Abstract
Objective
Neutrophils represent a prominent component of inflammatory joint effusions and are required for synovial inflammation in mouse models, but mechanisms are poorly understood. We developed a system to test the importance of production of specific factors by neutrophils in a mouse model of arthritis.
Methods
Neutrophil-deficient Gfi-1−/− mice were sub-lethally irradiated, then engrafted with donor bone-marrow cells (BMC), which resulted in production of mature neutrophils within two weeks. By reconstituting with BMC from mice lacking selected pro-inflammatory factors, mice specifically lacking these factors on neutrophils were generated. Arthritis was initiated by transfer of K/BxN serum to identify the role of defined neutrophil factors on arthritis incidence and severity.
Results
Neutrophils lacking the signaling chain of stimulatory Fc receptors (FcRγ −/−) were unable to elicit arthritis, but neutrophils lacking Fcγ RIII still did so. Neutrophils lacking the chemotactic or adhesion receptors C5aR or CD11a/LFA-1 also failed to initiate arthritis but could enter joints in which inflammation had been initiated by wild-type neutrophils. Neutrophils unable to produce interleukin-1 α and β (IL-1αβ −/−) or leukotrienes (5-LO−/−) produced arthritis of intermediate severity. Inability of neutrophils to make tumor necrosis factor (TNF), or to express receptors for TNF or IL-1, had no effect on arthritis.
Conclusion
A novel transfer system was developed to identify neutrophil production of FcRγ , C5aR, and CD11a/LFA-1 as critical components of autoantibody-mediated arthritis. Neutrophil production of IL-1 and leukotriene B4 likely contributes to inflammation but is not essential. Molecular requirements for neutrophil influx into joints become more permissive after inflammation is initiated.
Keywords: arthritis, neutrophils, mouse model, inflammation, autoantibodies
A variety of autoimmune responses in mice can lead to inflammatory arthritis, with histology resembling that of RA: expansion of type A and type B synoviocytes, angiogenesis, recruitment of lymphocytes to the synovium and neutrophils to the joint fluid, and destruction of cartilage and bone. Many of these arthritis models are dependent on breaking T cell tolerance, followed by the production of autoantibodies (1). The K/BxN model, characterized by autoimmunity to the glycolytic enzyme glucose-6-phosphate isomerase (GPI), has been particularly valuable because disease can be transferred into normal recipients with serum or IgG from arthritic mice (2), allowing isolated dissection of effector phase mechanisms downstream of autoantibody production. The effector phase can proceed independently of contributions from the adaptive immune system, since lymphocytes are dispensible (2), but it requires contributions from several cells of the innate immune system. Neutrophils appear to be absolutely necessary, with no contradictory data arising since the first report of this finding (3); requirements for mast cells (4, 5) and macrophages (6, 7), in contrast, may depend on the details of the experimental model and are thus more controversial. The effector phase involves several molecular mediators, including the cytokines IL-1 and TNF (8), the alternative pathway of complement and the receptor for C5a (9), the low-affinity Fc receptor Fcγ RIII (5, 9), the leukocyte adhesion molecule LFA-1 (10), and leukotriene B4 and its receptor BLT-1 (11, 12). In contrast, the effector phase is independent of the classical pathway of complement activation, membrane attack complex, and complement receptors CR-1, -2, and -3 (9, 13), the high-affinity Fc receptor, Fcγ RI (9), the IgE receptor, FcεR (14), the leukocyte adhesion molecule Mac-1 (10), and the oxidative burst of neutrophils (3). Similar requirements have been described for the antibody-mediated effector phase of collagen-induced arthritis (15–19).
On the other hand, it is not clear from the experiments summarized above how these molecular triggers are orchestrated, in particular which cell types require which signaling pathway for the unfolding of antibody-mediated arthritis. While it has been shown that mast cells require IL-1, FcRγ , and the C5aR (14, 20), the molecular details of neutrophil involvement remain less charted. Recently, roles for neutrophil expression of 5-lipoxygenase (5-LO, required for leukotriene synthesis) and BLT-1 in the K/BxN serum-transfer model were demonstrated by restoration of susceptibility to arthritis by transfer of normal neutrophils into strains genetically deficient in these two factors (11, 12). The importance of neutrophil expression of any of the other required factors listed above is unknown, but is plausible in many cases, given that they can express, or be affected by, most of the critical molecular components of the effector phase of autoantibody-mediated arthritis.
Regarding inflammatory cytokines, neutrophils can be induced to express IL-1α and β and to secrete IL-1β by stimulation with lipopolysaccharide (LPS), granulocyte-macrophage colony stimulating factor (GM-CSF) or IL-1 itself (21, 22). LPS or GM-CSF also induces the synthesis and release of TNF (23–25). When added to neutrophils in vitro, IL-1 promotes the synthesis of leukotriene B4 (26) and primes these cells for phagocytosis, degranulation, and superoxide generation in the presence of other stimuli (27–29). Both IL-1 and TNF enhance the bacteriocidal function of neutrophils (30), but expression of IL-1RI by neutrophils was recently shown to be dispensible for IL-1-dependent recruitment in vivo (31). Mouse neutrophils express both stimulatory (Fcγ RI, RII, and RIV) and inhibitory (RII) Fc receptors (32). Fcγ RIII is important for the binding of immune complexes and associated mobilization of calcium, activation of phospholipase A, and downstream effects such as the oxidative burst (33). As for factors involved in the migration of neutrophils out of the vasculature and into tissues, the integrin LFA-1 (CD11a/CD18) is important in their tight adhesion to vascular endothelium that precedes extravasation into tissue. Absence of CD18, leading to loss of LFA-1 and other adhesion molecules, is the cause of a well-described human immunodeficiency associated with chronic bacterial infections. The anaphylotoxin C5a is a potent chemoattractant for neutrophils (34), reduces their sensitivity to apoptosis (35), induces the oxidative burst, and promotes phagocytosis, degranulation (36, 37), increased expression of adhesion molecules (38), and production of macrophage migration inhibitory factor (MIF) (39).
To dissect the role of neutrophils in inflammatory arthritis, we have developed a transfer model to generate mice lacking expression of a given factor in this cell type. Mice with disruption of the gene for the transcription factor Gfi-1 have a selective defect in the ability to generate mature neutrophils (40). We first established that Gfi-1-deficient mice are, as expected, highly resistant to serum-transferred arthritis. Thus, we could use these mice as hosts for neutrophil precursors derived from a panel of mutant strains, thereby assessing which pathways need to be operative in neutrophils for the induction of antibody-mediated arthritis.
