Abstract
Various 2-benzylidene-6-(nitrobenzylidene)cyclohexanones were prepared as candidate cytotoxins in which the nitro group was located in the ortho, meta and para positions leading to series 1–3, respectively. The CC50 values towards human HSC-2 and HSC-4 oral squamous cell carcinomas as well as human HL-60 promyelocytic leukemic cells are in the low micromolar range in general. On the other hand, most of the compounds afforded clear evidence of being far less toxic towards human HGF gingival fibroblasts, HPC pulp cells and HPLF periodontal ligament fibroblasts which are non-malignant cells. Selectivity index (SI) figures were generated which are the ratios of the average CC50 values towards normal cells and the CC50 figure towards a malignant cell line. Huge SI values were obtained for many of the compounds. In particular 1c, 2f, 3c and 3g which have average SI values of >76, >38, 124 and 341, respectively, are clearly lead molecules affording direction for amplification of this area of study. A lead compound 1c caused internucleosomal DNA fragmentation and activation of caspase-3 in HL-60 cells but not in HSC-2 carcinomas. In a short-term toxicity study, doses up to and including 300 mg/kg of the majority of the compounds prepared in this study did not cause any mortalities to mice. Some guidelines for development of these tumor-selective cytotoxins are presented.
Keywords: Unsaturated ketones, Selective cytotoxicity, Structure1–activity relationships, Murine toxicity
1. Introduction
The major emphasis of this laboratory is the design, syntheses and bioevaluations of candidate antineoplastic agents. These compounds are principally conjugated unsaturated ketones which is a class of compounds known to have an affinity for thiols but possess far less propensity to interact with amines.1,2 Hence these enones may exert their cytotoxic properties without interfering with nucleic acids and hence be bereft of the genotoxic side effects of various anticancer drugs.3 Various in vitro studies revealed that conjugated enones lower thiol concentrations of malignant cells and this effect may be prevented by the addition of various thiols such as glutathione to the media.4,5
Previously a single enone pharmacophore was employed in the design of different series of compounds.6 An aryl ring with different substituents was normally attached to the α,β-unsaturated keto group so that the polarity of the olefinic atoms varies which may influence antineoplastic potencies.7 In addition, the use of different aryl substituents affects the hydrophobic and steric properties of the molecules. However recently several series of compounds have been prepared which contain the 1,5-diaryl-3- oxo-1,4-pentadienyl group (AR–CH=CHCOCH=CHAR) which is referred to hereafter as the dienone moiety. The reason for preparing this pharmacophore is that a number of tumours are more sensitive to chemical insult than some normal cells.8,9 Hence an initial reaction with cellular mercaptans followed by a second thiol alkylation may lead to greater toxicity in malignant cells than towards normal tissues. In other words, compounds which are capable of successive attacks in cells may display lethal effects preferentially in neoplasms. This theory is known as sequential cytotoxicity and has been described in detail10 while investigations based on this hypothesis have been undertaken.11,12
Several years ago, the decision was made to initiate a project in which compounds were designed so that olefinic carbon atoms which interact with thiols bore divergent electronic charges. In this way, a difference between the atomic charges of the olefinic carbon atoms exists13 which will enhance successive interactions with cellular thiols. In other words, one would predict that an initial reaction would take place at the more electropositive carbon atom followed by thiol alkylation on the remaining olefinic carbon. The design of the compounds is indicated in Figure 1A. Phase I of this project involved a preliminary investigation as to whether the compounds possessed cytotoxic properties. This work has been completed and the biodata reveal that the compounds inhibit the growth of human Molt 4/C8 and CEM T-lymphocytes and murine L1210 cells.13 On the basis of these results; Phase II of this study was initiated which addresses three issues. First, the crucial issues of whether the compounds exert preferential cytotoxicity to malignant cells compared to normal cells. Second, the mode of action of a lead compound to discern the way in which cytotoxicity is mediated. Third, if they are well tolerated in mammals or associated with any undesired toxicity. This report indicates the results of the Phase II evaluations.
Figure 1.
(A) Design of the compounds in series 1–3. (B) Specific compounds prepared.
2. Results
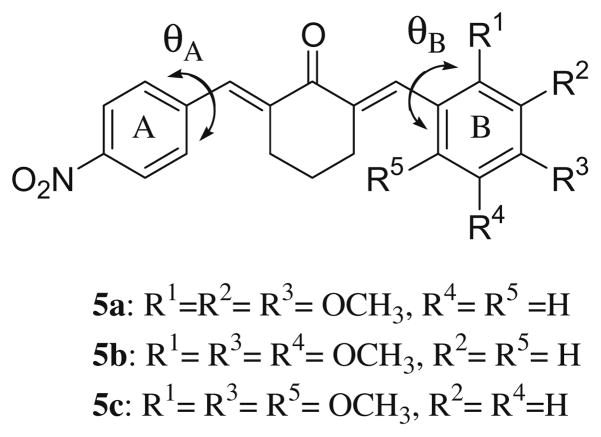
The compounds in series 1–4 which comprise the basis of this study are indicated in Figure 1B. The syntheses of 1–4 have been described previously13 where as 5a–c are new compounds. In general, condensation between a nitrobenzaldehyde and cyclohexanone led to the corresponding 2-(nitrobenzylidene)cyclohexanone which on reaction with a variety of aromatic aldehydes gave the desired products in series 1–3. Reaction of cyclohexanone and 2-nitrobenzaldehyde afforded the related aldol which on dehydration led to 4a. Conversion of cyclohexanone into the corresponding enamine followed by reaction with 3-nitrobenzaldehyde produced 4b. The olefinic double bonds in all of these compounds assumed the E configuration and the electron densities on the CB atoms are invariably lower than the CA atoms in series 1–3. In view of the biodata obtained from series 1–4 vide infra, 5a–c were prepared by condensing 2-(4-nitrobenzylidene)-cyclohexanone with various aryl aldehydes (see Fig. 2).
