Abstract
Mediators involved in the generation of pain in patients with cancer are poorly understood. Using a combined molecular, pharmacologic, behavioral, and genetic approach, we have identified a novel mechanism of cancer-dependent allodynia induced by protease-activated receptor 2 (PAR2). Here we show that human head and neck carcinoma cells have increased levels of proteolytic activity compared to normal human cell controls. Supernatant from human carcinoma cells, but not controls, caused marked and prolonged mechanical allodynia in mice, when administered into the hindpaw. This nociceptive effect was abolished by serine protease inhibition, diminished by mast cell-depletion and absent in PAR2-deficient mice. In addition, non-contact co-culture of trigeminal ganglion neurons with human head and neck carcinoma cells increased the proportion of neurons that exhibited PAR2-immunoreactivity. Our results point to a direct role for serine proteases and their receptor in the pathogenesis of cancer pain. This previously unrecognized cancer pain pathway has important therapeutic implications wherein serine protease inhibitors and PAR2 antagonists may be useful for the treatment of cancer pain.
Introduction
Pain is one of the problems that patients suffering with cancer fear most [16]. Cancer often produces severe pain and dysfunction secondary to mechanical hypersensitivity in humans [7,34,42,52]. Following the rapid development of opioid tolerance there are no pharmacologic agents available to treat intractable cancer pain. Given that cancer is often incurable, yet can cause significant pain, research should be focused on the management of cancer pain and improving quality of life. A major obstacle to the effective treatment of cancer pain is that the nociceptive mediators and their mechanisms of action are unknown. Nociceptive mediators secreted by the cancer and inflammatory cells within the cancer microenvironment are proposed to sensitize and activate primary afferents leading to pain [12,18,39]. Metabolic products of arachidonic acid are produced by a number of different cancer types, including head and neck squamous cell carcinoma (SCC) [29,57], and are well known to sensitize nociceptive primary afferents [6,58,59]. While medications including nonsteroidal anti-inflammatory drugs (NSAIDs) and cyclo-oxygenase-2 (COX-2) inhibitors prevent the production of arachidonic acid metabolites, these medications are often ineffective in alleviating cancer pain [41,55]. The poor efficacy of these drugs suggests that other peripheral nociceptive mediators contribute to the extreme intensity of cancer pain.
We sought to determine whether mediators released by human head and neck cancer cells produce hypersensitivity symptoms (mechanical allodynia) in animals that may occur in humans secondary to cancer pain. We focused our attention on proteases and their receptors because carcinomas and associated inflammatory cells (e.g. mast cells) in the cancer microenvironment produce and secrete proteases that are critical for carcinogenesis [2,61]. These proteases might act through protease-activated receptors (PARs), a family of G-protein coupled receptors (PAR1–4), whose activation by proteases expose a tethered ligand that binds peptide residues and initiates signal transduction [38,48,54]. PAR2 is of particular interest in peripheral nociception because it is expressed on nociceptive afferents, is preferentially activated by trypsin and related serine proteases, including mast cell tryptase, and its activation leads to the release of substance P (SP), which produces hyperalgesia [8,44,48,51]. However, the role of proteases and PAR2 in cancer pain is not known. Here we show evidence for a crucial role for serine proteases released by human head and neck cancer cells as mediators that generate hypersensitivity symptoms via a PAR2-dependent mechanism.
Methods
Cell culture
The supernatant of the human malignant head and neck SCC (HSC-3, ATCC, Manassas, VA) cell line was compared to the supernatant of the human normal oral keratinocyte (NK) cell line. The NK cell, the normal counterpart to the SCC cell, was chosen as the control: (1) to reduce the influence of normal cellular by-products in the supernatant and since (2) its proliferation in benign states such as squamous papillomas does not result in clinical pain behaviors. The human SCC and NK cell lines were cultured at 37°C with 5% CO2. Both cell lines were grown to confluence and then washed to remove all unattached cells. The media for both SCC and NK cell lines were replaced with Defined Keratinocyte–Serum Free Media (SFM) and then further incubated at 37°C with 5% CO2 for 72 hours prior to the protease activity, mast cell activity, or nociceptive behavioral assays.
Protease activity
Protease activity levels of supernatants derived from SCC and NK cultures were determined using a PDQ Protease Assay Kit (Athena Enzyme Systems) and microplate absorbance reader (Model 680, Bio-Rad Laboratories). The role of serine proteases, matrix metalloproteases, trypsin and tryptase in SCC-induced proteolysis was investigated by pre-incubating the SCC supernatant with a serine protease inhibitor (FUT-175, 50 μg/ml, BD Biosciences, San Jose, CA), matrix metalloprotease inhibitor (GM 6001, 100nM, Sigma-Aldrich, St. Louis, MO), soybean trypsin inhibitor (SBTI, 10μg/ml, Sigma-Aldrich, St. Louis, MO) or tryptase inhibitor (APC 366, 300μM, Tocris Bioscience, Ellisville, MO), respectively, for 30 minutes prior to performing the protease assay. The concentrations of the above protease inhibitors were selected based on their inhibitory efficacies in previous studies [5,20,23]. Standard trypsin reaction buffer (10mM Tris-HCl, pH 8.0, 400 μl) and supernatant test solution (SCC or NK supernatant, 100 μl) were added to vials containing the substrate matrix, the vials sealed tightly, and incubated at 37°C for 3 hours. To stop the reaction, 500 μl of 0.2N NaOH was added to each vial. The absorbance at 450 nm of the aqueous phase was measured spectrophotometrically. The proteolytic activity of the SCC and NK supernatants were standardized to the activity generated by known concentrations of trypsin.
Mouse cancer nociceptive models
Most experiments except where noted were performed on adult female wild-type (PAR2+/+) C57BL/6 mice (6–8 weeks old, Charles River Laboratories, Hollister, CA). The mice were housed in a temperature-controlled room on a 12:12 h light cycle with lights on from 07:00 to 19:00, with unrestricted access to food and water. Estrous cycles were not monitored. All procedures adhered to the guidelines of the Committee for Research and Ethical Issues of IASP [63] and were approved by the UCSF Committee on Animal Research, and researchers were trained under the Animal Welfare Assurance Program.
Proteases
Three separate groups of mice were used: SCC, NK and control (SFM) injection. In order to best ensure injection of standard supernatant concentrations of proteases, the supernatant from 5 confluent plates (3 mL/plate) pooled together after 72 hours of incubation (15 mL total volume) were used for all injection procedures. For the SCC group (n=5) and NK group (n=5), 50 μL of supernatant was deposited into the right hindpaw of each mouse using a 25-gauge, 5/8 in. long needle (Becton Dickinson & Co., Franklin Lakes, NJ). The same 50 μL volume of SFM was injected into the right paw of the control group (n=5). All three groups of mice were administered general anesthesia with isoflurane (Summit Medical Equipment Company, Bend, OR) for the injection. The role of serine proteases and matrix metalloproteases in SCC-induced nociception was next investigated by pre-incubating the SCC supernatant with FUT-175 (50 μg/ml) or GM 6001 (100nM), respectively, for 30 minutes prior to intraplantar injection (n=5/group). To determine whether the anti-nociceptive effect of FUT-175 is specific to carcinoma-associated serine protease inhibition, an additional set of experiments compared injection of FUT-175 (50 μg/ml) alone, injection of the non-protease noxious irritant capsaicin (0.1%, Calbiochem, La Jolla, CA) alone, and co-injection of FUT-175 (50 μg/ml) with capsaicin (0.1%).
PAR2
Adult PAR2-deficient (PAR2 -/-) mice (6-8 weeks old), originally from Jackson Laboratory (Bar Harbor, ME), were established on a C57BL/6 background together with their wild-type littermates at the UCSF Animal Care facility. In order to assess if the mechanism of carcinoma-induced nociception was trypsin and PAR2-dependent, 50 μL of human SCC supernatant alone or SCC supernatant pre-incubated with SBTI (10μg/ml) was injected into the right hindpaw of female wild-type (PAR2+/+) littermate mice (n=5/group). The nociceptive behavioral responses were then compared to responses in PAR2-deficient (PAR2 -/-) littermate mice (n=5). Wild-type littermates and PAR2-deficient mice were age- and sex-matched.
Mast cells
The contribution of tryptase and mast cells to carcinoma-induced nociception was investigated in two separate groups of mice: human SCC supernatant pre-incubated with APC (300μM) in naive mice and SCC supernatant in mast cell-depleted mice (n=5/group). Mast cell depletion was achieved by repeated administration of compound 48/80 (Sigma-Aldrich, St. Louis, MO) as described previously [31]. Compound 48/80 (0.1% w/v in saline) was administered intraperitoneally each morning and evening for 4 days. The dose used was 0.6 mg/kg for the first six injections and 1.2 mg/kg for the last two injections. On the fifth day, 50 μL of human SCC supernatant was injected into the right hindpaw of these mast cell-depleted mice. Following assessment of mechanical withdrawal-thresholds, mast cell-depleted mice were euthanized and specimens harvested from the left and right hindpaw skin for histological confirmation of mast cell-depletion using the protocol described below (data not shown).
