Abstract
Advances in optics, genetics, and chemistry have enabled the investigation of brain function at all levels, from intracellular signals to single synapses, whole cells, circuits, and behavior. Until recent years, these research tools have been utilized in an observational capacity: imaging neural activity with fluorescent reporters, for example, or correlating aberrant neural activity with loss-of-function and gain-of-function pharmacological or genetic manipulations. However, optics, genetics, and chemistry have now combined to yield a new strategy: using light to drive and halt neuronal activity with molecular specificity and millisecond precision. Photostimulation of neurons is noninvasive, has unmatched spatial and temporal resolution, and can be targeted to specific classes of neurons. The optical methods developed to date encompass a broad array of strategies, including photorelease of caged neurotransmitters, engineered light-gated receptors and channels, and naturally light-sensitive ion channels and pumps. In this review, we describe photostimulation methods, their applications, and opportunities for further advancement.
Keywords: photostimulation, optogenetics, SPARK, LiGluR, channelrhodopsin, halorhodopsin
INTRODUCTION
A central project in neuroscience is to understand how neural circuits process information, mediate perception, and drive behavior. The traditional approach has been to identify the neurons that are active in response to specific sensory stimuli or during motor, memory, or perceptual tasks, because those neurons are likely responsible for processing the relevant information. This has revealed much about the cells that are active during specific behavioral events. But a clearer picture of how these widely distributed and temporally complex patterns of activity create behavior could be achieved by selectively stimulating the cells in question, triggering their inputs, and ultimately playing back to these cells their patterns of activity.
Recent advances in optics, genetics, and chemistry have brought this closer to reality. Light can now be used to manipulate activity in live neurons and thus drive or halt neuronal signaling with cellular and molecular specificity and with millisecond precision. The spatiotemporal resolution of optical technology is on same scale as the submicron and submillisecond cellular processes that underlie neural activity. Light delivery is also noninvasive, allowing hands-off, remote control of neural activity and behavior.
Beyond optical resolution, additional specificity can be gained by using light-sensitive compounds that act selectively on a type of neuronal protein, such as a neurotransmitter receptor, as is the case with caged compounds. Genetic methods can also be employed to deliver light-sensitive targets to cells and subcellular compartments of interest. Although genetic targeting is currently limited to large classes of neurons, over the next decade many new promoter elements will be isolated and genetic switches developed, and this will refine the ability to express light-gated proteins to specific populations of cells. Combined with reporters of activity (such as calcium dyes or protein-based reporters), genetic targeting could one day make it possible to record activity in specific cell types and brain regions during perceptual tasks and then play back to subsets of those cells their natural activity to determine the functional contribution of the component parts to the behavior.
In this review, we focus on the methods of optically controlling specific cells and specific signals in the nervous system using light-sensitive compounds and both chemically engineered and naturally light-gated signaling proteins. The methods have been grouped into four categories on the basis of their strategic approaches: nongenetic methods, foreign receptors, engineered endogenous receptors, and opsins.
NONGENETIC METHODS FOR OPTICAL CONTROL
Direct Photostimulation
The simplest method for controlling neuronal activity uses only a light-delivery instrument and no other added chemicals or proteins. This was first demonstrated in 1957 by Pierce & Giese (70), who used short-wave UV light to reduce action potential amplitude in frog and crab nerves, which could then be restored with white light. In 1971, it was shown that action potential firing in Aplysia neurons could be induced with laser irradiation at 488 nm. In some cells, firing occurred during illumination, whereas in other cells, firing appeared as a response to stopping the illumination (32). In various preparations since then, neural activity has also been manipulated with laser illumination in the UV range at intensities near the damage threshold (5), with long-wavelength infrared light (45, 87), and with two-photon excitation (44). Improvements in efficacy by adding the Na+/K+ATPase inhibitor ouabain to the preparation (32), focusing the laser on the neuron somatic membrane or axon initial segment (44), and by loading cells with small-molecule dyes (30) have been reported. Although the mechanisms of action are not entirely clear, they may include localized heating that activates Na+ channels (86), indirect activation of Cl− channels (72), alteration of neurotransmitter concentrations (2, 85), or the production of oxygen free radicals that inhibit voltage-gated K+ channels and alter other ion transport pathways (25, 48). Stimulating neurons in this way may have great advantages for medical applications in humans, but the involvement of ambiguous signaling cascades can make this method particularly complicated for use in neuroscience research.
Caged Neurotransmitters and Calcium
A method to directly target the ion channels and receptors that control membrane voltage is photo-uncaging of a signaling molecule, such as a neurotransmitter or calcium, that has been rendered biologically inactive by a photocleavable protecting group (17, 88). The physiologically inert (caged) compound becomes functional when the protecting group is cleaved upon illumination with an appropriate wavelength of light and can then act on its cellular target (Figure 1a). Caged glutamate (Figure 2a), GABA, Ca2+, and others have been extensively used for controlling neuronal activity. For a review on this expansive topic, see Reference 28.
Figure 1.
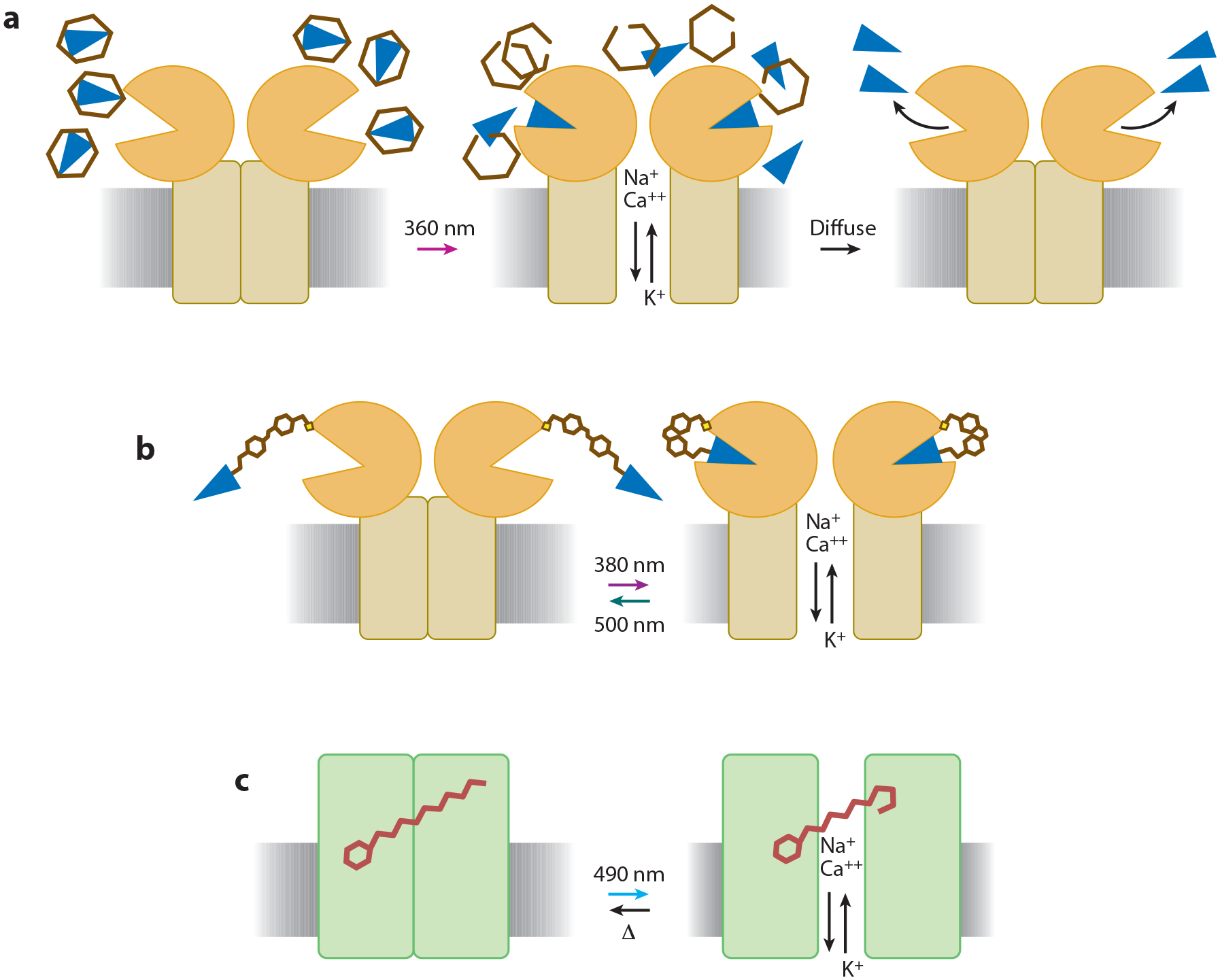
Photostimulation technology can be classified into three general categories. (a) Caged compounds can be released by a flash of light, allowing the liberated compound to act on endogenous or exogenous neuronal targets before diffusing away. (b) Neurotransmitter receptors can be engineered to become light sensitive when coupled to a synthetic photoswitchable molecule containing an analogue of the native neurotransmitter. (c) Naturally light-sensitive proteins can be expressed in neurons; these proteins interact with the chromophore retinal, which is native to the nervous system of many animals, to photoregulate membrane potential.
Figure 2.
