Abstract
Two novel methyl-substituted arachidonic acid derivatives were prepared in an enantioselective manner from commercially available chiral building blocks, and were found to be excellent templates for the development of (13S)-methyl-substituted anandamide analogues. One of the compounds synthesized, namely, (13S,5Z,8Z,11Z,14Z)-13-methyl-eicosa-5,8,11,14-tetraenoic acid N-(2-hy-droxyethyl)amide, is an endocannabinoid analogue with remarkably high affinity for the CB1 cannabinoid receptor.
Keywords: chirality, endocannabinoids, lipids, olefination, structure–activity relationships
Introduction
By the mid 1990s, the first two key endocannabinoids N-arachidonoylethanolamine (anandamide, Ia) and 2-arachidonoyl glycerol (Ib) were isolated and characterized as derivatives of the noncannabinergic arachidonic acid (Ic).[13] Compounds Ia and Ib act at the CB1 and CB2 receptors, two Gi/o-protein-coupled cannabinoid receptors (CBs)[4–8] known to modulate physiological and pathological processes, including nociception,[9–11] inflammation,[12] neuroprotec-tion,[13–15] feeding,[16, 17] memory,[18] anxiety,[19] and cell proliferation.[20, 21] The biological actions of Ia and Ib are terminated by a transport mechanism and enzymatic deactivation. In most tissues, compound Ia is metabolized hydrolytically by fatty acid amide hydrolase (FAAH),[22, 23] and Ib is metabolized by monoacylglycerol lipase (MAGL).[24, 25] However, recent investigations have demonstrated that oxidative enzymes of the arachidonate cascade, including lipoxygenases (LOX),[26–29] cytochrome P450,[28–31] and cyclooxygenase-2 (COX-2),[28, 29, 32–34] can transform Ia and Ib into eicosanoid-related bioactive products.
In addition to the hydrolytic metabolism of Ia and Ib, the alternative COX-2 metabolic route becomes important when FAAH or MAGL are inhibited and when endocannabinoid biosynthesis is activated following tissue damage.[35, 36] The first step in the COX-2 oxidative metabolism of Ic has been proposed to involve abstraction of the pro-(S) hydrogen from the C13 position by a tyrosyl radical.[37, 38] It has also been reported that mutation of Tyr385 prevents Ia oxygenation by COX-2, suggesting that, as in the case of Ic, metabolism of Ia is initiated by Tyr385-mediated hydrogen abstraction.[39]
The chemical structure of Ia can be divided into two major molecular fragments: 1) a polar head group and 2) a hydrophobic fragment comprising a nonconjugated tetraolefinic chain (with Z-configured double bonds) and an n-pentyl tail. Extensive structure–activity relationship (SAR) studies aimed at developing potent and metabolically stable analogues of Ia have focused on the polar head group and on the n-pentyl tail.[40–42] In this regard, earlier work from our laboratories has led to the synthesis of a high-affinity and hydrolytically stable analogue of Ia, (R)-methanandamide (Id).[43–47]
Unlike the polar head group and the n-pentyl tail, the nonconjugated tetraolefinic chain is essentially unexplored, and SAR studies seeking to probe unsaturation requirements have suggested that it plays a pivotal role in determining the bioactive conformation(s) of Ia and its congeners.[45, 49] Earlier computational and biophysical work by us[50] and others[51–54] on the conformational properties of anandamide has shown that the hydrophobic fragment is capable of assuming a variety of conformations in solution, which are generally characterized as hairpin (U shaped), partially extended (J shaped) and fully extended. Of these, the U- and the J-shaped conformations are believed to be responsible for CB receptor recognition.[50–54] Additionally, in the crystal structure of Ic bound in the COX active site of prostaglandin synthase-1, compound Ic adopts a partially extended L-shaped conformation.[55]
As a part of our ongoing program in cannabinoid medicinal chemistry, we focus on the development of novel endocannabinoid templates that possess high CB receptor binding affinity as well as metabolic stability to the action of the COX-2 enzyme. The novel analogues can provide additional insight into the stereoelectronic requirements for interaction with CB receptors, and aid in the discovery of more potent and selective cannabinergic drug candidates and COX-2 inhibitors. They can also serve as pharmacological tools towards understanding the connection between the endocannabinoid and COX-2 systems.
We report herein the design, synthesis, and preliminary biological data of two novel endocannabinoid molecular probes referred to as (13S)-methyl-substituted anandamide analogues (32 and 39, Schemes 1 and 6, respectively). Our synthetic strategy involves two approaches (methods A and B, Scheme 1) for the enantioselective construction of the cannabinergic skeleton. The first approach (method A) yields the final products in fewer synthetic steps, whereas the second approach (method B) facilitates the enantioselective introduction of different substituents at the C13 position. Biological testing results demonstrate that both analogues 32 and 39 are well recognized by the CB1 receptor. It is especially worthy of note that 32 is among the endocannabinoid analogues with the highest CB1 binding affinities known to date. A detailed SAR study as well as a full biological evaluation of the novel endocannabinoid analogues reported herein is underway.
Scheme 1.
Retrosynthetic analysis of (13S)-methylanandamide 32. TBDPS=tert-butyldiphenylsilyl.
Scheme 6.
Synthesis of the conformationally partially extended (13S)-methyl analogue 39. Reagents and conditions: a) KHMDS, THF, −78°C, 50 min, then 3, −98−0°C, 2.5 h, 58%; b) TBAF, CH3COOH, THF, 0°C to RT, 20 h, 76%; c) Dess–Martin periodinane, 0°C, 2h; d) CH3(CH2)5P+ Ph3Br, KHMDS, THF, 10°C, 40 min, then addition of 35, −98°C, 1 h, 58% from alcohol 34; e) KHMDS, THF, −78°C, 50 min, then 11, −98–0°C, 2.5 h, 21%; f) LiOH, THF/H2O, RT, 7 h, 82%; g) TBDPSCl, imidazole, CH3CN, 0°C, 30 min, 91%; h) Carbonyldiimidazole, THF, RT, 2 h, then 30, RT, 1 h, 97%; i) TBAF, CH3COOH, THF, 0°C to RT, 20 h, 75%.
Results and Discussion
Analogue design
Our approach for the design of the novel endocannabinoid analogues involved addition of a methyl substituent at position 13S of the lipophilic fragment (analogue 32, Scheme 1). It was hypothesized that the presence of a methyl substituent at C13 would not significantly affect the ability of the nonconjugated tetraolefinic chain to assume U- and/or J-shaped conformations, and thus would not significantly affect its bioactivity for CB1. In contrast, it seemed plausible that endocannabinoid analogues substituted at the C13S position might “shut off” the COX-2-mediated metabolism due to the absence of a hydrogen atom in the respective position. We also extended our design to include analogue 39 (Scheme 6), which can be considered to mimic the J- and/or L-shaped conformers of Ia and Ic, because of the conformational restriction imposed by the two triple bonds. The head group of the endogenous anandamide was incorporated in our templates.
Synthesis
Our retrosynthetic analysis identifies methyl ester 27 as the key intermediate from which (13S)-methylanandamide (32) would be generated through peptide coupling (Scheme 1).
Retrosynthetic disconnection (method A) of both the C11=C12 and C14=C15 double bonds in 27 generated three fragments: the phosphonium salts 20 and 8a, and the chiral aldehyde 3, which possesses the S configuration corresponding to the C13 stereogenic center of 27. In the synthetic direction, fragments 20, 8a, and 3 are joined by Wittig reactions. An alternative approach (method B) involves cleavage of the C11=C12 double bond, yielding phosphonium salt 20 and chiral aldehyde 11. Further disconnection at the C3=C4 double bond of 11 led to 8a and chiral methyl-substituted aldehyde 8, which can in turn be derived from the enantiomerically pure (R,R)-epoxide 6. The synthetic direction could thus be completed through Wittig reactions. Although method B involves more steps, it facilitates the enantioselective introduction of various substituents at the C13 position of Ia, through SN2-type reactions on the epoxide 6. Because of the similarity in the structures, a closely related retrosynthetic analysis could be envisaged for the conformationally partially extended (13S)-methyl analogue 39.
The syntheses of the required chiral aldehydes 3 and 11 are summarized in Scheme 2. Protection of the primary hydroxyl group in 1 as the TBDPS ether (2)[56] was followed by diisobutylaluminum hydride (DIBAL-H) reduction to give aldehyde 3[57] (61% yield) and alcohol 4[58] (37% yield). Conversion of 4 to 3 was carried out through Dess–Martin periodinane oxidation in 90% yield.[56] Enantiomerically pure (R,R)-epoxide 6 was synthesized in six steps starting from commercially available (−)-diethyl tartrate (5) by following our recently reported procedures.[59] Conversion of 6 to acetonide 7 involved the following steps:[60] 1) methylation of 6 with Me2CuLi, 2) benzyl ether cleavage by hydro-genolysis, and 3) treatment of the resulting triol with acetone, in the presence of p-toluenesulfonic acid.
Scheme 2.
