Abstract
Protein secretion from the endoplasmic reticulum (ER) requires the enzymatic activity of chaperones and oxidoreductases that fold polypeptides and form disulfide bonds within newly synthesized proteins. The best-characterized ER redox relay depends on the transfer of oxidizing equivalents from molecular oxygen through ER oxidoreductin 1 (Ero1) and protein disulfide isomerase to nascent polypeptides. The formation of disulfide bonds is, however, not the sole function of ER oxidoreductases, which are also important regulators of ER calcium homeostasis. Given the role of human Ero1α in the regulation of the calcium release by inositol 1,4,5-trisphosphate receptors during the onset of apoptosis, we hypothesized that Ero1α may have a redox-sensitive localization to specific domains of the ER. Our results show that within the ER, Ero1α is almost exclusively found on the mitochondria-associated membrane (MAM). The localization of Ero1α on the MAM is dependent on oxidizing conditions within the ER. Chemical reduction of the ER environment, but not ER stress in general leads to release of Ero1α from the MAM. In addition, the correct localization of Ero1α to the MAM also requires normoxic conditions, but not ongoing oxidative phosphorylation.
Keywords: Endoplasmic reticulum (ER), Mitochondria, Mitochondria-associated membrane (MAM), Oxidative protein folding, Ero1α
Introduction
The endoplasmic reticulum (ER) is the point of origin for protein secretion. To allow for this crucial function, the ER contains folding chaperones required for the production of secretion-competent, fully folded secretory proteins. For example, the hsp70 family member BiP/GRP78 is among the first chaperones to interact with hydrophobic surfaces of newly synthesized polypeptides. At the expense of ATP, Bip/GRP78 promotes the folding of these hydrophobic amino acids to the interior of the protein structure (Gething 1999; Hammond and Helenius 1994; Kim et al. 1992). The lectin chaperones calreticulin and calnexin also mediate the folding of newly synthesized proteins by cycling on and off their folding substrates, depending on the presence of a glucose linked to the high mannose sugar core (Caramelo and Parodi 2008; Michalak et al. 2009). Another important group of ER protein-folding enzymes is oxidoreductases that catalyze the formation of disulfide bonds. The best-known member of this group is protein disulfide isomerase (PDI), which recognizes unfolded hydrophobic amino acids on newly synthesized proteins (Freedman et al. 2002; Klappa et al. 1997). Following the docking with its substrates, PDI is able to oxidize reduced cysteines or isomerize pre-formed disulfide bonds through transient mixed disulfides with its CXXC active sites and substrates (Horibe et al. 2004; Walker and Gilbert 1995). A prerequisite for the catalytic activity of PDI is the presence of proteins of the Ero1 family within the ER (Cabibbo et al. 2000; Mezghrani et al. 2001; Sevier and Kaiser 2008). These oxidoreductases, represented by Ero1α and Ero1β in human cells, mediate the re-oxidation of PDI after its enzymatic reaction by transferring oxidative equivalents using their CXXCXXC active sites (Benham et al. 2000; Pagani et al. 2000). The oxidative power of Ero1 proteins originate from their flavin adenine dinucleotide (FAD) prosthetic group that transfers electrons from cysteines to molecular oxygen (Wang et al. 2009). Hypoxia, the absence of oxygen, impacts on the functioning of Ero1 proteins in two ways: First, Ero1α, but not Ero1β is a target of hypoxia-inducible factor (HIF-1α)-mediated transcription (Gess et al. 2003; May et al. 2005). Second, whereas this induction may result in the increased production of growth factors by tumor cells, in vitro experiments suggest that Ero1 proteins cannot promote PDI re-oxidation in the absence of oxygen (May et al. 2005; Tu and Weissman 2002). Another factor that impacts on the catalytic activity of Ero1 proteins is their correct localization to the ER. Both yeast and rice Ero1 proteins require association with the ER membrane for their functioning (Onda et al. 2009; Pagani et al. 2001). Human Ero1α requires the interaction with PDI and another oxidoreductase, ERp44, for its retention within the ER (Anelli et al. 2003; Otsu et al. 2006).
The ER is not a homogeneous organelle, but is composed of numerous domains, including the transitional ER that mediates ER export and the rough ER (rER) that houses the protein translation and import machinery (Voeltz et al. 2002). Besides the rER, the mitochondria-associated membrane (MAM) is most highly enriched in ER chaperones and oxidoreductases (Hayashi et al. 2009). The MAM is the location where phosphatidylserine biosynthesis and its transfer to mitochondria occur (Shiao et al. 1998; Stone and Vance 2000), but also contains calcium handling proteins, such as the inositol 1,4,5-trisphosphate receptor (IP3R) (Csordas et al. 1999; Hajnoczky et al. 2000). On the MAM, ER chaperones and oxidoreductases appear to regulate the ER calcium content. For instance, calnexin reversibly interacts with sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) 2b to block calcium import (Roderick et al. 2000). Similarly, calreticulin inhibits uptake of calcium by inhibiting the affinity for calcium of the SERCA2b pump, but also regulates IP3-induced calcium release (Camacho and Lechleiter 1995; John et al. 1998). In vivo, these functions of calreticulin may very well be more crucial for survival than its chaperone activity, since calreticulin-deficient cells have impaired Ca2+ homoeostasis, yet only a modest decrease in protein folding (Michalak et al. 2009; Molinari et al. 2004). Another example of a folding enzyme regulating ER calcium content is the oxidoreductase ERp44 that interacts with cysteines of the IP3R type 1, thereby inhibiting calcium transfer to mitochondria when ER conditions are reducing (Higo et al. 2005). Recent results suggest that Ero1α might also perform such a function, since Ero1α interacts with the IP3R and potentiates the release of calcium during ER stress (Li et al. 2009). This function of Ero1α could impact the interaction of the ER with mitochondria that depends on the formation of calcium hotspots on the cytoplasmic face of the ER (Yi et al. 2004) and the induction of apoptosis that critically depends on ER-mitochondria calcium communication (Mendes et al. 2005; Szalai et al. 1999). To increase our understanding of the functions of Ero1α during resting and stressed conditions, we examined its localization within the ER by subcellular fractionation and immunofluorescence microscopy. Our results show that Ero1α is highly enriched on the MAM, consistent with its role in the regulation of ER calcium homeostasis. Hypoxia leads to a rapid and eventually complete depletion of Ero1α from the MAM. Our findings suggest that hypoxic tumor cells that could display high levels of this oxidoreductase on their surface or in the space surrounding the tumor.
