Abstract
Objective
This study examines determinants of patient’s side effects from arthritis medication. Proposed predictors were patient’s beliefs about medications, objective disease activity, treatment regimen, psychiatric and rheumatoid arthritis symptoms.
Methods
In a longitudinal design 100 rheumatoid arthritis outpatients were investigated at baseline and again at 6-months after receiving both pharmacological and psychosocial treatment.
Results
Multivariate analyses showed no influence of disease status, type of treatment, psychiatric or arthritis symptoms on side effects. Heightened concerns about arthritis medication at baseline predicted side effects at baseline (partial correlation rp = .37, p < .001) and at 6-months (rp = .25, p < .001), after controlling for relevant disease and treatment related variables. In a cross-lagged panel analysis, prior experience with side effects from arthritis medication was ruled out as a cause of heightened concerns, indicating that negative beliefs contribute genuinely to side effects. A comparison of patients who did and did not start new medications showed no difference in side effects in patients with positive beliefs about medications, but led to significantly more side effects in patients with negative beliefs.
Conclusions
Patient’s beliefs about arthritis medication were stable and consistently associated with side effects. Patients with greater concerns about their arthritis medications are at higher risk for developing side effects, especially when starting new drugs. Identifying those patients is important to avoid premature drug discontinuation. Research into cause and preventability of negative attitudes to prescribed medicines is needed.
Keywords: rheumatoid arthritis, psychosocial factors, side effects, beliefs about medicine, patient expectation
Drug side effects place a considerable burden on health care systems, clinicians and patients. For example, the annual costs associated with adverse drug-related events in the US rose from $76.6 billion to over $177.4 billion between 1995 and 2000 1.
Side effects are generally defined as an action of a drug other than the one for which it is being used. They do not only include drug or drug-use induced adverse effects, but also side effects brought about by patient, treatment or doctor-related factors, such as beliefs and expectations. Most studies have focused on the incidence of relatively infrequent but medically serious adverse events, morbidity and mortality 2. However, the majority of side effects are non-serious and non-specific symptoms that are not clearly attributable to the pharmacologic action of a drug. Depending on the method of ascertainment and the patient population, they comprise 62% to 89% of all patient-reported adverse effects 3, 4. Non-specific symptoms that the patient perceives to be medication-related are frightening and distressing to patients, decrease quality of life 5, cause non-adherence 6, 7 and add to the cost of treatment. Such side effects are even seen in patients taking placebo rather than active drugs 8,9. Understanding the determinants of non-specific side effects would be particularly useful in managing patients with chronic diseases, as it may provide guidance in deciding whether to discontinue or adjust the dose of a medication and/or treat the symptom with another agent if the medication is viewed as medically essential.
Side effects to placebo (the nocebo phenomenon) are widely reported. In randomized controlled trials of arthritis therapy, the incidence and type of many side effects are similar in patients taking active drugs compared to those taking placebo 10, 11. In addition, there is a clinical impression that some patients are more likely to report and suffer from bothersome effects than others. This suggests that patient characteristics along with disease and medication variables affect side effect reporting 12. Side effect reporting has been associated with patient’s expectations, prior experiences with medication, gender, age, anxiety, and depression 3, 8, 13. One reliable measure of patient’s positive and negative expectations regarding their medications is the Beliefs about Medicines Questionnaire (BMQ) 14. This questionnaire assesses an individual’s perceptions of medicines prescribed for them on the basis of how they judge their personal need for the medication (Necessity beliefs) relative to their concerns about potential risks (Concerns). In studies across a range of illnesses including asthma, diabetes, cancer 15, depression 16, 17, HIV/Aids 18, 19 and rheumatoid arthritis (RA) 20, 21, medication beliefs are more strongly associated with adherence than socio-demographic or clinical factors, with low adherence related to doubts about personal needs and concerns about side effects. Specific concerns include commonly-held beliefs about the potential dangers of regular medication use like dependence or accumulated long-term effects 14, 22, they are prevalent even among patients who have not experienced side effects from medicines 19. However, little is known about the direct relationship of medication beliefs and side effects.
We hypothesized that patients with negative beliefs about their medications would report more side effects, and that the predictive value of those beliefs persists after taking into account objective measures of disease severity and the medication regimen. Furthermore we hypothesized that pre-existing beliefs about medicines would influence subsequent side effect reporting, as opposed to prior experience with side effects from arthritis medication generating negative beliefs about those medicines. A further aim was to determine whether beliefs about medicines have a specific effect on side effects when patients start with a newly prescribed drug. It was hypothesized that RA patients with heightened specific concerns about arthritis medications would experience more bothersome symptoms that they view to be caused by the new medication 23.
We believe this to be the first investigation of the nature, direction and course of these relationships in RA patients. Understanding these factors can inform clinical practice. If negative beliefs precede increased side effects reporting, needless termination or adjustment of dose, or additional medications to mitigate these nocebo effects might be averted.
Methods
Study Design
The present study was part of a randomized, controlled trial of behavioral interventions for RA. Eligible subjects, who gave informed consent in accordance with the requirements of our Institutional Review Board, were randomized to one of three treatments: Cognitive-behavior therapy, relaxation response training, or RA education. Throughout the study, all patients received medical care as usual from their rheumatologists.
Subjects and Setting
Subjects were identified through a patient registry and public announcements. Patients were included if they were between 18 and 75 years old; met American College of Rheumatology criteria for RA 24, and were fluent and literate in English. There were three exclusion criteria: Fibromyalgia; very serious medical co-morbidity likely to progress substantially or cause death in the ensuing 12 months; and receiving concurrent psychosocial treatment for RA.
