Abstract
Behavioral analgesic techniques such as distraction reduce pain in both clinical and experimental settings. Individuals differ in the magnitude of distraction-induced analgesia, and additional study is needed to identify the factors that influence the pain relieving effects of distraction. Catastrophizing, a set of negative emotional and cognitive processes, is widely recognized to be associated with increased reports of pain. We sought to evaluate the relationship between catastrophizing and distraction analgesia. Healthy participants completed three sessions in a randomized order. In one session (Pain Alone), pain was induced by topical application of a 10% capsaicin cream and simultaneous administration of a tonic heat stimulus. In another session (Pain + Distraction), identical capsaicin+heat application procedures were followed, but subjects played video games that required a high level of attention. During both sessions, verbal ratings of pain were obtained and participants rated their degree of catastrophizing. During the other session (Distraction Alone) subjects played the video games in the absence of any pain stimulus. Pain was rated significantly lower during the distraction session compared to the “pain alone” session. In addition, high catastrophizers rated pain significantly higher regardless of whether the subjects were distracted. Catastrophizing did not influence the overall degree of distraction analgesia; however, early in the session high catastrophizers had little distraction analgesia, though later in the session low and high catastrophizers rated pain similarly. These results suggest that both distraction and catastrophizing have substantial effects on experimental pain in normal subjects and these variables interact as a function of time.
Keywords: Experimental pain, Behavioral analgesia, Distraction, Catastrophizing, Capsaicin
Introduction
The pain-reducing effects of behavioral analgesic techniques, such as distraction, have valuable applications in clinical settings and have received considerable empirical support. Distraction has been shown to reduce reports of pain in laboratory settings, during medical procedures, and in the context of ongoing chronic pain[30;34;39]. Several neuroimaging studies[3;5;7;17;23;28;37] have shown that the reduction in pain ratings observed during distraction is associated with decreased activity in structures belonging to the thalamo-cortical ascending pain network (such as the thalamus, primary and secondary somatosensory cortices, insula and anterior cingulate cortex), thus suggesting that distraction analgesia is associated with objective neurophysiological changes. Activity in a number of brain regions increases during distraction analgesia, such as the cingulo-frontal cortex, periaquaeductal gray (PAG) and the posterior thalamus[44;50], potentially representing active mediators (i.e., a potential physiological “signature”) of this phenomenon. Moreover, endogenous opioids have been implicated as a key mechanistic factor in activating behavioral analgesic effects[2;43;44]. Endogenous opioids are central neurochemical modulators of multiple pain-inhibitory systems, and opioids such as beta-endorphins act both in the peripheral and central nervous systems to modulate incoming information related to noxious stimulation. Behavioral analgesic techniques such as distraction, therefore, engage neural mechanisms contributing to endogenous modulation of pain which appear to be similar to those involved in pharmacological analgesia.
Substantial individual differences have been observed in the magnitude of distraction-induced analgesia[6;20]. Understanding the mechanisms that influence the effectiveness of distraction and related cognitive-behavioral coping processes would be helpful in tailoring multidisciplinary interventions, particularly in patients suffering from pain disorders. Catastrophizing, a set of negative emotional/cognitive processes such as magnification, rumination and pessimism about pain sensations and feelings of helplessness when in pain, has a profound impact on both clinical and laboratory-induced pain responses. Generally, those reporting high levels of catastrophizing also report greater pain in response to controlled, laboratory-induced noxious stimuli, including heat (using a temporal summation paradigm)[13], cold pressor[11;12] and electrically-induced pain[16;35]. While the mechanisms underlying the influence of catastrophizing on pain have yet to be fully elucidated, theoretical work[41] as well as several empirical studies suggest that catastrophizing is associated with enhanced attention to pain[10;45]. To date, though, no laboratory research has examined the association between catastrophizing and individual differences in the magnitude or time course of distraction-induced analgesia. In this exploratory pilot study, we employed a model of tonic capsaicin pain and an engaging series of video games to evaluate the impact of catastrophizing on the changes in pain responses produced by distraction.
Methods
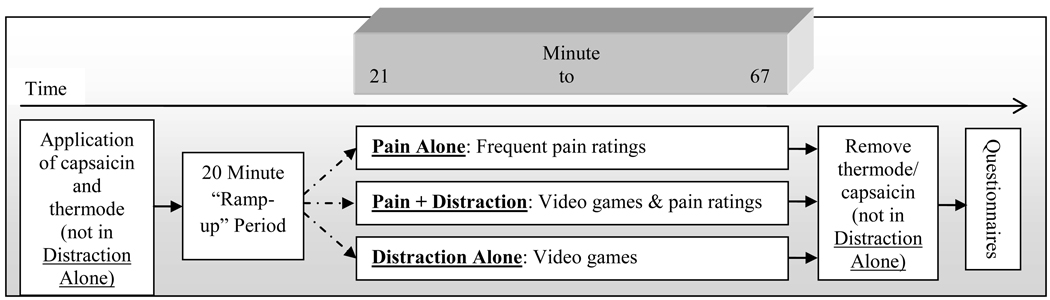
Subjects were recruited using flyers posted around a large medical institution and in the community. A total of 32 healthy individuals (49% female) participated in the study. All study-related procedures were approved by the Johns Hopkins University School of Medicine Institutional Review Board. Eligibility criteria included having no pain or medical/psychiatric disorders, current alcohol or drug abuse problems, or use of narcotics, antidepressants, anticonvulsants, and muscle relaxants. Verbal and written informed consent was obtained, after which participants underwent one of three testing sessions, depicted in Figure 1, in randomized order. Each subject participated in all 3 sessions, with intervals of at least one week.
