receptor. Following 1 h incubation at 37°C with these GHR monoclonal antibodies in microwells, goat anti-mouse IgG labeled with alkaline phosphatase was used as the secondary antibody for spectrophotometrical signal detection at 405 nm (open triangle GHR-103, filled circle GHR-106, open circle GHR-114). b ELISA to reveal the dose-dependent binding between GHR monoclonal antibodies and microwells coated with OC-3-VGH cancer cells. Experimental conditions are similar to those of Fig. 1a
Fig. 2.
a Inhibition of GnRH I on the binding of GHR-106 Mab (2 μg/ml) to microwells coated with OC-3-VGH cancer cells. The relative signal as percent of the maximum (in the absence of GnRH I) was plotted against GnRH I concentration (in Log Scale). b Inhibition of synthetic peptide of N1-29 amino acid residues of GnRH receptor (p-GnRHR) on the binding between GHR-106 (2 μg/ml) and microwells coated with OC-3-VGH cancer cells. The relative signal as percent of the maximum (in the absence of the peptide) was plotted against the peptide concentrations (in Log scale). The assay conditions are similar to those described in Fig. 1. c Western blot assay to reveal the protein band (s) of 60 KDa from OC-3-VGH cancer cell extract recognized by GHR-103 (Lane 1), GHR-106 (Lane 2), and GHR-114 (Lane 3). Normal mouse IgG was used as negative control (Lane 4)
Western blot assay was performed with GHR-103, GHR-106 and GHR-114 by using alkaline phosphatase-labeled goat anti-mouse IgG as the detecting probe. As shown in Fig. 2c, one single protein band from OC-3-VGH ovarian cancer cell extract was observed with molecular weight of approximately 60 KDa for all three anti-GnRH receptor Mabs [3]. Similar results were obtained with cell extract of other cancer cell lines listed in Table 1.
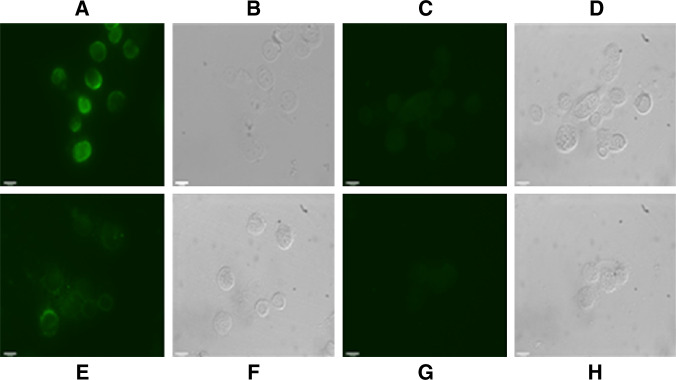
Indirect immunofluorescence assay was also employed to demonstrate the presence of GnRH receptor on the surface of selected cancer cells listed in Table 1. Typical examples are given in Fig. 3 with OC-3-VGH ovarian cancer cell line and C-33A cervical cancer cell line, respectively. The results of IIF assay clearly demonstrated that both cancer cell lines were positively stained with GHR106 as the primary antibody (Fig. 3a, e). The negative control using normal mouse IgG of the same concentration revealed no IIF staining (Fig. 3c, g).
Fig. 3.
