Abstract
Unambiguous examples of ecological causation of sexual dimorphism are rare, and the best evidence involves sexual differences in trophic morphology. We show that moderate female-biased sexual dimorphism in bill curvature is the ancestral condition in hermit hummingbirds (Phaethornithinae), and that it is greatly amplified in species such as Glaucis hirsutus and Phaethornis guy, where bills of females are 60 per cent more curved than bills of males. In contrast, bill curvature dimorphism is lost or reduced in a lineage of short-billed hermit species and in specialist Eutoxeres sicklebill hermits. In the hermits, males tend to be larger than females in the majority of species, although size dimorphism is typically small. Consistent with earlier studies of hummingbird feeding performance, both raw regressions of traits and phylogenetic independent contrasts supported the prediction that dimorphism in bill curvature of hermits is associated with longer bills. Some evidence indicates that differences between sexes of hermit hummingbirds are associated with differences in the use of food plants. We suggest that some hermit hummingbirds provide model organisms for studies of ecological causation of sexual dimorphism because their sexual dimorphism in bill curvature provides a diagnostic clue for the food plants that need to be monitored for studies of sexual differences in resource use.
Keywords: sexual dimorphism, hummingbird, resource partitioning, coevolution, foraging
I. Introduction
Evolutionary ecologists have traditionally used patterns of morphological variation among closely related species to make inferences about the kinds of ecological processes that may have caused them, such as competition and mutualism (e.g. Lack 1947; Faegri & van der Pijl 1971; Grant 1986). This tradition traces back to Charles Darwin and his classic observations of Darwin's finches, Geospiza (Darwin 1845). When he observed the tremendous variation in the size and shape of the bills of Geospiza finches, Darwin remarked that it was as though the bill of ‘one species had been taken and modified for different ends’ (Darwin 1845, p. 380). It was Darwin's reflections on morphological variation within and between finch species that led in part to his theory of evolution by natural selection. Since Darwin's time, studies of morphological variation within and between the bills of Darwin's finches have helped shape our understanding of the roles of natural selection and interspecific competition in resource partitioning by species and the evolution of differences between them via character displacement (Lack 1947; Grant 1986; Grant & Grant 2008).
Although evolutionary ecologists currently recognize competition as a force that can drive the evolution of differences between species through character displacement, the role of competition in driving the evolution of differences between sexes within a species is poorly understood (Hedrick & Temeles 1989; Shine 1989; Fairbairn et al. 2007), even though Darwin (1871) hypothesized that some sexual dimorphisms in animals resulted from ecological causes in the form of resource partitioning. A major problem is the lack of unambiguous evidence in support of his hypothesis, despite numerous examples of sexual differences in resource use (Temeles 1986; Shine 1989; Butler et al. 2000; Pearson et al. 2002). One reason for the paucity of evidence is that for the sexes of many species (e.g. predatory birds, snakes, mustelids), the ability to eat different prey often is a function of size (Storer 1966; Temeles 1985; Shine 1991; Dayan & Simberloff 1994). The positive relationships between body size, feeding morphology and prey size make it unclear whether ecological differences between the sexes are the cause or the consequence of sexual dimorphisms (Shine 1989).
Darwin was aware of the problem of attributing sexual dimorphisms to ecological causes, and in suggesting divergence in food utilization as a cause, he noted that such sexual differences should be confined to the feeding apparatus (Darwin 1871). The example Darwin used to illustrate this possible cause of sexual dimorphism was the New Zealand huia (Heterolocha acutirostris). Although the sexes were similar in body size and plumage, the bill of the male was short, thick and straight, whereas the bill of the female was long, slender and decurved (Selander 1966). Unfortunately, the huia went extinct in the early 1900s before its feeding ecology could be adequately studied.
Recent studies of the purple-throated carib hummingbird, Eulampis jugularis, provide strong evidence for Darwin's ecological causation hypothesis by linking sexual differences in trophic morphology to sexual differences in resource use (Temeles et al. 2000; Temeles & Kress 2003). Males are 25 per cent larger than females, yet females have bills that are 20 per cent longer and 40 per cent more curved than bills of males (Temeles et al. 2009). The sexual differences in bill size and shape of the purple-throated carib are associated with differences in the use, foraging efficiency and floral traits of their primary food plants (Temeles et al. 2000, 2005, 2009; Temeles & Kress 2003). Males are the primary visitor to Heliconia caribaea, which has short, straight flowers corresponding to their bills, whereas females are the primary visitor to Heliconia bihai, which has long, curved flowers corresponding to their bills. The long, curved bills of females give them much higher feeding efficiency than males at the long, curved flowers of H. bihai, whereas the short, straight bills of males prevent them from accessing nectar in many H. bihai flowers but confer higher feeding efficiency when hover-feeding at short, straight flowers (Temeles et al. 2009). Moreover, the extreme sexual dimorphism in bill morphology and the highly curved bills of female E. jugularis are unique among the Eulampis–Anthracothorax clade, supporting the hypothesis that sexual dimorphism in bill morphology evolved in response to its food plants.
The purple-throated carib and its Heliconia food plants provide some of the best evidence to date for ecological causation of a sexual dimorphism (Brown 2000; Altshuler & Clark 2003), but also raise the question of whether this evidence is simply an isolated case resulting from ecological release on islands or alternatively is more widespread than previously imagined. In this paper, we report on sexual dimorphism in bill size and shape of hermit hummingbirds.
Hermit hummingbirds (Phaethornithinae) exhibit relatively modest sexual dimorphism in size and in bill length in comparison to many members of the other subfamily of hummingbirds, the Trochilinae, which includes E. jugularis (Bleiweiss 1999). Many hermits, however, are characterized by highly curved bills, and a preliminary examination of museum specimens by one of us suggested that sexual dimorphism in bill curvature was present in some species (Temeles et al. 2009). Schuchmann (1999) also provided a qualitative assessment of sexual dimorphism in bill curvature of hermit hummingbirds, noting sexual differences in bill curvature in about 50 per cent of species. Although sexual dimorphism in bill length and body size of hummingbirds has been precisely studied (e.g. Bleiweiss 1999; Colwell 2000), the significance of sexual dimorphism in bill curvature was not previously considered.
First, we quantified the magnitude of sexual dimorphism in body size and bill size and shape in 30 species of hummingbirds, including 21 Phaethornithinae, from measurements of museum specimens. Second, following a published phylogeny of hummingbirds (McGuire et al. 2007, 2008), we quantified and examined patterns of sexual dimorphism in bill curvature in Phaethornithinae to determine whether curvature dimorphism was an ancestral or derived trait in this group. Third, based on findings of Temeles et al. (2009), we tested the prediction that sexual differences in bill curvature of hermits should be greatest for long-billed species. This prediction stems from recent studies demonstrating that feeding performances at short-straight or short-curved flowers were equivalent for hummingbirds of differing bill morphologies (Temeles et al. 2009). Only at long flowers were differences in bill morphology between hummingbirds significant for niche partitioning, with long, straight-billed birds having higher feeding efficiency at long, straight flowers, and long, curved-billed birds having higher feeding efficiency at long, curved flowers. We conclude by considering hypotheses for the evolution of bill dimorphism based on mating systems and ecological causes.