Figure 1.
Session Timeline
Pain Alone Session
The use of topical capsaicin with skin temperature control to induce pain tonically has been previously described[1]. In brief, a piece of thick, non-porous adhesive dressing with a 6.25 cm2 opening cut into it (used to standardize the area of capsaicin cream application) was applied to the skin at one of two sites (randomized) on the dorsum of the non-dominant hand. Approximately 0.35 g of 10% capsaicin cream were applied inside this opening and evenly spread on the skin. The area was then covered by Tegaderm transparent dressing (3M Health Care, St. Paul, MN, USA). Since topical capsaicin-induced pain varies strongly as a function of skin temperature, a Peltier-device heating element (Medoc US, Minneapolis, MN, USA) was strapped on top of the 6.25 cm2 area with Velcro wrist straps. This device was held at a constant temperature of 38°C during the session. This methodology produces pain that is rated, on average, as moderate in intensity, and which peaks at 15–25 min post-application and plateaus for approximately 1 hour afterwards[1;4]. Participants provided pain intensity ratings every 30 seconds (in order to sustain attention to the noxious stimulus) on a 0–100 computerized visual analogue scale (VAS) for 70 minutes and also provided verbal pain ratings every five minutes as in the Pain + Distraction Session. Following completion of the session, the capsaicin cream was removed from the skin.
Pain + Distraction Session
Identical capsaicin and thermal procedures were followed during this session. Twenty minutes following application, participants began the distraction task. This task consisted of 3 attention demanding arcade-style video games, (Chicken Invaders, Pacman and Bejeweled) each played for 15 minutes in a randomized order, with performance (i.e., scores) monitored and monetary incentives provided. These video games are conceptually similar to a visual Stroop task, in that they require sustained attention and working memory as subjects respond to a series of stimuli[36]. In light of findings suggesting that more engaging distraction tasks produce greater distraction analgesia[22], and given that previous studies of the effect of distraction on pain have used only fairly brief applications of pain and distraction (e.g., 1–4 minutes), we enhanced sustained engagement in the video game task by offering monetary incentives for successful performance (i.e., nominal increase in cash payment for higher scores). During this session, participants provided verbal pain intensity ratings once every 5 minutes as in the “Pain Alone” session; in this case, though, participants were instructed to rate their perceived pain while continuing to play the video games. Following completion of the session, the capsaicin cream was removed from the skin.
Distraction Alone Session
In another session, participants played the series of 3 video games, receiving monetary incentives based on their performance as in the “Distraction + Pain” session, but neither capsaicin nor thermal stimulation was applied to the skin. Because participants did not undergo a pain-induction procedure during this session, no pain ratings were obtained.
Questionnaires
Situational Catastrophizing Questionnaire (SCQ)
In light of research demonstrating the increased relevance of situational catastrophizing to pain responses in healthy participants[8;11;12], this measure was completed immediately following pain testing in both pain sessions. The Situational Catastrophizing questionnaire is an adaptation of the Pain Catastrophizing Scale[40] modified to more appropriately assess catastrophizing in response to laboratory pain. Variations of the questionnaire have been used to examine relationships between catastrophizing and experimentally induced pain.[11;18;35] The SCQ has been described more fully by Edwards et al [13]. Participants completed this questionnaire immediately following the pain induction procedures described above and were instructed to reference the pain procedure while answering.
Post Distraction Questions
At the end of each of the two distraction sessions, participants were asked to rate their level of attention focused on the video games on a 0–100 scale (0 = not at all distracted, 100 = completely distracted). Furthermore, during the Distraction + Pain session, participants also rated the degree to which playing the video games reduced the amount of pain they felt, as well as how much the pain interfered with performance on the video games.
Data Reduction and Analysis
Participants were split into low or high catastrophizing groups based on a median split of their situational catastrophizing scores (averaged across both pain sessions, bivariate correlation between sessions r = .8), as in previous studies by our group[13]. Following the 20-minute ramp-up period, pain ratings were averaged, by 15 minute increments, into early (minutes 21 to 35), middle (36 to 51) and late (52 to 67) ratings by session (see Figure 1). Repeated measures analysis of variance (ANOVA) was conducted on pain ratings during the Pain Alone and Pain + Distraction sessions in order to examine the effects of distraction on pain in low and high catastrophizers. Catastrophizing grouping served as a between-subjects independent variable, while Session and Time were within-subjects factors. ANOVAs were also conducted to evaluate the degree of attention to the video games reported by each group during the Pain + Distraction session and the Distraction Alone session. Finally, analyses examined participants’ self-reported distraction induced analgesia, perceived interference by pain on game performance, and actual game performance.
