Abstract
Biodegradable elastomeric scaffolds are of increasing interest for applications in soft tissue repair and regeneration, particularly in mechanically active settings. The rate at which such a scaffold should degrade for optimal outcomes, however, is not generally known and the ability to select from similar scaffolds that vary in degradation behavior to allow such optimization is limited. Our objective was to synthesize a family of biodegradable polyurethane elastomers where partial substitution of polyester segments with polycarbonate segments in the polymer backbone would lead to slower degradation behavior. Specifically, we synthesized poly(ester carbonate)urethane ureas (PECUUs) using a blended soft segment of poly(caprolactone) (PCL) and poly(1,6-hexamethylene carbonate) (PHC), a 1,4-diisocyanatobutane hard segment and chain extension with putrescine. Soft segment PCL/PHC molar ratios of 100/0, 75/25, 50/50, 25/75, and 0/100 were investigated. Polymer tensile strengths varied from 14-34 MPa with breaking strains of 660-875%, initial moduli of 8-24 MPa and 100% recovery after 10% strain. Increased PHC content was associated with softer, more distensible films. Scaffolds produced by salt leaching supported smooth muscle cell adhesion and growth in vitro. PECUU in aqueous buffer in vitro and subcutaneous implants in rats of PECUU scaffolds showed degradation slower than comparable poly(ester urethane)urea and faster than poly(carbonate urethane)urea. These slower degrading thermoplastic polyurethanes provide opportunities to investigate the role of relative degradation rates for mechanically supportive scaffolds in a variety of soft tissue repair and reconstructive procedures.
Keywords: elastomer, polyurethane, polycaprolactone, polycarbonate, biodegradation, scaffold
Introduction
Scaffolds that are mechanically compatible with the tissue to which they are applied can avoid potential problems with stress concentrations at tissue-scaffold interfaces (if too stiff) or scaffold mechanical failure (if too weak). For dynamic tissues, such mechanical compatibility becomes of greater importance, as evidenced by the interest in appropriately compliant vascular scaffolds [1, 2] and the general growth in interest in biodegradable elastomers for soft tissue repair in the biomaterials community [3, 4]. Commonly investigated biodegradable elatomers include poly(glycerol sebacate) [5,6], poly(hydroxyalkanoate)s [7], poly(ether ester)s [3, 8] and poly(trimethylene carbonate) based polymers [9, 10] and polyurethanes [4, 11]. The latter group is regularly synthesized from soft segments of aliphatic polyesters or polycarbonates, hard segments of aliphatic diisocyanates and potentially chain extension with diols or diamines that may possess hydrolytic or enzymatic lability [4, 12-14] Thermoplastic biodegradable polyurethanes also generally exhibit good processability and have been used to create porous or fibrous scaffolds in a variety of shapes using methods such as salt leaching [15, 16], phase separation [17, 18] and electrospinning [19, 20]. Further, these polymers are amenable to tuning of properties by adjusting the composition of the soft segment, hard segment or chain extender and also by adjusting the molar ratio of individual components.
Although not often directly addressed, in most instances where degradable scaffolds are being applied in vivo, investigators lack specific knowledge as to the optimal period over which the scaffold should be present. The trade offs between too short of a duration (e.g. mechanical failure) and too long (e.g. inappropriate tissue development, fibrosis) are recognized, but require the availability of broader biomaterial options to begin to explore these effects for various tissue applications. In work aimed at creating elastic scaffolds for soft tissue application, we have previously reported on a series of biodegradable poly(ester urethane) ureas (PEUUs) based on a poly(caprolactone) soft segment [21], a family of faster degrading poly(ether ester urethane)ureas (PEEUUs) where the soft segment was a poly(ether ester) triblock copolymer [22], and a third family where specific enzymatic liability was introduced by a elastase-specific peptide chain extender [23]. These polyurethanes provide a range of degradation behaviors, but are generally limited in that the slowest degradation behavior is exhibited by PEUU. Having access to similar degradable polyurethane elastomers where the degradation rate could be tuned to be slower than PEUU would allow investigation of elastomeric scaffold solutions to soft tissue repair where a longer presence is required of the material support.
Our objective in this report was to synthesize a group of degradable polyurethane elastomers where the partial substitution of polyester segments with polycarbonate segments in the polymer backbone would lead to slower degradation behavior. Specifically, we synthesized poly(ester carbonate)urethane ureas (PECUUs) using a blended soft segment of polycaprolactone (PCL) and poly(1,6-hexamethylene carbonate) (PHC) and a hard segment of 1,4-diisocyanatobutane (BDI) with chain extension by putrescine. Different molar ratios of PCL and PHC were investigated as soft segments. The chemical structure, mechanical properties, in vitro degradation and cytocompatibility of PECUU films were studied and porous PECUU scaffolds were generated using the salt leaching method. These scaffolds were characterized mechanically and for cytocompatibility and were then implanted into a rat subcutaneous model to examine in vivo degradation relative to polyurethane scaffolds lacking either polycarbonate or polyester in their backbone.
Materials and Methods
Materials
Polycaprolactone diol (PCL, Mn=2000, Sigma) and poly(1,6-hexamethylene carbonate) diol (PHC, Mn=2000, Sigma) were dried under vacuum at 50°C to remove the residual water before synthesis. 1,4-diisocyanatobutane (BDI, Sigma) and putrescine (Sigma) were distilled before usage. Dimethyl sulfoxide (DMSO, Sigma), N,N,-dimethylformamide (DMF, Sigma), 1,1,1,3,3,3-hexafluoroisopropanol (HFIP, Oakwood Products) and phosphate buffered saline (PBS, Lonza) were used as received. Stannous octoate (Sn(Oct)2, Sigma) was dried by adding molecular sieves.
Synthesis of poly(ester carbonate urethane)ureas
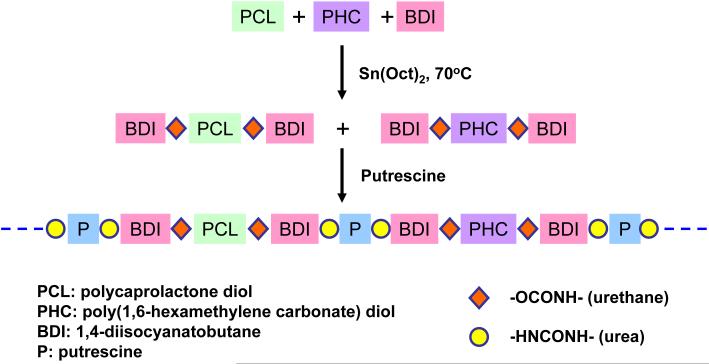
Poly(ester carbonate urethane)urea (PECUU) was synthesized from PCL, PHC and BDI using putrescine as a chain extender by a two-step solvent synthesis method (Figure 1). The (PCL+PHC):BDI:putrescine molar ratio was defined as 1:2:1. Briefly, variable molar ratios of PCL and PHC (PCL/PHC ratios of 100/0, 75/25, 50/50, 25/75 and 0/100) were completely dissolved in DMSO in a 3-neck flask with argon protection and then BDI was added to the solution, following 4 drops of Sn(Oct)2. The flask was placed in an oil bath at 70°C. After 3 h, the prepolymer solution was cooled at room temperature and then a putrescine/DMSO solution was added dropwise into the agitated solution. The final polymer solution concentration was controlled to be approximately 4% (w/v). Then the flask was placed in the oil bath and kept at 70°C overnight. The polymer was precipitated in an excess volume of cool deionized water and then dried in a vacuum at 60°C for 3 d. The polyurethane ureas synthesized from the different PCL/PHC molar ratios defined above are referred to as PEUU, PECUU 75/25, PECUU 50/50, PECUU 25/75 and PCUU, respectively. The yields of all final products were approximately 95%.
Figure 1.