Materials and methods
Antibodies and reagents
All chemicals were from Sigma (Oakville, ON), except for tunicamycin and thapsigargin (Enzo Life Sciences, Farmingdale, NY). The rabbit anti-calnexin antiserum was generated using a cytosolic peptide by Open Biosystems, Huntsville, AL. Antibodies against GAPDH were provided by Tom Hobman, Edmonton, AB; the monoclonal antiserum against Ero1α was a gift from Roberto Sitia, Milan, Italy. Antibodies to the human proteins Ero1α (polyclonal rabbit, Santa Cruz, Santa Cruz, CA), PDI, calreticulin, actin (ABR/Pierce, Golden, CO), β-tubulin, BiP/GRP78 (Calbiochem, San Diego, CA), βCOP (Genetex, San Antonio, TX), mitochondrial complex 2 (Mitosciences, Eugene, OR), and the myc-tag (Millipore, Billerica, MA) were purchased as indicated. HEK 293 and HeLa cells were from ECACC (Porton Down, UK) and cultured in Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA). Percoll was from GE Healthcare (Piscataway, NJ); Optiprep was from Axis-Shield (Dundee, Scotland). Mitotracker was from Invitrogen (Carlsbad, CA).
Western blotting, immunofluorescence microscopy
Western blotting was performed according to standard protocols using goat-anti mouse/rabbit secondary antibodies conjugated to Alexafluor 680/750 (Invitrogen, Carlsbad, CA) on an Odyssey infrared imaging system (LICOR, Lincoln, NE). Processing of samples for immunofluorescence microscopy was performed as follows: HEK 293 cells were grown on coverslips for 24 h and preloaded with Mitotracker for 30 min according to the manufacturer's instructions. Cells were washed with phosphate-buffered saline (PBS) containing 1 mM CaCl2 and 0.5 mM MgCl2 (PBS++) and fixed with 4% paraformaldehyde for 20 min. After washing with PBS++, cells were permeabilized for 1 min with 0.1% Triton X-100, 0.2% bovine serum albumin in PBS++. Immunofluorescence preparations with the anti-βCOP antibody involved fixation by incubation in methanol for 2 min at −20°C. Cells were then incubated with primary antibodies (1:100 dilution for PDI monoclonal and Ero1α polyclonal) and secondary antibodies in PBS++, 0.2% bovine serum albumin (BSA) for 1 h each, interrupted with three washes using PBS++. Secondary antibodies were AlexaFluor-conjugated 350 and 488 (Invitrogen, Carlsbad, CA) and used at a dilution of 1:2,000. Samples were mounted in Prolong AntiFade (Invitrogen, Carlsbad, CA). Images were obtained with an Axiocam on an Axioobserver microscope (Carl Zeiss, Jena, Germany) using a 100× plan-Apochromat lens. All images were iteratively deconvolved using the Axiovision 4 software. Deconvolved images were enhanced with Photoshop (Adobe, San Jose, CA) using the levels functions only, until reaching saturation in the most intense areas of the image.
Membrane fractionation
Mitochondria and light membranes were separated as follows. HEK 293 cells were homogenized with 15 passages through a ball-bearing homogenizer (Isobiotec, Heidelberg, Germany, ball clearance 18 μM) in homogenization buffer (0.25 M sucrose, 10 mM HEPES–NaOH, pH 7.4, 1 mM EDTA, 1 mM ethyline glycol tetraacetic acid (EGTA), containing Complete protease inhibitor, Roche, Mississauga, ON). The resulting homogenates were centrifuged for 10 min at 800×g to remove unbroken cells and nuclei. Post-nuclear supernatants were centrifuged for 10 min at 10,000×g to yield heavy membrane fractions (HM). The supernatants were then centrifuged for 60 min at 100,000×g to separate the cytosolic fraction and light membrane fraction.
Membranes of the ER (MAM and rER) were fractionated on a continuous OPTIPREP gradient using 25%, 20%, 15%, 10%, and 5% OPTIPREP. HEK 293 and HeLa cells, treated as indicated, were homogenized as indicated above. For non-reducing conditions, 10 mM N-ethylmaleimide was added. Cell debris and nuclei were pelleted by centrifugation at 1,000×g for 10 min. The post-nuclear supernatant was overlayed onto the continuous gradient and centrifuged at 32,700 rpm for 3 h at 4°C in a SW55Ti rotor (Beckman Coulter, Mississauga, ON). Six equal fractions were collected from the top of the gradient and precipitated with acetone. Fractions were probed on a Western blot for the following markers of ER domains according to Myhill et al. 2008: calnexin (mostly MAM), acyl-CoA/cholesterol acyltransferase (ACAT1, MAM), PDI, calreticulin, BiP/GRP78 (pan-ER), ERp57, eIF2α (rER), and mitochondrial complex 2 (mitochondria). Markers of the Golgi and the plasma membrane have also been described previously (Myhill et al. 2008).
Mitochondria were separated from the MAM as follows: HEK 293 cells were grown to confluency on 15 20-cm dishes and homogenized using a ball-bearing homogenizer as above in 4 ml isolation buffer (250 mM mannitol, 5 mM HEPES, pH 7.4, 0.5 mM EGTA, 0.1% BSA). Debris and nuclei were removed by 5 min centrifugation at 600×g in 15 ml Corex tubes in a JA 20 rotor. The supernatant was centrifuged at 8,500 rpm in a JA-12 rotor for 10 min to pellet crude mitochondria. Subsequently, microsomes were pelleted at 100,000×g for 1 h in a TLA120.2 rotor. The previously isolated mitochondria were resuspended in 1 ml isolation medium and layered on top of 8.5 ml Percoll isolation medium (225 mM d-mannitol, 25 mM HEPES, pH 7.4, 1 mM EGTA, and 20% Percoll (v/v)) in a 10-ml ultraclear polycarbonate Beckman tube. The tube was centrifuged for 30 min at 30,500 rpm (95,000×g) in a Ti-70 rotor with slow acceleration and deceleration, after which purified mitochondria (3/4 down the tube) and MAM were removed from the Percoll gradient (located above the mitochondria). Percoll was removed from the mitochondria fraction and the MAM fraction was diluted 5-fold with fresh isolation medium and re-centrifuged at 60,000 rpm in a TLA120.2 rotor for 1 h. Equal proportional amounts were loaded for all fractions.
Secretion assays
For secretion assays, HEK 293 cells were grown to confluency in 6-well dishes (900,000 cells/well) for 24 h in DMEM/10%FBS. Subsequently, the medium was exchanged to OPTIMEM for the indicated times and the medium was precipitated using acetone.