Results
Participants
A total of 39 healthy individuals were recruited to participate in this study. One individual moved, four participants stopped testing prior to completing either the Pain Alone or Pain + Distraction session due to intolerability, and an additional two participant’s data were dropped for being incomplete. Thus a total of 32 healthy participants completed all three testing sessions. Demographic data for the lower and higher catastrophizing groups are presented in Table 1. There were no significant differences in sex, age or ethnicity between the two groups.
Table 1.
Demographic variables
| Variable | Low Catastrophizers (n=17) | High Catastrophizers (n=15) |
|---|---|---|
| Age (SD) | 26.9 (7.5) | 26.8 (7.7) |
| Sex (% female) | 50% | 46.7% |
| Ethnicity (% non-Hispanic White) | 56% | 37% |
| Mean SCQ | 1.85 (1.2), | 8.97 (5.9) |
| Attn Score: Pain + Dist. | 86.22 (16.95) | 84.67 (8.98) |
| Attn Score: Dist. Alone | 87.96 (19.27) | 84.37 (15.91) |
| Perceived Reduction in Pain | 8.06 (1.43) | 7.62 (1.78) |
| Interference Score* | 1.39 (1.42) | 3.44 (2.34) |
p < 0.05.
SCQ = Situational Catastrophizing Questionnaire
Effects of capsaicin and heat
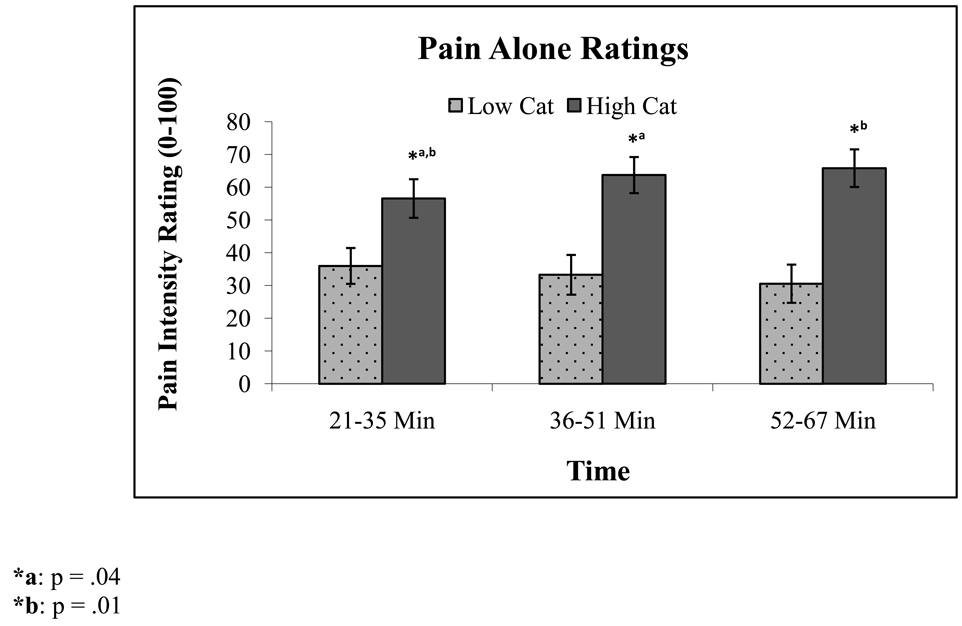
Participants higher in catastrophizing reported greater capsaicin-induced pain (M = 62.93, SD = 21.2) when compared to low catastrophizers (M = 33.27, SD = 22.8; F(1,30) = 14.38, p = 0.001) in the Pain Alone session and in the Pain + Distraction session (M = 51.14, SD = 26.976 vs. M = 23.49, SD = 14.36, respectively; F(1,30) = 13.55, p = .001), see Table 2 for pain ratings. In addition, a group by time interaction emerged during the Pain Alone session (F(2,29) = 5.44, p = .01) such that higher catastrophizers reported more pain with time, see Figure 2. Simple interaction contrasts revealed that during the Pain Alone session, in higher catastrophizers, ratings increased over time with early ratings being significantly lower than middle (F(1,14) = 5.04, p = 0.04) and late (F(1,14) = 7.76, p = 0.01) ratings; while ratings from middle to late did not significantly increase. In contrast, lower catastrophizers’ ratings’ marginally decreased from early- to mid- to late time points (p’s range from .08 and 0.1). Overall, catastrophizing scores were significantly lower during the Pain + Distraction session compared to the Pain Alone session (M = 4.52, SD = 5.9 vs. M = 6.26, SD = 6.2, respectively; p = .04). However, no interaction emerged between catastrophizing group and these sessions (p = .2), suggesting each group’s catastrophizing was reduced to a similar degree during the distraction session.
Table 2.