MATERIALS AND METHODS
Mice
KRN and K/BxN mice were generated and propagated as described (41). Gfi-1+/− mice, on a mixed C57Bl/6 (B6) and 129 genetic background (40), were a gift from S. Orkin (Children’s Hospital and Dana-Farber Cancer Institute, Harvard Medical School) and were bred to obtain Gfi-1−/− and littermate control mice. This colony was maintained on sulfamethoxizole/trimethoprim, which greatly improved the health of the Gfi-1−/− mice and led to a low mortality rate. Mice deficient in both IL-1α and β (42) and back-crossed to the B6 background were a gift from Y. Iwakura (U. of Tokyo). Mice deficient in C5aR (43) and back-crossed to the B6 background were a gift from C. Gerard (Children’s Hospital, Harvard Medical School); deficient in CD11a (44) and back-crossed to the B6 background were a gift from C. Ballantyne (Baylor College of Medicine); and lacking 5-LO (45) and back-crossed to B6 were a gift from B. Koller (U. of North Carolina). Mice deficient in IL-1RI (B6.129S7-Il1r1tm1Imx), TNF (B6;129S-Tnftm1Gkl), both TNF-RI and TNF-RII (B6;129S-Tnfrsf1atm1ImxTnfrsf1btm1Imx), or Fcγ RIII (B6.129P2-Fcgr3tm1Sjv) were purchased from the Jackson Laboratory, as were B6 mice congenic for CD45.1 (B6.SJL-PtprcaPepcb/BoyJ) and B6 or B6/129 intercrossed controls (B6129SF2/J). Mice deficient in FcRγ (B6.129P2-Fcer1gtm1RavN12) were purchased from Taconic. All mice were maintained under specific- pathogen-free conditions at Harvard Medical School facilities using an IACUC-approved protocol 03024.
Screening for Gfi-1 −/− mice
We developed a PCR-based screening procedure simpler than the one originally described (40). Tail DNA was amplified using standard conditions and the following primers: 5’ KO (Neo) GTAGAATTCCCCGCAAGAGGCCC, 3’ KO AGAACACCTGAGGGCGAGT, 5’ WT AGCCTGGGGACAGGTTTTAC, 3’ WT AACCTAAACCTGGCCGAACT. The wild type (WT) Gfi-1 allele appeared as a band of about 680 bases, and the disrupted allele as a band of about 450 bases.
Bone marrow reconstitution
Recipient mice were sub-lethally irradiated (4 Gy). Donor bone-marrow cells (BMC) were depleted of red blood cells (RBC) by hypotonic lysis, then incubated with a mixture of mature-lineage-binding biotinylated monoclonal antibodies (mAbs) [erythrocytes (Ter119), granulocytes (RB6-8C5), monocytes (M1/70), B cells (RA3-6B2), and T cells (KT3), all purified and biotinylated in-house], followed by streptavidin microbeads and magnetic depletion (MACS, Milenyi Biotech) per the manufacturer's instructions. Remaining cells were washed with phosphate-buffered saline (PBS) and injected intravenously (i.v.), 2–10 x 105 cells per recipient mouse. Two weeks later, the presence of mature neutrophils was confirmed by examination of blood smears, and arthritis was induced within a week of this evaluation.
In some experiments, circulating mature neutrophils were quantified 2 weeks after arthritis induction by hemacytometer count of > 100 WBC combined with flow cytometry to determine the percentage of WBC that were neutrophils, based on scatter profiles and Gr-1/MAC3 staining (see below). Mice that did not have circulating neutrophils at this time-point (25/84 = 30% of mice screened this way) uniformly did not develop arthritis beyond the very mild disease sometimes seen in unreconstituted Gfi-1 −/− mice, and were excluded from the analyses.
In other experiments, recipient mice were not irradiated and unfractionated bone marrow cells, equivalent to the amount obtained from the femurs and tibias of one donor B6 mouse, were injected i.v. daily for 4 days (denoted as days 0–3). K/BxN serum (see below) was co-injected i.v. with the cells on days 0 and 2.
Serum-transferred arthritis
Serum was collected from K/BxN mice at 7 weeks of age and stored at −20° C. Serum was injected intraperitoneally (i.p.) by our standard protocol: 0.15 ml on day 0 and again on day 2. The four paws were assessed by measurement of the right ankle using a precision caliper (Kafer dial thickness gauge with flat anvils, Long Island Indicator) and by a clinical index routinely used in our lab (46): 0–3 in each paw (thus 0–12 total score), where “0” indicates no swelling, “1” signifies either swelling confined to one or two digits or mild swelling of the larger structures, “3” indicates severe arthritis involving the wrist or ankle but extending along the dorsum of the paw to the bases of the digits, and “2” indicates severity intermediate between 1 and 3. Arthritis was evaluated at least twice per week, and results of different groups were compared by two-tailed T-tests, with P < 0.05 considered significant.
Flow cytometry
All steps were at room temperature (RT) or warmer to prevent neutrophil activation. Blood was collected into PBS/EDTA, and RBC were removed by dextran precipitation and hypotonic lysis. Remaining cells were treated with mAb 2.4G2 to block Fc receptors, then stained with various combinations of labeled mAbs and analyzed on a Coulter Epics XL-MCL instrument. Mature neutrophils were identified by the forward versus side scatter profile and by Gr-1hi and MAC3neg staining.
Synovial fluid cells were obtained by puncture of an inflamed ankle or wrist, with the fluid collected with a micropipette and diluted immediately into PBS containing 10 mM EDTA. Staining proceeded as above.
Staining reagents included: phycoerythrin (PE)-conjugated anti-Gr-1 (made in our laboratory using mAb RB6-8C5), fluorescein (FITC)-conjugated anti-MAC3 and anti-CD45.2, and biotinylated (Bio) anti-CD16/32, anti-CD45.1, and anti-CD45.2 (all from BD Pharmingen), anti-F4/80-FITC (Serotec), streptavidin-PE (Jackson Immunoresearch), and streptavidin-PE-Cy5 (DakoCytomation).
Immunofluorescent staining
Tissue containing the ankle and midfoot joints was skinned, then immediately frozen in OCT medium, stored at −80° C, then sectioned without decalcification using a tape-transfer method (4, 9, 47). Frozen sections were fixed in cold acetone, blocked with 2% bovine serum albumin (BSA) and 0.1% Tween 20, stained with anti-CD11b-FITC (eBioscience) and either anti-CD45.1-Bio or anti-CD45.2-Bio mAbs followed by streptavidin-PE, then mounted using GelMount (Biomeda) aqueous medium. Microscopy was performed using a Zeiss Axioplan 2 instrument equipped with a Spot RT Slider CCD camera (Diagnostic Instruments) and IPLab (Scanalytics) imaging software.
RESULTS
Gfi-1−/− mice are resistant to serum-transferred arthritis
The involvement of neutrophils in antibody-induced arthritis is well accepted, but it is worth noting that this contention rests mainly on experiments that relied on depletion of neutrophils by anti-Gr-1 antibody, now known to recognize other leukocyte subsets (3). We set out first to confirm an essential role for neutrophils using genetically deficient animals. Gfi1 encodes a zinc-finger transcription factor expressed in granulocyte and lymphocyte lineages. Mutation of this gene results in a profound defect in terminal differentiation of neutrophils; differentiation of lymphocytes is inefficient, but mature and functional B and T cells accumulate to normal levels (40, 48). Evaluation of toluidine-blue-stained sections of ankle joints revealed mast cells in the connective tissue, and the synovial lining looked grossly normal on hematoxylin/eosin (H+E)-stained sections (not shown). Experiments on bone-marrow chimeras (see below) confirmed the presence of CD11b-positive macrophages of host Gfi-1−/− origin in the synovial lining.
Healthy Gfi-1−/− mice 5–12 weeks of age were injected with K/BxN serum following our standard protocol, and as expected, proved to be highly resistant to arthritis. Most never presented with any disease whatsoever (clinical index 0), while a minority showed mild or very mild arthritis (maximum clinical index 1–4 on a scale of 0–12; Figure 1A).
Figure 1.