Synthetic scheme for poly(ester carbonate urethane)ureas.
Film casting and porous scaffold fabrication
The synthesized polymers were completely dissolved in HFIP after which a ~3% polymer solution was poured into a PTFE dish. After near complete HFIP evaporation, the film was dried in a vacuum oven at 60°C for 3 d to remove residual solvent. Initial solution volumes were selected to achieve film thicknesses of 80 μm.
For scaffold fabrication, polymer samples were completely dissolved in HFIP to obtain a 20% (w/v) solution. This solution (1 mL) was blended uniformly with 4.5 g salt particles (NaCl, Sigma), which had particle sizes ranging from 100-150 μm obtained by from serial treatment of obtained material with American standard sieves. The polymer/salt mixture was poured into a cylindrical glass mould. After complete solvent evaporation, the mixture was immersed in an excess of 30% ethanol solution to remove the salt particles from the scaffold. The ethanol solution was changed frequently over 2 d of immersion. The scaffold was then placed in pure deionized water to exchange the ethanol solution for 3 h, and then was frozen at −80 °C, followed by lyophilization for 2 d to obtain a porous scaffold for subsequent characterization, cell seeding or implantation.
Polymer and scaffold characterization
Fourier transform infrared (FTIR) spectra were obtained at room temperature with a Nicolet FTIR spectrometer. A polymer solution in DMF was cast directly onto the NaCl window with subsequent evaporation of DMF under IR irradiation. The glass transition temperature (Tg) and melting temperature (Tm) were determined by differential scanning calorimetry (DSC-60; Shimadzu) with a scanning rate of 20°C/min over a range of −100 to 200°C with nitrogen flow. The water contact angle (n=8 per polymer) in air was detected on the film surface using a sessile drop method on a UCA contact angle instrument (UCA optima, AST Products Inc.).
The polymer inherent viscosity was measured using an Ubbelohde viscometer at 22°C [24]. Each sample was dissolved in 15 mL HFIP at a concentration of 0.1 dL/100 mL and then filtered using a 0.45 μm polytetrafluoroethylene filter. Each sample was tested 5 times and the inherent viscosity was calculated as ln (tp/ts)/Cp, where tp represents the polymer solution flow time, ts represents the HFIP flow time and Cp is the polymer concentration.
Scaffold morphology was observed with scanning electronic microscopy (SEM, JEM-1011, JEOL). Porosity was measured using an ethanol displacement method [25], where a scaffold sample was immersed in a graduated cylinder containing a known volume of ethanol (V1). After 5 min, the total volume of ethanol and the ethanol-impregnated scaffold was recorded as V2. The ethanol-impregnated scaffold was removed from the cylinder and the residual ethanol volume was recorded as V3. The porosity of the scaffold was calculated as (V1-V3)/(V2-V3)*100%.
Mechanical testing
Strips (2 × 20 mm) were cut from the polymer films and mechanical properties were measured on an MTS Tytron 250 MicroForce Testing Workstation at room temperature. The crosshead speed was set at 10 mm/min according to ASTM D638-98. Four samples were tested for each polymer. The instant strain recovery was measured under the same conditions with samples marked at their distal ends, stretched to 10% strain and held for 1 min, and then released. This stretch cycle was repeated 3 times and then the length change was recorded after the release of tension. The original length (L0) and the length immediately after releasing the tension (L1) were measured by a caliper. Instant strain recovery was calculated as (1 - (L1 - L0)/L0) × 100%. The mechanical properties of porous scaffolds were also measured using the above protocol. Four samples were tested for each type of polymer scaffold.
In vitro degradation
Polymer samples having an initial weight of ~30 mg (W0) were cut from cast films and then immersed in 20 mL glass vials loaded with 10 mL PBS at 37°C. At predetermined time points, samples were removed, dried in a vacuum oven at 60°C for 3 d, then weighted (W1) and discarded. The percent mass remaining was calculated as W1/W0 × 100%. Further, the inherent viscosity (IV) of each polymer sample after 4 and 8 wk degradation time was measured using an Ubbelohde viscometer method as described above. The residual IV was calculated as IV1/IV0 × 100%, where IV0 represents the original inherent viscosity before degradation and IV1 represents the inherent viscosity after degradation.
In vitro cell culture
Polymer films were cut into 6 mm diameter disks using a standard punch and sterilized using 70% ethanol solution and UV irradiation in a laminar flow cell culture hood (Class II A/B3 Biological Safety Cabinet). Vascular smooth muscle cells previously isolated from Lewis rat aorta (RSMCs) were seeded on the surface of each polymer film in a 96-well cell culture plate for evaluating cellular attachment and cellular growth, using 2×104 and 5×103 cells respectively. The culture medium (DMEM supplemented with 10% fetal bovine serum and 5% penicillin/streptomycin solution; Lonza) was changed every 2 days. A mitochondrial activity assay (MTT assay, Sigma) was used as an indirect method to quantify cellular attachment at 1d and cellular numbers at 2, 3 and 4 d, with the presumption of similar metabolic activity across the surfaces and over time. Tissue cultured polystyrene (TCPS) was utilized as a positive control. To qualitatively verify the results based on the MTT assay, fluorescence microscopy was employed to visualize cells on the material surfaces.
Visualization of cell numbers and cellular morphology was performed by first fixing the cell seeded samples with 10% paraformaldehyde solution. After PBS rinsing, the sample was immersed in 0.5% Triton X-100 solution for 45 min, then rhodamine phalloidin (1:250, Invitrogen) was added to stain alpha-smooth muscle actin for 30 min. After rinsing 3 times with PBS, cell nuclei were stained by DRAQ5 (1:1000, Biostatus) for 1 h. Following another series of PBS rinses (3x), cellular morphology on the sample surface was observed with confocal laser scanning microscopy (Fluoview 500, Olympus).
For cell seeding into porous scaffolds, samples 1 mm thick were cut from the cylindrical porous scaffolds, followed by using a tissue punch to obtain 6 mm diameter disks. Each sample was sterilized by 70% ethanol solution immersion and UV irradiation for 1 h, followed by washing three times with sterilized PBS within 3 h. Finally, the sample was placed in the RSMC culture medium overnight for cell seeding. RMSCs (1 × 106 cells) were seeded into the scaffold by a filtration seeding method [26]. The cellularized scaffold was placed into a 12-well culture plate for 3 h and transferred to a spinner flask culture system (BELLCO Biotechnology) with 16 rpm stirring. To exchange culture medium, every third day 50% of the old medium was removed and the same volume of fresh medium was added. As was done for the films, the MTT assay was used to indirectly quantify cellular numbers in the scaffolds at 1 and 7 d. To qualitatively verify the MTT results and to visualize cell distribution in the scaffolds, at 1 and 7 d cellularized scaffolds were fixed in 10% paraformaldehyde solution, and then embedded in paraffin for histological sectioning. Sections were stained with hematoxylin and eosin (H&E) for cellular visualization.
In vivo subcutaneous placement
Adult female Lewis rats (Harlan Sprague Dawley, Indianapolis, IN, USA) weighing 200–250 g were used. The protocol followed National Institutes of Health (NIH) guidelines for animal care and was approved by the University of Pittsburgh Institutional Animal Care and Use Committee and Children’s Hospital of Pittsburgh Animal Research Care Committee. Anesthesia was induced with 3.0% isoflurane inhalation, followed by 1.5 % isoflurane inhalation for maintenance. The hair on the back of the rat was trimmed with an electrical clipper. Before skin incision, one dose of cefuroxime (100 mg/kg) as an antibiotic was administered intramuscularly for prophylaxis of surgical infection. The skin of the right back was cut with a 1.0 cm incision in the dorsal lumbar region, and a subcutaneous pocket was made. The scaffold (6 mm diam. × 200 μm thickness, prepared analogously to those above for cell culture) was secured in the pocket (1 scaffold per rat), using a 7–0 polypropylene with over-and-over peripheral sutures to affix it to the underlying muscle. Scaffolds made from PEUU, PECUU 50/50 and PCUU were implanted. The skin was then closed with 4–0 polyglactin absorbable suture (Vicryl, Ethicon). At 8 wk post-implantation (n=4 for each group), animals were sacrificed. After macroscopic photography of the material in situ, the material implant location was harvested and frozen in 2-methylbutane, which was pre-cooled in liquid nitrogen, followed by sectioning and staining with H&E for histological assessment.
