Abstract
Background and objectives: Membranoproliferative glomerulonephritis (MPGN) is an immune complex–mediated glomerulonephritis characterized by subendothelial and mesangial deposition of immune complexes. Autoimmune diseases and chronic infections, such as hepatitis C, are commonly recognized causes of MPGN; however, monoclonal gammopathy is a less widely recognized cause of MPGN.
Design, setting, participants, & measurements: We reviewed all renal biopsies of MPGN in Mayo Clinic patients during a 6-year period to determine the association of monoclonal gammopathy with MPGN. Results were correlated with electrophoresis studies and bone marrow biopsies to clarify the relationship between MPGN and gammopathies.
Results: Of 126 patients with MPGN, 20 did not have workup for hepatitis B or C. Of the remaining 106 patients, 25 (23.5%) were positive for hepatitis B or C. Of the 81 hepatitis-negative patients, 13 were not evaluated for gammopathies. Of the remaining 68 patients, 28 (41.1%) had serum and/or urine electrophoresis studies positive for monoclonal gammopathy. Serum immunofixation electrophoresis was the most sensitive method for diagnosing monoclonal gammopathy. Renal biopsy showed a membranoproliferative pattern of injury; immunofluorescence microscopy was often instrumental in diagnosing the underlying gammopathy. On the basis of the bone marrow biopsy, monoclonal gammopathy of undetermined significance was the most common entity associated with MPGN. Other, less common causes included multiple myeloma, low-grade B cell lymphoma, and chronic lymphocytic leukemia.
Conclusions: Monoclonal gammopathy is an important and common cause of MPGN; therefore, all patients with a diagnosis of MPGN should be evaluated for an underlying monoclonal gammopathy.
Membranoproliferative glomerulonephritis (MPGN) is an immune complex–mediated glomerulonephritis characterized by subendothelial and mesangial deposition of immune complexes. These complexes typically trigger activation of complement and a phase of acute injury in the glomerular capillaries and mesangium. The acute injury phase is followed by an inflammatory (cellular) phase with influx of inflammatory cells and proliferative glomerular changes, which evolves into a reparative phase in which new basement membranes are formed along capillary walls and in the mesangium, resulting in double contours and mesangial expansion, respectively (1,2).
On the basis of etiology, MPGN is classified as primary/idiopathic or secondary. Primary/idiopathic MPGN includes immune complex–mediated glomerulonephritis MPGN types I and III and has been the subject of reviews (3). MPGN type II, also known as dense-deposit disease, is not due to immune complex deposition but results from the dysregulation of the alternative pathway of the complement cascade and secondary persistent complement activation (4). Secondary MPGN is most commonly caused by an antecedent hepatitis B or C viral infection that results in persistent antigenemia with secondary antigen-antibody immune complex deposition in the glomerulus (5,6). Other chronic infectious causes include shunt nephritis, abscesses, and endocarditis (7–9). Autoimmune diseases such as systemic lupus erythematosus and occasionally Sjögren syndrome and rheumatoid arthritis are also associated with persistent circulating immune complexes and the consequent development of MPGN (10,11).
Less widely known, however, is the association of MPGN with monoclonal gammopathy. Monoclonal gammopathy is characterized by the proliferation of a single clone of Ig-producing lymphocytes or plasma cells that results in the circulation of monoclonal Igs. The clinical spectrum of diseases that is associated with monoclonal gammopathy includes monoclonal gammopathy of undetermined significance (MGUS), Waldenström macroglobulinemia, lymphoproliferative disorders, and multiple myeloma (MM) (12,13).
In the renal pathology service at the Mayo Clinic, we have noted an increasing number of cases of MPGN associated with monoclonal gammopathies. In this study, we analyzed renal biopsies of Mayo Clinic patients who had a diagnosis of MPGN during a 6-year period. Results were correlated with serum and urine electrophoresis studies and bone marrow biopsies to clarify the relationship between MPGN and monoclonal gammopathies.
Materials and Methods
Patient Selection and Renal Biopsy Evaluation
This study was conducted using a protocol approved by the institutional review board of the Mayo Clinic. To be eligible for this study, patients had to be seen at the Mayo Clinic and have a renal biopsy that showed MPGN. Each biopsy was studied by light microscopy, immunofluorescence, and electron microscopy (EM). Light microscopic examination included hematoxylin- and eosin-, trichrome-, periodic-acid Schiff–, and silver-stained sections; immunofluorescence studies were done with antibodies directed against IgG, IgA, IgM, C3, C1q, albumin, fibrinogen, and κ and λ light chains; and EM was included to resolve the presence of glomerular dense deposits.
Pertinent clinical and laboratory data were extracted from electronic databases and from the patient's medical record. All renal biopsies of MPGN associated with monoclonal gammopathies were retrieved, and light microscopy, immunofluorescence, and EM findings were reviewed again. Cases of light- or heavy-chain deposition disease and fibrillary and immunotactoid glomerulonephritis were excluded. Cases of MPGN from lupus and other autoimmune diseases were also excluded from the study as were cases of thrombotic angiopathy with an MPGN pattern of injury. It is likely that other causes of secondary MPGN, such as visceral abscesses, shunt infections, and endocarditis, may have been present in a small percentage of the hepatitis-negative and monoclonal gammopathy–negative MPGN cases.
Results
MPGN and Gammopathies
During the period of 2001 through 2006, a total of 126 cases of MPGN were diagnosed at Mayo Clinic. Twenty patients were not evaluated for possible hepatitis B or C virus infection and were excluded from the study. Of the remaining 106 patients, 25 (23.5%) were positive for hepatitis B or C or both (12 cases were positive for hepatitis B, 13 cases were positive for hepatitis C, and two cases were positive for both hepatitis B and C). The mean age of patients with hepatitis C and MPGN was 54 years. Their mean serum creatinine was 1.69 mg/dl, with an average proteinuria of 2.98 g/d. The mean C3 level was 52.6 mg/dl (normal 75 to 175 mg/dl), and mean C4 level was 18. 4 mg/dl (normal 14 to 40 mg/dl). Eight of the patients with hepatitis C showed positive results for cryoglobulins type II, and one showed type III cryoglobulin. Rheumatoid factor activity was present in six cases (studies were not done in five cases). Serum immunofixation electrophoresis studies showed that seven patients with hepatitis C had monoclonal IgM in the serum, and, of these, four also had polyclonal IgG in the serum. The clinical characteristics of patients with hepatitis C are shown in Table 1.
Table 1.