A. Resistance of Gfi-1−/− mice to arthritis induction by K/BxN serum. The maximum severity in each of 25 Gfi-1−/− mice is shown; bar = mean +/− SEM. B. Restoration of arthritis susceptibility in Gfi-1−/− mice by daily (days 0–3) injection of BMC. Results from four reconstituted mice and three Gfi-1−/− controls are shown. C–E. Restoration of circulating neutrophils, and susceptibility to arthritis, by BMC transfer into Gfi-1−/− mice. C. Gfi-1−/− mice were sub-lethally irradiated, then reconstituted with BMC from wild-type CD45.1 congenic donors. Circulating neutrophils were quantified over time. Results from four mice are shown, as is mean +/− SD of three wild-type mice (WT45.1) for comparison. D. Ankle tissue from reconstituted mice was stained for CD11b (a marker of macrophage-like synoviocytes) and either CD45.1 or CD45.2. CD11b co-localized with host-derived CD45.2 (right) but not donor-derived CD45.1 (left). E. Arthritis was induced in reconstituted Gfi-1−/− mice (Gfi-1 + WT) and monitored by clinical index and change in ankle thickness. Gfi-1−/− (as in panel A) and Gfi-1+/+ (WT) littermates served as controls. Points indicate means ± SEM, P < 0.001 between each pair of groups.
Sublethally irradiated Gfi-1−/− mice reconstituted with normal bone-marrow cells produce mature neutrophils and are sensitive to arthritis induction
Gfi-1−/− mice are profoundly deficient in production of mature neutrophils but also have defects in other leukocyte lineages (see Discussion). To test whether sensitivity to arthritis in Gfi-1−/− mice could be restored by neutrophil reconstitution, we injected bone-marrow cells (BMC) daily for four days, with K/BxN serum co-injected on the first and third days. Three out of four mice treated this way developed severe arthritis with the typical kinetics of serum-transferred arthritis (Figure 1B). In separate experiments, a single injection of BMC did not lead to production of donor-derived leukocytes (not shown). Together, these experiments indicated that the defect leading to arthritis resistance could be rapidly reversed by short-lived circulating cells, therefore presumably neutrophils.
Since this reconstitution strategy would have been prohibitively expensive for larger-scale experiments, we developed an alternative approach based on sublethal irradiation and donor BMC engraftment. Gfi-1−/− mice were irradiated (4 Gy), then reconstituted with 106 lineage-depleted bone-marrow cells from wild-type mice. Donors were congenic for the CD45.1 allele, allowing us to ascertain the origin of myeloid cells in the reconstituted mice. Numbers of blood neutrophils and other leukocytes of donor (CD45.1) or host (CD45.2) origin were followed over time. Neutrophils were of donor origin and, as shown in Figure 1C, they reached numbers comparable with those of unmanipulated mice by day 10–14. By day 14, there was significant chimerism among monocytes and lymphocytes in the same mice, with 59–69% and 72–86%, respectively, being of donor origin. Chimerism was also assessed in tissue macrophages around the ankle joint (type A synoviocytes) by immunofluorescent staining. In the three mice examined, staining of the synovial lining for CD11b co-localized with staining for CD45.2 (host hematopoietic cells) but not with CD45.1 (donor cells; Figure 1D). Mast cells could not readily be assessed by this method, but since they are highly radio-resistant (like tissue macrophages) were likely to be of host origin.
Reconstituted Gfi-1−/− mice were challenged with K/BxN serum, and proved highly susceptible to arthritis induction (36/39 mice), with average severity greater than that seen concomitantly in unreconstituted Gfi-1+/+ littermates (Figure 1E). The reason for this increase in severity is unclear; most likely it reflects either exuberant repopulation of the neutrophil compartment or the smaller size of Gfi-1−/− mice, which might lead to a higher concentration of the arthritogenic anti-GPI antibodies.
Thus, the neutrophil deficiency imparted by the Gfi-1 deficiency had a profound effect on arthritis sensitivity. Complementation of this defect by short-term reconstitution with bone-marrow-derived precursors provided a robust tool with which to test the genetic and molecular requirements in this reconstituting population.
Arthritis development does not require neutrophils to make, nor respond to, IL-1 or TNF
IL-1 and TNF are critical inflammatory cytokines in the K/BxN serum-transfer model as well as many other mouse models of rheumatoid arthritis (1, 8). Neutrophils can be induced to secrete both of these cytokines (21–25, 49). Mast cells are one important source of IL-1 in the serum-transfer model, but only very early in the course of disease, with another, unidentified, cell type needed to make it subsequently (14). In addition, IL-1 and TNF activate pro-inflammatory and microbicidal pathways in neutrophils, indicating the presence of functionally relevant receptors for these mediators (26–30).
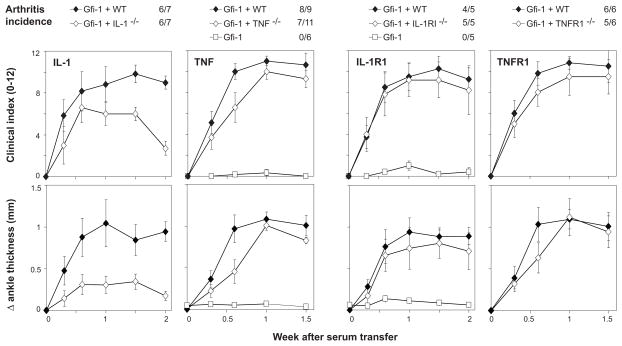
Mice with disruptions of the genes for IL-1 (both α and β ) or TNF were used as BMC donors for sub-lethally irradiated Gfi-1−/− recipients. These chimeras retained susceptibility to arthritis (Figure 2A). Mice reconstituted with cells lacking expression of IL-1α and β developed somewhat less severe arthritis than did control mice. Thus, although production of IL-1 by neutrophils is not absolutely required for arthritis, these cells might nevertheless be an important source of IL-1. In contrast, arthritis severity was not significantly reduced in mice receiving bone-marrow cells from TNF−/− donors.
Figure 2.
Arthritis severity in mice with neutrophils lacking IL-1, TNF, or their receptors. Gfi-1 −/− mice were sub-lethally irradiated, then reconstituted with lineage-depleted bone-marrow cells from donor mice lacking expression of IL-1α and β , TNF, IL-1RI or TNFRI and II, or from control (WT) mice. Arthritis was induced by K/BxN serum transfer starting 2 weeks after reconstitution, and incidence (clinical index ≥3 on multiple occasions) and severity (clinical index 0–12 or change in ankle thickness) were followed over the next 1.5 – 2 weeks. Unmanipulated Gfi-1−/−mice (Gfi–1) served as controls in some experiments. Each point indicates mean severity among all (4–8) arthritic mice in a group, with error bars indicating SEM. Comparing groups at 1.5 weeks after serum transfer: P < 0.01 comparing Gfi+IL-1αβ with Gfi-1+WT, P > 0.05 for other comparisons.
Mice with disruptions of the genes for IL-1RI or the TNF-Rs (both I and II) were also used as BMC donors for Gfi-1−/− recipients. Arthritis incidence and severity were indistinguishable whether normal, IL-1RI−/−, or TNF-R−/− reconstituting cells were used (Figure 2B). Thus, responsiveness to IL-1 and TNF is also not essential for neutrophil function in this model.