Statistical analysis
Results are displayed as the mean ± standard deviation. One-way ANOVA was utilized to evaluate the mechanical properties and cellular response using Neuman-Keuls testing for post hoc assessment of the differences between specific samples. Significance was considered to exist at p<0.05.
Results
Polymer synthesis and characterization
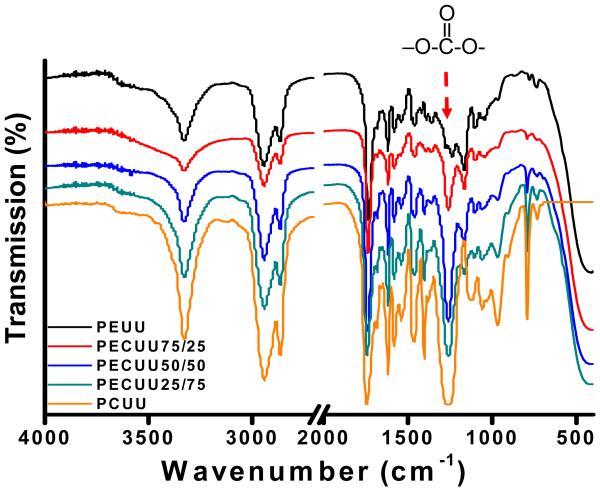
The FTIR spectra for the synthesized polymers are seen in Figure 2. The urethane and urea groups in polyurethanes were confirmed by a peak at 3342 cm−1, which was assigned to the hydrogen bonded N-H stretching from the urethane and urea groups. The asymmetric and symmetric stretching of CH2 was shown at 2948 cm−1 and 2859 cm−1, respectively. The absorbance peak at 1752 cm−1 was attributed to the carbonyl of the ester, carbonate, urethane and urea groups. A strong peak assigned to the –O-C-O- group of the carbonate group in PECUUs and PCUU was observed at 1260 cm−1 [13], while the peak was not present in the FTIR spectrum of PEUU. Another peak at 799 cm−1 assigned to out-of-plane carbonate bending was shown in the spectra of PECUUs and PCUU. Both of these absorbance peaks appear to increase with increased polymer carbonate content.
Figure 2.
FT-IR spectra of PEUU, PECUUs and PCUU.
From differential scanning calorimetry results (summarized in Table 1), all of the polymers showed low soft segment glass transition temperatures (−46 to −54 °C), but no obvious hard segment transitions were observed. The glass transition temperatures increased with increasing polycarbonate molar ratio when the polycarbonate content exceeded 50 % in the soft segment. PEUU with a semi-crystalline PCL soft segment had the highest melting temperature at 40 °C, while the melting temperature of PCUU with an amorphous PHC soft segment was not detectable. PECUUs had decreasing melt temperatures from 24°C to non-detectable with increasing PHC content. Polymer inherent viscosities are also shown in Table 1 and ranged from 0.80 to 1.38 dL/g.
Table 1. Polymer characterization.
| Tg (°C) |
Tm (°C) |
Tensile strength (MPa) |
Breaking strain (%) |
Initial modulus (MPa) |
Inherent viscosity (dL/g) |
Contact angle (°) |
Instant recovery (%) |
|
|---|---|---|---|---|---|---|---|---|
| PEUU | −54 | 40 | 34 ± 3 a | 660 ± 85 a | 24 ± 2 a | 1.38 | 80 ± 2 a | 99 ± 1 |
| PECUU75/25 | −54 | 24 | 21 ± 2 b | 667 ± 34 a | 14 ± 3 b | 1.32 | 81 ± 3 a | 99 ± 1 |
| PECUU50/50 | −54 | 16 | 16 ± 4 c | 685 ± 90 a | 13 ± 3 b | 0.96 | 76 ± 2 b | 100 ± 1 |
| PECUU25/75 | −51 | - | 16 ± 3 c | 827 ± 96 b | 9 ± 2 c | 0.80 | 76 ± 2 b | 99 ± 1 |
| PCUU | −46 | - | 14 ± 2 c | 875 ± 83 b | 8 ± 2 c | 0.80 | 73 ± 1 c | 100 ± 1 |
denote significantly different group
denote significantly different group
denote significantly different group
The water contact angle of the polymers was measured to characterize their surface hydrophilicity and showed a trend toward lower angles at higher PHC content. PEUU and PECUU 75/25 had the highest contact angles (p<0.05) while PCUU had the lowest contact angle (p<0.05).
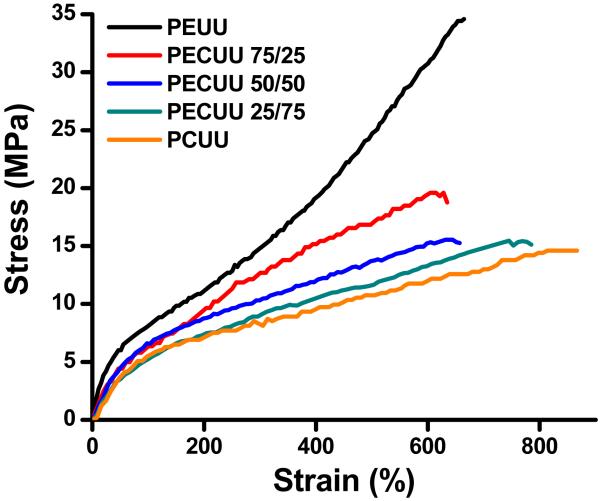
Typical stress-strain curves of PEUU, PECUUs and PCUU are shown in Figure 3 and the measured mechanical properties are summarized in Table 1. All of the synthesized polyurethanes were elastic, with tensile strengths ranging from 14 ± 2 to 34 ± 3 MPa, breaking strains from 660 ± 85 to 875 ± 83% and initial moduli from 8 ± 2 to 24 ± 2 MPa. The instant strain recovery for all polymers was ≥99% after 3 stretches at 10% strain. PEUU had both the highest tensile strength and initial modulus (p<0.05) and there was a trend towards reduced tensile strength and initial modulus with increasing PHC content. The breaking strains of PECUU 25/75 and PCUU were significantly higher than those of PEUU, PECUU 75/25 and PECUU 50/50 (p<0.05), whose breaking strains had no significant difference (p>0.05).
Figure 3.
Typical stress-strain curves for PEUU, PECUUs and PCUU films.
In vitro degradation in PBS
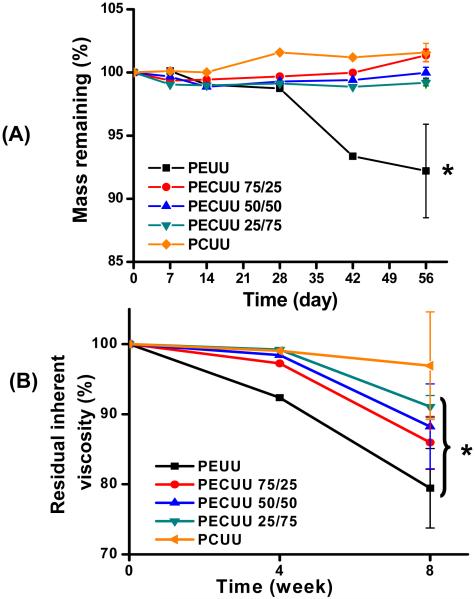
Polymer hydrolysis degradation was measured in PBS at 37°C over 8 wk and the mass remaining and inherent viscosity over this period are presented in Figure 4. Over a period of 56 days, PEUU exhibited a 9% mass loss, while all of the PECUUs and PCUU did not show detectable loss of mass (p<0.05 versus PEUU at 8 wk; p>0.05 versus initial time) (Figure 4A). As shown in Figure 4B, reductions in the inherent viscosity during in vitro degradation were present. At 4 wk, PEUU lost 8% of its original inherent viscosity (p<0.05 versus initial time), while the other polyurethanes exhibited no obvious inherent viscosity decrease. By 8 wk, PEUU had 80% of its original inherent viscosity, while PCUU had a value statistically similar to that at the initial time. PECUUs with increasing PHC content in soft segment had decreasing inherent viscosity percentages ranging from 85 to 91 % (p<0.05 versus initial time), which were between that of PEUU and PCUU.