Figure 2.
Structures of 5a–c.
All of the compounds in series 1–5 were evaluated against three human non-malignant cells, namely HGF gingival fibroblasts, HPC pulp cells and HPLF periodontal ligament fibroblasts and three malignant cells, namely HSC-2 and HSC-4 squamous cell carcinomas as well as HL-60 promyelocytic leukemia cells. These results are presented in Table 1. Mode of action studies revealed that a lead compound 1c caused internucleosomal DNA fragmentation (Fig. 3) and activation of caspase-3 in HL-60 cells but not in HSC-2 carcinomas (Fig. 4). Doses of 30, 100 and 300 mg/kg of 1a–c,f,g, 2a,b,d–g, 3a–g, 4a,b and 5a–c were injected intraperitoneally into mice and the animals were examined for mortalities and neurotoxicity at the end of 0.5 and 4 h.
Table 1.
Evaluation of 1a–g, 2a–g, 3a–g, 4a,b, 5a–c and melphalan against a panel of normal and malignant cell lines
| Compound | Human normal cells
|
Human malignant cells
|
Ave SI | log P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC50a (μM)
|
HSC-2
|
HSC-4
|
HL-60
|
|||||||||
| HGF | HPC | HPLF | Ave | CC50(μM)a | SIb | CC50(μM)a | SIb | CC50(μM)a | SIb | |||
| 1a | 93 | 16 | 26 | 45 | 3.8 | 12 | 3.2 | 14 | 3.7 | 12 | 13 | 4.70 |
| 1b | 82 | 11 | 67 | 53 | 2.7 | 20 | 2.2 | 24 | 3.0 | 18 | 21 | 4.86 |
| 1c | >400 | 400 | >400 | >400 | 3.7 | >108 | 6.1 | >66 | 7.6 | >53 | >76 | 5.38 |
| 1d | >400 | >400 | >400 | >400 | >400 | ~1.0 | >400 | ~1.0 | >400 | ~1.0 | ~1.0 | 4.80 |
| 1e | >400 | >400 | >400 | >400 | 11 | >36 | 40 | >10 | 11 | >36 | >27 | 5.15 |
| 1f | >400 | >400 | 386 | >395 | 11 | >36 | 24 | >17 | 26 | >15 | >23 | 4.76 |
| 1g | 44 | 35 | 79 | 53 | 5.9 | 9.0 | 6.5 | 8.2 | 3.0 | 18 | 12 | 4.33 |
| 2a | 25 | 14 | 18 | 19 | 3.7 | 5.1 | 2.4 | 7.9 | 3.0 | 6.3 | 6.4 | 4.90 |
| 2b | 42 | 38 | 36 | 39 | 2.2 | 18 | 2.5 | 16 | 1.0 | 39 | 24 | 5.07 |
| 2c | 39 | 18 | 38 | 32 | 2.2 | 15 | 2.3 | 14 | 3.2 | 10 | 13 | 5.58 |
| 2d | >400 | >400 | >400 | >400 | 32 | >13 | 71 | >5.6 | 30 | >13 | >11 | 5.00 |
| 2e | >400 | 192 | >400 | >331 | 17 | >20 | 45 | >7.4 | 38 | >8.7 | >12 | 5.35 |
| 2f | >400 | 33 | 354 | >262 | 4.8 | >55 | 11 | >24 | 7.2 | >36 | >38 | 4.96 |
| 2g | 336 | >400 | >400 | >379 | 73 | >5.2 | 92 | >4.1 | 59 | >6.4 | >5.2 | 4.53 |
| 3a | 371 | 123 | 282 | 259 | 4.9 | 53 | 9.2 | 28 | 20 | 13 | 31 | 4.93 |
| 3b | 326 | >400 | >400 | >375 | 19 | >20 | 15 | >25 | 32 | >12 | >19 | 5.09 |
| 3cc | 191 | 345 | 332 | 289 | 1.5 | 193 | 2.7 | 107 | 4.1 | 71 | 124 | 5.60 |
| 3d | >400 | >400 | >400 | >400 | 11 | >36 | >400 | ~1.0 | >400 | ~1.0 | ~13 | 5.03 |
| 3e | >400 | 92 | 348 | >280 | 6.6 | >42 | 13 | >22 | 27 | >10 | >25 | 5.38 |
| 3fc | >400 | >400 | >400 | >400 | >400 | ~1.0 | >400 | ~1.0 | >400 | ~1.0 | ~1.0 | 4.98 |
| 3gc | 336 | 347 | 378 | 354 | 0.76 | 466 | 1.5 | 236 | 1.1 | 322 | 341 | 4.56 |
| 4a | 218 | 117 | 103 | 146 | 28 | 5.2 | 29 | 5.0 | 27 | 5.4 | 5.2 | — |
| 4b | 74 | 49 | 56 | 60 | 6.6 | 9.1 | 18 | 3.3 | 5.8 | 10 | 7.5 | — |
| 5a | >400 | >400 | >400 | >400 | >400 | ~1.0 | >400 | ~1.0 | >400 | ~1.0 | ~1.0 | — |
| 5b | >400 | >400 | >400 | >400 | 67 | >6.0 | 250 | >1.6 | 400 | ~1.0 | ~2.9 | — |
| 5c | >400 | >400 | >400 | >400 | >400 | ~1.0 | >400 | ~1.0 | 11 | >36 | ~13 | — |
| Melphaland | >200 | >200 | >200 | >200 | 35 | >5.7 | 81 | >2.5 | 6.0 | >33 | >14 | — |
The CC50 value is the concentration of the compound which reduces the number of viable cells by 50% and was obtained from duplicate determinations. The differences between the CC50 figures in the two bioassays are less than 5%. The maximum concentration of each compound was 400 μM except solubility considerations necessitated the highest concentration of melphalan to be 200 μM.