Assessment
Side Effect Scale
We defined side effects as any bothersome symptom that the patient subjectively ascribed to their RA medication. Patients were asked about such side effects initially before the start of the study and at follow-up. They were asked: “How much have you been bothered by side effects?” (on a 5 point Likert-scale, from 1 = not at all, to 5 = a great deal), “Have side effects caused you to change intake or dose of your prescribed medications?”, Have you taken over-the-counter or non-prescription medicine to relieve any side-effects?”, and “Have you reported any medication side effects to your doctor?” (dichotomous formats). The 4 questions were integrated into a Side Effects Scale, by calculating the standardized sum-score. Additionally, patients who had reported adverse side effects were asked to list the most problematic symptoms in a free format.
Medication regimen
At each interview, patients enumerated their current RA medications, including analgesics, non-steroidal anti-inflammatory agents, COX-2 inhibitors, salicylates, disease-modifying anti-rheumatic drugs (DMARDs), biologic response modifiers, steroids, and antidepressants prescribed for pain. These medications were categorized hierarchically according to aggressiveness and degree of risk associated with them, and grouped into: symptomatic drugs, steroids, DMARDs, and biological response modifiers.
Beliefs about medicines were measured with the BMQ1 14, 15, 23, an established instrument for assessing people’s perceptions and expectations about medications. It contains a general and a specific section with two subscales each. The subscales of the BMQ-General are General Harm with 4 items (e.g., “Most medicines are addictive”, “Medicines do more harm than good”), and General Overuse with 4 items (e.g., “Doctors use too many medicines” and “If doctors had more time with patients they would prescribe fewer medicines”). The BMQ-Specific measures perceived risks as well as perceived benefits of prescribed medicines. Specific Concerns are measured with 6 items (e.g., “I sometimes worry about the long-term effects of my medicines”, “My medicines disrupt my life”); Specific Necessity with 5 items (e.g., “My health, at present, depends on my medicines”, “Without my medicines I would be very ill”). Items are rated on a Likert scale, from 1 = strongly disagree to 5 = strongly agree. Good reliability and validity have been established in psychiatric and medical ill populations 14.
Disease Activity was assessed with laboratory findings and a complete joint examination. Erythrocyte sedimentation rate (ESR), determined by the Westergren method 25, an acute phase reactant, was used to assess inflammation 26. Joint swelling was rated with a standardized, 28-joint examination by a rheumatologist 27. Each joint was rated by the ACR Glossary 4-point scale (0 = no swelling, 1 = detectable synovial thickening without loss of bony contours, 2 = loss of distinctness of bony contours, 3 = bulging synovial proliferation with cystic characteristics), and a total joint swelling score (JSS) calculated 28. This method yields reproducible results that are associated with ESR and immunochemical determinants 29. Joint swelling is a good index of overall disease activity 30.
Patient-reported symptoms of rheumatoid arthritis were collected in a standardized manner with a 14-item data collection form (the RA Symptom Questionnaire, RASQ) that replicates the standard review of arthritis symptoms, including pain, stiffness, swelling, restriction of movement, fatigue, poor appetite, sleeping problems, and malaise. The 14 questions are rated on a 10 cm visual analog scale from “no” distress (“0”) to the “worst possible” (“10”) and a total symptom score was computed.
Psychiatric symptoms were measured with the Rand Mental Health Inventory (MHI), a standard, widely used questionnaire. It collects common symptoms associated with the more prevalent mental disorders, and has high internal consistency and external validity 31. We employed the 18-item version, and report the summary scores for depression and anxiety (based on 4 and 5 items, respectively).
Statistical data analysis
Bivariate correlations
Bivariate association coefficients were calculated for the Side Effect Scale, the BMQ subscales, disease severity (Erytrocyte sedimentation rate, Swollen joint score), medication (symptomatic drugs, steroids, DMARDs, biologic response modifiers), disease symptoms (RASQ), psychopathology (depression, anxiety), and demographic variables (age, gender) at baseline and 6-month follow-up.
Covariate adjusted analyses
Multiple linear regression analyses were used to test the hypothesis that beliefs about medicines contribute independently to side effects at baseline. Power analyses for the specific increase in R2 in a multiple regression model with four predictors revealed a required total n = 77 to determine at least medium effects (f2 = .15, α = .05, 1− α = .80). After univariate testing of all four BMQ subscales, the Specific Concerns scale was included as main predictor. Objective measures of disease severity, the medication regimen, and additional factors that have been shown to increase incidence and negative impact of patient-reported side effects (i.e., higher levels of anxiety and depression, a younger age, being female) were included in the prediction models as covariates. Initially, the variance explained (R2) through each predictor alone was calculated in unadjusted models. Subsequently, three adjusted models were calculated. Our main predictor, the BMQ Specific Concerns scale, was included in each of the three models. Model 1 controlled for objective parameters of disease activity (erythrocyte sedimentation rate, swollen joint score); Model 2 for disease activity and patient’s medication regimen; and Model 3 controlled for disease activity, medication, gender, age, patient’s arthritis symptoms (RASQ), and psychiatric symptoms (depression, anxiety). Partial correlation coefficients (rp), adjusting for all variables in the respective model, were calculated as indicators of the independent influence of each predictor. For every model the cumulative amount of explained variance (R2) was determined as an indicator of the overall performance of the models. Furthermore, the additional amount of variance explained through Specific Concerns (ΔR2) was calculated to provide a measure for the relative importance of the main predictor.
Longitudinal analyses
The same series of sequential multiple regressions was repeated, still using the baseline BMQ Specific Concerns as the primary predictor, to predict side effects at the 6-month follow-up. Since regression analysis cannot rule out reverse pathways for predictors and criterion, a cross-lagged panel was employed to test the causal relationship of medication beliefs and side effects. Cross-lagged panel correlations are used to analyse theoretic process factor models by testing for spuriousness. If certain statistical assumptions are met (see Appendix A), statistical precedence can be demonstrated 32 by testing and comparing cross-lagged correlations from panel data 33.