2. Material and methods
(a). Morphology
Data on bill morphology and wing length were taken from 854 specimens in museum collections (E. J. Temeles 2006, personal communication). On average, 16 males and 13 females were measured for each species, and significant differences between species were assessed using t-tests within species. All specimens were adults as determined from plumage and the absence of bill corrugations (Ortiz-Crespo 1972; Schuchmann 1999). For each specimen, we measured (in millimetres) the length (chord) of the exposed culmen, total bill length (from bill tip to gape), arc length of the exposed culmen and wing chord. Previous studies of sexual dimorphism in hummingbird bills used either the length of the exposed culmen or total bill length as measures of bill length (Bleiweiss 1999; Colwell 2000). Such measures adequately quantify bill size for birds with straight bills, but they underestimate the amount of tissue devoted to the bill for birds with curved bills. Accordingly, we used arc length as a second measure of bill size. Arc length of the exposed culmen was measured by bending a flexible plastic ruler along the upper curve of the bill from the bill tip to the anterior extension of the feathers on the maxillary ramphotheca. We assessed curvature of the bill by relating it to the curvature of a circle. Mathematically, the curvature of a circle, K, is equal to the reciprocal of its radius, R, e.g. 1/R. The radius of a circle (millimetres) was determined from the equation R = (C/2)/sinA, where C = the length (chord) of the exposed culmen and A = the angle of declension (in radians; Bell 1956). Angle of declension was measured using a circular protractor by centring the base of the bill on zero and then aligning the bill tip with the degree reading (Temeles et al. 2009). We used only total bill length, arc length, bill curvature and wing length in our analyses.
To quantify sexual dimorphism within a species, we used the Lovich–Gibbons ‘two-step’ ratio (Lovich & Gibbons 1992; Stephens & Wiens 2009). This ratio is computed from the equation s.d. = (L/S − 1)×1 if the female is the larger sex, or s.d. = (L/S − 1)×(−1) if the male is the larger sex, where L is the average size of the larger sex and S is the average size of the smaller sex for that species. This ratio produces measures of sexual dimorphism that are continuous around zero, directional, easy to interpret and properly scaled across species differing in overall size (Stephens & Wiens 2009). Sexual dimorphism for each trait and for all species was calculated using species averages following measurements of museum specimens.
(b). Phylogenetic hypotheses and sensitivity analyses
Our analysis used the molecular phylogeny of McGuire et al. (2007, 2008), which was based on two protein-coding mitochondrial genes, their flanking tRNAs and two nuclear introns. Their phylogeny included 21 of the 34 species of hermit hummingbirds listed by Schuchmann (1999) representing four of the five hermit genera (Glaucis, Eutoxeres, Phaethornis and Threnetes). We thus focused on these species, and also included nine non-hermit (Trochilinae) species (table 1). In the McGuire et al. (2007, 2008) analysis, backbone relationships among the major lineages of hummingbirds (e.g. topazes, hermits, mangoes) were generally well resolved with high support values. However, an important exception involved relationships among three monophyletic groups: (i) topazes, (ii) hermits and (iii) the remaining monophyletic lineage including the mangoes, brilliants, coquettes, emeralds, mt. gems, bees and Patagona (see fig. 3 in the McGuire et al. (2007) study). These groups were represented by several taxa in the present study (table 1). Because relationships among these groups could affect inferences related to ancestral state reconstructions and independent contrasts, we created all possible alternative topologies of relationships among these three lineages, and performed character reconstructions and independent contrasts on all alternative topologies. In addition, because we were most interested in the hermits, we used a fourth topology that included only those taxa in Phaethornithinae (21 taxa); relationships in this tree were generally well supported and followed McGuire et al. (2007). Ancestral character reconstructions for body size and bill trait dimorphisms were inferred for ancestral species using squared-change parsimony as implemented in Mesquite, v. 2.6 (Maddison & Maddison 2009) for the topologies mentioned above.
Table 1.
Hermit and non-hermit hummingbird species included in this study, and population.
| topazes | hermits |
| Topaza pella (Guyana) | Eutoxeres aquila (Peru) |
| Florisuga mellivora (Panama) | Eutoxeres condamini (Peru) |
| Glaucis aeneus (Panama) | |
| mangoes | Glaucis hirsutus (Brazil) |
| E. jugularis (Dominica) | Threnetes leucurus (Brazil) |
| Chrysolampis mosquitus (Trinidad) | Threnetes ruckeri (Panama) |
| Phaethornis augusti (Venezuela) | |
| coquettes | Phaethornis bourcieri (Peru) |
| Heliangelus exortis (Colombia) | |
| Phaethornis hispidus (Peru) | |
| Phaethornis koepckeae (Peru) | |
| brilliants | Phaethornis philippii (Peru) |
| Eriocnemis luciani (Ecuador) | |
| Phaethornis anthophilus (Colombia) | |
| Phaethornis atrimentalis (Peru) | |
| mt. gems | Phaethornis ruber (Peru) |
| Eugenes fulgens (S.W. USA and Mexico) | Phaethornis griseogularis (Peru) |
| Phaethornis longuemareus (Mexico) | |
| bees | Phaethornis syrmatophorus (Peru) |
| Archilochus colubris (N.E. USA) | |
| Phaethornis guy (Panama) | |
| Phaethornis yaruqui (Colombia) | |
| emeralds | Phaethornis malaris (Bolivia) |
| Chlorostilbon melanorhynchus (Colombia) | |
| Phaethornis longirostris (Panama) |
(c). Sexual dimorphism and bill length
We used linear regression to assess the relationship between sexual dimorphism in bill curvature and average bill length (bill arc or bill chord). For the 30-taxon dataset (21 hermit species plus nine Trochilinae), phylogenetically independent contrasts were conducted for all three alternative topologies using the PDAP module, v. 1.14 (Midford et al. 2003) in Mesquite. All branch lengths were set to unity and diagnostic tests in PDAP were run to ensure that the contrasts were properly standardized (Garland et al. 1992; Diaz-Uriarte & Garland 1998). We tested the relationship between the variables by least-squares linear regression of the contrasts through the origin (Harvey & Pagel 1991; Garland et al. 1992). Significant contrast correlations support the hypothesis that evolutionary changes in one character are consistently related to changes in a second character across the phylogeny. To confirm that the inclusion of the nine non-hermit species did not affect our interpretation, we also repeated these analyses for the hermit-only, 21-taxon dataset.
