Abstract
In recent studies, we showed that exogenous hyaluronic acid oligomers (HA-o) stimulate functional endothelialization, though native long-chain HA is more bioinert and possibly more biocompatible. Thus, in this study, hydrogels containing high molecular weight (HMW) HA (1×106 Da) and HA oligomer mixtures (HA-o: 0.75–10 kDa) were created by crosslinking with divinyl sulfone (DVS). The incorporation of HA oligomers was found to compromise the physical and mechanical properties of the gels (rheology, apparent crosslinking density, swelling ratio, degradation) and to very mildly enhance inflammatory cell recruitment in vivo; increasing the DVS crosslinker content within the gels in general, had the opposite effect, though the relatively high concentration of DVS within these gels (necessary to create a solid gel) also stimulated a mild sub-cutaneous inflammatory response in vivo and VCAM-1 expression by ECs cultured atop; ICAM-expression levels remained very low irrespective extent of DVS crosslinking or HA-o content. The greatest EC attachment and proliferation (MTT assay) was observed on gels that contained the highest amount of HA-o. The study shows that the beneficial EC response to HA oligomers and biocompatibility of HA is mostly unaltered by their chemical derivatization and crosslinking into a hydrogel. However, the study also demonstrates that the relatively high concentrations of DVS, necessary to create solid gels, compromises their biocompatibility. Moreover, the poor mechanics of even these heavily crosslinked gels, in the context of vascular implantation, necessitates the investigation of other, more appropriate crosslinking agents. Alternately, the outcomes of this study may be used to guide an approach based on chemical immobilization and controlled surface-presentation of both bioactive HA oligomers and more biocompatible HMW HAon synthetic or tissue engineered grafts already in use, without the use of a crosslinker, so that improved, predictable, and functional endothelialization can be achieved, and the need to create a mechanically compliant biomaterial for standalone use, circumvented.
Keywords: Hyaluronic Acid, Endothelial Cells, Oligomers, Hydrogel, Divinyl Sulfone
INTRODUCTION
To overcome the poor or, alternatively, exaggerated vascular cell responses to synthetic materials, there has been a recent shift toward the development and use of “natural or tissue-based” biomaterial scaffolds that could potentially evoke more appropriate, and accelerated regenerative/healing responses by vascular cells. The extracellular matrix (ECM), once regarded simply as a structural scaffold, is now recognized as an important modulator of cell phenotype and function1. From a tissue engineering perspective, it is thus increasingly apparent that the presence of ECM molecules is vital to developing a biomechanical and biochemical environment that mimics the cellular surroundings within native tissues. One class of ECM molecules that is increasingly studied in the context of designing regenerative materials are glycosaminoglycans (GAGs). One such GAG, hyaluronic acid (HA), occurs naturally in connective tissues (e.g. skin) as a simple, linear molecule consisting of repeating dissacharide units of N-acetyl-D-glucosamine and D-glucuronic acid2. Most cells have the ability to synthesize HA at some point during their cell cycle, signifying that the molecule has vital function in several fundamental biological processes2.
From a biomaterials standpoint, HA has been increasingly recognized as an appropriate biomolecule to modulate tissue regeneration, since it can be chemically derivatized into biomaterials with little change to its inherent biologic properties. It is now known that HA, when physiologically degraded into smaller-sized fragments, facilitates wound healing by promoting angiogenesis3. HA fragments can, under specific circumstances, also incite early inflammation, which is critical to initiate wound healing, and then modulate later stages of the process to stabilize the matrix and reduce of long-term inflammation3. In an uncrosslinked state, HA is also highly biocompatible and has been shown to poorly elicit a foreign-body response upon cross-transplantation due to the high degree of structural homology that HA exhibits across species and tissue types2. In addition, HA is amenable to binding peptides, matrix proteins, and growth factors capable of further modulating cell responses4,5. Although the modes of interaction between HA and the human body are still incompletely understood, the favorable characteristics outlined above have assured its extensive use as a scaffolding biomaterial for tissue engineering applications, most recently for cartilage6 and dermal7 repair and regeneration. Our lab is currently investigating the potential use of HA as a vascular regenerative implant material. Since HA forms a significant (4–7% w/w) component of vascular ECM8, we hypothesize that HA modified biomaterials will provide biomechanical and biochemical signals to vascular cells to ensure a healthy physiologic-like phenotype.
Previously, we reported on the size-specific effects of HA on vascular ECs 9. Briefly, we found exogenous supplementation of a mixture of HA oligomers (HA-o), containing predominantly 6mers and 12mers, to stimulate EC proliferation, secretion of pro-angiogenic growth factors, and formation of micro-vascular networks. The chemical immobilization of these HA oligomer mixtures onto 2-D cellular substrates (glass) did not alter their impact on EC behavior differentially from when the same mixtures were exogenously delivered to ECs. This reinforced our hypothesis that chemical derivatization of HA oligomers does not necessarily alter their cellular interaction and hence, could likely be incorporated into cell-contacting biomaterials, with little or no detriment. However, under both modes of presentation, HA oligomer mixtures somewhat elevated EC expression of inflammatory CAMs. In addition, when HA oligomers are crosslinked, the resulting biomaterials are fluid-like and exhibit very poor handling properties. One way to address these problems, is to incorporate the bioactive HA-o within mechanically robust constructs containing chemically-crosslinked, highly biocompatible and rather bioinert, high molecular weight (HMW) HA (MW > 1 ×106 Da).
Previous studies with divinyl sulfone (DVS)-crosslinked HA hydrogels, composed of HMW HA alone, showed them to be bioinert and non-conducive to cell spreading and proliferation 10. However, we earlier showed that such gels could be bio-activated by controlled irradiation with UV light (λ = 254 nm), a process that randomly splices HMW HA on the gel surface to generate HA fragments of a range of sizes, including HA-o11. It was observed that vascular cells readily attached, spread, proliferated and generally exhibited a healthy phenotype, and normal functionality when cultured atop these bio-activated gels. Since the effects of UV light can be difficult to control, and can potentially cause random ionizations that are structurally disruptive, a better approach is to create ‘bioactivated’ gels containing bio-inert long-chain HA, necessary to maintain mechanical integrity and potentially provide a high degree of biocompatibility, and smaller, more cell-interactive HA fragments/HA-o. In pursuing this approach, it is however important to incorporate optimally-sized HA fragments, and modulate their content within the HA biomaterial such as to evoke desired cell responses, prevent exaggerated cell responses (e.g. activation or inflammation) to their presence, and deter adverse impacts on physical properties (e.g., ease of handling, mechanics, hydration, degradability) of the biomaterial. At this time, the incorporation of HA-o into biomaterials for enabling functional vascular endothelialization and tissue regeneration has not been thoroughly investigated. Particularly, the effects of crosslinking HMW HA/HA-o to obtain HA scaffolds with good handling properties, and the densities at which bioactive HA-o need to be presented on these biomaterials to achieve the desired EC responses are unknown. In the current study, we therefore specifically investigate the impact of divinyl sulfone (DVS) crosslinking of HMW HA and HA-o mixtures on the physical, mechanical and biological properties of the resulting biomaterials.
MATERIALS AND METHODS
Preparation of HA Oligomer Mixtures
HMW HA (HA 1500; MW 1.5 – 1.8 × 106; Sigma, St. Lewis, MO) was enzymatically digested to produce a mixture of HA oligomers (HA-o), as stated in a previous publication9. Briefly, HA 1500 (5 mg/ml) was digested with bovine testicular hyaluronidase, (4.5 mg/ml; 439 U/mg; Sigma) in a solution of digest buffer (150 mM NaCl, 100 mM CH3COONa, 1 mM Na2-EDTA, pH 5.0) at 37 °C for 48 h. The enzyme was then precipitated, its activity terminated by boiling (2 min), and cooled on ice. Following centrifugation (2800 rpm, 10 min), to separate the enzyme from the mixture, the supernatant was dialyzed in water (12 h) using dialysis cassettes (Pierce Biotechnology Inc.) with a MW cut-off of 2 kDa and then freeze-dried overnight to generate lyophilized HA-o. The mixture was analyzed by fluorophore assisted carbohydrate electrophoresis (FACE) as we have described in a prior publication9, 11.
Briefly, for FACE analysis, a 50 μg sample of the digest was dissolved in 40 μl of a 0.0125 M solution of the fluorescent dye 2-aminoacridone (AMAC, Sigma) prepared in acetic acid/DMSO (3:17 v/v), and incubated for 15 min in the dark. A 40 μl aliquot of an aqueous solution of 1.25 M sodium cyanoborohydride (Sigma) was then added to each sample and incubated (37 °C, 16 h, dark). Following this, 20 μl of glycerol was added to each sample. The standards (oligomer ladder, 2mer, 6mer; Associates of Cape Cod, East Falmouth, MA) were prepared in the same manner.