Neutrophils do not need to make β-leukotrienes for arthritis to develop
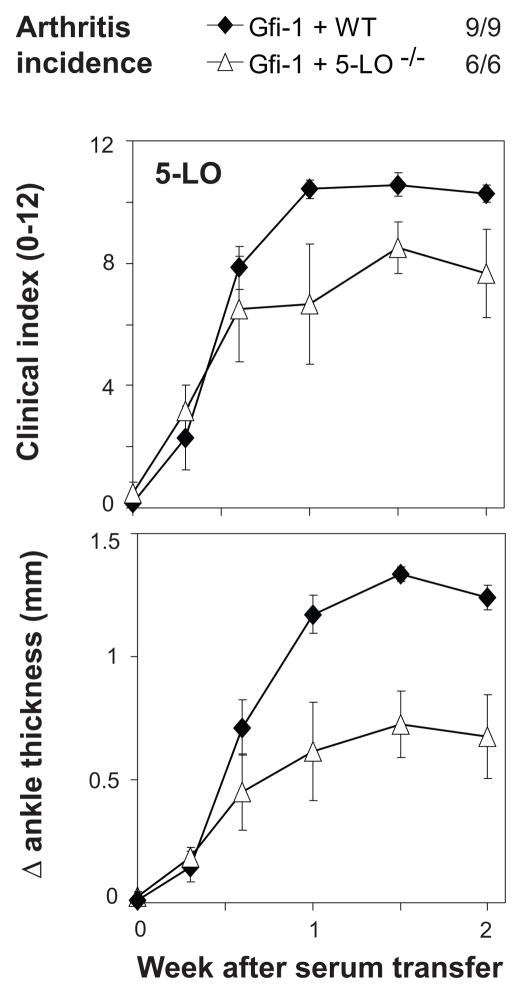
Leukotriene B4, a pro-inflammatory lipid mediator produced by an enzyme cascade that includes 5-lipoxygenase (5-LO), is also important in the K/BxN serum-transfer model. Production of 5-LO by neutrophils is sufficient to facilitate arthritis according to results on reconstitution of 5-LO−/− mice with wild-type mature neutrophils (11).
Thus, we expected that expression of 5-LO by neutrophils would be an absolute requirement as well. Surprisingly, Gfi-1−/− mice reconstituted with 5-LO−/− BMC were susceptible to arthritis, although perhaps with lower severity than that seen using normal BMC (Figure 3). Thus, neutrophil production of leukotriene B4 contributes to arthritis severity and is sufficient for arthritis induction, but it is not necessary.
Figure 3.
Arthritis induction in mice with neutrophils lacking 5-LO. Gfi-1−/− mice were sub-lethally irradiated, then reconstituted with bone-marrow cells from donor mice lacking expression of 5-LO, or from control (WT) mice. Arthritis was induced by serum transfer 2 weeks after reconstitution, and incidence (clinical index ≥3 on multiple occasions) and severity (clinical index 0–12 or change in ankle thickness) were followed for the next 2 weeks. Each point indicates mean severity in all (6–7) mice in a group, with error bars indicating SEM. Comparing groups at 1.5 weeks after serum transfer: P = 0.047 comparing Gfi-1+5-LO with Gfi-1+WT.
Arthritis requires neutrophils to signal via FcR, but not Fcγ RIII
Arthritis in the K/BxN serum-transfer model is mediated by immune complexes, and Fc receptors for IgG are critical for arthritis induction (5, 9). Neutrophils express both high-affinity (Fcγ RI) and low-affinity (Fcγ RIII and IV) Fc receptors with activating properties (32, 33).
Mice with disruption of the common γ signaling chain (used by Fcγ RI, Fcγ RIII, Fcγ RIV, FcεR, and other receptors) were used as BMC donors for Gfi-1−/− recipients. Neutrophil expression of FcRγ was required for arthritis development (Figure 4A). Because these chimeras remained resistant to arthritis, we confirmed that circulating neutrophils were reconstituted to normal levels in Gfi-1−/− mice that received FcRγ −/− BMC (not shown).
Figure 4.
Arthritis induction in mice with neutrophils lacking stimulatory Fc receptors. Gfi-1−/− mice were sub-lethally irradiated, then reconstituted with bone-marrow cells from donor mice lacking expression of either FcRγ (FcRg) or Fcγ RIII (FcgRIII), or from control (WT) mice. Unmanipulated Gfi-1−/− mice (Gfi-1) served as controls in some experiments. Arthritis was induced by serum transfer 2 weeks after reconstitution, and incidence (clinical index ≥3 on multiple occasions) and severity (clinical index 0–12 or change in ankle thickness) were followed for the next 2 weeks. Each point indicates mean severity among all (4–6) mice in a group, with error bars indicating SEM. Comparing groups at 1.5 weeks after serum transfer: P < 0.001 comparing Gfi-1+FcRγ with Gfi-1+WT, P > 0.05 comparing Gfi-1+Fcγ RIII with Gfi-1+WT.
Previous experiments have shown that Fcγ RIII is the main Fc receptor involved in K/BxN arthritis, although Fcγ RIII−/− mice, unlike FcRγ −/− mice, are not completely resistant to arthritis (5, 9). Given the absolute requirement for FcRγ in neutrophils, we surmised that Fcγ RIII would also be essential. Surprisingly, this proved not to be the case, as the transfer of Fcγ RIII-deficient neutrophil precursors still allowed for robust arthritis (Figure 4B). We confirmed by flow cytometric staining that the donor-derived neutrophils did show the profile of Fcγ RIII-deficient cells (not shown) and, additionally, two Fcγ RIII−/− mice from the same lot as the donors were injected concurrently with K/BxN serum and did not develop any clinical evidence of arthritis.
Thus, there is a requirement for Fc receptor signaling in neutrophils for arthritis development, but one that does not seem to require Fcγ RIII.
Neutrophils require expression of LFA-1 and C5aR to promote arthritis
Neutrophil extravasation requires adhesion to the endothelium, as well as chemotactic signals that promote both adhesion and subsequent migration into the underlying tissue. Among the many molecules involved in neutrophil extravasation, two are notable for also having been identified as essential in the K/BxN serum-transfer model: CD11a/CD18 (LFA-1), which is an integrin important in firm adhesion to ICAM-1 and other receptors on endothelial cells (10), and the receptor for the complement cleavage product C5a (C5aR), which is a potent chemotactic factor and activator of neutrophils (9, 34, 36, 38).
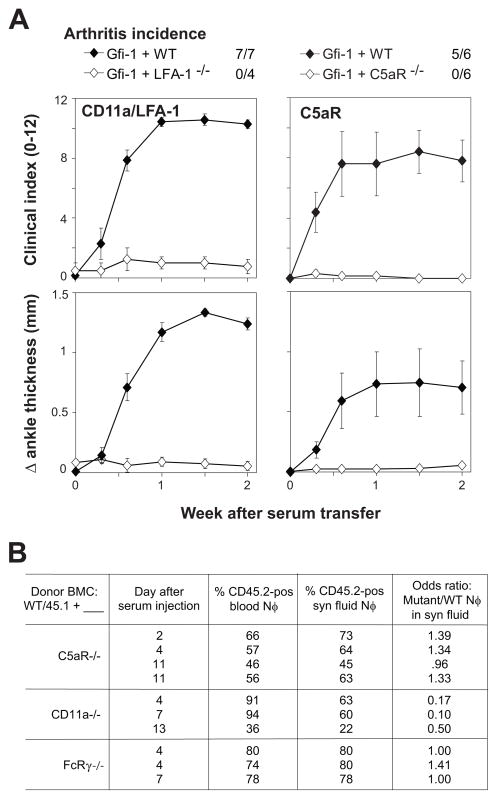
C5aR−/− mice and CD11a−/− mice were used as BMC donors as above. Gfi-1−/− mice reconstituted with these cells remained highly resistant to arthritis (Figure 5A). The presence of normal numbers of circulating neutrophils was confirmed in recipients of CD11a−/− cells. In addition, mixed chimeras (see below) confirmed the ability of either CD11a−/− or C5aR−/−precursors to generate numbers of circulating neutrophils similar to those of normal (CD45.1-marked) BMC.