Synthesis of chiral aldehydes 3 and 11. Reagents and conditions: a) TBDPSCl, imidazole, CH2Cl2, 0°C to RT, 1.5 h, 98%; b) DIBAL-H, CH2Cl2, −110–−90°C, 20 min, 61% for 3 and 37% for 4; c) Dess–Martin periodinane, 0°C to RT, 45 min, 90%; d) PCC, CH2Cl2, 4 Å molecular sieves, RT, 2.5 h, 93%; e) CH3(CH2)5P+Ph3Br−, KHMDS, THF, 10°C, 50 min, then 8, −98°C, 1 h, 50%; f) BF3•CH3COOH, MeOH, 0°C, 1 h, 84%; g) Pb(OAc)4, CH2Cl2−78°C, 1.5 h, 97%. PCC = pyridinium chlorochromate, KHMDS = potassium bis(trimethylsilyl)amide.
Oxidation of alcohol 7 with PCC in dry CH2Cl2 gave aldehyde 8 in excellent yield (93%).[61, 62] Combination of aldehyde 8 and the ylide derived from hexyltriphenylphosphonium bromide and KHMDS, at −98°C, resulted in the formation of exclusively the Z isomer 9 in 50% yield (J(3H,4H) = 10.4 Hz, see the Supporting Information). Mild deprotection of the ketal 9 by using the BF3•CH3COOH complex (84% yield), and lead tetraacetate mediated cleavage of the resulting 1,2-diol 10 at −78°C provided aldehyde 11 (97% yield), which was used immediately in a Wittig reaction with phosphonium salts 20 and 23.
The construction of the required alkenyl phosphonium salt 20 proceeded as shown in Scheme 3. Commercially available 3-butyn-1-ol (12) was converted into alcohol 18 through several modifications of a known sequence,[63a] in improved overall yield. Thus, TBDPS protection of 12 led to alkyne 13 (99% yield), which was subsequently treated with nBuLi and quenched with paraformaldehyde to give alcohol 14 in 73% yield. Conversion of 14 to bromide 15 was carried out by using the PPh3/CBr4 method (85% yield).
Scheme 3.
Synthesis of alkenyl phosphonium salt 20. Reagents and conditions: a) TBDPSCl, imidazole, THF, 0°C, 1.5 h, 99%; b) nBuLi, THF, 0°C, 1.5 h, then (CH2O)n −50°C to RT, 1.5 h, 73%; c) CBr4, Ph3P, CH2Cl2, 0°C to RT, 3 h, 85%; d) CH≡C-(CH2)3-COOMe, Cs2CO3, NaI, CuI, DMF, RT, 2.5 h, 95%; e) Ni(OAc)2, NaBH4, ethylenediamine, H2, MeOH, RT, 2h, 85%; f) TBAF, THF, 0°C to RT, 1.5 h, 93%; g) CBr4, Ph3P, CH2Cl2−25−0°C, 1.5 h, 96%; h) Ph3P, CH3CN, 72–75°C, 8 days, 95%. TBAF = tetrabutylammonium fluoride.
Copper-mediated cross-coupling of 15 with methyl 5-hexynoate in the presence of Cs2CO3 and NaI afforded diyne 16 (95% yield), which was partially hydrogenated over P-2 nickel catalyst to give the corresponding skipped Z diene 17 in 85% yield.[63] Deprotection with TBAF at 0°C and exposure of the resulting alcohol 18 to the PPh3/CBr4 system gave bromide 19 in 89% overall yield. Heating 19 (72–75°C) with PPh3 in dry CH3CN for 8 days afforded the target phosphonium salt 20 in 95% yield after purification. In the 1H NMR spectrum of 20, all four double bond protons are well separated with coupling constants J(5H,6H) and J(8H,9H) of less than 10.8 Hz (see the Supporting Information), which suggests a Z relationship between the hydrogen atoms in the 5H–6H and 8H–9H spin systems.
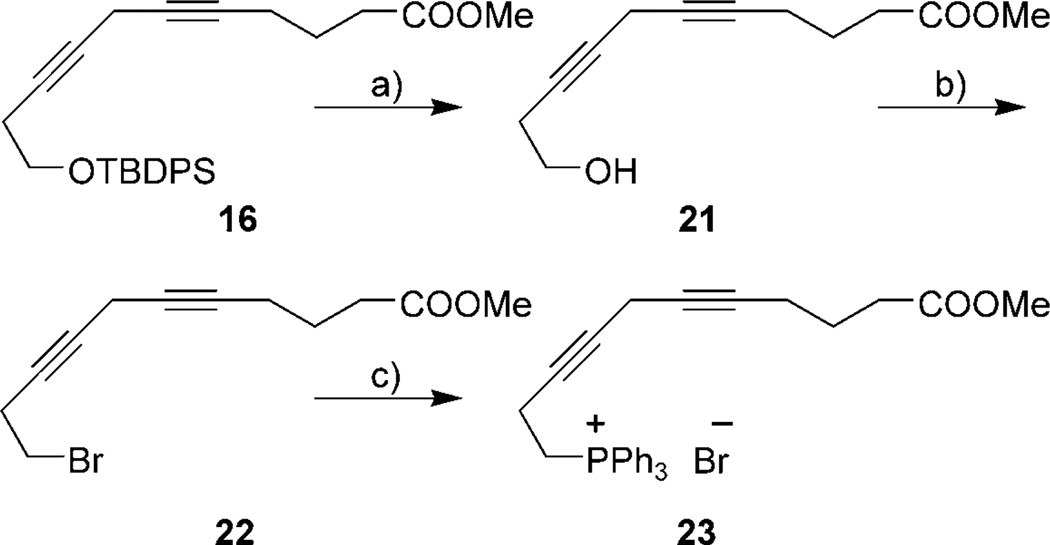
Similarly, the alkynylphosphonium salt 23 was produced in three steps (72% overall yield) from diyne 16 through desilylation, conversion of alcohol 21 to the bromide 22, and reaction with PPh3 (Scheme 4).
Scheme 4.
Synthesis of alkynyl phosphonium salt 23. Reagents and conditions: a) TBAF, THF, 0°C, 2 h, 84%; b) CBr4, Ph3P, CH2Cl2 −16°C, 2 h, 98%; c) Ph3P, CH3CN, 70–72°C, 7 days, 88%.
The assembly of the synthesized aldehydes 3 and 11 with phosphonium salt 20 into the (13S)-methyl anandamide (32) is shown in Scheme 5. Thus, treatment of 20 with KHMDS and coupling of the resulting ylide with aldehyde 3 at −115°C produced ester 24 (61% yield). Based on 1H NMR analysis, this Wittig olefination reaction afforded exclusively the Z olefin with J(11H,12H) = 10.8 Hz (the 1H NMR spectrum of 24 is available in the Supporting Information). Desilylation of 24 with TBAF gave alcohol 25 in 85% yield.
Scheme 5.
Synthesis of 32. Reagents and conditions: a) KHMDS, THF, −78–−60°C, 20 min, then 3, −115–0°C, 3 h, 61%; b) TBAF, THF, 0°C to RT, 1.5 h, 85%; c) Dess–Martin periodinane, 0°C to RT, 75 min; d) CH3-(CH2)5P+Ph3Br−, KHMDS, THF, 0°C, 40 min, then addition of 26, −115–−100°C, 50 min, 65% from alcohol 25; e) KHMDS, THF, −78–−60°C, 20 min, then 11, −98−0°C, 2.5 h, 22%; f) LiOH, THF/H2O, RT, 24 h, 86%; g) TBDPSCl, imidazole, CH3CN, 0°C, 30 min, 97%; h) carbonyldiimidazole, THF, RT, 2 h, then 30, RT, 1 h, 91%; i) TBAF, THF, 0°C to RT, 1 h, 88%.
The coupling constant between 11H and 12H of 25 (J(11H,12H) = 10.4 Hz) correlates well with Z stereochemistry of the C11=C12 double bond. Dess–Martin periodinane oxidation of 25 led to aldehyde 26, which was used immediately, without purification, in a Wittig reaction with hexyltriphenylphosphonium bromide, under salt-free conditions to give (13S)-methyl arachidonate 27 in 65% yield from 25. By using our second approach, phosphonium bromide 20 was treated with KHMDS at −78°C and the resulting phosphorane was coupled with aldehyde 11 at −98°C to give 27 in 22% yield. The structure of 27 was established by using 1D and 2D NMR methods (COSY, HSQC, HMBC, and NOESY; data are available in the Supporting Information). NOESY interactions between 13H and 16H confirm the Z stereochemistry for the newly formed double bond at the C14 position. Saponification of ester 27 with lithium hydroxide in THF/H2O led to acid 28 (86% yield), which was coupled with 2-(tert-butyldiphenylsilyloxy)ethanamine (30) to give amide 31 (91% yield) by using the carbonyldiimidazole activation procedure. Removal of the silyl protecting group was carried out by using TBAF in THF, and gave (13S)-methylanandamide (32) in 88% yield.