Impact of new medications
The specific influence of beliefs about medications in patients taking a newly prescribed drug was tested with planned contrasts. Patient’ scores on the BMQ Specific Concerns scale were dichotomised according to a median split (Specific Concern scores ≥ 17 were considered high); drug regimens were coded as changed if any of the four drug categories not taken at baseline were newly prescribed during the first 6-month of follow-up. Analysis of variance was used with the Side Effects Scale at 6 months as the outcome variable. Our predictors were the dichotomized baseline Specific Concerns scores, a binary variable indicating newly prescribed medications, and the interaction between these two variables. Significance of the interaction term would indicate that new medications lead to relatively more side effect reporting in patients with high concerns than in those with low concerns.
Missing data analyses
Descriptive data screening revealed the following missing data points on a-priori defined covariates: 4 missing values for swollen joint count at baseline, and 1 for RASQ; 31 missing values for swollen joint count at follow-up, 17 for ERS, and 1 for RASQ. We applied Little’s test 34 to test the assumption that data points were missing completely at random (MCAR), and received nonsignificant results (baseline: Chi-Square = 1.32, DF = 2, Sig. = .52; follow up: Chi-Square = 3.36, DF = 5, Sig. = .64) indicating that the hypothesis of MCAR should be maintained. Subsequently, these missings were imputed with multiple imputation algorisms.
Software
Data analyses were conducted using the SPSS Statistical Software Package (version 16.0; SPSS Inc., 2007). Missing data on covariates were imputed using NORM (version 2.03; 35); the Expectation Maximation algorithm was employed to compute starting values and Markov-Chain-Monte-Carlo methods to generate multiple imputations. Power analyses were conducted with G-Power 36. The cross-lagged analyses were conducted using the Mplus Software Package (version 5.1; 37).
Results
A total of 100 subjects were included in the analysis. Sixty-eight patients who started the trial before the BMQ was incorporated were excluded from analysis. Table 1 shows relevant demographic and disease characteristics. The cohort is predominantly female, highly educated, and employed. Of these 100 patients, 87 were followed-up at 6 months after treatment, constituting a retention rate of 87%.
Table 1.
Baseline demographic characteristics for n = 100 RA patients
| Patient Characteristic | Baseline |
|---|---|
| Age in years (range; M + SD) | 24 - 74; 53.0 (13.0) |
| Years since diagnose (range; M + SD) | 0.7 - 50; 13.9 (12.4) |
| Gender (female) | 90 |
| Race (white/ black/ other) | 80/ 11/ 9 |
| Education (high school/ college/ graduate school) | 35/ 25/ 40 |
| Marital status (married/ single/ widowed or divorced) | 49/ 22/ 29 |
| Currently employed | 57 |
Note. M = Mean; SD = standard deviation.
The most frequently reported side effects are presented in Table 2, along with descriptive statistics for the Side Effect Scale, BMQ, and patients’ medication regimens. Abdominal symptoms, including pain, indigestion, heartburn, nausea, skin reactions, rashes, and allergies were the most common side effects reported in free recall.
Table 2.
Descriptive statistics for side effects, medications, and beliefs about medicines at baseline and 6-month follow-up
| Variables | Patient numbers | |
|---|---|---|
| Baseline (n = 100) | Follow-up (n = 87) | |
| Side Effects Scale (M, SD, Cronbach’s alpha) | 4.31 ± 2.31, α=.81 | 2.24 ± 1.81, α=.84 |
| Bothered by side effects from arthritis medication (1-5), (M, SD) | 2.72 ± 1.33 | 1.68 ± 0.98 |
| Self-changed prescribed medication due to side effects | 51 (51 %) | 17 (19.5 %) |
| Taken non-prescribed medication to relieve side effects | 38 (38 %) | 15 (17.2 %) |
| Reported any side effects to your doctor | 70 (70 %) | 21 (27.7 %) |
| Incidence of side effects § | 77 (77%) | 45 (51.7%) |
| Type of side effects in free format # | ||
| Abdominal discomfort (stomach pain, cramps, etc.) | 27 (27 %) | 15 (17.2 %) |
| Skin reactions (rash, ulcer, sores, allergies) | 18 (18 %) | 7 (8.0 %) |
| Nausea | 15 (15 %) | 8 (9.2 %) |
| Weight loss/gain | 12 (12 %) | 3 (3.4 %) |
| Fatigue | 10 (10 %) | 5 (5.7 %) |
| Diarrhea, vomiting | 7 (7 %) | 1 (1.1 %) |
| Headache | 5 (5 %) | 5 (5.7 %) |
| Medication regimen | ||
| Symptomatic drugs | 73 (73 %) | 60 (68.9 %) |
| Steroids | 30 (30 %) | 25 (28.7 %) |
| DMARDs | 58 (58 %) | 45 (51.7 %) |
| Biologic response modifiers | 48 (48 %) | 44 (50.6 %) |
| New drug prescription within 6-months follow-up | - | 17 (19.5 %) |
| BMQ subscales (M, SD, Cronbach’s alpha) | ||
| General Harm (1-20) | 8.86 ± 2.83, α=.68 | 8.57 ± 2.47, α=.61 |
| General Overuse (1-20) | 11.56 ± 3.09, α=.74 | 11.21 ± 2.78, α=.69 |
| Specific Necessity (1-25) | 19.63 ± 3.77, α=.82 | 19.62 ± 3.72, α=.81 |
| Specific Concern (1-30) | 17.01 ± 4.29, α=.73 | 16.63 ± 3.73, α=.71 |
Note. M = Mean; SD = standard deviation
Incidence was defined as the proportion of patients who were at least mildly bothered by side effects or agreed to any of the other three side effect questions
Reported are side effects with a base rate > 5%; DMARDs = Disease modifying anti-rheumatic drugs; BMQ = Beliefs about Medicines Questionnaire.