Pain Alone and Pain + Distraction Mean Ratings and Standard Deviations
| Time | Low Catastrophizers (n=17) | High Catastrophizers (n=15) | ||
|---|---|---|---|---|
| Pain Alone (sd) | Pain + Distraction (sd) |
Pain Alone (sd) | Pain + Distraction (sd) |
|
| 21–35 Minutes | 35.98 (23.4) | 28.14 (15.6) | 57.42 (22.8) | 54.84 (25.8) |
| 36–51 Minutes | 33.27 (22.9) | 23.06 (16.6) | 64.6 (21.3) | 48.96 (29.8) |
| 52–67 Minutes | 30.55 (23.9) | 19.27 (15.1) | 66.76 (22.2) | 49.6 (29.4) |
Figure 2.
Pain intensity ratings in the Pain Alone session in lower vs. higher catastrophizers over time. Error bars represent SEM.
Effect of distraction on pain, performance, and pain interference
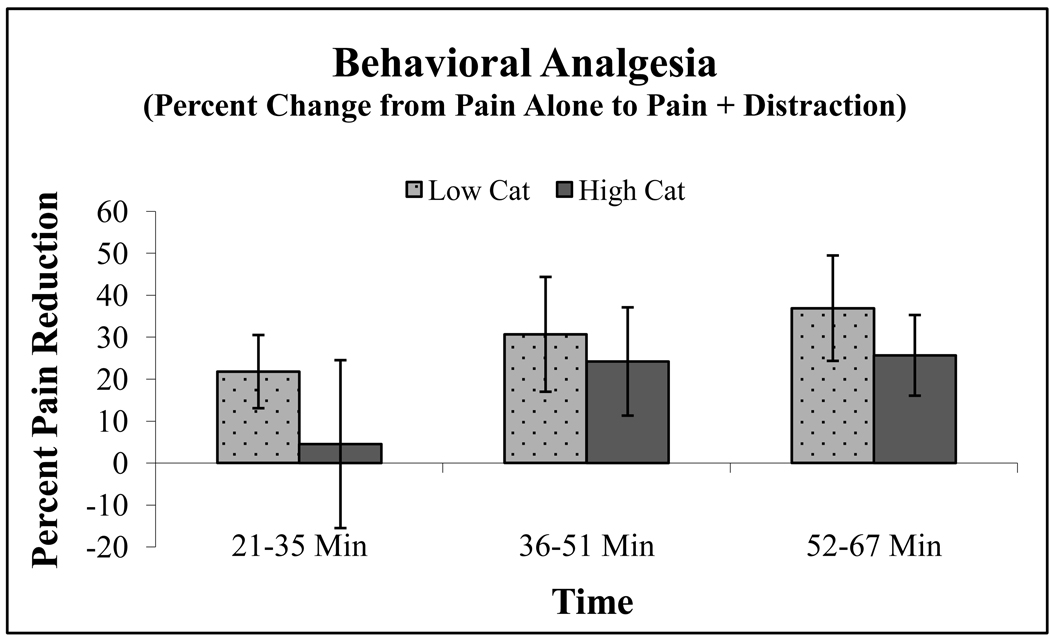
Distraction was associated with substantial reductions in pain ratings for all participants when compared to ratings of capsaicin pain alone (F(1,30) = 11.02, p = .002), a main effect of catastrophizing was also observed, as described above. A distraction by catastrophizing interaction was not observed, suggesting that overall, distraction analgesia was not more effective in one catastrophizing group over another. However, a 3-way interaction of Session × Time × Catastrophizing emerged (F(2,29) = 3.74, p = .036), revealing that distraction produced differing degrees of analgesia across the course of the session depending on whether the subject was a high or low catastrophizer. Examination of simple contrasts indicated that while low and high catastrophizers did not differ in percent pain reduction during the last 30 minutes of the Distraction session, only low catastrophizers achieved significant distraction analgesia during the first 15 minutes (t(16) = 2.12, p = .05). That is, the pain-reducing effects of distraction appeared to take longer to emerge in the higher catastrophizing group. These data are presented as percent change in pain (calculated using the pain ratings from the Pain Alone session as a reference) in Figure 3.
Figure 3.
Percent change in pain between Pain Alone and Pain + Distraction among lower vs. higher catastrophizers over time. Error bars represent SEM.
In order to further investigate the relationship between capsaicin pain ratings and behavioral analgesia, correlations were examined for the entire group and separately within each catastrophizing group. Averaged pain ratings during the Pain Alone session were marginally associated (r = 0.3; p = 0.1) with the percent change during behavioral analgesia (calculated by subtracting Pain + Distraction ratings from Pain Alone ratings divided by Pain Alone ratings) in the sample as a whole. Thus, there was a tendency for individuals who reported greater pain during capsaicin alone to also report greater distraction analgesia. However, when controlling for catastrophizing, Pain Alone ratings were significantly correlated with behavioral analgesia (r=.5; p = 0.004). Interestingly, no relationship between Pain Alone ratings and distraction analgesia emerged in the higher catastrophizing group (r = .06; p = .8), despite reporting a 20+% reduction in pain during the Pain + Distraction session relative to the Pain Alone session. Pain Alone ratings were significantly associated with distraction analgesia in the lower catastrophizers (r = .55; p = 0.02).