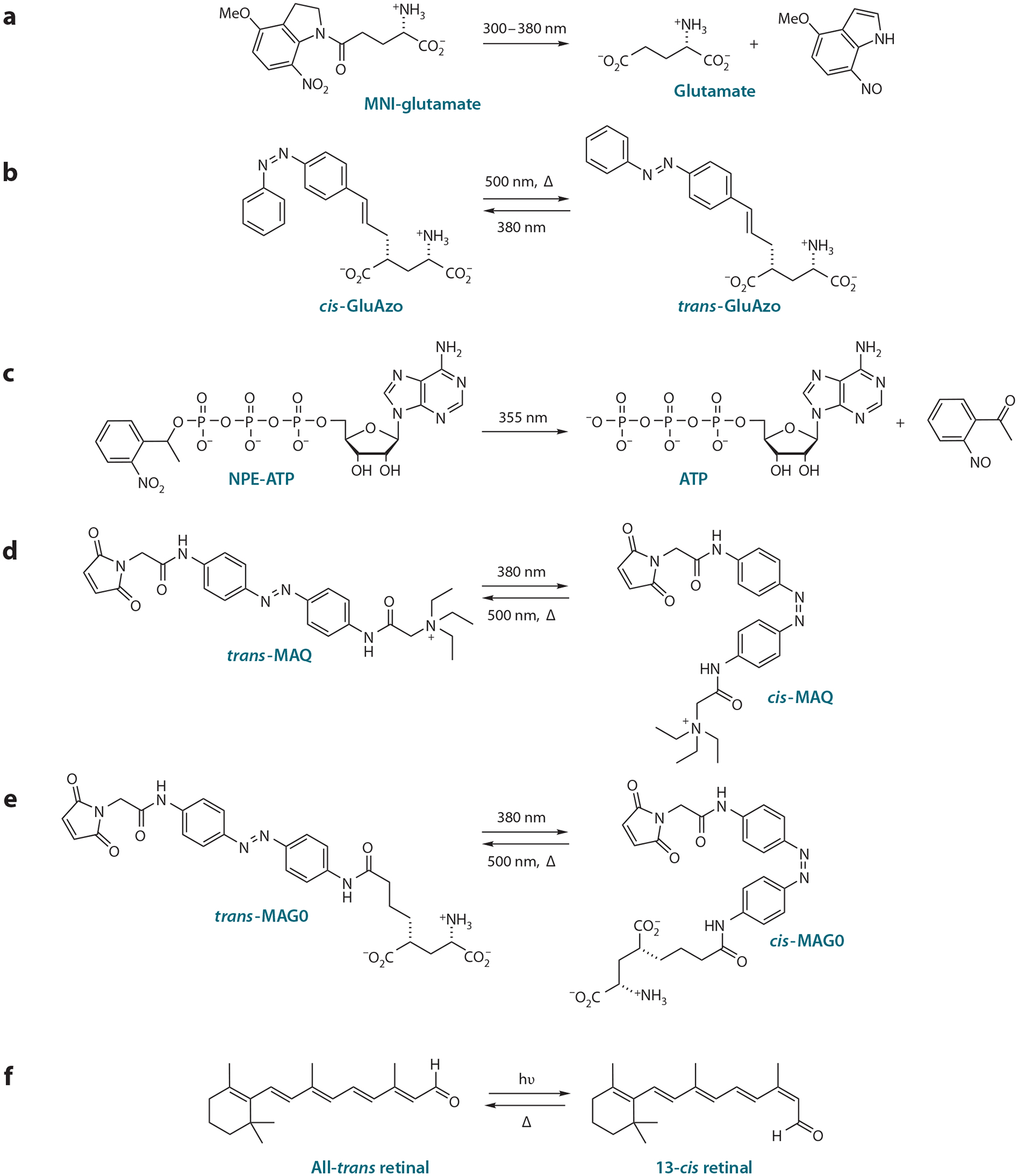
Light-sensitive compounds enable photostimulation of neurons. (a) Caged glutamate acts on native glutamate receptors after it is uncaged by a pulse of UV light. (b) GluAzo is a reversibly caged neurotransmitter acting on native glutamate receptors when photoisomerized to the active trans configuration. (c) Caged ATP acts on nonnative P2X2 receptors that can be expressed in invertebrate neurons that lack ATP receptors and do not use ATP as a transmitter. (d) The cis form of maleimide-azobenzene-quaternary ammonium (MAQ), when coupled to an engineered Shaker K+ channel, silences neuronal activity by unblocking the pore, a process that is reversed when photoisomerized to the trans form. (e) Maleimide-azobenzene-glutamate (MAG) attaches covalently to an introduced cysteine in glutamate receptors and activates them when it is photoisomerized to the active isoform. Depending on the attachment site, activation is by either cis or trans MAG. MAG0 is the shortest of three different versions of MAG. (f) Retinal-coupled proteins like channelrhodopsin and halorhodopsin become activated when all-trans retinal is converted to 13-cis retinal (at wavelengths dictated by the specific interactions between the chromophore and protein) and then relax to the inactive state in the dark. We thank Matthew Volgraf for drawing the chemical structures.
Reversibly Caged Signaling Molecules: Bis-Q, GluAzo, and XAQs
Photo-uncaging rapidly releases effector molecules by cleaving an accessory group with a brief pulse of light; this process is reversed only when the effector molecules diffuse away from the target. The process also liberates a cleaved by-product. An alternative to photo-uncaging is to attach the ligand to a photoisomerizable, rather than photocleavable, accessory group. The compound is driven from an inactive state to an active state by two different wavelengths of light, a process that can be reversed indefinitely. The photoisomerizable molecule azobenzene has been used successfully in reversibly caged compounds. Azobenzene undergoes a significant shift in shape and end-to-end length when changing between its elongated trans form and its compact, bent cis form. The peak absorption wavelengths that drive the molecule between the two configurations are spectrally well separated, typically 380 (trans to cis) and 500 nm (cis to trans), depending on the nature of the substituents attached to the azobenzene. The azobenzene’s trans configuration is stable in the dark indefinitely, whereas the higher-energy cis configuration can remain stable in the dark for long periods depending on the chemical groups attached to it.
This strategy has, so far, been applied to acetylcholine receptors (AChRs), glutamate receptors (GluRs), and potassium channels (K+ channels). Bis-Q consists of an azobenzene derivatized at both ends with a benzylic trimethylammonium ion, an agonist of AChRs (11). This molecule agonizes AChRs in the trans state and can be rapidly switched between cis and trans to reversibly control liganding (49). Two additional photoisomerizable compounds, similar in design to Bis-Q, enabled reversible antagonism of AchRs (50, 51).
GluAzo (Figure 2b) consists of a glutamate analogue coupled at the gamma-carbon to an azobenzene (84). When applied to cultured hippocampal neurons, trans-GluAzo activates iGluR5 and iGluR6, triggering action potentials that cease upon photoisomerization to the cis form. This can be repeated without the need for perfusion of additional compound. Intermediate wavelengths induce isomerization in a fraction of molecules, such that the concentration of active trans-GluAzo can be titrated by using stepped decreases in wavelength. The cis configuration is stable in the dark for many hours (t1/2 = 18 h). Thus, only a brief pulse of light at the proper wavelength is required to trigger photoisomerization, and the isomer remains stable until the reversing wavelength is applied.
Banghart et al. (10) recently developed a series of photoisomerizable molecules that block K+ channels from inside the cell. The molecules consist of aliphatic appendages of different hydrophobicity to facilitate transmembrane delivery, linked to an azobenzene and quaternary ammonium. By blocking the internal tetraethylammonium site of K+ channels in the trans configuration, the azobenzene quaternary ammonium compounds (XAQs) can reversibly depolarize neurons and trigger action potentials in cultured neurons.
Photoswitched Tethered Ligands for Native Channels: QBr and a Quaternary Ammonium PAL
Reversibly caged neurotransmitters include a photoisomerizable moiety and a ligand for activating the protein target. An extension of this approach is to convert the free ligand to a tethered one (i.e., a photoswitched tethered ligand, or PTL) by adding a reactive group that attaches to the protein of interest. Photoswitching between two isomers either presents or removes the ligand from its binding site. Because these molecules react with endogenous neuronal targets, their effect is exerted wherever those targets are located. The efficacy of tethered ligands appears to be high, presumably because the photosensitive linker can rapidly increase the local concentration of ligand at its binding site, which for azobenzene isomerization occurs on the femtosecond timescale (66).
At the same time that Bis-Q was invented, the tethered ligand approach was implemented in the nicotinic AChR (11), which has a loop of solvent-exposed cysteines near the ACh binding site. The photoswitch, QBr, contains one benzylic trimethylammonium ion for activating the receptor, an azobenzene for light sensitivity, and one electrophilic benzylic bromide for covalent reaction with an exposed cysteine. Reduction of disulfides with DTT is required to free a thiol for attachment to QBr. Once the photoswitch has attached to the receptor, any soluble compound remaining in solution can be washed away. Photoswitching from the cis to the trans isomer reversibly activates the receptor.
A tethered K+ channel blocker consisting of an azobenzene-linked quaternary ammonium with an acrylamide for attachment at a nucleophilic residue was designed to photosensitize K+ channels, acting as a photoaffinity label (PAL) (33). Binding of the quaternary ammonium (a high-affinity blocker) at the K+ channel pore promotes reaction of the acrylamide at residues that are approximately 20Å away, a process that is augmented ~11-fold in the dark, when the photoswitch is in the trans isomer. The isomer dependence of the conjugation reaction enables optical lithography, that is, patterning of photoswitch conjugation by shining the preferred wavelength in specific regions during attachment. PAL reacts with a large variety of K+ channels and was also later shown to function as an intracellular blocker of K+ channels (10). Reversible block using PAL produces light-dependent inhibition of action potential firing in neurons.