Figure 4.
(A) Mass remaining and (B) residual inherent viscosity of PEUU, PECUUs and PCUU films after PBS immersion at 37°C over a period of 8 wks. Error bars are only shown for the last time points for clarity. * = reduced inherent viscosity relative to time = 0 (p<0.05).
Cellular behavior on polymer films
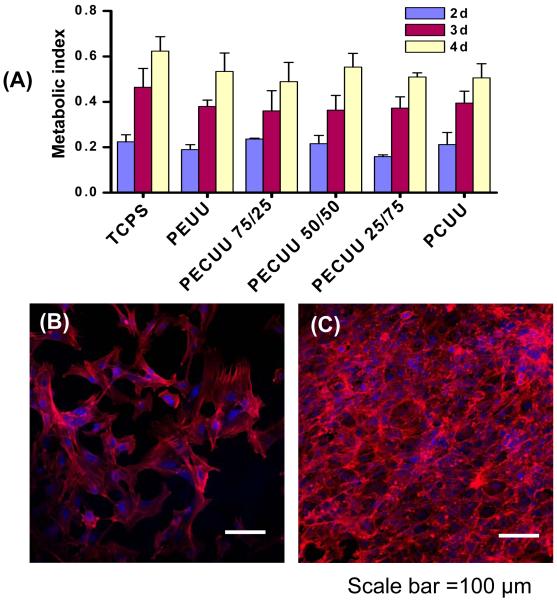
RSMCs were seeded on the surface of polymer films to evaluate cytocompatibility. At 1 d after cell seeding, the cellular metabolic index was slightly higher for each of the five polyurethanes than for the positive control TCPS surface, but these increased values were not significantly different than TCPS (data not shown). Visual inspection of the cellularized surfaces confirmed that the metabolic activity index was reflective of cell numbers and there were no apparent differences in cell numbers between the five polyurethanes and TCPS. Over a 4 d period, the metabolic index for the seeded RSMCs significantly increased for each of the polyurethane surfaces and for the positive control of TCPS (Figure 5A). There was no significant difference between surface types at 4 d. All cellularized surfaces were observed under confocal microscopy at 2 and 4 d and the images verified that the metabolic index was representative of trends in cell numbers. Cell morphology did not vary remarkably between the polyurethane surfaces and TCPS. Fluorescent micrographs for a representative polyurethane surface, PECUU 50/50, at 2 and 4 d are shown in Figures 5B and 5C.
Figure 5.
(A) Metabolic index to show RSMC behavior at 2, 3 and 4 d after cell seeding on PEUU, PECUUs and PCUU films (TCPS as control). Fluorescently stained RSMCs on the surface of a representative PECUU 50/50 film at (B) 2 and (C) 4 d of culture. Other surfaces were comparable. Alpha-smooth muscle actin is stained red with rhodamine phoallidin and cell nuclei are stained blue with DRAQ5. Scale bar = 100 μm.
Scaffold characterization
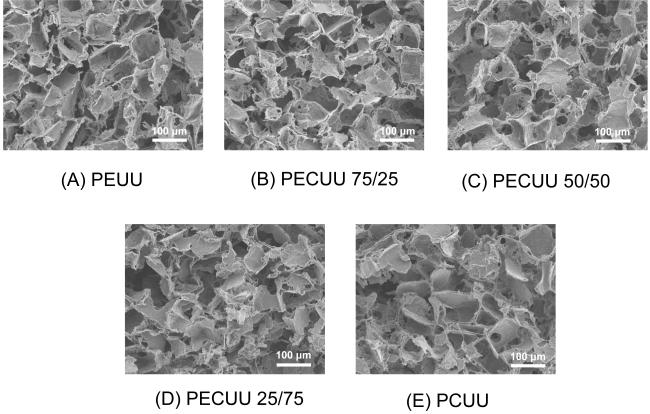
Scanning electron micrographs of each of the polyurethanes processed using salt leaching with 100-150 μm salt particles are seen in Figure 6. The cubic salt particle shaped pores are evident as are some larger pores formed due to stacking particles. The scaffolds did not have an obvious surface “skin” of reduced porosity and the porosity appeared to be even throughout the cross-sections obtained. The mechanical strengths of the porous scaffolds were much lower than for solid film as would be expected. From Table 2 it is seen that the PEUU scaffold had a tensile strength of 109 ±10 kPa, while for the PCUU scaffold this value was 33±17 kPa. The tensile strengths of PECUU 75/25, PECUU 50/50 and PECUU 25/75 scaffolds were distributed in order between these extremes with decreasing tensile strength with increasing polycarbonate diol content. All scaffolds qualitatively exhibited elastic behavior and had breaking strains ranging from 112 to 173%, with higher breaking strains associated with introduction of polycarbonate diol. The scaffold tensile property trends mirrored those found for the polymer films. The porosity of all scaffolds was in the range of 84-88% without significant differences.
Figure 6.
Electron micrographs of scaffold cross sections generated from (A) PEUU, (B) PECUU 75/25, (C) PECUU 50/50, (D) PECUU 25/75 and (E) PCUU using salt-leaching with salt particles ranging from 100 to 150 μm.
Table 2. Scaffold properties.
| Tensile strength (kPa) |
Breaking strain (%) |
Porosity (%) |
|
|---|---|---|---|
| PEUU | 109 ± 10 a | 112 ± 16 a | 87 ± 2 |
| PECUU75/25 | 72 ± 10 b | 131 ± 23 a,b | 84 ± 4 |
| PECUU50/50 | 49 ± 4 c | 156 ± 30 b | 85 ± 4 |
| PECUU25/75 | 35 ± 5 c | 173 ± 47 b | 88 ± 1 |
| PCUU | 33 ± 17 c | 166 ± 51 b | 85 ± 4 |
denote significantly different group
denote significantly different group
denote significantly different group
RSMC growth within scaffold
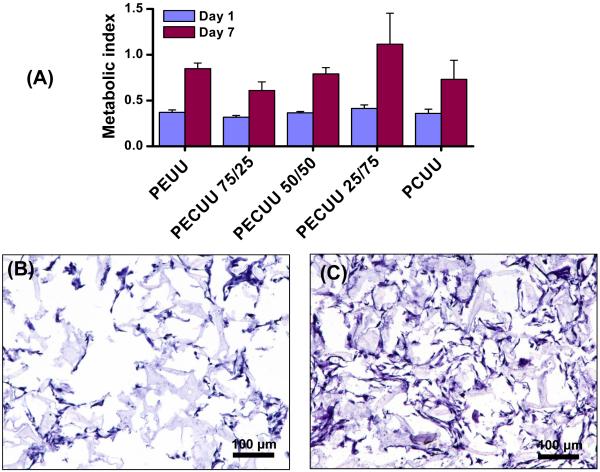
RSMCs were easily seeded within the porous scaffolds and proliferated during spinner flask culture for 7 d. As shown in Figure 7A, the metabolic index for the seeded RSMCs increased significantly within all porous scaffolds between the 1 and 7 d time points. There were no significant differences in the metabolic index between the polyurethane scaffolds at either day 1 or 7. Confirming that the metabolic index was generally reflective of cell numbers, H&E stained cross-sections showed comparable cellular deposition across scaffold type and an increase for each scaffold type between days 1 and 7. H&E cross section images from a representative PECUU 50/50 scaffold are seen in Figures 7B and 7C.