On the side effect scale, patients were moderately bothered by side effects from their arthritis medication at baseline (M = 2.72, SD = 1.33) and mildly bothered at 6-month follow-up (M = 1.68, SD = 0.98). At baseline, 51 patients (51%) reported having had their arthritis medication changed due to side effects, 38 (38%) reported they had taken over-the-counter medicines to relieve side effects, and 70 (70%) of the patients stated that they had reported side effects to their doctors. At 6-months follow-up these number were reduced to 17 (20%), 15 (17%), and 21 (28%) patients, respectively. The incidence of side effects, defined as the proportion of patients who reported to have been bothered by side effects (item score > 1) or answered “yes” to any of the other three side effect questions, was 77 patients (77%) at baseline and decreased to 45 patients (52%) at 6-month follow-up.
Internal consistencies (Cronbach`s alpha, see Table 2) were high for the Side Effect Scale (baseline: α = .81; follow up: α = .84), and moderate-to-high for the BMQ subscales (baseline: α = .68 to α = .82; follow up: α = .61 to α = .81). Two thirds of the patients took symptomatic drugs such as Acetaminophen at baseline and follow-up. Steroids were taken by nearly one third of patients, DMARDs and biologic response modifiers were each taken by half of the patients throughout the study period. Twenty-four of the patients started on one or more newly prescribed drugs within the half year follow-up period. These included 10 patients who started intake of symptomatic drugs, 6 patients starting with steroids, 4 with DMARDs, and 12 with biologic response modifiers.
Effects of the psychosocial treatments on reported side effects were tested with a repeated measures Generalized Linear Model. The standardized sum-score obtained from the Side Effect Scale was used as the outcome variable for the repeated measures ANOVA, with time entered as the within-subjects factor and the three treatment groups as between-subjects factor. While side effects showed a significant decline over time (F1,84 = 56.74; p < .001), the slopes for the three treatment groups did not show any differences (F2,84 = 4.39; p = .47). Hence, the three treatment arms had no differential effect on side effect reporting and for the subsequent analyses the subjects were pooled and analyzed jointly.
In our analyses of bivariate associations of the Side Effect Scale, the BMQ subscales, and all covariates at baseline and 6-months follow-up, side effects show the highest consistent correlations with the BMQ Specific Concern Scale (rbaseline = .39, p < .01; r6-months = .32, p < .01). Thus, patients holding stronger negative beliefs about their arthritis medication (e.g., “having to take arthritis medications worries me”, “they give me unpleasant side effects”, “I worry about becoming too dependent on them”) report more bothersome events and more severe consequences side effects. The other BMQ subscales showed no significant relationship to side effects (General Harm: rbaseline = −.02, p = .87; r6-months = .06, , p = .22; General Overuse: rbaseline = .12, , p = .22; r6-months = .01, , p = .22; Specific Necessity: rbaseline = .06, , p = .57; r6-months = .22, , p = .22)2. Consistent associations could be seen for the Side Effect Scale and patients’ age as well (rbaseline = −.21, p < .05; r6-months = −.43, p < .01). Younger patients describe significantly more side effects at baseline and follow-up. Furthermore, a higher prevalence of side effects was associated with use of steroids (r6-months = .27, p < .01) and higher anxiety (r6-months = .24, p < .01) at 6 months but not at baseline.
The results of the multiple regression analyses are presented in Table 3. Significant unadjusted effects on side effects at baseline and longitudinally are shown for age (R2 = .05, .18) and BMQ Specific Concern (R2 = .15, .10). Partial correlation coefficients show consistent moderate to large independent effects for Specific Concerns, in all three adjusted models, cross-sectionally and longitudinally. Furthermore, this subscale explained significant additional amounts of variance at each time-point, over and above all control variables, resulting in significant increases in R2 of 5 to 15% at baseline and from baseline to follow-up. The full model explained 25% of the variance in side effect reporting at baseline and 28% longitudinally.
Table 3.
Cross-sectional and longitudinal prediction of non-specific side effects with sequential multiple regression analyses
| Baseline Predictors | Unadjusted effect |
Model 1 R2 = .16** |
Model 2 R2 =.20** |
Model 3 R2 = .25** |
|---|---|---|---|---|
| Criterion: Side effects at baseline | r | rp | rp | rp |
| Age | −.21* | - | - | −.18 |
| Gender | −.14 | - | - | −.09 |
| Arthritis symptoms (RASQ) | .15 | - | - | .10 |
| Depression (MHI) | −.06 | - | - | −.10 |
| Anxiety (MHI) | −.03 | - | - | −.01 |
|
|
||||
| Symptomatic drugs | −.02 | - | −.06 | −.10 |
| Steroids | .14 | - | .09 | .08 |
| DMARDs | −.04 | - | .06 | .01 |
| Biologic response modifiers | .19# | - | .14 | .10 |
|
|
||||
| Erytrocyte sedimentation rate | .08 | .11 | .11 | .11 |
| Swollen joints count | .10 | .02 | .01 | −.06 |
|
|
||||
| BMQ Specific Concern | .39** | .39** | .39** | .37** |
| ΔR2 for BMQ Specific Concern | - | .15** | .14** | .12** |
| Baseline Predictors | Unadjusted effect |
Model 1 R2= .11** |
Model 2 R2= .14** |
Model 3 R2= .28** |
|---|---|---|---|---|
| Criterion: Side effects at 6-months follow-up |
r | rp | rp | rp |
| Age | −.42** | - | - | −.34** |
| Gender | −.10 | - | - | −.04 |
| Arthritis symptoms (RASQ) | .03 | - | - | .06 |
| Depression (MHI) | .18# | - | - | .03 |
| Anxiety (MHI) | .19# | - | - | .12 |
|
|
||||
| Symptomatic drugs | .02 | - | −.01 | −.003 |
| Steroids | .15 | - | .12 | .13 |
| DMARDs | .05 | - | .11 | .04 |
| Biologic response modifiers | .07 | - | .04 | .05 |
|
|
||||
| Erytrocyte sedimentation rate | .04 | .07 | .06 | .06 |
| Swollen joints count | .10 | .04 | .02 | −.03 |
|
|
||||
| BMQ Specific Concern | .32** | .32** | .34** | .25** |
| ΔR2 for BMQ Specific Concern | - | .10** | .11** | .05** |
Note. Model 1: adjusted for disease activity (i.e., Erytrocyte sedimentation rate, swollen joints count); Model 2: adjusted for disease activity, and medication (i.e., Symptomatic drugs, Steroids, DMARDs, Biologic response modifiers); Model 3: adjusted for disease activity, medication, demographics (i.e., age, gender), and symptoms (i.e., RASQ, depression, anxiety).