Indirect immunofluorescence staining (under UV light) was used to demonstrate the presence of GnRH receptor on the surface of cancer cells, including OC-3-VGH ovarian cancer cell line (a) and C-33A cervical cancer cell line (e), by using GHR106 (5 μg/ml) as the primary antibody. b and f are the corresponding cells visualized under the visible light, respectively. For the negative control, the corresponding cancer cells were stained with normal mouse IgG of the same concentration as the primary antibody. The results are shown in (c, d) for OC-3-VGH and (g, h) for C-33A, respectively (magnification ×100). Details of experimental conditions were described in the text
Induction of cellular apoptosis by TUNEL assay and MTT assay
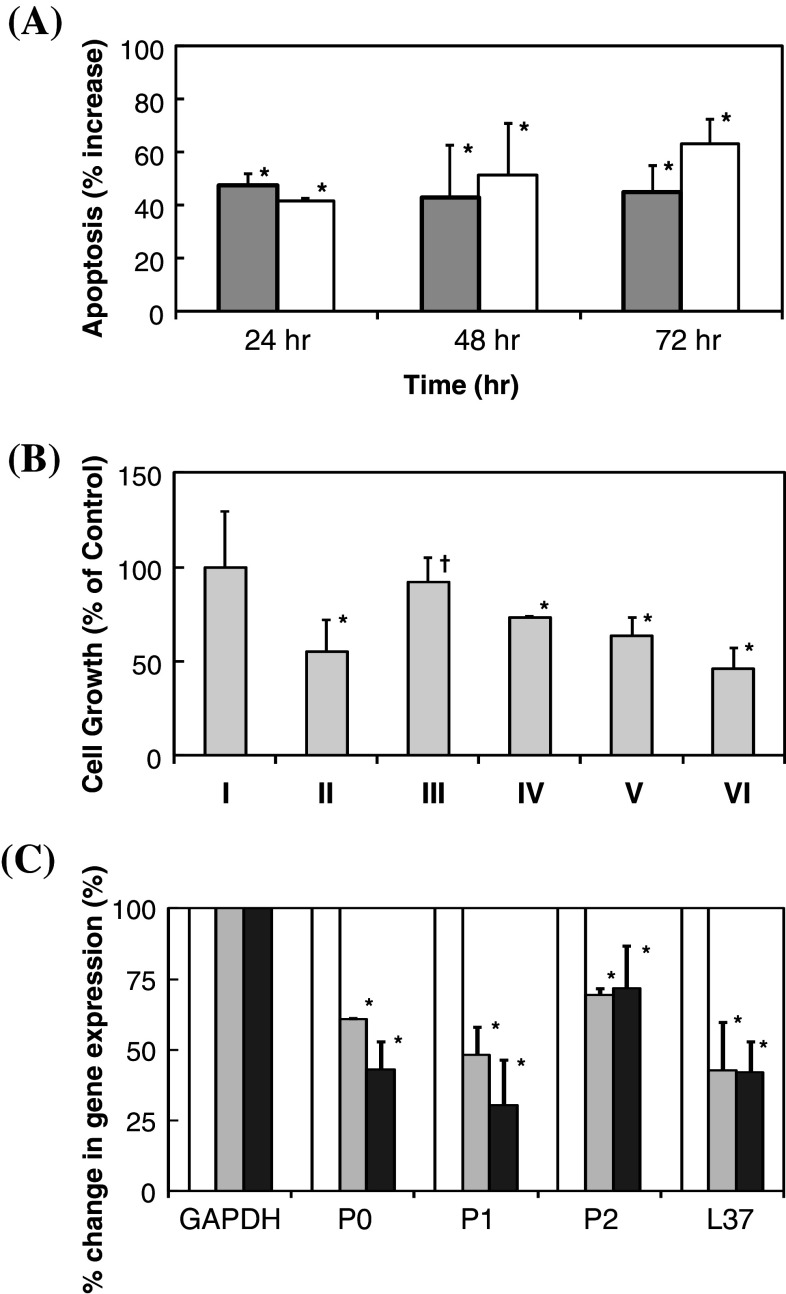
By employing TUNEL assay, inductions of apoptosis following treatments of cancer cells with GnRH I or GHR-106 were quantitatively determined. As shown in Fig. 4a, significant increases in apoptosis of treated cancer cells were detected upon the treatment with either 10 μg/ml of GHR-106 or 0.1 μg/ml of GnRH I. Induction of cellular apoptosis with GHR-106 was found to have little time-dependent increase from 24 to 72 h as compared to the negative control. By comparison, apoptotic induction with GnRH I treatments increased significantly from 40 to 65% from 24 to 72 h of incubation time.