GENETICALLY INTRODUCED FOREIGN RECEPTORS
Caged Capsaicin and ATP for Genetically Introduced Foreign Receptors
Caged compounds and tethered photoisomerizable compounds act on endogenous neuronal targets and require no genetic manipulation. Although this simplifies the procedure, is particularly useful when gene delivery is not feasible, and has the advantage of working through natural protein signaling machinery, it lacks the cellular specificity that can be obtained through genetic targeting to particular cell types or subcellular regions (Figure 3).
Figure 3.
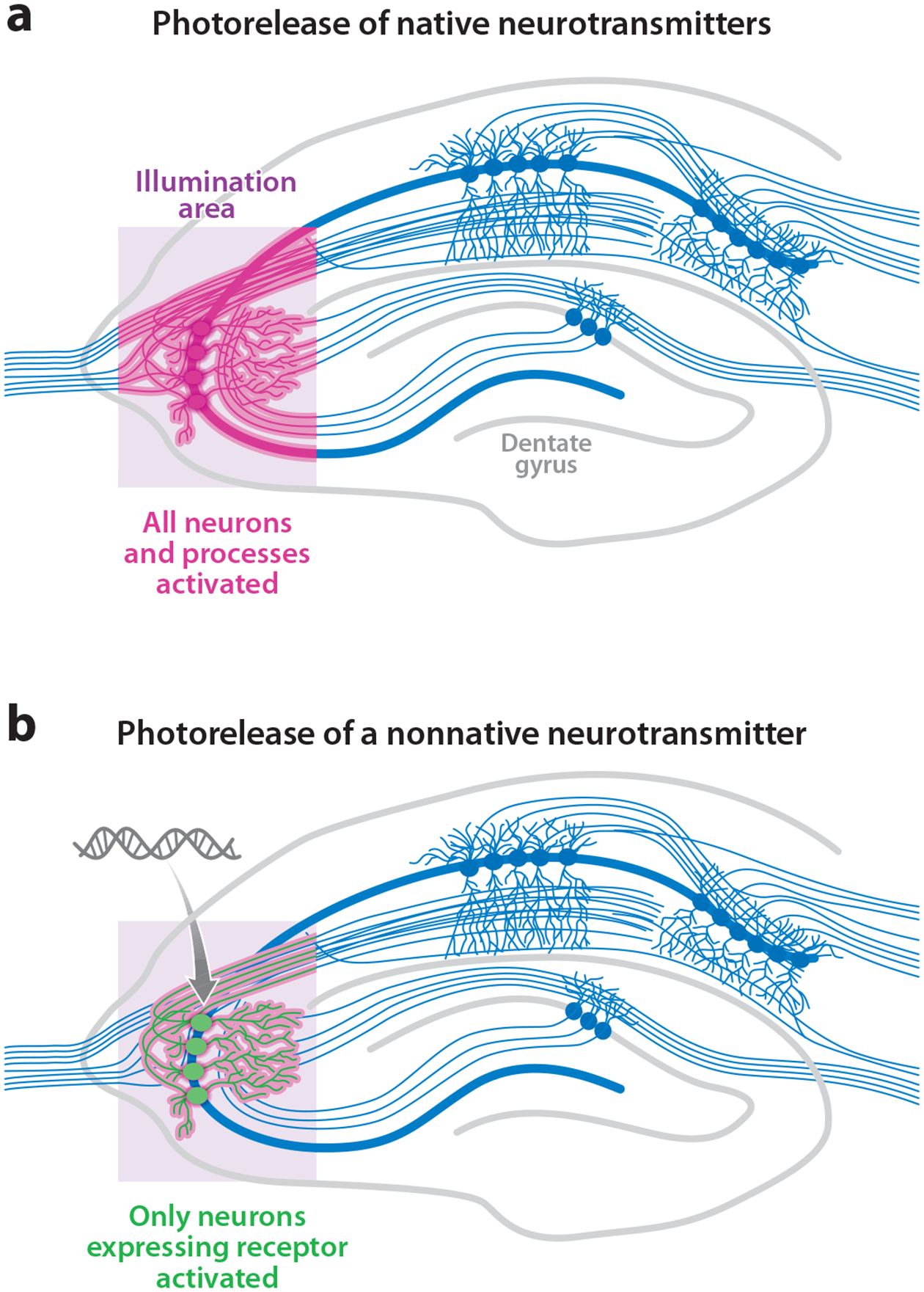
Photostimulation of neurons has additional genetic resolution when neurons are induced to be light sensitive by genetic targeting. (a) Photorelease of native neurotransmitters activates all the neurons and neuronal processes within the illumination region. (b) Photorelease of a nonnative transmitter activates only the neurons within the illumination region that are also expressing the exogenous transmitter receptor. This genetic specificity also exists for opsins and photoswitched tethered ligand-gated channels. Image modified after http://www.bris.ac.uk/Depts/Synaptic/info/pathway/figs/hippocampus.gif
An approach that combines photo-uncaging with genetically introduced foreign receptors was invented to address this issue. Two versions of this optogenetic strategy emerged, one using caged capsaicin and the other caged ATP (91). TRPV1 is a capsaicin-activated ionotropic receptor that, when activated, conducts a depolarizing Na+ and Ca2+ current. Photolysis of caged capsaicin evokes action potential firing in neurons overexpressing TRPV1 (91). TRPV1 is native to the nociceptive neurons in the peripheral nervous system (19) and is also present to some extent in the CNS (63), although exogenous overexpression of the channel appears necessary for capsaicin-evoked action potentials (91).
Caged ATP (Figure 2c) was the first photosensitive caged compound introduced for biological applications (46). Extending its utility, caged ATP is now used to directly photoactivate genetically introduced P2X2 receptors, which are ATP-gated ion channels that conduct depolarizing currents and can evoke action potentials when overexpressed in neurons (91). Although the presence of endogenous ATPsensitive channels (47) could limit the genetic resolution of this technique, it has been used successfully to photostimulate genetically targeted neurons in cell culture and in Drosophila melanogaster, which lack native P2X receptors (47), providing orthogonal and specific control. Transgenic fruit flies, expressing P2X2 in genetically defined populations of cells and microinjected with caged-ATP, have elicited light-induced escape maneuvers, altered locomotion, and courtship wing-beating patterns (Figure 4) (56, 22). Recently, this system was used to demonstrate that the activity of a small number of specific dopaminergic neurons that synapse in the mushroom body of the adult fly is sufficient to carry aversive reinforcement signals that create memory in olfactory conditioning (Figure 5) (21).
Figure 4.
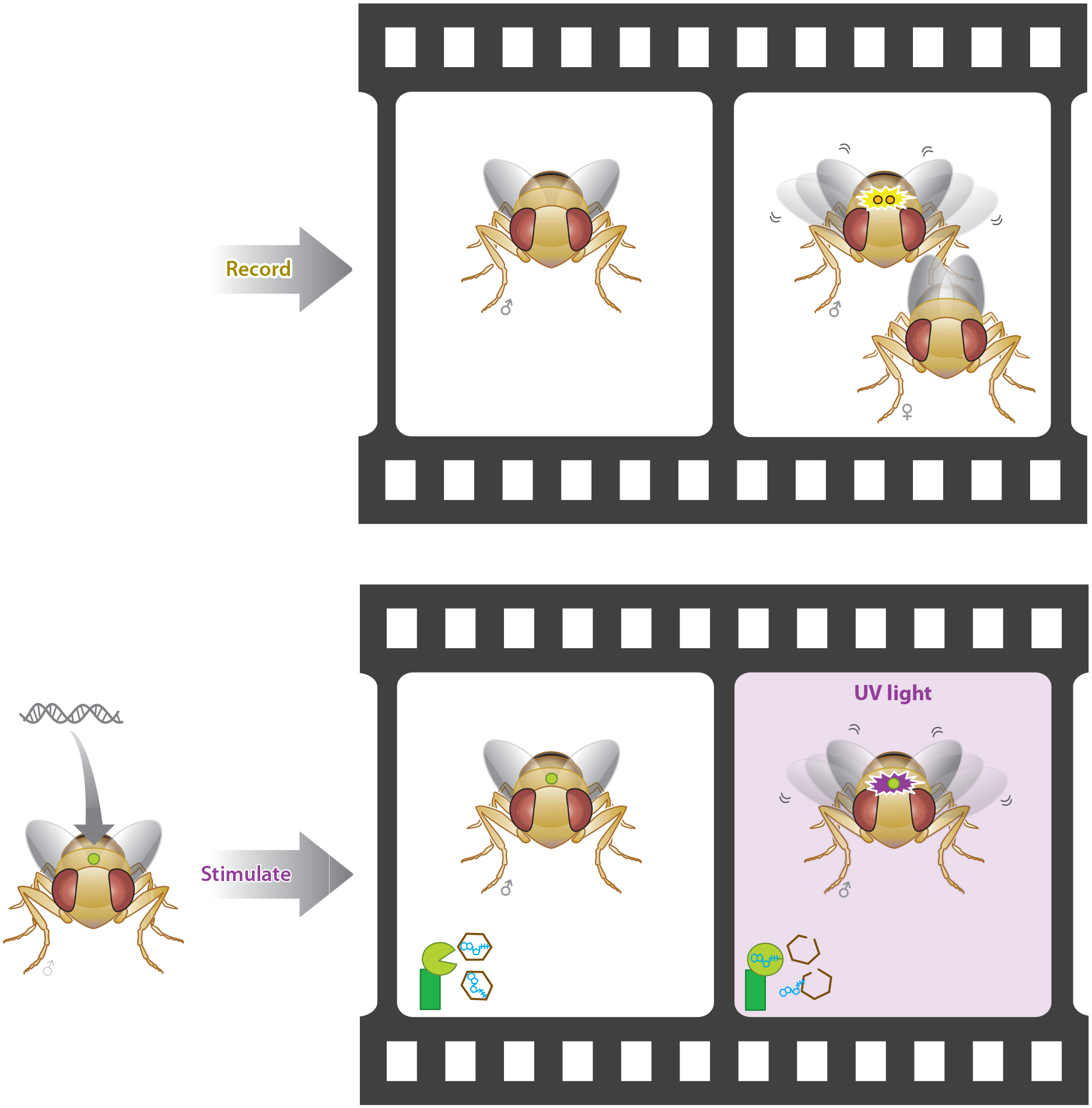
Genetic targeting of specific neural circuits enables optical gating of natural behaviors. In this example, generation of a courtship song involves wing vibrations and a complex behavior that engages many neural circuits. This neural activity can be recorded, and a subset of those neurons can then be stimulated to establish causation of the behavior using optogenetic stimulation (22).