3. Results
(a). Ancestral character state reconstruction
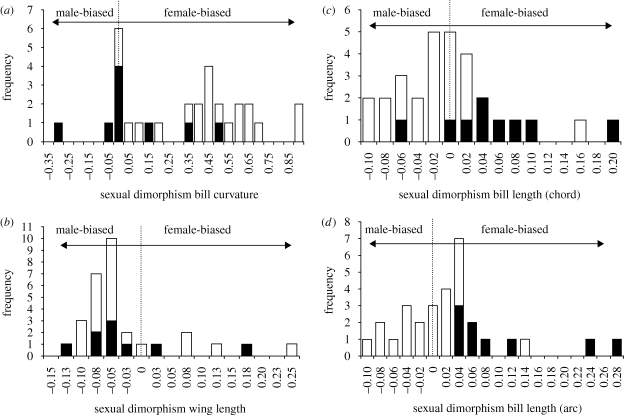
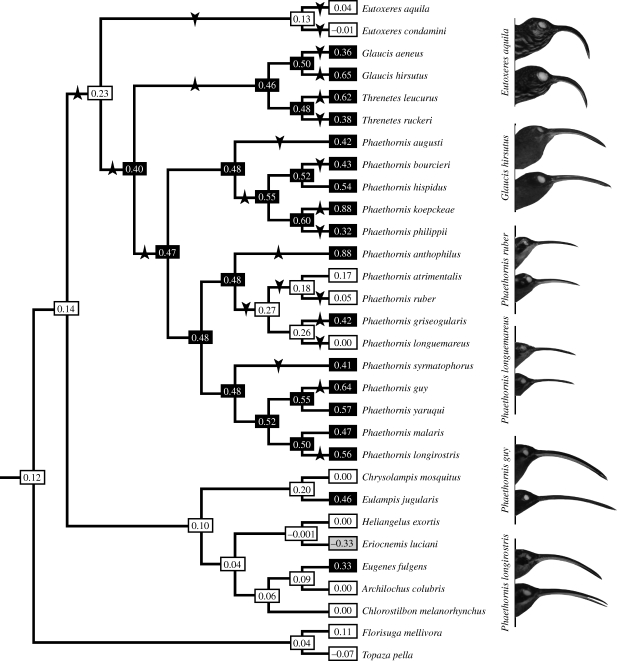
Female-biased bill curvature is widespread in the Phaethornithinae, and the majority of hermits studied had greater than 30 per cent female-biased curvature dimorphism (16 of 21 hermit species, 76%; figure 1a). Mapping sexual dimorphism in bill curvature onto McGuire et al.'s (2007, 2008) phylogeny indicates that moderate, female-biased sexual dimorphism is the ancestral character state in hermits (figure 2). This result was the case regardless of the topology used for ancestral character reconstructions (e.g. three alternatives based on McGuire et al. (2007, 2008), as well as the hermit-only topology; electronic supplementary material, table S1). Sexual dimorphism in bill curvature increased in the clade containing all hermits except Eutoxeres, in the ancestor of the Glaucis + Threnetes clade, and in the ancestor of Phaethornis (figure 2). Bill curvature dimorphism was lost or reduced in Eutoxeres and in the ancestor of the short-billed Phaethornis lineage (including P. atrimentalis, P. ruber and P. longuemareus).
Figure 1.
Sexual dimorphism in (a) bill curvature, (b) wing length, (c) bill chord and (d) bill arc for 30 hummingbird species included in the present study (21 Phaethornithinae and nine Trochilinae species). Positive values indicate female bias in dimorphism, whereas negative values indicate male bias. Phaethornithinae are indicated by open bars and Trochilinae by closed bars.
Figure 2.
Sexual dimorphism in bill curvature in Phaethornithinae. Values in boxes are bill curvature dimorphisms calculated for extant taxa or reconstructed for ancestral species (§2). Black shading indicates bill curvature dimorphism greater than 30%, whereas white boxes represent moderate (approx. 25%) or no bill curvature dimorphism. Note that the non-hermit Eriocnemis luciani is the only taxon with male-biased bill curvature dimorphism (grey shading). Arrows represent transitions (greater than 5%) in bill curvature dimorphism for hermit hummingbirds, which are either towards increasing female bias (arrows up) or decreasing female bias (arrows down). Photographs of species depict female above and male below. (Phylogeny adapted from McGuire et al. (2007, 2008).)
In contrast to the female-biased pattern of bill curvature dimorphism in hermits, sexual dimorphism in other traits was generally male-biased (figure 1b–d). Weak, male-biased sexual dimorphism in bill chord was present in the majority of ancestors in the Phaethornithinae and sexual dimorphism became more pronounced in derived taxa with the exception of a clade of short-billed hermits (electronic supplementary material, figure S1). This result replicates earlier findings that sexual dimorphism in bill length (chord) is male-biased within the Phaethornithinae (figure 1c; Bleiweiss 1999). Minimal female-biased bill arc dimorphism was the ancestral state in Phaethornithinae and was retained in Eutoxeres, and slightly reduced in Glaucis and Threnetes, reflecting the contribution of bill curvature to overall bill size. In contrast, male-biased sexual dimorphism in bill arc was ancestral in Phaethornis (electronic supplementary material, figure S2), but there was a reversal to female bias in the ancestor of P. griseogularis and P. longemareus. In general, bill arc dimorphism, like bill chord dimorphism, is male-biased within hermits (figure 1d).
Lastly, male-biased sexual dimorphism in wing length was the ancestral condition in hermits and was retained in all descendant lineages with the exception of small Phaethornis species (P. atrimentalis, P. ruber, P. griseogularis and P. longuemareus; electronic supplementary material, figure S3). This finding is consistent with earlier studies of sexual size dimorphism in hummingbirds that reported male-biased dimorphism in large species (>3 g) and female-biased dimorphism in small species (<3 g; Colwell 2000; figure 1b).