Clinical features of patients with MPGN and hepatitis C
| Patient | Age at time of SPEP (years) | Gender | SCr at Time of SPEP (mg/dl) | Proteinuria (g/24 h) | Serum Albumin (g/dl) | Urine Microscopy/hpf | SPEP | SIFE | UPEP | UIFE | RF | Cryo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | M | 3.8 | 7.2 | 3.5 | 4 to 10 RBCs, >25% DR, 3 to 10 WBCs, + for casts | Negative | Negative | ND | ND | ND | Type II |
| 2 | 51 | F | 0.8 | 0.1 | 3.4 | 1 to 20 RBCs, >25% DR, + for casts | Negative | Negative | ND | ND | 1370 | Type III |
| 3 | 71 | M | 1.3 | 3.9 | 3.3 | 3 to 40 RBCs, <25% DR later >25% DR, + for casts | Negative | Negative | Negative | Negative | ND | Type II |
| 4 | 56 | M | 1.5 | 0.4 | 3.1 | 4 to 10 RBCs, <25% DR, + for casts | Negative | Negative | Negative | Negative | <15 | Negative |
| 5 | 52 | M | 1.6 | 6.5 | 4.6 | 51 to 100 RBCs, >25% DR, + for casts | Negative | M IgM | Negative | M IgM κ | 272 | Negative |
| 6 | 50 | M | 1.5 | 3.0 | 2.8 | 4 to 10 RBCs, >25% DR, + for casts | Hypo | M IgM | Negative | Negative | ND | Negative |
| 7 | 52 | F | 2.1 | 1.7 | 4.6 | 11 to 20 RBCs, >25% DR, + for casts | Abnormal band in γ region | M IgM | ND | ND | 70 | Type II |
| 8 | 47 | M | 1.3 | 2.7 | 2.3 | 41 to 50 RBCs, <25% DR, + for casts | Negative | M IgM, P IgG | Negative | Negative | ND | Type II |
| 9 | 57 | M | 1.7 | 6.22 | 2.5 | 4 to 10 RBCs, >25% DR, + for casts | Polyclonal | M IgM, P IgG | ND | ND | ND | Type II |
| 10 | 46 | F | 5.4 | 3.6 | 4.1 | 4 to 10 RBCs, <25% DR | Polyclonal | Negative | ND | ND | ND | Type II |
| 11 | 55 | M | 1.6 | <0.2 | 2.7 | 41 to 50 RBCs, >25% DR, + for casts | Polyclonal | M IgM, P IgG | ND | ND | 347 | Type II |
| 12 | 48 | M | 1.5 | 3.9 | 4.1 | 4 to 10 RBCs, >25% DR + for casts | Polyclonal | Negative | ND | ND | 1170 | Type II |
| 13 | 53 | M | 1.6 | 0.6 | 3.0 | 51 to 100 RBCs, <25% DR, + for casts | Hypo | M IgM, P IgG | Negative | M IgM κ | ND | ND |
+ casts, positive for RBC casts; Cryo, cryoglobulins; DR, dysmorphic red cells; Hypo, hypogammaglobulinemia; M, monoclonal; ND, not done; P, polyclonal; RF, rheumatoid factor; SCr, serum creatinine; WBC, white blood cell.
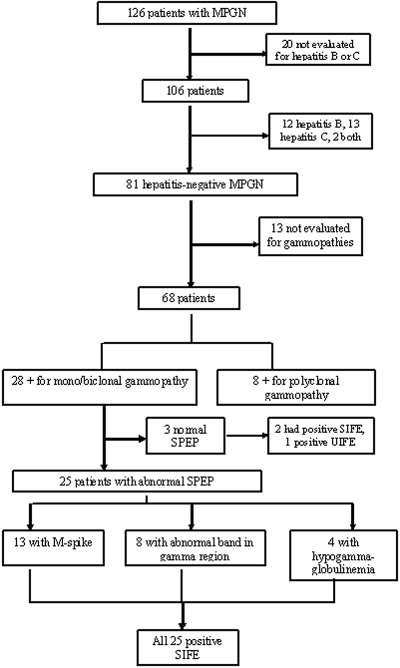
Among the 81 patients with hepatitis-negative MPGN, we next identified those who were positive for gammopathies on the basis of serum or urine protein electrophoresis and/or serum or urine immunofixation studies. Thirteen patients who were hepatitis negative had not been not evaluated for a gammopathy. Of the remaining 68 patients, 28 (41.1%) were positive for monoclonal/biclonal Igs and eight were positive for polyclonal Igs. The breakdown is shown in Figure 1.
Figure 1.
Summary of workup of patients with MPGN.
Clinical Characteristics of Hepatitis-Negative MPGN with Monoclonal Igs
There were 12 women and 16 men with monoclonal/biclonal Igs on electrophoresis and immunofixation studies. Mean age at time of diagnosis was 59.1 years (range 33 to 82 years), with all patients being older than 40 yr of age except for one 33-year-old woman, who had MM on bone marrow biopsy. Nearly all patients presented with renal insufficiency and significant proteinuria; most also had mild hypertension. The average serum creatinine was 2.49 mg/dl (range 1.0 to 6.4 mg/dl) with an average estimated creatinine clearance of 33.5 ml/min (range 6.0 to 62.0 ml/min). The mean C3 level was 72.3 mg/dl, and mean C4 level was 26.5 mg/dl, which was slightly higher than those seen in patients with hepatitis C–associated MPGN. In 12 (42.8%) cases, C3 levels were low; in 10 (35.7%) cases, it was within the normal range; and in six cases (21.5%), C3 levels were not done. In 11 (39.3%) cases, the C4 levels were below the normal range; in nine (32.1%) cases, C4 levels were within normal range; and in eight (28.6%) cases, C4 levels were not done. Average 24-hour urinary protein was 3.83 g/24 h (range 300 mg to 10 g/24 h). Urinalysis showed less than 4 red blood cells (RBCs)/high-power field (hpf) in three cases and 4 to 10 RBCs/hpf in four cases but >50 RBCs/hpf in the remaining cases. Dysmorphic RBCs were present in 23 patients, 17 of whom cases showed >25% dysmorphic cells. Cryoglobulins were positive in only three cases: Two showed type I cryoglobulin and one showed type II cryoglobulin (in seven cases, cryoglobulin studies were not done). The clinical features of MPGN cases associated with monoclonal and biclonal gammopathy are listed in Tables 2 and 3.
Table 2.