Conformationally partially extended (13S)-methyl analogue 39 was synthesized in a similar fashion, as depicted in Scheme 6. Thus, Wittig reaction of aldehyde 3 with phosphonium salt 23 in the presence of KHMDS gave exclusively the Z olefin 33 in 58% yield (J(11H 12H) = 10.8 Hz). De-protection with TBAF under neutral conditions (to suppress the formation of byproducts) gave 34 (76% yield). Aldehyde 35 was obtained from 34 through Dess–Martin periodinane oxidation and was used immediately in the next step. Wittig reaction of aldehyde 35 with hexyltriphenylphosphonium bromide produced ester 36 in 58% yield from 34. Alternatively, ester 36 could also be obtained in 21% yield through Wittig reaction of aldehyde 11 and alkynyl phosphonium salt 23. These Wittig olefination reactions led exclusively to the required Z geometry at the newly generated double bonds under the experimental conditions used. Subsequent saponification, coupling with protected ethanolamine 30 and deprotection by using the TBAF/CH3COOH system led to the target amide 39 in 60% overall yield from 36.
Receptor binding studies
Binding affinities of the newly synthesized analogues for CB1 and CB2 cannabinoid receptors were determined as described in the Experimental Section.[4, 44, 45, 47, 64, 65] For the CB1 receptor, binding data were obtained by using a rat brain membrane in the presence of phenylmethanesulfonyl fluoride (PMSF),[66, 67] a general serine protease inhibitor that is used to protect the analogues from the hydrolytic activity of fatty acid amide hydrolase (FAAH).[43, 67] Rat brain membranes have a high concentration of CB1 receptors without significant CB2 receptors present. CB2 receptor affinity was measured by using mouse spleen membranes, in which FAAH activity is absent and does not require pretreatment with PMSF. [3H]CP-55,940 was chosen as a competing ligand for the assays, because it has high affinity for both CB1 and CB2 receptors and is nonselective. It is one of the most widely used radioligands for characterizing both CB1 and CB2 cannabinoid receptors.[40–42] SAR, mutation, and computer modeling study results indicate that nonclassical cannabinoids (e.g., CP-55,940), classical cannabinoids, and anandamide share key binding motifs, whereas other classes of cannabinergic compounds, such as the aminoalkylindole WIN55212-2, might have different binding features.[53] The binding affinities (Ki values) of the novel endocannabinoid analogues are summarized in Table 1, in which the endogenous anandamide is included for comparison.
Table 1.
Affinities (Ki) of endocannabinoid analogues for CB1 and CB2 cannabinoid receptors (95% confidence limits).
| Compound | CB1 Ki [nm][a] with PMSF | CB2 Ki [nm][a] |
|---|---|---|
| anandamide | 61[b] | 1930[b] |
| 32 | 4.8±1.3 | 137±22 |
| 39 | 139±25 | 967±321 |
Affinities for CB1 and CB2 were determined by using rat brain (CB1) or mouse spleen (CB2) membranes and [3H]CP-55,940 as the radioligand following previously described procedures.[4, 44, 45, 47, 64, 65] Data were analyzed by using nonlinear regression analysis. Ki values were obtained from three independent experiments run in duplicate and are expressed as the mean of the three values.
Reported previously.[40]
It is apparent from the CB2 affinities reported herein that these analogues show selectivity for the CB1 receptor. Additionally, the backbone of the two ligands is well recognized by the CB1 receptor. In this regard, compound 32 is the most interesting, with a 13-fold higher affinity for CB1 over anandamide, a feature which places it among the endocannabinoid analogues with the highest CB1 binding affinities known to date. Furthermore, preliminary functional characterization of 32 using the cannabinoid-stimulated guanosine-5′-O-(3-[35S]thio)triphosphate ([35S]GTPγS) binding assay[68] indicates that 32 is a full agonist for the CB1 cannabinoid receptor.
Conclusion
We have designed and synthesized two novel endocannabinoid templates with potential resistance to oxidative metabolism by COX-2. The synthetic strategy developed involves two approaches for the enantioselective construction of the cannabinergic (13S)-methyl-substituted skeleton. The first approach yields the final products in 18–19 synthetic steps starting from commercially available 1. The second approach provides the targeted compounds in 19–20 synthetic steps from the key intermediate 6, which can in turn be prepared in six steps from commercially available 5. Although this synthetic route involves more steps, it facilitates the introduction of different substituents at the 13S position. Both approaches use Wittig reactions and peptide coupling as key steps.
Biological testing results show that the synthesized analogues are well recognized by the CB1 receptor, and 32 is one of the endocannabinoid analogues with the highest CB1-binding affinity known to date (Ki = 4.8 nm for CB1). In addition, preliminary functional characterization of 32 indicates that this analogue is a full agonist for the CB1 cannabinoid receptor. A detailed SAR study and a full biological evaluation of the two novel endocannabinoids described are currently underway.
Experimental Section
General
All reagents and solvents were purchased from Aldrich Chemical Company, unless otherwise specified, and used without further purification. All anhydrous reactions were performed under a static argon or nitrogen atmosphere in flame-dried glassware using scrupulously dry solvents. Flash column chromatography was carried out by using silica gel 60 (230–400 mesh). All compounds were demonstrated to be homogeneous by analytical TLC on precoated silica gel TLC plates (Merck, 60 F245 on glass, layer thickness 250 mm), and chromatograms were visualized by staining with phosphomolybdic acid. Melting points were determined on a micromelting point apparatus and are uncorrected. Optical rotations were recorded on a Perkin–Elmer 341 digital polarimeter. NMR spectra were recorded in CDCl3, unless otherwise stated, on Varian 300 (1H at 300 MHz, 13C at 75 MHz) and Varian 600 (1H at 600 MHz, 13C at 150 MHz) NMR spectrometers and chemical shifts are reported in units of δ relative to internal TMS. Multiplicities are indicated as br (broadened), s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), and coupling constants (J) are reported in hertz (Hz). Low- and high-resolution mass spectra were obtained in the School of Chemical Sciences, University of Illinois at Urbana-Champaign. Mass spectral data are reported in the form of m/z (intensity relative to base = 100). Elemental analyses were obtained in Baron Consulting, Milford, CT.
Compound 24
KHMDS (384 mg, 1.93 mmol) was added to a solution of 20 (1.09 g, 2.03 mmol) in dry THF (8 mL) at −78°C under an argon atmosphere. The mixture was stirred and allowed to warm from −78 to −60°C over 20min to ensure complete formation of the orange ylide, then the resulting mixture was cooled to −115°C A solution of aldehyde 3 (331 mg, 1.02 mmol) in dry THF (2 mL) was added dropwise. The reaction mixture was stirred for 35 min at −115°C, then warmed to 0°C over 1.5 h, and stirring was continued at that temperature for 1 h. The reaction mixture was cooled to −115°C and quenched with a saturated aqueous solution of sodium bicarbonate. The mixture was warmed to room temperature, extracted with diethyl ether, and the combined organic extracts were washed with brine, dried (Na2SO4), and concentrated in vacuo. Purification by flash column chromatography on silica gel (5–7% diethyl ether in hexane) gave 24 as a colorless oil (312 mg, 61% yield). (c = 0.00521 gmL−1 in CHCl3); 1H NMR (CDCl3, 600 MHz): δ=1.00 (d, J=7.2 Hz, 3H; CH-CH3), 1.05 (s, 9H; C(CH3)3), 1.70 (quintet, J=7.8 Hz, 2H; 3-H), 2.09 (dt, J=6.6, 6.6 Hz, 2H; 4-H), 2.31 (t, J= 7.8 Hz, 2H; 2-H), 2.67–2.74 (m, 2H; 10-H), 2.75–2.84 (m and t overlapping, 3H; 13-H, 7-H, including 2.77, t, J=6.0 Hz; 7-H), 3.46 (dd, J=10.2, 6.8 Hz, 1H; 14-H), 3.50 (dd, J=10.2, 6.3 Hz, 1H; 14-H), 3.66 (s, 3H; COOCH3), 5.21 (tdd, J=10.8, 9.6, 1.2 Hz, 1H; 12-H), 5.28–5.42 (m, 5H; 5-H, 6-H, 8-H, 9-H, 11-H), 7.34–7.38 (m, 4H; 3-H, 5-H, ArH), 7.39–7.44 (m, 2H; 4-H, ArH), 7.66 ppm (d, J=7.0 Hz, 4H; 2-H, 6-H, ArH); 13C NMR (CDCl3, 75 MHz): δ=17.4, 19.3, 24.8, 25.6, 25.9, 26.5, 26.8, 33.4, 34.7, 51.5 (OMe), 68.5 (CH2O), 127.5, 127.6, 128.0, 128.5, 128.9, 129.5, 133.1, 133.9, 135.6, 174.1 ppm (C=O); MS (ESI): m/z (%): 527.9 [M+Na]+ (24), 427.7 (12), 249.4 (100), 217.3 (47), 123.2 (23); MS (EI): m/z (%): 447 [M–C(CH3)3]+ (39), 213 (45), 199 (100), 183 (19), 133 (39), 91 (27), 77 (18); MS (ESI): m/z (%): 527 [M+Na]+(100); HRMS (ESI): m/z calcd for C32H44O3NaSi+Na+: 527.2957 [M+Na]+; found: 527.2954.