The letters SI indicate the selectivity index and is the quotient of the average CC50 figure of the three normal cells and the CC50 value of a malignant cell line.
With the exception of the result using HSC-4 cells, the data were reported previously26 [copyright (2005) by Elsevier].
The data were reported previously27 [copyright (2008) by Elsevier].
Figure 3.
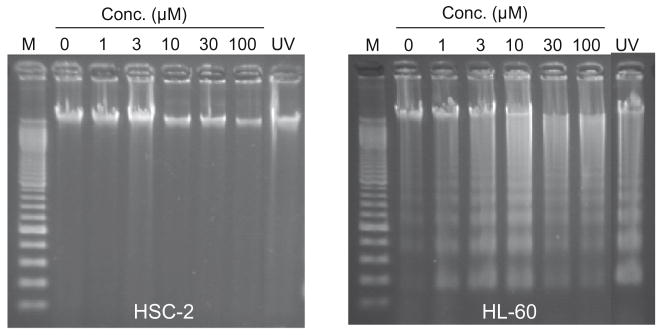
Evaluation of 1c on the induction of internucleosomal DNA fragmentation in HSC-2 and HL-60 cells after 6 h of incubation. M is the molecular weight marker of DNA.
Figure 4.
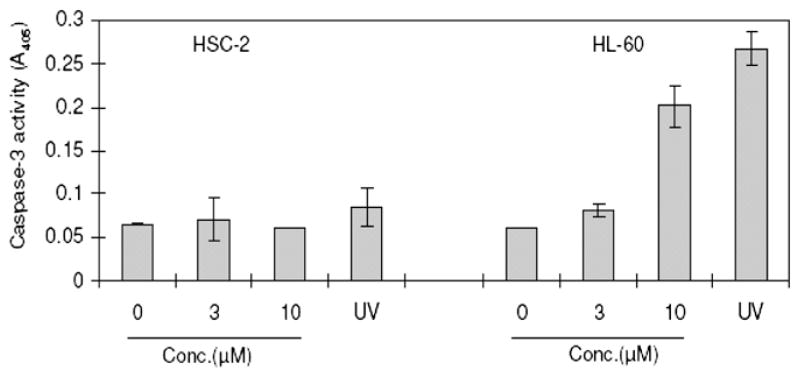
Effect of 1c on the activation of caspase-3 after 5 h incubation in HSC-2 and HL-60 cells. UV refers to ultraviolet radiation (6 J/m2/min) applied for 1 min followed by incubation of the cells for 3 h. The bar graphs represent the mean standard deviations (n = 3).
3. Discussion
The first issue to be resolved is whether the compounds in series 1–3 are well tolerated by HGF, HPC and HPLF normal cells. The percentages of the CC50 values of 1a–g, 2a–g and 3a–g which are in excess of 100 μM are 57 (12 out of 31), 52 (11 out of 21) and 95 (20 out of 21), respectively, indicating that the compounds in series 3 have lower unwanted toxic properties towards normal cells than the analogs in series 1 and 2. Overall 68% (43 out of 63) of the CC50 figures of 1–3 are greater than 100 μM and for the remaining compounds the average CC50 value is 42 μM. In general, therefore, these normal cells are not adversely affected by the dienones 1–3.
The biodata of the sensitivity of the human HSC-2, HSC-4 and HL-60 neoplastic cell lines to the compounds in series 1–3 are presented in Table 1 which reveal that, in general, these compounds are cytotoxic to oral HSC-2 and HSC-4 tumours as well as HL-60 cells (chosen as a positive control of apoptosis since this biochemical effect is readily induced in this cell line by many chemotherapeutic agents). There are three outliers, namely 1d, 3d and 3f which have CC50 values in excess of 400 μM in all three bioassays with the exception of 3d towards HSC-2 cells. The CC50 figures of all of the other compounds in series 1–3 are below 100 μM and, in fact, 48% and 59% of these CC50 values are less than 5 and 10 μM, respectively.