Figure 7.
(A) Metabolic index for RSMCs within PEUU, PECUU and PCUU scaffolds after 1 and 7 d spinner flask culture. H&E stained cross-sections of a representative PECUU 50/50 scaffold after (B) 1 and (C) 7 d spinner flask culture. Other scaffolds appeared similar. Scale bar = 100 μm.
In vivo subcutaneous placement
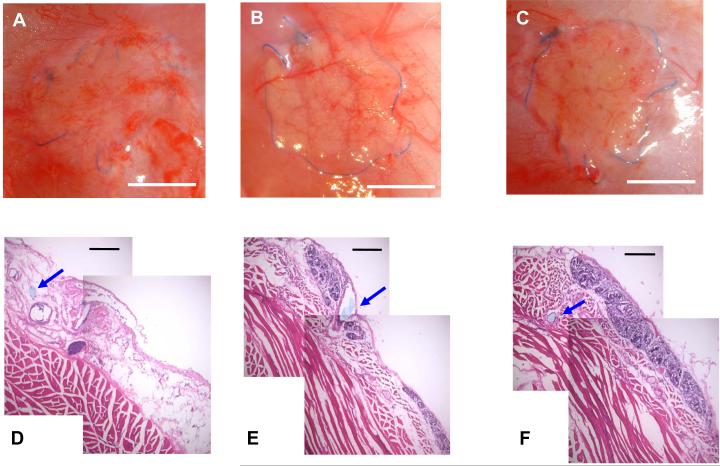
The in vivo degradation and tissue response to PEUU, PECUU 50/50 and PCUU scaffolds were evaluated in a rat subcutaneous model. No complications were encountered in the placement procedure or follow up period and the placement site was free from infection. In Figure 8A-C representative macroscopic images of each scaffold implant site is seen 8 wk following surgery. At this site the blue suture line used for the procedure provides a reference point for the original implant location. In all animals the majority of the PEUU scaffold was found to have degraded by simple inspection (Figure 8A), whereas for the PECUU 50/50 scaffold group remnant material existed sporadically, merging with native tissue (Figure 8B). The implanted PCUU scaffolds were found to still be apparent at the time of necropsy, retaining their circular form, but covered with connective tissue (Figure 8C). These macroscopic findings were better illustrated by histological imaging of tissue sections as shown in the typical images of Figures 8D-F. The suture line is used again here as a point of reference, appearing in cross-section and marked by a blue arrow. In Figure 8D, the majority of the PEUU scaffold was degraded, and loose connective tissue occupied the implant area with few observed putative macrophages. The remnant material existed sporadically. In Figure 8D, near the center of the image, the darker violet stained elliptical region would be consistent with a small area of remaining material and associated macrophages. For the PECUU 50/50 scaffolds, more remnant material was seen with darker violet staining of the putative infiltrating macrophages and fibroblasts (Figure 8E). For the PCUU scaffolds, largely continuous areas of remnant scaffold were found, infiltrated with darker violet labeling cells. Such an area is seen above the suture line in Figure 8F. The total scaffold area qualitatively appeared to be slightly reduced from the time of implant.
Figure 8.
Macroscopic view of rat subcutaneous implant sites for (A) PEUU, (B) PECUU 50/50 and (C) PCUU scaffolds after 8 wk. H&E stained cross-sections of the implanted area at 8 wk for (D) PEUU, (E) PECUU 50/50 and (F) PCUU scaffolds demonstrating the variable resorption behavior between scaffold types. Scale bar (A-C) = 5 mm and scale bar (D-F) = 200 μm. Blue arrows indicate suture area which was utilized along the scaffold edge to affix at the time of implantation.
Discussion
Biodegradable polyurethane elastomers have been applied in a variety of settings to aid the repair and reconstruction of mechanically active soft tissues, including blood vessels [27, 28], the cardiac wall [19, 29], cardiac valves [30], urinary bladder [31], and skin [32]. To achieve the desired polyurethane behavior, soft segment alteration is a popular and relatively simple approach. In previous studies single component polyesters, such as PCL [14, 21, 33], poly(lactide) (PLA) [34] and poly(hydroxybutyrate) (PHB) [35]), or polycarbonates, such as poly(1,6-hexyl 1,2-ethyl carbonate) [13, 36, 37], poly(1,6-hexamethylene carbonate) [38, 39] and poly(1,3-trimethylene carbonate) (PTMC) [24, 40], in the polyurethane backbone have been shown to increase lability. For further control over degradation rate and mechanical behavior, polyester copolymers, such as PCL-PEG-PCL [22, 41], PHB containing copolymers [42, 43], and PCL-co-PLA copolymers [44] have been studied. Alternatively, polycarbonate copolymers such as PTMC-co-PCL [24, 40] and PTMC-PEG-PTMC [45] have also been used to replace single component soft segments. Using a blended soft segment containing two polymers with different characteristics is another approach to achieve an elastomeric polyurethane with tunable mechanical properties and degradation rate [46-49]. In our studies, two commercially available synthetic polymer diols, including semi-crystalline polyester (PCL) and amorphous slowly degradable polycarbonate (PHC) were incorporated as a blended soft segment and examined over a range of molar ratios.
The thermal properties of the synthesized PECUUs showed glass transition temperatures (T) that in all cases were lower than −46°C (Table 1), resulting in polymers in a rubbery state at room temperature. Hard segment transitions were not found on the DSC curves for any of the polymers, indicating a low degree of phase separation. The low Tg were attributed to the soft segments, with Tg increasing when the PHC ratio in the soft segments exceeded 50%, due to the higher Tg of PHC than PCL. Only a single soft segment Tg was found for the PECUUs, suggesting good miscibility and strong interactions between PHC and PCL segments. The endothermic melting temperature peak was also attributed to the soft segments. Only PEUU, PECUU 75/25 and PECUU 50/50 showed detectable melting temperatures, with values decreasing with increasing PHC content (Table 1). PHC is an amorphous polymer without detectable melting temperature and has good miscibility with PCL, thus its presence likely interfered with PCL crystallization. Further, PCL crystallization domains would decrease with increasing PHC content, reducing and ultimately eliminating the melting temperature.
The hydrophilicity of the PECUUs was not markedly impacted by alteration of the PHC content in the polymers, although the water contact angle did drop significantly with increasing amounts of PHC. In earlier work with PEEUUs, increasing the hydrophilicity of the soft segment by increasing the relative amount of PEG versus PCL in a PCL-PEG-PCL triblock copolymer resulted in a more hydrophilic polymer that degraded more rapidly in buffer in direct relation to the increase in hydrophilicity [22]. While increased PHC content increased the polymer surface hydrophilicity (attributed to the increased presence of more hydrophilic carbonate groups [50]), in this case the degradation behavior was not increased and was in fact slower for PCUU relative to PEUU.
While our primary objective was to create a family of degradable polyurethanes that would provide slower degradation behavior than PEUU and the PEEUUs previously reported, an associated objective was that the synthesized PECUUs possess mechanical properties similar to PEUU. The PECUU and PCUU films and scaffolds generally varied significantly in their tensile mechanical properties from their PEUU counterparts; however this variance was not large. The tensile strength and initial moduli of synthetic polyurethanes tended to decrease with increasing PHC amount, while the breaking strains of PECUUs and PCUU increased after the amount of PHC exceeded 75%. PEUU and PECUU 75/25 exhibited higher tensile strengths possibly due to strain induced crystallization at higher levels of distension, which would be consistent with the stress-strain curve behavior seen in Figure 3, while this was not seen for PECUU 50/50, PECUU 25/75 and PCUU. These observations can be attributed to decreased intermolecular forces associated with decreased PCL crystallinity at lower PCL content.