Clinical features of hepatitis-negative patients with MPGN and MGUS
| Patient | Age (years) | Gender | BP (mmHg) | SCr (mg/dl) | CrCl (ml/min) | Proteinuria (g/24 h) | SPEP | SIFE | UPEP | UIFE | Cryo | κ FLC, λ FLC, κ/λ Ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 77 | F | 140/65 | 2.0 | 31 | 2.4 | Abnormal fraction | λ light chains | Negative | λ light chains | Negative | 1.76, 4.13, 0.43 |
| 2 | 59 | M | 143/87 | 1.6 | 57 | 1.5 | M spike | IgM κ | Negative | IgM κ | Type I | ND |
| 3 | 54 | F | 179/77 | 3.1 | 20 | 4.3 | M spike | IgM κ | Negative | IgM κ | Negative | 8.02, 3.49, 2.30 |
| 4 | 66 | M | 173/94 | 3.6 | 22 | 1.4 | M spike | IgG κ | Restricted migration | IgG κ | ND | ND |
| 5 | 48 | F | 165/92 | 3.1 | 42 | 4.6 | M spike | IgM κ | Negative | IgM κ | Negative | 4.34, 3.39, 1.28 |
| 6 | 58 | F | 154/80 | 5.2 | 11 | 1.4 | Abnormal fraction | IgG κ | Negative | Negative | Negative | 10.30, 6.77, 1.52 |
| 7 | 42 | F | 142/93 | 1.4 | 43 | 3.5 | M spike | IgG λ | M spike | IgG λ | ND | ND |
| 8 | 68 | M | 150/78 | 6.4 | 10 | 0.83 | M spike | IgG λ | M spike | IgG λ | ND | ND |
| 9 | 64 | M | 150/81 | 3.7 | 17 | 1.5 | M spike | IgM κ | Negative | IgM κ | Negative | ND |
| 10 | 60 | M | 92/67 | 3.0 | 22 | 3.0 | Negative | IgM κ | Negative | Negative | Negative | ND |
| 11 | 54 | M | 160/80 | 2.8 | 25 | 10.3 | M spike | IgG κ | M spike | IgG κ | ND | 2.060, 2.180, 0.945 |
| 12 | 53 | F | 130/90 | 1.0 | 62 | 0.18 | Abnormal fraction | IgG κ | Negative | Negative | Negative | 0.99, 1.60, 0.62 |
| 13 | 42 | M | 153/84 | 2.4 | 38 | 9.8 | M spike | IgM κ | Negative | IgM κ | Negative | 2.83, 0.97, 2.97 |
| 14 | 42 | M | 160/101 | 1.7 | 47 | 1.0 | Abnormal fraction | IgG κ | Negative | κ | Negative | 57.50, 2.86, 20.10 |
| 15 | 56 | F | 145/84 | 1.8 | 29 | 0.55 | Abnormal fraction | IgM κ | Negative | IgM κ | Negative | 3.22, 1.88, 1.78 |
| 16 | 58 | M | 140/85 | 1.7 | 51 | 2.5 | Hypo | IgM κ | Negative | Negative | Negative | 1.61, 1.45, 1.11 |
CrCl, creatinine clearance; Cryo, cryoglobulins; FLC, free light chains (reference range κ FLC 0.33 to 1.94 mg/dl, λ FLC 0.57 to 2.63, κ/λ ratio 0.26 to 1.65); Hypo, hypogammaglobulinemia; ND, not done; SCr, serum creatinine.
Table 3.
Clinical features of hepatitis-negative patients with MPGN and lymphoproliferative disorders or MM
| Patient | Age (years) | Gender | BP (mmHg) | SCr (mg/dl) | Proteinuria (g/24 h) | CrCl (ml/min) | SPEP | SIFE | UPEP | UIFE | Cryo | κ FLC, λ FLC, κ/λ Ratio | Clinical Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17 | 68 | F | 174/79 | 1.5 | 4.5 | 44 | Hypo | IgG and IgM λ | Negative | IgG λ | Negative | 1.86, 1.81, 1.03 | LGBCL |
| 18 | 43 | F | 155/93 | 2.2 | 4.8 | 36 | Abnormal fraction | IgG λ | M spike | λ | ND | 0.670, 63.400, 0.010 | MM |
| 19 | 70 | M | – | 5.6 | 5.3 | 6 | Negative | IgM κ and IgM λ | Negative | Negative | Negative | ND | CLL |
| 20 | 82 | M | 156/88 | 1.9 | 10.2 | 44 | Abnormal fraction | IgM κ and IgG λ | ND | IgG λ | Negative | 4.53, 2.56, 1.77 | LGBCL |
| 21 | 75 | M | 150/60 | 2.4 | 4.084 | 25 | Abnormal fraction | IgM and IgG κ | ND | IgG κ | Type I (IgG κ) | 5.21, 1.73, 3.01 | CLL |
| 22 | 33 | F | 112/80 | 1.7 | 0.311 | 35 | Negative | Negative | Negative | IgG κ | ND | 45.30, 2.64, 17.20 | MM |
| 23 | 63 | M | 111/67 | 1.3 | 5.216 | 32 | M spike | Monoclonal IgG κ | M spike | IgG κ | ND | 12.80, 17.40, 0.74 | MM |
| 24 | 71 | M | 130/70 | 1.5 | 1.815 | 40 | M spike | Monoclonal IgM κ | Negative | κ | Negative | 16.70, 0.89, 18.80 | LPL |
| 25 | 54 | M | 130/78 | 1.5 | 8.2 | 52 | M spike | Monoclonal IgG κ | M spike | IgG κ | Negative | 157.00, 1.61, 97.50 | MM |
| 26 | 77 | F | 155/81 | 1.3 | 8.3 | 42 | Hypo | Monoclonal IgM κ | Negative | IgM κ | Type II | ND | LGBCL |
| 27 | 60 | M | 145/87 | 2.3 | 2.9 | 31 | M spike | Monoclonal IgG λ | Negative | IgG λ | Negative | 3.17, 5.55, 0.57 | MM |
| 28 | 61 | F | 121/59 | 2.2 | 3.1 | 24 | Hypo | Monoclonal IgG κ | Negative | Negative | Negative | 1.46, 1.10, 1.33 | MM |
CrCl, creatinine clearance; Cryo, cryoglobulins; FLC, free light chains (reference range κ FLC 0.33 to 1.94 mg/dl, λ FLC 0.57 to 2.63 mg/dl, κ/λ ratio 0.26 to 1.65); Hypo, hypogammaglobulinemia; ND, not done, SCr, serum creatinine.
Serum/Urine Electrophoresis and Serum/Urine Immunofixation Results of Hepatitis-Negative MPGN Cases with Monoclonal Gammopathy
Serum protein electrophoresis (SPEP) was abnormal in 25 of 28 patients. Thirteen patients had M spikes, eight showed abnormal (oligoclonal) bands in the γ region, and four showed hypogammaglobulinemia. Three cases were negative on SPEP studies. Urine electrophoresis (UPEP) was completed in 26 of the 28 patients and was abnormal in only eight patients: Seven patients showed M spikes, and one showed restricted migration in the γ region. Of the seven patients with M spikes on UPEP studies, six also had M spikes on SPEP studies, whereas the remaining case showed abnormal bands on SPEP studies. In contrast, seven patients with positive M spike on SPEP studies had negative UPEP studies.