In order to ascertain if the electronic, hydrophobic or steric properties of the groups in ring B influence cytotoxic potencies, linear (l) and semilogarithmic (sl) plots were made between the CC50 values of 1a–c,e–g in the HSC-2 assay and the Hammett sigma, Hansch pi and molecular refractivity (MR) figures of the aryl substituents. In addition, logarithmic plots were constructed using MR constants. The experiment was repeated using the biodata in the HSC-4 test and then the results using HL-60 cells. A similar situation was undertaken with 2a–g and finally with 3a–c,e,g. The following correlations (p <0.05) and trends towards significance (p <0.1) were noted. A negative correlation was found between the CC50 figures of 1a–c,e,g towards HSC-2 cells and the σ values. The MR constants of the aryl substituents in series 2 correlated positively with the CC50 values obtained in the HSC-2, HSC-4 and HL-60 bioassays. On the other hand, a negative correlation was noted between the MR constants of 3a–c,e,g and the potencies towards HL-60 cells and there was a negative trend towards significance in the case of HSC-4 cells. No other correlations or trends to significance were noted. Thus in developing these compounds, increasing potencies may result from inserting groups into aryl ring B with large positive σ values in series 1, small substituents (low MR values) in series 2 and large groups (high MR constants) in series 3. Since no correlations were noted with π values, lipophilicity appears to be unimportant in reference to the cytotoxic potencies in series 1–3. This conclusion was reinforced when linear, semilogarithmic and logarithmic plots were made between the log P values of 1a–g, 2a–g and then 3a–g (which are presented in Table 1) and the CC50 values in each series against HSC-2 cells, then HSC-4 neoplasms and finally HL-60 cells. No correlations were found and only a trend towards a negative correlation between the CC50 figures of 2a–g in the HSC-2 screen and the log P values was noted (p <0.1).
In a further attempt to discern correlations between the electronic, hydrophobic and steric properties of the aryl substituents and cytotoxic potencies, multi-linear regression (MLR) analyses were undertaken. A data set was obtained for 21 compounds viz 1a–g, 2a–g and 3a–g and four physiochemical descriptors (σ, π, MR and log P). When the ring B is di or tri-substituted, the sum of individual parameters (Σσm,p, Σπ, ΣMR) was used. The results indicate that there were no statistical correlations between the cytotoxicity against HSC-2 and HSC-4 cells and the four physicochemical descriptors. However, a modest correlation was noted against the HL-60 cell line (Eq. 1). Efforts were made to improve this result by excluding one or more descriptors from the regression analysis which led to a lowering of the statistical quality observed in Eq. 1.
| (1) |
n = 21, r = 0.741, radj = 0.631, s = 0.61, F = 3.65, p = 0.023.
In this equation, n represents the number of data points, r is the correlation coefficient, radj is the adjusted r, s is the standard deviation of the regression equation, and the F value is related to the F-statistic analysis (Fisher test).
The cytotoxicity of the compounds in series 1–5 is considered to be mediated principally by reactions at the olefinic carbon atoms with cellular thiols. In order to gain an understanding of the effect of different aryl substituents on the electron densities at the olefinic carbon atoms, the 13C NMR spectra of the representative enones 1a–c,e–g were determined (1d was omitted due to the absence of specific CC50 values). The 13C NMR absorptions at CA (as designated in Fig. 1A) are in the range of 132.53–133.11 while at CB, the relevant figures are 136.62–138.17 (the precise values of individual compounds are listed in the Experimental Section). These data reveal that the CB carbon atoms are more electron deficient than the CA atoms and are the loci of the initial interactions with thiols. Linear and logarithmic plots were constructed between the 13C chemical shifts of the CB atoms the CC50 values of 1a–c,e–g in the HSC-2, HSC-4 and HL-60 bioassays. No correlations or trends to significance were noted although the logarithmic plot between the CC50 figures in the HSC-2 assay provided a p value of 0.125 (a positive correlation). One may conclude that the spectroscopic data reveal a marked disparity in the electron densities on the olefinic carbon atoms which did not correlate significantly with cytotoxic potencies.