Ratings of the self-reported degree of attention focused on the video games in both sessions, as well as perceived distraction-induced analgesia, did not differ as a function of catastrophizing (p’s > 0.05; see table 1). Somewhat surprisingly, a significant difference emerged in interference ratings, such that high catastrophizers perceived that capsaicin pain more strongly interfered with their video game performance when compared to low catastrophizers (p = 0.004). However, actual video game performance scores did not differ by catastrophizing group on any of the three video games in either the Pain + Distraction session or the Distraction Alone session (p’s > 0.05). Similarly, no significant differences were observed between catastrophizing groups on performance scores during the first video game played, suggesting that performance was comparable even early in the distraction task.
Discussion
Distraction was associated with substantial reductions in capsaicin pain ratings. While distraction analgesia has been shown to be effective in a variety of settings and under a number of pain paradigms[27], this study is among the first to demonstrate distraction analgesia over a longer period of time. Our findings also suggest differences between low and high catastrophizers in the process of distraction-induced analgesia. We originally hypothesized that high catastrophizers would not achieve the same level of distraction analgesia as low catastrophizers; though overall these levels did not significantly differ. However, high catastrophizers were delayed in experiencing the pain-reducing effects of distraction. One potential explanation for this difference may be type 1 error. Another explanation by which higher catastrophizers may experience greater pain is through exaggerated attentional engagement with pain stimuli[41], and difficulty disengaging attention from pain, although one would expect that this effect would be reflected in game performance. Hoffman and colleagues have conducted a series of virtual reality distraction studies, suggesting attentional mechanisms play a key role in this form of behavioral analgesia[23–25]. For example, they found increased analgesic effects when the distraction experienced was more interactive[25] and deficits in performance while engaged in virtual reality during a divided attention task[21]. A few studies have shown that catastrophic thinking is associated with an inability to divert attention from pain-related thoughts, feelings and sensations. For example, in a chronic pain population, catastrophizing has been shown to moderate the effects of pain focusing strategies[6]. In a study using a painful and non-painful (tone) cueing paradigm, Van Damme and colleagues found that pain catastrophizing was related to retarded disengagement from painful stimuli in healthy adults[45]. Our findings do not appear to be due to the level of attention focused on the distraction task, as no differences emerged in self-reported attentional engagement or performance toward the video games between low and high catastrophizers. However, participants did not rate attention to capsaicin pain nor the change in attention toward their pain following engagement in the distraction task, although again one would expect this would be reflected in game performance. Another possible explanation for the smaller magnitude of distraction analgesia early on in higher catastrophizing individuals may be that distracting from more intense pain stimuli is more difficult than from milder stimuli. However, the pain experienced in the Pain Alone session was not significantly associated with distraction analgesia in higher catastrophizers. If greater pain ratings among the higher catastrophizing group led to less distraction analgesia, one would expect this relationship to be negative. Another potential explanation may be that the distraction task produced reduced catastrophizing; however, this reduction appeared similar between the two groups. Another explanation for this difference may be related to reward. Monetary incentives have been shown to affect pain ratings in some studies[31], and not in others[29]. Nevertheless, it is possible that the monetary incentives provided in this study were reflected in the reduction in pain scores during the pain + distraction session. It is also possible that this incentive affected ratings in the lower catastrophizing group more so than the higher catastrophizing group.
Of note, higher catastrophizers believed that capsaicin pain interfered more with their video game performance than their lower catastrophizing counterparts. However, they did not have significantly different videogame performance scores during the pain + distraction session when compared to the distraction alone session. Nor were their scores significantly different from low catastrophizers in either of the distraction sessions. Some debate exists within the literature over the nature of catastrophizing, attentional performance, and pain perception[46–49]. Some research suggests that high catastrophizers exhibit poorer performance on cognitive tasks (used to assess attentional interference) during intermittent painful stimuli when compared to low catastrophizers[47], while others have not found decrements in performance under painful conditions[49]. The focus of the current analyses was not to capture the potentially disruptive effects of pain on performance per se, but to characterize the relationship between catastrophizing and distraction analgesia. While the current study was not designed to fully characterize the complex interrelationships between attention, performance and pain among catastrophizers, it is interesting that high catastrophizers perceived their performance to be inhibited by the experimental pain stimulus. It remains unclear whether this difference stems from different expectations regarding performance, a negative response bias to pain related stimuli, or other factors. This would be an interesting target for future study.
Preliminary studies suggest that distraction analgesia may be mediated in part by endogenous opioids[27]. Neuroimaging studies have compared brain activity to pain alone versus brain activity when pain is paired with a simultaneous distraction task, showing significant effects in pain-related brain regions. For example, Valet and colleagues[44] found that a Stroop distractor task (presented during noxious stimulation) reduced pain intensity and unpleasantness and reduced neural activity in multiple pain-related brain areas relative to identical noxious stimulation under non-distracted conditions. The Pain + Distraction condition, relative to the Pain Alone condition, increased activation in a number of areas, including the cingulo-frontal cortex, as well as the periaqueductal gray (PAG) and the posterior thalamus. The authors suggest that distraction activates a cingulo-frontal network of cortical circuitry that gates pain modulation by exerting descending influences on the PAG and posterior thalamus[44]. Other fMRI studies have found similar results [3;43]. In reviewing cognitive modulation of pain, Villemure and Bushnell[50] discuss several studies implicating central nervous system processing in attentional pain modulation both in non-human animal and human studies; they propose a pain-modulatory pathway descending from the frontal cortex to the amygdala, through PAG, the rostral ventral medulla and spinal cord dorsal horn. Taken together, these studies suggest that cognitive modulation of pain by changes in attention may be instantiated by changes in pain-related brain activation among pathways rich in endogenous opioidergic innervation.