(b). Relationship between bill dimorphism and bill length
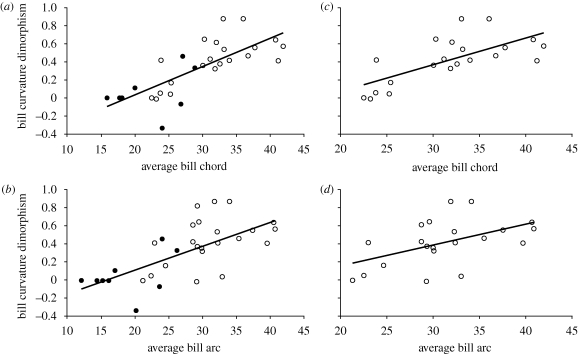
Studies of hummingbird feeding performance indicated that differences in performance are most pronounced among birds of differing bill curvatures at long flowers of varying curvatures (Temeles et al. 2009). Accordingly, we predicted that if sexual dimorphism in bill curvature evolved to allow the sexes to partition floral resources, such dimorphism should be most pronounced in long-billed hermit species, because only for these species would such dimorphism result in an exclusive or near-exclusive niche. Results were consistent with our prediction. Sexual dimorphism in bill curvature was significantly related to both measurements of bill length for uncorrected and phylogenetically independent contrasts (figure 3a,b). Because bill length dimorphism is female-biased in non-hermit species, which tend to have short bills, and male-biased in hermit species (figure 1c,d), which tend to have long bills (Bleiweiss 1999), and because bill curvature dimorphism tends to be absent or reduced in non-hermit species (figure 1a), these significant relationships could be driven by differences between hermit and non-hermit taxa, rather than by evolutionary forces within the hermit subfamily. When non-hermits were excluded from these analyses, sexual dimorphism in bill curvature was significantly related to bill length for both uncorrected and phylogenetically independent contrasts (figure 3c,d). Thus, within hermits, as bill length increases between species, so does sexual dimorphism in bill curvature.
Figure 3.
Sexual dimorphism in bill curvature becomes increasingly more female-biased with increasing bill length, as measured by both (a,c) average bill chord and (b,d) average bill arc for (a,b) all species and (c,d) hermit hummingbirds only. Regressions of raw variables were performed in JMP, v. 5.0.1a (SAS Institute, 1989–2002) and phylogenetically independent contrasts (PIC) were carried out in Mesquite, v. 2.6 (Maddison & Maddison 2009). (a) y = 0.0309x − 0.5794; r2 = 0.5705; F1,28 = 37.19, p < 0.0001; PIC: r > 0.482, p ≤ 0.007. (b) y = 0.0262x − 0.4113; r2 = 0.4768; F1,28 = 25.51, p < 0.0001; PIC: r > 0.453, p ≤ 0.015. (c) y = 0.0294x − 0.5147; r2 = 0.4763; F1,19 = 17.3, p = 0.0005; PIC: r = 0.565, p = 0.008. (d) y = 0.0234x − 0.3121; r2 = 0.2621, F1,19 = 6.75, p = 0.0177; PIC: r = 0.472, p = 0.03.
4. Discussion
As shown here, most but not all hermit hummingbirds are sexually dimorphic in bill morphology and body size. In a phylogenetic analysis of sexual size dimorphism in 154 species of diverse hummingbirds (i.e. both hermits and non-hermits), Colwell (2000) found that hummingbirds exhibited Rensch's rule, with females larger than males in small species, and males larger than females in large species. Following Colwell (2000), we assessed allometry in body size dimorphism by regressing the natural logarithms of female wing length versus male wing length for our analysis of hummingbirds (62% of all Phaethornithinae). Although the regression for the 21 species of hermits had a slope less than unity (F1,19 = 272.1, p < 0.0001, slope = 0.65) consistent with Rensch's rule, we also analysed the relationship between male and female wing lengths using a phylogenetically independent constrast. In agreement with Colwell's (2000) findings from an analysis of both hummingbird subfamilies, the relationship between male and female wing lengths persisted following a phylogenetically independent contrast (r = 0.89, p < 0.0001, slope = 0.64). To account for this pattern, Colwell offered a hypothesis based on sexual selection and energetics acting on males, and reproductive constraints acting on females. Specifically, he argued that for species having promiscuous or polygynous mating systems, sexual selection acting through male–male competition should favour large male size when resources are sufficient. For species in which resources are limiting and reproductive behaviour is energetically expensive, sexual selection may favour smaller male size. Variation in female size within and across hummingbird species, on the other hand, may be constrained owing to the costs of incubation and reproduction (Payne 1984; Colwell 2000). Comparative studies of the energetics of male–male competition in male-larger and male-smaller species are needed to test his hypothesis. In particular, for hermit hummingbirds, comparison of species in the female-size-biased Phaethornis clade (P. atrimentalis, P. ruber, P. griseogularis and P. longuemareus) with their closest male-size-biased relative (P. anthophilus) is warranted (electronic supplementary material, figure S3).
We also observed pronounced sexual dimorphism in bill chord, bill arc and especially bill curvature in many hermit species; indeed, 67 per cent of the hermit species in our study had statistically significant bill length dimorphism as determined by t-tests within species. Likewise, 85 per cent of the hermits in our study had significant female-biased curvature dimorphism as determined by t-tests within species. Both Bleiweiss (1999) and Colwell (2000) also noted significant sexual dimorphism in bill length of many hummingbird species. Such pronounced sexual dimorphism in bill morphology of hermits raises two questions: (i) what mechanisms favour the evolution of bill dimorphism in hermits? and (ii) why is dimorphism in bill curvature so pronounced in hermits?
(a). Foraging behaviour, mating systems and the evolution of bill dimorphism
In an analysis of 166 hummingbird species, Bleiweiss (1999) found that sexual differences in bill length were common, with some species exhibiting male-biased sexual dimorphism and others exhibiting female-biased sexual dimorphism. He suggested that mating and social behaviours, as well as feeding ecology, shaped these patterns. Among species in which males have the shorter bills (typically non-hermits; figure 1c,d), males defend and monopolize floral resources (Pitelka 1942; Stiles 1973; Temeles et al. 2005). Females of such species visit male territories for feeding and mating, or feed from undefended patches of flowers. Given their subordinate status relative to males, natural selection may have favoured a longer bill for females of these species to broaden their range of food plants, especially plants having flowers with lengths inaccessible to males. His hypothesis receives support from field and laboratory studies of sexual differences in hummingbird feeding performance (Temeles & Roberts 1993; Temeles et al. 2005, 2009). In contrast, Bleiweiss (1999) noted that among species in which males have the longer bill, such as hermits (figure 1c,d), males are often non-territorial and exhibit a lek mating system (Snow 1972, 1977; Stiles & Wolf 1979). He argued that male attendance at the lek reduces their priority of access to nectar resources and suggested that such a constraint might select for longer bills in lekking males to improve their abilities in exploitative competition.
One prediction stemming from Bleiweiss's (1999) hypothesis is that spatial segregation of the sexes caused by male lekking would reduce male priority of access to nectar resources, and hence favour the evolution of male-biased bill length, whereas breeding aggregations that lack separation of the sexes, such as harem polygyny, would not. In support of his prediction, he noted that males of many Phaethornis species, which lek, have longer bills than females, whereas males of Glaucis species, which exhibit harem polygyny, have bills approximately the same length as females.