RASQ = Rheumatoid arthritis symptom questionnaire; MHI = Mental health inventory; DMARDs = Disease-modifying anti-rheumatic drugs; BMQ = Beliefs about Medicines Questionnaire
Dummy codes entered for gender (1 = female; 2 = male).
rp = partial correlation coefficients, adjusting for all variables in the respective model
p<0.10
p<0.05
p<0.01
ΔR2 = change in explained variance through integration of BMQ specific concerns with significance of F change; R2 = Cumulative amount of explained variance through all predictors in the model.
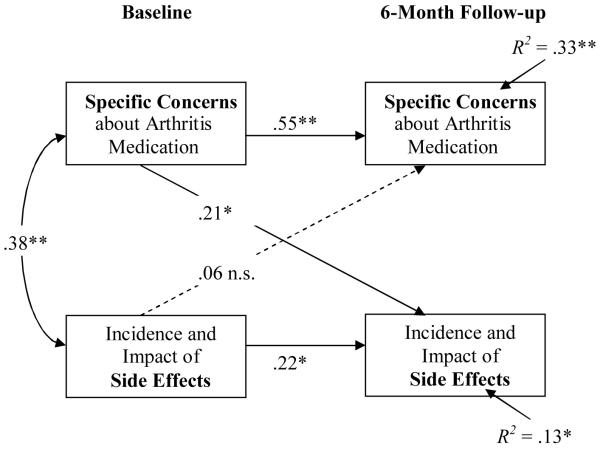
The results of the cross-lagged analysis are depicted in Figure 1 (see Appendix A for tests of statistical assumptions). Covariates showing significant bivariate correlations with side effects (i.e., age at baseline and 6 months, steroids and anxiety at 6 months) were partialed out of the respective cross-lagged correlations. The coefficient for the beliefs – side effects path was significant and of moderate size (rSC Baseline/ AE Follow-up = .21, p(two-tailed) = .032), while the side effects – beliefs path was nonsignificant (rAE Baseline/SC Follow-up = .06, p(two-tailed) = .46); differences were significant in the hypothesized direction (zPearson-Filon = 3.03).
Figure 1.
Cross-lagged panel analyses of patient-reported non-specific side effects (Side Effect Scale) and Concerns about medicines (BMQ Specific Concerns) over the course of the study.
Note. The Side Effect Scale was standardized within baseline, and 6-month follow-up measurements, in order to exclude variance due to mean changes in side effect reporting over time; age, intake of steroids and anxiety were partialed out of all included data; BMQ = Beliefs about Medicines Questionnaire; *p<0.05, ** p<0.01.
The planned contrast for the interaction of BMQ Specific Concern with changes in medication regimen regarding side effects at follow-up was significant (t(83) = 1.77, p = .04). Thus, heightened concerns about arthritis medication led to increased side effect reporting in general and specifically in those 17 patients who started with newly prescribed arthritis medication within the study period (see Figure 2).
Figure 2.
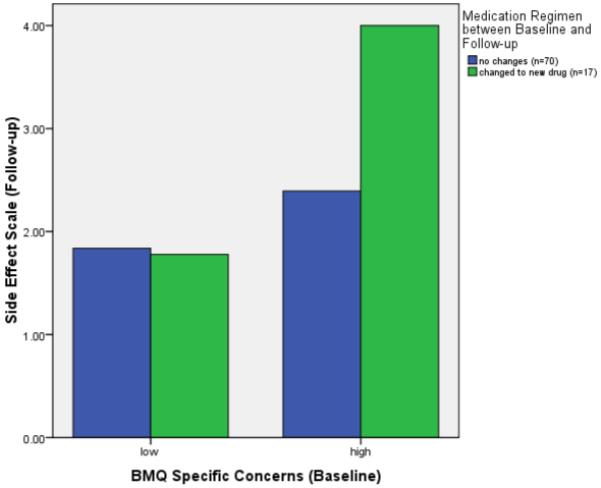
Side effects at 6 month follow-up as a function of BMQ Specific Concerns and changes in medication regimen.
Note. BMQ = Beliefs about Medicines Questionnaire. Scores for the BMQ Specific Concerns Scale are dichotomized with a median split (scores ≥ 17 considered high). Included are n = 87 subjects with follow-up data for the Side Effect Scale.
To analyse the sensitivity of the documented results to selective dropout, we employed intention-to-treat analyses. Side effects data were imputed for the 13 patients who dropped out at 6 month follow up, with a best-case and a worst-case criterion; imputing the minimum and the maximum score of the side effect scale, respectively. We tested the MCAR assumption and received a nonsignificant result (Chi-Square = 1.438, DF = 1, Sig. = .230) indicating that the hypothesis of MCAR should be maintained. Following these imputations we re-ran the sequential multiple regression analyses. Former results were maintained with the best-case imputation (Model 3: R2= .24, p = .01; ΔR2 for BMQ Specific Concern = .07, p = .005), but not maintained with the worst-case assumption (Model 3: R2= .06ns; ΔR2 for BMQ Specific Concern = .02 ns). This indicates that especially improvements in the side effect scale could be explained by medication concerns. Conversely, when all dropouts were considered as maximally impaired by side effects (worst-case), concerns about medicines no longer had a significant additional variance-explaining effect. Even though results were only stable for the best-case assumption, the fact that the side effect data of patients who dropped out at follow-up were MCAR, proofs it unlikely that systematic effects related to side effect reporting caused treatment drop out.