The discernment of whether the compounds in series 1–3 display greater toxicity to neoplasms than normal cells was achieved as follows. In patients afflicted with cancer, the malignant cells are often surrounded by a number of different normal tissues. Hence in order to simulate clinical conditions, a comparison was made between the cytotoxicity of a compound to a malignant cell line compared to the average CC50 value towards three normal cell lines. The figure generated is referred to as the selectivity index (SI) value of the compound and these data are listed in Table 1. A SI figure of 10 was arbitrarily chosen as an indicator of marked selectivity. The results of the outliers 1d, 3d and 3f were removed from consideration and for the other compounds in series 1–3, this criterion was observed in 80% of the SI data. Hence the 2-benzylidene-6-(nitrobenzylidene) cyclohexanones are a novel cluster of cytotoxins with a substantial predilection for greater toxicity towards neoplasms than normal cells. In particular, the compounds demonstrating the highest selective toxicity to malignant cells (average SI values in parentheses) are 1c(>76), 2f (>38), 3c (124) and 3g (341), all of which clearly serve as lead molecules. A comparison was made between 4a and 4b and the analogs in series 1 and 2, respectively, in terms of their potencies towards HSC-2, HSC-4 and HL-60 malignant cells and SI values. With the exception of the outlier 1d, in general the CC50 figures of 4a are higher than obtained for series 1 while the SI values are invariably lower. The enone 4b is less potent than the analogs in series 2 in approximately half of the comparisons made while the SI figures are generally lower than was observed for 2a–g. Thus the addition of an arylidene group to a nitrobenzylidenecyclohexanone leads, in general, to increases in both potencies to malignant cells as well as SI values.
Conjugated enones alkylate cellular thiols, vide supra, and comparisons were made between the potencies and selective toxicity of a clinically used alkylating agent melphalan and the compounds in series 1–3. In the case of melphalan, the CC50 figures are higher and the SI values lower in 68% and 65%, respectively, of the comparisons made. This observation reinforces the decision to develop these compounds further.
An important issue to resolve which will affect the way in which the project may be amplified is whether the positions of the three methoxy groups in 3g control cytotoxic potencies and SI values. Hence three structural isomers of 3g were prepared namely 5a–c and their bioevaluation is presented in Table 1. These compounds are less toxic to human normal cells than 3g. However apart from the lethal effects of 5c towards HL-60 cells, significant potency among 5a–c towards the neoplasms is lacking. One ortho methoxy group is present in 5a and 5b while two are present in 5c and hence the possibility exists that these groups will increase the torsion angles (θB) between aryl ring B and the adjacent olefinic group. However models reveal that the θB angles of 5a–c are 54.5°, −54.8° and 74.8°, respectively, compared to a figure of −51.9 for 3g.11 The different substituents in ring B have little effect on the θA values (the torsion angles between ring A and the attached olefinic group) which for 3g and 5a–c are 51.2,11 −48.0, 51.1 and −48.1, respectively. Hence the interplanar angles θA and θB appear to contribute little to the disparity in cytotoxicity between 3g and the closely related structural analogs 5a–c. However while the π and MR values of 3g are the same as 5a–c, there are huge differences in the Σσvalues of 3g (−0.03) on one hand and 5a (−0.37), 5b (−0.37) and 5c (−0.71) on the other hand which likely contributes significantly to the different potencies. In addition, similar electronic factors likely account for the low cytotoxicity of 3d (σp = −0.83) and 3f (σp = −0.27).
A mode of action study was undertaken using a representative compound 1c. This compound was chosen based on its cytototic potency and especially its large SI values. Incubation of 1c with HL-60 cells induced the internucleosomal DNA fragmentation (Fig. 3) and concentration-dependent activation of caspase-3 (Fig. 4) by 1c, suggesting the occurrence of apoptosis via the mitochondrial pathway. On the other hand, there is no evidence from the data provided in Figs. 3 and 4 that 1c causes apoptosis in HSC-2 carcinomas. Hence the lethality of 1c to this cell line is mediated by interference with other biochemical mechanisms. Thus the modes of action are dependent on the cell line under consideration which in turn gives rise to the enormous SI values displayed by many of these novel cytotoxins.
The final segment of this study involved the determination of the tolerability of the majority of the compounds in series 1–5 in mice. This investigation is important since many anticancer agents are highly toxic to mammals, for example, the LD50 of melphalan in mice is 4.0 mg/kg14 and neurotoxicity is a side effect of certain anticancer drugs such as oxaliplatin and etoposide.15 Doses of 30, 100 and 300 mg/kg of 1a–c,f,g, 2a,b,d–g, 3a–g,4a,b and 5a–c were injected intraperitoneally into mice and the animals examined for mortalities and neurotoxicity after 0.5 and 4 h. None of the compounds caused any deaths to the animals. Mice receiving doses of 1a,b,f,g, 2e,g, 3a,c,f and 5a,c did not display any neurotoxic symptoms. One of these dienones, namely 3c, was identified earlier as a lead compound since it has an average SI value of 124 and the absence of overt mammalian toxicity enhances its status for analog development. Some neurotoxicity was observed in the remaining compounds, specific details of which are given in the Experimental section. Marginal neurotoxicity was noted in less than half of the animals examined in the case of 2a,b,d,f, 3b,d,g, 4a,b and 5b after 0.5 but not 4 h. Neurological deficit occurred in half or more mice receiving 300 mg/kg of 1c after 4 h and both 2a and 3e when observed after 0.5 h. The conclusion to be drawn from this short-term toxicity evaluation of the majority of the compounds prepared in this study is that these dienones are well tolerated in mice.
