Abstract
The Src family kinases (SFKs) have been proposed to play stimulatory and inhibitory roles in platelet activation. The mechanisms for these apparently contradictory roles are unclear. Here we show that SFK, mainly Lyn, is important in stimulating a common signaling pathway leading to secretion of platelet granules. Lyn knock-out or an isoform-nonselective SFK inhibitor, PP2, inhibited platelet secretion of both dense and α granules and the secretion-dependent platelet aggregation induced by thrombin, collagen, and thromboxane A2. The inhibitory effect of Lyn knock-out on platelet aggregation was reversed by supplementing granule content ADP, indicating that the primary role of Lyn is to stimulate granule secretion. Inhibitory effect of PP2 on platelet aggregation induced by thrombin and thromboxane A2 were also reversed by supplementing ADP. Furthermore, PP2 treatment or Lyn knock-out diminished agonist-induced Akt activation and cyclic GMP production. The inhibitory effect of PP2 or Lyn knock-out on platelet response can be corrected by supplementing cyclic GMP. These data indicate that Lyn stimulates platelet secretion by activating the phosphoinositide 3-kinase-Akt-nitric oxide (NO)-cyclic GMP pathway and also provide an explanation why Lyn can both stimulate and inhibit platelet activation.
Keywords: ADP, Akt PKB, Collagen, Platelet, Secretion, Thrombin
Introduction
Platelets play a critical role in thrombosis and hemostasis. At sites of vascular injury, platelets are activated by adhesive proteins, such as collagen and von Willebrand factor, and soluble platelet agonists such as thrombin, thromboxane A2 (TXA2),2 and ADP (1). Collagen-induced platelet responses can be induced by multiple collagen receptors, including glycoprotein VI (GPVI)/Fc receptor γ (FcRγ) complex, integrin α2β1, glycoprotein IV, etc. (2, 3). GPVI/FcRγ signals via the immunoreceptor tyrosine-based activation motif (ITAM)-Syk signaling pathway, and GPVI signaling can be stimulated by GPVI-selective agonists, collagen-related peptide (CRP), and convulxin (4–6). von Willebrand factor activates platelets via the platelet glycoprotein Ib-IX (GPIb-IX)-mediated signaling. Soluble agonists activate platelets mainly via G-protein-coupled receptor (GPCR) signaling pathways. Among GPCRs, thrombin receptors (protease-activated receptors 1 and 4) and TXA2 receptor signal mainly via the Gq and G13-coupled pathways (7–12). Platelet activation includes a series of rapid positive feedback loops that greatly amplify activation signals and enable robust platelet recruitment and stabilization of thrombi at the site of vascular injury. An important mechanism of this response amplification is the secretion of granule contents, which is required for full platelet responses induced by low concentrations of agonists or “weak” agonists. One of the important substances secreted from dense granules is ADP, which induces integrin activation and platelet aggregation mainly through the P2Y1 (Gq-coupled) and P2Y12 (Gi-coupled) ADP receptors (13). The signaling pathways leading to platelet granule secretion are not totally clear but are believed to involve protein kinase C and calcium-dependent signaling pathways and require the stimulation of formation of the SNARE complex, which mediates fusion between granules (vesicles) and plasma membranes (14, 15). We have recently discovered that platelet granule secretion can be stimulated via the phosphoinositide 3-kinase (PI3K)-Akt-nitric oxide (NO)-cGMP pathway (16–20). It has also been shown that granule secretion in leukocytes and neuronal cells can also be stimulated via the NO-cGMP pathway (21–23).
Src family kinases (SFKs) are a group of closely related nonreceptor protein tyrosine kinases. There are at least six different Src family tyrosine kinases that are expressed in platelets, Fgr, Fyn, Lck, Lyn, Src, and Yes (24). The roles of SFK in platelet activation have been complex and controversial. For example, c-Src binds to the cytoplasmic domain of the integrin β3 subunit and plays an important role in integrin αIIbβ3-dependent outside-in signaling (25–28). On the contrary, Lyn was reported to inhibit integrin outside-in signals in platelets (29). In the GPIb-IX pathway, however, Lyn has been shown to play stimulatory roles in the GPIb-IX-mediated early signaling, leading to integrin activation and integrin-dependent stable adhesion to von Willebrand factor under shear stress (30–33). Lyn and Fyn have been shown to constitutively bind to the cytoplasmic domain of collagen receptor, GPVI (34, 35), and are involved in the collagen/GPVI-induced tyrosine phosphorylation of the FcRγ ITAM, leading to activation of the tyrosine kinase Syk and its downstream enzymes (36). However, Lyn knock-out platelets have also been shown to potentiate platelet aggregation and secretion induced by GPVI-selective agonists (37), suggesting the dual roles of Lyn in GPVI-mediated platelet activation. Similarly controversial, although one study suggests that SFKs are not required in thrombin-induced platelet aggregation (38), other studies indicate that SFK stimulates platelet activation induced by thrombin (39) and ADP (40, 41). The controversies about the roles of Lyn in platelet activation are in a way similar to the controversies about the role of the NO-cGMP pathway in platelet activation in that both stimulatory and inhibitory roles of this enzyme have been reported. However, it is unclear what the mechanisms responsible for these contradictory roles of SFK are in different receptor-mediated platelet activation pathways.
In this study, we demonstrate that SFK, mainly Lyn, plays an important primary role in stimulating platelet secretion of dense and α-granules, and this primary role of Lyn is responsible for its stimulation and amplification of platelet aggregation during platelet activation induced by low concentrations of platelet agonists or weak agonists. Furthermore, we have provided data indicating that one mechanism by which SFK (Lyn) stimulates platelet secretion is its activation of the PI3K-Akt-eNOS-cGMP-PKG pathway. Because the role of the cGMP pathway in platelet activation is known to be biphasic (inhibitory and stimulatory), our results also provide a potential explanation for the controversy on the dual role of Lyn in stimulating and inhibiting platelet activation.
EXPERIMENTAL PROCEDURES
Materials
The TXA2 analog U46619 and Src inhibitor PP2 were purchased from Calbiochem. α-Thrombin was from Enzyme Research Laboratories (South Bend, IN). Collagen and luciferase/luciferin reagent were from Chrono-log (Havertown, PA). A rabbit polyclonal antibody against a recombinant human Akt 1 fragment (amino acid residues 345–480) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and a rabbit polyclonal antibody against phosphorylated Ser473 of Akt was from Cell Signaling Technology. CRP was kindly provided by Dr. Debra K. Newman (Blood Research Institute, Blood Center of Milwaukee, WI). The TXB2 EIA kit was from Assay Designs (Ann Arbor, MI). The cGMP enzyme immunoassay kit was from Amersham Biosciences.