Bleiweiss (1999) did not examine sexual dimorphism in bill curvature, however. As noted here, even though sexes of G. hirsutus do not differ appreciably in bill length, they exhibit pronounced differences in bill curvature, with bills of females being about 60 per cent more curved than bills of males. Such sexual differences in curvature also occur in other hermit species with variable mating systems (e.g. Threnetes; Stiles & Wolf 1979). The partitioning of floral resources by curvature as well as by length suggests that mating system per se may not be the driving factor for the evolution of sexual dimorphism in bills of hermit hummingbirds, especially as some lekking species exhibit little dimorphism, or even female-biased dimorphism, in bill length (figure 1c,d and electronic supplementary material, figures S1 and S2).
We thus offer three possible mechanisms by which feeding ecology could drive the evolution of sexual dimorphism in bill morphology of hermit hummingbirds and note that they are not mutually exclusive. First, male hermit hummingbirds may lack priority of access to floral resources regardless of their mating system (i.e. both lekking and non-lekking, in contrast to Bleiweiss (1999)), and sexual dimorphism in bill length or curvature may reduce exploitative competition between the sexes by allowing males to use a different suite of floral resources than females. Second, sexual dimorphism in hermit hummingbird bill morphology may be driven by male dominance, as in non-hermit hummingbirds, and exclusion of subordinate females from floral resources by dominant males may have led to the evolution of curved bills in females to partition the niche by allowing them to use different plant species than males (Stiles 1973; Bleiweiss 1999; Temeles et al. 2000; Taylor & White 2007). Third, sexual dimorphism in hermit hummingbird bill morphology may result from either interference or exploitative competition between the sexes during the non-breeding season, when resources might be most limiting (Temeles et al. 2009). Tests of these hypotheses require determination of the timing of sexual differences in resource use (i.e. during the breeding season or non-breeding season), the degree to which mating systems constrain male foraging behaviour, and the degree to which one sex dominates the other through interference competition.
(b). Other explanations for bill dimorphism
Limited observations of breeding female trochiline hummingbirds Calypte anna, Panterpe insignis and E. jugularis suggest that females spend more time than males engaged in arthropod feeding (Wolf & Stiles 1970; Stiles 1973; Remsen et al. 1986; Chavez-Ramirez & Dowd 1992). Consequently, arthropod feeding may act as a selective force on bill morphology of females. Studies of foraging time budgets by Stiles (1995), however, suggest that floral nectar resources are both the primary food source and the primary evolutionary constraint on hummingbird bill morphology. More specifically, a hummingbird's bill morphology as driven by flower-feeding dictates its mode of arthropod feeding rather than the reverse (Stiles 1995; Rodríguez-Flores & Stiles 2005).
Alternatively, sexual dimorphism in bill morphology of hermit hummingbirds may be driven by other aspects of male and female reproductive roles, such as fighting in males or nest building in females (Temeles et al. 2009). Nest structure is highly conserved in hermit hummingbirds, however (Schuchmann 1999). In addition, species of hermit hummingbirds differ greatly in bill shape, from species with nearly straight bills (e.g. Ramphodon naevius, P. boucieri) to species with curved bills and extremely curved bills (e.g. P. guy, G. hirsutus, Eutoxeres species). If male–male competition (i.e. fighting) favours the evolution of straight bills in male hummingbirds, then why males of some hermit species have curved bills requires additional explanation. Similarly, if nest building favours the evolution of curved bills in female hummingbirds, then why females of some hermit species have straight bills requires explanation. These hypotheses (e.g. male fighting and female nest building) do not seem likely, especially given the close match between bill morphology and floral morphology observed for some hummingbird species (see below).
Finally, sexual dimorphism in bill morphology may serve as a cue for sexual recognition (Wolf 1975). Many hermit hummingbird species are somewhat dimorphic in plumage, however, and many also exhibit sexual dimorphism in size (Schuchmann 1999; this study). As with the alternatives discussed above, sexual recognition is a less parsimonious explanation for sexual dimorphism in bill morphology of hermit hummingbirds, given the numerous examples of close matching between bill and flower morphology for many species (Stiles 1975; Feinsinger 1976; Wolf et al. 1976; Snow & Snow 1980; Stein 1992; Temeles et al. 2000; Taylor & White 2007).
(c). Why do hermit hummingbirds exhibit sexual dimorphism in curvature?
Hermits comprise a small subfamily of hummingbirds (approx. 10% of all hummingbird species) and yet are remarkable for the number of species that exhibit sexual dimorphism in bill curvature as well as for the number of species that exhibit curved bills. Their sexual dimorphism and resource partitioning owing to intersexual competition are most probably a consequence of ecological character release stemming from an evolutionary radiation into a vacant, curved-flower niche consisting of many Heliconia and other plant species with curved floral tubes (e.g. Bolnick & Doebeli 2003; Butler et al. 2007). Hermits dominate the lowland understory curved-flower niche, which suggests that this niche may have been unoccupied when they entered it. This hypothesis is supported by molecular data placing the emergence of hermits and Heliconia at approximately the same time, 18–22 Myr ago (Bleiweiss 1998; Kress & Specht 2005).
Our finding that those hermit hummingbird species with the longest bills also exhibited the greatest sexual dimorphism in bill curvature (figure 3) is consistent with the hypothesis of character release and resulting reduced interspecific competition, because only at long flower lengths does bill curvature dimorphism result in exclusive or nearly exclusive niches. Short-billed species (P. ruber, P. longuemareus, P. atrimentalis) exhibited little sexual differences in bill length or curvature, presumably because short flowers are accessible by longer-billed birds, unless they are extremely curved (Temeles et al. 2009). The accessibility of short-straight and short-curved flowers by longer-billed hummingbirds offers an explanation for the highly curved bills of sicklebill hermit hummingbirds (E. aquila and E. condamini) as well as for their reduced sexual dimorphism in bill length and curvature. The bills of these birds, as well as the tubes of the flowers of their food plants, are decurved at nearly a 90° angle from the horizontal (Gill 1978; Stein 1992). Such extreme curvature would exclude longer-billed birds, especially if the floral tubes were inflexible (Temeles et al. 2009). Specialization on moderately long, highly curved flowers may constrain the niche of sicklebill hummingbirds, reducing their sexual dimorphism in bill morphology as a result.
Although hermits dominate the lowland understory curved-flowered niche, other, non-hermit hummingbirds appear to have replaced them where they are absent and also have converged on a similar pattern of sexual dimorphism in bill morphology. In the Lesser Antilles where hermit hummingbirds are absent, female purple-throated caribs (E. jugularis) take their place as the traplining pollinator of long, curved flowers, especially heliconias (Lack 1973; Feinsinger & Colwell 1978). The extreme sexual dimorphism in bill morphology of E. jugularis is likewise attributed to competition between the sexes and ecological release (Temeles et al. 2000; Temeles & Kress 2003). Similarly, on mainland Central and South America, Lafresnaya lafresnayi and some Campylopterus species inhabit montane environments lacking hermits and have Phaethorninae-like bill morphologies and ecologies (Schuchmann 1999).