Results
For most experiments, we used HEK 293 cells, which express high levels of Ero1α. Since over-expression of ER proteins can lead to their mis-localization within the expansive ER network (Registre et al. 2004), we decided to exclusively analyze the distribution of endogenous Ero1α. To test whether Ero1α localizes to a specific sub-domain of the ER, we first fractionated cellular membranes into heavy and light membranes (Fig. 1a). This fractionation protocol showed that the majority of the Ero1α signal was found on heavy membranes that comprise mitochondria, the rER and the MAM (Myhill et al. 2008). We next analyzed the distribution of Ero1α on a Percoll gradient, which can distinguish between MAM and mitochondria localization in liver tissue (Stone and Vance 2000) and cell lines (Wieckowski et al. 2009). On the Percoll gradient, membranes isolated as crude mitochondria are resolved into pure mitochondria that are largely devoid of ER markers and a MAM fraction that is enriched in calnexin and acyl-CoA/cholesterol acyltransferase 1 (ACAT1, Fig. 1b, (Myhill et al. 2008; Rusinol et al. 1994). Conversely, mitochondrial complex 2 is found in the crude and pure mitochondria fraction, but not the MAM fraction (Fig. 1b). This fractionation showed that Ero1α was present virtually exclusively on the MAM in HEK 293 cells (Fig. 1b). We complemented this analysis with our MAM isolation protocol based on Optiprep that separates the MAM from other domains of the ER, such as the rER and the late secretory pathway, but does not distinguish between the MAM and mitochondria (Myhill et al. 2008; Roth et al. 2010). On the Optiprep gradient, markers of the MAM peak at the bottom in fraction 6, whereas ER proteins that are not enriched on the MAM, such as PDI are found in all fractions, including fractions containing the rER markers ERp57 and eIF2α (Fig. 1c, e). With this protocol, we confirmed that in HeLa and HEK 293 cells, the bulk of the Ero1α signal co-fractionated with markers of the MAM, but not the rER or the late secretory pathway (Fig. 1c). When analyzing our fractions for the presence of the characteristic oxidative states of Ero1α, we found that fully oxidized OX1 and OX2 Ero1α was found at the bottom of the gradient, whereas the late secretory pathway contained a mixture between oxidized and reduced Ero1α (Fig. 1d). We confirmed that the localization of Ero1α on our biochemical fractionations was unique by comparing its distribution in HeLa and HEK 293 cells to the chaperones and oxidoreductases calreticulin, ERp57, ERp44, PDI, and BiP/GRP78. Of these chaperones, BiP/GRP78 was the only one which showed a significant enrichment on fractions of the MAM. Conversely, PDI and, in particular, ERp57 showed high amounts on fractions of the rER (Fig. 1c, e). The distribution of ERp44 was unique, but entirely consistent with the functions described for this oxidoreductase: it is predominantly found in fractions co-migrating with the ERGIC and cis-Golgi marker βCOP, where it functions as a scavenger of proteins with exposed thiols (Anelli et al. 2003), but also on the MAM, where it regulates the activity of the IP3R (Higo et al. 2005). Therefore, our three subcellular fractionation protocols showed that Ero1α is highly enriched on the MAM, in contrast to numerous other ER oxidoreductases and chaperones that are present in higher amounts on the rER.
Fig. 1.
Ero1α co-fractionates with the MAM in HEK 293 and HeLa cells. a Ero1α fractionates into heavy membranes. Membranes from HEK 293 cells were fractionated into low (HM) and high speed pellets (LM), which were analyzed by Western blotting for complex 2 (mitochondria), ACAT1 (MAM), calnexin (rER/MAM), PDI (all ER), and Ero1α. b Ero1α distribution between mitochondria and the MAM. HEK 293 homogenates were fractionated into cytosol (Cyt.), microsomes (Micro), crude mitochondria (MC), purified mitochondria (MP), and MAM according to Materials and methods. Marker proteins indicate mitochondria (complex 2), MAM (ACAT1), and ER membranes (PDI and calnexin). c Ero1α distribution within ER domains. HeLa and HEK 293 cell homogenates were fractionated on a discontinuous 10–30% Optiprep gradient. Marker proteins indicate mitochondria (complex 2), MAM (ACAT1, calnexin), pan-ER (PDI), and Ero1α. Lane numbers refer to the Optiprep fractions. The localization of Ero1α from three independent experiments is quantified in Fig. 3a. d Presence of Ero1α oxidative forms on domains of the secretory pathway. HEK293 homogenates were fractionated and analyzed under non-reducing conditions. Ero1α was detected by Western blot. e Analysis of the ER domain distribution of selected components of the ER protein folding machinery and the Golgi complex. HEK 293 cell homogenates were fractionated on a discontinuous 10–30% Optiprep gradient. eIF2α indicates the position of the rER (fractions 3 and 4), β-COP indicates the cis-Golgi (fraction 2), ACAT1 indicates the MAM (fractions 5 and 6). Calreticulin (CRT), BiP/GRP78 (BiP), ERp44, and ERp57 were analyzed for their intra-ER location
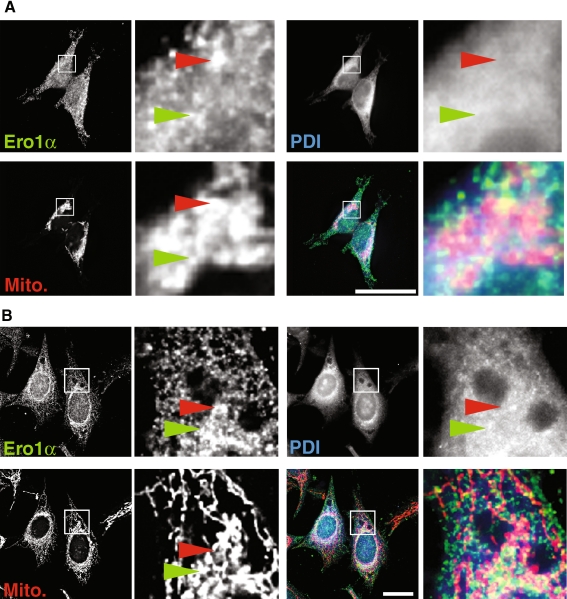
To further examine the localization of Ero1α, we analyzed the distribution of this oxidoreductase in HEK 293 and HeLa cells by immunofluorescence microscopy. Ero1α and PDI showed distinct distributions within HEK 293 (Fig. 2a) and HeLa cells (Fig. 2b). Whereas PDI localized to a dispersed reticular staining pattern that concentrated on the nuclear envelope in both cell lines (Fig. 2a, b), the staining pattern of Ero1α was patchier and tended to show more apposition with mitochondria (compare green arrowheads for PDI/Ero1α/mitochondria overlap and red arrowheads for Ero1α/mitochondria overlap in Fig. 2a, b). As typical for proteins of the MAM, this close apposition did not translate into full overlap between Ero1α and mitochondria (Myhill et al. 2008; Rizzuto et al. 1998). Together, these results are consistent with the fractionation data that showed that Ero1α and PDI have a distinct intracellular localization.