Platelet Aggregation and Secretion
For studies using human platelets, fresh blood was drawn by venipuncture from healthy volunteers (performed in the General Clinical Research Centers at the University of Illinois Medical Center and University of Kentucky Medical Center). Institutional Review Board approval was obtained from the University of Illinois at Chicago and the University of Kentucky, and informed consent was provided to volunteers according to the Declaration of Helsinki. Blood was anticoagulated with one-seventh volume of ACD (85 mm trisodium citrate, 110 mm dextrose, and 78 mm citric acid). Platelets were washed with CGS buffer (0.12 m sodium chloride, 0.0129 m trisodium citrate, and 0.03 m d-glucose, pH 6.5), resuspended in modified Tyrode's solution at 3 × 108/ml, and allowed to incubate at 22 °C for 1–2 h as described previously (42). Platelet aggregation was measured by detecting changes in light transmission. Platelet secretion was determined by measuring the release of ATP using luciferin/luciferase reagent (Chrono-lume). Luciferin/luciferase reagent (12 μl) was added to 238 μl of washed platelet suspension within 1 min before stimulation. Platelet aggregation and secretion were recorded in real time in a Chrono-log lumiaggregometer at 37 °C with stirring (1000 rpm). To examine the effects of PP2, washed platelets were preincubated with PP2 (10 μm) or DMSO (0.1%) for 2 min prior to the addition of the agonists.
Mouse Platelet Preparation
Lyn knock-out mice were generated as described previously (43) and were backcrossed to C57BL/6J background. C57BL/6J mice (obtained from Jackson Laboratory (Bar Harbor, ME)) or wild type littermates from Lyn heterozygote breeding were used as controls. Fyn knock-out mice were obtained from Jackson Laboratory. Mice were bred and maintained in the University of Illinois Animal Care Facility and in the University of Kentucky Animal Care Facility following institutional and National Institutes of Health guidelines after approval by the Animal Care Committee. Washed platelets from Lyn knock-out and wild type mice were prepared as described previously (16). Briefly, male and female mice (6–8 weeks) were anesthetized by intraperitoneal injection of pentobarbital. Whole blood from homozygous Lyn knock-out and wild type mice was collected from the inferior vena cava using one-seventh volume of ACD (85 mm trisodium citrate, 83 mm dextrose, and 21 mm citric acid) as anticoagulant. For each experiment, blood was pooled from five or six mice of each genotype. Platelets were then washed twice with CGS, resuspended in modified Tyrode's buffer at 3 × 108/ml, and incubated for 2 h at 22 °C before use.
P-selectin Expression
Washed platelets from healthy human donors were resuspended in Tyrode's buffer and incubated at 37 °C for 2 h before use. Platelets were preincubated with or without PP2 or DMSO for 2 min at 37 °C. The platelets were then incubated with different agonists for 5 min at 37 °C and fixed by adding paraformaldehyde (final concentration of 1%). The platelets were incubated with a monoclonal anti-human P-selectin antibody, SZ51. After washing, the platelets were further incubated with a fluorescein isothiocyanate-conjugated goat anti-mouse Ig antibody. P-selectin expression was analyzed using a FACSCalibur flow cytometer. To determine the role of Lyn in P-selectin expression induced by platelet agonists, washed platelets from Lyn knock-out mice or wild type controls were stimulated with platelet agonists for 5 min at 37 °C and fixed by adding paraformaldehyde. Platelets were then incubated with a fluorescein isothiocyanate-conjugated anti-mouse P-selectin antibody at 22 °C for 30 min. P-selectin expression was analyzed by flow cytometer.
Measurement of cGMP Levels
Washed platelets (3 × 108/ml) in 400 μl of Tyrode's buffer were stirred at 37 °C after the addition of control buffer, thrombin (0.05 unit/ml), U46619 (500 nm). To determine the effect of PP2 on cGMP production, platelets were preincubated with PP2 (10 μm) or DMSO at 37 °C for 2 min and then exposed to the platelet agonists. To determine the role of Lyn in cGMP production induced by platelet agonists, washed platelets from Lyn knock-out mice or wild type controls were stimulated with platelet agonists for 5 min at 37 °C. The reaction was stopped by the addition of 400 μl of ice-cold 12% (w/v) trichloroacetic acid. Samples were mixed and centrifuged at 2000 × g for 15 min at 4 °C. The supernatant was removed and washed four times with 5 volumes of water-saturated diethyl ether and then lyophilized. cGMP levels were measured using a cGMP enzyme immunoassay kit from Amersham Biosciences.
Western Blot Analysis of Akt Phosphorylation in Platelets
Washed platelets (3 × 108/ml) were resuspended in modified Tyrode's buffer and incubated at room temperature for 1 h before use. Platelets were stimulated with collagen (0.5 μg/ml) or thrombin (0.05 unit/ml) in a platelet aggregometer at 37 °C for 5 min and then solubilized in SDS-PAGE sample buffer. To examine the effect of PP2 on Akt phosphorylation, platelets were preincubated with PP2 or DMSO at 37 °C for 2 min prior to the addition of the platelet agonists. Platelet lysates were analyzed by SDS-PAGE on 4–15% gradient gel and electrotransferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% nonfat dry milk in TBS (20 mm Tris-HCl, 150 mm NaCl, pH 7.5) and incubated with a polyclonal anti-Akt antibody or a polyclonal antibody specific for the phosphorylated Ser473 of Akt (Cell Signaling) for 2 h at 22 °C. After three washes in TBS containing 0.05% Tween 20, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (0.5 μg/ml) for 45 min. After further washing, reactions were visualized using an Amersham Biosciences enhanced chemiluminescence kit.
RESULTS
Effect of an SFK Inhibitor, PP2, on Platelet Secretion and Aggregation Induced by G-protein-coupled Receptor Agonists TXA2 and α-Thrombin
To investigate the role of SFK in platelet secretion and aggregation induced by GPCR-dependent agonists, we examined the effect of an SFK inhibitor, PP2, on ATP release and platelet aggregation induced by a stable TXA2 analog U46619 and α-thrombin. At a low concentration of U46619 (250 nm), human platelet aggregation and ATP secretion were both abolished by PP2 (Fig. 1, A and E). However, at a higher concentration of U46619 (500 nm), platelet aggregation was partially inhibited by PP2, showing one-wave aggregation that is characteristic of a lack of secretion-dependent secondary aggregation response. Indeed, ATP secretion induced by this concentration of U46619 was inhibited by PP2 (Fig. 1, B and E). These results suggest that PP2 inhibits platelet secretion and secretion-dependent platelet aggregation induced by U46619. Similarly, PP2 inhibited ATP release and the second wave of platelet aggregation induced by a low concentration of α-thrombin (0.05 unit/ml) (Fig. 1, C and F). However, at a higher concentration of thrombin (0.1 unit/ml), PP2 only partially inhibited ATP release, and consequently, platelet aggregation was not affected (Fig. 1, D and F), suggesting that the inhibitory effect of PP2 on platelet aggregation is also secretion-dependent. To exclude possible nonspecific effects of PP2 on platelet secretion and aggregation, we also obtained data indicating that a structural analogue of PP2, PP3, which does not inhibit SFK, did not affect platelet aggregation and secretion induced by U46619 (Fig. 2A) or thrombin (data not shown). These data indicate that SFK is an important mediator but not the only mediator in platelet-dense granule secretion induced by GPCR-dependent agonists, TXA2 and thrombin.
FIGURE 1.