Serum immunofixation electrophoresis (SIFE) studies were done on all 28 patients and was positive in 27 patients, all of whom had monoclonal/biclonal bands. There was only one negative case on SIFE studies. In addition, two of three patients with negative SPEP studies had monoclonal/biclonal bands on SIFE. Urine immunofixation electrophoresis (UIFE) studies were done on all 28 patients. Twenty-two patients showed monoclonal/biclonal bands, and six had negative UIFE studies. Of the single patient whose SPEP and SIFE studies were negative, UIFE studies showed monoclonal bands.
To summarize, of the 28 patients with monoclonal and/or biclonal gammopathy, 24 were positive for monoclonal gammopathies and four were positive for biclonal gammopathies. Of the 24 monoclonal gammopathies, 10 were positive for IgM κ, nine were positive for IgG κ, four were positive for IgG λ, and one had only λ light chains. No patient was positive for IgM λ. These findings are summarized in Tables 4 and 5.
Table 4.
Summary of SPEP, UPEP, SIFE, and UIFE results of hepatitis-negative patients with MPGN
| Parameter | SPEP | UPEP | SIFE | UIFE |
|---|---|---|---|---|
| M spike | 13 | 7 | ||
| Monoclonal/biclonal bands | 27 | 22 | ||
| Abnormal bands in γ region | 8 | Negative | ||
| Hypogammaglobulinemia | 4 | 1 | ||
| Negative | 3 | 18 | 1 | 6 |
| Total | 28 | 26 | 28 | 28 |
Table 5.
Summary of monoclonal/biclonal Igs of hepatitis-negative patients with MPGN
| Type of Igs | No. of cases (N = 28) |
|---|---|
| IgM κ | 10 |
| IgG λ | 4 |
| IgG κ | 9 |
| Biclonal | 4 |
| Light chain only | 1 |
Bone Marrow Biopsy Results of Hepatitis-Negative MPGN Cases with Monoclonal Gammopathy
Bone marrow biopsy was done on all 28 hepatitis-negative cases that showed monoclonal or biclonal bands on electrophoresis studies. Of the 28 cases, 16 were classified as monoclonal gammopathy of undetermined significance (MGUS) on the basis of the definition of MGUS as the presence of an M protein on electrophoresis studies in a patient with a marrow biopsy showing <10% plasma cells and with no evidence for a lymphoproliferative disorder or end organ damage (12,13). Two patients with MGUS subsequently converted to MM, and one patient with MGUS converted to chronic lymphocytic leukemia (CLL). Light chain (κ or λ) clonality was based on flow cytometry analysis. In eight cases of MGUS, plasma cells or lymphocytes showed light-chain restriction that correlated with the electrophoresis studies. Surprising, in one case, plasma cells showed κ light chain restriction that correlated with the SIFE results, but the lymphocytes showed λ light-chain restriction. In the remaining eight cases, light-chain restriction was not present (six cases) or the studies were not done (two cases).
Bone marrow biopsy of the remaining 12 cases showed the following results: Two cases showed CLL, one showed lymphoplasmacytic lymphoma (LPL)/Waldenström macroglobulinemia, three showed low-grade B cell lymphoma (LGBCL) not further classifiable, and six patients showed MM. In 11 cases, plasma cells and/or lymphocytes showed light-chain restriction that correlated with the electrophoresis studies. In the remaining one case, light-chain restriction was not evident. Bone marrow biopsy results are listed in Table 6.
Table 6.
Summary of bone marrow biopsy results of hepatitis-negative MPGN cases showing monoclonal bands on electrophoresis studies
| Patient | Cellularity | Plasma Cell % | PC Light Chain | Lymphoid Infiltrate | Lymphocyte Light Chain | % Lymphoid Involvement | Flow Cytometry | Clinical Diagnosis |
|---|---|---|---|---|---|---|---|---|
| 1 | Increased | 5 | κ | No | No | Negative | MGUS | |
| 2 | Normal | <5 | ND | No | ND | <5 | ND | MGUS |
| 3 | Normal | <5 | No | No | No | – | Negative | MGUS |
| 4 | Decreased | 8 | ND | No | ND | – | ND | MGUS |
| 5 | Normal | <5 | ND | No | κ | <5 | No | MGUS |
| 6 | Increased | 5 | No | No | No | – | Negative | MGUS |
| 7 | Normal | 5 | λ | No | ND | – | PC | MGUS |
| 8 | Normal | 5 to 10 | λ | No | No | – | PC | MGUS |
| 9 | Normal | <5 | κ | Yes | λ | <5 | PC, Ly | MGUS |
| 10 | Decreased | <5 | No | No | No | – | Negative | MGUS |
| 11 | Normal | <5 | No | No | No | – | Negative | MGUS |
| 12 | Normal | 5 to 10 | κ | No | No | – | PC | MGUS |
| 13 | Decreased | <5 | No | No | No | – | Negative | MGUS |
| 14 | Decreased | 5 to 10 | κ | No | No | – | PC | MGUS |
| 15 | Decreased | <5 | No | No | No | – | Negative | MGUS |
| 16 | Normal | <5 | No | Yes | κ | 30 | Ly | MGUS |
| 17 | Normal | <5 | No | Yes | λ | <5 | Ly | LGBCL |
| 18 | Normal | 15 | λ | No | ND | – | PC | MM |
| 19 | Increased | <5 | No | Yes | κ | 30 | Ly | CLL |
| 20 | Increased | <5 | No | Yes | κ | 20 to 30 | Ly | LGBCL |
| 21 | Increased | <5 | No | Yes | κ | 80 | Ly | CLL |
| 22 | Increased | 12 | κ | No | No | – | PC | MM |
| 23 | Normal | 10 to 15 | κ | No | ND | – | PC | MM |
| 24 | Normal | 5 | κ | Yes | κ | 10 | PC, Ly | LPL |
| 25 | Normal | 20 | κ | No | No | – | PC | MM |
| 26 | Increased | <5 | No | Yes | κ | 5 to 10 | Ly | LGBCL |
| 27 | Normal | 10 | λ | No | No | – | PC | MM |
| 28 | Increased | 70 to 80 | κ | No | No | – | PC | MM |
CMPD, chronic myeloproliferative disease; Ly, lymphocytes; ND, not done; PC, plasma cell.