Fig. 2.
Intracellular localization of Ero1α to the vicinity of mitochondria. a HEK 293 cells were grown on coverslips for 24 h and processed for immunofluorescence microscopy. Ero1α was detected with a rabbit polyclonal antibody, PDI with a mouse monoclonal antiserum and mitochondria were visualized with Mitotracker. Immunofluorescence images were acquired and deconvolved. Inserts show a magnified area, indicated by white frames on the bigger pictures. The red arrowheads point out Ero1α/mitochondria overlap, whereas the green arrowheads point out triple PDI/Ero1α/mitochondria overlap. Scale bar = 25 μm. A representative image is shown. b HeLa cells were processed and imaged as in (a)
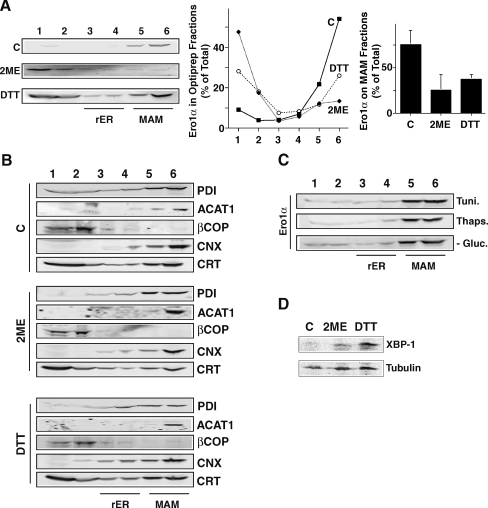
The localization of ER proteins to the MAM is a regulatable mechanism, as shown in the case of calnexin that can relocate from the MAM to the rER under conditions of extended ER stress (N. Myhill and T. Simmen unpublished observations). Another example is the cytosolic connector protein PACS-2 that temporarily shifts from light membranes to membranes of the MAM and mitochondria during ER stress (Simmen et al. 2005). We hypothesized that Ero1α, being an ER luminal protein, would exhibit intra-ER motility. To test this idea, we first treated HEK 293 cells with reducing agents known to lead to secretion of over-expressed, fully reduced Ero1α via a disruption of the Ero1α ERp44 retention mechanism (Anelli et al. 2002, 2003). As expected, treatment of cells with 1 mM 2-mercaptoethanol (2ME) and 1 mM dithiothreitol (DTT) for 2 h led to a shift of the Ero1α signal on the Optiprep gradient that monitors the localization of Ero1α among the MAM, the rER, the Golgi, and the plasma membrane. Under these conditions, the amount of Ero1α on the MAM decreased from roughly 75% to approximately 25% of the total signal, whereas its amounts on fractions of the late secretory pathway increased reciprocally (Fig. 3a). We verified whether the properties of secretory pathway membranes remained the same in this experiment by confirming the sustained presence of PDI, ACAT1, and βCOP in their respective fractions (Fig. 3b). Since we could not detect significant relocation for calnexin or calreticulin either, our results mean that during our time frame, only Ero1α shifted away from the MAM (Fig. 3b). Moreover, Ero1α did not relocate from the mere exposure to ER stress, as indicated by the gradients of homogenates from HEK 293 cells exposed to tunicamycin, thapsigargin, or glucose deprivation (Fig. 3c). Nevertheless, this incubation with reducing agents led to the induction of XBP-1 at the protein level within our 2-h time frame (Fig. 3d). Therefore, the complete reduction of the ER environment, but not the exposure of cells to ER stress led to the relocation of Ero1α from the MAM to cellular fractions of the late secretory pathway.
Fig. 3.
Ero1α MAM retention is redox-sensitive. a Optiprep fractionation of HEK 293 control cells and cells treated for 2 h with 1 mM 2ME and 1 mM DTT. Three independent experiments were probed for Ero1α and quantified by LICOR. The individual amounts of Ero1α per fraction are plotted in the middle graph (error bars omitted for clarity, but shown on the right panel). Right panel: the amount of Ero1α on the MAM was determined as the sum of signal found within fractions 5 and 6 (n = 3). b Optiprep fractionation of HEK 293 cells as in a. Western blots were probed for calnexin (CNX) and calreticulin (CRT). c Optiprep fractionation of HEK 293 cells treated with the ER stressors tunicamycin (Tuni., 10 μM), thapsigargin (Thaps., 1 μM), and in medium depleted of glucose, but supplemented with galactose (-Gluc., see Materials and methods). d Protein levels of the ER stress associated transcription factor XBP-1 after 2 h treatment with reducing agents. Loading equalized using α-tubulin as a loading control
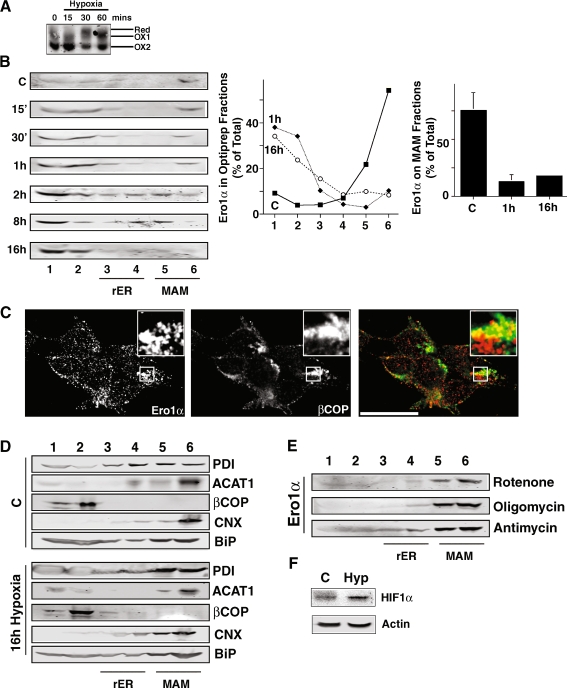
Since Ero1α requires oxygen for its enzymatic reaction (Tu and Weissman 2002), we examined whether or not the deprivation of atmospheric oxygen during hypoxia influenced its localization. Thus, we incubated HEK 293 cells in a hypoxic chamber containing 1% oxygen. Since reducing agents such as 2ME or DTT cause a shift of the oxidation state of Ero1α from the fully oxidized OX1 and OX2 forms to the reduced form, we first aimed to determine whether hypoxia could have a similar effect (Anelli et al. 2002). Indeed, the incubation in hypoxic atmosphere temporarily transformed a portion of Ero1α from the fully oxidized OX2 to the fully reduced form after 30 min of hypoxia (Fig. 4a). Moreover, starting as soon as 15 min after the exposure to