Fig. 4.
a Percent increases in apoptosis in response to treatments of cultured OC-3-VGH cancer cells with 10 μg/ml of GHR-106 Mab (gray) and 0.1 μg/ml of GnRH I (white) for 24, 48 and 72 h. Percent increase in apoptosis of cancer cells was determined by subtracting spontaneous apoptosis obtained from incubation with normal mouse IgG of the same concentration or without hormone treatment for the same incubation period (all data revealed statistical significance at P < 0.05); b MTT assay to reveal dose-dependent anti-proliferative effects of I Negative control, II GnRH I (0.1 μg/ml) (P < 0.05), III Antide (0.1 μg/ml) (P > 0.05), IV Antide (0.2 μg/ml) (P < 0.05), V GHR-106 (10 μg/ml) (P < 0.05) and VI GHR-106 (20 μg/ml) Mab, respectively, for 48 h on the cultured cancer cells. Relative absorbance at 560 nm was normalized with negative control (NC) without treatment; and c down regulations of mRNA expression of four selected ribosomal proteins (P0, P1, P2 and L37) upon treatment of OC-3-VGH cancer cells with GHR-106 Mab (10 μg/ml) (gray) or GnRH I (0.1 μg/ml) (black) for an incubation period of 24 h. mRNA expression of GAPDH gene was used as the internal control and treated as 100%. Percent changes in RT-PCR signals which represent levels of P0, P1, P2, and L37 ribosomal proteins are presented with respect to GAPDH (100%) at 0 and 24 h (all data revealed statistical a significance at P < 0.05)
Anti-proliferative effects of GnRH I and its antagonist as well as GHR-106 Mab on the growth of cultured cancer cells were also studied by MTT assay. The results of this assay are presented in Fig. 4b. Both GnRH and GHR-106 were shown to significantly inhibit the growth of cultured cancer cells upon incubation for 48 h with the hormone or with Mab. Similarly, antide, a GnRH antagonist was also shown to induce apoptosis although to a much less extent. Induction of apoptosis by GHR-106 on cancer cells also revealed a dose-dependence (Fig. 4b).
Effect of antibody or hormone treatments on the mRNA expressions of selected ribosomal proteins
Four ribosomal proteins P0, P1, P2, and L37 were selected for semi-quantitative analysis of mRNA expressions. The expression of mRNA of GAPDH gene was used as the internal control. Upon 24 h treatment with GHR-106, it was clearly indicated that there were significant decreases in gene expressions of these four ribosomal proteins. The results of such semi-quantitative analysis are presented in Fig. 4c. Messenger RNA expressions of these genes of cultured cells without GHR-106 treatment served as the internal negative control and those with GnRH treatment served as positive control.
Expressions of GnRH receptor mRNA in different cancer cell lines
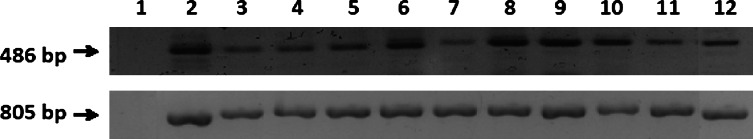
Thirty-three different human cancer cell lines were employed to investigate the mRNA expressions of GnRH receptor by using RT-PCR. The results of some cell lines are displayed in Fig. 5 and summarized in Table 1. So far, only 1 out of 33 cell lines, Jurkat (T-cell leukemia), shows little or no evidence of GnRH receptor mRNA expressions. To demonstrate the consistency regarding the expressions of GnRH receptor at mRNA and protein levels, Western blot and IIF assay of some cancer cell lines were performed. By IIF, some of the cancer cell lines were stained positively, when GHR-106 Mab was used as the primary antibody probe (see Table 1). Typical results of IIF assay with OC-3-VGH ovarian cancer cell line and others (C-33A cervical cancer) are presented in Fig. 3 and described in the previous section. Similarly, by Western blot assay, the majority of the tested cancer cell lines were shown to reveal similar protein bands of 60 KDa which corresponds to the molecular weight of human GnRH receptor in OC-3-VGH cancer cells (Fig. 2c), when either of the three established Mabs was used as the probe (Table 1).
Fig. 5.