Compound 25
TBAF (0.8 mL, 0.8 mmol, 1m solution in THF) was added dropwise to a stirred solution of 24 (290 mg, 0.575 mmol) in dry THF (12 mL), at 0°C under an argon atmosphere. Stirring was continued for 10 min at 0°C and for 1.5 h at room temperature. The reaction mixture was quenched with a saturated aqueous solution of NH4Cl at 0°C and extracted with AcOEt. The combined organic extracts were washed with brine, dried (Na2SO4), and concentrated in vacuo at 37°C. The crude oil was purified by flash column chromatography on silica gel (40% ethyl acetate in hexane) to afford 25 as a colorless viscous liquid (130 mg, 85% yield). (c = 0.00067 gmL−1 in CHCl3); 1H NMR (CDCl3, 600 MHz): δ=0.90 (d, J=6.6 Hz, 3H; CH-CH3), 1.54 (br s, 1H; OH), 1.64 (quintet, J=7.8 Hz, 2H; 3-H), 2.04 (dt, J=6.6, 6.6 Hz, 2H; 4-H), 2.26 (t, J=7.8 Hz, 2H; 2-H), 2.67 (m as septet, J= 7.8 Hz, 1H; 13-H), 2.73 (t, J=5.4 Hz, 2H; 7-H), 2.75–2.85 (m, 2H; 10-H), 3.29 (dd, J=10.2, 7.8 Hz, 1H; 14-H), 3.42 (dd, J=10.2, 6.0 Hz, 1H; 14-H), 3.60 (s, 3H; COOCH3), 5.10 (tdd, J=10.4, 9.6, 1.8 Hz, 1H; 12-H), 5.26–5.36 (m, 4H; 5-H, 6-H, 8-H, 9-H), 5.43 ppm (dtd, J=10.4, 7.8, 1.2 Hz, 1H; 11-H; 13C NMR (CDCl3, 150 MHz): δ=16.95, 24.75, 25.62, 26.04, 26.56, 33.43, 34.95, 51.55 (OCH3), 67.64 (CH2OH), 128.05, 128.32, 128.77, 128.93, 129.94, 132.53, 174.12 ppm (C=O); MS (EI): m/z (%): 266 [M]+ (2), 248 (7), 236 (16), 175 (9), 161 (17), 147 (18), 133 (37), 119 (32), 107 (58), 93 (89), 79 (100); MS (ESI): m/z (%): 289 [M+Na]+ (100), 267 [M+H]+ (38), 249 (41), 217 (39); HRMS (EI): m/z calcd for C16H26O3: 266.1882 [M]+; found: 266.1890.
Compound 26
Dess–Martin periodiane (DMP; 271 mg, 0.639 mmol) was added to a solution of alcohol 25 (100 mg, 0.376 mmol) in dry CH2Cl2 (8 mL) at 0°C under an argon atmosphere. The resulting suspension was warmed to room temperature and stirred for 45 min. An additional amount of DMP (112 mg, 0.263 mmol) was added at 0°C and stirring was continued for 30 min at room temperature to ensure total consumption of alcohol 25. The reaction mixture was quenched by adding a 1:1 (v/v) mixture of a 10% aqueous solution of Na2S2O3 and a saturated aqueous solution of NaHCO3, and diluted with diethyl ether. The slurry was filtered through Celite, the layers were separated and the aqueous layer was extracted with diethyl ether. The combined organic extracts were washed with a saturated aqueous solution of NaHCO3, brine, and dried (Na2SO4). Concentration in vacuo at 37–40°C provided the sensitive crude product 26 as a colorless oil, which was used in the next step immediately. 1H NMR (CDCl3, 600 MHz): δ=1.18 (d, J=6.6 Hz, 3H; CH-(CH3)), 1.69 (quintet, J=7.8 Hz, 2H; CH2-CH2-CH2-COO), 2.10 (dt, J= 6.6, 6.6 Hz, 2H; CH2-CH2-CH2-COO), 2.31 (t, J=7.8 Hz, 2H; CH2-COO), 2.78 (t, J=5.8 Hz, 2H; CH=CH-CH2-CH=CH), 2.83 (ddd, J= 16.0, 8.1, 7.3 Hz, 1H; CH2-CH=CH-CH(CH3)), 2.89 (ddd, J=16.0, 8.1, 7.2 Hz, 1H; CH2-CH=CH-CH(CH3)), 3.37 (m as quintet, J=8.4 Hz, 1H; CH(CH3)), 3.65 (s, 3H; COOCH3), 5.24 (tdd, J=10.5, 9.5, 1.8 Hz, 1H; CH=CH-CH(CH3)), 5.31–5.44 (m, 4H; CH=CH-CH2-CH=CH), 5.64 (dtd, J=10.5, 7.8, 1.2 Hz, 1H; CH=CH-CH(CH3)), 9.52 ppm (d, J= 1.2 Hz, 1H; CHO).
Compound 27
Method A (from aldehyde 26)
KHMDS (291 mg, 1.46 mmol) was added to a stirred solution of hexyltriphenylphosphonium bromide (641 mg, 1.50 mmol) in dry THF (21 mL) at 0°C under an argon atmosphere. The mixture was stirred for 40 min at 0°C to ensure complete formation of the orange ylide, then cooled to −115°C A solution of crude aldehyde 26 in dry THF (3 mL) was added dropwise, the reaction mixture was stirred for 50 min at −115–100°C, and then quenched by the addition of a saturated aqueous solution of sodium bicarbonate. The mixture was warmed to room temperature, extracted with diethyl ether, and the combined organic extracts were washed with brine, dried (Na2SO4), and concentrated in vacuo. Purification by flash column chromatography on silica gel (2–4% diethyl ether in hexane) gave ester 27 as a colorless oil (81 mg, 65% yield from alcohol 25). (c = 0.00225 gmL−1 in CHCl3); 1H NMR (CDCl3, 600 MHz): δ=0.89 (t, J=7.2 Hz, 3H; 20-H), 1.01 (d, J=6.6 Hz, 3H; CHCH3), 1.24–1.33 (m, 4H; 18-H, 19-H), 1.36 (sextet, J=7.2 Hz, 2H; 17-H), 1.71 (quintet, J=7.8 Hz, 2H; 3-H), 2.02–2.09 (m, 2H; 16-H), 2.11 (dt, J=6.8, 6.8 Hz, 2H; 4-H), 2.32 (t, J=7.8 Hz, 2H; 2-H), 2.75–2.84 (ddd and t overlapping, 3H; 10-H and 7-H; including 2.80, t, J=6.6 Hz, 2H; 7-H), 2.87 (ddd, J=15.6, 7.5, 6.5 Hz, 1H; 10-H), 3.46 (ddq as sextet, J=7.5 Hz, 1H; 13-H), 3.67 (s, 3H; COOCH3), 5.20–5.30 (m, 4H; 11-H, 12-H, 14-H, 15-H), 5.43–5.32 ppm (m, 4H; 5-H, 6-H, 8-H, 9-H); 13C NMR (CDCl3, 150 MHz): δ=14.03 (C-20), 22.01 (C-13-CH3), 22.56 (C-19), 24.76 (C-3), 25.59 (C-7), 25.81 (C-10), 26.53 (C-4), 27.48 (C-16), 29.44 (C-17), 30.50 (C-13), 31.55 (C-18), 33.42 (C-2), 51.48 (−OCH3), 125.61 (C-11), 128.07 (C-15), 128.24 (C-5), 128.35 (C-9), 128.84 (C-8 or C-6), 128.88 (C-6 or C-8), 134.12 (C-14), 135.02 (C-12), 174.00 ppm (C=O); MS (EI): m/z (%): 332 [M] +(9), 248 (16), 217 (12), 164 (13), 147 (11), 133 (23), 119 (29), 107 (43), 93 (100), 91 (44), 81 (58); MS (ESI): m/z (%): 355 [M+Na]+ (100), 333 [M+H]+ (64), 217 (48), 199 (43), 170 (36); HRMS (EI): m/z calcd for C22H36O2: 332.2715 [M]+; found: 332.2722.
Method B (from aldehyde 11)
The synthesis was carried out as described for 24, using phosphonium salt 20 (167 mg, 0.31 mmol), KHMDS (58 mg, 0.29 mmol), and aldehyde 11 (53 mg, 0.34 mmol). The reaction was completed in 2.5 h at −98–0°C to give 27 (25 mg, 22% yield).