the hypoxic environment, Ero1α shifted from the MAM to membranes of the late secretory pathway (Fig. 4b). This translocation appeared complete after about 2 h of incubation in hypoxia, when HIF1α protein expression started to increase (Fig. 4f). Under hypoxic conditions, the amount of Ero1α on the MAM decreased from about 75% of total to about 20%. Since we detected Ero1α in fractions 1 and 2 of our Optiprep gradient, we examined whether this translated into an overlap with βCOP. Indeed, our immunofluorescence experiments showed accumulations of paranuclear staining for Ero1α at 16 h hypoxia, which however only partially overlapped with the crescent-shaped βCOP pattern (Fig. 4c). Under control conditions, we could not detect accumulations (Fig. 2 and data not shown). Interestingly, we neither detected relocation of other ER protein folding chaperones including PDI, calnexin, and BiP/GRP78 nor of the MAM marker ACAT1 and the cis-Golgi marker βCOP during the incubation under hypoxic conditions (Fig. 4d). The Ero1α relocation that occurred under hypoxia was not dependent on the correct functioning of mitochondria, since neither the electron transport chain inhibitors rotenone and antimycin, nor the ATP synthase inhibitor oligomycin when added for 2 h led to a shift of Ero1α from the MAM to membranes of the late secretory pathway (Fig. 4e). Our results therefore show that oxygen supply is required to maintain the normal redox state of Ero1α and its retention on the MAM.
Fig. 4.
Ero1α MAM retention is oxygen-sensitive. a Analysis of the Ero1α oxidation state when incubated under hypoxic conditions (1% oxygen) for the indicated times. Lines point to fully reduced Ero1α (red) and the two oxidized forms OX1 and OX2. B. Optiprep fractionation of HEK 293 control cells and cells incubated for the indicated times (in minutes and hours, see numbers on the left) in hypoxic environment (1% oxygen). Three independent experiments were probed for Ero1α and quantified by LICOR. The individual amounts of Ero1α per fraction at the 1 and 16 h time points are plotted in the middle graph (error bars omitted for clarity, but shown on the right panel). Right panel: the amount of Ero1α on the MAM was determined as the sum of signal found within fractions 5 and 6 (n = 3). c Immunofluorescence co-localization of Ero1α and βCOP at 16 h of hypoxia. HEK 293 cells were grown on coverslips for 24 h, followed by incubation in hypoxia for 16 h and processed for immunofluorescence microscopy. Ero1α was detected with a rabbit polyclonal antibody and βCOP with a mouse monoclonal antiserum. Immunofluorescence images were acquired and deconvolved. Inserts show a magnified area, indicated by white frames on the bigger pictures. Scale bar = 25 μm. A representative image is shown. Partial overlap is seen in the magnified area, but also on numerous spots elsewhere. d Optiprep fractionation of HEK 293 cells as in (a). Western blots were probed for calnexin (CNX) and BiP/GRP78 (BiP). e Optiprep fractionation of HEK 293 cells treated for 2 h with the mitochondrial stressors rotenone (1 μM), oligomycin (5 μM), and antimycin (100 μM). f HIF1α protein levels after 2 h in hypoxic environment (Hyp, 1% oxygen). Loading equalized using actin as a loading control
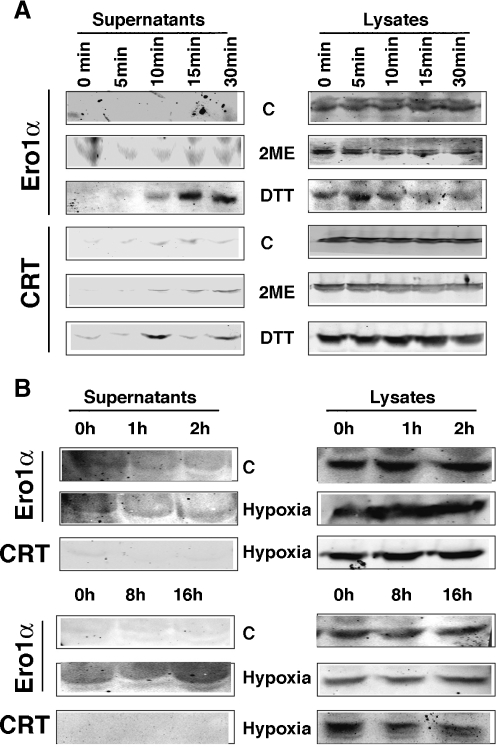
Our fractionation and immunofluorescence experiments did not allow us to conclusively determine the destination to where Ero1α migrates under conditions of disrupted ER redox or low oxygen. Therefore, we tested the hypothesis that Ero1α undergoes secretion by probing the supernatants of DTT- and 2ME-treated HEK 293 cells for Ero1α. As demonstrated earlier for over-expressed Ero1α, the incubation of cells with the reducing agent 2ME, and even more remarkably with DTT, led to the secretion of Ero1α starting at 10 min of incubation (Fig. 5a). Consistent with a slight increase of the calreticulin signal in late secretory pathway membranes (Fig. 3b), we also detected some secretion of calreticulin under these conditions (Fig. 5a). These results suggested that hypoxia leads to secretion of endogenous Ero1α. We tested this possibility, but only detected secretion of endogenous Ero1α, but not calreticulin, after long periods of hypoxia, i.e., after 16 h (Fig. 5c) despite the induction of HIF1α at the protein level starting at 2 h of hypoxia (Fig. 4f). Together, our results show that Ero1α, which is normally confined to the MAM, leaves that domain of the ER and enters the secretory pathway, resulting in a partial co-localization with βCOP, but ultimately leading to its secretion under conditions of disrupted cellular redox and prolonged hypoxia.
Fig. 5.