PCR products (486 bp) using GnRHR1 primers to indicate the expression of GnRHR1 chain in cancer cell lines as follows: lane 1 negative control showed no band, lane 2 Calu6, lane 3 HCT 115, lane 4 H441, lane 5 Hep3B, lane 6 HT-29, lane 7 SW-48, lane 8 SK-OV-3, lane 9 MMRU, lane 10 MRC-5, lane 11 MCF7, and lane 12 OC-3-VGH. Expression of housekeeping gene GAPDH (805 bp) is also presented to check the integrity of cDNA from each cancer cell line and to normalize the cDNA concentrations
Discussions
In a previous study, we reported generations and partial characterizations of several Mabs against a synthetic peptide derived from N1-29 amino acid residues in the extracellular domains of human GnRH receptor [2]. They were shown to react with human GnRH receptor on the surface of some cancer cells and pituitary tissues by immunohistochemical staining, Western blot assay, and RT-PCR method [2]. In this study, additional Mabs were generated against extracellular domain peptides (N1-29) and characterized with respect to their specificity, affinity as well as functional activities to cancer cells. One of these Mabs, designated as GHR-106, was selected and reported with more detailed studies due to its high specificity and affinity to the extracellular domain (N1-29) of GnRH receptor, which is present in many normal and cancer tissues in humans [12]. Judging from the dose-dependent inhibition of GHR-106 binding to OC-3-VGH cancer cells by GnRH, it can be concluded that GHR-106 and GnRH compete for the same binding sites(s) localized on the extracellular domains of GnRH receptor (Fig. 2).
After the amino acid sequence of human GnRH receptor was elucidated through molecular cloning, the extracellular domains of this G-protein coupled receptor were deduced [30]. Specific Mabs against the extracellular domains of GnRH receptor have been developed and characterized previously by us and others [2, 22–25]. However, none was used to demonstrate their anti-proliferative effects on cancer cells. Even though polyclonal antibodies against selected synthetic peptides of the extracellular domains (N5-17) of mouse GnRH receptor were found to have similar effects [24, 25], the molecular target specificity of the raised antisera was never established. Therefore, our studies presented in this communication should be the first to demonstrate unequivocally the anti-proliferative effects on cancer cells in vitro by anti-GnRH receptor-specific Mabs, similar to those induced by numerous GnRH analogs currently used clinically. However, compared to decapeptide GnRH analogs, the antibodies generally have a much longer half life in circulations [31].
Similar to GnRH or its analogs, GHR-106 was found to induce apoptosis in cultured cancer cells in vitro, when analyzed by TUNEL assay (Fig. 4a). The anti-proliferative effect of GHR-106 on the cultured cancer cells was also demonstrated by MTT assay. Both GnRH and GHR-106 showed significant growth inhibitions of cultured cancer cells (Fig. 4b). This experimental result was consistent with that of the TUNEL assay. Furthermore, significant dose-dependent inhibitions of cancer cell growth by GHR-106 and GnRH analog such as antide were also observed.
From our present studies, the inhibitory effects of anti-GnRH receptor Mabs on the growth of many different cancer cells were similar to those by GnRH I and its antagonist, antide. However, the mechanism of growth inhibitions to cancer cells by GnRH or its analogs is still unknown in spite of numerous previous investigations and hypotheses proposed by others [6–8, 12–19]. Most likely, the growth inhibitions of cancer cells are caused by the down regulation of epidermal growth factor and c-fos upon binding with GnRH receptor by GnRH analogs or by anti-GnRH receptor Mabs [32]. The work is in progress to explore this possibility in our laboratory.
Both GnRH and GHR-106 were shown to down regulate gene expressions of typical ribosomal proteins including P0, P1, P2 and L37, when GAPDH gene expression served as the internal control (Fig. 4c). Down regulations of gene expressions for selected ribosomal proteins have been reported previously by others [7]. However, our present results are not consistent with those reported by others [7]. In our study, all selected ribosomal markers were shown to be down-regulated when compared to that of GAPDH. Furthermore, both GnRH and GHR-106 revealed similar down-regulatory effects on all of the selected ribosomal proteins. This discrepancy could be due to the differences in cancer cell lines employed in the present study and the culture conditions.
When RT-PCR was used to study the gene expressions of GnRH receptor in many cancer cell lines in humans, only 1 out of 33 revealed little or no expression of GnRH receptor mRNA (Fig. 5; Table 1). The results of this analysis indicate that expressions of GnRH receptor in human cancer cell lines take place in a relatively high incidence and abundance with very few exceptions [12]. Furthermore, Western blot and IIF assay were performed to some cancer cell lines to indicate the consistency regarding the mRNA and protein expressions of GnRH receptor in this study.
Recently, it has been reported that different transcript forms of GnRH receptor I mRNA were identified in human benign prostatic hyperplasia [29]. The primers selected in our studies [2] covers the sequences from 1944 of Exon I and 2479 of Exon 2. So far, no variant forms were found either in pituitary tissues or in the cancer cell lines studied [2]. Although one cannot rule out the possibility of the expressions of different variant forms of GnRH receptor I mRNA, our study seem to indicate widespread expressions of GnRH receptor I among different human cancer cell lines.