Behavioral testing of paw withdrawal threshold
Testing was performed by an observer blinded to the experimental groups during the afternoon portion of the circadian cycle (07:00–19:00 h) only between 14:00 and 17:00. Mice were placed in a plastic cage with a wire mesh floor which allowed access to the paws. The cage consists of 6 cubicles and allows for a maximum of 6 mice to be tested consecutively. Mice were placed into the cage starting at the left-most cubicle and proceeding to the right. One hour was allowed for cage exploration and grooming activities prior to testing. The mice were then tested in the sequence that they were loaded into the cage. The area tested was the mid-plantar right hindpaw using an electronic von Frey anesthesiometer (IITC Life Sciences) as we have previously described [21]. A positive response was noted if the paw was sharply withdrawn and if there was an immediate flinching upon application of an increasing force with the electronic von Frey rigid probe tip. The withdrawal threshold was defined as the force (g) that was sufficient to elicit the above withdrawal response. The mechanical stimuli were presented at least 1 min apart to allow resolution of previous stimuli. Once testing for a mouse was complete the mouse in the adjacent cubicle was tested. The baseline paw withdrawal threshold (g; average of three trials) was measured 30 and 15 min prior to injection. Supernatant (SCC or NK) or SFM (50 μL) was then injected in the mid-plantar right hind paw of each animal. Paw withdrawal thresholds (g) were then measured every 5, 10, 15, 30, 45, 60, 90, 120 and 180 min post-injection and compared to the baseline paw withdrawal threshold for each animal prior to injection.
Mast Cell Activity
Human SCC supernatant-induced changes in mast cell number and activity in the mouse hindpaw were assessed with a similar method as described previously [2]. For this purpose, two separate groups of mice were studied: human SCC and control (NK) supernatant injection. For the SCC (n=15) and control (n=15) groups, 50 μL of human SCC or NK supernatant was injected into the right hindpaw of each mouse using the same procedure as in the above mouse cancer nociceptive models. Mice were then euthanized at different time points (15, 45 and 180 minutes post-injection) and a specimen of the right hindpaw skin was obtained and fixed in chloroform-free Carnoy's fixative (American Master Tech Scientific, Lodi, CA) to ensure the preservation of the glycosaminoglycan binding sites of the different types of granules corresponding to the different mast cell phenotypes. Following fixation, three samples of each specimen were obtained by cross-sectioning at equidistant points. All the samples were embedded in paraffin and serially sectioned at a thickness of 6 μm. After brief rehydration in distilled water, sections were stained with 0.1% Alcian Blue 8GX (Fisher Scientific, Pittsburgh, PA) in 0.7 N HC1 for 30 min, rinsed in 0.7 N HC1 and further stained for 2 min in 0.5% Safranin O (Fisher Scientific, Pittsburgh, PA) in 0.125 N HCI [40]. The sections were then rinsed briefly in distilled water and taken rapidly through 70% ethanol and absolute ethanol into xylene. All stains were freshly prepared each day. Mast cell counts in the dermis were performed by direct counting at 200× magnification in 0.79 mm2 fields by an observer blinded to the experimental groups. For mast cell activity assessment, two categories of mast cells were considered: inactive and active. Safranin stains high-sulphated substances red whereas alcian blue stains low-sulphated heparin blue. The mast cells that stained uniformly red with safranin but did not stain with alcian blue were considered inactive, while those that stained with alcian blue alone or mixed alcian blue–safranin staining were considered active (Figure 3a).
Figure 3. Mast cells mediate human carcinoma-induced cancer pain.
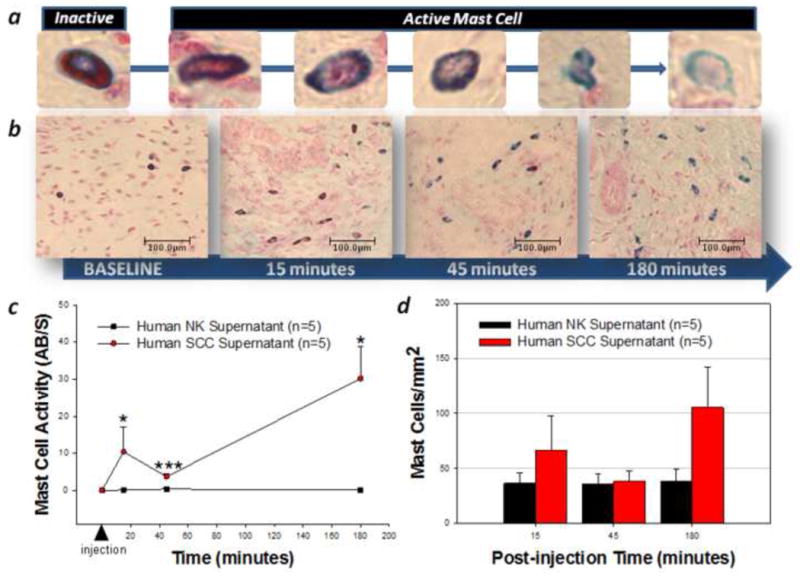
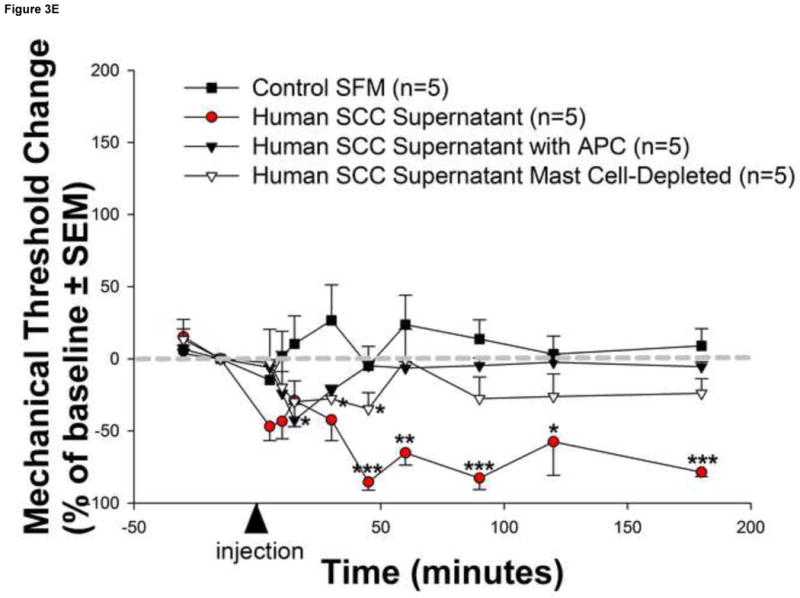
Human squamous cell carcinoma (SCC) supernatant-induced changes in mast cell number and activity in the mouse hindpaw were assessed with a differential Alcian Blue/Safranin staining method. Mast cells stained uniformly red with safranin but not with alcian blue are inactive, while those that stained with alcian blue alone or mixed are active (a). Mast cells were persistently activated by human SCC supernatant as depicted in (b) images (200× magnification) of representative sections from 5 distinct animals at 15, 45 and 180 minutes post-injection and (c) graphically as the ratio of active/non-active (AB/S) mast cells over time. However, there was no change in the total mast cell count in the dermis (P>0.05, RM ANOVA) (d). The magnitude and duration of human SCC supernatant–induced reduction in withdrawal-thresholds was attenuated in (e) mast cell-depleted mice (white triangles) as well as in naive mice injected with human SCC supernatant pre-incubated with the tryptase inhibitor APC (black triangles) (*P<0.05, **P<0.01, ***P<0.001, RM ANOVA, Bonferroni or Dunn's as appropriate).
Immunofluorescence
Human SCC
Human SCC cells were grown on cover slips at 37°C overnight. After the growth media was removed, the cells were washed with PBS and fixed in cold acetone for 10 min. Incubation with the primary rabbit polyclonal anti-trypsin antibody 1:500 (H-101, Santa Cruz Biotechnology, Santa Cruz, CA) was performed at room temperature for 2 h followed by the incubation with the secondary anti-rabbit Texas Red-conjugated antibody 1:500 (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 1 h at room temperature. The nuclei were stained with Hoechst 33342 (Molecular Probes, Carlsbad, CA). The cover slips were mounted on slides in Gel-Mount (Biomeda Corp., Foster City, CA) and visualized on a Nikon Eclipse E600 microscope using epifluorescence. The images were captured and analyzed with a RT Spot Camera and RT Spot Software (Diagnostics Instruments, Inc., Sterling Heights, MI). Controls for trypsin included pre-incubation of the primary antibody for 24h at 4°C with 20-fold excess immunogenic peptide (pre-absorption control) before staining, replacement of the primary antibody with similarly diluted normal rabbit serum, omission of the primary antibody and the omission of both primary and secondary antibodies during incubation.