Renal Biopsy Results of MPGN Cases with Gammopathy
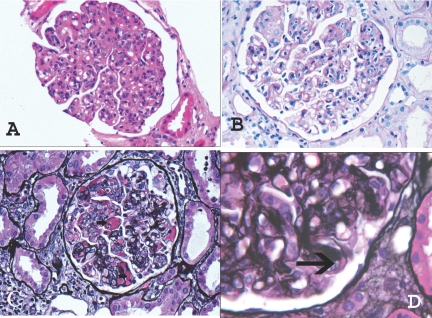
Light microscopic examination of renal biopsies of the 28 patients who had MPGN with monoclonal or biclonal gammopathy showed a membranoproliferative pattern of injury. The glomeruli appeared enlarged and hypercellular, with an expanded mesangium that showed an increase in matrix material and cellularity, the latter mostly as a result of mononuclear cells. Glomerular basement membranes were thickened, and many capillary loops showed subendothelial expansion with cellular elements, eosinophilic deposits, and new basement membrane formation resulting in double contours. These changes were easily appreciated on periodic-acid Schiff and silver stains (Figure 2). Many capillary loops showed prominent endocapillary proliferation with mononuclear cells and neutrophils that resulted in a distinctly lobular accentuation of the glomerular tufts. Small cellular crescents were noted in three of 28 biopsies, and features suggestive of cryoglobulinemia such as eosinophilic material in capillary lumina (immune microthrombi) were found in four biopsies of MPGN associated with MGUS, one case of MPGN associated LGBCL, and one case of LPL. Focal global glomerulosclerosis was present in nearly all biopsies, with the amount of sclerosis ranging from 0 to 90% with a mean of 15%. The interstitium showed varying degrees of tubular atrophy and interstitial fibrosis that ranged from 0 to 100%, with a mean of 20%. Arteries and arterioles often showed mild to moderate sclerosis.
Figure 2.
Representative light microscopy showing MPGN. (A) Hematoxylin- and eosin-stained section showing a hypercellular glomerulus with lobular accentuation of the glomerular tufts. (B) Periodic-acid Schiff–stained section showing mesangial expansion, endocapillary proliferation, and thickened glomerular basement membranes. (C) Silver stain showing cryoglobulins (arrow) in the lumen and immune deposits along the capillary walls. (D) Silver stain showing double contours (arrow) along the capillary wall. Magnifications: ×40 in A through C; ×60 in D.
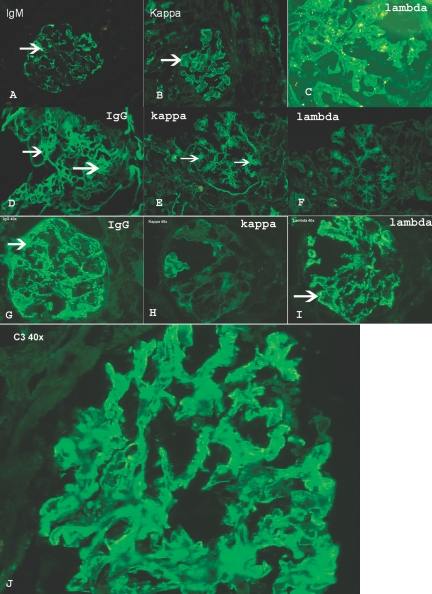
Immunofluorescence microscopy showed granular immune deposits in the mesangium and/or along the capillary walls in 20 biopsies (Figure 3) and correlated with immunofixation electrophoresis results (in two cases, IgG was noted in the mesangium and along capillary walls, but light-chain restriction was not documented). In most biopsies, the deposits were more prominent along the capillary walls than in the mesangium, whereas in few others the reverse was true. Three biopsies did not contain glomeruli or contained only globally sclerotic glomeruli, and in five biopsies significant immune deposits were not noted; however, three of the five negative cases showed C3 along the capillary walls. Tubular deposits of IgG or IgM were not present.
Figure 3.
Immunofluorescence microscopy. Each panel represents one case. (A) Granular capillary wall staining for IgM. (B) Negative staining for λ light chains. (C) Positive staining for κ light chains. (D) Mesangial staining for IgG. (E) Positive mesangial staining for κ light chains. (F) Negative staining for λ light chains. (G) Granular capillary wall staining for IgG. (H) Negative staining for κ light chains. (I) Positive staining for λ light chains. (J) C3 staining along capillary walls.
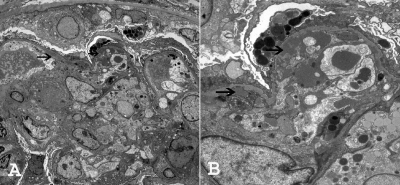
EM was performed in 26 of 28 cases; in one case, tissue that was submitted for EM studies contained only sclerosed glomeruli, and in one case glomeruli were not present. Ultrastructural examination showed thickening of the capillary walls with subendothelial deposits in all 26 cases (Figure 4). Cellular interposition and new basement membrane formation with double contours were also present. The deposits were granular, and substructures were typically absent. In four biopsies, scattered subepithelial deposits could be identified. The mesangium also contained electron-dense deposits in 21 biopsies. Podocytes showed segmental effacement of the foot processes, and many of the capillary loops showed leukocyte infiltration. Tubuloreticular structures were absent in the endothelial cells. Tubular deposits were not present. Renal biopsy findings are summarized in Table 7.
Figure 4.
EM ultrastructural studies show subendothelial deposits (arrows), with new basement formation resulting in double contours. Magnifications: ×1850 in A; ×5800 in B.
Table 7.