Similar to GnRH and its analogs, GHR-103, GHR-106 and GHR-114 react specifically with the GnRH receptor localized on the surface of cancer cells. This could result in the induction of cellular apoptosis of cancer cells. However, GHR-103, GHR-106 and GHR-114 are Mabs and have a relatively longer half life in circulation when compared with that of GnRH or its analogs (days vs. hours) [31, 33]. Therefore, the humanized form of these antibodies may have advantages over the hormone or hormone analogs with respect to the therapeutic treatments of a variety of cancers in humans. Moreover, GHR Mabs may also behave as long acting GnRH analogs in hormonal actions, when fertility regulations or human reproductive functions are taken into considerations. The potential mechanism of anti-proliferative effect [4, 17] of GHR-103 and GHR-106 on different cancer cells are under investigations. Further in vivo studies are also in progress to assess these two potential therapeutic applications in humans.
Acknowledgments
The Industry R&D Fellowship (IRDF, #360647) from NSERC (Natural Sciences and Engineering Research Council of Canada) to BG was acknowledged. This project was supported in parts by Vancouver Biotech Ltd (#080201). The NSERC and IRAP support of the following co-op students and research assistants are also acknowledged: C. Zhang, D.Tsui, E. Laflamme, A. Spreeuw and K. Huang. Proof reading of this manuscript by I. Wang is acknowledged.
Conflict of interest statement
GL is co-founder of Vancouver Biotech Ltd.
Abbreviations
- ELISA
Enzyme-linked immunosorbent assay
- FITC
Fluorescein isothiocyanate
- GAPDH
Glyceraldehyde phosphate dehydrogenase
- GnRH I
Type I gonadotropin-releasing hormone (a decapeptide: pGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2)
- IIF
Indirect immunofluorescence
- Mab
Monoclonal antibody
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- p-GnRHR
A synthetic peptide corresponding to N1–29 amino acid residues in the extracellular domain of human GnRH receptor
- KLH
Keyhole limpet hemocyanin
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
References
- 1.Clayton RN. Gonadotropin-releasing hormone: its action and receptors. J Encrinol. 1989;120:11–19. doi: 10.1677/joe.0.1200011. [DOI] [PubMed] [Google Scholar]
- 2.Lee GCY, Ho J, Chow SN, Yasojima K, Schwab C, McGeer PL. Immunoidentification of gonadotropin releasing hormone receptor in human sperm, pituitary and cancer cells. Am J Reprod Immunol. 2000;44:170–177. doi: 10.1111/j.8755-8920.2000.440307.x. [DOI] [PubMed] [Google Scholar]
- 3.Chien CH, Chen CH, Lee CYG, Chang TC, Chen RJ, Chow SN. Detection of gonadotropin-releasing hormone receptor and its mRNA in primary human epithelial ovarian cancers. Int J Gynecol Cancer. 2004;14:451–458. doi: 10.1111/j.1048-891x.2004.014304.x. [DOI] [PubMed] [Google Scholar]
- 4.Kang SK, Choi K-C, Yang H-S, Leung PCK. Potential role of gonadotrophin-releasing hormone (GnRH)-I and GnRH-II in the ovary and ovarian cancer. Endocr Relat Cancer. 2003;10:169–177. doi: 10.1677/erc.0.0100169. [DOI] [PubMed] [Google Scholar]
- 5.Choi J-H, Gilks CB, Auersperg N, Leung PCK. Immunolocalization of gonadotropin-releasing hormone GnRH-I, GnRH-II, and Type I GnRH receptor during follicular development in the human ovary. J Clin Endocrinol Metab. 2006;91:4562–4570. doi: 10.1210/jc.2006-1147. [DOI] [PubMed] [Google Scholar]