Emerging evidence suggests that high levels of catastrophizing may also impact on endogenous opioid pain-control systems in a generally negative manner. Although inconsistent, several studies find that elevated catastrophizing is associated with greater need for post-operative opioid analgesics to control post-surgical pain[26] and reduced analgesic benefit of pentazocine, especially among men[14], suggesting that opioids may produce less analgesic benefit in individuals who reported higher levels of catastrophizing. In addition, indirect evidence supporting the hypothesis that catastrophizing interferes with effective functioning of endogenous opioid systems can be derived from several recently-published psychophysical studies in which high-catastrophizing healthy participants demonstrated less effective functioning of endogenous pain-inhibitory systems, measured using a counter-irritation paradigm[19;51]. Such counter-irritation analgesic effects had been shown to be opioid-mediated in prior studies utilizing competitive antagonists such as naloxone [52]. While these studies are generally cross-sectional in nature, they provide some indication that catastrophizing may interfere with endogenous opioid analgesic systems.
Several limitations should be considered when interpreting the results of the present study. First, all participants were young, healthy individuals undergoing laboratory-based pain induction procedures. Thus, the relevance of this work to acute clinical pain and persistent pain conditions is unknown. However, a number of studies have provided evidence for the clinical relevance of laboratory pain induction procedures[15], and hyperalgesic responses to topical capsaicin have been specifically associated with clinical pain and tenderness in patients with fibromyalgia and rheumatoid arthritis[32;33]. Moreover, several studies note that distraction techniques may be quite useful in acute pain settings, particularly in children[9;38], although some controversy has been raised over the efficacy of distraction in chronic pain conditions[42]. Another limitation relates to the use of only a single type of experimental pain stimulus. It is possible that the association between catastrophizing and pain responses, or between catastrophizing and distraction’s analgesic effects, could vary as a function of the type, intensity, and/or duration of noxious stimulus presented. Additionally, we do not have data addressing the potential mechanisms by which catastrophizing might shape the pain-reducing effects of distraction. In particular, the opioid-catastrophizing relationship described above is speculative as we did not assess the functioning of endogenous opioid systems (e.g., using a naloxone challenge).
Despite these limitations, our findings provide evidence of increased pain over time in high catastrophizers, as well as a delayed onset of distraction-induced analgesia. This pattern of findings suggests the possibility that high catastrophizers are less equipped to mobilize opioid-dependent pain inhibitory processes. Longitudinal studies utilizing other assessment methods will be necessary to determine whether catastrophizing interferes with opioid mediated analgesic processes. Future studies might benefit from examination of the differential physiological effects of catastrophizing and distraction to gain a more thorough understanding of the mechanisms contributing to the differential analgesic effects of distraction depending on catastrophizing. Manipulations involving naloxone or another opioid antagonist may be a useful next step in elucidating the underlying mechanisms by which catastrophizing shapes the experience of pain and analgesia. Clinically, it is noteworthy that it may be useful to start a distraction task earlier in higher catastrophizing patients in order to allow time for these analgesic processes to engage; alternatively, these patients may need to have appropriate expectations established prior to implementing distraction as a pain-reducing strategy. Collectively, additional study seems warranted to characterize the potentially complex relationships between catastrophizing and a variety of pain-reducing interventions.
Acknowledgements
This work was supported by NIH grants AT001433 and F32 NS063624
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest.