Discussion
In this prospective study of RA outpatients, patients’ beliefs about arthritis medication influenced side effects after adjusting for disease activity, types of medications, and levels of prior experience with side effects. Patients with greater concerns about their medication experienced more side effects, both cross-sectional and longitudinal. Those starting new medications during the study period were more likely to suffer side effects if they had negative perceptions about medicines. Furthermore, those patients reported more side effects to their doctors, took more non-prescribed medications to relieve side effects, and more often adjusted their own prescribed medication due to side effects. The prospective influence of medication beliefs (specific concerns) on side effects justifies further work to gain a better understanding of why some patients start out with a more negative attitude and greater concerns about prescribed medicines than others.
Higher concern about arthritis medication was the strongest predictor of adverse side effects, over and above the influences of objective disease activity, medication regimen, symptomatic and socio-demographic factors. The only other significant influence was age, with younger RA patients reporting more adverse symptoms throughout the study period. Whether this is due to differing expectations, increased awareness or sensitivity or other factors still needs to be clarified. To our knowledge only one other study has investigated determinants of side effect reporting in RA, and it did not study beliefs about medicines 12. In this study, Barsky et al. documented increased reporting of side effects in patients with hypochondriacal attitudes, an amplifying somatic style, and a tendency to somatize. These effects persist after controlling for disease activity.
Over 6 months, we documented incidence rates of patient-reported side effects from arthritis medication of 77% and 52%, accompanied by mild to moderate levels of distress, consistent with rates reported in previous studies of RA 21, 38. Surprisingly, side effects decreased considerably over the 6 month study period, while none of the three psychosocial treatments involved any specific interventions on dealing with medication side effects. They comprised of general information, relaxation training and cognitive behavioral skills, which when applied to discomfort, distress an disability caused by side effects, can reduce side effect related distress and even incidence of them. Especially, the non-specific medication side effects, which are idiosyncratic in the sense that they are caused more by patient related factors than drug specific ones, should be susceptible to psychosocial therapy induced amelioration.
In the present study, patients changed their prescribed medication regimen on their own 20 - 51% of the time, corresponding with non-adherence rates of 20 - 70% reported in the literature 39. An inconsistent relationship has been found between medication adherence and objective measures of disease activity in RA 20, 39, while the importance of psychosocial and demographic factor such as self-efficacy 40, beliefs about medicines 20, and age 41 have been demonstrated. In our study the prevalence and perceived distress from side effects were closely related to patient-initiated changes in arthritis medication (i.e., a possible index of non-adherence).
Previous studies have shown that pretreatment beliefs about medicines (necessity beliefs and concerns) influence uptake and adherence to prescribed medicines 20, 21. The present study suggests that medication concerns may also influence the experience of side effects. RA patients who are especially concerned about their arthritis medications, or who expect side effects, are at greater risk of experiencing them. Furthermore, when starting a new drug these patients are more likely to change their medication or discontinue treatment. In such cases a change in the regimen might actually not be indicated since the benefit of an effective agent might be obscured by non-specific side effects that are more the product of the patients’ attitudes, beliefs and concerns than of the drug itself. Previous reports have documented similarly high rates of medication concerns in RA patients 21, 42, one qualitative study showed the importance of patient’s expectancy of side effects on side effect reporting in RA 43. However, unlike these important observations which were cross-sectional, thereby making causality uncertain, ours is the first prospective study of these relationships.
This study has several limitations. The patients included in this study had a rather high educational level and they were all participants in a study offering psychosocial treatment for rheumatoid arthritis patients. Thus, it is unclear whether the results would apply to a completely unselected sample of arthritis patients. A replication of the analyses in a sample of arthritis outpatients receiving just pharmacological treatment might be warranted. Moreover, the naturalistic design of the pharmacologic treatment allowed us to monitor medication regimens but not to control them. As a result, the sample included a number of patients starting new agents during the study and may not be generalizable to populations with fewer changes in regimens or dosing. A potential selection bias might result from studying only 100 from the originally 168 participants, but we judge this unlikely as the BMQ was simply unavailable for the first 68 participants. Finally, our side effect data collection form comprising investigator-derived questions without pre-tested psychometric properties constitutes a limitation. Since reliable and validated patient-report side effect scales are not yet standard in clinical practice 44, we used a face-valid and standardized data collection form which essentially replicated what rheumatologists ask their patients in routine practice. Replications of our analyses using a structured and validated side effect scale 45 are needed. Furthermore, in analyzing the impact of expectations about medications in future studies, it would be interesting to distinguish more clearly between specific and non-specific side effects (i.e., with pharmacological ratings).
Implications
In this study of RA patients, individual concerns about arthritis medication had a clinically meaningful influence on patient-reported side effects, even in patients who had been chronically ill for an average of 12 years. Thus, side effects may be as much a characteristic of the person experiencing them, as of their medical condition or drug treatment.
These findings highlight the importance of patients’ beliefs 3 and they are useful to identify, early in the course of treatment, patients at high risk for non-specific side effects and modify clinical care. Exploring patient’s individual attitudes towards medications proactively or being alert to their potential concerns and addressing them might help the patient take a more active role in their health care and deal with side effects. Moreover, efforts to identify and adapt negative beliefs about medicines early in the course of pharmacological treatment may preempt occurrence of non-specific side effects and associated noncompliance to pharmacological treatment.
Acknowledgments
Supported by research grant R01 AR 4701401 from the National Institute of Arthritis & Musculoskeletal and Skin Diseases.