- 6.So W-K, Cheng J-C, Poon S-L, Leung PCK. Gonadotropin-releasing hormone and ovarian cancer: a functional and mechanistic overview. FEBS J. 2008;275:5496–5511. doi: 10.1111/j.1742-4658.2008.06679.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen A, Kaganovsky E, Rahimipour S, Ben-Aroya N, Okon E, Koch Y. Two forms of gonadotropin-releasing hormone (GnRH) are expressed in human breast tissue and overexpressed in breast cancer: a putative mechanism for the antiproliferative effect of GnRH by down-regulation of acidic ribosomal phosphoproteins P1 and P2. Cancer Res. 2002;62:1036–1044. [PubMed] [Google Scholar]
- 8.Pati D, Habibi HR. Inhibition of human heptocarcinoma cell proliferation by mammalian and fish gonadotropin releasing hormones. Endocrin. 1995;136:75–84. doi: 10.1210/en.136.1.75. [DOI] [PubMed] [Google Scholar]
- 9.Choi KC, Auersperg N, Leung PCK. Expression and anti-proliferative effect of a second form of gonadotropin-releasing hormone in normal and neoplastic ovarian surface epithelial cells. J Clin Endocrinol Metab. 2001;86:5075–5078. doi: 10.1210/jc.86.10.5075. [DOI] [PubMed] [Google Scholar]
- 10.Marelli MM, Moretti RM, Mai S, Januszkiewicz-Caulier J, Motta M, Limonta P. Type I gonadotropin-releasing hormone receptor mediates the antiproliferative effects of GnRH-II on prostate cancer cells. J Clin Endocrinol Metab. 2009;94:1761–1767. doi: 10.1210/jc.2008-1741. [DOI] [PubMed] [Google Scholar]
- 11.Tsai N-M, Hsieh R-H, Au H-K, Shieh M-J, Huang S-Y, Tzeng C-R. Effects of gonadotrophin-releasing hormone agonist on apoptosis of granulose cells. Ann N Y Acad Sci. 2005;1042:531–537. doi: 10.1196/annals.1338.065. [DOI] [PubMed] [Google Scholar]
- 12.Nagy A, Schally AV. Targeting of cytotoxic luteinizing hormone-releasing hormone analogs to breast, ovarian, endometrial, and prostate cancers. Biol Reprod. 2005;73:851–859. doi: 10.1095/biolreprod.105.043489. [DOI] [PubMed] [Google Scholar]
- 13.Szepeshazi K, Schally AV, Treszi A, Seitz S, Halmos G. Therapy of experimental hepatic cancers with cytotoxic peptide analogs targeted to receptors for luteinizing hormone-releasing hormone, somatostatin or bombesin. Anti Cancer Drugs. 2008;19:349–358. doi: 10.1097/CAD.0b013e3282f9adce. [DOI] [PubMed] [Google Scholar]
- 14.Stangelberger A, Schally AV, Djavan B. New treatment approaches for prostate cancer based on peptide analogues. Eur Urol. 2008;53:890–900. doi: 10.1016/j.eururo.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Emons G, Gruendker C, Guenthert AR, Westphalen S, Kavanagh J, Verschraegen C. GnRH antagonists in the treatment of gynecological and breast cancers. Endocr Relat Cancer. 2003;10:291–299. doi: 10.1677/erc.0.0100291. [DOI] [PubMed] [Google Scholar]
- 16.Gnananpragasam VJ, Darby S, Khan MM, Lock WG, Robson CN, Leung HY. Evidence that prostate gonadotropin-releasing hormone receptors mediate an anti-tumourigenic response to analogue therapy in hormone refractory prostate cancer. J Pathol. 2005;206:205–213. doi: 10.1002/path.1767. [DOI] [PubMed] [Google Scholar]
- 17.Choi J-H, Choi K-C, Auersperg N, Leung PCK. Differential regulation of two forms of gonadotropin-releasing hormone messenger ribonucleic acid by gonadotropins in human immortalized ovarian surface epithelium and ovarian cancer cells. Endocr Relat Cancer. 2006;13:641–651. doi: 10.1677/erc.1.01057. [DOI] [PubMed] [Google Scholar]
- 18.Weckermann D, Harzmann R. Hormone therapy in prostate cancer: LHRH antagonists versus LHRH analogues. Eur Urol. 2004;46:279–284. doi: 10.1016/j.eururo.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Huhtaniemi L, White R, McArdle CA, Persson B-E. Will GnRH antagonists improve prostate cancer treatment? Trends Endocrinol Met. 2008;20:43–50. doi: 10.1016/j.tem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Reichert J, Pavlou A. Monoclonal antibodies market. Nat Rev Drug Discov. 2004;3:383–384. doi: 10.1038/nrd1386. [DOI] [PubMed] [Google Scholar]