Comparing the film tensile behavior from ref # [22] for PEEUU films and the PECUU and PCUU film behavior of the current report, tensile strength and breaking strain were comparable, although the latter was consistently higher for the PECUUs relative to the PEEUUs. For scaffolds the PECUUs and PCUU had markedly lower tensile strengths than the PEEUU scaffolds reported in [17], although this can be attributed to the differences in processing. For the carbonate containing polyurethanes salt-leaching was used, whereas for the PEEUUs thermally induced phase separation was employed. While the former is more readily applied across polymer types due to broader solvent options, at high porosities the thermally induced separation process provides stronger scaffolds for the families of polyurethanes that we have studied. Thermally induced phase separation was not readily achieved with the PECUUs due to limited solubility of the PECUUs in DMSO. The PECUU and PCUU scaffold mechanical properties should be able to be increased by increasing the polymer concentration and polymer/salt particle ratio and decreasing the pore size [51, 52], although trade offs will exist in terms of cell placement and ingrowth for the scaffold. Additionally, we have shown that the PECUUs are readily processed into fibrous scaffolds using electrospinning (data not shown).
From a basic cytotoxicity and cytocompatibility perspective, PECUU and PCUU films appeared to be non-cytotoxic and to support smooth muscle cell adhesion and proliferation in vitro at a level similar to tissue culture polystyrene and PEUU films. Scaffolds generated from the PECUUs and PCUU supported smooth muscle cell seeding and growth in a comparable fashion to PEUU and without differences between scaffold types. The latter result suggests that the slight differences found in hydrophilicity did not appear to impact basic cell behavior. In contrast, for PEEUUs it was reported that increasing hydrophilicity (and degradation rate) was associated with reduced cell adhesion and slower cell growth, an effect that could be offset with surface modification [22].
In vitro hydrolytic degradation studies in PBS showed no measurable mass loss for PECUU and PCUU films over an 8 wk period, but decreasing inherent viscosity reflected decreasing molecular weight with increased incubation time for the PECUUs. This degradation behavior was attributed to the relatively higher hydrolysis rate of ester bond compared to carbonate bond. The ester bond containing PECUUs therefore degraded faster than PCUU that contains only carbonate bond. Polyester hydrolytic degradation might also be locally accelerated by acidic degradation product generation upon ester cleavage, while carbonate degradation would not be expected to provide this effect given the unstable weak acidic degradation products which readily decompose into the alcohol. [53] Generally while the slight increase in hydrophilicity might be expected to increase ester bond susceptibility to cleavage, the reduced number of ester bonds, the reduced acidic byproducts and the reduced susceptibility of the carbonate bond to hydrolysis would be expected to slow degradation of the PECUUs and PCUU inversely with ester bond content.
The 8 wk in vivo subcutaneous implantation of PEUU, PECUU 50/50 and PCUU scaffolds further demonstrated the trend of slower degradation with increased polycarbonate introduction. However, with implantation the scaffolds are exposed to a markedly more complex aqueous environment than PBS, most notably with enzymatic exposure associated with the acute foreign body response. Santerre, Labow and colleagues have performed numerous studies examining enzyme degradative effects on polyurethanes and most notably have reported that cholesterol esterase and monocyte-specific esterase secreted from monocyte-derived macrophages can accelerate the degradation of poly(carbonate urethane) synthesized from a PHC soft segment, hexane diisocyanate hard segment and butanediol chain extender [38, 54]. Our finding that the majority of the PEUU porous scaffold degraded over an 8 wk period when implanted subcutaneously generally agrees with earlier reports in which this material was placed in rats as a full wall thickness replacement in the right ventricular outflow tract or as an epicardial patch after myocardial infarction [29, 55]. In those studies the PEUU was processed with thermally induced phase separation but showed similar near complete resorption at 8 wk (epicardial) and 12 wk (right ventricle). These earlier animal studies show markedly accelerated degradation in vivo relative to in vitro studies on the same scaffolds [17], an effect attributed at least in part to macrophage-associated enzyme release and phagocytic activity.
The relatively greater amount of scaffold consistently observed at 8 wk for PECUU 50/50 relative to PEUU, and for PCUU relative to PECUU 50/50, qualitatively confirmed that the synthesized polymers could provide the desired extension of degradation behavior in vivo from PEUU, at least in the subcutaneous setting. Combined with the availability of faster degrading PEEUU scaffolds, and accepting that some moderate differences in mechanical properties exist, a range of polyurethanes have been defined where relative degradation rates can be selected that are faster or slower than the PEUU studied previously in a variety of cardiac and vascular applications [20, 28, 29, 55]. These materials thus allow for the general investigation of hypotheses regarding the role of mechanical support duration in tissue repair and regeneration efforts, with the aforementioned caveat with respect to some initial mechanical variability and the potential impact of different degradation products and varied surface properties.
While the reported materials offer some promising opportunities, several limitations of this report should be noted. First, the examination period for in vitro degradation behavior could be further extended. This would be expected to ultimately result in a better separation of the mass and viscosity loss behavior of the studied polymers and might show, for instance, specific differences between the PECUUs. Similarly, while the in vivo behavior showed marked differences between the three examined polymers, further studies at multiple time points could be conducted for different PECUU compositions to evaluate differences in resorption rates. Another limitation is that mechanical properties were only examined at the initial time point in vitro and not over the 8 wk degradation period. While the intrinsic viscosity dropped for the PECUUs and PEUU over the 8 wk period, mechanical properties were not quantified temporally, although substantial changes were not noted in handling. For a more extended in vitro degradation study, characterization of mechanical deterioration would be warranted. Finally, only subcutaneous implants were studied as opposed to applying the scaffolds in a more functional setting and in vivo toxicity assessment was not pursued beyond observational assessments of the implant location. By applying a series of variably degrading polyurethane scaffolds in a load bearing setting, with or without cell loading, one could assess both in vivo resorption of the scaffold and the loss, maintenance or gain in scaffold site mechanical properties as polymer degradation and tissue remodeling proceed.
Conclusions
A series of degradable poly(ester carbonate urethane)ureas was successfully synthesized by employing soft segments resulting from a range of PCL/PHC blends, diisocyanatobutane hard segments and chain extension with putrescine. The synthesized polyurethanes exhibited in vitro and in vivo degradation behavior that was slower than comparable poly(ester urethane)urea and faster than poly(carbonate urethane)urea, mechanical properties generally attractive for soft tissue applications and processability to form porous scaffolds supportive of cell seeding and growth. These slower degrading thermoplastic polyurethanes provide opportunities to investigate the role of relative degradation rates for mechanically supportive scaffolds in a variety of soft tissue repair and reconstructive procedures.