Renal biopsy findings of hepatitis-negative patients with MPGN and monoclonal gammopathy
| Patient | Pattern of Glomerular Injury | Total Glomeruli/Globally Sclerosed Glomeruli | Tubulointerstitial Scarring (%) | Immunofluorescence Studies | EM | Clinical Diagnosis |
|---|---|---|---|---|---|---|
| 1 | MPGN | 18/1 | 10 | IgM, λ | DC, SU, Mes. | MGUS |
| 2 | MPGN | 16/0 | 10 | Negative, C3 | DC, SU, Mes | MGUS |
| 3 | MPGN | 17/2 | 30 | IgM, κ | DC, SU | MGUS |
| 4 | MPGN | 6/1 | 20 | IgM, κ | DC, SU, Mes | MGUS |
| 5 | MPGN | 6/1 | 0 | IgM, κ | DC, SU, Mes | MGUS |
| 6 | MPGN | 16/0 | 10 | Negative, C3 | DC, SU, Mes | MGUS |
| 7 | MPGN | 26/0 | 10 | IgG, λ | DC, SU, Mes | MGUS |
| 8 | MPGN | 21/18 | 100 | Global sclerosis | DC, SU, Mes | MGUS |
| 9 | MPGN | 8/1 | 0 | IgM, κ | DC, SU, Mes | MGUS |
| 10 | MPGN | 33/5 | 20 | IgM, κ | DC, SU, Mes | MGUS |
| 11 | MPGN | 8/0 | 10 | IgG, κ | DC, SU, Mes | MGUS |
| 12 | MPGN | 19/4 | 10 | IgG, κ | DC, SU, Mes | MGUS |
| 13 | MPGN | 18/0 | 0 | No glomeruli | DC, SU, Mes | MGUS |
| 14 | MPGN | 16/0 | 5 to 10 | IgG | DC, SU, Mes | MGUS |
| 15 | MPGN | 13/2 | 20 | IgM, κ | SU, Mes | MGUS |
| 16 | MPGN | 13/2 | 10 | IgM, κ | No glomeruli | MGUS |
| 17 | MPGN | 15/2 | 5 | IgG, IgM, λ | DC, SU, SE, Mes | LGBCL |
| 18 | MPGN | 18/2 | 10 | Negative | DC, SU | MM |
| 19 | MPGN | 10/7 | 90 | IgM, κ | ND | CLL |
| 20 | MPGN | 6/1 | 15 to 20 | IgM, κ | DC, SU, Mes | LGBCL |
| 21 | MPGN | 9/3 | 25 | C3 | DC, SU, Mes | CLL |
| 22 | MPGN | 4/0 | 0 | Negative | DC, SU, Mes | MM |
| 23 | MPGN | 16/10 | 50 | IgG, κ | DC, SU, Mes | MM |
| 24 | MPGN | 5/0 | 0 | IgM, κ | DC, SU, Mes | LPL |
| 25 | MPGN | 14/5 | 50 | IgG | DC, SU | MM |
| 26 | MPGN | 20/2 | 20 | IgM, κ | DC, SU | LGBCL |
| 27 | MPGN | 4/0 | 10 | No glomeruli | DC, SU | MM |
| 28 | MPGN | 14/2 | 0 | IgG, κ | DC, SU | MM |
DC, double contours; Mes, mesangial electron-dense deposits; SE, subepithelial electron-dense deposits; SU, subendothelial electron-dense deposits.
Treatment and Follow-up of Monoclonal/Biclonal Gammopathy–Associated MPGN
Treatment of the 16 cases of MGUS was as follows: Three patients were treated with prednisone and cyclophosphamide; three patients were treated with prednisone and rituximab (see next paragraph); one patient was treated with prednisone alone; one patient was treated with prednisone and mycophenolate mofetil (MMF); one patient was treated with MMF alone; one patient was treated with dexamethasone; one patient was treated with prednisone, MMF, cyclosporine, and rituximab (see next paragraph); and five patients did not get any specific treatment. Treatment of patients with MM included melphalan, dexamethasone, cyclophosphamide, lenalidomide, and stem cell transplantation. Treatment for patients with lymphoproliferative diseases included prednisone and MMF, prednisone and cyclophosphamide, and rituximab.
Three patients with MGUS (3, 13, and 15) were treated with prednisone and rituximab. In these patients, proteinuria decreased from 4.3, 9.8, and 0.5 g/24 h at baseline to <0.3 g/24 h in all patients. Baseline serum creatinine improved from 3.1 to 1.3 mg/dl in patient 3, from 2.4 o 1.4 mg/dl in patient 13, and from 1.8 to 1.1 mg/dl in patient 15. One patient was treated with prednisone for 6 months followed by MMF for 12 months without a response. The patient was then started on cyclosporine, but, because of adverse effects, it was discontinued after 3 weeks of treatment. The patient was then treated with rituximab. Proteinuria decreased from a baseline of 10 to 4 g/24 h 5 months after rituximab treatment. Serum creatinine decreased from 1.6 to 1.4 mg/dl during the same period. The patient was subsequently lost to follow-up.
Follow-up at 2 Years
Renal function of the 16 patients with MGUS was as follows: The creatinine was stable in six patients (serum creatinine range 1.2 to 1.7 mg/dl), two patients had gradual decline of renal function (serum creatinine 2.8 and 3.2 mg/dl), two patients were on hemodialysis within months of the renal biopsy, and follow-up was not available for six patients.
Patients with MPGN and MM did not do well, except for two patient, one of whom received a stem cell transplant followed by a kidney transplant (serum creatinine 1.7 mg/dl), and one of whom had MGUS that converted to MM (serum creatinine 1.8 mg/dl). Of the remaining four patients, three died and one had a serum creatinine of 3.8 mg/dl and was subsequently lost to follow-up.
Of the six patients with MPGN and lymphoproliferative disorders, two had stable renal function, one had a gradual decline of renal function (creatinine 2.7 mg/dl), one died, and two were lost to follow-up. Of the two patients with stable renal function, one had LPL and the second had LGBCL.
Discussion
Monoclonal gammopathy is defined as an excessive secretion of Ig (whole molecule or subunits) that results from abnormal clonal proliferation of plasma cells or B lymphocytes. Dysproteinemia and plasma cell dyscrasia are alternative terms used for monoclonal gammopathy. The majority of kidney diseases in monoclonal gammopathy are secondary to deposition of light chains (κ or λ) and not heavy chains or intact Igs (12). These include myeloma kidney (cast nephropathy), AL amyloidosis, and light-chain deposition disease (13). The spectrum of renal lesions associated with monoclonal gammopathy is extensive (14) and depends on the physiochemical properties of the Ig produced. Of the 2603 native renal biopsies done at the Mayo Clinic from 2001 through 2006, 239 showed AL amyloidosis (9.16%), 30 showed myeloma kidney (1.3%), 48 showed light-chain deposition disease (1.8%), and only one showed heavy-chain deposition disease. As discussed in the Results section, during this period, 126 (4.8%) cases showed MPGN, 28 (1.07%) of which were hepatitis negative and were associated with monoclonal gammopathy. It should be pointed out that although light-chain deposition with an MPGN pattern of injury is a recognized entity, MPGN secondary to intact monoclonal Igs is poorly recognized. We completed a retrospective study of renal biopsies diagnosed as MPGN during a 6-year period beginning in 2001. Of these cases, we considered the workup complete for 81 patients. In this cohort, we were surprised to find that monoclonal/biclonal gammopathies were more frequently diagnosed than hepatitis (28 [34.5%] versus 25 [30%] patients).
Light-chain deposition in the mesangium and along the glomerular and tubular basement membranes (light-chain deposition disease) is a well-documented cause of MPGN (15,16), and we found that deposition of monoclonal IgG or IgM (with κ or λ light-chain restriction) in the mesangium and along glomerular basement membranes resulted in a similar pattern of renal injury. Tubular deposits of IgG or IgM were not present. We hypothesize that deposition of monoclonal Ig in the mesangium and along the capillary walls activates complement to cause acute injury to the glomerular capillary walls and mesangium. Proliferative and reparative changes then develop. This pathologic process is supported by the frequent co-localization of C3 with the monoclonal Igs in the mesangium and along capillary walls (Figure 2J).