4. Conclusions
Series 1–3 are novel tumor-specific cytotoxins many of which have huge SI values. In particular 1c, 2f, 3c and 3g are clearly lead molecules. In some instances correlations were noted between various physicochemical parameters and cytotoxic potencies. Thus in series 1, the IC50 values diminished as the magnitude of the Σ value increased. On the other hand, the overall electron-releasing effect of the substituents in ring B of 5a–c likely contributed significantly to their lack of marked antineoplastic potencies. Cytotoxicity is favoured by the placement of small groups in ring B in series 2 and large substituents in series 3. A lead compound 1c caused apoptosis in HL-60 cells but not in HSC-2 carcinomas. In general the compounds are well tolerated in mice.
5. Experimental
5.1. Synthesis of compounds
Melting points are recorded in degrees Celsius on a Gallenkamp apparatus and are uncorrected. 1H and 13C NMR spectra were recorded on a Bruker AMX 500 FT instrument while elemental analyses were obtained using an Elementer analyzer.
5.1.1. Synthesis of the compounds in series 1–5
The methodology for the preparation of the nitrobenzylidenecyclohexanones in series 1–4 has been described previously.13 The general procedure for the synthesis of 5a–c is as follows. Dry hydrogen chloride was passed into a mixture of 2-(4-nitrobenzylidene) cyclohexanone (0.005 mol), which was prepared by a literature procedure,16 the appropriate trimethoxybenzaldehyde (0.005 mol), chloroform(5 mL) and diethyl ether (40 mL). The reaction mixture was stirred at room temperature for 6–8 h and the resultant solid was collected and crystallized from chloroform/ether (1:5).
5.1.1.1. 6-(4-Nitrobenzylidene)-2-(2,3,4-trimethoxybenzylidene) cyclohexanone (5a)
Mp 176 °C; yield 72%. 1H NMR (CDCl3): δ 1.82 (p, 2H, CH2), 2.90 (m, 4H, 2 × CH2), 3.90 (s, 3H, OCH3), 3.92 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 6.71 (d, 1H, Ar-H, J = 8.73 Hz), 7.11 (d, 1H, Ar-H, J = 8.69 Hz), 7.59 (d, 2H, Ar-H, J = 8.67 Hz), 7.79 (s, 1H, =CH),7.98 (s, 1H, =CH), 8.27 (d, 2H, Ar-H, J = 8.73 Hz). Anal. Calcd for C23H23NO6: C, 67.47; H, 5.66; N, 3.42. Found: C, 67.40; H, 5.43; N, 3.43.
5.1.1.2. 6-(4-Nitrobenzylidene)-2-(2,4,5-trimethoxybenzylidene) cyclohexanone (5b)
Mp 163 °C; yield 64%. 1H NMR (CDCl3): δ 1.84 (p, 2H, CH2), 2.92 (m, 4H, 2 × CH2), 3.89 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 6.57 (s, 1H, Ar-H), 6.96 (s, 1H, Ar-H), 7.60 (d, 2H, Ar-H, J = 8.60 Hz), 7.79 (s, 1H, =CH), 8.08 (s, 1H, =CH), 8.28 (d, 2H, Ar-H, J = 8.68 Hz). Anal. Calcd for C23H23NO6: C, 67.47; H, 5.66; N, 3.42. Found: C, 67.33; H, 5.66; N, 3.33.
5.1.1.3. 6-(4-Nitrobenzylidene)-2-(2,4,6-trimethoxybenzylidene) cyclohexanone (5c)
Mp 159 °C; yield 58%. 1H NMR (CDCl3): δ 1.75 (p, 2H, CH2), 2.51 (t, 2H, CH2), 2.89 (t, 2H, CH2), 3.83 (s, 6H, 2 × OCH3), 3.86 (s, 3H, OCH3), 6.17 (s, 2H, Ar-H), 7.58 (d, 2H, Ar-H, J = 8.66 Hz), 7.73 (s, 1H, =CH), 7.77 (s, 1H, =CH), 8.26 (d, 2H, Ar-H, J = 8.75 Hz). Anal. Calcd for C23H23NO6: C, 67.47; H, 5.66; N, 3.42. Found: C, 67.32; H, 5.56; N, 3.42.
5.2. Structure–activity relationships
The constants used for various atoms and groups in series 1–3 (σ, π and MR values are given in parentheses) were taken from a reference source17 and are as follows: hydrogen (0.00, 0.00, 1.03), 4-fluoro (0.06, 0.14, 0.92), 4-chloro (0.23, 0.71, 6.03), 4-dimethylamino (−0.83, 0.18, 15.55), 4-methyl (−0.17, 0.56, 5.65), 4-methoxy (−0.27, −0.02, 7.87) and 3,4,5-trimethoxy (−0.03, −0.06, 23.61). The MR value of the hydrogen atom is 1.03 and not 0.00. Hence in the case of the monosubstituted compounds in series 1–3 viz. b–f, 2.06 (2 × 1.03) was added to the MR figure of the substituent and the MR value of the unsubstituted compound is 3.09 (3 × 1.03). The linear, semilogarithmic and logarithmic plots were constructed using a statistical software package.18 The following correlations (p <0.05) were noted [nature of the plots viz. linear (l), semilogarithmic (sl) or logarithmic (log)/cell line under consideration, in parentheses]: 1a–c,e,g versus σ (l, sl/HSC-2 cells), 2a–g versus MR (l, sl, log/HSC-2, HSC-4, HL-60 cells) and 3a–c,e,g versus MR [sl, log/HL-60 cells]. A trend to significance (p <0.1) was noted as follows namely 3a–c,e,g versus MR (sl, log/HSC-4 cells) and 2a– g versus log P (sl/HSC-2). The MLR analyses used a commercial software package.18
5.3. 13C NMR determinations
Concentrations of 40 μM of 1a–c,e–g in deuterated chloroform were prepared and the 13C NMR spectra determined at room temperature. The chemical shifts of the olefinic carbon atoms were assigned with the help of HMQC experiments. The chemical shifts of these compounds at carbon atoms CA and CB, respectively, are as follows: 1a: 133.00, 138.06; 1b: 133.10, 136.88; 1c: 133.11, 136.62; 1e: 132.75, 137.93; 1f 132.53, 138.17; 1g: 132.99, 137.89, respectively.