For electrophoresis, all 8 lanes of a polyacrylamide MONO® gel (Glyko, San Leandro, CA) were loaded simultaneously with 4 μl of sample/standard and run with MONO® gel running buffer (Glyko) at < 10 °C, as described previously (Calabro et al. 2000). Samples were electrophoresed at a constant 500 V with a starting current of 25 mA/gel and a final current of 10 mA/gel for 80 min. After electrophoresis, the gels were illuminated with UV-B light (λ = 365 nm) in a FluorChem 8900 (Alpha Innotech, San Leandro, CA) and band intensities quantified and compared to an HA oligomer ladder (10–20mer; 1915–3811 Da) and commercially procured HA 2mer (397 Da) and HA 6mer (1156 Da; Associates of Cape Cod, East Falmouth, MA) to determine the size range of HA fragments within the digest. Using this method, we previously reported the enzymatic digest to contain 33.3 ± 2.4% w/w of HA 6mers and 39.2 ± 2.7% w/w of HA 12mers, with oligomers of closely-related sizes forming the balance 9.
Hydrogel Formulation
HA hydrogels crosslinked with DVS (DVS-HA) were formulated using methods loosely based on a previously described protocol 9. Briefly, HA 1500 with added HA-o (0, 5, 10, 20% w/w) was completely dissolved at a concentration of 45 mg/ml in a 1:4 v/v solution of 1M sodium hydroxide (NaOH; to maintain a high pH): 1M sodium chloride (NaCl; to increase the dissolution rate of HA), pH 13.0. Thorough mixing of the resulting viscous solution was achieved by repeated transfer of the mixture between two sterile syringes (Beckton Dickenson, Franklin Lakes, NJ) through a 3-way stopcock (Kimble Kontes, Vineland, NJ). The mixture was then centrifuged for 5 min at 1000 g to remove air bubbles and aliquoted into cylindrical molds (for rheology, compression: 2 cm2, 0.5 ml; for all other analytical techniques: 0.79 cm2, 0.2 ml). The aliquoted mixtures were then homogenously crosslinked by adding DVS (Sigma; Density = 1.177 g/ml) directly into the aliquoted HA solutions at either of two concentrations (1:1 or 1:2 w/w DVS:HA), which translated to added volumes of 7.6 μl and 3.8 μl per gel. A spatula was used to mix DVS rapidly into the solution before a significant increase in viscosity of the solution was observed. The solution was allowed to solidify into a gel over 2 hr through radical addition crosslinking reaction between the vinyl groups of DVS and hydroxyl groups of HA. The crosslinked gels were thoroughly washed in DI water (3 cycles; 2 hr/cycle) to leach out unreacted DVS, which can be potentially cytotoxic21. The gels were then finally equilibrated in sterile phosphate-buffered saline (PBS). There were therefore a total of 8 formulations (4 oligomer concentrations × 2 crosslinker densities). The dimensions (height and diameter) of the swollen cylindrical hydrogels, as crosslinked within molds of 0.79 cm2- area, were measured with a digital caliper (Fisher, Pittsburgh, PA).
Fluorescence – Based Detection of HA Oligomers within Gels
HA oligomers (HA-o) were fluorescently labeled prior to incorporation within the hydrogel to visualize the extent of their retention within. HA-o was dissolved (1.25 mg/ml) in a 0.0125 M solution of the fluorescent dye 2-aminoacridone (AMAC, Sigma) prepared in acetic acid/DMSO (3:17 v/v), and incubated for 15 min in the dark. An equivalent amount of 1.25 M sodium cyanoborohydride (Sigma) was then added and incubated (37 °C, 16 h, dark). The fluorescently labeled-HA-o was then recovered and purified by precipitation in acetone and redissolution in DI water (3 cycles), and finally precipitated in acetone and freeze-dried in the dark. Fluorescent HA-o was then incorporated into DVS-crosslinked hydrogels, as described previously. The fluorescence intensities (λ = 365 nm) emanating from the gels (n = 4), were quantified at regular intervals over 21 days of incubation in PBS (37°C), using a FluorChem 8900 (Alpha Innotech, San Leandro, CA). Gels were imaged at a constant exposure time of 200 msec.
Apparent Crosslinking Density
The structural integrity of DVS-HA is primarily maintained by DVS crosslinks, bonding the HA strands to one another and limiting their freedom of motion. The incorporation of HA oligomers may reduce the effectiveness of these crosslinks, diminishing the overall mechanical properties of the gel. In addition, the DVS may not be effective in crosslinking 100% of the HA crosslinking sites. Accordingly, we deemed it necessary to define a parameter termed ‘apparent crosslinking’, which is a parameter that describes the effective crosslinking within each gel formulation calculated from the gel response to uniaxial compression tests. Briefly, cylindrical gel samples (8 mm diameter) were punched out of a larger gel using a 8-mm-diameter corneal trephine (BRI, Malden, MA) and were compressed without constraining the edges (unconfined compression testing) on a DMA Q800 (TA Instruments, New Castle, DE). The gels were subject to an initial force of 0.05 N and were then compressed at a rate of 20% strain/min (n = 8). All tests were performed in air, though the gels were kept hydrated in PBS while being compressed. Stress/strain curves were developed according to the following formula developed by Flory 13.
| (Eq 1) |
where σ – uniaxial compressive stress (Pa), P – universal gas constant (J/mol K), T – temperature (K), φ2,x – polymer volume fraction post-crosslinking, φ2,s – polymer volume fraction swollen, νe/Vo – apparent crosslinking density (mol/cm3), and α – compressed fraction. The initial slope of the curve (0 – 30% strain) was used to estimate the apparent crosslinking density of the gel.
Rheology
To further characterize the impact of crosslinking and oligomer incorporation on hydrogel mechanics, rheological oscillatory shear stress experiments were performed. The strength or stiffness was experimentally determined by measuring the storage (G′) and loss (G″) moduli. An AR G2 rheometer (TA Instruments, New Castle, DE) was used in the parallel plate geometry, with a 25-mm plate and constant normal force of 0.2 N. A deformation angle of 1 mrad was maintained throughout each frequency sweep of 0.01–10 Hz (n = 4).
Swelling Ratio
Swelling tests were performed to study the effects of HA-o content and DVS concentration on the bulk hydrodynamic properties of the gels. Fully hydrated gels were blotted to remove excess PBS and the weight of the swollen samples were recorded using a sensitive balance (OH AUS, Pine Brook, NJ). The gels were then freeze-dried and weighed again. The swelling ratio (unitless) was calculated by the following formula (n = 4).
| (Eq 2) |
where Ws is the swollen mass of the gel (mg), and Wd is the dry mass of the gel (mg). Gel mass rather than volume was used to estimate swelling since the dried gels are irregular, highly porous, and collapsed, which renders estimation of their volume very difficult.
In Vitro Degradation
In vitro enzymatic degradation of the hydrogels was measured as a function of time by incubating the DVS-crosslinked gels in testicular hyaluronidase and monitoring the remaining dry mass of the hydrogel. The gels were initially soaked in digest buffer (pH 5.0) overnight until swelling equilibrium was attained. Bovine testicular hyaluronidase (Sigma) in digest buffer (see section of HA-o preparation; 2 mL of 50 U/ml) was then added to each gel and incubated for up to 8 h at 37 °C with mild mixing on a platform shaker. At each assay time point (0, 2, 4, 6, and 8 h), gels (n = 3) were recovered, lyophilized, weighed, and then replaced in a fresh aliquot of the enzyme solution and incubated until the next assay time point. This degradation profile was fitted according to first-order degradation kinetics using non-linear regression to estimate gel degradation rates.
| (Eq 3) |
where C(t) is the dry mass of the gel at time t (mg), Co is the initial dry mass of the gel (mg), k is the degradation rate (h−1), t is time (h).
In Vivo Biocompatibility
The biocompatibility of the hydrogels was determined by subcutaneous implantation in rats. Prior to implantation, hydrogels were sterilized in 95% v/v ethanol (Sigma) for 2 h and then re-hydrated in sterile 1×PBS overnight. Sprague-Dawley rats (~250 g) were anesthetized (0.01 ml/g intramuscular injection of 4% chloral hydrate; Sigma), shaved, and a 5-cm incision made in the skin along the spine. Blunt dissection was used to form a pocket between the skin and muscle, and muscle surface was cleared of fascia. Hydrogels and matrigel (Sigma) were placed directly into these pockets (8 implants/animal; n = 7). After implantation, the surgical incision was closed with 4-0 silk suture with a FS-2 cutting needle (Ethicon, Piscataway, NJ). One additional rat was surgically manipulated to create 3 subcutaneous pockets, but were not implanted with any biomaterials (sham treatment controls).