Figure 5.
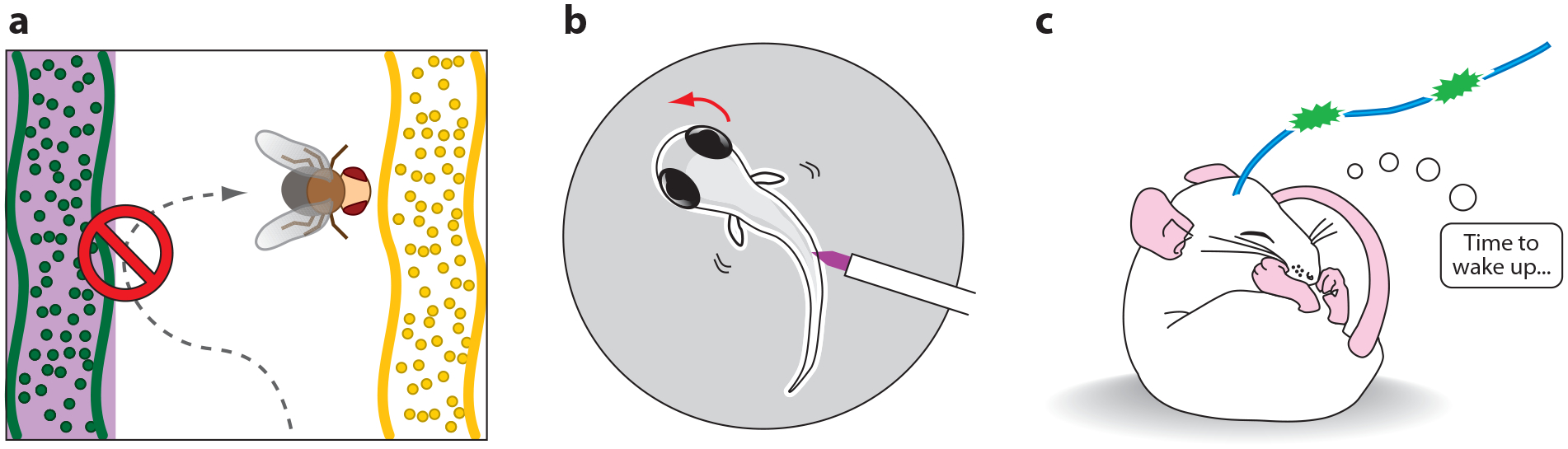
Optogenetic strategies for photostimulation. (a) A fly learns that an odor is aversive when the odor is paired with optical activation of P2X2 receptors in circumscribed dopaminergic neurons (21). (b) A zebrafish engages in a directed C-turn escape maneuver upon optical stimulation of LiGluR in defined sensory neurons (89). (c) A mouse wakes up when hypocretin neurons in the hypothalamus expressing channelrhodopsin are optically stimulated (1).
GENETICALLY ENGINEERED LIGHT-GATED CHANNELS AND RECEPTORS
Another approach combines the speed, efficacy, and bi-directionality of the PTL system with the genetic resolution of cell-specific targeting. This is accomplished by using a PTL that does not functionalize native channels or receptors, but that does functionalize a genetically engineered protein that can be targeted to particular neurons or subcellular regions using specific promoters and signaling motifs. The strategy to date has been to introduce a cysteine point mutation near the protein’s ligand-binding site that serves as a point of attachment for a cysteine-reactive PTL. Unlike the caged compounds and broadly reactive PTLs that act on many or all members of an ion channel or receptor family, such PTLs are specific only for the cysteine-engineered channel or receptor.
SPARK and D-SPARK: Light-Gated K+ Channels
SPARK (synthetic photoisomerizable azobenzene-regulated K+ channel) was the first genetically targeted PTL-based system invented and also the first optogenetic system for silencing neuronal activity (9). The PTL consists of maleimide (a cysteine-reactive moiety for covalent attachment), azobenzene (for light sensitivity), and quaternary ammonium (a K+ channel pore blocker) (MAQ) (Figure 2d). Once attached to a Shaker K+ channel at an introduced cysteine residue, conduction through the channel is blocked by 500-nm light (corresponding to the extended trans isoform of the PTL) and unblocked by 380 nm light (the retracted cis isoform). The Shaker K+ channel was also modified to increase its functional range by eliminating fast inactivation, reducing slow inactivation, and shifting the voltage dependence so that for practical purposes the channel is constitutively open. Action potentials in neurons that express this modified K+ channel and have been treated with the PTL are reversibly inhibited under 380-nm light and resume normal firing under 500-nm light (or in the dark, which also corresponds to the elongated trans configuration). The relatively large, ~20 pS conductance of the Shaker K+ channel (42), combined with robust expression in mammalian neurons, makes this an effective tool for optically inhibiting action potential firing (9).
Recognizing that a single point mutation in the ion selectivity filter of Shaker could significantly increase its permeability to Na+ (41), SPARK was later modified to function as a trigger for optically depolarizing neurons and inducing action potential firing (20). This modified version of SPARK (or D-SPARK, for depolarizing) allows Na+ conduction into neurons when the pore is unblocked by 380-nm illumination, triggering action potentials that cease upon illumination at 500 nm. Both SPARK and D-SPARK use the same PTL, so that in practice the two channels could be targeted to different populations of neurons by genetic methods and then equipped with light sensitivity after treatment with the same PTL.
LiGluR: Light-Gated Kainate-Type Glutamate Receptor/Channel
An extension of this strategy is the light-gated glutamate receptor LiGluR (Figure 1b). LiGluR is an ionotropic glutamate receptor (iGluR6) rendered light sensitive through genetic engineering and modification with a PTL (83). The PTL, consisting of maleimide, azobenzene, and glutamate (MAG), can be conjugated to a cysteine residue that is introduced into the clamshell-like ligand-binding domain of iGluR6. Under 380-nm illumination, the PTL photoisomerizes to the bent cis configuration and directs its glutamate end toward the binding site, activating the receptor. With 500-nm light, the agonist is withdrawn and the receptor deactivated. When the mutant iGluR6 is expressed in neurons and labeled with the PTL, photoisomerization to cis causes a depolarization and triggers the firing of action potentials. Single action potentials can be triggered by millisecond-long pulses of ~380-nm light, with high fidelity at rates reaching the firing capacity of the neuron (>50 Hz) (79). The fast kinetics of iGluR6 [τ on = 0.12–0.22 ms (52)] and its large conductance [25 pS for homomeric iGluR6Q (78)] make it a particularly suitable candidate for rapid, robust optical control of neural activity.
Numano et al. (67) utilized the modular nature of the LiGluR system to create converse light sensitivity, such that the receptor is activated by 500-nm light and deactivated by 380-nm light. This was accomplished by shortening the length of the PTL (MAG0, Figure 2e) and relocating the anchoring cysteine residue. When expressed in cultured hippocampal neurons, action potentials are reliably evoked by pulses of ~500-nm light. Conveniently, this shorter PTL, when conjugated to iGluR6 containing a cysteine at the original LiGluR position, functions in the same manner as the original LiGluR design. Thus, the two iGluR6 mutants can be genetically targeted to different populations of neurons and then treated with the same PTL, allowing activity to be triggered in one group of cells by 380-nm light and in another group of cells by 500-nm light. This kind of opposition may have utility in trying to reconstitute contrast in sensory systems, such as ON and OFF retinal cells.