Effects of the SFK inhibitor PP2 on GPCR-induced ATP secretion and platelet aggregation. A–D, washed human platelets in modified Tyrode's buffer (3 × 108/ml) were preincubated with PP2 (10 μm) or DMSO control (0.1%) at 37 °C for 2 min and then exposed to various concentrations of U46619 (A and B) or thrombin (C and D) and simultaneously recorded for ATP secretion and aggregation. The aggregation and ATP release traces are representatives of at least three different experiments. E and F, aggregation and secretion results in the experiments described in A–D were quantitated. Secretion values were normalized with respect to platelets pretreated with DMSO and stimulated with the lowest concentration of U46619 (U) (E) or thrombin (Throm) (F).
FIGURE 2.
Specificity of the effect of PP2 on platelet secretion and aggregation and restoration of the platelet aggregation by exogenous ADP in the PP2-treated platelets. A, washed human platelets in modified Tyrode's buffer (3 × 108/ml) were preincubated with DMSO (0.1%), PP2 (10 μm), or an analog control for PP2, PP3 (10 μm), which does not inhibit SFK at 37 °C for 2 min, and then exposed to U46619 (500 nm). B, washed human platelets were preincubated with DMSO or PP2 (10 μm) (PP2) for 2 min at 37 °C and then stimulated with thrombin (0.025 unit/ml). PP2-treated platelets were also stimulated with thrombin followed by 0.5 μm ADP (PP2+ADP). Platelets were also stimulated with ADP alone (0.5 μm) (ADP). C, quantitative data (mean ± S.D.) from four experiments as described in B. D, washed human platelets were preincubated with DMSO or PP2 (10 μm) for 2 min at 37 °C and then stimulated with U46619 (250 nm). PP2-treated platelets were also stimulated with U46619 followed by 0.5 μm ADP. Platelets were also stimulated with 0.5 μm ADP alone.
Inhibition of ADP Secretion Is Responsible for Decreased Platelet Aggregation Induced by α-Thrombin and U46619 in PP2-treated Platelets
To further verify whether the inhibitory effect of PP2 on the second wave of platelet aggregation resulted from its inhibitory effect on secretion, we examined whether supplementing granule content ADP could rescue the second wave of platelet aggregation in PP2-treated human platelets. Exogenous ADP (0.5 μm) alone did not induce platelet aggregation in washed platelets (Fig. 2B). Thrombin-induced platelet aggregation was inhibited by PP2. Adding exogenous ADP at a concentration (0.5 μm) below the concentrations of ADP released by low dose thrombin (44) significantly reversed the inhibitory effect of PP2 and restored platelet aggregation induced by thrombin (Fig. 2, B and C). Similarly, exogenous ADP also restored the second wave of aggregation of PP2-treated platelets induced by low dose U46619 (Fig. 2D). These data suggest that the inhibitory effect of PP2 on thrombin- and TXA2-induced platelet aggregation results from its inhibition of platelet secretion of dense granule ADP.
Role of SFK in Platelet Secretion of α-Granules
To determine whether SFK also plays a role in platelet secretion of α-granules, we investigated the effect of PP2 on surface expression of P-selectin induced by platelet agonists. Expression of P-selectin was examined by flow cytometry using a monoclonal anti-human P-selectin antibody SZ51. Fig. 3 shows that PP2 abolished P-selectin expression induced by U46619. P-selectin expression induced by thrombin was partially but significantly inhibited by PP2. Thus, SFK plays an important role in platelet secretion from α-granules induced by TXA2 and thrombin.
FIGURE 3.
The inhibitory effect of PP2 on P-selectin expression. A and B, washed human platelets in modified Tyrode's buffer were preincubated with PP2 (10 μm) or DMSO for 2 min at 37 °C and then stimulated with U46619 (U) (250 nm) or thrombin (T) (0.025 unit/ml) for 5 min at 37 °C with stirring and subsequently fixed with paraformaldehyde. Fixed platelets were incubated with a monoclonal anti-human P-selectin antibody, SZ51, or negative control mouse IgG (M IgG) for 30 min at 22 °C. After washing once with PBS, platelets were further incubated with a fluorescein isothiocyanate-conjugated rabbit anti-mouse Ig antibody. Surface expression of P-selectin was analyzed using flow cytometry. Data from a representative experiment are shown in A. Quantitative results from three experiments are expressed as the P-selectin expression index (fluorescence intensity of platelets stimulated with an agonist/fluorescence intensity of unstimulated platelets) (B).
Role of Lyn in Platelet Granule Secretion
Lyn is one of the major SFK members expressed in platelets. To investigate whether Lyn is responsible for the role of SFK in platelet secretion induced by U46619 and thrombin, we compared agonist-induced platelet ATP release and aggregation between Lyn-deficient platelets and wild type controls. Thrombin-induced ATP release and aggregation induced by low dose concentrations of α-thrombin were significantly inhibited in the Lyn-deficient platelets (Fig. 4, A and B), which is consistent with a previous report showing inhibitory effect of Lyn knock-out on γ-thrombin-induced platelet response (39). Also, P-selectin expression induced by thrombin was significantly inhibited in Lyn knock-out platelets compared with wild type platelets (Fig. 4, C and D). To determine whether the defect in platelet secretion signaling is responsible for the defective integrin-dependent platelet aggregation response of Lyn knock-out, Lyn knock-out platelets were stimulated with thrombin with the supplementation of low concentrations of dense granule content ADP. Indeed, ADP completely corrected the defect in platelet aggregation (Fig. 4, E and F). These results indicate that an important role of Lyn in platelet activation is to mediate granule secretion signaling and that Lyn is the major SFK member responsible for mediating platelet secretion.
FIGURE 4.
The inhibitory effect of Lyn deficiency on platelet aggregation and secretion. A, washed platelets from Lyn+/+ or Lyn−/− mice were stimulated with thrombin (0.025 units/ml) in a lumiaggregometer at 37 °C. Real-time ATP secretion and platelet aggregation were simultaneously recorded. B, aggregation and secretion results for the experiments described in A were quantitated (n = 3). Secretion values were normalized with respect to platelets from Lyn+/+ mice. C and D, washed platelets from Lyn+/+ (C) or Lyn−/− mice (D) were incubated with various concentrations of thrombin for 5 min at 37 °C with stirring and then fixed with paraformaldehyde. Fixed platelets were incubated with a fluorescein isothiocyanate-labeled monoclonal anti-mouse P-selectin antibody, SZ51, for 30 min at 22 °C, and analyzed using flow cytometry. The P-selectin expression index (median of fluorescence intensity with a certain concentration of thrombin/median of fluorescence intensity of unstimulated platelets (control)) is also shown. E, aggregation traces of platelets from either Lyn knock-out mice (Lyn−/−) or wild type controls (Lyn+/+) stimulated with thrombin or thrombin plus 0.5 μm ADP (Lyn−/−+ADP). F, aggregation results for the experiments described in E were quantitated (n = 3). WT, wild type.