Disruption of cellular redox and oxygen supply leads to Ero1α secretion. a Ero1α content of cellular growth medium (supernatants) treated for the indicated times with the indicated reducing agents (1 mM 2ME, 1 mM DTT). Ero1α secretion, detected by the sharp increase in the growth medium (supernatants), is observed after 15 min. b Ero1α content of cellular growth medium (supernatants) incubated for the indicated times in hypoxic atmosphere. Ero1α secretion is observed after 16 h. For both a and b, calreticulin (CRT) serves as a secretion control
Discussion
Our results show for the first time the specific enrichment of an ER oxidoreductase to the MAM. The enrichment of Ero1α on the MAM appears to be specific for this ER oxidative folding enzyme, since PDI, ERp57 and the chaperones BiP/GRP78 and calreticulin do not show such an enrichment. Nevertheless, all of these proteins are found to some extent on the MAM (Fig. 1), thus still allowing them to perform calcium-regulatory roles on this domain of the ER. In contrast to calnexin, another MAM-enriched ER folding enzyme, the localization of Ero1α to this signaling hub of the ER is highly susceptible to the redox state of the ER and to oxygen supply, since incubation in reducing milieu and under hypoxic conditions lead to the rapid depletion of Ero1α from the MAM (Figs. 3 and 4). Subsequently, this oxidoreductase appears in the growth medium of cultured cells, in a much more pronounced way than other ER chaperones such as calreticulin (Fig. 5a, c). Interestingly, the relocation of Ero1α happens typically after 1–2 h of treatment, much earlier than the induction of transcriptional responses to reducing milieu and hypoxia (Fig. 5b, d). A determining factor for the Ero1α shift appears to be the Ero1α oxidation state. Characteristically, Ero1α appears in two oxidized states, OX1 and OX2, which are sensitive to incubation with DTT (Anelli et al. 2002). We detected a partial, temporary shift of the Ero1α oxidation state to the reduced form after 30 min of hypoxia (Fig. 4a), correlating with the mixed ratio of oxidized and reduced Ero1α in fractions of the late secretory pathway (Fig. 1d) and indicating that, as for incubation with DTT, hypoxia leads to a disruption of the thiol-mediated retention mechanism of Ero1α (Anelli et al. 2003).
The localization of Ero1α to the MAM is surprising for two reasons. First, Ero1α is expected to play an important role in the re-oxidation of the thioredoxin-related oxidoreductase PDI, which is classically thought to occur on the rER (Fassio and Sitia 2002; Sevier and Kaiser 2008). The almost exclusive presence of Ero1α on the MAM suggests that Ero1α-mediated PDI re-oxidation occurs on the MAM, rather than on the rER. Such a scenario would require the transport of PDI/peptide or at least PDI/Ero1α complexes from the rER to the MAM, so far unobserved. However, the shuttling of PDI/Ero1α complexes into the vicinity of mitochondria would result in the higher availability of mitochondrial ATP and FAD, both of which are required for efficient oxidative protein folding (Papp et al. 2005). Alternatively, PDI could be re-oxidized by the other Ero1 family member, Ero1β, which is present in non-detectable levels in HEK 293 and HeLa cells (Dias-Gunasekara et al. 2005, 2006). Since PDI is found abundantly in all domains of the ER, such a mechanism definitely remains a possibility. Clearly, the formation of PDI/Ero1α complexes is not a determinant of PDI localization, since none of the conditions described in this paper led to a significant relocalization of PDI. Instead, PDI, like calreticulin and BiP, could predominantly depend on the Lys-Asp-Glu-Leu (KDEL)-receptor for its ER retention. Notably, Ero1α lacks the KDEL sequence at its C-terminus. ERp57 and ERp44 show yet other types localizations of intra-ER localization patterns, suggesting the presence of additional intra-ER localization mechanisms. Consistent with their role in the regulation of ER calcium homeostasis, both oxidoreductases show, nevertheless, portions of their respective signals on the MAM.
Second, hypoxia leads to the induction of expression of Ero1α (Gess et al. 2003; May et al. 2005), which, according to our results, is subsequently secreted. One possible explanation for these observations is that hypoxic cells need to compensate for constant loss of Ero1α during hypoxia. Alternatively, Ero1α may perform other functions outside the cell during conditions of prolonged hypoxia.
With our results, the classes of identified proteins on the MAM increases by another type, the oxidoreductases. In fact, Ero1α emerges quite surprisingly as one of the best-known MAM markers to date. Supporting our data, Ero1α has also been implicated in the regulation of ER-mitochondria calcium communication by promoting IP3R-mediated calcium efflux during ER stress (Li et al. 2009). Since the IP3Rs are enriched themselves enriched on the MAM (Hajnoczky et al. 2000), our results raise the possibility that the majority of human Ero1α, at least in the tumor-derived HEK 293 and HeLa cells, might regulate IP3R-mediated calcium signaling on the MAM. In this case, Ero1α would not be the first ER oxidative protein chaperone involved in the regulation of ER calcium homeostasis. These observations have a precedent in the functions of calreticulin (Michalak et al. 2009; Molinari et al. 2004), but the presence of a calcium binding domain within Ero1α itself also supports such a possibility. Further research is required to address these possibilities and also to clarify the functional relationship between the two human Ero1 protein family members.
Despite Ero1α being an ER luminal protein, the targeting of Ero1α to the MAM is quite stringent (>75%), compared with the chaperone calnexin, whose targeting mechanism to the MAM we have previously analyzed (about 2/3 of calnexin localizes to the MAM under resting conditions, Myhill et al. 2008). Similar to calnexin, the relatively low, but detectable amounts of Ero1α on the rER might be sufficient to perform its enzymatic role in ER oxidative protein folding. Further elucidation of the Ero1α targeting mechanism to the MAM will provide more insight into this question. What are the possible mechanisms by which Ero1α is targeted to the MAM? In the case of calnexin, interaction with the cytosolic connector protein PACS-2 contributes to its localization to the MAM (Myhill et al. 2008). Such a mechanism cannot account for the MAM-localization of Ero1α directly, since Ero1α is not in contact with the cytosolic face of the ER. Besides the known interaction of Ero1α with PDI and ERp44, interaction with an as-yet unknown receptor may mediate retention of Ero1α on the MAM. Another possibility could be a tight association of Ero1α with the MAM membrane, which has been shown to exhibit specific lipid composition (Sano et al. 2009). Similar to the characterization of the rice Ero1α ER targeting mechanism (Onda et al. 2009), we expect a detailed analysis of the Ero1α peptide sequence to yield information concerning a novel targeting mechanism for ER oxidoreductases and chaperones to the MAM. We further expect such a targeting mechanism to have important implications on cell survival and protein secretion.