The slow thermal relaxation of azobenzene from cis to trans makes it possible to trigger sustained trains of action potentials after only a brief pulse of light and therefore probe animal behavior in the dark, that is, under conditions in which the illumination does not act as a visual hindrance (Figure 6) (79). By targeting expression to specific neurons using the UAS-Gal4 system in zebrafish, Scott et al. (75) selectively expressed LiGluR in the sensory neurons that mediate the touch-evoked escape response. Long illumination with weak light followed by darkness produced a loss of response to a mechanical poke, suggesting that light-driven excitatory channels could be used to numb neurons via depolarization block or circuit adaptation (79). A strong pulse of light focused along the spine (and away from the eyes) could drive a directed escape away from the optical poke (89). LiGluR was also used in an intersectional optogenetic search in zebrafish to screen for neurons whose activation triggers a specific behavior (89).
Figure 6.
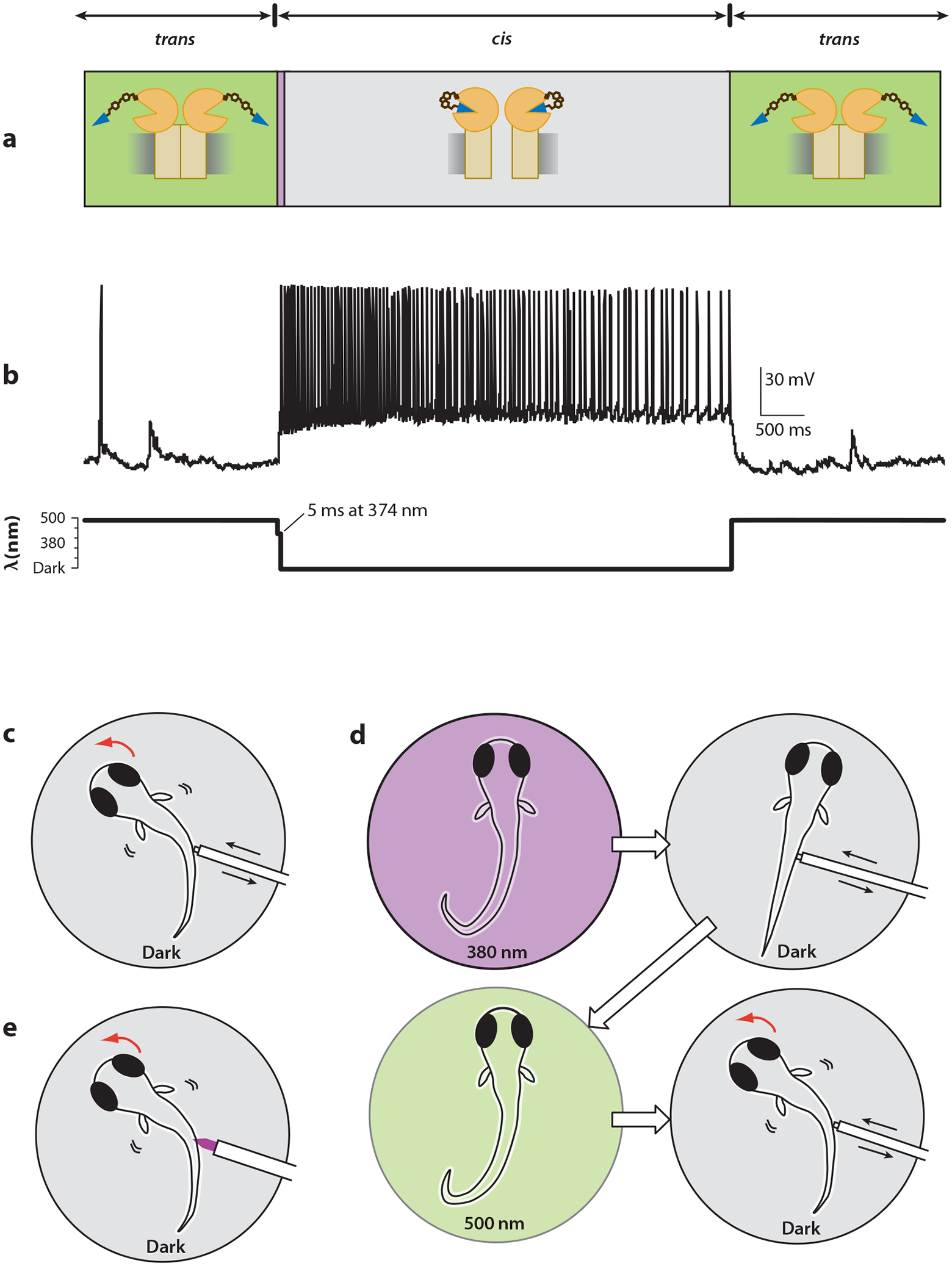
Some photostimulation methods allow activity to persist in the dark, enabling observation of behaviors that would otherwise be perturbed by constant illumination. (a) LiGluR remains activated (for tens of minutes) after brief exposure to ~380-nm light (34). The same is true of some ChR2 mutants, though for shorter times (12). (b) A hippocampal neuron expressing LiGluR fires action potentials when illuminated at 374 nm for 5 ms and then continues to fire in the dark until 488-nm light restores native activity. (c) Zebrafish naturally respond to a mechanical poke in the side by making a directed C-turn to escape the stimulus. (d) Illumination at ~380 nm followed by a long period of darkness induces a numbing of the escape reflex, which is then restored after exposure to ~500-nm light (79). (e) A focused optical poke with high-intensity 380 nm prevents light from entering the eyes and induces the same C-turn escape maneuver as in panel c (89).
OPSIN-BASED TRIGGERS OF NEURONAL ACTIVITY
The ability to optically control neuronal activity has been powerfully augmented by both of the above-described classes of genetically targeted light-sensitive channels: foreign receptors gated by photo-uncaged transmitters, and engineered receptors and channels controlled by cysteine-conjugated PTLs. However, these two approaches share the common obstacle of delivery of a synthetic chemical (caged transmitter or PTL). Although this may not be difficult for in vitro applications and some in vivo approaches (such as head injection of a caged transmitter into flies or mixing a PTL into the zebrafish water), there is great appeal in the simplicity of a method that would not require an added chemical.
This has become possible with the advent of opsin-based systems for optical manipulation (Figure 1c). In these systems, photoisomerization of the chromophore retinal (Figure 2f) triggers a functional change in the protein (opsin) to which it is coupled, be that protein a GPCR, ion channel, or pump. Retinal naturally exists as a mediator of visual responses and phototaxis in a variety of organisms and is readily available in the vertebrate nervous system. After one photocycle, the bleached retinal either dissociates from the protein and is replaced by unbleached chromophore [as in vertebrate visual rhodopsins (61)], or remains attached and is then photoconverted or biochemically converted back to the active form [as in Drosophila visual rhodopsin (43) and microbial rhodopsins (77)].
chARGe
The first opsin approach for the optical stimulation of neurons was to heterologously express in neurons three proteins from the Drosophila visual phototransduction cascade: rhodopsin, its cognate α-subunit, and arrestin-2 (90). This system, chARGe (arrestin, rhodopsin, and Gα), activates endogenous cation channels in the plasma membrane, leading to depolarization of the neuron and firing of action potentials. The chromophore, 11-cis retinal, is photoisomerized by 400- to 600-nm light into all-trans retinal, Gα then activates nonselective cation channels, and arrestin catalyzes regeneration of 11-cis retinal. Absorption of a single photon is amplified through Gα signaling, leading to the opening of multiple cation channels. In electrophysiology experiments with cultured hippocampal neurons, cells were pretreated with all-trans retinal, which is presumably converted to useful chromophore through the photoisomerase capability of chARGe. This finding could prove to be a big advantage for use in vivo, because studies with other rhodopsins have shown that endogenous levels of all-trans retinal are sufficiently available in vertebrates (93). This approach has the disadvantage that it requires delivery of three genes as well as sufficient levels of endogenous cation channels, and it appears to have kinetics that are somewhat slow, triggering action potentials on the order of seconds in cultured neurons.
Melanopsin
Two similar photosensory proteins from the mammalian visual system, human melanopsin and mouse melanopsin, when expressed in murine Neuro-2a cells or HEK293 cells, enable light-evoked depolarizing currents, do not require coexpression with exogenous Gα or arrestin, and may (62) or may not (71) require pretreatment with exogenous 9-cis or 11-cis retinal. Evidence suggests that mammalian melanopsin may have intrinsic photoisomerase activity, accounting for its minimal need for exogenous retinal and apparent ability to independently regenerate the active chromophore (60). When melanopsin is ectopically expressed in retinal ganglion cells, brief pulses of light induce long trains of action potentials (57). The use of melanopsin as a light-sensitive trigger elsewhere in the nervous system has not yet been demonstrated. Like chARGe, the activity of melanopsin may be too slow for some applications, but because of amplification through G-protein signaling, these systems could prove useful under conditions of low-intensity light.
RO4
Expression of rat rhodopsin 4 (RO4) in neurons and illumination with 475-nm light hyperpolarize cells by signaling through native Gi/o to endogenous GIRK channels and voltage-gated Ca2+ channels (24, 27, 54). In cultured hippocampal neurons, light induced a hyperpolarization of 9 mV, inhibited ~50% of action potentials, and reduced excitatory postsynaptic current amplitude by 40%. RO4 also modulated spontaneous activity in chick spinal cord in vitro and in ovo. As with chARGe, cells were pretreated with all-trans retinal before in vitro experiments. Even though RO4 requires a replenishing supply of 11-cis retinal or 9-cis retinal to replace the all-trans retinal that dissociates from the protein after photoisomerization, endogenous levels of retinal may have been sufficient in ovo (53).