Role of SFK in Collagen-induced Platelet Secretion
Unlike the GPCR-coupled soluble agonists, adhesive protein collagen mediates platelet adhesion and activation via multiple platelet collagen receptors and their signaling pathways, including the GPVI-ITAM pathway and integrin α2β1. Several members of SFK have been suggested to play a role in early GPVI-ITAM signaling (45). Indeed, PP2 totally abolished collagen-induced ATP release (Fig. 5A) and P-selectin expression (Fig. 5B) and also totally abolished collagen-induced platelet aggregation. However, although collagen-induced FcRγ phosphorylation in Lyn knock-out platelets appeared slightly reduced at an early time point, as previously reported (37) (supplemental Fig. 1), platelet activation induced by GPVI-specific agonist CRP was not reduced (and was even enhanced) in Lyn knock-out platelets (37) (data not shown), suggesting that Lyn-independent ITAM phosphorylation is sufficient for full-scale platelet response. In contrast, under our experimental conditions, low dose collagen-induced ATP release and aggregation were markedly but partially reduced in the Lyn-deficient platelets (Fig. 5C). These data indicate that Lyn is important in collagen-induced platelet secretion and aggregation, and this role of Lyn in collagen-induced platelet aggregation appears to be distinct from the role of SFK in the GPVI-ITAM pathway Consistent with this notion, we show that, although inhibition of collagen-induced platelet aggregation by PP2 was not rescued by the addition of exogenous ADP (data not shown), exogenous ADP reversed inhibition of Lyn deficiency on platelet aggregation induced by collagen (Fig. 5, D and E). These results suggest that an important role of Lyn in platelet activation induced by collagen is to mediate ADP secretion. Previously, it has been shown that SFKs are important in stimulating TXA2 synthesis (40, 46), which is important in collagen-induced platelet aggregation. We found that collagen-induced TXA2 production was totally abolished by PP2 in human platelets, which was not corrected by ADP supplementation (data not shown). Thus, our data suggest that although some SFK members are involved in the proximal GPVI-dependent ITAM signaling leading to TXA2 synthesis and platelet activation, Lyn plays an important role in a distinct collagen signaling pathway mediating granule secretion in response to low dose collagen.
FIGURE 5.
Effects of PP2 and Lyn knock-out on collagen-induced platelet secretion and aggregation. A, washed human platelets were preincubated with PP2 or DMSO for 2 min and then exposed to collagen in a lumiaggregometer at 37 °C. Real-time ATP secretion and platelet aggregation were simultaneously recorded. B, washed human platelets were preincubated with PP2 or DMSO for 2 min and then stimulated with collagen (Coll) (0.5 μg/ml) for 5 min at 37 °C with stirring and then analyzed for P-selectin expression by flow cytometric analysis of the binding of a monoclonal anti-human P-selectin antibody, SZ51. C, aggregation and ATP secretion traces of washed platelets from Lyn+/+ or Lyn−/− mice stimulated with collagen (0.5 μg/ml) in a lumiaggregometer at 37 °C. D, aggregation traces of Lyn−/− or Lyn+/+ mouse platelets stimulated with collagen (0.5 μg/ml) or collagen plus 0.5 μm ADP (Lyn−/−+ADP). E, quantitative data (mean ± S.D.) from three experiments as described in D. WT, wild type.
The Role of Fyn in Platelet Activation
Platelets also express Fyn. However, Fyn is not required for thrombin-induced platelet aggregation and secretion (39) (data not shown). To investigate whether Fyn is involved in platelet secretion and aggregation induced by U46619 and collagen, we compared agonist-induced platelet ATP release and aggregation between Fyn-deficient platelets and wild type controls. ATP release and aggregation induced by either low dose concentrations of U46619 (250 nm) or collagen (0.5 μg/ml) were not significantly affected in the Fyn-deficient platelets (Fig. 6), suggesting that Fyn is not required for platelet secretion and aggregation induced by these platelet agonists.
FIGURE 6.
The effect of Fyn deficiency on platelet aggregation and secretion. A, washed platelets from Fyn+/+ or Fyn−/− mice were stimulated with collagen (0.5 μg/ml) in a lumiaggregometer at 37 °C. Real-time ATP secretion and platelet aggregation were simultaneously recorded. B, washed platelets from Fyn+/+ or Fyn−/− mice were stimulated with U46619 (250 nm) in a lumiaggregometer at 37 °C. Real-time ATP secretion and platelet aggregation were simultaneously recorded. C and D, aggregation (C) and secretion (D) results for the experiments described A and B were quantitated (n = 3). Secretion values were normalized with respect to wild type platelets stimulated with collagen. U, U46616; Coll, collagen.
Role of SFK in Platelet Agonist-induced Akt Phosphorylation and cGMP Production
In platelets and other cell types, SFKs have been suggested to be important in agonist-mediated PI3K activation and signaling (39, 47). We have recently reported that the PI3K-Akt-eNOS-cGMP-PKG signaling pathway stimulates platelet secretion (18–20). Thus, we hypothesized that SFK mediates platelet secretion by activating the PI3K-Akt-NO-cGMP pathway. To test this hypothesis, we examined the effects of PP2 on Akt phosphorylation and intracellular cGMP elevation induced by GPCR-dependent agonist thrombin and by collagen in human platelets. Akt phosphorylation induced by either collagen (Fig. 7, A and B) or thrombin (Fig. 7, C and D) was nearly completely inhibited by PP2, indicating a critical role for SFK in the agonist stimulation of the PI3K-Akt pathway. Interestingly, although collagen-induced cGMP elevation was completely inhibited by PP2 (Fig. 7E), thrombin-induced cGMP elevation was only partially but significantly blocked by PP2 (Fig. 7F), suggesting that although SFK plays an important role in agonist-stimulated cGMP elevation, there is a SFK-independent signaling pathway leading to cGMP synthesis. These data indicate that SFK plays an important role in the activation of the PI3K-Akt-NO-cGMP pathway in platelets, but thrombin may also stimulate cGMP elevation via an SFK-independent pathway.
FIGURE 7.
The effect of PP2 on Akt phosphorylation and cGMP production. A, washed human platelets were preincubated with PP2 (10 μm) or DMSO for 2 min at 37 °C. Platelets were then stimulated with collagen (Coll) in the aggregometer for 5 min at 37 °C and solubilized with SDS-PAGE sample buffer. Phosphorylation of Akt (p-Akt) was detected by Western blotting with an antibody specifically recognizing the phosphorylated Ser473 site in Akt. Equal sample loading was assessed by Western blotting using an anti-Akt antibody. B, densitometry measurements from results in A. Values were normalized with respect to sample with stimulation for each immunoblot and are expressed as relative phosphorylation (mean ± S.D. from three separate experiments). Statistical significance was determined using Student's t test. C, washed human platelets were preincubated with PP2 (10 μm) or DMSO (0.1%) for 2 min at 37 °C. Platelets were then stimulated with thrombin in the aggregometer for 5 min at 37 °C and solubilized with SDS-PAGE sample buffer. Phosphorylation of Akt was detected by Western blotting. D, densitometry measurements from the results in C. Values were normalized with the value obtained with stimulated platelets for each immunoblot and are expressed as relative phosphorylation. E and F, washed human platelets were preincubated for 2 min at 37 °C with DMSO or PP2 (10 μm). The platelets were then stimulated with collagen (0.5 μg/ml) (E) or thrombin (0.05 units/ml) (F) in a platelet aggregometer for 5 min. cGMP concentrations were determined using a cGMP enzyme immunoassay kit. Results are expressed as mean ± S.D. (n = 3) (p < 0.01).