Murine trigeminal ganglion and human SCC non-contact co-culture
Trigeminal ganglia were dissected aseptically from adult female wild-type C57BL/6 mice (6–8 weeks old, Jackson Laboratory) and washed with cold (4°C) modified Hanks' balanced salt solution (mHBSS). Ganglia were diced into small pieces and incubated in 3 ml mHBSS with 0.1% collagenase (Type XI-S, Sigma-Aldrich) for 20–40 min at 37 °C. Individual cells were dissociated by triturating them through a fine pipette, followed by a 10 min incubation at 37 °C with 10 μg/ml DNase I (Type IV, Sigma-Aldrich) in DMEM/F-12 (1:1) medium (Invitrogen Corp.). Cells were then centrifuged three times with DMEM/F-12 for 5 min at 1500 rpm. Cells were plated on poly-D-lysine-coated glass coverslips and cultured in DMEM/F-12 supplemented with 10% fetal bovine serum for 48 h prior to co-culture with human SCC. For the non-contact co-culture, a coverslip with pre-plated murine trigeminal neurons was combined in one non-treated culture dish with a coverslip of pre-plated human SCC cells. Cells were maintained in 3 ml of fresh DMEM/F-12 at 37°C in a humidified atmosphere of 5% CO2. The role of serine proteases, matrix metalloproteases, trypsin and tryptase in SCC modulation of trigeminal neuronal PAR2-immunoreactivity was investigated by incubating the co-cultures with FUT-175 (50 μg/ml), GM 6001 (100nM), SBTI (10μg/ml), or APC (300μM), respectively, in vitro for 48 h before use. No physical contact occurred between neurons and SCC cells during this time.
Following 48 h of non-contact co-culture, the trigeminal neurons were prepared with the same methodology as with the above human SCC immunofluorescence study. The murine trigeminal neurons were incubated with the primary rabbit polyclonal anti-PAR2 antibody 1:500 (H-99, Santa Cruz Biotechnology) at room temperature for 2 h followed by incubation with the secondary anti-rabbit Texas Red-conjugated antibody 1:500 (Jackson ImmunoResearch Laboratories) for 1 h at room temperature. The nuclei were stained with Hoechst 33342 (Molecular Probes) and the cover slips mounted on slides in Gel-Mount (Biomeda Corp.) and visualized on a Nikon Eclipse E600 microscope using epifluorescence. The PAR2-immunoreactivity for trigeminal neurons cultured alone for 48 h (control) was compared to that in non-contact co-culture with human SCC for 48 h by an observer blinded to the experimental groups using a methodology that we have previously described [21]. Briefly, the colored fluorescent images of trigeminal neurons were converted to grayscale using RT Spot Software and the fluorescence emitted by each trigeminal neuron was quantified by Scion Image software (Alpha version 4.0.3.2, Scion Corporation, Frederick, MD) as the average gray value per pixel in the selected trigeminal neuron. The gray value per pixel ranges between 0 and 256, with higher values indicating higher intensities of fluorescence. A value of 256 indicates that all of the pixels in the selected image are expressing maximum gray value. To prevent the skewing of data by using absolute values, fluorescence values were calculated as a percentage of 256. Only trigeminal neurons that did not overlap with other cells and had a visible nucleus were used for image analysis. Controls for PAR2 included the replacement of the primary antibody with similarly diluted normal rabbit serum, omission of the primary antibody and the omission of both primary and secondary antibodies. In addition, as a negative control, incubation of. trigeminal neurons cultured from adult female PAR2-deficient (PAR2 -/-) littermate mice (6–8 weeks old, Jackson Laboratory) with H-99 failed to demonstrate PAR2-immunoreactivity. Although the human SCC cells in the co-culture condition were maintained partially in neuronal medium and not entirely in the medium routinely used to prepare cells or supernatant for in vivo behavioral assays, we tested and determined that the change in medium did not alter their ability to produce mechanical allodynia in animals (data not shown).
Statistical analysis
Data are reported as mean±SE. RM ANOVA and RM ANOVA-on-ranks were used as appropriate (P<0.05 considered to reflect statistical significance).
Results
Human cancer cells produce and secrete high levels of proteases
To evaluate the potential role for proteases in cancer pain, we performed immunohistochemistry for the serine protease trypsin in human SCC and measured the proteolytic activity levels in the supernatant of these cells. Human SCC produces trypsin in abundance as evidenced by the homogeneous cytoplasmic trypsin-immunoreactivity in Figure 1a. These highly malignant human cancer cells produce and secrete proteases into the cancer microenvironment (Figure 1b-c). The proteolytic activity levels in the malignant cell supernatant (2.64±0.41 BAEE units/mL) was significantly elevated compared to human normal keratinocyte (NK) control supernatant (0.00±0.00 BAEE units/mL, P<0.01). The attenuation in human SCC-induced proteolysis by pre-incubation with GM 6001 (0.07±0.07 BAEE units/mL, P<0.01) and FUT-175 (0.35±0.14 BAEE units/mL, P<0.01) suggests that both matrix metalloproteases and serine proteases, respectively, contribute to this increased proteolytic activity. Trypsin and tryptase are candidate serine proteases inhibited by FUT-175 since pre-incubation with trypsin (SBTI, 0.31±0.08 BAEE units/mL, P<0.01) and tryptase (APC, 0.05±0.05 BAEE units/mL, P<0.01) inhibitors also attenuate SCC supernatant-induced proteolysis (RM ANOVA, Holm-Sidak).
Figure 1. Elevated levels of proteolytic activity in human cancer.
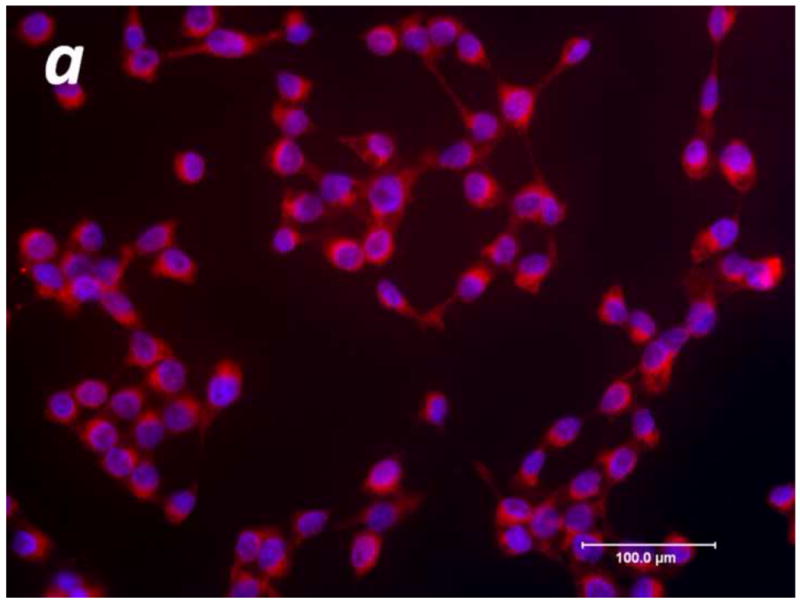
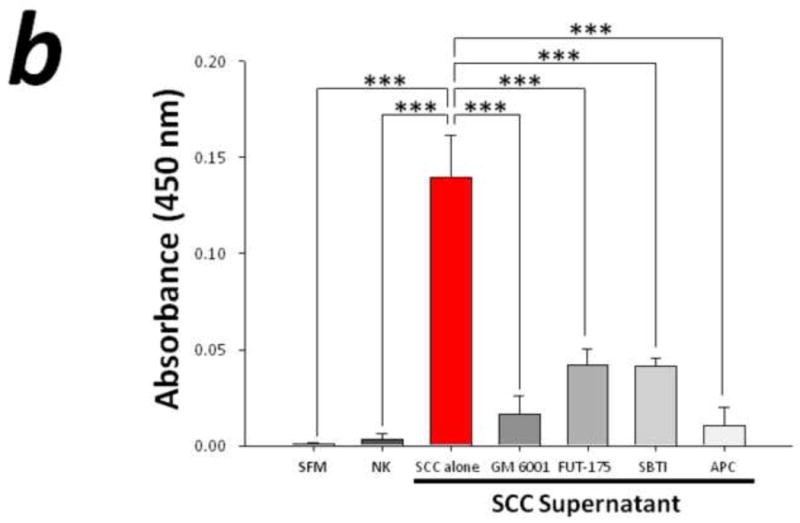
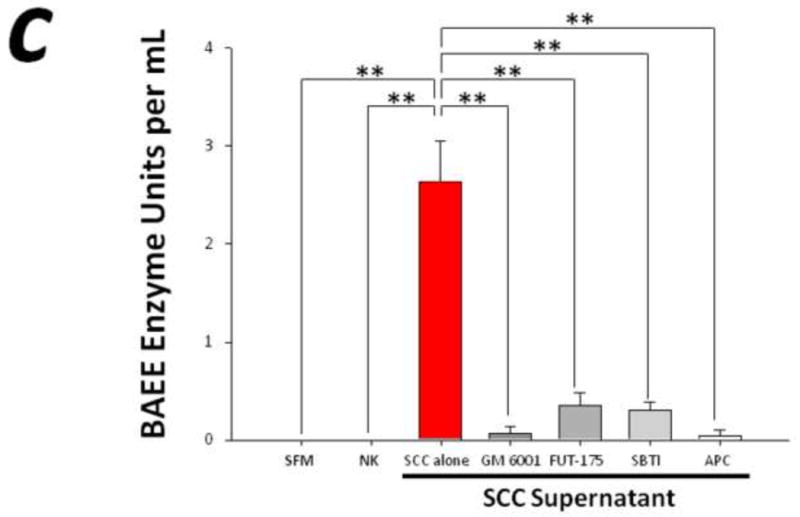
(a) Immunofluorescence of human squamous cell carcinoma (SCC) cells revealed homogeneous cytoplasmic trypsin staining, (b) and (c) Proteolytic activity of human SCC supernatant was markedly elevated compared to control serum free media (SFM) and human normal keratinocyte (NK) supernatant. Matrix metalloproteases and serine proteases contribute to this increased proteolytic activity as suggested by the attenuation in SCC-induced proteolysis by pre-incubation with GM 6001 and FUT-175, respectively. In particular, both trypsin and tryptase serine proteases significantly modulate SCC-induced proteolysis as demonstrated by pre-incubation with trypsin (SBTI) and tryptase (APC) inhibitors (**P<0.01, ***P<0.001, RM ANOVA, Holm-Sidak).