An important finding of this study is the association of MPGN with MGUS. We found that 16 of 28 patients with monoclonal/biclonal gammopathies were classified as having MGUS on bone marrow biopsy. The diagnosis of MGUS requires a serum monoclonal paraprotein band of <30 g/L, a bone marrow biopsy that shows <10% plasma cells, absence of lytic lesions, anemia and hypercalcemia, and absence of end organ damage. It is the most common plasma cell disorder recognized and is a potential precursor for MM (17–19). In one study, patients with MGUS often showed renal disease unrelated to the monoclonal Igs. The most common entities included diabetic glomerulosclerosis and FSGS (20); however, we now show that a small subset of patients with MGUS have renal involvement secondary to monoclonal gammopathy with development of MPGN. In light of these findings, we believe that in patients with MPGN, the monoclonal gammopathy should not be called of “unknown significance” and should be called “monoclonal gammopathy–related or monoclonal gammopathy–associated MPGN.”
The incidence of MGUS increases in the older population: It is present in 3% of people who are older than 50 years and in 5% who are older than 70 years (21). At the Mayo Clinic, 50% of patients with monoclonal gammopathy have MGUS, with IgG constituting the most common type of M protein (17). Why more patients with MGUS do not develop MPGN is unknown but may be related to the presence of a specific subtype of monoclonal Ig, such as IgG3 or the M protein value at diagnosis, because this is the most important predictor of progression to a plasma cell disorder (22).
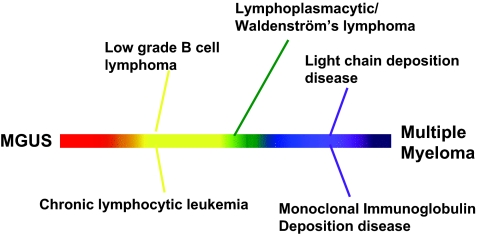
This study also shows that in addition to MGUS, MPGN with monoclonal gammopathy can be seen in the setting of other lymphoplasmacytic diseases, including LGBCL, CLL, and MM (Figure 5). Furthermore, MPGN may often be the first sign of the underlying lymphoplasmacytic disorder.
Figure 5.
Summary of plasma cell and lymphoproliferative disorders associated with MPGN.
SPEP is a sensitive and rapid screening method to detect the M protein but should always be followed by SIFE to confirm the presence or absence of M protein and determine the specific type of monoclonal Ig. SIFE will also detect small amounts of M protein that can be missed by SPEP (17,18,21). In this study, SIFE was the most sensitive test for the detection of monoclonal proteins. Cryoglobulins, both monoclonal and polyclonal, are also an important secondary cause of MPGN and were present in only three of 28 patients with monoclonal gammopathy. Thus, it is important to recognize that although cryoglobulin deposition in the glomeruli can result in MPGN, cryoglobulin formation is not at all essential for MPGN that results from monoclonal gammopathy.
These studies are also important because, for many patients, it was the renal biopsy, particularly the immunofluorescence findings, that dictated the subsequent evaluation for a gammopathy with electrophoresis studies, free light-chain assays, and a bone marrow biopsy. It is also important to recognize that although most biopsies showed capillary wall deposits, in a few cases, the deposits were more prominent in the mesangium (Figure 3, D, E, and F). In one interesting case, serum electrophoresis studies were negative even though the renal biopsy suggested MPGN secondary to monoclonal gammopathy. A few months after the biopsy, serum immunofixation results returned positive for a monoclonal gammopathy.
Nasr and colleagues (23,24) recently described an entity of proliferative glomerulonephritis associated with IgG deposition. In their study, the deposits were composed of monoclonal IgG3; the cases may belong to a subgroup of the monoclonal gammopathies (i.e., the monoclonal IgG deposits) that we have noted in our study. Circulating monoclonal proteins were identified in 30% of their cases. Bone marrow biopsy was performed in only 22 of 37 cases; only one patient showed MM. They did not ascribe the lesions to MGUS, lymphoproliferative disease, or MM. It is likely that few of our MPGN cases with monoclonal IgG belonged to the IgG3 subtype. Although IgG3 subtype may be present in some of the cases of monoclonal IgG deposits, we believe that capillary wall deposition of other monoclonal Igs including monoclonal IgM, other monoclonal IgG subtypes, and monoclonal IgA all can result in an MPGN pattern of injury. In our renal biopsy consultative practice at Mayo Clinic, we have seen a few cases of monoclonal IgA–related MPGN (these cases were not included in our study because they were not Mayo Clinic patients). To confirm that the deposits were monoclonal, we had repeated the immunofluorescence studies in many cases; as a result, most of the frozen tissue was used up. We could do the subtyping in only six of the 13 cases of monoclonal IgG deposits: In two cases the deposits stained for IgG1, one case was equivocal, and in three cases glomeruli were not present. Thus, it is important to recognize that although IgG3 monoclonal deposits can result in MPGN pattern of injury, other monoclonal deposits, including IgM, IgA, and other subtypes of IgG, all can result in MPGN.
Recognition of monoclonal gammopathy–associated MPGN is particularly important in the transplant setting. We recently assessed renal allograft protocol biopsies in patients with MPGN to determine the incidence and risk factors for recurrent disease. Patients with monoclonal gammopathy have a particularly high incidence of MPGN recurrence (66.7%) as compared with patients without monoclonal proteins (30%) (25). Recent data from our group also showed that kidney transplantation in patients with ESRD secondary to light-chain deposition disease or a monoclonal gammopathy with fibrillary deposits, both of which can be associated with an MPGN pattern of injury, are associated with a high recurrence rate, with the transplant biopsy also commonly showing an MPGN pattern of injury (26,27).
There is no standard treatment for patients with MPGN associated with a monoclonal gammopathy. Conservative as well as immunosuppressive therapy with the use of corticosteroids (alone or in combination with an alkylating agent), thalidomide, bortezomib (Velcade), MMF, cyclosporine, and rituximab all have been used in a small number of patients with variable outcomes (23,24). Prospective, controlled studies in a larger cohort of patients with MPGN and monoclonal gammopathy are needed to ascertain optimal therapy. At the present time, treatment decisions will have to be made purely on the basis of clinical experience.
Conclusions
Many patients with MPGN have an underlying monoclonal gammopathy. All renal biopsies should be analyzed with anti–light-chain antibodies to detect a possible underlying monoclonal gammopathy. Furthermore, it is imperative that patients with MPGN undergo a full workup for gammopathies, which should include serum and urine electrophoresis and immunofixation studies and free light-chain assays if conventional electrophoresis studies are negative. If positive, then a bone marrow biopsy including immunophenotyping studies and flow studies is warranted to determine whether the monoclonal gammopathy is due to MGUS or to underlying plasma cell myeloma or lymphoproliferative disorder. We also recommend judicious use of the term “idiopathic” MPGN because it is likely that an underlying cause can be found in almost every case of MPGN. Last, there is urgent need for standardized treatment for MPGN associated with monoclonal gammopathy that should focus on the underlying cause of the gammopathy (28).