Role of Lyn in Platelet Agonist-induced cGMP Elevation and Akt Phosphorylation
To determine whether Lyn is the major SFK responsible for the activation of cGMP-PKG pathway, we compared agonist-induced cGMP elevation between Lyn-deficient platelets and wild type controls. The intracellular levels of cGMP induced by thrombin or U46619 were significantly but partially inhibited in Lyn-deficient platelets (Fig. 8A). The collagen-induced platelet cGMP elevation was almost completely inhibited in Lyn knock-out platelets. Similarly, Akt phosphorylation induced by either collagen or thrombin was reduced in the Lyn-deficient platelets (Fig. 8, B and C). These results are consistent with the results obtained with PP2, indicating that Lyn is a major SFK responsible for agonist-induced activation of the cGMP pathway.
FIGURE 8.
The effect of Lyn deficiency on Akt phosphorylation and cGMP production. A, washed platelets from Lyn+/+ or Lyn−/− mice were stimulated with collagen (0.5 μg/ml), thrombin (0.05 units/ml), or U46619 (500 nm) in a platelet aggregometer at 37 °C for 5 min. cGMP concentrations were determined using a cGMP enzyme immunoassay kit. Results are expressed as mean ± S.D. (n = 3). B and C, washed platelets from Lyn+/+ or Lyn−/− mice were stimulated with collagen (B) or thrombin (C) in the aggregometer for 5 min at 37 °C and solubilized with SDS-PAGE sample buffer. Phosphorylation of Akt was detected by Western blotting. Values were normalized with the value of stimulated wild type platelets for each immunoblot and are expressed as relative phosphorylation (mean ± S.D. from three separate experiments).
Inhibition of Platelet Secretion and Aggregation by PP2 and Lyn Knock-out Is Rescued by cGMP
If SFK (Lyn)-stimulated platelet secretion is mediated via the cGMP pathway, the inhibitory effect of PP2 or Lyn knock-out on platelet activation should be reversed by supplementing exogenous cGMP. Indeed, the inhibitory effect of PP2 on thrombin-induced aggregation was significantly reversed by adding a low concentration of membrane-permeable cGMP analog, 8-bromo-cGMP (5 μm) (Fig. 9, A and B), but not the membrane-impermeable cGMP (data not shown). 8-Bromo-cGMP alone did not induce any platelet aggregation and secretion (data not shown) (17). 8-Bromo-cGMP also partially but significantly reversed the inhibitory effect of PP2 on thrombin-induced platelet granule secretion (Fig. 9C). Similarly, the defect of Lyn knock-out platelets in platelet secretion and aggregation was also partially corrected by supplementing 8-bromo-cGMP (Fig. 9D). These results, together with the finding that inhibition of SFK by PP2 or Lyn knock-out significantly diminished cGMP elevation, indicate that the cGMP-dependent signaling pathway is an important signaling pathway that mediates the role of SFK in stimulating platelet secretion.
FIGURE 9.
8-Bromo-cGMP rescues platelet aggregation and secretion in PP2-treated platelets. A, washed human platelets in modified Tyrode's buffer (3 × 108/ml) were preincubated with DMSO (control) or PP2 (10 μm) for 2 min at 37 °C and then stimulated with thrombin (0.025 units/ml), followed by the addition of 8-bromo-cGMP (5 μm) (PP2+cGMP) or buffer (PP2). B, quantitative data (mean ± S.D.) on the percentage of aggregation (light transmission) from three experiments as described in A. C, 6 min after the addition of thrombin, luciferase/luciferin reagent was added to the platelet suspension to measure the total amount of ATP released. The data represent the mean ± S.D. of ATP release from three experiments. D, platelets from Lyn knock-out mice were stimulated with thrombin (Lyn−/−) or thrombin followed by the addition of 8-bromo-cGMP (Lyn−/−+Br-cGMP). Platelets from wild type controls were also stimulated with thrombin (Lyn+/+).
Inhibition of cGMP Elevation by Lyn Deficiency Is Reversed by Exogenous ADP
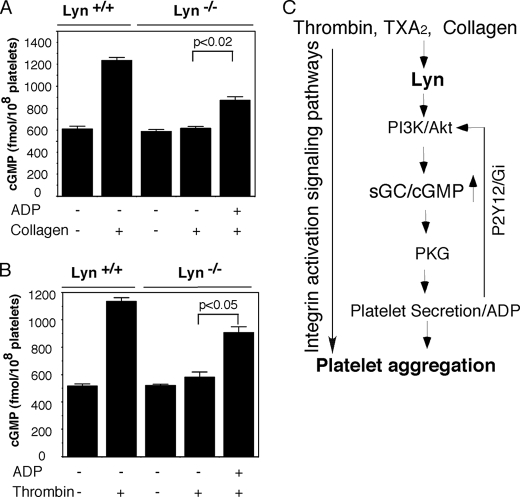
It has been previously reported that the ADP receptor, P2Y12-mediated signaling pathway leads to activation of Akt in platelets, which activates eNOS-dependent NO synthesis and cGMP elevation. Therefore, we determined whether the inhibitory effect of Lyn deficiency on cGMP elevation induced by collagen or thrombin could be reversed by the addition of exogenous ADP. We found that the addition of ADP (0.5 μm) partially reversed the inhibitory effect of Lyn knock-out on the cGMP elevation in the collagen- or thrombin-stimulated mouse platelets. ADP alone at 0.5 μm did not affect intracellular cGMP levels in the wild type or Lyn-deficient platelets (data not shown). These results suggest that ADP, possibly via the P2Y12 pathway, induces a Lyn-independent positive feedback loop amplifying cGMP elevation and platelet secretion (Fig. 10). Thus, ADP and cGMP signaling pathways mutually amplify each other, leading to promotion of platelet response to low doses of agonists.
FIGURE 10.
Exogenous ADP rescues cGMP in Lyn-deficient platelets. A and B, washed platelets (3 × 108/ml) from Lyn+/+ or Lyn−/− mice were stimulated with collagen in the aggregometer for 5 min at 37 °C. They were stimulated with collagen (0.5 μg/ml) (A) or thrombin (0.025 unit/ml) (B) with or without the addition of ADP (0.5 μm). cGMP concentrations were determined using a cGMP enzyme immunoassay kit. Results are expressed as mean ± S.D. (n = 3). C, a positive feedback loop regulating platelet secretion. Platelet agonists, thrombin or collagen, bind to their receptors, which, by activating the SFK pathway, induce the activation of the PI3K/Akt pathway. PI3K/Akt induces platelet secretion via PKG. Secreted ADP activates the PI3K/Akt pathway through its Gi-coupled P2Y12 receptor, which further induces cGMP elevation.
DISCUSSION
In this study, we demonstrate that SFK, mainly Lyn, plays an important role in a GPCR- and collagen-induced common signaling pathway mediating platelet secretion from dense granules and α-granules. We further demonstrate that Lyn-mediated stimulation of platelet granule secretion is the mechanism responsible for the SFK role in promoting and stabilizing platelet aggregation induced by Gq and G13-coupled agonists, such as thrombin and TXA2. Furthermore, we show that the PI3K-Akt-NO-cGMP pathway is an important downstream signaling pathway of Lyn-mediated platelet granule secretion.