At 3 weeks, the hydrogels and the tissue capsules, if any, were explanted from the subcutaneous pockets, fixed in 4% v/v paraformaldehyde (Sigma) and soaked for 1 h intervals in 30% w/v sucrose, 1:1 30% sucrose: optimal cutting temperature (OCT; Sakura, Torrance, CA) compound and finally pure OCT. The explants were then embedded within OCT solution, frozen on dry ice and stored at −80°C. Prior to sectioning, frozen blocks were acclimated to −20°C (overnight) and cryosectioned perpendicular to the skin and muscle surfaces. The 8-μm thick sections were transferred onto HistoBond® glass slides (VWR, West Chester, PA) and stained with Haematoxylin and Eosin to detect cell/tissue accumulation towards or within the implant, or in the case of sham controls, the presence of any fibrous tissue mass. The tissue sections were imaged on a Leica DM IRB microscope equipped with a JVC TK-C1380 color camera (Leica, Allendale, NJ). In addition, immunofluorescence methods were used to detect the collagen, and hence presence of a fibrous capsule within the tissue mass surrounding the implant. Sections, 15 μm thick, were initially quenched with 1% v/v phosphomolybdic acid (Sigma) to eliminate autofluorescence from collagen and then incubated with a primary antibody for collagen I for 1 h (rabbit vs. rat collagen I; 1:100 v/v in PBS; Chemicon, Temecula, CA). A solution of donkey serum (5% v/v in PBS; VWR) was added to the sections as a blocking agent (20 minutes) to prevent nonspecific binding of the secondary antibody. The sections were then treated with a FITC-conjugated secondary antibody (donkey vs. rabbit IgG; 1:500 v/v in PBS; Chemicon) for 1 h. Draq 5 (1:2000 v/v in PBS, 10 min; Biostatus, Leicestershire, UK) was used to fluorescently label the cell nuclei and visualize the cell density in the region surrounding the implant. Fluorescently labeled cultures were imaged on a TCS SP2 AOBS confocal microscope (Leica) using the z-axis function to image sections 5 μm apart. The vertical stack of images was then overlaid to create a composite.
Cell Culture
Hydrogels were initially sterilized in 95% v/v ethanol (2 h) and re-equilibrated in sterile PBS prior to seeding of rat aortic endothelial cells (passage 6; Cell Applications, Inc., San Diego, CA) onto the gel surface. The cells were cultured in MCDB-131 medium supplemented with 10% v/v FBS (Invitrogen, Carlsbad, CA), 1% v/v penicillin-streptomycin (Invitrogen), 50 μg/ml EC growth supplement (Beckton Dickinson, Franklin Lakes, NJ), 4 mM L-glutamine (Invitrogen), and 30 U/ml heparin (Sigma). Spent medium was replaced thrice weekly. Possibly due to the high water content of the gels and high anionicity of component HA, the adherent ECs were found to be unable to fully spread upon seeding. Previous studies have shown protein adsorption onto hydrogel surfaces enhances cellular affinity and interaction with the underlying bulk material10. Therefore, we sought to address this issue by adsorbing a mixture of adhesive proteins (Matrigel; BD Biosciences), similar to the composition of the vascular basement membrane, onto the hydrogel surfaces by incubating each gel in 3 ml of a dilute matrigel solution (10 μg/ml of PBS; 4 h) prior to EC seeding.
Protein Adsorption Assay
The protein content in the bulk coating solution of matrigel was quantified using a DC protein assay (Bio-Rad, Hercules, CA), before and after incubation with hydrogel formulations, to estimate loss via adsorption onto the respective gel surfaces. Briefly, 100 μl of bulk matrigel solution was combined with 500 μl of reagent A and 4 ml of reagent B of the assay kit, and incubated for 15 min. A 200-μl aliquot of the reacted sample solutions was pipetted into micro-well plates and their absorbances were measured at λ = 750 nm. Background absorbance from control wells containing PBS only, was subtracted from the sample absorbance measurements. The detected absorbencies (n = 4) were compared to standards prepared with bovine plasma gamma globulin (Bio-Rad) to estimate the depletion of proteins from the matrigel stock solution, and thus calculate the total amount of protein deposited onto the DVS-HA hydrogels.
Fluorescence Detection of Cell Viability
Calcein acetoxymethyl ester (Calcein AM; VWR) was used to fluorescently label live (metabolically-active) cells when adhered atop HA hydrogels, so as to assess their morphology; dead cells were lost into the culture medium and thus not labeled for. ECs were seeded onto the HA gel surfaces at a density of 2 ×104 cells/gel and cultured for 1 week prior to calcein AM detection. Cells were then incubated with calcein AM (8×10−3 mg/ml) in Hank’s Buffered Salt Solution (HBSS; 45 min) and fixed in 4% w/v paraformaldehyde (Sigma). Surface-adherent ECs were imaged on a Leica TCS SP2 AOBS confocal microscope (n = 4/gel formulation).
MTT Assay for EC Proliferation
EC proliferation on gels was quantified using a colorimetric MTT assay, described previously by Denizot and Lang 14. ECs were seeded onto the gels at a density of 1 ×104 and cultured for 2 wks. The number of live, attached cells on each set of samples was quantified at 1 and 14 days, respectively, after seeding. At the end of the culture period, medium was aspirated from the wells, and the gels with adherent cells were transferred to fresh wells. Gels were briefly rinsed with 1×PBS to remove unattached cells. A 2-ml aliquot of 2-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyl tetrazolium bromide (MTT) reagent (1 mg/mL in phenol red-free DMEMF12) was added to each gel and incubated for 3 h at 37°C. Each sample was then overlaid with 2 ml of n-propanol and mixed for 15 min on a rotary shaker. The formazan product produced by live, attached cells upon uptake of the MTT reagent, was extracted by the propanol (Sigma), 200 μl of the extract was pipetted into micro-well plates, and the absorbance was measured at λ= 550 nm (n = 4). Background absorbance from control wells containing no cells was subtracted from the sample absorbance measurements. For generation of a standard curve for calibration of the measured absorbancies, between cells were seeded at known densities, between 5 × 103 and 3 × 105/well in 6-well polystyrene culture plates, allowed to attach overnight, and then quantified with the MTT assay.
Immunolabeling for EC CAM Expression
Expression levels of CAM (ICAM-1, VCAM-1) by ECs cultured atop the various HA hydrogel formulations were compared by immunofluorescence and quantified by calculating the mean fluorescence intensity (MFI) on a per cell basis. ECs were seeded onto the gels at 2 ×104 and cultured for 1 wk prior to analysis. The cells were then fixed with 4% w/v paraformaldehyde (Sigma) and incubated at 4 °C for 1 h with fluorescein isothiocyanate (FITC)-conjugated mouse anti-rat CD54 (ICAM-1; Abcam, Cambridge, MA) and Alexa 488-conjugated mouse anti-rat CD106 (VCAM-1; Biolegend, San Diego, CA) diluted 1:100 v/v in PBS-azide. Draq 5 (1:2000 in PBS, 10 min; Biostatus) was also used to fluorescently label the cell nuclei in order to count the number of cells within each image. The cells were imaged on a TCS SP2 AOBS confocal microscope (n = 9 regions/gel) at a constant gain and offset for FITC (650, 0.8). MFI per cell was quantified using Image J software.
Statistical Analysis
All experiments were performed in triplicate unless otherwise mentioned. Statistical significance between and within groups was determined by using a one-way ANOVA test with SPSS software (SPSS Inc., Chicago, IL). An alpha level of 0.05 was used for all statistical tests. Quantitative results are reported as mean ± standard deviation.
RESULTS
HMW HA and HA oligomers (HA-o) were combined into hydrogels by crosslinking with DVS, resulting in 8 total gel formulations: 2 crosslinking concentrations (1:1, 1:2 w/w DVS:HA) ×4 oligomer concentrations (0, 5, 10, 20% w/w HA-o/HA). Table I shows the measured dimensions of the hydrated hydrogels crosslinked in 0.79 cm2 molds. The HA oligomer (HA-o) content appeared to minimally affect hydrogel size with limited increases in height (h) and diameter (d). On the other hand, increasing the DVS to HA ratio drastically reduced the size of the gels.
Table 1. Size of Hydrated DVS-HA Hydrogels.