Compound 28
A 1 m aqueous solution of LiOH (0.2 mL) was added to a stirred solution of 27 (34 mg, 0.102 mmol) in dry THF (1 mL) at room temperature, under an argon atmosphere. Stirring was continued for 24 h, then the reaction mixture was acidified with a 5% aqueous solution of HCl to pH 3, and lipophilic products were extracted with Et2O. The combined organic extracts were washed with brine, and dried (Na2SO4). Concentration in vacuo at 37–39°C gave acid 28 as a colorless oil (28 mg, 86% yield), which was used in the next step without further purification. 1H NMR (CDCl3, 600 MHz): δ=0.89 (t, J=6.6 Hz, 3H; 20-H), 1.01 (d, J=7.2 Hz, 3H; CH-CH3), 1.24−1.33 (m, 4H; 18-H, 19-H), 1.36 (sextet, J=7.2 Hz, 2H; 17-H), 1.72 (quintet, J=7.8 Hz, 2H; 3-H), 2.01–210 (m, 2H; 16-H), 2.14 (dt, J=7.2, 7.2 Hz, 2H; 4-H), 2.37 (t, J=7.8 Hz, 2H; 2-H), 2.76–2.85 (ddd and t overlapping, 3H; 10-H and 7-H; including 2.81, t, J=6.3 Hz, 2H; 7-H), 2.86 (ddd, J=15.7, 7.5, 6.5 Hz, 1H; 10-H), 3.46 (ddq as sextet, J=7.2 Hz, 1H; 13-H), 5.20–5.31 (m, 4H; 11-H, 12-H, 14-H, 15-H), 5.32–5.45 (m, 4H; 5-H, 6-H, 8-H, 9-H), 10.55 ppm (br s, 1H; COOH); MS (EI): m/z (%): 318 [M]+ (4), 248 (6), 220 (15), 191 (9), 164 (12), 133 (23), 119 (38), 107 (46), 93 (100), 81 (67); HRMS (EI): m/z calcd for C21H34O2: 318.2559 [M]+; found: 318.2551.
Compound 31
A mixture of acid 28 (22 mg, 0.069 mmol), and fresh car-bonyldiimidazole (23 mg, 0.138 mmol) in dry THF (1 mL) was stirred for 2 h at room temperature under an argon atmosphere. A solution of protected ethanolamine 30 (62 mg, 0.208 mmol) in THF (0.5 mL) was added. The reaction mixture was stirred for 1 h and then diluted with water and ethyl acetate. The organic layer was separated and the aqueous layer was extracted with AcOEt. The combined organic extracts were washed with brine, dried (Na2SO4), and concentrated in vacuo. The crude product was purified by flash column chromatography on silica gel (25% acetone in hexane) to give 31 as a colorless oil (37 mg, 91% yield). 1H NMR (CDCl3, 600 MHz): δ=0.88 (t, J=6.9 Hz, 3H; 20-H), 1.02 (d, J=6.6 Hz, 3H; CH-CH3), 1.07 (s, 9H; C(CH3)3), 1.24–1.31 (m, 4H; 18-H, 19-H), 1.35 (sextet, J=7.2 Hz, 2H; 17-H), 1.69 (quintet, J=7.2 Hz, 2H; 3-H), 2.02–2.09 (m, 2H; 16-H), 2.12 (dt, J=7.2, 7.2 Hz, 2H; 4-H), 2.13 (t, J= 7.6 Hz, 2H; 2-H), 2.75–2.84 (ddd and t overlapping, 3H; 10-H and 7-H; including 2.81, t, J=6.0 Hz, 2H; 7-H), 2.86 (ddd, J=15.7, 7.5, 6.5 Hz, 1H; 10-H), 3.40 (dt, J=5.4, 5.4 Hz, 2H; CH2-NH), 3.45 (sextet, J= 7.5 Hz, 1H; 13-H), 3.75 (t, J=5.4 Hz, 2H; CH2-OTBDPS), 5.18–5.31 (m, 4H; 11-H, 12-H, 14-H, 15-H), 5.32–5.44 (m, 4H; 5-H, 6-H, 8-H, 9-H), 5.73 (br s, 1H; NH), 7.39 (t, J=7.8 Hz, 4H; 3-H, 5-H, ArH), 7.44 (t, J=7.8 Hz, 2H; 4-H, ArH), 7.64 ppm (d, J=7.8 Hz, 4H; 2-H, 6-H, ArH); MS (EI): m/z (%): 599 [M]+ (3), 542 (100), 296 (15), 276 (9), 242 (38), 199 (89), 164 (35), 91 (24); HRMS (EI): m/z calcd for C39H57NO2Si: 599.4159 [M]+; found: 599.4165.
Compound 32
The synthesis was carried out as described for 18, using 31 (32 mg, 0.053 mmol) and TBAF (0.07 mL, 0.07 mmol, 1 m solution in THF) in dry THF (2 mL). The reaction was completed in 1 h and the resulting crude oil was purified by flash column chromatography on silica gel (57:40:3 ethyl acetate/hexane/MeOH) to afford 32 (17 mg, 88% yield) as a colorless oil. (c = 0.0009 gmL−1 in CHCl3); 1H NMR (CDCl3, 600 MHz): δ=0.89 (t, J=6.9 Hz, 3H; 20-H), 1.02 (d, J=7.2 Hz, 3H; CH-CH3), 1.24–1.33 (m, 4H; 18-H, 19-H), 1.35 (sextet, J=7.2 Hz, 2H; 17-H), 1.73 (quintet, J=7.2 Hz, 2H; 3-H), 2.06 (nonet, J=6.3 Hz, 2H; 16-H), 2.12 (dt, J=7.2, 7.2 Hz, 2H; 4-H), 2.22 (t, J= 7.6 Hz, 2H; 2-H), 2.54 (br s, 1H; OH), 2.76–2.84 (ddd and t, overlapping, 3H; 10-H and 7-H, including 2.81, t, J=6.0 Hz, 2H; 7-H), 2.87 (ddd, J= 15.0, 7.5, 6.3 Hz, 1H; 10-H), 3.42 (dt, J=5.2, 5.2 Hz, 2H, CH2N), 3.46 (sextet, J=7.5 Hz, 1H; 13-H), 3.73 (t, J=5.2 Hz, 2H; CH2O), 5.20–5.31 (m, 4H; 11-H, 12-H, 14-H, 15-H), 5.44–5.32 (m, 4H; 5-H, 6-H, 8-H, 9-H), 5.88 ppm (br s, 1H; NH); 13C NMR (CDCl3, 150 MHz): δ=14.09 (C-20), 22.04 (C-13-CH3), 22.59 (C-19), 25.44 (C-3), 25.61 (C-7), 25.84 (C-10), 26.61 (C-4), 27.51 (C-16), 29.46 (C-17), 30.50 (C-13), 31.57 (C-18), 35.92 (C-2), 42.48 (NH-CH2), 62.70 (-CH2-OH), 125.61 (C-11), 128.10 (C-9), 128.26 (C-15), 128.43 (C-6), 128.84 (C-5), 129.02 (C-8), 134.12 (C-14), 135.10 (C-12), 174.16 ppm (C=O); MS (EI): m/z (%): 361 [M]+ (2), 328 (12), 218 (9), 178 (14), 125 (20), 103 (33), 85 (100); HRMS (EI): m/z calcd for C23H39NO2: 361.2981 [M]+; found: 361.2973; elemental analysis calcd (%) for C23H39NO2: C 76.40, H 10.87, N 3.87; found: C 76.15, H 10.59, N 4.16.
Compound 33
The synthesis was carried out as described for 24, using phosphonium salt 23 (217 mg, 0.41 mmol), KHMDS (75 mg, 0.38 mmol), and aldehyde 3 (94 mg, 0.29 mmol) in anhydrous THF (0.8 mL). The reaction was completed in 2.5 h at −98–0°C to give 33 (84 mg, 58% yield). (c = 0.00550gmL−1 in CHCl3). 1H NMR (CDCl3, 300MHz): δ=0.98 (d, J=6.6 Hz, 3H; CH-CH3), 1.04 (s, 9H; Si(Ph)2C(CH3)3), 1.80 (quintet, J=7.2 Hz, 2H; 3-H), 2.23 (tt, J=6.9, 2.4 Hz, 2H; 4-H), 2.42 (t, J=7.5 Hz, 2H; 2-H), 2.63 (m, 1H; 13-H), 2.74–2.86 (m, 1H; 10-H), 2.86–2.98 (m, 1H; 10-H), 3.08 (quintet, J=2.4 Hz, 2H; 7-H), 3.45 (d, J= 6.3 Hz, 2H; 14-H), 3.67 (s, 3H; COOCH3), 5.24 (tdd, J=10.8, 9.6, 0.9 Hz, 1H; 12-H), 5.41 (dtd, J=10.8, 6.3, 0.5 Hz, 1H; 11-H), 7.33–7.46 (m, 6H; 3-H, 4-H, 5-H, ArH), 7.68–7.62 ppm (m, 4H; 2-H, 6-H, ArH); 13C NMR (CDCl3, 75 MHz): δ=9.7, 17.2, 17.4, 18.2, 19.3, 23.9, 26.8, 26.9, 32.8, 34.7, 51.5 (OCH3), 68.3 (CH2O), 74.2 (C≡C), 75.3 (C≡C), 78.8 (C≡C), 79.1 (C≡C), 124.7, 127.6, 129.5, 133.8, 134.3, 135.6, 173.6 ppm (C=O); MS (ESI): m/z (%): 523 [M+Na]+ (100), 423 (6), 270 (14).