ChR1 and ChR2
A phylogenetically distant class of opsins are the channelrhodopsins, retinal-coupled ion channels that are native to the unicellular green alga Chlamydomonas reinhardtii. Opening of these ion channels results directly from photoisomerization of a covalently bound retinal chromophore, rather than through a slower G-protein-signaling cascade, as in the above-described rhodopsins. Channelrhodopsin-1 (ChR1) and channelrhodopsin-2 (ChR2) are both cation channels that permeate Na+, K+, H+, and Ca2+ in a pH-dependent manner (14, 65). They are activated by photoisomerization of all-trans retinal to 13-cis retinal, which optimally occurs at the peak absorption wavelength of ~475 nm (8, 14, 29, 65). After photoisomerization, the covalently bound retinal spontaneously relaxes to all-trans in the dark, providing closure of the ion channel and regeneration of the chromophore.
Both ChR1 and ChR2 in heterologous cellular systems have been studied extensively, but the larger conductance of ChR2 [approximately 40 fS to 1 pS (8, 31, 58, 65)] and reversal potential near 0 mV make it well suited for depolarizing neurons and evoking action potentials in vitro (16, 53), in ovo (53), and in vivo (6, 13, 15, 23, 64, 74). Conveniently, the chromophore all-trans retinal, which is required for photoswitching and possibly also proper folding and membrane insertion of the protein (40), naturally exists at sufficient levels in cultured and intact vertebrate neural tissue, although not in D. melanogaster or Caenorhabditis elegans (64, 74, 93). ChR2 responds to two-photon illumination at 920 nm, capable of evoking action potentials in dissociated rat superior cervical ganglion neurons (73). ChR2 has been utilized in diverse applications such as mapping long-range cortical projections (68), electrophysio-logically tagging neuronal populations for identification in vivo (55), restoring breathing in a rat model of spinal cord injury (4), inducing dopaminergic behavioral conditioning in mice (81), identifying a role for fast-spiking cells in gamma oscillations associated with sensory responses (18), dissecting circuit elements associated with gamma oscillations in cortical signaling (76), and manipulating activity in the macaque frontal cortex (39).
Illumination of ChR2 triggers cation flux with a rapid τ on of 0.2 to 2 ms; however, relaxation in the dark to the nonconducting state is substantially slower with a τ off of 10 to 20 ms (12, 58, 65), which can make it difficult to trigger action potentials in rapid succession. ChR1-ChR2 chimeras with improved properties were created to improve fidelity at high firing frequencies (58), whereas in other work mutations were introduced to create even slower off-kinetics, such that the channel would function at practical timescales as bi-stable and require absorption of a photon of a different wavelength to return to the nonconducting state (12). ChR2 has undergone several other human-made alterations, from truncating unnecessary transmembrane domains (which were replaced with a fluorescent protein tag) to increasing photocurrent amplitude (64, 37). Much effort has also been directed at boosting expression to increase whole-cell currents.
VChR1 and VChR2
In an effort to find a spectrally distinct version of channelrhodopsin that could be used along-side ChR2 to allow independent optical control of two populations of neurons, additional proteins with sequence homology to ChR1 and ChR2 were identified in the genome of the spheroidal alga Volvox carteri (29, 92). Reflecting their sequence similarity to ChR1 and ChR2, these newly identified channelrhodopsins were termed VChR1 and VChR2. Because the spectral properties of VChR2 are similar to those of ChR2 (8, 29), VChR2 has not been developed as a tool for use in neurons. VChR1, on the other hand, has an action spectrum that peaks at 535 nm, with substantial absorption at even longer (and shorter) wavelengths. Driving action potentials at 589 nm in VChR1-expressing cells and at 406 nm in ChR2-expressing cells allows spectral separation, particularly in ChR2-expressing cells, which appear completely in-sensitive to 589-nm light. Future adjustments to increase the kinetics of activation and deactivation may lead to great improvements in firing fidelity (58), and narrowing or further red-shifting the absorption spectrum will probably make this a useful tool that can be combined with other optical triggers and reporters.
Halo, or NpHR
Complementing the toolbox of excitatory opsin-based neural triggers is halorhodopsin (Halo, or NpHR), a light-sensitive chloride pump from the archaebacterium Natronomonas pharaonis. Illumination near the peak absorption wavelength of 577 nm (26) generates hyperpolarizing Cl− currents that silence activity in cultured neurons (38, 94), in brain slices (80, 94, 96), in inner retinal neurons (95), in C. elegans motor neurons and muscles (59, 94), in zebrafish (7), and in rodent models of Parkinson’s disease (35). As a pump, halorhodopsin can drive hyperpolarizing Cl− currents regardless of the cell’s resting potential or reversal potential for Cl− ions. Strong hyperpolarizations are followed by rebound excitation (7). As with the channelrhodopsins, the chromophore all-trans retinal is converted to 13-cis retinal by the absorption of a photon. With halorhodopsin, each absorbed photon induces the transport of one Cl− ion across the cell membrane (69, 82); therefore, high light intensities provide the most robust neuronal silencing (94, 96). Although there is some over-lap in the action spectra between halorhodopsin and channelrhodopsin-2, this can be mitigated by tuning the light intensity and wavelengths, and neurons coexpressing these two constructs can be alternately hyperpolarized and depolarized by yellow light and blue light, respectively. In addition to codon optimization for mammalian use, the pump has been modified for improved trafficking with a signal peptide and ER export sequence (36, 96). Arch and Mac, two light-driven proton pumps from Halorubrum sodomense and Leptosphaeria maculans, respectively, efficiently silence neural activity and may prove to be valuable optogenetic tools that sidestep some of the drawbacks associated with halorhodopsin (22a).
COMMENTS
Optical tools for controlling neuronal activity are being rapidly invented and gaining widespread use. Each one of the optical strategies described above has advantages and disadvantages, depending on the application. When genetically targeting the neurons of interest is not an option or not wanted, photostimulation with caged compounds, reversibly caged ligands, and photochromic ligands with affinity-labeling capabilities can be effective. Some of these molecules have selectivity for specific receptors and channels, but generally this approach causes widespread stimulation through-out the area of illumination (Figure 3).
Cell-specific photostimulation using genetic targeting (Figure 5) can be achieved in several ways: (a) by expressing a foreign receptor in a cell-specific manner and photo-uncaging its cognate foreign ligand, (b) by selectively conjugating PTLs to genetically targeted receptors and channels that have an introduced cysteine residue for attachment, and (c) by genetically introducing opsin proteins that couple to native channels or are themselves channels or electrogenic pumps and utilize a photoswitch, retinal, native to the vertebrate nervous system. All these systems are amenable to genetic engineering to tune the properties and the manner for activating or inhibiting the cell. The PTL-based systems can also be tuned by altering the chemical PTL. The PTL approach may have the greatest versatility, with the potential to be adapted to any native protein that can be activated or blocked by a ligand on a tether. The opsin-based approaches have the greatest ease of use, and there is always a possibility that other naturally light-sensitive proteins that can mimic the function of neuronal regulatory proteins will be discovered. Moreover, chimeras of opsins and the related GPCRs have yielded light-gated GPCRs (3), providing optical access to a large number of neuronal regulatory pathways.
Optical approaches for controlling neuronal activity will be greatly enhanced by further advances in cell-type-specific gene delivery and in patterned, penetrating, focused, and rapidly switching light delivery. New PTLs and light-gated proteins will likely make it possible to control multiple neural signals in parallel, just as it is becoming easier to detect simultaneous neural signals with an expanding rainbow of protein-based fluorescent sensors.
Azobenzene:
a photoisomerizable chemical moiety that changes configuration from an extended trans state to a bent cis state, thereby repositioning an attached ligand in PTLs or reversibly caged ligands
PTL:
photoswitchable tethered ligand
Photoswitchable affinity label (PAL):
a PTL that labels native ion channels
Optogenetics:
a photostimulation technique in which the light-sensitive target is introduced genetically
iGluR6Q:
he unedited version of iGluR6 that is more calcium permeative and has a higher single channel conductance than the edited (R) version
PROGRESS IN OPTOGENETICS BUILDS ON COMPLEMENTARY TECHNOLOGIES.
Optical control of neuronal signaling will become more sophisticated with advancing technology in complementary fields. The following papers are recommended for further information on the latest advances in a few of the fields that are expected to have a significant impact on photostimulation and optogenetics in the near future.