Our data support the conclusion that SFK, mainly Lyn, plays an important role in mediating platelet secretion of dense granules and α-granules. These data are consistent with the results of Cho et al. who reported that γ-thrombin-induced platelet aggregation and secretion were reduced by Lyn knock-out (39). However, the data of Cho et al. do not differentiate whether the role of Lyn is in the agonist-induced signaling leading to aggregation and thus subsequently affects aggregation-dependent granule secretion or in the signaling leading to secretion, which subsequently affects the amplification of platelet aggregation. We show that platelet secretion induced by α-thrombin, U46619, or collagen was inhibited by PP2 and in Lyn knock-out platelets and that the inhibitory effect of PP2 and Lyn-knock-out on platelet aggregation induced by these GPCR agonists can be significantly reversed by supplementing low concentrations of dense granule content ADP, indicating that inhibition by Lyn knock-out on platelet aggregation is consequent to its inhibitory effect on granule secretion. The addition of ADP did not completely reverse the inhibitory effect of PP2, suggesting that other secreted granule contents and/or a secretion-independent pathway may also be involved in SFK-mediated platelet activation.
The role of SFK in platelet activation induced by Gq/G13-coupled agonists, such as thrombin and TXA2, appears to be distinct from that in adhesion protein (collagen or GPIb-IX)-induced platelet activation. The role of SFK (Lyn) as well as the cGMP pathway in GPIb-IX signaling appears to be proximal to the receptor and is important in the stable platelet adhesion to von Willebrand factor under shear stress, independent of ADP secretion (17, 32, 33). It is recognized that SFK is important in the collagen-induced activation of the ITAM pathway because SFK mediates tyrosine phosphorylation of the ITAM motif of FcRγ required for recruiting the downstream kinase Syk, which is required for collagen-induced platelet secretion and aggregation. Thus, the SFK inhibitor PP2 inhibited collagen receptor-proximal signaling responses, which can activate both ADP secretion-dependent and -independent downstream signaling. Consequently, the inhibitory effect of PP2 cannot be corrected by supplementing ADP (data not shown). In contrast, Lyn is important in a collagen-induced signaling mechanism that appears to be distinct from the early GPVI/ITAM pathway induced by the GPVI-specific agonist, CRP, because Lyn knock-out showed reduced platelet secretion and aggregation in response to collagen but enhanced response to CRP (37) (data not shown). Furthermore, the decreased collagen-induced platelet aggregation in Lyn knock-out platelets can be reversed by ADP supplementation, suggesting a granule secretion-dependent role for Lyn. In contrast to collagen-induced platelet activation, both PP2 and Lyn knock-out had little effect on primary platelet aggregation induced by GPCR-coupled platelet agonists, such as thrombin and TXA2, but inhibited platelet granule secretion and thus the secretion-dependent second wave of platelet aggregation. The inhibitory effect of both PP2 and Lyn knock-out on platelet aggregation induced by Gq/G13-coupled agonists, thrombin and TXA2, was rescued by supplementing ADP, which mainly activates Gi signaling pathway (in addition to Gq, which is already activated by thrombin or TXA2). Also, the effect of ADP in correcting the inhibition of platelet aggregation by PP2 was abolished by the Gi-coupled P2Y12 antagonist 2-methylthioadenosine 5′-monophosphate triethylammonium salt (data not shown). Thus, Lyn appears to be a major SFK member responsible for the role of SFK in platelet activation induced by low concentrations of Gq/G13-coupled agonists, such as thrombin and TXA2, which is to mediate platelet granule secretion. Secreted ADP, by activating the Gi pathway, greatly amplifies the platelet activation signals, including the cGMP signal, inducing the second wave of platelet aggregation and stabilizing the platelet thrombus. Our finding that SFK (Lyn) plays an important role in mediating platelet secretion represents a significant advance in our understanding of the roles of SFK in platelet activation and the mechanism of platelet secretion signaling. It is important to note that platelet aggregation induced by a high dose of thrombin does not require secretion of ADP (Fig. 1). Therefore, a low dose of thrombin was used in this study. Due to various responses to thrombin in different donors, variable concentrations of thrombin (0.025–0.05 units/ml) were used to achieve an optimal secretion-dependent platelet aggregation in which the role of Lyn manifests.
Platelet agonist-induced granule secretion involves both aggregation-dependent and aggregation-independent pathways. Aggregation-dependent platelet secretion involves integrin outside-in signaling. SFK, particularly c-Src, have been shown to play critical roles in the outside-in signaling, leading to platelet spreading. However, we believe that the main role of Lyn in platelet secretion is not due to integrin outside-in signaling for the following reasons. First, P-selectin expression induced by thrombin, U46619, or collagen is significantly but only partially inhibited by the integrin inhibitor, RGDS peptide, suggesting a partial dependence on integrin signaling. In contrast, P-selectin expression is almost completely abolished by PP2, suggesting that SFK is important in mediating both integrin-dependent and integrin-independent granule secretion. Second, although Lyn is the major SFK member responsible for mediating agonist-induced platelet secretion, Lyn has been reported to negatively regulate integrin αIIbβ3-mediated outside-in signaling in platelets (29) and thus is unlikely to be responsible for promoting integrin outside-in signaling leading to platelet secretion. Instead, c-Src has been identified as the SFK member that is critical for the outside-in signaling of integrin αIIbβ3 in platelets (25–28). Therefore, although our data do not exclude the possibility that certain SFK isoforms such as c-Src are important in integrin outside-in signaling and consequent responses, our data indicate that the SFK Lyn stimulates a platelet secretion signaling pathway that is distinct from the integrin outside-in signaling.