Serine proteases secreted by human cancer induce pain in a PAR2-dependent manner
In order to determine whether human cancer cells release proteases that produce cancer pain in patients, we examined the effects of human SCC supernatant on hindpaw withdrawal-thresholds in wild-type (PAR2+/+) mice. This experimental approach addresses the cancer-associated proteases that are necessary and sufficient to cause cancer pain and avoids confounding anatomic factors such as compression, vascular occlusion and cancer infiltration. Significant mechanical allodynia, as indicated by reduction in withdrawal-thresholds in response to mechanical stimulation, began at 45 minutes post-injection of human SCC supernatant and lasted the entire 3-hour observation period (Figure 2a). In contrast, the control human NK supernatant had no behavioral effect. There were no differences in baseline withdrawal-thresholds for mice in the control serum free media (SFM, 4.09±0.28 g), NK supernatant (4.65±0.69 g), and SCC supernatant (4.64±0.54 g) groups (P>0.05, RM ANOVA).
Figure 2. Human cancer cells induce serine protease and PAR2-dependent cancer pain.
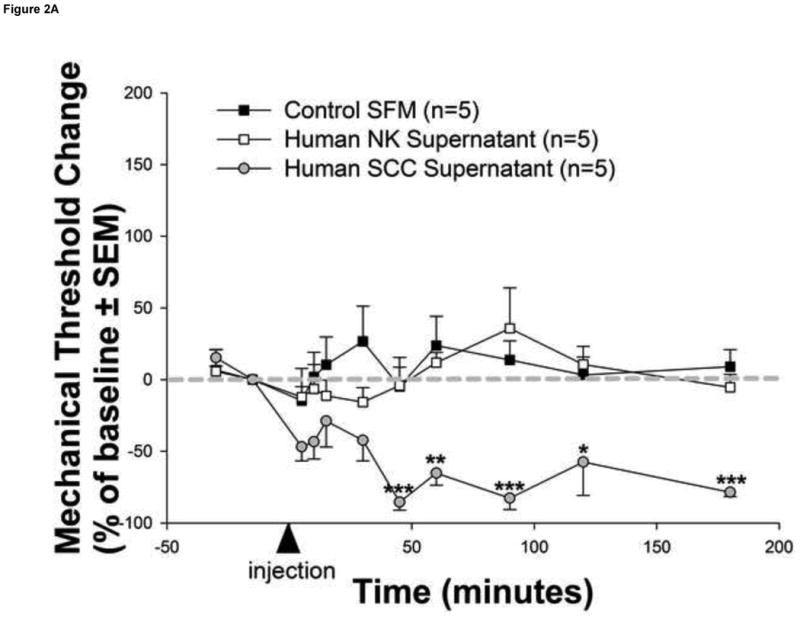
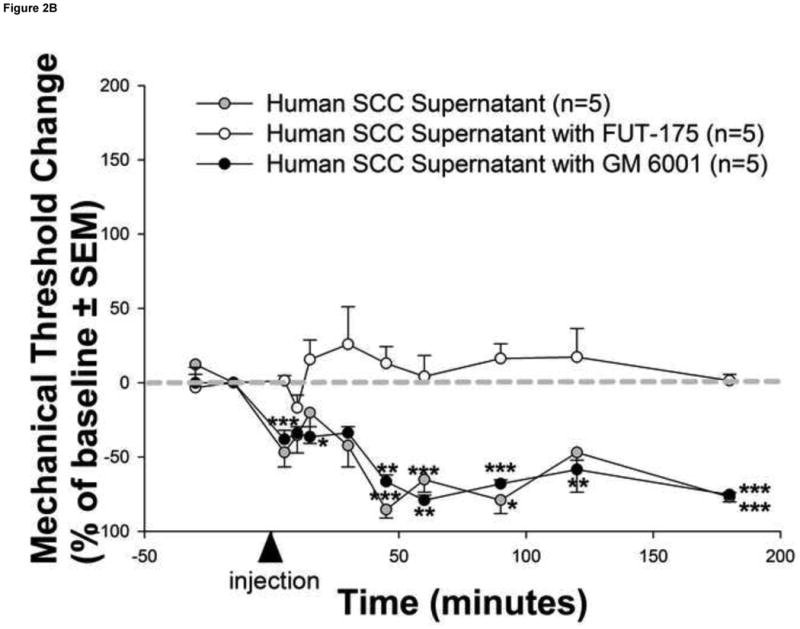
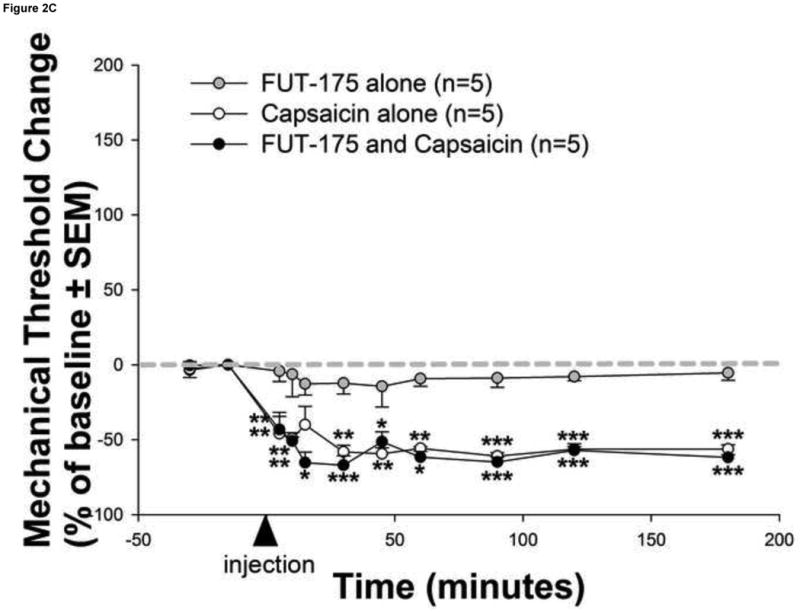
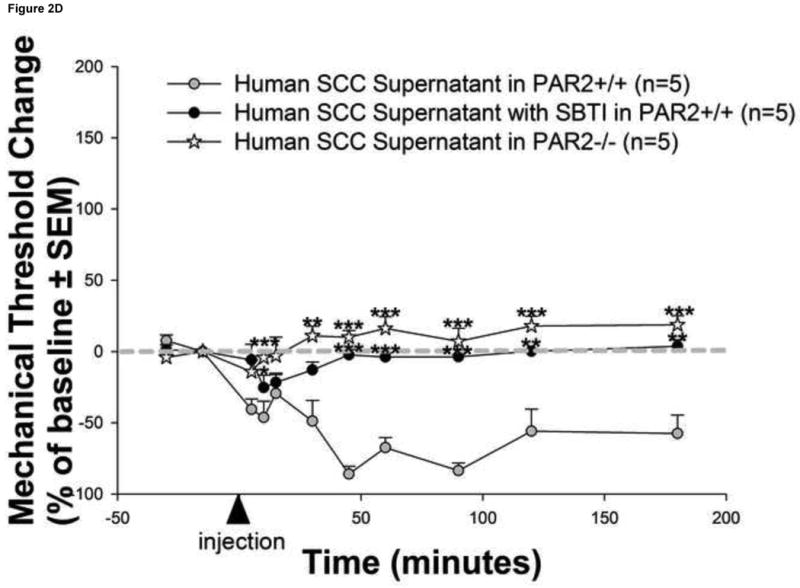
(a) Injection of human squamous cell carcinoma (SCC) supernatant into the hindpaw of mice produced marked and prolonged reductions in mechanical withdrawal-thresholds (grey circles). These nociceptive responses are mediated by serine proteases since (b) pre-incubation of human SCC supernatant with the serine protease inhibitor, FUT-175, abolished human SCC supernatant-induced reductions in withdrawal-thresholds (white circles). In contrast, pre-incubation with the matrix metalloprotease inhibitor, GM 6001, had no effect on SCC supernatant-induced reductions in withdrawal-thresholds (black circles). The anti-nociceptive effect of FUT-175 is specific to SCC-associated serine protease inhibition since (c) injection of FUT-175 alone into the hindpaw of mice did not affect baseline mechanical withdrawal-thresholds (grey circles) and, in contrast to its attenuation of SCC supernatant-induced mechanical allodynia, co-injection of FUT-175 with 0.1% capsaicin (black circles) did not affect capsaicin-induced reductions in mechanical withdrawal-thresholds (white circles). This cancer-induced nociceptive mechanism is trypsin and PAR2-dependent since (d) human SCC supernatant-induced mechanical withdrawal-threshold reductions were markedly attenuated when pre-incubated with trypsin inhibitor (SBTI) and injected in wild-type (PAR2+/+) littermate mice (black circles) and abolished when injected alone in PAR2-/- mice (white stars) (*P<0.05, **P<0.01, ***P<0.001, RM ANOVA, Holm-Sidak, Bonferroni or Dunn's as appropriate).