Increasing the amount of DVS within the gels resulted in reduced gel sizes and an opaque coloration. Measuring the dimensions of the DVS-HA hydrogels showed that both height (h) and diameter (d) of the cylindrical gels also slightly increased with HA oligomer content.
| HA-o w/w |
|||||
|---|---|---|---|---|---|
| DVS:HA w/w | 0% | 5% | 10% | 20% | |
| 1:1 | h | 0.350 | 0.358 | 0.368 | 0.372 |
| d | 1.386 | 1.418 | 1.458 | 1.474 | |
| 1:2 | h | 0.433 | 0.451 | 0.463 | 0.474 |
| d | 1.716 | 1.787 | 1.835 | 1.876 | |
Incorporation of HA Oligomers into Hydrogels
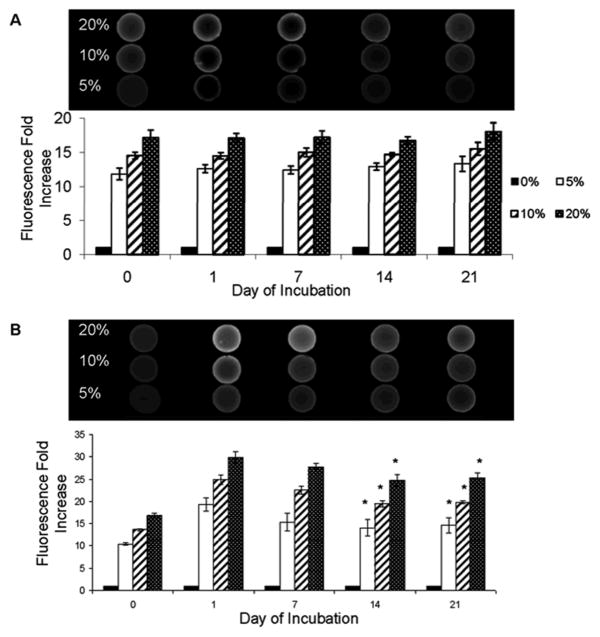
Irrespective of crosslinker content, higher HA-o content within the DVS-HA gels corresponded with increased fluorescence intensity (Figure 1). However, gels containing 1:1 w/w ratios of DVS and HA fluoresced to a greater degree than those containing 1:2 w/w ratios of DVS and HA. The fluorescence intensity levels of 1:1 DVS-HA was unchanged over 21 days of incubation in PBS. Gels containing 1:2 w/w ratios of DVS to HA showed a sharp increase in fluorescence intensity between day 0 and 1; the fluorescence intensity mildly decreased between day 1 and 14, but remained constant thereafter.
Figure 1.
Mean Fluorescence Intensities (MFI) due to HA oligomers (HA-o) embedded within DVS-HA gels. The oligomer content within 1:1 w/w DVS-HA (A) appeared to remain constant over the 21 days of incubation whereas the oligomer content of 1:2 w/w DVS-HA (B) decreased slightly before reaching a plateau. However, within both DVS concentration groups, the differences in fluorescence intensities between gels containing different amounts of HA oligomers were maintained across time points [* denotes statistical significance of differences deemed for a p-value < 0.05 in comparison to day 1].
Hydrogel Crosslinking and Swelling
The apparent crosslinking density and swelling ratio of the various DVS-HA gel formulations were used to estimate the accomplished degree of crosslinking. The results show that apparent crosslinking density (Table II) and swelling ratio (Figure 2) are dependent on both concentrations of DVS and presence of HA-o. For a given DVS concentration, the incorporation of HA-o into the hydrogels increased their swelling capacity over gels containing only HA 1500. On the other hand, when other DVS concentration remained unchanged, gels containing higher HA-o content exhibited lower apparent crosslinking densities. For each HA-o concentration, increasing the DVS amounts within the gels reduced their ability to swell and increased their apparent crosslinking density. For all gel formulations, the apparent crosslinking densities were much lower than that theoretically-calculated for each assuming 100% of the incorporated DVS was actually utilized in crosslinking; the overall crosslinking efficiency was calculated to be 10–15% for 1:1 w/w DVS-HA and 15 – 20% for 1:2 w/w DVS-HA.
Table 2. Apparent Crosslinking Density of DVS-HA.
Overall, 1:1 w/w DVS-HA gels (A) exhibited a higher apparent crosslinking density than 1:2 w/w DVS-HA gels (B). The addition of HA oligomers (HA-o) into the hydrogels reduced their apparent crosslinking density. These values were approximately 10 – 20% of the estimated theoretical values assuming that all the provided DVS was involved in crosslinking.
| 1:1 w/w DVS:HA νe (mol/cm3 × 106) |
1:2 w/w DVS:HA νe (mol/cm3 × 106) |
|||
|---|---|---|---|---|
| HA-o w/w | Theoretical | Measured | Theoretical | Measured |
| 0% | 143.37 | 21.35 ± 0.85 | 37.87 | 7.17 ± 1.71 |
| 5% | 133.91 | 18.77 ± 1.11 | 33.53 | 6.59 ± 0.76 |
| 10% | 123.16 | 16.77 ± 0.71 | 30.95 | 6.35 ± 0.78 |
| 20% | 119.24 | 12.19 ± 0.76 | 28.96 | 4.72 ± 0.36 |
Figure 2.
Swelling ratios (mg wet weight/mg dry weight) of DVS-HA hydrogels. Incorporation of HA oligomers within the DVS-HA hydrogels mildly increased their swelling capacity irrespective of their crosslinking density. Irrespective of HA oligomer content, the swelling ratio was dramatically lower for the more crosslinked gels. [* denotes statistical significance of differences deemed for a p-value < 0.05 in comparison to HA oligomer-free gels within the respective groups of gels].
Hydrogel Stiffness and Resistance to Degradation
Rheological analyses quantified the viscous and elastic responses of DVS-HA gels as a function of their HA-o and DVS content, providing a relative indication of their stiffness. In all cases, the storage moduli (G′) and loss moduli (G″) were generally independent of frequency, with the G′ values always higher than G″ (Figure 3). The stiffness (G′) of the gels correlated directly with DVS content and inversely with HA-o content.
Figure 3.

Viscoelastic properties of DVS-HA. The storage moduli (G′) in all cases were greater than the loss moduli (G″). Increasing the concentration of DVS crosslinker within the hydrogels (i.e., 1:1 w/w (A) vs. 1:2 w/w (B) DVS-HA gels), resulted in a greater G′ and overall stiffness of the gels. The addition of HA oligomers, however, reduced the storage moduli indicating lowered gel stiffness.
When incubated in a solutions containing super-physiologic concentrations of bovine testicular hyaluronidase, the 1:1 w/w DVS-HA gels (Figure 4A, Table III) degraded more slowly than 1: 2 w/w DVS-HA gels incorporating identical HA-o content (Figure 4B, Table III); within each group of gels, gel degradation rates increased with HA-o content.
Figure 4.

Degradation of DVS-HA in vitro. Hydrogels containing greater amounts of DVS crosslinker, i.e., the 1:1 w/w DVS-HA gels (A) exhibited greater stability against degradation by testicular hyaluronidase than the less crosslinked gel formulations, i.e., vs. 1:2 w/w (B) DVS-HA. However, increasing the concentration of HA oligomer content within each of these gel formulations enhanced the degradation rate of the gels.
Table 3. Degradation Rate of DVS-HA gel formulations In Vitro.
A greater DVS concentration, i.e. 1:1 w/w of DVS to HA (A) vs. a 1:2 w/w ratio (B) within DVS-HA gels, and increasing the content of HA oligomers (HA-o), resulted in a higher rate of degradation when incubated with super-physiologic concentrations of testicular hyaluronidase enzyme. ‘k’ represents the reaction rate constant (hr−1).
| 1:1 w/w DVS:HA |
1:2 w/w DVS:HA |
|||
|---|---|---|---|---|
| HA-o w/w | k (hr-1) | pvalue | k(hr-1) | pvalue |
| 0% | 0.0103 | <0.00001 | 0.068 | 0.0045 |
| 5% | 0.014 | <0.00001 | 0.0708 | 0.016 |
| 10% | 0.0136 | 0.0042 | 0.0969 | 0.02 |
| 20% | 0.0221 | 0.0016 | 0.1227 | 0.018 |
Hydrogel Biocompatibility
In all cases, H&E staining revealed distinct rings of cellularized tissue around the implants though the capsule was minimal around some samples,. The thicknesses of these highly cellularized regions appeared to be greater around the more crosslinked gels and those incorporating greater HA-o content (Figure 5). Though the density of collagen matrix immediately surrounding the implants was less than that further a field, the cell density was much greater, suggesting that these cells were not collagen-generating fibroblasts (Figure 5). Also, unlike fibroblasts, these cells neither appeared flattened, nor aligned with the collagen fibers, further reinforcing our belief that they were inflammatory cells. As can be seen in Figures 5, the more crosslinked gels stimulated much greater inflammatory cell recruitment (see darkened region near implant). The densities of cells in the tissue further afield from the gel implants were visually similar for all gel formulations; these cells also appeared more flattened and fibroblast-like compared to the cells closer to the implants. Immunofluorescence studies (Figure 6) confirmed these results in that greater cellularity (blue) was noted in the region immediately surrounding all the implants, than further afield, and that the thickness of this layer was greater around gels that contained (a) greater DVS content and (b) higher oligomer content. These studies also confirmed lower densities of collagen matrix (green) in the regions immediately surrounding the implants than regions further afield. Very little tissue infiltration was observed within DVS-HA gels, as compared to matrigel plugs (Figure 5). In particular, the implants that contained 1:1 w/w DVS-HA were consistently void of any inward tissue projections. Sham controls generated no visible fibrous tissue deposition except in one case where a very thin, minute, and irregular semblance of a fibrous mass was seen.