Disclosures
None.
Acknowledgments
This research was supported in part by National Institutes of Health grant DK074409 to S.S. and R.J.H.S.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Smith KD, Alpers CE: Pathogenic mechanisms in membranoproliferative glomerulonephritis. Curr Opin Nephrol Hypertens 14: 396– 403, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Rennke H: Secondary membranoproliferative glomerulonephritis. Kidney Int 47: 643– 656, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Hewins PS, Smith RJH, Savage CO. Idiopathic membranoproliferative glomerulonephritis. In: Therapy in Nephrology and Hypertension, 3rd Ed., edited by Wilcox CS, Berl T, Himmelfarb J, Mitch WE, Murphy B, Salant DJ, Yu ASL.Philadelphia, Saunders/Elsevier, 2008, pp 249– 256 [Google Scholar]
- 4.Smith RJ, Alexander J, Barlow PN, Botto M, Cassavant TL, Cook HT, de Cordoba SR, Hageman GS, Jokiranta TS, Kimberling WJ, Lambris JD, Lanning LD, Levidiotis V, Licht C, Lutz HU, Meri S, Pickering MC, Quigg RJ, Rops AL, Salant DJ, Sethi S, Thurman JM, Tully HF, Tully SP, van der Vlag J, Walker PD, Wurzner R, Zipfel PF, Dense Deposit Disease Focus Group: New approaches to the treatment of dense deposit disease. J Am Soc Nephrol 18: 2447– 2456, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamabe H, Johnson RJ, Gretch DR, Fukushi K, Osawa H, Miyata M, Inuma H, Sasaki T, Kaizuka M, Tamura N: Hepatitis C virus infection and membranoproliferative glomerulonephritis in Japan. J Am Soc Nephrol 6: 220– 223, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Alpers CE, Smith KD: Cryoglobulinemia and renal disease. Curr Opin Nephrol Hypertens 17: 243– 249, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Hulton SA, Risdon RA, Dillon MJ: Mesangiocapillary glomerulonephritis associated with meningococcal meningitis, C3 nephritic factor and persistently low complement C3 and C5. Pediatr Nephrol 6: 239– 243, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Boseman P, Lewin M, Dillon J, Sethi S: Marfan syndrome, MPGN, and bacterial endocarditis. Am J Kidney Dis 51: 697– 701, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Vella J, Carmody M, Campbell E, Browne O, Doyle G, Donohoe J: Glomerulonephritis after ventriculo-atrial shunt. QJM 88: 911– 918, 1995 [PubMed] [Google Scholar]
- 10.Adam F, Torun D, Bolat F, Zumrutdal A, Sezer S, Ozdemir FN: Acute renal failure due to mesangial proliferative glomerulonephritis in a pregnant woman with primary Sjögren's syndrome. Clin Rheumatol 25: 75– 79, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi L-M, Makino H, Moura LA, Nagata M: The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 65: 521– 530, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Sanders P, Herrera G: Monoclonal immunoglobulin light chain-related renal diseases. Semin Nephrol 13: 324– 341, 1993 [PubMed] [Google Scholar]
- 13.Leung N, Rajkumar SV: Renal manifestations of plasma cell disorders. Am J Kidney Dis 50: 155– 165, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Audard V, Georges B, Vanhille P, Toly C, Deroure B, Fakhouri F, Cuvelier R, Belenfant X, Surin B, Aucouturier P, Mougenot B, Ronco P: Renal lesions associated with IgM-secreting monoclonal proliferations: Revisiting the disease spectrum. Clin J Am Soc Nephrol 3: 1339– 1349, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salant DJ, Sanchorawala V, D'Agati VD: A Case of atypical light chain deposition disease: Diagnosis and treatment. Clin J Am Soc Nephrol 2: 858– 867, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Mutluay R, Aki SZ, Erten Y, Konca C, Yagci M, Barit G, Sindel S: Membranoproliferative glomerulonephritis and light-chain nephropathy in association with chronic lymphocytic leukemia. Clin Nephrol 70: 527– 531, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, Dispenzieri A, Katzmann JA, Melton LJ, 3rd: Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 354: 1362– 1369, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, Melton LJ, 3rd: A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med 346: 564– 569, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Robert A. Kyle SV: Monoclonal gammopathy of undetermined significance. Br J Haematol 134: 573– 589, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Paisit P, Monica PR, Robert GH, Scott S, Fogo A: Monoclonal gammopathy: Significance and possible causality in renal disease. Am J Kidney Dis 42: 87– 95, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Kyle RA, Rajkumar SV: Monoclonal gammopathy of undetermined significance and smouldering multiple myeloma: Emphasis on risk factors for progression. Br J Haematol 139: 730– 743, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Rajkumar S, Kyle R, Therneau T, Clark R, Bradwell A, Melton J: Presence of monoclonal free light chains in the serum predicts risk of progression in monoclonal gammopathy of undetermined significance. Br J Haematol 127: 308– 310, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Nasr SH, Satoskar A, Markowitz GS, Valeri AM, Appel GB, Stokes MB, Nadasdy T, D'Agati VD: Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol 20: 2055– 2064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nasr SH, Markowitz GS, Stokes MB, Seshan SV, Valderrama E, Appel GB, Aucouturier P, D'Agati VD: Proliferative glomerulonephritis with monoclonal IgG deposits: A distinct entity mimicking immune-complex glomerulonephritis. Kidney Int 65: 85– 96, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Lorenz EC, Sethi S, Leung N, Dispenzieri A, Fervenza FC, Cosio FG: Recurrent membranoproliferative glomerulonephritis after kidney transplantation. Kidney Int February3, 2010. [ epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Czarnecki PG, Lager DJ, Leung N, Dispenzieri A, Cosio FG, Fervenza FC: Long-term outcome of kidney transplantation in patients with fibrillary glomerulonephritis or monoclonal gammopathy with fibrillary deposits. Kidney Int 75: 420– 427, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Leung N, Lager DJ, Gertz MA, Wilson K, Kanakiriya S, Fervenza FC: Long-term outcome of renal transplantation in light-chain deposition disease. Am J Kidney Dis 43: 147– 153, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Vilayur E, Trevillian P, Walsh M: Monoclonal gammopathy and glomerulopathy associated with chronic lymphocytic leukemia. Nat Clin Pract Nephrol 5: 54– 58, 2009 [DOI] [PubMed] [Google Scholar]