Acknowledgements
Financial support for this work was provided by the National Institutes of Health (grant #HL069368). We also thank Jennifer Debarr and Deanna Rhoads for histological sectioning and staining.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lee SJ, Liu J, Oh SH, Soker S, Atala A, Yoo JJ. Development of a composite vascular scaffolding system that withstands physiological vascular conditions. Biomaterials. 2008;29:2891–8. doi: 10.1016/j.biomaterials.2008.03.032. [DOI] [PubMed] [Google Scholar]
- [2].Crapo PM, Wang Y. Physiologic compliance in engineered small-diameter arterial constructs based on an elastomeric substrate. Biomaterials. 2010;31:1626–35. doi: 10.1016/j.biomaterials.2009.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Webb AR, Yang J, Ameer GA. Biodegradable polyester elastomers in tissue engineering. Expert Opin Biol Ther. 2004;4:801–12. doi: 10.1517/14712598.4.6.801. [DOI] [PubMed] [Google Scholar]
- [4].Guelcher SA. Biodegradable polyurethanes: synthesis and applications in regenerative medicine. Tissue Eng Part B Rev. 2008;14:3–17. doi: 10.1089/teb.2007.0133. [DOI] [PubMed] [Google Scholar]
- [5].Wang YD, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat Biotechnol. 2002;20:602–6. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- [6].Yang J, Webb A, Ameer G. Novel citric acid-based biodegradable elastomers for tissue engineering. Adv Mater. 2004;16:511–6. [Google Scholar]
- [7].Sodian R, Sperling JS, Martin DP, Egozy A, Stock U, Mayer JE, et al. Fabrication of a trileaflet heart valve scaffold from a polyhydroxyalkanoate biopolyester for use in tissue engineering. Tissue Eng. 2000;6:183–8. doi: 10.1089/107632700320793. [DOI] [PubMed] [Google Scholar]
- [8].Cohn D, Salomon AH. Designing biodegradable multiblock PCL/PLA thermoplastic elastomers. Biomaterials. 2005;26:2297–305. doi: 10.1016/j.biomaterials.2004.07.052. [DOI] [PubMed] [Google Scholar]
- [9].Engelberg I, Kohn J. Physio-mechanical properties of degradable polymers used in medical applications: A comparative study. Biomaterials. 1991;12:292–304. doi: 10.1016/0142-9612(91)90037-b. [DOI] [PubMed] [Google Scholar]
- [10].Pego AP, Poot AA, Grijpma DW, Feijen J. Biodegradable elastomeric scaffolds for soft tissue engineering. J Control Release. 2003;87:69–79. doi: 10.1016/s0168-3659(02)00351-6. [DOI] [PubMed] [Google Scholar]
- [11].Rockwood DN, Woodhouse KA, Fromstein JD, Chase DB, Rabolt JF. Characterization of biodegradable polyurethane microfibers for tissue engineering. J Biomater Sci Polym Ed. 2007;18:743–58. doi: 10.1163/156856207781034115. [DOI] [PubMed] [Google Scholar]
- [12].Guelcher SA, Gallagher KM, Didier JE, Klinedinst DB, Doctor JS, Goldstein AS, et al. Synthesis of biocompatible segmented polyurethanes from aliphatic diisocyanates and diurea diol chain extenders. Acta Biomater. 2005;1:471–84. doi: 10.1016/j.actbio.2005.02.007. [DOI] [PubMed] [Google Scholar]
- [13].Szelest-Lewandowska A, Masiulanis B, Szymonowicz M, Pielka S, Paluch D. Modified polycarbonate urethane: synthesis, properties and biological investigation in vitro. J Biomed Mater Res A. 2007;82:509–20. doi: 10.1002/jbm.a.31357. [DOI] [PubMed] [Google Scholar]
- [14].Skarja GA, Woodhouse KA. Synthesis and characterization of degradable polyurethane elastomers containing an amino acid-based chain extender. J Biomater Sci Polym Edn. 1998;9:271–95. doi: 10.1163/156856298x00659. [DOI] [PubMed] [Google Scholar]
- [15].Spaans CJ, Rienstr O, de Groot JH, Veth RPH, Pennings AJ. Solvent-free fabrication of micro-porous polyurethane amide and polyurethane-urea scaffolds for repair and replacement of the knee-joint meniscus. Biomaterials. 2000;21:2453–60. doi: 10.1016/s0142-9612(00)00113-7. [DOI] [PubMed] [Google Scholar]
- [16].Guelcher SA, Srinivasan A, Dumas JE, Didier JE, McBride S, Hollinger JO. Synthesis, mechanical properties, biocompatibility, and biodegradation of polyurethane networks from lysine polyisocyanates. Biomaterials. 2008;29:1762–75. doi: 10.1016/j.biomaterials.2007.12.046. [DOI] [PubMed] [Google Scholar]
- [17].Guan J, Fujimoto KL, Sacks MS, Wagner WR. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials. 2005;26:3961–71. doi: 10.1016/j.biomaterials.2004.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rowlands AS, Lim SA, Martin D, Cooper-White JJ. Polyurethane/poly(lactic-co-glycolic) acid composite scaffolds fabricated by thermally induced phase separation. Biomaterials. 2007;28:2109–21. doi: 10.1016/j.biomaterials.2006.12.032. [DOI] [PubMed] [Google Scholar]
- [19].Fromstein JD, Zandstra PW, Alperin C, Rockwood D, Rabolt JF, Woodhouse KA. Seeding bioreactor-produced embryonic stem cell-derived cardiomyocytes on different porous, degradable, polyurethane scaffolds reveals the effect of scaffold architecture on cell morphology. Tissue Eng Part A. 2008;14:369–78. doi: 10.1089/tea.2006.0410. [DOI] [PubMed] [Google Scholar]
- [20].Hong Y, Ye SH, Nieponice A, Soletti L, Vorp DA, Wagner WR. A small diameter, fibrous vascular conduit generated from a poly(ester urethane)urea and phospholipid polymer blend. Biomaterials. 2009;30:2457–67. doi: 10.1016/j.biomaterials.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Guan J, Sacks MS, Beckman EJ, Wagner WR. Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. J Biomed Mater Res. 2002;61:493–503. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- [22].Guan J, Sacks MS, Beckman EJ, Wagner WR. Biodegradable poly(ether ester urethane)urea elastomers based on poly(ether ester) triblock copolymers and putrescine: synthesis, characterization and cytocompatibility. Biomaterials. 2004;25:85–96. doi: 10.1016/s0142-9612(03)00476-9. [DOI] [PubMed] [Google Scholar]
- [23].Guan J, Wagner WR. Synthesis, characterization and cytocompatibility of polyurethaneurea elastomers with designed elastase sensitivity. Biomacromolecules. 2005;6:2833–42. doi: 10.1021/bm0503322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Asplund JO, Bowden T, Mathisen T, Hilborn J. Synthesis of highly elastic biodegradable poly(urethane urea) Biomacromolecules. 2007;8:905–11. doi: 10.1021/bm061058u. [DOI] [PubMed] [Google Scholar]
- [25].Zhang R, Ma PX. Poly(alpha-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J Biomed Mater Res. 1999;44:446–55. doi: 10.1002/(sici)1097-4636(19990315)44:4<446::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- [26].Li Y, Ma T, Kniss DA, Lasky LC, Yang ST. Effects of filtration seeding on cell density, spatial distribution, and proliferation in nonwoven fibrous matrices. Biotechnol Prog. 2001;17:935–44. doi: 10.1021/bp0100878. [DOI] [PubMed] [Google Scholar]
- [27].Hofmann A, Ritz U, Verrier S, Eglin D, Alini M, Fuchs S, et al. The effect of human osteoblasts on proliferation and neo-vessel formation of human umbilical vein endothelial cells in a long-term 3D co-culture on polyurethane scaffolds. Biomaterials. 2008;29:4217–26. doi: 10.1016/j.biomaterials.2008.07.024. [DOI] [PubMed] [Google Scholar]