In order to assess whether the behavioral pain response was due to proteolytic activity in the supernatant, we repeated the experiments with the human SCC supernatant in the presence of the serine protease inhibitor FUT-175 or the matrix metalloprotease inhibitor GM 6001 (Figure 2b). Pre-incubation of human SCC supernatant with FUT-175 (50 μg/ml) prior to injection prevented human SCC-induced mechanical allodynia confirming the nociceptive effect of carcinoma-associated serine proteases. In contrast, pre-incubation of SCC supernatant with GM 6001 (100nM) prior to injection had no effect on SCC-induced mechanical allodynia. There were no differences in baseline withdrawal-thresholds for mice in SCC supernatant with FUT-175 (4.02±0.47 g), SCC supernatant with GM 6001 (4.06±0.24 g), and SCC supernatant alone (4.95±0.66 g) groups (P>0.05, RM ANOVA).
The anti-nociceptive specificity of FUT-175 to SCC-associated serine protease inhibition was further studied by examining FUT-175 effects on mechanical allodynia induced by the non-protease noxious irritant capsaicin (Figure 2c). Injection of FUT-175 (50 μg/ml) alone into the hindpaw of mice did not affect baseline mechanical withdrawal-thresholds confirming that FUT-175 does not possess anesthetic properties in naïve mice. Moreover, the finding that co-injection of FUT-175 (50 μg/ml) with capsaicin (0.1%) does not alter capsaicin-induced mechanical allodynia further confirms the anti-nociceptive specificity of FUT-175 to cancer-associated serine protease inhibition. There were no differences in baseline withdrawal-thresholds for mice in FUT-175 alone (3.82±0.16 g), capsaicin alone (4.11±0.14 g), and FUT-175/capsaicin (3.80±0.22 g) groups (P>0.05, RM ANOVA).
To confirm that the behavioral pain responses induced by human SCC supernatant were mediated specifically by PAR2 and not another receptor, we tested mechanical allodynia in mice lacking the gene encoding PAR2. In contrast to the prolonged reduction in mechanical withdrawal-thresholds in wild-type (PAR2+/+) littermate mice induced by human SCC supernatant, PAR2-deficient (PAR2-/-) mice were unaffected by human SCC supernatant suggesting a PAR2-dependent mechanism in the production of cancer pain (Figure 2d). In addition, this cancer-induced nociceptive mechanism is mediated by the carcinoma-associated serine protease trypsin since human SCC supernatant-induced mechanical withdrawal-threshold reductions were significantly attenuated when pre-incubated with SBTI (10μg/ml) prior to injection in wild-type (PAR2+/+) littermate mice. There were no differences in baseline withdrawal-thresholds in the SCC supernatant alone (4.01±0.23 g), SCC supernatant pre-incubated with SBTI (3.99±0.05 g) and PAR2-/- (3.87±0.34 g) groups (P>0.05, RM ANOVA).
Mast cell degranulation mediates human cancer-induced pain
Mast cells produce and secrete high levels of the serine protease tryptase into the cancer microenvironment when activated. We investigated whether mast cell activation contributes to cancer pain. We found that human SCC supernatant activates mast cells and induces mechanical allodynia that is attenuated in mast cell-depleted mice (Figure 3). Mast cell activation is significantly increased following injection of human SCC supernatant compared to control human NK supernatant and persists for the entire 3-hour observation period (Figure 3a-c). However, there was no change in the total mast cell count in the dermis (Figure 3d) suggesting that activation of resident dermal mast cells rather than recruitment of new mast cells contributes to cancer-induced pain. The duration and magnitude of human SCC supernatant–induced mechanical allodynia was significantly attenuated in mast cell-depleted mice At 60 minutes post-injection of human SCC supernatant, the withdrawal-thresholds were no longer different from baseline values suggesting that mast cell degranulation contributes to the maintenance of human SCC-induced mechanical allodynia (Figure 3e). Mast cell tryptase is the key mediator involved in this process since the duration and magnitude of SCC supernatant-induced mechanical allodynia was similarly attenuated in naive mice injected with SCC supernatant pre-incubated with the tryptase inhibitor APC (300μM). There were no differences in baseline withdrawal-thresholds for mice in the control SFM (4.09±0.28 g), SCC supernatant (4.64±0.54 g), SCC supernatant pre-incubated with APC (4.39±0.16 g), and SCC supernatant in mast cell-depleted (4.07±0.25 g) groups (P>0.05, RM ANOVA). Taken together, the above findings suggest that mast cell degranulation aggravates but is not necessary for human carcinoma-induced pain.
Chronic exposure to serine proteases secreted by human cancer up-regulates PAR2 levels in peripheral nerves
To determine whether mediators produced and secreted by the cancer leads to changes in gene expression in the neurons in the cancer microenvironment, a novel non-contact co-culture system of murine trigeminal ganglion neurons with human head and neck carcinoma cells was created. This unique experimental condition allowed us to study the cancer-associated mediators that are necessary and sufficient to modulate neuronal cell expression and avoids confounding factors associated with direct cell contact and cancer infiltration. While this assay does not represent all of the cellular components within the in vivo cancer microenvironment, it allows us to measure the effect of human cancer mediators on neuronal PAR2 expression in a controlled environment. Co-culture of trigeminal neurons with human SCC in vitro for 48 h increased the proportion of neurons expressing PAR2-immunoreactivity compared to trigeminal neurons cultured alone (81.9±1.9% vs. 32.4±2.0%, P<0.05, Figure 4). Cancer-associated serine proteases, trypsin in particular, likely contribute to increased neuronal PAR2-immunoreactivity since incubating the co-cultures with FUT-175 (50 μg/ml) or SBTI (10μg/ml) attenuated neuronal PAR2 expression (P<0.05, RM ANOVA-on-ranks, Tukey test). These results suggest that the persistent bombardment of sensory pain fibers supplying the cancer microenvironment by serine proteases up-regulates peripheral PAR2 on these sensory cells.
Figure 4. PAR2-immunoreactivity increased in trigeminal neurons co-cultured with human cancer cells.