Figure 5.
Arthritis induction in mice with neutrophils lacking C5aR or CD11a/LFA-1, and influx of these mutant neutrophils into inflamed joints. A. Gfi-1−/− mice were sub-lethally irradiated, then reconstituted with bone-marrow cells from donor mice lacking expression of either C5aR or CD11a (LFA-1), or from control (WT) mice. Arthritis was induced by serum transfer 2 weeks after reconstitution, and incidence (clinical index ≥3 on multiple occasions) and severity (clinical index 0–12 or change in ankle thickness) were followed for the next 2 weeks. Each point indicates mean severity among all (4–7) mice in a group, with error bars indicating SEM. Comparing groups at 1.5 weeks after serum transfer: P < 0.001 comparing either Gfi-1+C5aR or Gfi-1+CD11a/LFA-1 with Gfi-1+WT. B. Gfi-1−/− mice were sub-lethally irradiated, then reconstituted with mixtures of wild-type (CD45.1) and mutant (CD45.2) bone-marrow cells. Blood and synovial fluid were harvested from arthritic mice 2–13 days after serum transfer, and the percentages of neutrophils of wild-type and mutant origin were determined by flow cytometry. Groups of odds ratios obtained in multiple trials were compared by ANOVA and Tukey-Kramer multiple comparisons test: p < 0.01 comparing CD11a−/− with either C5aR−/− or FcRγ −/−, p = NS comparing C5aR−/− with FcRγ −/−
That neutrophils required expression of adhesion molecules and chemoattractant receptors led to the question of whether those molecules would still be required for neutrophils to enter the joint if inflammation were to be initiated by normal neutrophils. Gfi-1−/− mice were reconstituted with 1:1 mixtures of BMC from gene-disrupted (CD45.2) and wild-type (CD45.1) mice, then challenged with K/BxN serum. Such mice routinely developed severe arthritis. Joint fluid and blood were collected and the percentages of neutrophils of wild type and mutant origin were determined. Neutrophils deficient in C5aR entered the joint space as efficiently as did wild-type neutrophils (Figure 5B). CD11a−/− neutrophils emigrated at somewhat lower efficiency but were nevertheless found in the inflamed synovial fluid. As an additional control, FcRγ −/− neutrophils were also evaluated and, as expected, migrated as efficiently as did wild-type neutrophils.
Thus, although neutrophil expression of LFA-1 and C5aR is required to initiate inflammatory arthritis, these surface receptors are not absolutely essential for neutrophil egress into inflamed synovium. Signaling through either of these receptors may still be necessary.
DISCUSSION
Exploiting a system entailing transfer of bone-marrow precursor cells into a neutrophil-deficient strain, we have found that neutrophil expression of FcRγ , C5aR, and CD11a/LFA-1 is required for the induction of inflammatory arthritis by anti-GPI Abs, but that synthesis of Fcγ RIII, IL-1α and β , TNF, IL-1RI, TNF-RI and II, and 5-LO by this cell type is not required. For the required factors, mixed chimeras containing normal and mutant donor cells were used to confirm that the mutant precursor cells were able to produce mature neutrophils at normal numbers, and to demonstrate that neutrophils lacking C5aR or CD11a/LFA-1 were competent to enter the inflamed synovial fluid within a few days of arthritis onset. Among the non-essential neutrophil-made factors, only IL-1αβ and 5-LO showed some influence on the severity of arthritis.
Production of 5-LO by neutrophils is sufficient to facilitate arthritis, since reconstitution of 5-LO−/− mice with wild-type mature neutrophils restored susceptibility (11). Thus, we were surprised to find that Gfi-1−/− mice reconstituted with 5-LO−/− BMC were susceptible to arthritis, although perhaps with lower severity than that seen using normal BMC. However, these findings remain consistent with the published findings (11) by the following reasoning: neutrophil production of leukotriene B4 contributes to arthritis severity and is sufficient for arthritis induction, but it is not completely necessary.
The necessity of FcRγ signaling by neutrophils was expected, but the dispensability of Fcγ RIII was surprising, given that mice lacking this molecule are moderately arthritis-resistant (9). Thus, Fcγ RIII is apparently more important on another cell type, and signaling through another FcRγ-dependent receptor (instead of or in addition to Fcγ RIII) is required on neutrophils. Certainly, either Fcγ RI or RIV could deliver an activating signal, but neither of these receptors binds well to IgG1, the predominant isotype of anti-GPI Ab in K/BxN serum (32). Alternatively, other receptors that use the FcRγ chain for signaling, such as paired immunoglobulin-like receptor (PIR)-A (50, 51), leukocyte immunoglobulin-like receptors (LIRs) (52), dendritic cell immunoactivating receptor (DCAR) (53), and/or osteoclast-associated receptor (OSCAR) (54), could be required.
The ability of neutrophils lacking either C5aR or CD11a/LFA-1 to enter the synovial fluid once inflammation is initiated by normal neutrophils is strikingly similar to what has been reported for BLT-1−/− neutrophils (12). Thus, the factors that can perpetuate neutrophil extravasation seem to be more redundant than those that are required to initiate it. It remains unclear whether any of these receptors is required for neutrophil activation above and beyond extravasation.
The adoptive transfer model described herein appears to produce mature neutrophils of strictly donor origin. Those conclusions based on strong differences in arthritis susceptibility are unlikely to be weakened by the presence of chimerism in non-neutrophil leukocyte lineages; the development of severe arthritis in mixed chimeras of normal and mutant BMC also argues against such an issue. However, less striking findings, such as the decreased severity of arthritis associated with neutrophil deficiency in IL-1 or 5-LO, should be interpreted more cautiously.
It is also important to consider the possibility that Gfi-1−/− mice might have other defects in non-neutrophil lineages relevant to arthritis that might be corrected by the protocol we have developed. We addressed this issue directly by repeated infusion of large numbers of normal BMC into non-irradiated Gfi-1−/− mice, which immediately restored susceptibility to arthritis (Figure 1B). Since a single injection of BMC into non-irradiated Gfi-1−/− recipients did not lead to engraftment of precursors capable of producing neutrophils over time, these findings strongly suggested that short-lived bone-marrow-derived cells introduced into the circulation, therefore most plausibly neutrophils or short-lived precursors of neutrophils, were capable of correcting the defect in Gfi-1−/− mice. Complementation of defects in other lineages seems unlikely to have played an important role on other grounds as well. First, although Gfi-1−/− mice are known to have aberrant dendritic cell (DC) function (55) and decreased numbers and altered function of lymphocytes (48, 56–58), K/BxN serum readily induced arthritis in lymphocyte-deficient RAG1−/−mice (2) and in mice depleted of DC using a CD11c-associated toxin (59). Second, our findings cannot be explained by simple reconstitution of a defective mast cell population because when mast cells were used to reconstitute W/Wv mice they had to express not only FcRγ and C5aR, but also IL-1 for arthritis to develop (14, 20); additionally, mast cell engraftment is not apparent until more than 7 weeks after transfer of precursors (60).