Acknowledgements
Work in the Simmen laboratory was supported by Alberta Health Services (Bridge Grant #24170) and by the NCIC/CCSRI (Grant #17291) EML and MDB are supported by ACRI studentships. T. S. is supported by the Alberta Heritage Foundation for Medical Research (200500396). The authors thank Richard Rachubinski and Roberto Sitia for comments, support, and reagents.
Abbreviations
- 2ME
2-mercaptoethanol
- BiP/GRP78
Immunoglobulin binding protein/glucose-regulated protein of 78 kDa
- Ero1α
ER oxidoreductin 1
- HEK
Human embryonic kidney
- MAM
Mitochondria-associated membrane
- PACS-2
Phospho-furin acidic cluster sorting protein 2
References
- Anelli T, Alessio M, Mezghrani A, Simmen T, Talamo F, Bachi A, Sitia R. ERp44, a novel endoplasmic reticulum folding assistant of the thioredoxin family. EMBO J. 2002;21:835–844. doi: 10.1093/emboj/21.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anelli T, Alessio M, Bachi A, Bergamelli L, Bertoli G, Camerini S, Mezghrani A, Ruffato E, Simmen T, Sitia R. Thiol-mediated protein retention in the endoplasmic reticulum: the role of ERp44. EMBO J. 2003;22:5015–5022. doi: 10.1093/emboj/cdg491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham AM, Cabibbo A, Fassio A, Bulleid N, Sitia R, Braakman I. The CXXCXXC motif determines the folding, structure and stability of human Ero1-L alpha. Embo J. 2000;19:4493–4502. doi: 10.1093/emboj/19.17.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabibbo A, Pagani M, Fabbri M, Rocchi M, Farmery MR, Bulleid NJ, Sitia R. ERO1-L, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J Biol Chem. 2000;275:4827–4833. doi: 10.1074/jbc.275.7.4827. [DOI] [PubMed] [Google Scholar]
- Camacho P, Lechleiter JD. Calreticulin inhibits repetitive intracellular Ca2+ waves. Cell. 1995;82:765–771. doi: 10.1016/0092-8674(95)90473-5. [DOI] [PubMed] [Google Scholar]
- Caramelo JJ, Parodi AJ. Getting in and out from calnexin/calreticulin cycles. J Biol Chem. 2008;283:10221–10225. doi: 10.1074/jbc.R700048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Thomas AP, Hajnoczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. Embo J. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Gunasekara S, Gubbens J, Lith M, Dunne C, Williams JA, Kataky R, Scoones D, Lapthorn A, Bulleid NJ, Benham AM. Tissue-specific expression and dimerization of the endoplasmic reticulum oxidoreductase Ero1beta. J Biol Chem. 2005;280:33066–33075. doi: 10.1074/jbc.M505023200. [DOI] [PubMed] [Google Scholar]
- Dias-Gunasekara S, Lith M, Williams JA, Kataky R, Benham AM. Mutations in the FAD binding domain cause stress-induced misoxidation of the endoplasmic reticulum oxidoreductase Ero1beta. J Biol Chem. 2006;281:25018–25025. doi: 10.1074/jbc.M602354200. [DOI] [PubMed] [Google Scholar]
- Fassio A, Sitia R. Formation, isomerisation and reduction of disulphide bonds during protein quality control in the endoplasmic reticulum. Histochem Cell Biol. 2002;117:151–157. doi: 10.1007/s00418-001-0364-0. [DOI] [PubMed] [Google Scholar]
- Freedman RB, Klappa P, Ruddock LW. Model peptide substrates and ligands in analysis of action of mammalian protein disulfide-isomerase. Methods Enzymol. 2002;348:342–354. doi: 10.1016/S0076-6879(02)48653-3. [DOI] [PubMed] [Google Scholar]
- Gess B, Hofbauer KH, Wenger RH, Lohaus C, Meyer HE, Kurtz A. The cellular oxygen tension regulates expression of the endoplasmic oxidoreductase ERO1-Lalpha. Eur J Biochem. 2003;270:2228–2235. doi: 10.1046/j.1432-1033.2003.03590.x. [DOI] [PubMed] [Google Scholar]
- Gething MJ. Role and regulation of the ER chaperone BiP. Semin Cell Dev Biol. 1999;10:465–472. doi: 10.1006/scdb.1999.0318. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Csordas G, Madesh M, Pacher P. The machinery of local Ca2+ signalling between sarco-endoplasmic reticulum and mitochondria. J Physiol. 2000;529(Pt 1):69–81. doi: 10.1111/j.1469-7793.2000.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Helenius A. Folding of VSV G protein: sequential interaction with BiP and calnexin. Science. 1994;266:456–458. doi: 10.1126/science.7939687. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T, Mikoshiba K. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell. 2005;120:85–98. doi: 10.1016/j.cell.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Horibe T, Gomi M, Iguchi D, Ito H, Kitamura Y, Masuoka T, Tsujimoto I, Kimura T, Kikuchi M. Different contributions of the three CXXC motifs of human protein-disulfide isomerase-related protein to isomerase activity and oxidative refolding. J Biol Chem. 2004;279:4604–4611. doi: 10.1074/jbc.M310922200. [DOI] [PubMed] [Google Scholar]
- John LM, Lechleiter JD, Camacho P. Differential modulation of SERCA2 isoforms by calreticulin. J Cell Biol. 1998;142:963–973. doi: 10.1083/jcb.142.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PS, Bole D, Arvan P. Transient aggregation of nascent thyroglobulin in the endoplasmic reticulum: relationship to the molecular chaperone, BiP. J Cell Biol. 1992;118:541–549. doi: 10.1083/jcb.118.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klappa P, Hawkins HC, Freedman RB. Interactions between protein disulphide isomerase and peptides. Eur J Biochem. 1997;248:37–42. doi: 10.1111/j.1432-1033.1997.t01-1-00037.x. [DOI] [PubMed] [Google Scholar]
- Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May D, Itin A, Gal O, Kalinski H, Feinstein E, Keshet E. Ero1-L alpha plays a key role in a HIF-1-mediated pathway to improve disulfide bond formation and VEGF secretion under hypoxia: implication for cancer. Oncogene. 2005;24:1011–1020. doi: 10.1038/sj.onc.1208325. [DOI] [PubMed] [Google Scholar]
- Mendes CC, Gomes DA, Thompson M, Souto NC, Goes TS, Goes AM, Rodrigues MA, Gomez MV, Nathanson MH, Leite MF. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biol Chem. 2005;280:40892–40900. doi: 10.1074/jbc.M506623200. [DOI] [PubMed] [Google Scholar]