(d). Directions for further research
Of the three hypotheses that Charles Darwin presented for the evolution of sexual dimorphism—sexual selection, fecundity selection and ecological causation—ecological causation has been both the most challenging to test and the most difficult to support. Part of the problem is that it has been difficult to unambiguously link sexual differences in trophic morphology to sexual differences in resource use. Another problem is that it is inherently more difficult to link sexual differences in resource use to competition and sexual differences in morphology than it is to manipulate tail lengths of birds or fishes to examine sexual selection, or quantify egg production to examine fecundity selection (Shine 1989). Many species of hermit hummingbirds, as well as a few non-hermits (E. jugularis, L. lafresnayi and Campylopterus species), provide model organisms for studies of ecological causation of sexual dimorphism because their sexual dimorphism in bill curvature provides a diagnostic clue for the food plants that need to be monitored for studies of sexual differences in resource use. Preliminary studies support the hypothesis that differences in bill morphology between the sexes are associated with differences in resource use. In a study conducted in Costa Rica, Taylor & White (2007) found that males of P. guy accounted for 96 per cent of visits by this species to Heliconia beckneri, which has straight flowers, whereas females and juveniles of P. guy accounted for 96 per cent of visits by this species to Heliconia tortuosa, which has curved flowers. The difference between the sexes in the use of these plants is consistent with our finding that P. guy has significant female-biased sexual dimorphism in bill curvature (figures 2 and 4). Similarly, straight-billed male G. hirsutus and P. guy were the sole visitors to the straight-flower Heliconia hirsuta, whereas curved-billed female G. hirsutus and P. guy visited the curved-flowered H. bihai (figure 4; E. J. Temeles 2006, personal communication).
Figure 4.
Bill and flower correspondence in two species of hermit hummingbirds. (a) Phaethornis guy. A straight-billed male bird pictured with H. beckneri (above) and a female curve-billed bird with a flower of curved H. tortuosa (below). (b) Glaucis hirsutus. A male straight-billed bird with H. hirsuta (above) and a female curve-billed bird with a curved flower from H. bihai (below). Photographs of H. beckneri and H. tortuosa from Taylor (2005); other photographs by E. Temeles.
Not all of the hermit species we studied had statistically significant differences in bill curvature between the sexes (E. condamini, P. longuemareus, P. ruber), and in E. aquila and P. atrimentalis, sexual differences in bill curvature, although significant, were reduced relative to many hermit species (figure 2). In these species, sexual dimorphism in bill curvature may be a consequence of evolutionary history and may lack functional significance. Nonetheless, as noted above, sexual differences in resource use have been observed for some hermit species, indicating that morphological differences are in fact associated with differences in resource use. Glaucis hirsutus, P. guy and P. longisrostis, all of which exhibit pronounced sexual dimorphism in bill curvature, represent promising species for studies of the relationship between bill dimorphism, resource use and mating system, because G. hirsutus exhibits harem polygyny (Snow 1973) whereas the two Phaethornis species are lekkers (Snow 1972, 1977). Studies of P. superciliosus are warranted as well, because this species reputedly exhibits male-biased sexual dimorphism in bill curvature, which is uncharacteristic of hermits (Schuchmann 1999). Observations should focus on plants in hermit communities that have straight or curved flowers with long corollas, because those are most probably the drivers of resource partitioning via bill dimorphism; that is, studies of feeding performance predict that sexes should differ in feeding performance and the use of straight or curved flowers with long corollas, but may have similar feeding performance and hence overlap in their use of straight or curved flowers with short corollas (Temeles et al. 2009). Furthermore, many hermit species (e.g. P. longirostris, P. superciliosus and G. hirsutus) exhibit geographical variation in bill morphology (Hinkelmann 1996; E. J. Temeles 2006, personal communication), and studies of these species across their range may provide insights into the role of ecology in the evolution of bill dimorphism by linking such dimorphism to geographical changes in food plants (e.g. Temeles & Kress 2003).
The occurrence of sexual dimorphism in bill curvature within many hermit species, which seems to be unambiguously associated with their feeding ecology, suggests that sexual dimorphism in bill length in some species is also likely to result from ecological causes. Thus, studies of species with extreme sexual differences in bill length (e.g. P. yaruqui) might provide evidence for sexual partitioning along this resource axis. Finally, studies of sexually dimorphic species such as L. lafesnayi and Campylopterus species that occupy hermit-like niches in high altitude, hermit-free communities may offer valuable evidence for convergent evolution of sexual dimorphism in bill curvature in order to partition floral resources.
(e). Concluding remarks
As shown here, sexual dimorphism in bill curvature is widespread in hermit hummingbirds (figures 1 and 2). For some species, this dimorphism has been associated with sexual differences in resource use (figure 4; Taylor & White 2007). A similar association between sexual dimorphism in bill curvature and sexual differences in resource use has been documented precisely for the non-hermit species E. jugularis (Temeles et al. 2000, 2005, 2009). Sexual dimorphism in trophic morphology occurs in a number of other animal taxa, including musetlids (Dayan & Simberloff 1994), snakes (Shine 1991), birds of prey (Temeles 1985) and even some Darwin's finches (Grant & Grant 2003), although the connections between sexual dimorphism in trophic morphology, sexual differences in resource use and ecological causation are less clear. Given the unambiguous association between sexual dimorphism in bill morphology and sexual differences in resource use for some hummingbird species, we suggest that researchers consider ecological causation as a mechanism for the evolution of sexual differences for any species in which the sexes differ in trophic morphology, or for which differences in size may relate to differences in resource use.
Acknowledgements
We thank the curators and staff at the following institutions for allowing us to examine specimens in their collections: J. V. Remsen Jr and S. W. Cardiff, Louisiana State University Museum of Natural Science, J. Bates and D. Willard, Chicago Field Museum and G. R. Graves and J. P. Dean, Smithsonian Institution National Museum of Natural History. J. A. McGuire, J. V. Remsen Jr and J. Taylor kindly provided us with preprints of manuscripts or copies of dissertations. We thank two anonymous reviewers for comments. This research was supported by a Smithsonian Senior Fellowship to E.J.T., Amherst College, and NSF grants IBN-0078483, DEB-0614218 and DEB-0843364.
Footnotes
One contribution of 13 to a Theme Issue ‘Darwin's Galápagos finches in modern evolutionary biology’.