Figure 5.
H&E staining of sub-cutaneous DVS-HA gel implants. Light microscopy images (A) showed a distinct, darkened ring of inflammatory cells (see arrows) to surround all gel implants. The thickness of this highly cellular region appeared to be increase with the extent of DVS-crosslinking within the gels. The more crosslinked hydrogels, i.e., the 1:1 w/w DVS-HA gels stimulated an enhanced inflammatory response from the surrounding tissue that was greater than both the less crosslinked gels, i.e., the 1:2 w/w DVS-HA gels, and matrigel plugs. Likewise, gels containing greater HA oligomer (HA-o) content, appeared to incite a greater inflammatory response, though these effects were muted compared to the impact of DVS crosslinking. The macroscopic images (B) show very little tissue infiltration within DVS-HA, as compared to the matrigel control. The defect containing the 1:1 w/w DVS-HA gel implants were consistently void of inward tissue projections, though some tissue projections (see arrows) were observed in those defects that contained the 1:2 w/w DVS-HA gels.
Figure 6.
Immunofluorescence analysis of fibrous mass surrounding implants. Gels containing greater crosslinker (DVS) content (1:1 DVS-HA vs. 1:2 DVS-HA) prompted greater cellularity in the region immediately surrounding them though the fibrous mass in this region, shown to be collagen I (green), appeared far less dense than in regions further afield. This suggested that the cells near the implant are likely not collagen-synthesizing fibroblasts, but rather inflammatory cell recruits. The recruitment of the inflammatory cells (blue) appeared to be enhanced by increasing HA oligomer (HA-o) content.
Protein Adsorption
The total protein content within the bulk matrigel-coating suspension decreased following incubation with DVS-HA gels indicating successful adsorption of protein onto the hydrogel surfaces (Figure 7). The total amount of protein adsorbed onto 1:2 w/w DVS-HA gels was greater than the 1:1 w/w DVS-HA gels but when adjusted for increased swelling, and therefore increased surface area (SA) of 1:2 w/w DVS-HA (SA = 3.8 ± 0.3 cm2) relative to 1:1 w/w DVS-HA gels (2.4 ± 0.1 cm2), the adsorbed protein density was found to be identical on both gel formulations. Likewise, regardless of crosslinker amounts, matrigel adsorption on the gels was independent of the content of incorporated HA oligomers (HA-o).
Figure 7.
Matrigel adsorption onto DVS-HA gels. A drop in protein content within the bulk coating suspension was observed indicating protein adsorption onto the gel surfaces. Calculations revealed that the density of adsorbed protein was the same on all DVS-HA gels, regardless of crosslinker density or HA oligomer content. [* denotes statistical significance of difference vs. no gel condition, deemed for a p-value of < 0.05].
EC Morphology and Proliferation
ECs cultured atop the matrigel-adsorbed DVS-HA gels appeared spread, and exhibited a natural cobblestone morphology when visualized after calcein AM staining (Figure 8). The cells appeared morphologically similar to those cultured on fibronectin-coated glass (A) but did not form tubes as did cells cultured on matrigel substrates (B). The ECs appeared as isolated clusters on 1:2 w/w DVS-HA gels (G-J), while more homogenously distributed atop 1:1 w/w DVS-HA gels (C-F). All gel formulations, irrespective of DVS and HA-o content, adsorbed identical amount of matrigel on a per unit area basis, therefore, any differences in cell proliferation between the gel formulations were purely due differences in HA-o content and/or DVS concentration. In absence of any oligomers, EC proliferation on 1:1 DVS-HA hydrogels was greater than the less crosslinked 1:2 w/w DVS-HA gels (Figure 8). This difference was maintained even when the HA oligomers were incorporated into the gels. Within each crosslinking group, EC proliferation increased in direct correlation with incorporated HA-o amounts. However, even on the gels incorporating the highest amounts of HA-o (i.e. 20%), EC proliferation over 14 days of culture was much slower relative to those ECs cultured on matrigel and fibronectin substrates.
Figure 8.
Morphology and proliferation of ECs cultured on matrigel-adsorbed DVS-HA gels. The incorporation of HA oligomers within the gels enhanced EC adherence onto their surfaces. The ECs appeared to spread and exhibited natural cobblestone morphology similar to cells cultured on fibronectin (A), but did not form tubular projections as did ECs cultured on matrigel alone (B). The ECs formed isolated clusters atop 1:2 w/w DVS-HA gels (C-F), while they were more uniformly distributed atop the more crosslinked 1:1 w/w DVS-HA gels (G-J). The 1:1 w/w DVS-HA gels stimulated greater EC proliferation than 1:2 w/w DVS-HA gels, as did increased HA oligomer content within either gel type. In all cases, the surface-embedded HA oligomers were able to interact with ECs to enhance their proliferation. However, EC proliferation levels on these gels were overall lower than those cultured on matrigel and fibronectin substrates. [* denotes statistical significance of differences in proliferation ratios relative to HA-o-free gels within the respective crosslinking groups, deemed for a p-value < 0.05].
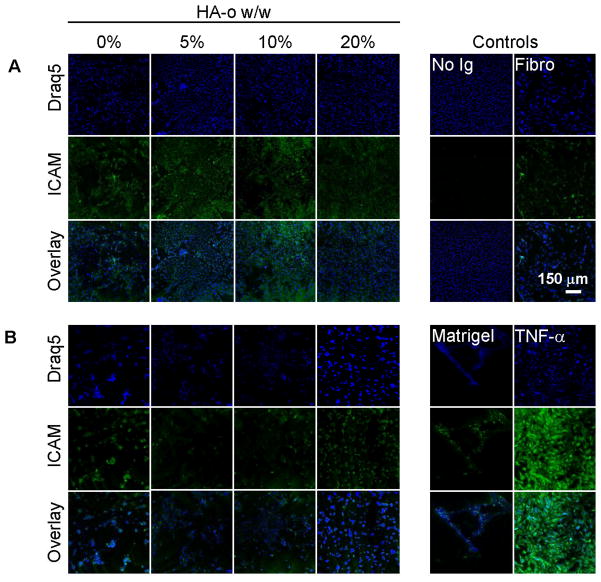
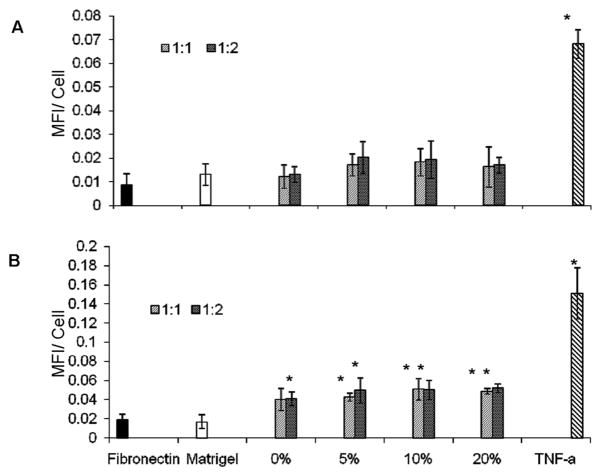
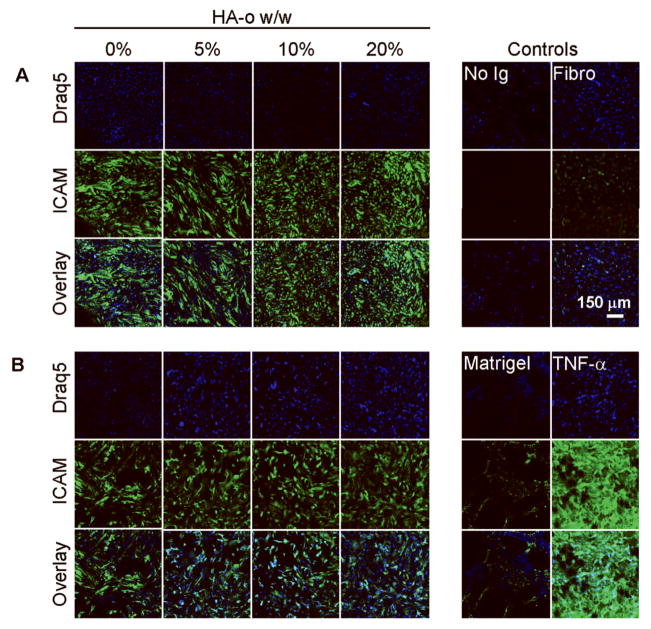
CAM Expression by Cultured ECs
A visual assessment of the fluorescence micrographs (Figure 9) indicated that ICAM expression levels by ECs cultured atop matrigel-adsorbed DVS-HA are similar to ECs grown on fibronectin and matrigel substrates, and far less intense than TNF-α-stimulated ECs. Quantification of the fluorescence intensities due to ICAM expression confirmed this observation and additionally showed EC ICAM expression to remain unchanged upon incorporation of HA-o into DVS-HA gels (Figure 11). VCAM-1 expression levels (Figures 10, 11) were also unaffected by HA-o content within DVS-HA gels and remained much more attenuated in comparison to TNF-α stimulated ECs. However, VCAM-1 expression of these ECs was somewhat greater than the ECs cultured on matrigel and fibronectin substrates.