Despite the importance of granule secretion in platelet function, signaling pathways regulating platelet granule secretion are not well defined (48). Recently, we have discovered that the NO-cGMP-PKG pathway stimulates platelet secretion (17–19). These results are consistent with the report that insulin stimulates secretion of ATP from dense granule via the NO-cGMP-PKG pathway (49) and with the findings that the NO-cGMP-PKG pathway stimulates exocytosis in leukocytes and neuronal cells (21–23). The cGMP-dependent granule secretion is associated with the PKG-dependent association between syntaxin 2 VAMP3 (vesicle-associated membrane protein 3) (49) in platelets and phosphorylation of soluble SNAP (N-ethylmaleimide-sensitive factor-attached protein)-23, and syntaxins 2 and 4 in leukocytes (21). However, the stimulatory effect cGMP in platelet activation was not repeated by some studies (50, 51). These discrepancies between our results and the data from Marshall et al. (50) and Morrell et al. (51) could be attributed to different experimental conditions because the platelets used in these reports are insensitive to low concentrations of platelet agonists, such as thrombin (50, 51). Because the role of cGMP in platelet activation is mainly to amplify low dose agonist-induced platelet activation, it is not surprising that these investigators failed to show the stimulatory role of cGMP. Although we are the first to systematically demonstrate the stimulatory roles and pathways of NO and cGMP in platelet aggregation and secretion, the phenomenon of stimulatory effects of cGMP analogs on platelet activation was reported as early as in 1976 (52) but was submerged by the opposition and thus not publicly confirmed by other laboratories until our reports in 2003 (17). Furthermore, PI3K and Akt are known to promote platelet secretion and secretion-dependent platelet aggregation (16, 20, 53, 54), and the effects of PI3K and Akt are mediated via the NO-sGC/cGMP-PKG pathway (20, 32). In this study, we further show that 1) the SFK inhibitor, PP2, inhibited agonist-induced Akt phosphorylation, indicating that SFK is important in GPCR-mediated activation of the PI3K-Akt signaling pathway; 2) PP2 and Lyn knock-out also inhibited thrombin- and collagen-induced increases in the intracellular cGMP levels in platelets, indicating that SFK is upstream of the NO-cGMP signaling pathway; 3) supplementation of low concentrations of 8-bromo-cGMP corrected the platelet aggregation defect caused by PP2 and Lyn knock-out and partially but significantly reversed the inhibitory effect of PP2 on platelet granule secretion, indicating that SFK (Lyn)-mediated activation of the cGMP signaling pathway plays an important role in the stimulatory effect of SFK (Lyn) on platelet secretion and aggregation; and 4) similar to PP2, the specific sGC inhibitor ODQ inhibited platelet aggregation in response to low dose thrombin (19). ODQ also inhibited cGMP production induced by thrombin in human platelets. Thus, although our data suggest that SFK is also important in other platelet signaling pathways, our data reveal that SFK, mainly Lyn, sequentially activates the PI3K-Akt pathway and NO-cGMP-PKG pathway, leading to platelet granule secretion and consequently the second wave of platelet aggregation. There have been reports that sGC can be activated through Src kinase-dependent phosphorylation of the sGC β1 subunit in a nitric-oxide synthase/NO-independent manner (55, 56). Therefore, our data could not exclude the possibility that SFK (Lyn)-dependent phosphorylation and activation of sGC might also be involved in the activation of the cGMP/PKG pathway in response to platelet agonists.
Interestingly, although the Lyn-dependent cGMP pathway stimulates ADP secretion, ADP, via P2Y12, also stimulates PI3K and Akt (16, 57) and partially reverses the defects in cGMP production in Lyn knock-out platelets (Fig. 10D), suggesting that there is a positive feedback loop in which cGMP and ADP mutually amplify each other. The Lyn-independent activation of cGMP synthesis is probably mediated by Gβγ-mediated PI3Kγ and Akt activation induced via P2Y12 (16). Therefore, our results reveal not only a Lyn-dependent cGMP pathway leading to the platelet granule secretion pathway but also a positive amplification mechanism in which cGMP promotes ADP secretion and ADP increases cGMP synthesis, leading to greatly increased granule secretion and stabilization of platelet aggregation in the presence of low concentrations of agonists.
Supplementary Material
This work was supported in part by National Institutes of Health (NIH), NHLBI, Grants HL68819, HL62350, and 080264 (to X. D.) and by NIH/National Center for Research Resources Centers of Biomedical Research Excellence in Obesity and Cardiovascular Disease Grant P20 RR021954-01A1. This work was also supported by American Heart Association (AHA) National Scientist Development Grant 0430095N and AHA Midwest Affiliate Grand-in-aid 0855698G (to Z. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- TXA2
- thromboxane A2
- GPVI
- glycoprotein VI
- FcRγ
- Fc receptor γ
- ITAM
- immunoreceptor tyrosine-based activation motif
- CRP
- collagen-related peptide
- GPIb-IX
- glycoprotein Ib-IX
- GPCR
- G-protein-coupled receptor
- SNARE
- soluble NSF attachment receptors
- PI3K
- phosphatidylinositol 3-kinase
- cGMP
- cyclic GMP
- eNOS
- endothelial nitric-oxide synthase
- SFK
- Src family kinase
- PKG
- cGMP/cGMP-dependent protein kinase
- ODQ
- 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one.
REFERENCES
- 1.Ruggeri Z. M. (2002) Nat. Med. 8, 1227–1234 [DOI] [PubMed] [Google Scholar]
- 2.Herr A. B., Farndale R. W. (2009) J. Biol. Chem. 284, 19781–19785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tandon N. N., Kralisz U., Jamieson G. A. (1989) J. Biol. Chem. 264, 7576–7583 [PubMed] [Google Scholar]
- 4.Clemetson K. J., Clemetson J. M. (2001) Thromb. Haemost. 86, 189–197 [PubMed] [Google Scholar]
- 5.Moroi M., Jung S. M., Okuma M., Shinmyozu K. (1989) J. Clin. Invest. 84, 1440–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poole A., Gibbins J. M., Turner M., van Vugt M. J., van de Winkel J. G., Saito T., Tybulewicz V. L., Watson S. P. (1997) EMBO J. 16, 2333–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Offermanns S., Toombs C. F., Hu Y. H., Simon M. I. (1997) Nature 389, 183–186 [DOI] [PubMed] [Google Scholar]
- 8.Sambrano G. R., Weiss E. J., Zheng Y. W., Huang W., Coughlin S. R. (2001) Nature 413, 74–78 [DOI] [PubMed] [Google Scholar]