Reference List
- Anderson WS, Sheth RN, Bencherif B, Frost JJ, Campbell JN. Naloxone increases pain induced by topical capsaicin in healthy human volunteers. Pain. 2002;99:207–216. doi: 10.1016/s0304-3959(02)00103-3. [DOI] [PubMed] [Google Scholar]
- Bandura A, Cioffi D, Taylor CB, Brouillard ME. Perceived self-efficacy in coping with cognitive stressors and opioid activation. J Pers Soc Psychol. 1988;55:479–488. doi: 10.1037//0022-3514.55.3.479. [DOI] [PubMed] [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- Bencherif B, Fuchs PN, Sheth R, Dannals RF, Campbell JN, Frost JJ. Pain activation of human supraspinal opioid pathways as demonstrated by [11C]-carfentanil and positron emission tomography (PET) Pain. 2002;99:589–598. doi: 10.1016/S0304-3959(02)00266-X. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15:293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- Buck R, Morley S. A daily process design study of attentional pain control strategies in the self-management of cancer pain. Eur J Pain. 2006;10:385–398. doi: 10.1016/j.ejpain.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen J, Carrier B. Pain perception: Is there a role for primary somatosensory cortex? Proc Natl Acad Sci U S A. 1999;96:7705–7709. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CM, Kronfli T, Buenaver LF, Haythornthwaite JA, Smith MT, Edwards RR. In vivo vs. standard catastrophizing in multiple pain measures among healthy, TMD and arthritis patients. Journal of Pain. 2008;9(4):56. Ref Type: Abstract. [Google Scholar]
- Cohen LL. Behavioral approaches to anxiety and pain management for pediatric venous access. Pediatrics. 2008;122 Suppl 3:S134–S139. doi: 10.1542/peds.2008-1055f. [DOI] [PubMed] [Google Scholar]
- Crombez G, Eccleston C, van den BA, Van HB, Goubert L. The effects of catastrophic thinking about pain on attentional interference by pain: no mediation of negative affectivity in healthy volunteers and in patients with low back pain. Pain Res Manag. 2002;7:31–39. doi: 10.1155/2002/576792. [DOI] [PubMed] [Google Scholar]
- Dixon KE, Thorn BE, Ward LC. An evaluation of sex differences in psychological and physiological responses to experimentally-induced pain: a path analytic description. Pain. 2004;112:188–196. doi: 10.1016/j.pain.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Campbell CM, Fillingim RB. Catastrophizing and experimental pain sensitivity: only in vivo reports of catastrophic cognitions correlate with pain responses. J Pain. 2005;6:338–339. doi: 10.1016/j.jpain.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain. 2006;22:730–737. doi: 10.1097/01.ajp.0000210914.72794.bc. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Hastie BA, Ness TJ, Glover TL, Campbell CM, Staud R. Sex-related psychological predictors of baseline pain perception and analgesic responses to pentazocine. Biol Psychol. 2005;69:97–112. doi: 10.1016/j.biopsycho.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Lautenbacher S. The Importance of Quantitative Sensory Testing in the Clinical Setting. In: Lautenbacher S, Fillingim RB, editors. Pathophysiology of pain perception. New York: Kluwer Academic Plenum Publishers; 2004. [Google Scholar]
- France CR, France JL, Al'Absi M, Ring C, McIntyre D. Catastrophizing is related to pain ratings, but not nociceptive flexion reflex threshold. Pain. 2002;99:459–463. doi: 10.1016/s0304-3959(02)00235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenstein UN, Richter W, McIntyre MC, Remy F. Distraction modulates anterior cingulate gyrus activations during the cold pressor test. Neuroimage. 2001;14:827–836. doi: 10.1006/nimg.2001.0883. [DOI] [PubMed] [Google Scholar]
- Geisser ME, Robinson ME, Pickren WE. Differences in cognitive coping strategies among pain-sensitive and pain-tolerant individuals on the cold pressor test. Beh Ther. 1992;23:31–42. [Google Scholar]
- Goodin BR, McGuire L, Allshouse M, Stapleton L, Haythornthwaite JA, Burns N, Mayes LA, Edwards RR. Associations between catastrophizing and endogenous pain-inhibitory processes: sex differences. J Pain. 2009;10:180–190. doi: 10.1016/j.jpain.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Hadjistavropoulos HD, Hadjistavropoulos T, Quine A. Health anxiety moderates the effects of distraction versus attention to pain. Behav Res Ther. 2000;38:425–438. doi: 10.1016/s0005-7967(99)00044-3. [DOI] [PubMed] [Google Scholar]
- Hoffman HG, Garcia-Palacios A, Kapa V, Beecher J, Sharar SR. Immersive virtual reality for reducing experimental ischemic pain. International Journal of Human-Computer Interaction. 2003;15:469–486. [Google Scholar]
- Hoffman HG, Richards T, Coda B, Richards A, Sharar SR. The illusion of presence in immersive virtual reality during an fMRI brain scan. Cyberpsychol Behav. 2003;6:127–131. doi: 10.1089/109493103321640310. [DOI] [PubMed] [Google Scholar]
- Hoffman HG, Richards TL, Coda B, Bills AR, Blough D, Richards AL, Sharar SR. Modulation of thermal pain-related brain activity with virtual reality: evidence from fMRI. Neuroreport. 2004;15:1245–1248. doi: 10.1097/01.wnr.0000127826.73576.91. [DOI] [PubMed] [Google Scholar]
- Hoffman HG, Richards TL, Van OT, Coda BA, Jensen MP, Blough DK, Sharar SR. The analgesic effects of opioids and immersive virtual reality distraction: evidence from subjective and functional brain imaging assessments. Anesth Analg. 2007;105:1776–1783. doi: 10.1213/01.ane.0000270205.45146.db. table. [DOI] [PubMed] [Google Scholar]