Appendix A
Tests of statistical assumptions of the cross-lagged panel analysis
To insure appropriate causal interpretation of panel data, we tested whether three statistical assumptions were met: instrument reliability, synchronicity of measurement (or simultaneous measurement of variables over time), and stationarity (i.e., consistency in strength and direction of relationships) 33. First, to examine reliability, we computed internal consistencies and test-retest correlations (i.e., autocorrelations) of the study variables. Satisfying internal consistencies and significant autocorrelations resulted for the BMQ Specific Concern (SC) (αSC Baseline = .73; αSC Follow-up = .71; rSC Baseline/ Follow-up = .61, p < .001) and the Side Effect Scale (SES) (αSES Baseline = .81; αSES Follow-up = .84; rSES Baseline/ Follow-up = .22, p = .027). Note that the autocorrelation of the Side Effect Scale is significant and small, while internal consistencies are high, indicating a high sensitivity of the scale. Second, regarding synchronicity we achieved simultaneous assessment of beliefs about medicines and side effects during each research visit. Third, we examined stationarity by comparing the correlations between BMQ Specific Concern and the Side Effect Scale at baseline and follow-up. Pearson-Filon comparisons 32 indicated that the synchronous correlations did not differ significantly (rSES/SC Baseline = .39, p < .001; rSES/SC Follow-up = .37, p < .001; zPearson-Filon = 0.84). Finally, statistical dominance of the beliefs – side effects relationship versus the side effects – beliefs relationship was analyzed by comparing the cross-lagged correlations.
Footnotes
©Professor Rob Horne, rob.horne@pharmacy.ac.uk
Interestingly, BMQ Specific Concern showed high correlations with General Harm (rbaseline = .60, r6-months = .39, ps < .01) and General Overuse (rbaseline = .46, r6-months = .45, ps < .01), but not with Specific Necessity.
References
- 1.Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc. 2001;41:192–9. doi: 10.1016/s1086-5802(16)31229-3. [DOI] [PubMed] [Google Scholar]
- 2.Zed PJ, Abu-Laban RB, Balen RM, et al. Incidence, severity and preventability of medication-related visits to the emergency department: a prospective study. CMAJ. 2008;178:1563–9. doi: 10.1503/cmaj.071594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barsky AJ, Saintfort R, Rogers MP, Borus JF. Nonspecific Medication Side Effects and the Nocebo Phenomenon. JAMA. 2002;287:622–7. doi: 10.1001/jama.287.5.622. [DOI] [PubMed] [Google Scholar]
- 4.Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289:1107–16. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 5.Larsen EB, Gerlach J. Subjective experience of treatment, side effects, mental state and quality of life in chronic schizophrenic out-patients treated with depot neuroleptics. Acta Psychiatr Scand. 1996;93:381–8. doi: 10.1111/j.1600-0447.1996.tb10664.x. [DOI] [PubMed] [Google Scholar]
- 6.FitzGerald M, Chan CK, Boulet LP. Medication use, asthma control and oropharyngeal side effects in a population of canadian asthma patients. Proc Am Thorac Soc. 2006;3:A593. [Google Scholar]
- 7.Mohr D, Likosky W, Boudewyn A. Side effects profile and adherence in the treatment of multile sclerosis with interferon beta-1a. Mult Scler. 1998;4:487–9. doi: 10.1177/135245859800400605. et a. [DOI] [PubMed] [Google Scholar]
- 8.Rief W, Avorn J, Barsky AJ. Medication-attributed adverse effects in placebo groups. Arch Intern Med. 2006;166:155–60. doi: 10.1001/archinte.166.2.155. [DOI] [PubMed] [Google Scholar]
- 9.Rief W, Nestoriuc Y, von Lilienfeld-Toal A, et al. Do Placebos in tricyclic antidepressant trials induce more side effects than placebos in SSRI trials? - A systematic review. Drug Safety. doi: 10.2165/11316580-000000000-00000. in press. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly P, Lloyd K, Campbell H. Indomethacin in rheumatoid arthritis: an evaluation of its anti-inflammatory and side effects. Br Med J. 1967;1:69–75. doi: 10.1136/bmj.1.5532.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Enverdingen AA, Jacobs JWG, Siewertsz van Reesema DR, Bijlsma JWJ. Low-dose Prednisone therapy for patients with early active rheumatoid arthritis: clinical efficacy, disease-modifying properties, and side effects. Ann Intren Med. 2002;136:1–12. doi: 10.7326/0003-4819-136-1-200201010-00006. [DOI] [PubMed] [Google Scholar]
- 12.Barsky AJ, Orav JE, Ahern DK, Rogers MP, Gruen SD, Liang MH. Somatic style and symptom reporting in rheumatoid arthritis. Psychosomatics. 1999;40:396–403. doi: 10.1016/s0033-3182(99)71204-1. [DOI] [PubMed] [Google Scholar]
- 13.Rief W, Nestoriuc Y, Hofmann SG. The power of expectation - understanding the placebo and nocebo phenomenon. Social and Personality Psychology Compass. 2008;2(4):1624–37. [Google Scholar]
- 14.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychology & Health. 1999;14(1):1. [Google Scholar]
- 15.Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555–67. doi: 10.1016/s0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 16.Aikens JE, Neas DE, Nau DP, Klinkman MS, Schwenk TL. Adherence to maintenance-phase antidepressant medication as a function of patient beliefs about medication. Ann Fam Med. 2005;3:23–30. doi: 10.1370/afm.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunot V, Horne R, Leese MN, Churchill RC. A cohort study of adherence to antidepressants in primary care: The influence of antidepressant concerns and treatment preferences. Prim Care Companion J Clin Psychiatry. 2007;9:91–9. doi: 10.4088/pcc.v09n0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez J, Penedo F, Llabre M, et al. Physical symptoms, beliefs about medications, negative mood, and long-term HIV medication adherence. Ann Behav Med. 2007;34:46–55. doi: 10.1007/BF02879920. [DOI] [PubMed] [Google Scholar]