Compound 34
Acetic acid (0.1 mL) and TBAF (0.73 mL, 0.73 mmol, 1 m solution in THF) were added sequentially to a stirred solution of 33 (81 mg, 0.16 mmol) in dry THF (3.2 mL), at 0°C under an argon atmosphere. Stirring was continued for 10 min at 0°C and for 20 h at room temperature. The reaction mixture was quenched by the addition of a saturated aqueous solution of NH4Cl at 0°C, then extracted with AcOEt. The combined organic extracts were washed with a saturated aqueous solution of NaHCO3 and brine, dried (Na2SO4), and concentrated in vacuo at 37°C. Purification by flash column chromatography on silica gel (40% ethyl acetate in hexane) gave 34 as a colorless viscous liquid (32 mg, 76% yield). (c = 0.00265 gmL−1 in CHCl3); 1H NMR (CDCl3, 300 MHz): δ=0.96 (d, J=6.9 Hz, 3H; CH-CH3), 1.80 (quintet, J=7.2 Hz, 2H; 3-H), 2.22 (tt, J=6.9, 2.4 Hz, 2H; 4-H), 2.42 (t, J= 7.2 Hz, 2H; 2-H), 2.70 (m, 1H; 13-H), 2.96 (m as d, J=7.2 Hz, 2H; 10-H), 3.10 (quintet, J=2.4 Hz, 2H; 7-H), 3.36 (dd, J=10.5, 8.1 Hz, 1H; 14-H), 3.50 (dd, J=10.5, 5.7 Hz, 1H; 14-H), 3.67 (s, 3H; COOCH3), 5.23 (tdd, J=10.9, 9.5, 0.9 Hz, 1H; 12-H), 5.57 ppm (dtd, J=10.9, 6.3, 0.5 Hz, 1H; 11-H); 13C NMR (CDCl3, 75 MHz): δ=9.7, 16.7, 17.5, 18.2, 23.8, 32.8, 34.9, 51.5 (OCH3), 67.5 (CH2O), 74.5 (C≡C), 75.2 (C≡C), 78.5 (C≡ C), 79.2 (C≡C), 126.3 (CH=CH), 133.9 (CH=CH), 173.7 ppm (C=O); MS (ESI): m/z (%): 285 [M+Na]+(40), 263 [M+H]+ (12), 202 (100), 186 (24).
Compound 35
The synthesis was carried out as described for 26, using alcohol 34 (32 mg, 0.12 mmol) and Dess–Martin periodinane (155 mg, 0.37 mmol) in anhydrous CH2Cl2 (2.4 mL). The reaction was completed in 2h at 0°C, and the sensitive aldehyde 35 was used in the next step without further purification. 1H NMR (CDCl3, 300 MHz): δ=1.18 (d, J= 6.6 Hz, 3H; CH-CH3), 1.80 (quintet, J=7.2 Hz, 2H; 3-H), 2.22 (tt, J= 6.9, 2.4 Hz, 2H; 4-H), 2.42 (t, J=7.2 Hz, 2H; 2-H), 2.82 (m, 1H; 13-H), 2.98 (m as d, J=7.1 Hz, 2H; 10-H), 3.10 (quintet, J=2.4 Hz, 2H; 7-H), 3.25–3.52 (m, 2H; 14-H), 3.67 (s, 3H; COOCH3), 5.32 (dd, J=10.8, 10.0 Hz, 1H; 12-H), 5.71 (td, J=10.8, 6.3 Hz, 1H; 11-H), 9.55 ppm (s, 1H; CHO).
Compound 36
Method A (from aldehyde 35)
The synthesis was carried out as described for 27 (method A), using hexyltriphenylphosphonium bromide (156 mg, 0.37 mmol), KHMDS (70 mg, 0.35 mmol), and aldehyde 35 (31 mg, 0.12 mmol). The reaction was completed in 1 h at −98°C to give 36 as a viscous oil (23 mg, 58% yield). (c = 0.00185 gmL−1 in CHCl3); 1H NMR (CDCl3, 300 MHz): δ=0.88 (t, J=6.6 Hz, 3H; 20-H), 1.00 (d, J=6.9 Hz, 3H; CH-CH3), 1.22–1.38 (m, 6H; 17-H, 18-H, 19-H), 1.80 (quintet, J=7.5 Hz, 2H; 3-H), 2.03 (dt, J=6.9, 6.9 Hz, 2H; 16-H), 2.22 (tt, J=6.9, 2.4 Hz, 2H; 4-H), 2.42 (t, J=7.5 Hz, 2H; 2-H), 2.94 (m, 2H; 10-H), 3.10 (quintet, J=2.4 Hz, 2H; 7-H), 3.39 (sextet, J=7.2 Hz, 1H; 13-H), 3.66 (s, 3H; COOCH3), 5.35–5.15 ppm (m, 4H; 11-H, 12-H, 14-H, 15-H); 13C NMR (CDCl3, 75 MHz): δ=9.7, 14.1, 17.3, 18.2, 21.8, 22.6, 23.9, 27.4, 29.4, 30.4, 31.5, 32.8, 51.5 (OCH3), 74.2 (C≡C), 75.3 (C≡ C), 78.7 (C≡C), 79.1 (C≡C), 122.3 (CH=CH), 128.6 (CH=CH), 133.6 (CH=CH), 136.1 (CH=CH), 173.6 ppm (C=O).
Method B (from aldehyde 11)
The synthesis was carried out as described for 24, using phosphonium salt 23 (425 mg, 0.77 mmol), KHMDS (144 mg, 0.72 mmol), and aldehyde 11 (131 mg, 0.85 mmol). The reaction was completed in 2.5 h at −98–0°C to give 36 (50 mg, 21% yield).
Compound 37
The synthesis was carried out as described for 28, using 36 (23 mg, 0.07 mmol) and a 1m aqueous solution of LiOH (0.14 mL, 0.14 mmol) in THF (0.25 mL). The reaction was completed in 7 h at room temperature to give 37 as a viscous oil (18 mg, 82% yield). (c = 0.00226 gmL–1 in CHCl3); 1H NMR (CDCl3, 300 MHz): δ=0.88 (t, J=6.6 Hz, 3H; 20-H), 1.01 (d, J=6.9 Hz, 3H; CH-CH3), 1.23–1.38 (m, 6H; 17-H, 18-H, 19-H), 1.82 (quintet, J=7.5 Hz, 2H; 3-H), 2.04 (dt, J=6.9, 6.9 Hz, 2H; 16-H), 2.25 (tt, J=6.9, 2.4 Hz, 2H; 4-H), 2.48 (t, J=7.5 Hz, 2H; 2-H), 2.95 (m, 2H; 10-H), 3.11 (quintet, J=2.4 Hz, 2H; 7-H), 3.39 (m as sextet, J=7.0 Hz, 1H; 13-H), 5.15–5.36 ppm (m, 4H; 11-H, 12-H, 14-H, 15-H); 13C NMR (CDCl3, 75 MHz): δ=9.7, 14.1, 17.3, 18.1, 21.8, 22.6, 23.5, 27.5, 29.4, 30.4, 31.5, 32.7, 74.2 (C≡C), 75.5 (C≡C), 78.7 (C≡C), 79.0 (C≡C), 122.3 (CH=CH), 128.6 (CH=CH), 133.6 (CH= CH), 136.1 (CH=CH), 179.1 ppm (C=O); MS (ESI): m/z (%): 337 [M+Na]+ (100), 315 [M+H]+ (4), 271 (16), 239 (44).
Compound 38
The synthesis was carried out as described for 31, using 37 (6 mg, 0.02 mmol), carbonyldiimidazole (6 mg, 0.04 mmol), and protected ethanolamine 30 (11 mg, 0.04 mmol) in dry THF (0.45 mL) to give 38 as a viscous oil (11 mg, 97% yield). 1H NMR (CDCl3, 300 MHz): δ=0.88 (t, J=6.9 Hz, 3H; 20-H), 1.01 (d, J=6.6 Hz, 3H; CH-CH3), 1.07 (s, 9H; Si(Ph)2C(CH3)3), 1.22–1.38 (m, 6H; 17-H, 18-H, 19-H), 1.79 (quintet, J=7.2 Hz, 2H; 3-H), 2.04 (dt, J=6.7, 6.7 Hz, 2H; 16-H), 2.17–2.28 (t and tt overlapping, 4H; 2-H, 4-H; including 2.23, t, J=7.5 Hz; 2-H), 2.94 (m, 2H; 10-H), 3.11 (quintet, J=2.4 Hz, 2H; 7-H), 3.34–3.45 (dt and sextet overlapping, 3H; 13-H, CH2-NH; including 3.39, dt, J=5.1, 5.1 Hz; CH2-NH), 3.74 (t, J=5.1 Hz, 2H; CH2OTBDPS), 5.12–5.34 (m, 4H; 11-H, 12-H, 14-H, 15-H), 5.76 (br s, 1H; NH), 7.34–7.46 (m, 6H; 3-H, 4-H, 5-H, ArH), 7.60–7.68 (m, 4H; 2-H, 6-H, ArH); MS (ESI): m/z (%): 618 [M+Na]+ (36), 596 [M+H]+ (38), 518 (100), 505 (44), 287 (96), 253 (52), 219 (44), 69 (38).