- 21.Takimoto CH, Calvo E (2008) Principles of oncologic pharmacotherapy. In: Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (eds) Cancer management: a multidisciplinary approach, 11th edn. http://www.cancernetwork.com/web/10165. Accessed April 2009
- 22.Karande AA, Rajeshwari K, Schol DJ, Hilgers JHM. Establishment of immunological probes to study human gonadotropin-releasing hormone receptors. Mol Cell Endocrinol. 1995;114:51–56. doi: 10.1016/0303-7207(95)03641-J. [DOI] [PubMed] [Google Scholar]
- 23.Rajeshwari K, Karande AA. Molecular mimicry by antidiotypic monoclonal antibody to gonadotropin releasing hormone. Immunol Invest. 1999;28:103–114. doi: 10.3109/08820139909061140. [DOI] [PubMed] [Google Scholar]
- 24.Asirvatham AL, Johnson GA, Belden EL, Van Kirk EA, Moss GE, Murdoch WJ. Immunization of mice against a synthetic N-terminal extracellular domain Gonadotropin-releasing hormone receptor peptide: evidence for a direct uterine effect. Am J Reprod Immunol. 1994;32:95–100. doi: 10.1111/j.1600-0897.1994.tb01099.x. [DOI] [PubMed] [Google Scholar]
- 25.Ackerman RC, Johnson GA, Van Kirk EA, Asirvatham AL, Murdoch WJ. Induction of apoptotic or lytic death in an ovarian adenocarcinoma cell line by antibodies generated against a synthetic N-terminal extracellular domain gonadotropin-releasing hormone receptor peptide. Cancer Lett. 1994;81:177–184. doi: 10.1016/0304-3835(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 26.Lee CYG, Chen KW, Sheu FS, Tsang A, Chao KC, Ng HT. Studies of a tumor- associated antigen, COX-1, recognized by a monoclonal antibody. Cancer Immunol Immunother. 1992;35:19–26. doi: 10.1007/BF01741050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CYG, Wong E, Richter DE, Menge AL. Monoclonal antibodies to human sperm antigens II. J Reprod Immunol. 1984;6:227–238. doi: 10.1016/0165-0378(84)90011-1. [DOI] [PubMed] [Google Scholar]
- 28.Freed KA, Brennecke SP, Moses EK. Gene expression of the constant region of the heavy chain of immunoglobulin G is down-regulated in human decidua in association with preeclampsia. J Reprod Immunol. 2005;68:105–120. doi: 10.1016/j.jri.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Rozsa B, Juhasz A, Treszl A, Toth G, Flasko T, Dezso B, Block N, Schally A, Halmos G. Expression of mRNA for human type-I LHRH receptor transcript forms in human benign prostatic hyperplasia. Int J Oncol. 2009;35:1053–1059. doi: 10.3892/ijo_00000420. [DOI] [PubMed] [Google Scholar]
- 30.Gruendker C, Voelker P, Schulz K-D, Emons G. Luteinizing hormone-releasing hormone agonist triptorelin and antagonist cetrorelix inhibit EGF-induced c-fos expression in human gynecological cancers. Gynecol Oncol. 2000;78:194–202. doi: 10.1006/gyno.2000.5863. [DOI] [PubMed] [Google Scholar]
- 31.Chi L, Zhou W, Golembo M, Illing N, Millar RP, Sealfon SC. Cloning and characterization of the human GnRH receptor. Mol Cell Endocrinol. 1993;91:R1–R6. doi: 10.1016/0303-7207(93)90278-R. [DOI] [PubMed] [Google Scholar]
- 32.Margolin K, Gordon MS, Holmgran E, Gaudreault J, Novotny W, Fyfe G, Adelman D, Stalter S, Breed J. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19:851–856. doi: 10.1200/JCO.2001.19.3.851. [DOI] [PubMed] [Google Scholar]
- 33.Periti P, Mazzei T, Mini E. Clinical pharmacokinetics of depot Leuprorelin. Clin Pharmacokin. 2002;41:485–504. doi: 10.2165/00003088-200241070-00003. [DOI] [PubMed] [Google Scholar]