References
- Altshuler D. L., Clark C. J.2003Darwin's hummingbirds. Science 300, 588–589 (doi:10.1126/science.1084477) [DOI] [PubMed] [Google Scholar]
- Bell J.19562619. Tangent, chord theorem. Math. Gaz. 40, 211–212 (doi:10.2307/3608819) [Google Scholar]
- Bleiweiss R.1998Tempo and mode of hummingbird evolution. Biol. J. Linn. Soc. 65, 63–76 (doi:10.1111/j.1095-8312.1998.tb00351) [Google Scholar]
- Bleiweiss R.1999Joint effects of feeding and breeding behaviour on trophic dimorphism in hummingbirds. Proc. R. Soc. Lond. B 266, 2491–2497 (doi:10.1098/rspb.1999.0951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick D. I., Doebeli M.2003Sexual dimorphism and adaptive speciation: two sides of the same ecological coin. Evolution 57, 2433–2449 (doi:10.1554/02-595) [DOI] [PubMed] [Google Scholar]
- Brown K.2000Ecology: food fight drives evolution. Science 289, 369–371 (doi:10.1126/science.289.5478.369) [DOI] [PubMed] [Google Scholar]
- Butler M. A., Schoener T. W., Losos J. B.2000The relationship between sexual size dimorphism and habitat use in Greater Antillean Anolis lizards. Evolution 54, 259–272 (doi:10.1554/0014-3820(2000)054[0259:TRBSSD]2.0.CO;2) [PubMed] [Google Scholar]
- Butler M. A., Sawyer S. A., Losos J. B.2007Sexual dimorphism and adaptive radiation in Anolis lizards. Nature 447, 202–205 (doi:10.1038/nature05774) [DOI] [PubMed] [Google Scholar]
- Chavez-Ramirez F., Dowd M.1992Arthropod feeding by two Dominican hummingbird species. Wilson Bull. 104, 743–747 [Google Scholar]
- Colwell R. K.2000Rensch's rule crosses the line: convergent allometry of sexual size dimorphism in hummingbirds and flower mites. Am. Nat. 156, 495–510 (doi:10.1086/303406) [DOI] [PubMed] [Google Scholar]
- Darwin C. R.1845Journal of researches into the natural history and geology of the countries visited during the voyage of H.M.S. Beagle round the world, under the Command of Capt. Fitz Roy, R.N., 2nd edn.London, UK: John Murray [Google Scholar]
- Darwin C. R.1871The descent of man and selection in relation to sex London, UK: John Murray [Google Scholar]
- Dayan T., Simberloff D.1994Character displacement, sexual dimorphism, and morphological variation among British and Irish mustelids. Ecology 75, 1063–1073 (doi:10.2307/1939430) [Google Scholar]
- Diaz-Uriarte R., Garland T.1998Effects of branch length errors on the performance of phylogenetically independent contrasts. Syst. Biol. 47, 654–672 (doi:10.1080/10635159826065) [DOI] [PubMed] [Google Scholar]
- Faegri K., van der Pijl L.1971The principles of pollination ecology, 2nd edn.Oxford, New York: Pergamon Press [Google Scholar]
- Fairbairn D. J., Blanckenhorn W. U., Szekely T.2007Sex, size, and gender roles: evolutionary studies of sexual dimorphism Oxford, UK: Oxford University Press [Google Scholar]
- Feinsinger P.1976Organization of a tropical guide of nectarivorous birds. Ecol. Monogr. 46, 257–291 (doi:10.2307/1942255) [Google Scholar]
- Feinsinger P., Colwell R. K.1978Community organization among neotropical nectar-feeding birds. Am. Zool. 18, 779–795 (doi:10.1093/icb/18.4.779) [Google Scholar]
- Garland T., Jr, Harvey P. H., Ives A. R.1992Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol. 41, 18–32 (doi:10.1093/sysbio/41.1.18) [Google Scholar]
- Gill F. B.1978Ecological fitting: use of floral nectar in Heliconia stilesii Daniels by three species of hermit hummingbirds. Condor 89, 779–787 (doi:10.2307/1368525) [Google Scholar]
- Grant P. R.1986Ecology and evolution of Darwin's finches Princeton, NJ: Princeton University Press [Google Scholar]
- Grant P. R., Grant B. R.2003Reversed sexual dimorphism in the beak of a finch. Ibis 145, 341–343 (doi:10.1046/j.1474-919X.2003.00157.x) [Google Scholar]
- Grant P. R., Grant B. R.2008How and why species multiply: the radiation of Darwin's finches Princeton, NJ: Princeton University Press [Google Scholar]
- Harvey P. H., Pagel M. D.1991The comparative method in evolutionary biology Oxford, UK: Oxford University Press [Google Scholar]
- Hedrick A. V., Temeles E. J.1989The evolution of sexual dimorphism in animals: hypotheses and tests. Trends Ecol. Evol. 4, 136–138 (doi:10.1016/0169-5347(89)90212-7) [DOI] [PubMed] [Google Scholar]
- Hinkelmann C.1996Systematics and geographic variation in long-tailed hermit hummingbirds, the Phaethornis superciliosus/ malaris/ longirostris species group (Trochilidae) with notes on their biogeography. Orn. Neotrop. 7, 119–148 [Google Scholar]
- Kress W. J., Specht C. D.2005Between Cancer and Capricorn: phylogeny, evolution, and ecology of the tropical Zingiberales. In Proc. Symp. on plant diversity and complexity patterns—local, regional and global dimensions (eds Friis I., Balslev H.). Copenhagen, Denmark: Biologiske Skrifter, Royal Danish Academy of Sciences and Letters [Google Scholar]
- Lack D.1947Darwin's finches Cambridge, UK: Cambridge University Press [Google Scholar]
- Lack D.1973The numbers of species of hummingbirds in the West Indies. Evolution 27, 326–337 (doi:10.2307/2406972) [DOI] [PubMed] [Google Scholar]
- Lovich J. E., Gibbons J. W.1992A review of techniques for quantifying sexual size dimorphism. Growth Develop. Aging 56, 269–281 [PubMed] [Google Scholar]
- Maddison W. P., Maddison D. R.2009Mesquite: a modular system for evolutionary analysis, v. 2.5 See http://mesquiteproject.org [Google Scholar]
- McGuire J. A., Witt C. C., Altshuler D. L., Remsen J. V., Jr2007Phylogenetic systematics and biogeography of hummingbirds: Bayesian and maximum likelihood analyses of partitioned data and selection of an appropriate partitioning strategy. Syst. Biol. 56, 837–856 (doi:10.1080/10635150701656360) [DOI] [PubMed] [Google Scholar]