Compound 39
The synthesis was carried out as described for 34, using 38 (11 mg, 0.02 mmol), acetic acid (0.4 mL) and TBAF (0.1 mL, 0.1 mmol, 1 m solution in THF) in dry THF (0.4 mL). The reaction was completed in 20 h and the crude oil was purified by flash column chromatography on silica gel (ethyl acetate) to give 39 (5 mg, 75% yield) as a viscous oil. (c = 0.00201 gmL–1 in CHCl3); 1H NMR (CDCl3, 300 MHz): δ=0.88 (t, J=6.9 Hz, 3H; 20-H), 1.01 (d, J=6.9 Hz, 3H; CH-CH3), 1.21–1.39 (m, 6H; 17-H, 18-H, 19-H), 1.82 (quintet, J=7.2 Hz, 2H; 3-H), 2.05 (dt, J=6.9, 6.9 Hz, 2H; 16-H), 2.23 (tt, J=6.9, 2.4 Hz, 2H; 4-H), 2.33 (t, J=7.5 Hz, 2H; 2-H), 2.95 (m, 2H; 10-H), 3.11 (quintet, J= 2.4 Hz, 2H; 7-H), 3.35–3.48 (dt and sextet overlapping, 3H; 13-H, CH2-NH; including 3.42, dt, J=5.1, 5.1 Hz; CH2-NH), 3.72 (t, J=5.1 Hz, 2H; CH2O), 5.14–5.40 (m, 4H; 11-H, 12-H, 14-H, 15-H), 5.99 (br s, 1H; NH); 13C NMR (CDCl3 75 MHz): δ=9.7, 14.1, 17.3, 18.0, 21.8, 22.6, 24.3, 27.4, 29.4, 30.4, 31.5, 35.1, 42.5 (CH2NH), 62.6 (CH2O), 74.3 (C≡C), 75.5 (C≡ C), 78.8 (C≡C), 79.3 (C≡C), 122.2 (CH=CH), 128.7 (CH=CH), 133.5 (CH=CH), 136.2 (CH=CH), 173.7 ppm (C=O); MS (ESI): m/z (%): 358 [M+H]+, (100), 255 (9), 198 (12); HRMS (ESI): m/z calcd for C23H36NO2 [M+H]+: 358.2746; found: 358.2751; elemental analysis calcd (%) for C23H35NO2: C 77.27, H 9.87, N 3.92; found: C 77.62, H 10.09, N 4.11.
Radioligand binding assay
For CB1, rat forebrain membranes were prepared according to the procedure of Dodd et al.[69] The binding of the novel anandamide analogues to the cannabinoid receptor was assessed as previously described,[4, 44, 45, 47, 64, 65] except that the membranes were pre-treated with PMSF. Membranes, previously frozen at −80°C, were thawed on ice. Three volumes of TME buffer (25 mm Tris-HCl buffer, 5 mm MgCl2, and 1 mm ethylenediaminetetraacetic acid (EDTA), pH 7.4) containing 150 µm PMSF (freshly prepared in 2-propanol as a 100 mm stock solution) were added to the stirred suspension. The suspension was incubated at 4°C, then after 15 min, a second addition of PMSF stock brought the concentration to 300 mm PMSF, and the mixture was incubated for another 15 min. At the end of the second 15 min incubation, the membranes were pelleted and washed three times with TME to remove unreacted PMSF. The pretreated membranes were subsequently used in the binding assay described next. The resulting PMSF-treated membrane (approximately 30 µg) was incubated in a silanized 96-well microtiter plate with TME containing 0.1% essentially fatty acid-free bovine serum albumin (BSA), 0.76 nm [3H]CP-55,940, and various concentrations of anandamide analogues in a final volume of 200 mL. The binding assay was performed at 30°C for 1 h with gentle agitation. The resultant material was transferred to Unifilter GF/B filter plates, and unbound ligand was removed by using a Packard Filtermate-96 Cell Harvester (Perkin–Elmer Packard, Shelton, CT). Filter plates were washed four times with ice-cold wash buffer (50 mm Tris, 5 mm MgCl2 containing 0.5% BSA, pH 7.4). Radioactivity was determined by using a Packard Top-Count. Data collected from three independent experiments performed with duplicate determinations were normalized between 100 and 0% specific binding for [3H]CP-55,940, determined using buffer and 100 nm CP-55,940, respectively. The normalized data were analyzed using nonlinear regression to yield IC50 values. Data from at least three independent experiments performed in duplicate were used to calculate IC50 values, which were converted to Ki values by using the assumptions of Cheng and Prusoff.[70]
For CB2 receptor binding studies, membranes were prepared from frozen mouse spleen essentially according to the procedure of Dodd et al.[69] Silanized centrifuge tubes were used throughout to minimize receptor loss due to absorption. The CB2 binding assay was conducted in the same manner as for CB1.
Supplementary Material
Acknowledgements
This work was supported by EURODESY MEST-CT2005—020575 and by a grant from the National Institute on Drug Abuse, grant number DA007215.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/chem.200902880. It contains experimental procedures physical properties and spectroscopic data for compounds 2–4, 8–11, 13–23 and 30 as well as 1H NMR COSY HSQC HMBC and NOESY spectra of compound 27 and a 1H NMR spectrum of compound 24
References
- 1.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 2.T Sugiura, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. Biochem. Biophys. Res. Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 3.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Biochem. Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 4.Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. Mol. Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 5.Munro S, Thomas KL, Abu-Shaar M. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 6.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 7.Howlett AC. Mol. Pharmacol. 1985;27:429–436. [PubMed] [Google Scholar]
- 8.Pertwee RG. Pharmacol. Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- 9.Calignano A, La Rana G, Giuffrida A, Piomelli D. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- 10.Walker JM, Huang SM, Strangman NM, Tsou K, Sanudo-Pena MC. Proc. Natl. Acad. Sci. USA. 1999;96:12198–12203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cravatt BF, Lichtman AH. J. Neurobiol. 2004;61:149–160. doi: 10.1002/neu.20080. [DOI] [PubMed] [Google Scholar]
- 12.Massa F, Marsicano G, Hermann H, Cannich A, Monory K, Cravatt BF, Ferri GL, Sibaev A, Storr M, Lutz B. J. Clin. Invest. 2004;113:1202–1209. doi: 10.1172/JCI19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowler CJ. Brain Res. Rev. 2003;41:26–43. doi: 10.1016/s0165-0173(02)00218-7. [DOI] [PubMed] [Google Scholar]
- 14.Karanian DA, Brown QB, Makriyannis A, Kosten TA, Bahr BA. J. Neurosci. 2005;25:7813–7820. doi: 10.1523/JNEUROSCI.2347-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, Shohami E. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- 16.Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, Nava F, Piomelli D, Rodriguez de Fonseca F. J. Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams CM, Kirkham TC. Physiol. Behav. 2002;76:241–250. doi: 10.1016/s0031-9384(02)00725-4. [DOI] [PubMed] [Google Scholar]
- 18.Mallet PE, Beninger RJ. Psychopharmacology. 1998;140:11–19. doi: 10.1007/s002130050733. [DOI] [PubMed] [Google Scholar]
- 19.Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Nat. Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 20.Melck D, Rueda D, Galve-Roberh I, De Petrocellis L, Guzman M, Di Marzo V. FEBS Lett. 1999;463:235–240. doi: 10.1016/s0014-5793(99)01639-7. [DOI] [PubMed] [Google Scholar]
- 21.Yamaji K, Sarker KP, Kawahara K, Iino S, Yamakuchi M, Abeyama K, Hashiguchi T, Maruyama I. Thromb. Haemost. 2003;89:875–884. [PubMed] [Google Scholar]
- 22.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 23.Bracey MH, Hanson MA, Masuda KR, Stevens RC, Cravatt BF. Science. 2002;298:1793–1796. doi: 10.1126/science.1076535. [DOI] [PubMed] [Google Scholar]