- Mezghrani A, Fassio A, Benham A, Simmen T, Braakman I, Sitia R. Manipulation of oxidative protein folding and PDI redox state in mammalian cells. EMBO J. 2001;20:6288–6296. doi: 10.1093/emboj/20.22.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J. 2009;417:651–666. doi: 10.1042/BJ20081847. [DOI] [PubMed] [Google Scholar]
- Molinari M, Eriksson KK, Calanca V, Galli C, Cresswell P, Michalak M, Helenius A. Contrasting functions of calreticulin and calnexin in glycoprotein folding and ER quality control. Mol Cell. 2004;13:125–135. doi: 10.1016/S1097-2765(03)00494-5. [DOI] [PubMed] [Google Scholar]
- Myhill N, Lynes EM, Nanji JA, Blagoveshchenskaya AD, Fei H, Carmine Simmen K, Cooper TJ, Thomas G, Simmen T. The subcellular distribution of calnexin is mediated by PACS-2. Mol Biol Cell. 2008;19:2777–2788. doi: 10.1091/mbc.E07-10-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda Y, Kumamaru T, Kawagoe Y. ER membrane-localized oxidoreductase Ero1 is required for disulfide bond formation in the rice endosperm. Proc Natl Acad Sci USA. 2009;106:14156–14161. doi: 10.1073/pnas.0904429106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu M, Bertoli G, Fagioli C, Guerini-Rocco E, Nerini-Molteni S, Ruffato E, Sitia R. Dynamic retention of Ero1alpha and Ero1beta in the endoplasmic reticulum by interactions with PDI and ERp44. Antioxid Redox Signal. 2006;8:274–282. doi: 10.1089/ars.2006.8.274. [DOI] [PubMed] [Google Scholar]
- Pagani M, Fabbri M, Benedetti C, Fassio A, Pilati S, Bulleid NJ, Cabibbo A, Sitia R. Endoplasmic reticulum oxidoreductin 1-lbeta (ERO1-Lbeta), a human gene induced in the course of the unfolded protein response. J Biol Chem. 2000;275:23685–23692. doi: 10.1074/jbc.M003061200. [DOI] [PubMed] [Google Scholar]
- Pagani M, Pilati S, Bertoli G, Valsasina B, Sitia R. The C-terminal domain of yeast Ero1p mediates membrane localization and is essential for function. FEBS Lett. 2001;508:117–120. doi: 10.1016/S0014-5793(01)03034-4. [DOI] [PubMed] [Google Scholar]
- Papp E, Nardai G, Mandl J, Banhegyi G, Csermely P. FAD oxidizes the ERO1-PDI electron transfer chain: the role of membrane integrity. Biochem Biophys Res Commun. 2005;338:938–945. doi: 10.1016/j.bbrc.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Registre M, Goetz JG, St Pierre P, Pang H, Lagace M, Bouvier M, Le PU, Nabi IR. The gene product of the gp78/AMFR ubiquitin E3 ligase cDNA is selectively recognized by the 3F3A antibody within a subdomain of the endoplasmic reticulum. Biochem Biophys Res Commun. 2004;320:1316–1322. doi: 10.1016/j.bbrc.2004.06.089. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Roderick HL, Lechleiter JD, Camacho P. Cytosolic phosphorylation of calnexin controls intracellular Ca(2+) oscillations via an interaction with SERCA2b. J Cell Biol. 2000;149:1235–1248. doi: 10.1083/jcb.149.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D, Lynes E, Riemer J, Hansen HG, Althaus N, Simmen T, Ellgaard L. A di-arginine motif contributes to the ER-localization of the type I transmembrane ER oxidoreductase TMX4. Biochem J. 2009;425:195–205. doi: 10.1042/BJ20091064. [DOI] [PubMed] [Google Scholar]
- Rusinol AE, Cui Z, Chen MH, Vance JE. A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. J Biol Chem. 1994;269:27494–27502. [PubMed] [Google Scholar]
- Sano R, Annunziata I, Patterson A, Moshiach S, Gomero E, Opferman J, Forte M, d'Azzo A. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol Cell. 2009;36:500–511. doi: 10.1016/j.molcel.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevier CS, Kaiser CA. Ero1 and redox homeostasis in the endoplasmic reticulum. Biochim Biophys Acta. 2008;1783:549–556. doi: 10.1016/j.bbamcr.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Shiao YJ, Balcerzak B, Vance JE. A mitochondrial membrane protein is required for translocation of phosphatidylserine from mitochondria-associated membranes to mitochondria. Biochem J. 1998;331(Pt 1):217–223. doi: 10.1042/bj3310217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen T, Aslan JE, Blagoveshchenskaya AD, Thomas L, Wan L, Xiang Y, Feliciangeli SF, Hung CH, Crump CM, Thomas G. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J. 2005;24:717–729. doi: 10.1038/sj.emboj.7600559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SJ, Vance JE. Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J Biol Chem. 2000;275:34534–34540. doi: 10.1074/jbc.M002865200. [DOI] [PubMed] [Google Scholar]
- Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. Embo J. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu BP, Weissman JS. The FAD- and O(2)-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol Cell. 2002;10:983–994. doi: 10.1016/S1097-2765(02)00696-2. [DOI] [PubMed] [Google Scholar]
- Voeltz GK, Rolls MM, Rapoport TA. Structural organization of the endoplasmic reticulum. EMBO Rep. 2002;3:944–950. doi: 10.1093/embo-reports/kvf202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KW, Gilbert HF. Oxidation of kinetically trapped thiols by protein disulfide isomerase. Biochemistry. 1995;34:13642–13650. doi: 10.1021/bi00041a045. [DOI] [PubMed] [Google Scholar]
- Wang L, Li SJ, Sidhu A, Zhu L, Liang Y, Freedman RB, Wang CC. Reconstitution of human Ero1-L alpha/protein-disulfide isomerase oxidative folding pathway in vitro. Position-dependent differences in role between the a and a' domains of protein-disulfide isomerase. J Biol Chem. 2009;284:199–206. doi: 10.1074/jbc.M806645200. [DOI] [PubMed] [Google Scholar]
- Wieckowski MR, Giorgi C, Lebiedzinska M, Duszynski J, Pinton P. Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat Protoc. 2009;4:1582–1590. doi: 10.1038/nprot.2009.151. [DOI] [PubMed] [Google Scholar]
- Yi M, Weaver D, Hajnoczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]