Digital Light Projection (DLP)/Digital Micromirror Device (DMD) arrays that can direct light simultaneously at multiple targets with submicron resolution:
Wang S, Szobota S, Wang Y, Volgraf M, Liu Z, et al. 2007. All optical platform for parallel, remote and spatiotemporal control of neuronal activity. NanoLetters 7:3859–63
Wyart C, Del Bene F, Warp E, Scott EK, Trauner D, et al. 2009. Optogenetic dissection of a behavioral module in the vertebrate spinal cord. Nature 461(7262):407–10
Holographic methods for pointing light:
Lutz C, Otis TS, DeSars V, Charpak S, DiGregorio DA, Emiliani V. 2008. Holographic photolysis of caged neurotransmitters. Nat. Methods 5:821–27
Papagiakoumou E, De Sars V, Oron D, Emiliani V. 2008. Patterned two-photon illumination by spatiotemporal shaping of ultrashort pulses. Optics Express 16:22039–47
Optics for photostimulating and imaging structures in the brains of living mice:
Wilt BA, Burns LD, Wei Ho ET, Ghosh KK, Mukamel EA, Schnitzer MJ. 2009. Advances in light microscopy for neuroscience. Annu. Rev. Neurosci. 32:435–506
Drug-delivery pumps, nanoparticles, and time-release substrates for long-term delivery of compounds in vivo:
Agarwal A, Lariya N, Saraogi G, Dubey N, Agrawal H, Agrawal GP. 2009. Nanoparticles as novel carrier for brain delivery: a review. Curr. Pharm. Des. 15(8):917–25
Elman NM, Patta Y, Scott AW, Masi B, Ho Duc HL, Cima MJ. 2009. The next generation of drug-delivery microdevices. Clin. Pharmacol. Ther. 85(5):544–47
Genetic methods in neuroscience:
Luo L, Callaway EM, Svoboda K. 2008. Genetic dissection of neural circuits. Neuron 57(5):634–60
Scott EK. 2009. The Gal4/UAS toolbox in zebrafish: new approaches for defining behavioral circuits. J. Neurochem. 110(2):441–56
ACKNOWLEDGMENTS
This work was supported by the NIH Nanomedicine Development Center for the Optical Control of Biological Function (5PN2EY018241). We’d also like to acknowledge the many investigators whose important work and key contributions could not be cited here due to space restrictions.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. 2007. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450(7168):420–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed NA, Radwan NM, Ibrahim KM, Khedr ME, El Aziz MA, Khadrawy YA. 2008. Effect of three different intensities of infrared laser energy on the levels of amino acid neurotransmitters in the cortex and hippocampus of rat brain. Photomed. Laser Surg 26(5):479–88 [DOI] [PubMed] [Google Scholar]
- 3.Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. 2009. Temporally precise in vivo control of intracellular signalling. Nature 458(7241):1025–29 [DOI] [PubMed] [Google Scholar]
- 4.Alilain WJ, Li X, Horn KP, Dhingra R, Dick TE, et al. 2008. Light-induced rescue of breathing after spinal cord injury. J. Neurosci 28(46):11862–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allégre G, Avrillier S, Albe-Fessard D. 1994. Stimulation in the rat of a nerve fiber bundle by a short UV pulse from an excimer laser. Neurosci. Lett 180(2):261–64 [DOI] [PubMed] [Google Scholar]
- 6.Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, et al. 2007. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54(2):205–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arrenberg AB, Del Bene F, Baier H. 2009. Optical control of zebrafish behavior with halorhodopsin. Proc. Natl. Acad. Sci. USA 106:17968–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bamann C, Kirsch T, Nagel G, Bamberg E. 2008. Spectral characteristics of the photocycle of channelrhodopsin-2 and its implication for channel function. J. Mol. Biol 375(3):686–94 [DOI] [PubMed] [Google Scholar]
- 9.Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. 2004. Light-activated ion channels for remote control of neuronal firing. Nat. Neurosci 7(12):1381–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banghart MR, Mourot A, Fortin DL, Yao JZ, Kramer RH, Trauner D. 2009. Photochromic blockers of voltage-gated potassium channels. Angew. Chem. Angew. Chem. Int. Ed. Engl 48(48):9097–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartels E, Wassermann NH, Erlanger BF. 1971. Photochromic activators of the acetylcholine receptor. Proc. Natl. Acad. Sci. USA 68(8):1820–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. 2009. Bi-stable neural state switches. Nat. Neurosci 12(2):229–34 [DOI] [PubMed] [Google Scholar]
- 13.Bernstein JG, Han X, Henninger MA, Ko EY, Qian X, et al. 2008. Prosthetic systems for therapeutic optical activation and silencing of genetically-targeted neurons. Proc. Soc. Photo-Opt. Instrum. Eng 6854:68540H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berthold P, Tsunoda SP, Ernst OP, Mages W, Gradmann D, Hegemann P. 2008. Channelrhodopsin-1 initiates phototaxis and photophobic responses in Chlamydomonas by immediate light-induced depolarization. Plant Cell 20(6):1665–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, et al. 2006. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron 50(1):23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. 2005. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci 8(9):1263–68 [DOI] [PubMed] [Google Scholar]
- 17.Callaway EM, Katz LC. 1993. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc. Natl. Acad. Sci. USA 90(16):7661–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, et al. 2009. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459(7247):663–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. 1997. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389(6653):816–24 [DOI] [PubMed] [Google Scholar]
- 20.Chambers JJ, Banghart MR, Trauner D, Kramer RH. 2006. Light-induced depolarization of neurons using a modified Shaker K+ channel and a molecular photoswitch. J. Neurophysiol 96:2792–96 [DOI] [PubMed] [Google Scholar]
- 21.Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, et al. 2009. Writing memories with light-addressable reinforcement circuitry. Cell 139:405–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clyne JD, Miesenböck G. 2008. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell 133(2):354–63 [DOI] [PubMed] [Google Scholar]
- 22a.Chow BY, Han X, Dobry AS, Qian X, Chuong AS, et al. 2010. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463(7277):98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douglass AD, Kraves S, Deisseroth K, Schier AF, Engert F. 2008. Escape behavior elicited by single, channelrhodopsin-2-evoked spikes in zebrafish somatosensory neurons. Curr. Biol 18(15):1133–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Downes GB, Gautam N. 1999. The G protein subunit gene families. Genomics 62(3):544–52 [DOI] [PubMed] [Google Scholar]
- 25.Duprat F, Guillemare E, Romey G, Fink M, Lesage F, et al. 1995. Susceptibility of cloned K+ channels to reactive oxygen species. Proc. Natl. Acad. Sci. USA 92(25):11796–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duschl A, Lanyi JK, Zimányi L. 1990. Properties and photochemistry of a halorhodopsin from the haloalkalophile, Natronobacterium pharaonis. J. Biol. Chem 265(3):1261–67 [PubMed] [Google Scholar]
- 27.Ebrey T, Koutalos Y. 2001. Vertebrate photoreceptors. Prog. Retin. Eye Res 20(1):49–94 [DOI] [PubMed] [Google Scholar]
- 28.Ellis-Davies GC. 2007. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat. Methods 4(8):619–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ernst OP, Sánchez Murcia PA, Daldrop P, Tsunoda SP, Kateriya S, Hegemann P. 2008. Photoactivation of channelrhodopsin. J. Biol. Chem 283(3):1637–43 [DOI] [PubMed] [Google Scholar]
- 30.Farber IC, Grinvald A. 1983. Identification of presynaptic neurons by laser photostimulation. Science 222(4627):1025–27 [DOI] [PubMed] [Google Scholar]
- 31.Feldbauer K, Zimmermann D, Pintschovius V, Spitz J, Bamann C, Bamberg E. 2009. Channelrhodopsin-2 is a leaky proton pump. Proc. Natl. Acad. Sci. USA 106(30):12317–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fork RL. 1971. Laser stimulation of nerve cells in Aplysia. Science 171(974):907–8 [DOI] [PubMed] [Google Scholar]
- 33.Fortin DL, Banghart MR, Dunn TW, Borges K, Wagenaar DA, et al. 2008. Photochemical control of endogenous ion channels and cellular excitability. Nat. Methods 5(4):331–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorostiza P, Volgraf M, Numano R, Szobota S, Trauner D, Isacoff EY. 2007. Mechanisms of photoswitch conjugation and light activation of an ionotropic glutamate receptor. Proc. Natl. Acad. Sci. USA 104(26):10865–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. 2009. Optical deconstruction of Parkinsonian neural circuitry. Science 324(5925):354–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gradinaru V, Thompson KR, Deisseroth K. 2008. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 36(1–4):129–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, et al. 2007. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J. Neurosci 27(52):14231–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han X, Boyden ES. 2007. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PloS ONE 2(3):e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han X, Qian X, Bernstein JG, Zhou HH, Franzesi GT, et al. 2009. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron 62(2):191–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haupts U, Tittor J, Oesterhelt D. 1999. Closing in on bacteriorhodopsin: progress in understanding the molecule. Annu. Rev. Biophys. Biomol. Struct 28:367–99 [DOI] [PubMed] [Google Scholar]
- 41.Heginbotham L, Lu Z, Abramson T, MacKinnon R. 1994. Mutations in the K+ channel signature sequence. Biophys. J 66(4):1061–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heginbotham L, MacKinnon R. 1993. Conduction properties of the cloned Shaker K+ channel. Biophys. J 65(5):2089–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hillman P, Hochstein S, Minke B. 1983. Transduction in invertebrate photoreceptors: role of pigment bistability. Physiol. Rev 63(2):668–772 [DOI] [PubMed] [Google Scholar]