- 19.Horne R, Cooper V, Gellaitry G, Date HL, Fisher M. Patients’ perceptions of Highly Active Antiretroviral Therapy in relation to treatment uptake and adherence: The utility of the Necessity-Concerns Framework. JAIDS. 2007;45:334–41. doi: 10.1097/QAI.0b013e31806910e3. [DOI] [PubMed] [Google Scholar]
- 20.Treharne GJ, Lyons AC, Kitas GD. Medication adherence in rheumatoid arthritis: effects of psychosocial factors. Psychology, Health & Medicine. 2004;9:337–49. [Google Scholar]
- 21.Neame R, Hammond A. Beliefs about medications: a questionnaire survey of people with rheumatoid arthritis. Rheumatology. 2005;44(6):762–7. doi: 10.1093/rheumatology/keh587. [DOI] [PubMed] [Google Scholar]
- 22.Clifford S, Barber N, Horne R. Understanding different beliefs held by adherers, unintentional nonadherers and intentional nonadherers: Application of the Necessity-Concerns Framework. J Psychosom Res. 2008;64:41–6. doi: 10.1016/j.jpsychores.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Horne R. Patients’ beliefs about treatment: The hidden determinant of treatment outcome? J Psychosom Res. 1999;47:491–5. doi: 10.1016/s0022-3999(99)00058-6. [DOI] [PubMed] [Google Scholar]
- 24.Arnett FC, Edworthy S, Bloch DA, et al. The American Rheumatism Association l987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 25.Sox HC, Liang MH. The erythrocyte sedimentation rate. Guidelines for rational use. Ann Intern Med. 1986;104:515–23. doi: 10.7326/0003-4819-104-4-515. [DOI] [PubMed] [Google Scholar]
- 26.Ward M. Relative sensitivity to change of the erythrocyte sedimentation rate and serum C-reactive protein concentration in rheumatoid arthritis. J Rheumatol. 2003;31:884–95. [PubMed] [Google Scholar]
- 27.Smolen JS, Breedveld FC, Eberl G, Jones I, Leeming M, Wylie GL. Validity and reliability of the twenty-eight-joint count for the assessment of rheumatoid arthritis activity. Arthr Rheum. 1995;38:38–43. doi: 10.1002/art.1780380106. [DOI] [PubMed] [Google Scholar]
- 28.ARA Glossary Committee. New York NCAIL . Vol I: Signs and Symptoms [monograph] Contact Associates International Ltd.; New York, NY: 1982. Dictionary of the Rheumatic Diseases. 1982. [Google Scholar]
- 29.Prevoo MLL, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LBA, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Arthr Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 30.van der Heijde DM, van’t Hof MA, van Riel PL. Validity of single variables and composite indices for measuring disease activity in rheumatoid arthritis. Ann Rheum Dis. 1992;51:177–81. doi: 10.1136/ard.51.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hays RD, Stewart AL. The structure of self-reported health in chronic disease patients. J Consult Clin Psychol. 1990;2:22–30. [Google Scholar]
- 32.Kenny DA. Cross-lagged panel correlation: A test for spuriousness. Psychol Bull. 1975;82(6):887–903. [Google Scholar]
- 33.Kenny DA, Harackiewick JM. Cross-Lagged Panel Correlation: Practice and Promise. J Appl Psychol. 1979;64(4):372–9. [Google Scholar]
- 34.Little RJA. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83:1198–202. [Google Scholar]
- 35.Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–77. [PubMed] [Google Scholar]
- 36.Erdfelder E, Faul F, Buchner A. GPOWER: A general power analysis program. Behavior Research Methods, Instruments & Computers. 1996;28:1–11. [Google Scholar]
- 37.Muthen LK, Muthen BO. Mplus User’s Guide. Muthen & Muthen; Los Angeles, CA: 1998. [Google Scholar]
- 38.Berry D, Bradlow A, Bersellini E. Perceptions of the risks and benefits of medicines in patients with rheumatoid arthritis and other painful musculoskeletal conditions. Rheumatology. 2004;43:901–5. doi: 10.1093/rheumatology/keh196. [DOI] [PubMed] [Google Scholar]
- 39.de Klerk E, van der Heijde DM, Landewe R, van der Tempel H, Urquhart J, van der Linden S. Patient compliance in rheumatoid arthrits, polymyalgia rheumatica, and gout. J Rheumatol. 2003;30:44–54. [PubMed] [Google Scholar]
- 40.Brus H, van de Laar M, Taal E, Rasker J, Wiegman O. Determinants of compliance with medication in patients with rheumatoid arthrits: the importance of self-efficacy expectations. Patient Education and Counseling. 1999;36:57–64. doi: 10.1016/s0738-3991(98)00087-1. [DOI] [PubMed] [Google Scholar]
- 41.Park DC, Hertzog C, Leventhal H, et al. Medication adherence in rheumatoid arthritis patients: Older is wiser. J Am Geriat Soc. 1999;47:172–83. doi: 10.1111/j.1532-5415.1999.tb04575.x. [DOI] [PubMed] [Google Scholar]
- 42.Kumar K, Gordon C, Toescu V, et al. Beliefs about medicines in patients with rheumatoid arthritis and systemic lupus erythematosus: a comparison between patients of South Asian and White British origin. Rheumatology. 2008;47(5):690–7. doi: 10.1093/rheumatology/ken050. [DOI] [PubMed] [Google Scholar]
- 43.Goodacre LJ, Goodacre JA. Factors influencing the beliefs of patients with rheumatoid arthritis regarding disease modifying medication. Rheumatology. 2004;43:583–6. doi: 10.1093/rheumatology/keh116. [DOI] [PubMed] [Google Scholar]
- 44.Foster JM, van der Molen T, Caeser M, Hannaford P. The use of questionnaires for measuring patient-reported side effects of drugs: its importance and methodological challenges. Pharmacoepide Drug Saf. 2008;17(3):278–96. doi: 10.1002/pds.1533. [DOI] [PubMed] [Google Scholar]
- 45.Rief W, Barsky AJ, Glombiewski JA, Nestoriuc Y, Glaesmer H, Brähler E. Assessing generic side effects in clinical trials: Reference data from the general population. doi: 10.1002/pds.2067. submitted. [DOI] [PubMed] [Google Scholar]