These findings need to be considered in the context of several pieces of existing data, some of which are controversial. In particular, the importance of mast cells has been questioned, since one mast-cell-deficient strain (W/Wv) is arthritis-resistant (4, 5), whereas another (Wsh) is sensitive (61). Both of these strains have multiple other hematologic abnormalities that could, respectively, decrease or increase susceptibility to arthritis (62). We find the data on reconstitution of W/Wv mice with mast cell precursors (4, 14) to provide compelling support for an important role for mast cells, although in certain settings they are not essential. Data which we will include in a revised model include: (i) an overall requirement for FcRγ and Fcγ RIII, but not Fcγ RI or FcεR, for arthritis development (9, 14); (ii) importance of mast cell expression of IL-1, FcRγ and C5aR (14, 20); (iii) the ability of exogenous IL-1 to circumvent the requirement for mast cells, but the necessity for another cell type to make this cytokine to perpetuate arthritis for more than a few days (14); (iv) a requirement for neutrophils to express BLT-1 in order to initiate arthritis (12); (v) an overall need for 5-LO, and restoration of sensitivity to arthritis by its expression only in neutrophils (11); (vi) a requirement for neutrophils and mast cells in the early phase of vascular permeability that precedes arthritis, and a requirement for FcRγ in this phase but only on radioresistant cells (63); (vii) dispensability of C5aR, BLT-1, TNF, IL-1 in this early vascular phase (63).
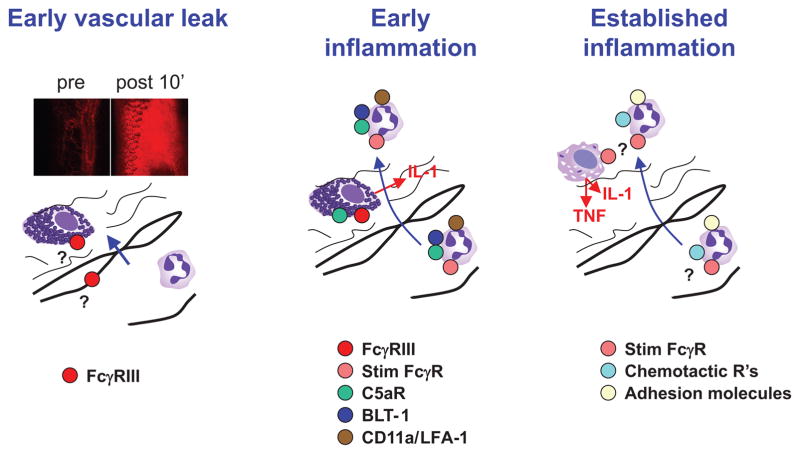
The current findings lead to the following revised model of serum-transferred arthritis, which remains consistent with previously described findings (Figure 6). First, IgG-containing immune complexes interact with radioresistant Fcγ RIII-expressing cells, likely including mast cells, both near to and distant from the joint, to generate vasoactive amines that promote the extravasation of immune complexes and plasma proteins including complement; neutrophils are required in this phase, but their role is unclear (63). Second, immune complexes are deposited in joint tissues and activate complement, leading to the activation of mast cells to produce IL-1 (14), and perhaps resident synovial macrophages to produce other important mediators. Neutrophils are recruited and activated via C5aR, BLT-1 (12), and one or more FcRγ-dependent receptors, and participate in both tissue damage and perpetuation of inflammation by secretion of IL-1, β-leukotrienes (11) and probably other mediators. Based on studies in human synovial tissue, macrophages are likely to be a major source of both IL-1 and TNF once inflammation has been initiated, but this has not been formally demonstrated in mice. Third, within a few days of the initiation of inflammation, there is considerable redundancy in the neutrophil receptors required for influx, with LFA-1, C5aR, and BLT-1 all individually dispensible (12); whether these receptors remain essential for neutrophil activation is unknown.
Figure 6.
Model of arthritis induction by K/BxN serum, with emphasis on role of neutrophils. The phase of early vascular leak (shown by extravasation of a fluorescent probe 10 minutes after serum injection) requires neutrophils and mast cells, but neutrophil requirements are unclear, since stimulatory FcR's must only be expressed on radio-resistant cells. For inflammation to be initiated at clinically-detectable levels, neutrophils require the expression of stimulatory FcR's, C5aR, CD11a/LFA-1, and BLT-1, and mast cells require expression of stimulatory FcR's, C5aR, and IL-1, based on the current and previously-published work. Once inflammation is established in otherwise-resistant mice (e.g., by wild-type neutrophils or injection of exogenous IL-1), then neutrophils may still enter the joint space despite lacking expression of the individual receptors required to initiate disease. Macrophages derived from circulating monocytes are likely a major source of IL-1 and TNF at this point, but this is uncertain. Photos of vascular leak appear courtesy of B. Binstadt; drawings of leukocytes were copied from www.wikipedia.org.
Reconstitution of Gfi-1−/− mice might prove useful in assessing the role of neutrophils in other models of inflammatory arthritis as well as in models of other diseases, such as cutaneous vasculitis (Arthus reaction), anti-phospholipid-antibody-induced pregnancy loss (64), and glomerulonephritis induced by anti-myeloperoxidase antibodies (65).
Acknowledgments
We are grateful to Drs. C. Ballantyne, C. Gerard, Y. Iwakura, B. Koller, and S. Orkin for mice; T. Bowman for assistance with histology; J. LaVecchio and G. Buruzala for help with flow cytometry; and C. Laplace for graphics.
Support: This work was supported by grants from the National Institutes of Health (NIH) (R01-AR-46580 and P01-AI65858), by Young Chair funds to D.M. and C.B., and by Joslin’s National Institute of Diabetes and Digestive and Kidney Diseases-funded Diabetes and Endocrinology Research Center core facilities. P.A.M. was supported by an Abbott Scholar Award for Rheumatology Research and an Arthritis Investigator Award from the Arthritis Foundation.