5.4. Molecular modeling
Models of the compounds in series 5 were built using a BIOMEDCACHE program.19 The conformational analysis was undertaken using the CONFLEX procedure20 which generates low energy conformers. This program incorporates down-stream, reservoir-filling, corner flap, edge flip and stepwise rotation as well as a pre-check. The structures generated are optimized using a modified MM2 molecular mechanics program. The heat of formation of various conformations was calculated using the PM3 Hamiltonian approach in MOPAC 2002 in order to obtain the global minimum energy as the true optimized geometry.
5.5. Bioevaluations
5.5.1. Cytotoxicity assays
The examination of the compounds in series 1–5 towards normal HGF, HPC and HPLF cells as well as HSC-2, HSC-4 and HL-60 neoplasms was accomplished using a literature procedure21 which has been summarized recently.22 In brief, the cells were incubated with different concentrations of the compounds for 24 h and after the appropriate workup, the CC50 values were determined from dose–response curves.
5.5.2. Mode of action study
The methodology for evaluating 1c for the induction of internucleosomal DNA fragmentation and caspase-3 activation has been described previously.23
5.5.3. Murine toxicity study
The evaluation of 1a–c,f,g, 2a,b,d–g, 3a–g,4a,b and 5a–c for mortalities and neurotoxicity in mice was undertaken by the National Institute of Neurological Disorders, and Stroke, USA according to their protocols.24 No deaths were observed. The evaluation of 3c,f,g in this screen has been recorded previously25 which revealed that 3c,f demonstrated no neurotoxicity while a dose of 100 mg/kg of 3g caused neurological deficit in 2/8 animals when observed after 0.5 h. Neurotoxicity was observed after administration of the following compounds (dose in mg/kg, number of animals demonstrating neurological deficit/number of treated mice in parentheses viz. 2a (100, 2/8; 300, 2/4), 2b (100, 1/8), 2d (100, 1/8; 300,1/4), 2f (30,1/4), 3b (100, 1/8), 3d (100, 2/8); 3e (300,3/4), 4a (300,1/4), 4b (300, 1/4) and 5b (100, 1/8; 300, 1/4). After 4 h, neurotoxicity was observed in one of two mice who received a dose of 300 mg/kg of 1c. The animals were housed, fed and handled using the protocols described in the ‘Guide for the Care and Use of Laboratory Animals’ which is published by the National Research Council.
Acknowledgments
The authors thank the Canadian Institutes of Health Research for an operating grant to J. R. Dimmock and the Ministry of Education, Science, Sports and Culture of Japan for a Grant-in-Aid (No. 19592156) to H. Sakagami. The National Institute of Neurological Disorders and Stroke, USA undertook the in vivo experimentation with mice which is recorded with gratitude. Our appreciation is extended to the Iranian Ministry of Health and Medical Education who provided financial support for A. Doroudi while H. I. Gul was supported by a visiting scholar grant (NATO-B2) distributed by the Scientific and Technical Research Council of Turkey (TUBITAK).