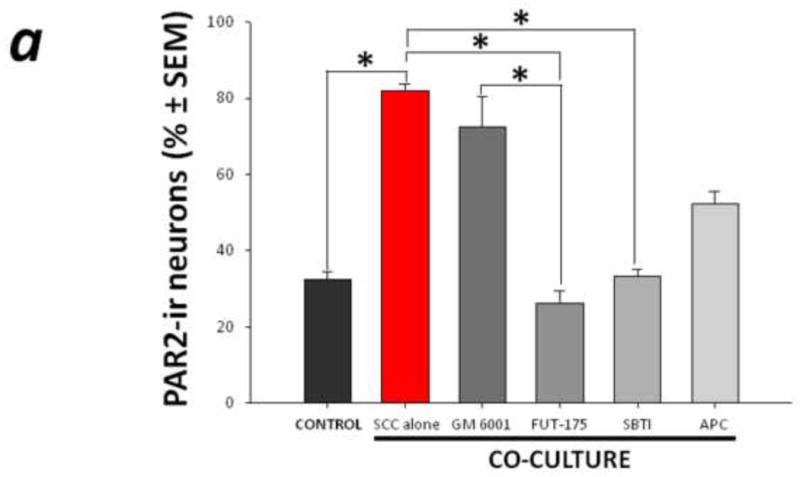
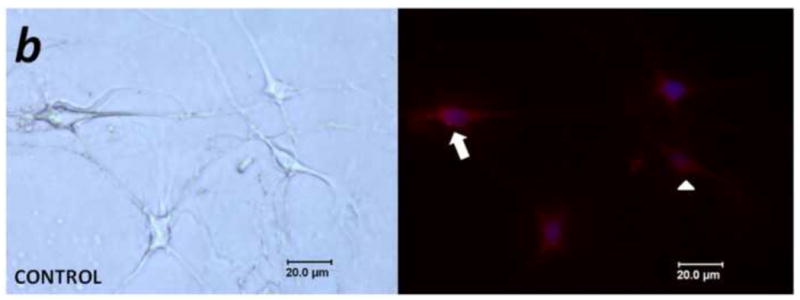
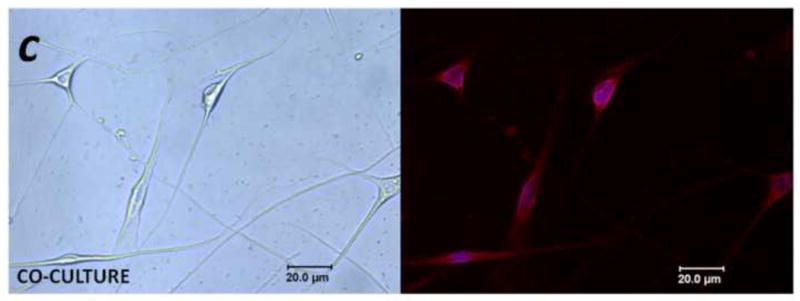
(a) Non-contact co-culture of trigeminal ganglion neurons with head and neck carcinoma cells (SCC) for 48 h increased the proportion of neurons that exhibited PAR2-immunoreactivity. The increased neuronal PAR2-immunoreactivity was attenuated in the presence of the serine protease inhibitor FUT-175 and trypsin inhibitor SBTI (*P<0.05, RM ANOVA-on-ranks, Tukey test). (b) and (c) Representative bright field (left panel) and corresponding PAR2 immunofluorescence (right panel) images of trigeminal ganglion neurons maintained in vitro for 48 h in control (b) and co-culture (c) conditions. In (b), arrow designates a PAR2-immunoreactive neuron, and the arrowhead indicates a non-labeled neuron.
Discussion
In this study we show that proteases are released in greater amounts from human cancer cells compared with human normal cells. Moreover we show that this elevated proteolytic activity is responsible for the generation of mechanical hypersensitivity through a mechanism involving the activation of PAR2. These results demonstrate that PAR2 represents an important novel receptor mediating cancer pain. Mast cells within the cancer microenvironment potentiate and prolong protease-induced cancer pain.
Proteases are critical to carcinogenesis and PAR2 mediates a number of these processes. We have now demonstrated the role of PAR2 in cancer pain. Cancer-associated trypsin has been identified in cancers such as ovarian carcinoma, pancreatic cancer, hepatocellular and cholangiocarcinomas, lung neoplasms, colorectal cancers, fibrosarcoma, erythroleukemia, gastric cancer and oral cancer [46]. Trypsin and mast cell tryptase promote cancer cell proliferation in melanoma, and esophageal, gastric and colon carcinoma via PAR2 activation on the cancer cells [10,26,46,61]. More recently, a similar mechanism of mast cell mediated-induction of cancer cell proliferation has been demonstrated in oral carcinomas [2]. Administration of trypsin or mast cell tryptase, both endogenous serine proteases, into peripheral tissues produces neurogenic inflammation, nocifensive behaviors and spinal Fos up-regulation in a PAR2-dependent manner [24,25,27,32,50,51,60]. If serine proteases such as trypsin from cancer cells or tryptase from mast cells are released into the cancer microenvironment, these proteases would likely contribute to cancer pain. While cancer cells are likely the primary source of proteases in the cancer microenvironment, other sources of locally secreted serine proteases amplify the pain-producing pathway. Epithelial cells surrounding the cancer are a likely source of additional trypsin [35]. Blood vessels surrounding cancers such as gastric carcinoma [36] express trypsin as do fibroblasts in the surrounding stroma of oral carcinoma [46]. Trypsin is also found in serum at nanomolar concentrations and may diffuse from blood to cancer cells. Elevated serum trypsin levels have been found in gastric carcinoma patients [26], suggesting that serum may be an important source of trypsin in some cancer patients.
In the present study, we show proteases capable of activating PAR2 on sensory neurons are secreted into the supernatants of human cancer cells. A variety of pain mediators including prostaglandins, ATP, bradykinin, cytokines, chemokines, nerve growth factor, and several vascular factors including endothelin 1 and vascular endothelial growth factor may be released by cancer and associated cells to either excite or sensitize nociceptive primary afferents [49]. We show here that of all the potential mediators released from cancer cells, serine proteases are the principle contributors to pain induced by mechanical stimulation since the serine-protease inhibitor FUT-175 abolished these nociceptive responses. Proteases might be less susceptible to degradation than other mediators in our study conditions and therefore account for most of the nociceptive effects we observed. The onset of significant mechanical allodynia induced by human cancer cell supernatant in the present study is in agreement with the delayed onset of mechanical allodynia evoked by injection of a PAR2-specific agonist into the mouse hindpaw demonstrated previously [8]. Although early mast cell degranulation occurs within 15 minutes following human SCC supernatant injection, appreciable mechanical allodynia may not develop following activation of PAR2 alone since as a G-protein coupled receptor, its sensitizing effect may be dependent upon functional interactions with other nociceptive receptors. Thus serine proteases might play a crucial role in cancer pain by sensitizing primary sensory neurons to other mediators. PAR2 activation has been shown to sensitize other peripheral nociceptive receptors such as TRPV1, TRPV4, NMDA and P2X3 [1,8,19,30,62]. In the case of cancer, this nociceptive pathway is especially likely and may provide an explanation for the apparent paradox in which the majority of hyperalgesia produced by PAR2 activation has been thought to be centrally mediated by prostaglandins [60] yet there is poor efficacy for NSAIDs and COX-2 inhibitors in alleviating cancer pain [41,55]. Recent evidence in which systemic administration of indomethacin, a non-selective COX inhibitor failed to alter trypsin-induced nociception in the mouse paw further supports this mechanism [50].
Mast cells degranulate when activated [43]. Our results point to mast cell activation and degranulation as a critical step in determining the level and duration of cancer pain. Injection of human carcinoma supernatants did not increase the number of mast cells in the cancer microenvironment but it did lead to increased activation and degranulation of resident dermal mast cells within the cancer microenvironment. In addition, our findings suggest a major role for mast cell activation in the pain response induced by human carcinoma supernatants. We used chronic compound 48/80 treatment to deplete mast cells of their contents which significantly attenuated the human carcinoma supernatant-induced mechanical allodynia. Thus one or more mast cell products may contribute to peripheral sensitization following human cancer cell release of serine proteases. Our results are in agreement with the recent finding of partial inhibition of trypsin-induced nociception with mast cell depletion [50]. Tryptase from mast cells in the cancer microenvironment, in addition to trypsin, likely activate PAR2 on primary afferents. The continual release of serine proteases from cancer and associated cells in the cancer microenvironment could produce ongoing excitation of primary nociceptive afferents and contribute to spontaneous pain and persistent mechanical allodynia in cancer patients. PAR2 has been shown to be up-regulated following various inflammatory and ischaemic insults [11,28,47,56] and in certain disease states including cancer [9,15,33,45]. Various mediators such as interleukin-1β, tumour necrosis factor-α, trypsin and specific PAR2 agonists have been demonstrated to up-regulate PAR2 [53]. Thus the persistent bombardment of nociceptive afferents supplying the cancer environment by serine proteases as well as other cancer-associated mediators may result in the up-regulation of peripheral PAR2 as suggested by our novel non-contact co-culture of trigeminal neurons with human carcinoma cells. PAR2 up-regulation may represent a phenotypic change in peripheral nociceptive afferents resulting in alterations in pain processing and the transition from acute to chronic cancer pain. Our results show for the first time that serine proteases such as trypsin from cancer cells and tryptase from mast cells, both of which can activate PAR2, contribute to cancer pain. The absence of human carcinoma supernatant-induced mechanical allodynia in mice lacking PAR2 clearly demonstrates the involvement of PAR2 in this cancer pain pathway and represent a potential analgesic target.
Our findings potentially have profound implications in the clinical management of cancer patients. Other than survival, the most important concern of many cancer patients is pain [37]. Pain is rated as the worst symptom by head and neck cancer patients [22] and the intensity of cancer pain significantly increases with disease progression, particularly during the final months of life [3]. While opiates may be initially effective in ameliorating cancer pain, they are associated with numerous adverse side effects, including tolerance [4] and immunosuppression [13,14]. Unfortunately, as the opiate dose escalates and tolerance develops, there are few, if any, alternative analgesic regimens available for intractable cancer pain. Naturally occurring PAR2 agonists such as trypsin and tryptase not only facilitate and perpetuate tissue damage and invasion during carcinogenesis but also contribute to cancer-induced pain by direct effects on PAR2 located on primary afferents. In this context, the strong inhibitory effect of a serine protease inhibitor on supernatant-evoked mechanical hypersensitivity seen in the present study would not only confirm the importance of protease activity in the development of cancer pain but also imply its therapeutic benefit in treatment of clinical cancer pain. The levels of intervention that can be envisaged for cancer pain therapy are drugs that target: (a) growth, development and migration of cancer and mast cells; (b) cancer or mast cell activation and secretion; (c) cancer or mast cell mediators. Since we have provided the first evidence that serine protease inhibitors produce antinociception, the targeting of specific serine proteases such as trypsin and mast cell tryptase in the cancer microenvironment may be a novel approach to the treatment of cancer pain. In addition, the targeting of peripheral PAR2 may provide a non-opioid rationale for pain therapy.