- [28].Nieponice A, Soletti L, Guan J, Hong Y, Maul T, Gharaibeh B, et al. In-vivo assessment of a tissue-engineered vascular graft combining a biodegradable elastomeric scaffold and muscle-derived stem cells in a rat model. Tissue Eng Part A. doi: 10.1089/ten.tea.2009.0427. Doi:10.1089/ten.TEA.2009.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fujimoto KL, Tobita K, Merryman WD, Guan J, Momoi N, Stolz DB, et al. An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J Am Coll Cardiol. 2007;49:2292–300. doi: 10.1016/j.jacc.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hayashida K, Kanda K, Yaku H, Ando J, Nakayama Y. Development of an in vivo tissue-engineered, autologous heart valve (the biovalve): preparation of a prototype model. J Thorac Cardiovasc Surg. 2007;134:152–9. doi: 10.1016/j.jtcvs.2007.01.087. [DOI] [PubMed] [Google Scholar]
- [31].Danielsson C, Ruault S, Simonet M, Neuenschwander P, Frey P. Polyesterurethane foam scaffold for smooth muscle cell tissue engineering. Biomaterials. 2006;27:1410–5. doi: 10.1016/j.biomaterials.2005.08.026. [DOI] [PubMed] [Google Scholar]
- [32].Li B, Davidson JM, Guelcher SA. The effect of the local delivery of platelet-derived growth factor from reactive two-component polyurethane scaffolds on the healing in rat skin excisional wounds. Biomaterials. 2009;30:3486–94. doi: 10.1016/j.biomaterials.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Spaans CJ, de Groot JH, Dekens FG, Pennings AJ. High molecular weight polyurethanes and a polyurethane urea based on 1,4-butanediisocyanate. Polym Bull. 1998;41:131–8. [Google Scholar]
- [34].Storey RF, Hickey TP. Degradable polyurethane networks based on D, L-lactide, glycolide, epsilon-caprolactone, and trimethylene carbonate homopolyester and copolyester triols. Polymer. 1994;35:830–8. [Google Scholar]
- [35].Saad B, Matter S, Ciardelli G, Uhlschmid GK, Welti M, Neuenschwander P, et al. Interactions of osteoblasts and macrophages with biodegradable and highly porous polyesterurethane foam and its degradation products. J Biomed Mater Res. 1996;32:355–66. doi: 10.1002/(SICI)1097-4636(199611)32:3<355::AID-JBM8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- [36].Tang YW, Labow RS, Santerre JP. Enzyme-induced biodegradation of polycarbonate-polyurethanes: dependence on hard-segment chemistry. J Biomed Mater Res. 2001;57:597–611. doi: 10.1002/1097-4636(20011215)57:4<597::aid-jbm1207>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- [37].Tang YW, Labow RS, Santerre JP. Enzyme-induced biodegradation of polycarbonate polyurethanes: dependence on hard-segment concentration. J Biomed Mater Res. 2001;56:516–28. doi: 10.1002/1097-4636(20010915)56:4<516::aid-jbm1123>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- [38].Matheson LA, McBane JE, Malowany JI, Santerre JP, Labow RS. Is cell culture stressful? Effects of degradable and non-degradable culture surfaces on U937 cell function. BioTechniques. 2007;42:744–50. doi: 10.2144/000112460. [DOI] [PubMed] [Google Scholar]
- [39].Eceiza A, Martin MD, de la Caba K, Kortaberria G, Gabilondo N, Corcuera MA, et al. Thermoplastic polyurethane elastomers based on polycarbonate diols with different soft segment molecular weight and chemical structure: mechanical and thermal properties. Polym Eng Sci. 2008;48:297–306. [Google Scholar]
- [40].Asplund B, Aulin C, Bowden T, Eriksson N, Mathisen T, Bjursten LM, et al. In vitro degradation and in vivo biocompatibility study of a new linear poly(urethane urea) J Biomed Mater Res B Appl Biomater. 2008;86:45–55. doi: 10.1002/jbm.b.30986. [DOI] [PubMed] [Google Scholar]
- [41].Cohn D, Stern T, Gonzalez MF, Epstein J. Biodegradable poly(ethylene oxide)/poly(epsilon-caprolactone) multiblock copolymers. J Biomed Mater Res. 2002;59:273–81. doi: 10.1002/jbm.1242. [DOI] [PubMed] [Google Scholar]
- [42].Lendlein A, Neuenschwander P, Suter UW. Tissue-compatible multiblock copolymers for medical applications, controllable in degradation rate and mechanical properties. Macromol Chem Phys. 1998;199:2785–96. [Google Scholar]
- [43].Loh XJ, Tan KK, Li X, Li J. The in vitro hydrolysis of poly(ester urethane)s consisting of poly[(R)-3-hydroxybutyrate] and poly(ethylene glycol) Biomaterials. 2006;27:1841–50. doi: 10.1016/j.biomaterials.2005.10.038. [DOI] [PubMed] [Google Scholar]
- [44].Kylma J, Seppala JV. Synthesis and characterization of a biodegradable thermoplastic poly(ester-urethane) elastomer. Macromolecules. 1997;30:2876–82. [Google Scholar]
- [45].Wang F, Li Z, Lannutti JL, Wagner WR, Guan J. Synthesis, characterization and surface modification of low moduli poly(ether carbonate urethane)ureas for soft tissue engineering. Acta Biomater. 2009;5:2901–12. doi: 10.1016/j.actbio.2009.04.016. [DOI] [PubMed] [Google Scholar]
- [46].Fromstein JD, Woodhouse KA. Elastomeric biodegradable polyurethane blends for soft tissue engineering. J Biomater Sci Polymr Edn. 2002;13:391–406. doi: 10.1163/156856202320253929. [DOI] [PubMed] [Google Scholar]
- [47].Li X, Loh XJ, Wang K, He C, Li J. Poly(ester urethane)s consisting of poly[(R)-3-hydroxybutyrate] and poly(ethylene glycol) as candidate biomaterials: characterization and mechanical property study. Biomacromolecules. 2005;6:2740–7. doi: 10.1021/bm050234g. [DOI] [PubMed] [Google Scholar]
- [48].Borkenhagen M, Stoll RC, Neuenschwander P, Suter UW, Aebischer P. In vivo performance of a new biodegradable polyester urethane system used as a nerve guidance channel. Biomaterials. 1998;19:2155–65. doi: 10.1016/s0142-9612(98)00122-7. [DOI] [PubMed] [Google Scholar]
- [49].Raghunath J, Georgiou G, Armitage D, Nazhat SN, Sales KM, Bulter PE, et al. Degradation studies on biodegradable nanocomposite based on polycaprolactone/polycarbonate (80:20%) polyhedral oligomeric silsesquioxane. J Biomed Mater Res. 2009;91A:834–44. doi: 10.1002/jbm.a.32335. [DOI] [PubMed] [Google Scholar]
- [50].Harris RF, Joseph MD, Davidson C, Deporter CD, Dais VA. Polyurethane elastomers based on molecular weight advanced poly(ethylene ether carbonate) diols. I. comparison to commercial diols. J Appl Polym Sci. 1990;41:487–507. [Google Scholar]
- [51].Pego AP, Siebum B, Luyn van MJA, Gallego y Van Seijen XJ, Poot AA, Grijpma DW, et al. Preparation of degradable porous structures based on 1,3-trimethylene carbonate and D,L-lactide (Co)polymers for heart tissue engineering. Tissue Eng. 2003;9:981–4. doi: 10.1089/107632703322495628. [DOI] [PubMed] [Google Scholar]
- [52].Ma PX, Choi JW. Biodegradable polymer scaffolds with well-defined. Tissue Eng. 2001;7:23–33. doi: 10.1089/107632701300003269. [DOI] [PubMed] [Google Scholar]
- [53].Kuran W, Sobczak, Listos T, Debek C, Florjanczyk New route to oligocarbonate diols suitable for the synthesis of polyurethane elastomers. Polymer. 2000;41:8531–41. [Google Scholar]
- [54].Santerre JP, Woodhouse K, Laroche G, Labow RS. Understanding the biodegradation of polyurethanes: from classical implants to tissue engineering materials. Biomaterials. 2005;26:7457–70. doi: 10.1016/j.biomaterials.2005.05.079. [DOI] [PubMed] [Google Scholar]
- [55].Fujimoto KL, Guan J, Oshima H, Sakai T, Wagner WR. In vivo evaluation of a porous, elastic, biodegradable patch for reconstructive cardiac procedures. Ann Thorac Surg. 2007;83:648–54. doi: 10.1016/j.athoracsur.2006.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]