Figure 9.
ICAM-1 expression of ECs cultured on DVS-HA gels. ICAM-1 expression (green) by ECs (nuclei appear blue) were very similar across the DVS-HA gel formulations. ICAM-1 expression in these cases was also similar to that that expressed by ECs cultured on fibronectin and matrigel substrates, but much lower than that expressed by TNF-α-stimulated ECs.
Figure 11.
Mean fluorescence intensities (MFI) due to ICAM-1 (A) and VCAM-1 (B) expression by ECs cultured on DVS-HA. The data confirms visual observations that the fluorescence due to EC expression of both CAMs remained the same irrespective of the formulation of the substrate DVS-HA gels. While the intensity of fluorescence due to ICAM-1 expression by ECs cultured on the gels was similar to that expressed by ECs cultured on fibronectin and matrigel, fluorescence intensities due to VCAM-1 expression were slightly elevated. Both ICAM-1 and VCAM-1 expression levels by ECs cultured on the DVS-HA gels (all formulations) were however much lower than ECs stimulated with TNF-α. [* denotes statistical significance of differences of MFI vs. ECs cultured on fibronectin, deemed for a p-value of < 0.05].
Figure 10.
VCAM-1 expression of ECs cultured on DVS-HA. ECs (nuclei in blue) cultured on the various DVS-HA gel formulations expressed similar levels of VCAM-1 (green), which were much lower than when ECs were stimulated with TNF-α, but were somewhat elevated over EC cultures atop fibronectin and matrigel substrates.
DISCUSSION
In order to create a HA biomaterial capable of surviving long-term implantation in vivo, it must be converted into a stable, biocompatible, insoluble form with good handling properties and mechanics appropriate to the site of implantation. Chemical modification has been well recognized as a means to enhance the biostability and mechanical properties of native HA. Two possible methods to modify HA are through derivatization and cross-linking, both of which can be achieved through reactions between the available functional groups of HA (-COOH, -OH). In the current study, we chose to use DVS to crosslink the hydroxyl (-OH) groups on the HA chains under alkaline conditions to yield stable hydrogels containing sulfonyl-bis-ethyl linkages. Such crosslinking produces a network of HA chains that is no longer water-soluble. Limited studies in the past have suggested that crosslinked HA gels containing a low concentration of DVS-HA retain the biologic characteristics of un-crosslinked HA, especially their high biocompatibility 15. DVS-HA exhibit significant flexibility in their mechanical properties and rheology, ideal for a wide variety of medical applications. For this reason, a number of clinical products approved by the Food and Drug Administration based on this formulation have been generated for use as post-surgical anti-adhesive films, ocular fillings, and joint lubricants, among others 16,17. However, it is to be noted that all these applications do not involve substantial cell interactions with the biomaterial, and instead capitalize on the poor cell binding properties of DVS-HA. In other words, the applications have capitalized on the physical and rheological characteristics of these formulations rather than their biologic interaction with host cell types.
Previous studies have suggested that DVS-HA gels containing long-chain HA (MW>1 ×106 Da) interact poorly with cells11, 12, which can be detrimental to our intended use of these materials as cellular scaffolds for tissue regeneration. This has been attributed variously to their physical properties (porosity, pore size, extreme hydrophilicity), chemical characteristics (anionicity, degree of hydration, crosslinker), and biologic composition (long-chain vs. fragmented HA). Of these parameters, the size of component HA chains seem to most critically influence cell response. Native long-chain HA has been implicated in cell excluding mechanisms, whereas HA fragments, especially HA oligomers, have been known to elicit enhanced cell responses, although these may be exaggerated and undesirable 18. The use of HA gels as tissue-engineering scaffolds may thus necessitate a need to optimize gel composition, derivatization and crosslinking chemistries, and post-formulation tailoring strategies to more closely modulate the physical and biologic characteristics necessary to elicit ideal cell responses To enhance cell attachment, we have previously developed two techniques to micro-texture the gel surface by controlled exposure to UV light and γ-irradiation11, 12. We demonstrated that both UV treatment and γ-irradiation alter the surface topography to create a less-uniform and ridged surface, more conducive to cell adherence and spreading. However, the greater impetus to ready cell adherence and proliferation on these irradiated gels was found to be shorter-sized HA fragments generated on the gel surfaces by scission/de-polymerization of long-chain HA by random ionization caused by irradiation. Since the effects of UV and γ-irradiation are highly variable, such methods are neither closely replicatable nor easily controlled, so that gels with predictable and bioactivity and other biologic and physical properties are difficult to create. Accordingly, we decided to explore the possibility of directly incorporating HA oligomers into DVS-HA scaffolds thereby influencing cell behavior in a predictable and controlled manner.
In this study we explore the concept of incorporating bioactive HA oligomers into DVS-crosslinked solutions of long-chain HA, to create standalone biomaterials for use as vascular scaffolds capable of functional and complete endothelialization. As mentioned above, studies have shown that DVS-crosslinked HA biomaterials containing only HMW HA do not support EC attachment possibly due to the extremely hydrophilic nature and anionicity of HMW HA which renders the surface thermodynamically unfavorable for cell interaction10. In contrast, we found that exogenous HA oligomers (HA-o), primarily 6mers and 12mers, stimulate proliferation, angiogenesis and the secretion of angiogenic growth factors by ECs cultured in vitro. Further, immobilization of derivatized HA-o mixtures onto 2-D cellular substrates did not alter their modulation of EC behavior, reinforcing our hypothesis that HA-o may be chemically crosslinked into a hydrogel and yet retain their innate biologic properties. However, in both presentation modalities (exogenous vs. surface-immobilized), the HA-o somewhat enhanced EC expression of inflammatory cell adhesion molecules (CAMs). We reason that we may be able to circumvent these problems by incorporating bioactive HA-o within crosslinked constructs containing bioinert, HMW HA, which would serve to temper EC activation by HA oligomers, while stimulating the adhesion and proliferation of ECs. In addition, the presence of HMW HA in crosslinked form would allow us to create solid biomaterials that may be handled easily, unlike the fluid mixtures that result when HA oligomers alone are crosslinked. For the same reason, high HA-o content within DVS-HA gels can compromise their physical and mechanical characteristics. Thus, we seek to investigate impact of HA-o content on the gel mechanical properties in addition to their biologic effects (e.g., endothelialization, biocompatibility).
In order to determine the initial incorporation and long-term retention of HA-o within DVS-HA, the fluorescence intensity of AMAC-tagged HA-o within the gels was quantified over time. Greater HA-o concentrations within the gels resulted in higher fluorescence intensities at all analysis time points indicating that a difference in HA-o content was maintained between the different formulations. The intensity of fluorescence emanating from 1:1 w/w DVS-HA gels was consistently lower than the 1:2 w/w DVS-HA gels which may be due to lack of complete penetration of the UV through the more crosslinked gels during imaging. The lower swelling exhibited by 1:1 w/w DVS-HA gels in comparison to 1:2 w/w DVS-HA gels suggested lower water content and, therefore, higher polymer density. Thus in imaging 1:1 w/w DVS-HA gels under UV light, it is possible that more UV rays were reflected by the denser polymer configuration reducing the detected fluorescence intensity relative to 1:2 w/w DVS-HA gels. In support of this hypothesis, we observe that fluorescence from the 1:2 w/w DVS-HA gels are similar to 1:1 w/w DVS-HA gels immediately after crosslinking (day 0), but higher following 1 day of incubation in PBS, during which time the 1:2 w/w DVS-HA gels swelled substantially. With regard to 1:1 w/w DVS-HA gels, the fluorescence intensity was maintained constant through the entire period of incubation in PBS, suggesting significant retention of the incorporated HA-o. On the other hand, an overall loss in fluorescence was observed from the 1:2 w/w DVS-HA gels over time. We believe that DVS crosslinking within these gels may have been insufficient and thus incapable of linking all of the added HA-o. Since polymerization of the HA solution into a highly viscous/solid hydrogel occurred very rapidly (< 2 minutes), mixing the crosslinker and the HA solution prior to casting was not an option in our studies. Rather, the DVS crosslinker was added to the HA solution, then mixed rapidly until it became highly viscous, after which point of time, the solution was allowed to cure for 2 hours. It is quite possible that the rapid gelling of the HA solution, and the short, allowable mixing time both limited diffusion of the crosslinker through the HA solution, thus contributing to the very low crosslinking efficiencies. Due to this, it is likely some un-crosslinked HA-o was merely physically entrapped within the DVS-HA gels during the crosslinking process and leached out gradually during subsequent incubation/rinsing in PBS.