Acknowledgments
We thank L. Prentice and J. Zhang for technical assistance. This work was supported by a grant from the NIH/NIDCR R21 DE018561.
Footnotes
The authors declare no conflicts of interest for this study.
The results point to a direct role for serine proteases and the PAR2 receptor in the pathogenesis of cancer pain.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas-Lopez C, Vanner S, Vergnolle N, Bunnett NW. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cε- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575:555–571. doi: 10.1113/jphysiol.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aromando RF, Pérez MA, Heber EM, Trivillin VA, Tomasi VH, Schwint AE, Itoiz ME. Potential role of mast cells in hamster cheek pouch carcinogenesis. Oral Oncol. 2008;44:1080–1087. doi: 10.1016/j.oraloncology.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Bjordal K, Ahlner-Elmqvist M, Hammerlid E, Boysen M, Evensen JF, Biörklund A, Jannert M, Westin T, Kaasa S. A prospective study of quality of life in head and neck cancer patients. Part II: Longitudinal data. Laryngoscope. 2001;111:1440–1452. doi: 10.1097/00005537-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Brasseur L. Review of current pharmacologic treatment of pain. Drugs. 1997;53:10–17. doi: 10.2165/00003495-199700532-00005. [DOI] [PubMed] [Google Scholar]
- 5.Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, Sharkey KA, Ferraz JG, Shaffer E, Vergnolle N. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Tanner K, Levine JD. Mechanical sensitization of cutaneous C-fiber nociceptors by prostaglandin E2 in the rat. Neurosci Lett. 1999;267:105–108. doi: 10.1016/s0304-3940(99)00345-6. [DOI] [PubMed] [Google Scholar]
- 7.Connelly ST, Schmidt BL. Evaluation of pain in patients with oral squamous cell carcinoma. J Pain. 2004;5:505–510. doi: 10.1016/j.jpain.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, Tominaga M, Noguchi K. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J Neurosci. 2004;24:4293–4299. doi: 10.1523/JNEUROSCI.0454-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Andrea MR, Derian CK, Santulli RJ, Andrade-Gordon P. Differential expression of protease-activated receptors-1 and -2 in stromal fibroblasts of normal, benign, and malignant human tissues. Am J Pathol. 2001;158:2031–2041. doi: 10.1016/S0002-9440(10)64675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darmoul D, Marie JC, Devaud H, Gratio V, Laburthe M. Initiation of human colon cancer cell proliferation by trypsin acting at protease-activated receptor-2. British Journal of Cancer. 2001;85:772–779. doi: 10.1054/bjoc.2001.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dattilio A, Vizzard MA. Up-regulation of protease activated receptors in bladder after cyclophosphamide induced cystitis and colocalization with capsaicin receptor (VR1) in bladder nerve fibers. J Urol. 2005;173:635–639. doi: 10.1097/01.ju.0000143191.55468.1d. [DOI] [PubMed] [Google Scholar]
- 12.Diener KM. Bisphosphonates for controlling pain from metastatic bone disease. Am J Health Syst Pharm. 1996;53:1917–1927. doi: 10.1093/ajhp/53.16.1917. [DOI] [PubMed] [Google Scholar]
- 13.Eisenstein TK, Hilburger ME. Opioid modulation of immune responses: effects on phagocyte and lymphoid cell populations. J Neuroimmunol. 1998;83:36–44. doi: 10.1016/s0165-5728(97)00219-1. [DOI] [PubMed] [Google Scholar]
- 14.Eisenstein TK, Rogers TJ, Meissler JJ, Adler MW, Hilburger ME. Morphine depresses macrophage numbers and function in mouse spleens. Adv Exp Med Biol. 1998;437:33–41. doi: 10.1007/978-1-4615-5347-2_4. [DOI] [PubMed] [Google Scholar]
- 15.Ferrell WR, Lockhart JC, Kelso EB, Dunning L, Plevin R, Meek SE, Smith AJ, Hunter GD, McLean JS, McGarry F, Ramage R, Jiang L, Kanke T, Kawagoe J. Essential role for proteinase activated receptor-2 in arthritis. J Clin Invest. 2003;111:35–41. doi: 10.1172/JCI16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foley KM, Gelband H. Improving palliative care for cancer: Summary and recommendations. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 17.Fujii K, Imamura S. Cell surface proteolysis by serine proteinases enhances RGD-sensitive melanoma cell adhesion on fibrinogen and vitronectin. Exp Cell Res. 1995;220:201–211. doi: 10.1006/excr.1995.1307. [DOI] [PubMed] [Google Scholar]
- 18.Fujita T, Matsui M, Takaku K, Uetake H, Ichikawa W, Taketo MM, Sugihara K. Size- and invasion-dependent increase in cyclooxygenase 2 levels in human colorectal carcinomas. Cancer Res. 1998;58:4823–4826. [PubMed] [Google Scholar]
- 19.Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, Altier C, Cenac N, Zamponi GW, Bautista-Cruz F, Lopez CB, Joseph EK, Levine JD, Liedtke W, Vanner S, Vergnolle N, Geppetti P, Bunnett NW. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grobelny D, Poncz L, Galardy RE. Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeriginosa elastase by peptide hydroxamic acids. Biochemistry. 1992;31:7152–7154. doi: 10.1021/bi00146a017. [DOI] [PubMed] [Google Scholar]
- 21.Guerrero AV, Quang P, Dekker N, Jordan RC, Schmidt BL. Peripheral cannabinoids attenuate carcinoma-induced nociception in mice. Neurosci Lett. 2008;433:77–81. doi: 10.1016/j.neulet.2007.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammerlid E, Bjordal K, Ahlner-Elmqvist M, Boysen M, Evensen JF, Biörklund A, Jannert M, Kaasa S, Sullivan M, Westin T. A prospective study of quality of life in head and neck cancer patients. Part I: at diagnosis. Laryngoscope. 2001;111:669–680. doi: 10.1097/00005537-200104000-00021. [DOI] [PubMed] [Google Scholar]
- 23.He S, Aslam A, Gaça MD, He Y, Buckley MG, Hollenberg MD, Walls AF. Inhibitors of tryptase as mast cell-stabilizing agents in the human airways: effects of tryptase and other agonists of proteinase-activated receptor 2 on histamine release. J Pharmacol Exp Ther. 2004;309:119–126. doi: 10.1124/jpet.103.061291. [DOI] [PubMed] [Google Scholar]
- 24.Hoogerwerf WA, Zou L, Shenoy M, Sun D, Micci MA, Lee-Hellmich H, Xiao SY, Winston JH, Pasricha PJ. The proteinase-activated receptor 2 is involved in nociception. J Neurosci. 2001;21:9036–9042. doi: 10.1523/JNEUROSCI.21-22-09036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoogerwerf WA, Shenoy M, Winston JH, Xiao SY, He Z, Pasricha PJ. Trypsin mediates nociception via the proteinase-activated receptor 2: a potentially novel role in pancreatic pain. Gastroenterology. 2004;127:883–891. doi: 10.1053/j.gastro.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Ichikawa Y, Koshikawa N, Hasegawa S, Ishikawa T, Momiyama N, Kunizaki C, Takahashi M, Moriwaki Y, Akiyama H, Yamaoka H, Yanoma S, Tsuburaya A, Nagashima Y, Shimada H, Miyazaki K. Marked increase of trypsin(ogen) in serum of linitis plastica (gastric cancer, borrmann 4) patients. Clin Cancer Res. 2000;6:1385–1388. [PubMed] [Google Scholar]
- 27.Ishikura H, Nishimura S, Matsunami M, Tsujiuchi T, Ishiki T, Sekiguchi F, Naruse M, Nakatani T, Kamanaka Y, Kawabata A. The proteinase inhibitor camostat mesilate suppresses pancreatic pain in rodents. Life Sci. 2007;80:1999–2004. doi: 10.1016/j.lfs.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 28.Jin G, Hayashi T, Kawagoe J, Takizawa T, Nagata T, Nagano I, Syoji M, Abe K. Deficiency of PAR-2 gene increases acute focal ischemic brain injury. J Cereb Blood Flow Metab. 2005;25:302–313. doi: 10.1038/sj.jcbfm.9600021. [DOI] [PubMed] [Google Scholar]
- 29.Jung TT, Berlinger NT, Juhn SK. Prostaglandins in squamous cell carcinoma of the head and neck: a preliminary study. Laryngoscope. 1985;95:307–312. doi: 10.1288/00005537-198503000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Kawabata A, Kawao N, Itoh H, Shimada C, Takebe K, Kuroda R, Masuko T, Kataoka K, Ogawa S. Role of N-methyl-D-aspartate receptors and the nitric oxide pathway in nociception/hyperalgesia elicited by protease-activated receptor-2 activation in mice and rats. Neurosci Lett. 2002;329:349–353. doi: 10.1016/s0304-3940(02)00702-4. [DOI] [PubMed] [Google Scholar]