References
- 1.Monach PA, Benoist C, Mathis D. The role of antibodies in mouse models of rheumatoid arthritis, and relevance to human disease. Adv Immunol. 2004;82:217–48. doi: 10.1016/S0065-2776(04)82005-4. [DOI] [PubMed] [Google Scholar]
- 2.Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10(4):451–61. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 3.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001;167(3):1601–8. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 4.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297(5587):1689–92. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 5.Corr M, Crain B. The role of FcgammaR signaling in the K/B x N serum transfer model of arthritis. J Immunol. 2002;169(11):6604–9. doi: 10.4049/jimmunol.169.11.6604. [DOI] [PubMed] [Google Scholar]
- 6.Bruhns P, Samuelsson A, Pollard JW, Ravetch JV. Colony-stimulating factor-1-dependent macrophages are responsible for IVIG protection in antibody-induced autoimmune disease. Immunity. 2003;18(4):573–81. doi: 10.1016/s1074-7613(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 7.Solomon S, Rajasekaran N, Jeisy-Walder E, Snapper SB, Illges H. A crucial role for macrophages in the pathology of K/B x N serum-induced arthritis. Eur J Immunol. 2005;35(10):3064–73. doi: 10.1002/eji.200526167. [DOI] [PubMed] [Google Scholar]
- 8.Ji H, Pettit A, Ohmura K, Ortiz-Lopez A, Duchatelle V, Degott C, et al. Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induced arthritis. J Exp Med. 2002;196(1):77–85. doi: 10.1084/jem.20020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16(2):157–68. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 10.Watts GM, Beurskens FJ, Martin-Padura I, Ballantyne CM, Klickstein LB, Brenner MB, et al. Manifestations of inflammatory arthritis are critically dependent on LFA-1. J Immunol. 2005;174(6):3668–75. doi: 10.4049/jimmunol.174.6.3668. [DOI] [PubMed] [Google Scholar]
- 11.Chen M, Lam BK, Kanaoka Y, Nigrovic PA, Audoly LP, Austen KF, et al. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J Exp Med. 2006;203(4):837–42. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim ND, Chou RC, Seung E, Tager AM, Luster AD. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med. 2006;203(4):829–35. doi: 10.1084/jem.20052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon S, Kolb C, Mohanty S, Jeisy-Walder E, Preyer R, Schollhorn V, et al. Transmission of antibody-induced arthritis is independent of complement component 4 (C4) and the complement receptors 1 and 2 (CD21/35) Eur J Immunol. 2002;32(3):644–51. doi: 10.1002/1521-4141(200203)32:3<644::AID-IMMU644>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Nigrovic PA, Binstadt BA, Monach PA, Johnsen A, Gurish M, Iwakura Y, et al. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL-1. Proc Natl Acad Sci U S A. 2007;104(7):2325–30. doi: 10.1073/pnas.0610852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagari T, Doi H, Shimozato T. The importance of IL-1 beta and TNF-alpha, and the noninvolvement of IL-6, in the development of monoclonal antibody-induced arthritis. J Immunol. 2002;169(3):1459–66. doi: 10.4049/jimmunol.169.3.1459. [DOI] [PubMed] [Google Scholar]
- 16.Kagari T, Tanaka D, Doi H, Shimozato T. Essential role of Fc gamma receptors in antitype II collagen antibody-induced arthritis. J Immunol. 2003;170(8):4318–24. doi: 10.4049/jimmunol.170.8.4318. [DOI] [PubMed] [Google Scholar]
- 17.Grant EP, Picarella D, Burwell T, Delaney T, Croci A, Avitahl N, et al. Essential role for the C5a receptor in regulating the effector phase of synovial infiltration and joint destruction in experimental arthritis. J Exp Med. 2002;196(11):1461–71. doi: 10.1084/jem.20020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson WC, Brown PS, Pitcock JA, Townes AS. Passive transfer studies with type II collagen antibody in B10. D2/old and new line and C57Bl/6 normal and beige (Chediak-Higashi) strains: evidence of important roles for C5 and multiple inflammatory cell types in the development of erosive arthritis. Arthritis Rheum. 1987;30(4):460–5. doi: 10.1002/art.1780300418. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka D, Kagari T, Doi H, Shimozato T. Essential role of neutrophils in anti-type II collagen antibody and lipopolysaccharide-induced arthritis. Immunology. 2006;119(2):195–202. doi: 10.1111/j.1365-2567.2006.02424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nigrovic PA, Lu B, Chaletsky B, Gerard N, Gerard C, Kepley C, et al. The anaphylatoxin C5a promotes chemokine production by mast cells and enables participation in experimental inflammatory arthritis. Arthritis Rheum. 2008;59:S503–4. [Google Scholar]
- 21.Lindemann A, Riedel D, Oster W, Meuer SC, Blohm D, Mertelsmann RH, et al. Granulocyte/macrophage colony-stimulating factor induces interleukin 1 production by human polymorphonuclear neutrophils. J Immunol. 1988;140(3):837–9. [PubMed] [Google Scholar]
- 22.Marucha PT, Zeff RA, Kreutzer DL. Cytokine regulation of IL–1 beta gene expression in the human polymorphonuclear leukocyte. J Immunol. 1990;145(9):2932–7. [PubMed] [Google Scholar]
- 23.Dubravec DB, Spriggs DR, Mannick JA, Rodrick ML. Circulating human peripheral blood granulocytes synthesize and secrete tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1990;87(17):6758–61. doi: 10.1073/pnas.87.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djeu JY, Serbousek D, Blanchard DK. Release of tumor necrosis factor by human polymorphonuclear leukocytes. Blood. 1990;76(7):1405–9. [PubMed] [Google Scholar]
- 25.Lindemann A, Riedel D, Oster W, Ziegler-Heitbrock HW, Mertelsmann R, Herrmann F. Granulocyte-macrophage colony-stimulating factor induces cytokine secretion by human polymorphonuclear leukocytes. J Clin Invest. 1989;83(4):1308–12. doi: 10.1172/JCI114016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Borish L, Rosenbaum R, McDonald B, Rosenwasser LJ. Recombinant interleukin-1 beta interacts with high-affinity receptors to activate neutrophil leukotriene B4 synthesis. Inflammation. 1990;14(2):151–62. doi: 10.1007/BF00917454. [DOI] [PubMed] [Google Scholar]
- 27.Bergenfeldt M, Linder C, Ohlsson K. Stimulation of human polymorphonuclear leukocytes by recombinant human interleukin-1 beta. Biol Chem Hoppe Seyler. 1992;373(5):255–60. doi: 10.1515/bchm3.1992.373.1.255. [DOI] [PubMed] [Google Scholar]
- 28.Moxey-Mims MM, Simms HH, Frank MM, Lin EY, Gaither TA. The effects of IL-1, IL-2, and tumor necrosis factor on polymorphonuclear leukocyte Fc gamma receptor-mediated phagocytosis. IL-2 down-regulates the effect of tumor necrosis factor. J Immunol. 1991;147(6):1823–30. [PubMed] [Google Scholar]
- 29.Dularay B, Elson CJ, Clements-Jewery S, Damais C, Lando D. Recombinant human interleukin-1 beta primes human polymorphonuclear leukocytes for stimulus-induced myeloperoxidase release. J Leukoc Biol. 1990;47(2):158–63. doi: 10.1002/jlb.47.2.158. [DOI] [PubMed] [Google Scholar]
- 30.Simms HH, D'Amico R. Studies on polymorphonuclear leukocyte bactericidal function: the role of exogenous cytokines. Shock. 1997;7(2):84–9. doi: 10.1097/00024382-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13(7):851–6. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 32.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24(1):19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Strohmeier GR, Brunkhorst BA, Seetoo KF, Meshulam T, Bernardo J, Simons ER. Role of the Fc gamma R subclasses Fc gamma RII and Fc gamma RIII in the activation of human neutrophils by low and high valency immune complexes. J Leukoc Biol. 1995;58(4):415–22. doi: 10.1002/jlb.58.4.415. [DOI] [PubMed] [Google Scholar]