- 9.Thomas D. W., Mannon R. B., Mannon P. J., Latour A., Oliver J. A., Hoffman M., Smithies O., Koller B. H., Coffman T. M. (1998) J. Clin. Invest. 102, 1994–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster C. J., Prosser D. M., Agans J. M., Zhai Y., Smith M. D., Lachowicz J. E., Zhang F. L., Gustafson E., Monsma F. J., Jr., Wiekowski M. T., Abbondanzo S. J., Cook D. N., Bayne M. L., Lira S. A., Chintala M. S. (2001) J. Clin. Invest. 107, 1591–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Léon C., Hechler B., Freund M., Eckly A., Vial C., Ohlmann P., Dierich A., LeMeur M., Cazenave J. P., Gachet C. (1999) J. Clin. Invest. 104, 1731–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djellas Y., Manganello J. M., Antonakis K., Le Breton G. C. (1999) J. Biol. Chem. 274, 14325–14330 [DOI] [PubMed] [Google Scholar]
- 13.Kahner B. N., Shankar H., Murugappan S., Prasad G. L., Kunapuli S. P. (2006) J. Thromb. Haemost. 4, 2317–2326 [DOI] [PubMed] [Google Scholar]
- 14.Lemons P. P., Chen D., Bernstein A. M., Bennett M. K., Whiteheart S. W. (1997) Blood 90, 1490–1500 [PubMed] [Google Scholar]
- 15.Ren Q., Barber H. K., Crawford G. L., Karim Z. A., Zhao C., Choi W., Wang C. C., Hong W., Whiteheart S. W. (2007) Mol. Biol. Cell 18, 24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z., Zhang G., Le Breton G. C., Gao X., Malik A. B., Du X. (2003) J. Biol. Chem. 278, 30725–30731 [DOI] [PubMed] [Google Scholar]
- 17.Li Z., Xi X., Gu M., Feil R., Ye R. D., Eigenthaler M., Hofmann F., Du X. (2003) Cell 112, 77–86 [DOI] [PubMed] [Google Scholar]
- 18.Li Z., Zhang G., Marjanovic J. A., Ruan C., Du X. (2004) J. Biol. Chem. 279, 42469–42475 [DOI] [PubMed] [Google Scholar]
- 19.Marjanovic J. A., Li Z., Stojanovic A., Du X. (2005) J. Biol. Chem. 280, 37430–37438 [DOI] [PubMed] [Google Scholar]
- 20.Stojanovic A., Marjanovic J. A., Brovkovych V. M., Peng X., Hay N., Skidgel R. A., Du X. (2006) J. Biol. Chem. 281, 16333–16339 [DOI] [PubMed] [Google Scholar]
- 21.Nanamori M., Chen J., Du X., Ye R. D. (2007) J. Immunol. 178, 416–427 [DOI] [PubMed] [Google Scholar]
- 22.Rettori V., Belova N., Dees W. L., Nyberg C. L., Gimeno M., McCann S. M. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 10130–10134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wildemann B., Bicker G. (1999) J. Neurobiol. 39, 337–346 [DOI] [PubMed] [Google Scholar]
- 24.Pestina T. I., Stenberg P. E., Druker B. J., Steward S. A., Hutson N. K., Barrie R. J., Jackson C. W. (1997) Arterioscler. Thromb. Vasc. Biol. 17, 3278–3285 [DOI] [PubMed] [Google Scholar]
- 25.Arias-Salgado E. G., Lizano S., Sarkar S., Brugge J. S., Ginsberg M. H., Shattil S. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13298–13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Virgilio M., Kiosses W. B., Shattil S. J. (2004) J. Cell Biol. 165, 305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arias-Salgado E. G., Haj F., Dubois C., Moran B., Kasirer-Friede A., Furie B. C., Furie B., Neel B. G., Shattil S. J. (2005) J. Cell Biol. 170, 837–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flevaris P., Stojanovic A., Gong H., Chishti A., Welch E., Du X. (2007) J. Cell Biol. 179, 553–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maxwell M. J., Yuan Y., Anderson K. E., Hibbs M. L., Salem H. H., Jackson S. P. (2004) J. Biol. Chem. 279, 32196–32204 [DOI] [PubMed] [Google Scholar]
- 30.Du X. (2007) Curr. Opin. Hematol. 14, 262–269 [DOI] [PubMed] [Google Scholar]
- 31.Liu J., Pestina T. I., Berndt M. C., Jackson C. W., Gartner T. K. (2005) Blood 106, 2750–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin H., Stojanovic A., Hay N., Du X. (2008) Blood 111, 658–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin H., Liu J., Li Z., Berndt M. C., Lowell C. A., Du X. (2008) Blood 112, 1139–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki-Inoue K., Tulasne D., Shen Y., Bori-Sanz T., Inoue O., Jung S. M., Moroi M., Andrews R. K., Berndt M. C., Watson S. P. (2002) J. Biol. Chem. 277, 21561–21566 [DOI] [PubMed] [Google Scholar]
- 35.Locke D., Liu C., Peng X., Chen H., Kahn M. L. (2003) J. Biol. Chem. 278, 15441–15448 [DOI] [PubMed] [Google Scholar]
- 36.Ezumi Y., Shindoh K., Tsuji M., Takayama H. (1998) J. Exp. Med. 188, 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quek L. S., Pasquet J. M., Hers I., Cornall R., Knight G., Barnes M., Hibbs M. L., Dunn A. R., Lowell C. A., Watson S. P. (2000) Blood 96, 4246–4253 [PubMed] [Google Scholar]
- 38.Briddon S. J., Watson S. P. (1999) Biochem. J. 338, 203–209 [PMC free article] [PubMed] [Google Scholar]
- 39.Cho M. J., Pestina T. I., Steward S. A., Lowell C. A., Jackson C. W., Gartner T. K. (2002) Blood 99, 2442–2447 [DOI] [PubMed] [Google Scholar]
- 40.Shankar H., Kahner B. N., Prabhakar J., Lakhani P., Kim S., Kunapuli S. P. (2006) Blood 108, 3027–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorsam R. T., Kim S., Murugappan S., Rachoor S., Shankar H., Jin J., Kunapuli S. P. (2005) Blood 105, 2749–2756 [DOI] [PubMed] [Google Scholar]
- 42.Li Z., Xi X., Du X. (2001) J. Biol. Chem. 276, 42226–42232 [DOI] [PubMed] [Google Scholar]
- 43.Chan V. W., Meng F., Soriano P., DeFranco A. L., Lowell C. A. (1997) Immunity 7, 69–81 [DOI] [PubMed] [Google Scholar]
- 44.Flevaris P., Li Z., Zhang G., Zheng Y., Liu J., Du X. (2009) Blood 113, 893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watson S. P., Auger J. M., McCarty O. J., Pearce A. C. (2005) J. Thromb. Haemost. 3, 1752–1762 [DOI] [PubMed] [Google Scholar]
- 46.Garcia A., Quinton T. M., Dorsam R. T., Kunapuli S. P. (2005) Blood 106, 3410–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thamilselvan V., Craig D. H., Basson M. D. (2007) FASEB J. 21, 1730–1741 [DOI] [PubMed] [Google Scholar]
- 48.Reed G. L., Fitzgerald M. L., Polgár J. (2000) Blood 96, 3334–3342 [PubMed] [Google Scholar]
- 49.Randriamboavonjy V., Schrader J., Busse R., Fleming I. (2004) J. Exp. Med. 199, 347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marshall S. J., Senis Y. A., Auger J. M., Feil R., Hofmann F., Salmon G., Peterson J. T., Burslem F., Watson S. P. (2004) Blood 103, 2601–2609 [DOI] [PubMed] [Google Scholar]
- 51.Morrell C. N., Matsushita K., Chiles K., Scharpf R. B., Yamakuchi M., Mason R. J., Bergmeier W., Mankowski J. L., Baldwin W. M., 3rd, Faraday N., Lowenstein C. J. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 3782–3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiang T. M., Dixit S. N., Kang A. H. (1976) J. Lab. Clin. Med. 88, 215–221 [PubMed] [Google Scholar]
- 53.Woulfe D., Jiang H., Morgans A., Monks R., Birnbaum M., Brass L. F. (2004) J. Clin. Invest. 113, 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J., De S., Damron D. S., Chen W. S., Hay N., Byzova T. V. (2004) Blood 104, 1703–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gambaryan S., Kobsar A., Hartmann S., Birschmann I., Kuhlencordt P. J., Müller-Esterl W., Lohmann S. M., Walter U. (2008) J. Thromb. Haemost. 6, 1376–1384 [DOI] [PubMed] [Google Scholar]
- 56.Riba R., Patel B., Aburima A., Naseem K. M. (2008) J. Thromb. Haemost. 6, 2121–2131 [DOI] [PubMed] [Google Scholar]
- 57.Kim S., Jin J., Kunapuli S. P. (2004) J. Biol. Chem. 279, 4186–4195 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.