References and notes
- 1.Pati HN, Das U, Sharma RK, Dimmock JR. Mini-Rev Med Chem. 2007;7:131. doi: 10.2174/138955707779802642. [DOI] [PubMed] [Google Scholar]
- 2.Dimmock JR, Raghavan SK, Logan BM, Bigam GE. Eur J Med Chem. 1983;18:248. [Google Scholar]
- 3.Okey AB, Harper PA. In: Principles of Medical Pharmacology. 7. Kalant H, Grant DM, Mitchell J, editors. Elsevier; Toronto, Canada: 2007. p. 902. [Google Scholar]
- 4.Kamiya D, Uchihata Y, Ichikawa E, Kato K, Umezawa K. Bioorg Med Chem. 2005;15:1111. doi: 10.1016/j.bmcl.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 5.Samudio I, Konopleva M, Hail N, Jr, Shi YX, McQueen T, Hsu T, Evans R, Honda T, Gubble GW, Sporn M, Gilbert HF, Sabe S, Andreeff M. J Biol Chem. 2005;280:36273. doi: 10.1074/jbc.M507518200. [DOI] [PubMed] [Google Scholar]
- 6.Dimmock JR, Taylor WG. J Pharm Sci. 1975;64:241. doi: 10.1002/jps.2600640210. [DOI] [PubMed] [Google Scholar]
- 7.Dimmock JR, Smith LM, Smith PJ. Can J Chem. 1980;58:984. [Google Scholar]
- 8.Siemann DW, Beyers KL. Br J Cancer. 1993;68:1071. doi: 10.1038/bjc.1993.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.del Olmo M, Alouso-Varona A, Castro B, Calle Y, Bilbao P, Palomares T. Melanoma Res. 2000;10:103. [PubMed] [Google Scholar]
- 10.Dimmock JR, Sidhu KK, Chen M, Reid RS, Allen TM, Kao GY, Truitt GA. Eur J Med Chem. 1993;28:313. [Google Scholar]
- 11.Dimmock JR, Padmanilayam MP, Zello GA, Quail JW, Oloo EO, Prisciak JS, Kraatz HB, Cherkasov A, Lee JS, Allen TM, Santos CL, Manavathu EK, De Clercq E, Balzarini J, Stables JP. Eur J Med Chem. 2002;37:813. doi: 10.1016/s0223-5234(02)01402-2. [DOI] [PubMed] [Google Scholar]
- 12.Dimmock JR, Kandepu NM, Nazarali AJ, Motaganahalli NL, Kowalchuk TP, Pugazhenthi U, Prisciak JS, Quail JW, Allen TM, LeClerc R, Santos CL, De Clercq E, Balzarini J. J Med Chem. 2000;43:3933. doi: 10.1021/jm000058o. [DOI] [PubMed] [Google Scholar]
- 13.Das U, Doroudi A, Das S, Bandy B, Balzarini J, De Clercq E, Dimmock JR. Bioorg Med Chem. 2008;16:6261. doi: 10.1016/j.bmc.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinn ER, Milne GWA. Fundam Appl Toxicol. 1986;6:270. doi: 10.1016/0272-0590(86)90240-x. [DOI] [PubMed] [Google Scholar]
- 15.Chen EX, Moore MJ. In: Principles of Medical Pharmacology. 7. Kalant H, Grant DM, Mitchell J, editors. Elsevier; Toronto, Canada: 2007. p. 777. [Google Scholar]
- 16.Vieweg H, Wagner G. Pharmazie. 1979;34:785. [PubMed] [Google Scholar]
- 17.Hansch C, Leo AJ. Substituent Constants for Correlation Analysis in Chemistry and Biology. John Wiley and Sons; New York: 1979. p. 49.p. 50. [Google Scholar]
- 18.Statistical Package for Social Sciences, SPSS for Windows, Release 17.0. SPSS Inc; Chicago, IL: 2008. [Google Scholar]
- 19.BIOMEDCACHE 6.1 Windows, BioMedCache. Fujitsu America Inc; 2003. [Google Scholar]
- 20.Goto H, Osawa E. J Chem Soc, Perkin Trans 2: Phys Org Chem. 1993;2:187. [Google Scholar]
- 21.Motohashi N, Wakabayashi H, Kurihara T, Fukushima H, Yamada T, Kawase M, Sohara Y, Tani S, Shirataki Y, Sakagami H, Satoh K, Nakashima H, Molnár A, Spengler G, Gyémánt N, Ugocsai K, Molnár J. Phytother Res. 2004;18:212. doi: 10.1002/ptr.1426. [DOI] [PubMed] [Google Scholar]
- 22.Das U, Kawase M, Sakagami H, Ideo A, Shimada J, Molnár J, Baráth Z, Bata Z, Dimmock JR. Bioorg Med Chem. 2007;15:3373. doi: 10.1016/j.bmc.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Sekine T, Takahashi J, Nishishiro M, Arai A, Wakabayashi H, Kurihara T, Kobayashi M, Hashimoto K, Kikuchi H, Katayama T, Kanda Y, Kunii S, Motohashi N, Sakagami H. Anticancer Res. 2007;27:133. [PubMed] [Google Scholar]
- 24.Stables JP, Kupferberg HJ. In: Molecular and Cellular Targets for Antiepileptic Drugs. Vanzini G, Tanganelli P, Avoli M, editors. John Libbey and Company Ltd; London: 1997. pp. 191–198. [Google Scholar]
- 25.Das U, Gul HI, Alcorn J, Shrivastav A, George T, Sharma RK, Nienaber KH, De Clercq E, Balzarini J, Kawase M, Kan N, Tanaka T, Tani S, Werbovetz KA, Yakovich AJ, Manavathu EK, Stables JP, Dimmock JR. Eur J Med Chem. 2006;41:577. doi: 10.1016/j.ejmech.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Dimmock JR, Das U, Gul HI, Kawase M, Sakagami H, Baráth Z, Ocsovsky I, Molnár J. Bioorg Med Chem. 2005;15:1633. doi: 10.1016/j.bmcl.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 27.Pati HN, Das U, Quail JW, Kawase M, Sakagami H, Dimmock JR. Eur J Med Chem. 2008;43:1. doi: 10.1016/j.ejmech.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]