Relative to synthetic materials, biomaterials fabricated from naturally-occurring molecules such as HA are more susceptible to degradation within the body due to the presence of enzymes that specifically target these molecules. Thus, we sought to investigate if crosslinking HA with DVS and incorporation of HA-o into the gels would inhibit its enzymatic depolymerization. When incubated with super-physiologic concentrations of testicular hyaluronidase enzyme, the degradation rates of the DVS-HA gels were found to be influenced by their swelling capacity (enzyme concentration within the gels) and apparent crosslinking density (HA bonding with the gels). Since hydrogels containing lower concentrations of DVS crosslinker and higher amounts of HA-o exhibited enhanced capacity to hydrate and swell, it is possible that they also imbibed a higher amount of enzyme from the bulk solution into the gel interior resulting in accelerated degradation; the additional stability provided by more robust crosslinking of HA likely also contributed to the relatively lower susceptibility to enzymatic breakdown. Additionally, these hydrogels possessed greater surface areas enhancing the degree of enzymatic attack from the bulk solution.
Rheological analysis quantitatively estimated the viscous and elastic responses of DVS-HA gels. Both the storage moduli (G′) and loss moduli (G″) were found to be independent of frequency. The G′ values were always higher than G″, typical of “strong hydrogels”, whose response to oscillating frequency more closely resembles a solid than a fluid. By increasing the concentration of DVS within these gels we were able to obtain G′ (1400 – 300 Pa) and G″ (10–40 Pa) that were much higher than commercially available DVS-HA products for soft tissue augmentation; the G′ and G″ of Hylaform® (a dermal filler) are 185 Pa and 21 Pa, respectively, as measured at 3.0 Hz 22. However, vascular regenerative applications demand far more resilient biomaterials then does skin due to the dynamic and strenuous environment of blood vessel tissues. A completely sulfated form of hyaluronic acid (HYAFF®), which has shown potential as a regenerative vascular grafting material, but not particularly conducive to endothelialization, exhibits G′ and G″ values of ~ 420 kPa and 17 kPa, respectively, at 1 Hz 19, 20. The high strength of this and other such vascular grafting biomaterials indicates that our DVS-HA hydrogels would not by themselves be suitable for use as a vascular scaffolding materials, but rather must be composited with other existing natural or synthetic graft materials, at least from the standpoint of surviving the forces experienced in a vascular environment.
Following sub-cutaneous implantation in rats, inflammatory cells were found to be recruited around the gel implants, regardless of their formulation. Hydrogels containing greater DVS-crosslinker densities stimulated an enhanced inflammatory response from the surrounding tissue. Free-form DVS is known to be toxic; vinyl sulfones have been shown to selectively inhibit glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which is an important ATP-generating enzyme in glycolysis 21. However, it has been shown to be biocompatible when incorporated into hydrogels as a crosslinker for HA at low concentrations such as that in our 1:2 w/w DVS: HA hydrogel formulations we, and within commercially available products 19. Here we show that the higher DVS concentrations within HA gels, required from the standpoint of imparting good biomaterial handling properties and improved mechanics, results in an exaggerated inflammatory tissue response. The addition of HA-o also appeared to increase the inflammatory response. HA is known to interact with multiple cellular receptors, including CD44, receptor for HA mediated motility (RHAMM), and toll-like receptor 4 (TRL4) 4. However, the cellular response is molecular weight dependant. It has been proposed that HMW HA interacts with cellular receptors in a polyvalent manner preventing receptor-receptor contact and inhibiting intracellular signaling pathways, thereby maintaining an anti-inflammaotry and immunosuppressive nature. In contrast, HA oligomers interact with cellular receptors in a monovalent manner allowing receptor clustering and multi-fragment binding stimulating a multitude of cell signaling cascades23, which may have contributed to the mild increase in cellularity surrounding implants with higher concentrations of HA-o 23.
In ascertaining the source of the inflammatory response, i.e., the surgical procedure itself or the implanted materials, it may be useful to consider that the surgical procedure and postoperative treatment in all the rats was the same. In this light, and in consideration of the established bioinertness of long-chain HA incorporating DVS-crosslinked HA gels (clinically used as tissue spacers following surgery), the minimal inflammatory response observed seen around HA-o-free 1: 2 w/w DVS-HA gels might be deemed to represent the basal inflammatory response of the rat to the surgical procedure itself. The additional inflammatory responses noted on increasing DVS crosslinker amounts and/or HA-o content within the gels are however likely due to the differences in material formulation.
The region around the implants also contained much less collagen I than further afield, strongly suggesting that the dense population of cells in this region are inflammatory, and not collagen-producing fibroblasts. The primary cell types that respond to subcutaneous implants are typically fibroblasts and inflammatory cells. Fibroblast attempt to isolate the implant by surrounding it with a collagen I-rich fibrous capsule, while inflammatory cells degrade/digest the implant. The lack of collagen I adjacent to the implant indicates an absence of fibroblastic activity. In addition, very little tissue infiltrated into the DVS-HA gels, relative to matrigel controls. The defect containing 1:1 w/w DVS-HA gel implant remained consistently void of inward tissue projections, though, some tissue projections were observed in 1:2 w/w DVS-HA gels, possibly due to the lower crosslinking density (lowered stiffness) allowing cells to infiltrate.
The goal of this work was to incorporate HA oligomers into a DVS-crosslinked gel containing HMW HA in order to promote EC attachment/proliferation on the hydrogel surface, while tempering the inflammatory cell response to HA oligomers by the presence of HMW HA. We found that ECs, cultured in vitro, attached onto gels with incorporated HA-o, and the HA-o content appeared to enhance this attachment. However, the EC maintained a rounded morphology throughout the culture period. Two factors known to influence cell morphology are surface charge and substrate hydrophilicity. Cells which typically are negatively-charged, adhere far less strongly to substrates containing acidic or neutral groups than to those with basic (positively charged) groups 24. In addition, the DVS-HA gels are very hydrophilic in nature and therefore contain high water content. Previous studies have shown that such extremely hydrophilic surfaces are thermodynamically unfavorable for cell attachment 25. As a cell-binding ligand, and via its ability to enhance surface roughness, HA-o was able to encourage EC adherence. However, the extreme anionicity of HMW HA and excessive water content within the DVS-HA gels likely dissuaded the ECs from spreading.
Matrigel is a mixture of predominantly laminin and collagen IV, a substrate similar to the natural basement membrane of blood vessels. In order to promote EC spreading, a low concentration of matrigel was adsorbed onto the hydrogel surfaces, which allowed the ECs to spread and exhibit their natural morphology while interacting with the HMW HA and HA oligomers also present on the hydrogel surface. Matrigel was successfully adsorbed onto the hydrogel surfaces and the Dc protein assay showed the concentration remained constant (~12 μg/cm2) on all hydrogel formulations. The natural cobblestone morphology of ECs on matrigel adsorbed DVS-HA indicate the adsorption process did not alter the conformation of laminin and collagen IV, a common problem with synthetic materials (i.e. polymethylmethacrylate). However, ECs remained fairly clustered in isolated locations on 1:2 w/w DVS-HA. This is likely due to the higher water content and anionicity of 1:2 w/w DVS-HA, which isolates cell attachment to specific regions of the hydogel surface. It is interesting to note that compared to ECs cultured on pure matrigel, those cultured on matrigel-coated HA gels, free of HA oligomers, exhibited much lower levels of proliferation. This suggests that the low-density matrigel surface coatings atop the HA gels primarily serve to facilitate cell adherence and morphology, and do not necessarily impact EC proliferation. On the other hand, the increase in EC adherence and proliferation as a direct function of HA-o content suggests that the ECs were able to interact with the embedded HA-o and as a result, up-regulate proliferation. Therefore, we can conclude that the incorporation of HA-o into vascular constructs benefits endothelialization by the promotion of EC proliferation. We believe the level of proliferation did not attain that of fibronectin and matrigel due to the vast excess of HMW HA on the hydrogel surface, which suppresses the effects of the HA oligomers.