- 44.Hirase H, Nikolenko V, Goldberg JH, Yuste R. 2002. Multiphoton stimulation of neurons. J. Neurobiol 51(3):237–47 [DOI] [PubMed] [Google Scholar]
- 45.Izzo AD, Richter CP, Jansen ED, Walsh JT Jr. 2006. Laser stimulation of the auditory nerve. Lasers Surg. Med 38(8):745–53 [DOI] [PubMed] [Google Scholar]
- 46.Kaplan JH, Forbush B, Hoffman JF. 1978. Caged-ATP, a photolabile source of ATP. Biophys. J 21(3):A72 [Google Scholar]
- 47.Khakh BS, North RA. 2006. P2X receptors as cell-surface ATP sensors in health and disease. Nature 442(7102):527–32 [DOI] [PubMed] [Google Scholar]
- 48.Kourie JI. 1998. Interaction of reactive oxygen species with ion transport mechanisms. Am. J. Physiol 275(Pt. 1):C1–24 [DOI] [PubMed] [Google Scholar]
- 49.Lester HA, Chang HW. 1977. Response of acetylcholine receptors to rapid photochemically produced increases in agonist concentration. Nature 266(5600):373–74 [DOI] [PubMed] [Google Scholar]
- 50.Lester HA, Nass MM, Krouse ME, Nerbonne JM, Wassermann NH, Erlanger BF. 1980. Electrophysiological experiments with photoisomerizable cholinergic compounds: review and progress report. Ann. NY Acad. Sci 346:475–90 [DOI] [PubMed] [Google Scholar]
- 51.Lester HA, Nerbonne JM. 1982. Physiological and pharmacological manipulations with light flashes. Annu. Rev. Biophys. Bioeng 11:151–75 [DOI] [PubMed] [Google Scholar]
- 52.Li G, Oswald RE, Niu L. 2003. Channel-opening kinetics of GluR6 kainate receptor. Biochemistry 42(42):12367–75 [DOI] [PubMed] [Google Scholar]
- 53.Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, et al. 2005. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl. Acad. Sci. USA 102(49):17816–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X, Hümmer A, Han J, Xie M, Melnik-Martinez K, et al. 2005. G protein beta2 subunit-derived peptides for inhibition and induction of G protein pathways. Examination of voltage-gated Ca2+ and G protein inwardly rectifying K+ channels. J. Biol. Chem 280(25):23945–59 [DOI] [PubMed] [Google Scholar]
- 55.Lima SQ, Hromádka T, Znamenskiy P, Zador AM. 2009. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS One 4(7):e6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lima SQ, Miesenböck G. 2005. Remote control of behavior through genetically targeted photostimulation of neurons. Cell 121(1):141–52 [DOI] [PubMed] [Google Scholar]
- 57.Lin B, Koizumi A, Tanaka N, Panda S, Masland RH. 2008. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc. Natl. Acad. Sci. USA 105(41):16009–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin JY, Lin MZ, Steinbach P, Tsien RY. 2009. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys. J 96(5):1803–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Q, Hollopeter G, Jorgensen EM. 2009. Graded synaptic transmission at the Caenorhabditis elegans neuromuscular junction. Proc. Natl. Acad. Sci. USA 106(26):10823–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lucas RJ. 2006. Chromophore regeneration: Melanopsin does its own thing. Proc. Natl. Acad. Sci. USA 103(27):10153–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matthews RG, Hubbard R, Brown PK, Wald G. 1963. Tautomeric forms of metarhodopsin. J. Gen. Physiol 47:215–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. 2005. Addition of human melanopsin renders mammalian cells photoresponsive. Nature 433(7027):741–45 [DOI] [PubMed] [Google Scholar]
- 63.Mezey E, Tóth ZE, Cortright DN, Arzubi MK, Krause JE, et al. 2000. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. USA 97(7):3655–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. 2005. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol 15(24):2279–84 [DOI] [PubMed] [Google Scholar]
- 65.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, et al. 2003. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 100(24):13940–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagele T, Hoche R, Zinth W, Wachtveitl J. 1997. Femtosecond photoisomerization of cis-azobenzene. Chem. Phys. Lett 272(5–6):489–95 [Google Scholar]
- 67.Numano R, Szobota S, Lau AY, Gorostiza P, Volgraf M, et al. 2009. Nanosculpting reversed wavelength sensitivity into a photoswitchable iGluR. Proc. Natl. Acad. Sci. USA 106(16):6814–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petreanu L, Huber D, Sobczyk A, Svoboda K. 2007. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat. Neurosci 10(5):663–68 [DOI] [PubMed] [Google Scholar]
- 69.Pfisterer C, Gruia A, Fischer S. 2009. The mechanism of photo-energy storage in the halorhodopsin chloride pump. J. Biol. Chem 284(20):13562–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pierce S, Giese AC. 1957. Photoreversal of ultraviolet injury to frog and crab nerves. J. Cell Physiol 49(2):303–17 [DOI] [PubMed] [Google Scholar]
- 71.Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, et al. 2005. Induction of photosensitivity by heterologous expression of melanopsin. Nature 433(7027):745–49 [DOI] [PubMed] [Google Scholar]
- 72.Reece PJ, Dholakia K, Thomas RC, Cottrell GA. 2008. Green laser light (532 nm) activates a chloride current in the C1 neuron of Helix aspersa. Neurosci. Lett 433(3):265–69 [DOI] [PubMed] [Google Scholar]
- 73.Rickgauer JP, Tank DW. 2009. Two-photon excitation of channelrhodopsin-2 at saturation. Proc. Natl. Acad. Sci. USA 106:15025–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schroll C, Riemensperger T, Bucher D, Ehmer J, Völler T, et al. 2006. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol 16(17):1741–47 [DOI] [PubMed] [Google Scholar]
- 75.Scott EK, Mason L, Arrenberg AB, Ziv L, Gosse NJ, et al. 2007. Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat. Methods 4:323–26 [DOI] [PubMed] [Google Scholar]
- 76.Sohal VS, Zhang F, Yizhar O, Deisseroth K. 2009. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459(7247):698–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spudich JL, Yang CS, Jung KH, Spudich EN. 2000. Retinylidene proteins: structures and functions from archaea to humans. Annu. Rev. Cell. Dev. Biol 16:365–92 [DOI] [PubMed] [Google Scholar]
- 78.Swanson GT, Feldmeyer D, Kaneda M, Cull-Candy SG. 1996. Effect of RNA editing and subunit co-assembly single-channel properties of recombinant kainate receptors. J. Physiol 492(Pt. 1):129–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Szobota S, Gorostiza P, Del Bene F, Wyart C, Fortin DL, et al. 2007. Remote control of neuronal activity with a light-gated glutamate receptor. Neuron 54(4):535–45 [DOI] [PubMed] [Google Scholar]
- 80.Tønnesen J, Sørensen AT, Deisseroth K, Lundberg C, Kokaia M. 2009. Optogenetic control of epileptiform activity. Proc. Natl. Acad. Sci. USA 106(29):12162–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, et al. 2009. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324(5930):1080–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Váró G, Needleman R, Lanyi JK. 1995. Light-driven chloride ion transport by halorhodopsin from Natronobacterium pharaonis. 2. Chloride release and uptake, protein conformation change, and thermo-dynamics. Biochemistry 34(44):14500–7 [DOI] [PubMed] [Google Scholar]
- 83.Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D. 2006. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat. Chem. Biol 2(1):47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Volgraf M, Gorostiza P, Szobota S, Helix MR, Isacoff EY, Trauner D. 2007. Reversibly caged glutamate: a photochromic agonist of ionotropic glutamate receptors. J. Am. Chem. Soc 129(2):260–61 [DOI] [PubMed] [Google Scholar]
- 85.Wade PD, Taylor J, Siekevitz P. 1988. Mammalian cerebral cortical tissue responds to low-intensity visible light. Proc. Natl. Acad. Sci. USA 85(23):9322–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wells J, Kao C, Konrad P, Milner T, Kim J, et al. 2007. Biophysical mechanisms of transient optical stimulation of peripheral nerve. Biophys. J 93(7):2567–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wells J, Kao C, Mariappan K, Albea J, Jansen ED, et al. 2005. Optical stimulation of neural tissue in vivo. Opt. Lett 30(5):504–6 [DOI] [PubMed] [Google Scholar]
- 88.Wilcox M, Viola RW, Johnson KW, Billington AP, Carpenter BK, et al. 1990. Synthesis of photolabile precursors of amino acid neurotransmitters. J. Org. Chem 55(5):1585–89 [Google Scholar]
- 89.Wyart C, Del Bene F, Warp E, Scott EK, Trauner D, et al. 2009. Optogenetic dissection of a behavioral module in the vertebrate spinal cord. Nature 461(7262):407–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zemelman BV, Lee GA, Ng M, Miesenböck G. 2002. Selective photostimulation of genetically chARGed neurons. Neuron 33(1):15–22 [DOI] [PubMed] [Google Scholar]
- 91.Zemelman BV, Nesnas N, Lee GA, Miesenböck G. 2003. Photochemical gating of heterologous ion channels: remote control over genetically designated populations of neurons. Proc. Natl. Acad. Sci. USA 100(3):1352–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang F, Prigge M, Beyriére F, Tsunoda SP, Mattis J, et al. 2008. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat. Neurosci 11(6):631–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang F, Wang LP, Boyden ES, Deisseroth K. 2006. Channelrhodopsin-2 and optical control of excitable cells. Nat. Methods 3(10):785–92 [DOI] [PubMed] [Google Scholar]
- 94.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, et al. 2007. Multimodal fast optical interrogation of neural circuitry. Nature 446(7136):633–39 [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Ivanova E, Bi A, Pan ZH. 2009. Ectopic expression of multiple microbial rhodopsins restores ON and OFF light responses in retinas with photoreceptor degeneration. J. Neurosci 29(29):9186–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao S, Cunha C, Zhang F, Liu Q, Gloss B, et al. 2008. Improved expression of halorhodopsin for light-induced silencing of neuronal activity. Brain Cell Biol. 36(1–4):141–54 [DOI] [PMC free article] [PubMed] [Google Scholar]