- Hoffman HG, Sharar SR, Coda B, Everett JJ, Ciol M, Richards T, Patterson DR. Manipulating presence influences the magnitude of virtual reality analgesia. Pain. 2004;111:162–168. doi: 10.1016/j.pain.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Jacobsen PB, Butler RW. Relation of cognitive coping and catastrophizing to acute pain and analgesic use following breast cancer surgery. J Behav Med. 1996;19:17–29. doi: 10.1007/BF01858172. [DOI] [PubMed] [Google Scholar]
- Johnson MH. How does distraction work in the management of pain? Curr Pain Headache Rep. 2005;9:90–95. doi: 10.1007/s11916-005-0044-1. [DOI] [PubMed] [Google Scholar]
- Longe SE, Wise R, Bantick S, Lloyd D, Johansen-Berg H, McGlone F, Tracey I. Counter-stimulatory effects on pain perception and processing are significantly altered by attention: an fMRI study. Neuroreport. 2001;12:2021–2025. doi: 10.1097/00001756-200107030-00047. [DOI] [PubMed] [Google Scholar]
- Lowery D, Fillingim RB, Wright RA. Sex differences and incentive effects on perceptual and cardiovascular responses to cold pressor pain. Psychosom Med. 2003;65:284–291. doi: 10.1097/01.psy.0000033127.11561.78. [DOI] [PubMed] [Google Scholar]
- McCaul KD, Haugtvedt C. Attention,distraction and cold pressor pain. J Personal Soc Psychol. 1995;43:154–162. doi: 10.1037//0022-3514.43.1.154. [DOI] [PubMed] [Google Scholar]
- McCue R, Lawrence J, Knutson B, Mackey S. Cumulative effects of reward and loss on pain evaluation. Journal of Pain. 2009;10(4) Supplement 1:S25. Ref Type: Abstract. [Google Scholar]
- Morris V, Cruwys S, Kidd B. Increased capsaicin-induced secondary hyperalgesia as a marker of abnormal sensory activity in patients with fibromyalgia. Neuroscience Letters. 1998;250:205–207. doi: 10.1016/s0304-3940(98)00443-1. [DOI] [PubMed] [Google Scholar]
- Morris VH, Cruwys SC, Kidd BL. Characterisation of capsaicin-induced mechanical hyperalgesia as a marker for altered nociceptive processing in patients with rheumatoid arthritis. Pain. 1997;71:179–186. doi: 10.1016/s0304-3959(97)03361-7. [DOI] [PubMed] [Google Scholar]
- Piira T, Taplin JE, Goodenough B, von Baeyer CL. Cognitive-behavioural predictors of children's tolerance of laboratory-induced pain: implications for clinical assessment and future directions. Behav Res Ther. 2002;40:571–584. doi: 10.1016/s0005-7967(01)00073-0. [DOI] [PubMed] [Google Scholar]
- Rhudy JL, Maynard LJ, Russell JL. Does in vivo catastrophizing engage descending modulation of spinal nociception? J Pain. 2007;8:325–333. doi: 10.1016/j.jpain.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Roelofs J, Peters ML, Vlaeyen JW. The modified Stroop paradigm as a measure of selective attention towards pain-related information in patients with chronic low back pain. Psychol Rep. 2003;92:707–715. doi: 10.2466/pr0.2003.92.3.707. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mikulis DJ, Davis KD. Cognitive modulation of pain-related brain responses depends on behavioral strategy. Pain. 2004;112:48–58. doi: 10.1016/j.pain.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Sharar SR, Miller W, Teeley A, Soltani M, Hoffman HG, Jensen MP, Patterson DR. Applications of virtual reality for pain management in burn-injured patients. Expert Rev Neurother. 2008;8:1667–1674. doi: 10.1586/14737175.8.11.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D, Chabal C, Griffith J, Rausch M, Steele B. A clinical trial of distraction techniques for pain and anxiety control during cataract surgery. Insight. 2004;29:13–16. [PubMed] [Google Scholar]
- Sullivan MJ, Bishop S, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychological Assessment. 1995;7:524–532. [Google Scholar]
- Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- Thorn BE. Cognitive Therapy for Chronic Pain: A Step-by-step Guide. Guilford Press; 2004. [Google Scholar]
- Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002;22:2748–2752. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, Erhard P, Tolle TR. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain--an fMRI analysis. Pain. 2004;109:399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Van DS, Crombez G, Eccleston C. Disengagement from pain: the role of catastrophic thinking about pain. Pain. 2004;107:70–76. doi: 10.1016/j.pain.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Vancleef LM, Peters ML. The interruptive effect of pain on attention. J Pain. 2006;7:21–22. doi: 10.1016/j.jpain.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Vancleef LM, Peters ML. Pain catastrophizing, but not injury/illness sensitivity or anxiety sensitivity, enhances attentional interference by pain. J Pain. 2006;7:23–30. doi: 10.1016/j.jpain.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Veldhuijzen DS. Pain and attention: a discussion of two studies. J Pain. 2006;7:31. doi: 10.1016/j.jpain.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Veldhuijzen DS, Kenemans JL, de Bruin CM, Olivier B, Volkerts ER. Pain and attention: attentional disruption or distraction? J Pain. 2006;7:11–20. doi: 10.1016/j.jpain.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195–199. doi: 10.1016/S0304-3959(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Weissman-Fogel I, Sprecher E, Pud D. Effects of catastrophizing on pain perception and pain modulation. Exp Brain Res. 2008;186:79–85. doi: 10.1007/s00221-007-1206-7. [DOI] [PubMed] [Google Scholar]
- Willer JC, Le Bars D, De Broucker T. Diffuse noxious inhibitory controls in man: involvement of an opioidergic link. European Journal of Pharmacology. 1990;182:347–355. doi: 10.1016/0014-2999(90)90293-f. [DOI] [PubMed] [Google Scholar]