- 24.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Proc. Natl. Acad. Sci. USA. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandevoorde S, Lambert DM. Curr. Pharm. Des. 2005;11:2647–2668. doi: 10.2174/1381612054546914. [DOI] [PubMed] [Google Scholar]
- 26.Ueda N, Yamamoto K, Yamamoto S, Tokunaga T, Shirakawa E, Shinkai H, Ogawa M, Sato T, Kudo I, Inoue K. Biochim. Biophys. Acta. 1995;1254:127–134. doi: 10.1016/0005-2760(94)00170-4. [DOI] [PubMed] [Google Scholar]
- 27.Hampson AJ, Hill WA, Zan-Phillips M, Makriyannis A, Leung E, Eglen RM, Bornheim LM. Biochim. Biophys. Acta. 1995;1259:173–179. doi: 10.1016/0005-2760(95)00157-8. [DOI] [PubMed] [Google Scholar]
- 28.Kozak KR, Marnett LJ. Prostaglandins Leukotrienes Essent. Fatty Acids. 2002;66:211–220. doi: 10.1054/plef.2001.0359. [DOI] [PubMed] [Google Scholar]
- 29.Di Marzo V. Pharmacol. Res. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Snider NT, Sikora MJ, Sridar C, Feuerstein TJ, Rae JM, Hollenberg PF. J. Pharmacol. Exp. Ther. 2008;327:538–545. doi: 10.1124/jpet.108.141796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bornheim LM, Kim KY, Chen B, Correira MA. Biochem. Pharmacol. 1995;50:677–686. doi: 10.1016/0006-2952(95)00177-2. [DOI] [PubMed] [Google Scholar]
- 32.Yu M, Ives D, Ramesha CS. J. Biol. Chem. 1997;272:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]
- 33.Kozak KR, Rowlinson SW, Marnet LJ. J. Biol. Chem. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- 34.Kozak KR, Crews BC, Morrow JD, Wang L-H, Ma YH, Weinander R, Jakobsson P-J, Marnett LJ. J. Biol. Chem. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- 35.Jhaveri MD, Richardson D, Chapman V. Br. J. Pharmacol. 2007;152:624–632. doi: 10.1038/sj.bjp.0707433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fowler CJ. Br. J. Pharmacol. 2007;152:594–601. doi: 10.1038/sj.bjp.0707379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamberg M, Samuelsson B. J. Biol. Chem. 1967;242:5336–5343. [PubMed] [Google Scholar]
- 38.Peng S, Okeley NM, Tsai A-L, Wu G, Kulmacz RJ, van der Donk WA. J. Am. Chem. Soc. 2002;124:10785–10796. doi: 10.1021/ja026880u. [DOI] [PubMed] [Google Scholar]
- 39.Kozak KR, Prusakiewicz JJ, Rowlinson SW, Prudhomme DR, Marnett LJ. Biochemistry. 2003;42:9041–9049. doi: 10.1021/bi034471k. [DOI] [PubMed] [Google Scholar]
- 40.Thakur GA, Nikas SP, Li C, Makriyannis A. In: Handbook of Experimental Pharmacology. Pertwee RG, editor. Springer, New York: 2005. pp. 209–246. [DOI] [PubMed] [Google Scholar]
- 41.Thakur GA, Nikas SP, Makriyannis A. Mini-Rev. Med. Chem. 2005;5:631–640. doi: 10.2174/1389557054368772. [DOI] [PubMed] [Google Scholar]
- 42.Pavlopoulos S, Thakur GA, Nikas SP, Makriyannis A. Curr. Pharm. Des. 2006;12:1751–1769. doi: 10.2174/138161206776873743. [DOI] [PubMed] [Google Scholar]
- 43.Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertw-ee RG, Makriyannis A. J. Med. Chem. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- 44.Khanolkar AD, Abadji V, Lin S, Hill WAG, Taha G, Abou-zid K, Meng Z, Fan P, Makriyannis A. J. Med. Chem. 1996;39:4515–4519. doi: 10.1021/jm960152y. [DOI] [PubMed] [Google Scholar]
- 45.Lin S, Khanolkar AD, Fan P, Goutopoulos A, Qin C, Papahadjis D, Makriyannis A. J. Med. Chem. 1998;41:5353–5361. doi: 10.1021/jm970257g. [DOI] [PubMed] [Google Scholar]
- 46.Goutopoulos A, Fan P, Khanolkar AD, Xie XQ, Lin S, Makriyannis A. Bioorg. Med. Chem. 2001;9:1673–1684. doi: 10.1016/s0968-0896(01)00088-8. [DOI] [PubMed] [Google Scholar]
- 47.Li C, Xu W, Vadivel SK, Fan P, Makriyannis A. J. Med. Chem. 2005;48:6423–6429. doi: 10.1021/jm050272i. [DOI] [PubMed] [Google Scholar]
- 48.Yao F, Li C, Vadivel SK, Bowman AL, Makriyannis A. Bioorg. Med. Chem. Lett. 2008;18:5912–5915. doi: 10.1016/j.bmcl.2008.07.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheskin T, Hanus L, Slager J, Vogel Z, Mechoulam R. J. Med. Chem. 1997;40:659–667. doi: 10.1021/jm960752x. [DOI] [PubMed] [Google Scholar]
- 50.Tian X, Guo J, Yao F, Yang D-P, Makriyannis A. J. Biol. Chem. 2005;280:29788–29795. doi: 10.1074/jbc.M502925200. [DOI] [PubMed] [Google Scholar]
- 51.Barnett-Norris J, Hurst DP, Lynch DL, Guarnieri F, Makriyannis A, Reggio PH. J. Med. Chem. 2002;45:3649–3659. doi: 10.1021/jm0200761. [DOI] [PubMed] [Google Scholar]
- 52.van der Stelt M, van Kuik JA, Bari M, van Zadelhoff G, Leeflang BR, Veldink GA, Finazzi-Agro A, Vliegenthart JFG, Maccarrone M. J. Med. Chem. 2002;45:3709–3720. doi: 10.1021/jm020818q. [DOI] [PubMed] [Google Scholar]
- 53.Reggio PH. Curr. Pharm. Des. 2003;9:1607–1633. doi: 10.2174/1381612033454577. [DOI] [PubMed] [Google Scholar]
- 54.Barnett-Norris J, Guarnieri F, Hurst DP, Reggio PH. J. Med. Chem. 1998;41:4861–4872. doi: 10.1021/jm9803471. [DOI] [PubMed] [Google Scholar]
- 55.Malkowski MG, Ginell SL, Smith WL, Garavito RM. Science. 2000;289:1933–1937. doi: 10.1126/science.289.5486.1933. [DOI] [PubMed] [Google Scholar]
- 56.Zylstra EJ, She MW-L, Salamant WA, Leahy JW. Synlett. 2007:623–627. [Google Scholar]
- 57.Cossy J, Bauer D, Bellosta V. Tetrahedron. 2002;58:5909–5922. [Google Scholar]
- 58.Guan Y, Wu J, Sun L, Dai W-M. J. Org. Chem. 2007;72:4953–4960. doi: 10.1021/jo070624o. [DOI] [PubMed] [Google Scholar]
- 59.Papahatjis DP, Kourouli T, Nahmias VR. Lett. Org. Chem. 2006;3:45–47. [Google Scholar]
- 60.Nicolaou KC, Papahatjis DP, Claremon DA, Dolle RE., III. J. Am. Chem. Soc. 1981;103:6967–6969. [Google Scholar]
- 61.Corey EJ, Suggs JW. Tetrahedron Lett. 1975;16:2647–2650. [Google Scholar]
- 62.Nicolaou KC, Daines RA, Uenishi J, Li WS, Papahatjis DP, Chakraborty TK. J. Am. Chem. Soc. 1988;110:4672–4685. [Google Scholar]
- 63.a Bourne C, Roy S, Wiley JL, Martin BR, Thomas BF, Mahadevan A, Razdan RK. Bioorg. Med. Chem. 2007;15:7850–7864. doi: 10.1016/j.bmc.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Brown CA, Ahuja VK. J. Org. Chem. 1973;38:2226–2230. [Google Scholar]; c Brown CA, Ahuja VK. J. Chem. Soc. Chem. Commun. 1973;15:553–554. [Google Scholar]
- 64.Papahatjis DP, Nikas SP, Kourouli T, Chari R, Xu W, Pertwee RG, Makriyannis A. J. Med. Chem. 2003;46:3221–3229. doi: 10.1021/jm020558c. [DOI] [PubMed] [Google Scholar]
- 65.Papahatjis DP, Nahmias VR, Nikas SP, Andreou T, Alapafuja SO, Tsotinis A, Guo J, Fan P, Makriyannis A. J. Med. Chem. 2007;50:4048–4060. doi: 10.1021/jm070121a. [DOI] [PubMed] [Google Scholar]
- 66.Deutsch DG, Chin SA. Biochem. Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- 67.Desarnaud F, Cadas H, Piomelli D. J. Biol. Chem. 1995;270:6030–6035. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- 68.Breivogel CS, Selley DE, Childers SR. J. Biol. Chem. 1998;273:16865–16873. doi: 10.1074/jbc.273.27.16865. [DOI] [PubMed] [Google Scholar]
- 69.Dodd PR, Hardy JA, Oakley AE, Edwardson JA, Perry EK, Delaunoy J-P. Brain Res. 1981;226:107–118. doi: 10.1016/0006-8993(81)91086-6. [DOI] [PubMed] [Google Scholar]
- 70.Cheng YC, Prusoff WH. Biochem. Pharmacol. 1973;22:3099–3102. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.