- 34.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–52. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 35.Perianayagam MC, Balakrishnan VS, King AJ, Pereira BJ, Jaber BL. C5a delays apoptosis of human neutrophils by a phosphatidylinositol 3-kinase-signaling pathway. Kidney Int. 2002;61(2):456–63. doi: 10.1046/j.1523-1755.2002.00139.x. [DOI] [PubMed] [Google Scholar]
- 36.Sacks T, Moldow CF, Craddock PR, Bowers TK, Jacob HS. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978;61(5):1161–7. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mollnes TE, Brekke OL, Fung M, Fure H, Christiansen D, Bergseth G, et al. Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin–based human whole blood model of inflammation. Blood. 2002;100(5):1869–77. [PubMed] [Google Scholar]
- 38.Guo RF, Riedemann NC, Laudes IJ, Sarma VJ, Kunkel RG, Dilley KA, et al. Altered neutrophil trafficking during sepsis. J Immunol. 2002;169(1):307–14. doi: 10.4049/jimmunol.169.1.307. [DOI] [PubMed] [Google Scholar]
- 39.Riedemann NC, Guo RF, Gao H, Sun L, Hoesel M, Hollmann TJ, et al. Regulatory role of C5a on macrophage migration inhibitory factor release from neutrophils. J Immunol. 2004;173(2):1355–9. doi: 10.4049/jimmunol.173.2.1355. [DOI] [PubMed] [Google Scholar]
- 40.Hock H, Hamblen MJ, Rooke HM, Traver D, Bronson RT, Cameron S, et al. Intrinsic requirement for zinc finger transcription factor Gfi-1 in neutrophil differentiation. Immunity. 2003;18(1):109–20. doi: 10.1016/s1074-7613(02)00501-0. [DOI] [PubMed] [Google Scholar]
- 41.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87(5):811–22. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 42.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, et al. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187(9):1463–75. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hopken UE, Lu B, Gerard NP, Gerard C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature. 1996;383(6595):86–9. doi: 10.1038/383086a0. [DOI] [PubMed] [Google Scholar]
- 44.Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, et al. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163(9):5029–38. [PubMed] [Google Scholar]
- 45.Goulet JL, Snouwaert JN, Latour AM, Coffman TM, Koller BH. Altered inflammatory responses in leukotriene-deficient mice. Proc Natl Acad Sci U S A. 1994;91(26):12852–6. doi: 10.1073/pnas.91.26.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monach P, Hattori K, Huang H, Hyatt E, Morse J, Nguyen L, et al. The K/BxN mouse model of inflammatory arthritis: theory and practice. Methods Mol Med. 2007;136:269–82. doi: 10.1007/978-1-59745-402-5_20. [DOI] [PubMed] [Google Scholar]
- 47.Monach PA, Verschoor A, Jacobs JP, Carroll MC, Wagers AJ, Benoist C, et al. Circulating C3 is necessary and sufficient for induction of autoantibody-mediated arthritis in a mouse model. Arthritis Rheum. 2007;56(9):2968–74. doi: 10.1002/art.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hock H, Orkin SH. Zinc-finger transcription factor Gfi-1: versatile regulator of lymphocytes, neutrophils and hematopoietic stem cells. Curr Opin Hematol. 2006;13(1):1–6. doi: 10.1097/01.moh.0000190111.85284.8f. [DOI] [PubMed] [Google Scholar]
- 49.Lord PC, Wilmoth LM, Mizel SB, McCall CE. Expression of interleukin-1 alpha and beta genes by human blood polymorphonuclear leukocytes. J Clin Invest. 1991;87(4):1312–21. doi: 10.1172/JCI115134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maeda A, Kurosaki M, Kurosaki T. Paired immunoglobulin-like receptor (PIR)-A is involved in activating mast cells through its association with Fc receptor gamma chain. J Exp Med. 1998;188(5):991–5. doi: 10.1084/jem.188.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ono M, Yuasa T, Ra C, Takai T. Stimulatory function of paired immunoglobulin-like receptor-A in mast cell line by associating with subunits common to Fc receptors. J Biol Chem. 1999;274(42):30288–96. doi: 10.1074/jbc.274.42.30288. [DOI] [PubMed] [Google Scholar]
- 52.Lopez-Botet M, Bellon T, Llano M, Navarro F, Garcia P, de Miguel M. Paired inhibitory and triggering NK cell receptors for HLA class I molecules. Hum Immunol. 2000;61(1):7–17. doi: 10.1016/s0198-8859(99)00161-5. [DOI] [PubMed] [Google Scholar]
- 53.Kanazawa N, Tashiro K, Inaba K, Miyachi Y. Dendritic cell immunoactivating receptor, a novel C-type lectin immunoreceptor, acts as an activating receptor through association with Fc receptor gamma chain. J Biol Chem. 2003;278(35):32645–52. doi: 10.1074/jbc.M304226200. [DOI] [PubMed] [Google Scholar]
- 54.Merck E, Gaillard C, Gorman DM, Montero-Julian F, Durand I, Zurawski SM, et al. OSCAR is an FcRgamma-associated receptor that is expressed by myeloid cells and is involved in antigen presentation and activation of human dendritic cells. Blood. 2004;104(5):1386–95. doi: 10.1182/blood-2004-03-0850. [DOI] [PubMed] [Google Scholar]
- 55.Rathinam C, Geffers R, Yucel R, Buer J, Welte K, Moroy T, et al. The transcriptional repressor Gfi1 controls STAT3-dependent dendritic cell development and function. Immunity. 2005;22(6):717–28. doi: 10.1016/j.immuni.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 56.Rathinam C, Klein C. Transcriptional repressor Gfi1 integrates cytokine-receptor signals controlling B-cell differentiation. PLoS One. 2007;2(3):e306. doi: 10.1371/journal.pone.0000306. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Zhu J, Jankovic D, Grinberg A, Guo L, Paul WE. Gfi-1 plays an important role in IL-2-mediated Th2 cell expansion. Proc Natl Acad Sci U S A. 2006;103(48):18214–9. doi: 10.1073/pnas.0608981103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu J, Davidson TS, Wei G, Jankovic D, Cui K, Schones DE, et al. Down-regulation of Gfi-1 expression by TGF-beta is important for differentiation of Th17 and CD103+ inducible regulatory T cells. J Exp Med. 2009;206(2):329–41. doi: 10.1084/jem.20081666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu HJ, Sawaya H, Binstadt B, Brickelmaier M, Blasius A, Gorelik L, et al. Inflammatory arthritis can be reined in by CpG-induced DC-NK cell cross talk. J Exp Med. 2007;204(8):1911–22. doi: 10.1084/jem.20070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52(2):447–52. [PubMed] [Google Scholar]
- 61.Zhou JS, Xing W, Friend DS, Austen KF, Katz HR. Mast cell deficiency in Kit(W-sh) mice does not impair antibody-mediated arthritis. J Exp Med. 2007;204(12):2797–802. doi: 10.1084/jem.20071391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nigrovic PA, Gray DH, Jones T, Hallgren J, Kuo FC, Chaletzky B, et al. Genetic inversion in mast cell-deficient (W(sh)) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol. 2008;173(6):1693–701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Binstadt BA, Patel PR, Alencar H, Nigrovic PA, Lee DM, Mahmood U, et al. Particularities of the vasculature can promote the organ specificity of autoimmune attack. Nat Immunol. 2006;7(3):284–92. doi: 10.1038/ni1306. [DOI] [PubMed] [Google Scholar]
- 64.Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112(11):1644–54. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao H, Heeringa P, Liu Z, Huugen D, Hu P, Maeda N, et al. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol. 2005;167(1):39–45. doi: 10.1016/S0002-9440(10)62951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]