- 31.Kawabata A, Kuroda R, Minami T, Kataoka K, Taneda M. Increased vascular permeability by a specific agonist of protease-activated receptor-2 in rat hindpaw. Br J Pharmacol. 1998;125:419–422. doi: 10.1038/sj.bjp.0702063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelso EB, Lockhart JC, Hembrough T, Dunning L, Plevin R, Hollenberg MD, Sommerhoff CP, McLean JS, Ferrell WR. Therapeutic promise of proteinase-activated receptor-2 antagonism in joint inflammation. J Pharmacol Exp Ther. 2006;316:1017–1024. doi: 10.1124/jpet.105.093807. [DOI] [PubMed] [Google Scholar]
- 33.Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewar GA, Thompson PJ. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. 2001;108:797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- 34.Kolokythas A, Connelly ST, Schmidt BL. Validation of the University of California San Francisco oral cancer pain questionnaire. J Pain. 2007;8:950–953. doi: 10.1016/j.jpain.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koshikawa N, Hasegawa S, Nagashima Y, Mitsuhashi K, Tsubota Y, Miyata S, Miyagi Y, Yasumitsu H, Miyazaki K. Expression of trypsin by epithelial cells of various tissues, leukocytes, and neurons in human and mouse. Am J Pathol. 1998;153:937–944. doi: 10.1016/S0002-9440(10)65635-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koshikawa N, Nagashima Y, Miyagi Y, Mizushima H, Yanoma S, Yasumitsu H, Miyazaki K. Expression of trypsin in vascular endothelial cells. FEBS Lett. 1997;409:442–448. doi: 10.1016/s0014-5793(97)00565-6. [DOI] [PubMed] [Google Scholar]
- 37.List MA, Stracks J, Colangelo L, Butler P, Ganzenko N, Lundy D, Sullivan P, Haraf D, Kies M, Goodwin W, Vokes EE. How do head and neck cancer patients prioritize treatment outcomes before initiating treatment? J Clin Oncol. 2000;18:877–884. doi: 10.1200/JCO.2000.18.4.877. [DOI] [PubMed] [Google Scholar]
- 38.MacFarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptor. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- 39.Mantyh PW, Clohisy DR, Koltzenburg M, Hunt SP. Molecular mechanisms of cancer pain. Nature Rev Cancer. 2002;2:201–209. doi: 10.1038/nrc747. [DOI] [PubMed] [Google Scholar]
- 40.Mayrhofer G. Fixation and staining of granules in mucosal mast cells and intraepithelial lymphocytes in the rat jejunum with specimen reference to the relationship between the acid glycosaminoglycans in the two cell types. Histochem J. 1980;12:513–526. doi: 10.1007/BF01011925. [DOI] [PubMed] [Google Scholar]
- 41.Mercadante S, Portenoy RK. Opioid poorly-responsive cancer pain. Part 3. Clinical strategies to improve opioid responsiveness. J Pain Symptom Manage. 2001;21:338–354. doi: 10.1016/s0885-3924(01)00250-0. [DOI] [PubMed] [Google Scholar]
- 42.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69:1–18. doi: 10.1016/s0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- 43.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 44.Noorbakhsh F, Vergnolle N, Hollenberg MD, Power C. Proteinase-activated receptors in the nervous system. Nat Rev Neurosci. 2003;4:981–990. doi: 10.1038/nrn1255. [DOI] [PubMed] [Google Scholar]
- 45.Noorbakhsh F, Vergnolle N, McArthur JC, Silva C, Vodjgani M, Andrade-Gordon P, Hollenberg MD, Power C. Proteinase activated receptor-2 induction by neuroinflammation prevents neuronal death during HIV infection. J Immunol. 2005;174:7320–7329. doi: 10.4049/jimmunol.174.11.7320. [DOI] [PubMed] [Google Scholar]
- 46.Nyberg P, Ylipalosaari M, Sorsa T, Salo T. Trypsins and their role in carcinoma growth. Exp Cell Res. 2006;312:1219–1228. doi: 10.1016/j.yexcr.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 47.Nystedt S, Ramakrishnan V, Sundelin J. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells. Comparison with the thrombin receptor. J Biol Chem. 1996;271:14910–14915. doi: 10.1074/jbc.271.25.14910. [DOI] [PubMed] [Google Scholar]
- 48.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 49.Pacharinsak C, Beitz A. Animal models of cancer pain. Comp Med. 2008;58:220–233. [PMC free article] [PubMed] [Google Scholar]
- 50.Paszcuk AF, Quintão NL, Fernandes ES, Juliano L, Chapman K, Andrade-Gordon P, Campos MM, Vergnolle N, Calixto JB. Mechanisms underlying the nociceptive and inflammatory responses induced by trypsin in the mouse paw. Eur J Pharmacol. 2008;581:204–215. doi: 10.1016/j.ejphar.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 51.Patwardhan AM, Diogenes A, Berg KA, Fehrenbacher JC, Clarke WP, Akopian AN, Hargreaves KM. PAR-2 agonists activate trigeminal nociceptors and induce functional competence in the delta opioid receptor. Pain. 2006;125:114–124. doi: 10.1016/j.pain.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81:129–134. doi: 10.1016/s0304-3959(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 53.Ritchie E, Saka M, Mackenzie C, Drummond R, Wheeler-Jones C, Kanke T, Plevin R. Cytokine upregulation of proteinase-activated-receptors 2 and 4 expression mediated by p38 MAP kinase and inhibitory kappa B kinase beta in human endothelial cells. Br J Pharmacol. 2007;150:1044–1054. doi: 10.1038/sj.bjp.0707150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidlin F, Bunnett NW. Protease-activated receptors: how proteases signal to cells. Curr Opin Pharmacol. 2001;1:575–582. doi: 10.1016/s1471-4892(01)00099-6. [DOI] [PubMed] [Google Scholar]
- 55.Sorge J. The lesson from cancer pain. Eur J Pain. 2000;4:3–7. [PubMed] [Google Scholar]
- 56.Striggow F, Riek-Burchardt M, Kiesel A, Schmidt W, Henrich-Noack P, Breder J, Krug M, Reymann KG, Reiser G. Four different types of protease-activated receptors are widely expressed in the brain and are upregulated in hippocampus by severe ischemia. Eur J Neurosci. 2001;14:595–608. doi: 10.1046/j.0953-816x.2001.01676.x. [DOI] [PubMed] [Google Scholar]
- 57.Sumitani K, Kamijo R, Toyoshima T, Nakanishi Y, Takizawa K, Hatori M, Nagumo M. Specific inhibition of cyclooxygenase-2 results in inhibition of proliferation of oral cancer cell lines via suppression of prostaglandin E2 production. J Oral Pathol Med. 2001;30:41–47. doi: 10.1034/j.1600-0714.2001.300107.x. [DOI] [PubMed] [Google Scholar]
- 58.Taiwo YO, Goetzl EJ, Levine JD. Hyperalgesia onset latency suggests a hierarchy of action. Brain Res. 1987;423:333–337. doi: 10.1016/0006-8993(87)90858-4. [DOI] [PubMed] [Google Scholar]
- 59.Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neurosci. 1989;32:577–580. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- 60.Vergnolle N, Bunnett NW, Sharkey KA, Brussee V, Compton SJ, Grady EF, Cirino G, Gerard N, Basbaum AI, Andrade-Gordon P, Hollenberg MD, Wallace JL. Proteinase-activated receptor-2 and hyperalgesia: A novel pain pathway. Nat Med. 2001;7:821–826. doi: 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- 61.Yoshii M, Jikuhara A, Mori S, Iwagaki H, Takahashi HK, Nishibori M, Tanaka N. Mast cell tryptase stimulates DLD-1 carcinoma through prostaglandin and MAP kinase-dependent manners. J Pharmacol Sci. 2005;98:450–458. doi: 10.1254/jphs.fpj05002x. [DOI] [PubMed] [Google Scholar]
- 62.Zhu WJ, Dai Y, Fukuoka T, Yamanaka H, Kobayashi K, Obata K, Wang S, Noguchi K. Agonist of proteinase-activated receptor 2 increases painful behavior produced by alpha, beta-methylene adenosine 5′-triphosphate. Neuroreport. 2006;17:1257–1261. doi: 10.1097/01.wnr.0000230518.31833.5d. [DOI] [PubMed] [Google Scholar]
- 63.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