The level of ICAM-1 expression by the ECs was also suppressed by HMW HA. HA oligomers elevated both ICAM-1 and VCAM-1 expression of ECs when presented as an exogenous supplement or immobilized surface but when these oligomers were embedded within HMW HA, EC ICAM-1 expression remained similar to DVS-HA without HA oligomers and the fibronectin control. VCAM-1 expression, on the other hand, was elevated on all DVS-HA hydrogels irrespective of HA oligomer concentration. This may be due to the high concentration of DVS within these hydrogels, which was also found to stimulate an exaggerated inflammatory response in the subcutaneous in vivo model.
CONCLUSIONS
In this study, we investigated hydrogels composed of HMW HA and HA oligomers, as potential biomaterials for the regeneration of luminal ECs of blood vessels. We showed the mechanical (degradation, viscoelasticity) and physical (crosslinking density, swelling) properties of these hydrogel can be adjusted by varying the crosslinker and oligomer densities within them. However, the overall strength of these hydrogels is too low for vascular applications as a stand-alone material and therefore, can only be recommended for use as a composite with other more mechanically-compliant. The presence of oligomers within DVS-HA seemed to enhance EC attachment and proliferation, supporting the notion that HA oligomers are more conducive to EC growth than other forms of HA. However, the high concentration of DVS, required to impart good handling properties of these gels, appears to be somewhat non-biocompatible in eliciting mild inflammatory responses and enhanced VCAM-1 expression by ECs. Therefore, another crosslinker may be more appropriate to use, which could result in better mechanics and improved biocompatibility. If no such crosslinker exists, a better approach might be to abandon the idea of a standalone HA vascular graft materials, and as we have described previously, chemically derivatize and immobilize both HA oligomers and HMW HA in a controlled manner onto the lumenal surface of existing synthetic or tissue engineered grafts, so that improved, predictable, and functional endothelialization may be achieved, while eliminating the need to address the biomechanics of the graft material at large. This method would also eliminate the need for crosslinking HA, which would likely circumvent the problems associated with crosslinker-mediated toxicity or inflammation.
Acknowledgments
This study was supported by grants from the National Institutes of Health (EB 006078-01A1, CO6RR01882, P20RR016461) and the American Heart Association (SDG 0335085N). The authors would like to thank Mr. Jonathan Kuo and Dr. Hai Yao of the Department of Bioengineering at Clemson University for their assistance in rheologically characterizing the HA hydrogels.
References
- 1.Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121(Pt 3):255–64. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 2.Tan SW, Johns MR, Greenfield PF. Hyaluronic acid--a versatile biopolymer. Aust J Biotechnol. 1990;4(1):38–43. [PubMed] [Google Scholar]
- 3.Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7(2):79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 4.Morra M. Engineering of biomaterials surfaces by hyaluronan. Biomacromolecules. 2005;6(3):1205–23. doi: 10.1021/bm049346i. [DOI] [PubMed] [Google Scholar]
- 5.Wei YT, Tian WM, Yu X, Cui FZ, Hou SP, Xu QY, Lee IS. Hyaluronic acid hydrogels with IKVAV peptides for tissue repair and axonal regeneration in an injured rat brain. Biomed Mater. 2007;2(3):S142–6. doi: 10.1088/1748-6041/2/3/S11. [DOI] [PubMed] [Google Scholar]
- 6.Pavesio A, Abatangelo G, Borrione A, Brocchetta D, Hollander AP, Kon E, Torasso F, Zanasi S, Marcacci M. Hyaluronan-based scaffolds (Hyalograft C) in the treatment of knee cartilage defects: preliminary clinical findings. Novartis Found Symp. 2003;249:203–17. discussion 229–33, 234–8, 239–41. [PubMed] [Google Scholar]
- 7.Price RD, Myers S, Leigh IM, Navsaria HA. The role of hyaluronic acid in wound healing: assessment of clinical evidence. Am J Clin Dermatol. 2005;6(6):393–402. doi: 10.2165/00128071-200506060-00006. [DOI] [PubMed] [Google Scholar]
- 8.Murata K, Yokoyama Y. High hyaluronic acid and low dermatan sulfate contents in human pulmonary arteries compared to in the aorta. Blood Vessels. 1988;25(1):1–11. doi: 10.1159/000158716. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim S, Ramamurthi A. Hyaluronic acid cues for functional endothelialization of vascular constructs. J Tissue Eng Regen Med. 2008;2(1):22–32. doi: 10.1002/term.61. [DOI] [PubMed] [Google Scholar]
- 10.Ramamurthi A, Vesely I. Smooth Muscle Adhesion on Crosslinked Hyaluronan Gels. J Biomed Mat Res. 2002;60:196–205. doi: 10.1002/jbm.10061. [DOI] [PubMed] [Google Scholar]
- 11.Ramamurthi A, Vesely I. Ultraviolet light-induced modification of crosslinked hyaluronan gels. J Biomed Mat Res. 2003;66A(2):317–29. doi: 10.1002/jbm.a.10588. [DOI] [PubMed] [Google Scholar]
- 12.Srinivas A, Ramamurthi A. Effects of gamma-irradiation on physical and biologic properties of crosslinked hyaluronan tissue engineering scaffolds. Tissue Eng. 2007;13(3):447–59. doi: 10.1089/ten.2006.0196. [DOI] [PubMed] [Google Scholar]
- 13.Flory P. Principles of polymer chemistry. Ithica, NY: Cornell University Press; 1953. [Google Scholar]
- 14.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89(2):271–7. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 15.Manna F, Dentini M, Desideri P, De Pita O, Mortilla E, Maras B. Comparative chemical evaluation of two commercially available derivatives of hyaluronic acid (hylaform from rooster combs and restylane from streptococcus) used for soft tissue augmentation. J Eur Acad Dermatol Venereol. 1999;13(3):183–92. [PubMed] [Google Scholar]
- 16.Arshinoff S. The use of ophthalmic viscosurgical devices in cataract surgery. In: Kennedy J, Phillips G, Williams P, Hascall V, editors. Hyaluronan: Biomedical, Medical, and Clinical Aspects. Cambridge, UK: Woodhead Publishing Limited; 2002. pp. 119–128. [Google Scholar]
- 17.Weiss C. Hyaluronan and hylan in the treatment of osteoarthritis. In: Kennedy J, Phillipps G, Williams P, Hascall V, editors. Hyaluronan: Biomedical, Medical, and Clinical Aspects. Cambridge, UK: Woodhead Publishing Limited; 2002. pp. 467–482. [Google Scholar]
- 18.Prestwich G, Luo Y, Kirker K, Ziebell M, Shelby J. Hyaluronan biomaterials for targeted drug delivery and wound healing. In: Kennedy J, Phillipps G, Williams P, Hascall V, editors. Hyaluronan: Biomedical, Medical, and Clinical Aspects. Cambridge, UK: Woodhead Publishing Limited; 2002. pp. 277–284. [Google Scholar]
- 19.Borzacchiello A, Mayol L, Ramires PA, Pastorello A, Di Bartolo C, Ambrosio L, Milella E. Structural and rheological characterization of hyaluronic acid–based scaffolds for adipose tissue engineering. Biomaterials. 2007;28(30):4399–408. doi: 10.1016/j.biomaterials.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Lepidi S, Abatangelo G, Vindigni V, Deriu GP, Zavan B, Tonello C, Cortivo R. In vivo regeneration of small-diameter (2 mm) arteries using a polymer scaffold. Faseb J. 2006;20(1):103–5. doi: 10.1096/fj.05-4802fje. [DOI] [PubMed] [Google Scholar]
- 21.Sok DE, Choi DS, Kim YB, Lee YH, Cha SH. Selective inactivation of glyceraldehyde-3-phosphate dehydrogenase by vinyl sulfones. Biochem Biophys Res Commun. 1993;195(3):1224–9. doi: 10.1006/bbrc.1993.2175. [DOI] [PubMed] [Google Scholar]
- 22.Monheit GD. Hylaform: a new hyaluronic acid filler. Facial Plast Surg. 2004;20(2):153–5. doi: 10.1055/s-2004-861757. [DOI] [PubMed] [Google Scholar]
- 23.Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000;275(35):26967–75. doi: 10.1074/jbc.M002527200. [DOI] [PubMed] [Google Scholar]
- 24.Koyano T, Minoura N, Nagura M, Kobayashi K. Attachment and growth of cultured fibroblast cells on PVA/chitosan-blended hydrogels. J Biomed Mater Res. 1998;39(3):486–90. doi: 10.1002/(sici)1097-4636(19980305)39:3<486::aid-jbm20>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]