- McGuire J. A., Witt C. C., Remsen J. V., Jr, Dudley R., Altshuler D. L.2008A higher-level taxonomy for hummingbirds. J. Ornithol. 150, 155–165 (doi:10.1007/s10336-008-0330-x) [Google Scholar]
- Midford P. E., Garland T., Jr, Maddison W. P.2003PDAP package of Mesquite, v. 1.14 [Google Scholar]
- Ortiz-Crespo F. I.1972A new method to separate immature and adult hummingbirds. Auk 89, 851–857 [Google Scholar]
- Payne R. B.1984Sexual selection, lek behavior, and sexual size dimorphism in birds. Ornithol. Monogr. 33, 1–52 [Google Scholar]
- Pearson D., Shine R., How R.2002Sex-specific niche partitioning and sexual size dimorphism in Australian pythons (Morelia spilota imbricta). Biol. J. Linn. Soc. 77, 113–125 (doi:10.1046/j.1095-8312.1999.00075.x) [Google Scholar]
- Pitelka F. A.1942Territoriality and related problems in North American hummingbirds. Condor 44, 189–204 (doi:10.2307/1364129) [Google Scholar]
- Remsen J. V., Jr, Stiles F. G., Scott P. E.1986Frequency of arthropods in stomachs of tropical hummingbirds. Auk 103, 436–441 [Google Scholar]
- Rodríguez-Flores C. I., Stiles F. G.2005Ánalisis ecomorfológico de una comunidad de colibríes ermitaños (Trochilidae, Phaethorninae) y sus flores en la amazonía colombiana. Ornitol. Colomb. 3, 7–27 [Google Scholar]
- Schuchmann L.1999Family Trochilidae (hummingbirds). In Handbook of the birds of the world, vol. 5: Barn owls to hummingbirds (eds del Hoyo J., Elliott A., Sargatal J.), pp. 468–680 Barcelona, Spain: Lynx Edicions [Google Scholar]
- Selander R. K.1966Sexual dimorphism and differential niche utilization in birds. Condor 68, 113–151 (doi:10.2307/1365712) [Google Scholar]
- Shine R.1989Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Quart. Rev. Biol. 64, 419–461 (doi:10.1086/416458) [DOI] [PubMed] [Google Scholar]
- Shine R.1991Intersexual dietary divergence and the evolution of sexual dimorphism in snakes. Am. Nat. 138, 103–122 (doi:10.1086/285207) [Google Scholar]
- Snow B. K.1972Lek behaviour and breeding of Guy's hermit hummingbird Phaethornis guy. Ibis 116, 278–297 (doi:10.1111/j.1474-919X.1974.tb00125.x) [Google Scholar]
- Snow B. K.1973Social organization of the hairy hermit Glaucis hirsuta. Ardea 61, 94–105 [Google Scholar]
- Snow B. K.1977Comparison of the leks of Guy's hermit hummingbird Phaethornis guy in Costa Rica and Trinidad. Ibis 119, 211–214 (doi:10.1111/j.1474-919X.1977.tb03543.x) [Google Scholar]
- Snow D. W., Snow B. K.1980Relationships between hummingbirds and flowers in the Andes of Columbia. Bull. Brit. Mus. Nat. Hist. (Zool.) 38, 105–139 [Google Scholar]
- Stein B. A.1992Sicklebill hummingbirds, ants, and flowers. BioScience 42, 27–33 (doi:10.2307/1311625) [Google Scholar]
- Stephens P. R., Wiens J. J.2009Evolution of sexual size dimorphisms in Emydid turtles: ecological dimorphism, Rensch's rule, and sympatric divergence. Evolution 63, 910–925 (doi:10.1111/j.1558-5646.2008.00597.x) [DOI] [PubMed] [Google Scholar]
- Stiles F. G.1973Food supply and the annual cycle of the Anna hummingbird. Univ. Calif. Publ. Zool. 97, 1–109 [Google Scholar]
- Stiles F. G.1975Ecology, flowering phenology and hummingbird pollination of some Costa Rican Heliconia species. Ecology 56, 285–301 (doi:10.2307/1934961) [Google Scholar]
- Stiles F. G.1995Behavioral, ecological and morphological correlates of foraging for arthropods by the hummingbirds of a tropical wet forest. Condor 97, 853–878 (doi:10.2307/1369527) [Google Scholar]
- Stiles F. G., Wolf L. L.1979Ecology and evolution of lek mating behavior in the long-tailed hermit hummingbird. Ornithol. Monogr. 27, 1–78 [Google Scholar]
- Storer R. W.1966Sexual dimorphism and food habits of three North American accipiters. Auk 83, 423–436 [Google Scholar]
- Taylor J.2005Observations on the pollination ecology of sympatric populations of Heliconia beckneri and H. tortuosa in southern Costa Rica. Dissertation, University of Glasgow [Google Scholar]
- Taylor J., White S. A.2007Observations of hummingbird feeding behavior at flowers of Heliconia beckneri and H. tortuosa in southern Costa Rica. Ornitol. Neotrop. 18, 133–138 [Google Scholar]
- Temeles E. J.1985Sexual size dimorphism of bird-eating hawks: the effect of prey vulnerability. Am. Nat. 125, 485–499 (doi:10.1086/284357) [Google Scholar]
- Temeles E. J.1986Reversed sexual size dimorphism: effect on resource defense and foraging behaviors of nonbreeding northern harriers. Auk 103, 70–78 [Google Scholar]
- Temeles E. J., Kress W. J.2003Adaptation in a plant–hummingbird association. Science 300, 630–633 (doi:10.1126/science.1080003) [DOI] [PubMed] [Google Scholar]
- Temeles E. J., Roberts W. M.1993Effect of sexual dimorphism in bill length on foraging behavior: an experimental analysis of hummingbirds. Oecologia 94, 87–94 (doi:10.1007/BF00317307) [DOI] [PubMed] [Google Scholar]
- Temeles E. J., Pan I. L., Brennan J. L., Horwitt J. N.2000Evidence for ecological causation of sexual dimorphism in a hummingbird. Science 289, 441–443 (doi:10.1126/science.289.5478.441) [DOI] [PubMed] [Google Scholar]
- Temeles E. J., Goldman R. S., Kudla A. U.2005Foraging and territory economics of sexually dimorphic purple-throated caribs (Eulampis jugularis) on three Heliconia morphs. Auk 122, 187–204 (doi:10.1642/0004-8038(2005)122[0187:FATEOS]2.0.CO;2) [Google Scholar]
- Temeles E. J., Koulouris C. R., Sander S. E., Kress W. J.2009Effect of flower shape and size on foraging performance and trade-offs in a tropical hummingbird. Ecology 90, 1147–1161 (doi:10.1890/08-0695.1) [DOI] [PubMed] [Google Scholar]
- Wolf L. L.1975Female territoriality in the purple-throated carib. Auk 92, 511–522 [Google Scholar]
- Wolf L. L., Stiles F. G.1970Evolution of pair cooperation in a tropical hummingbird. Evolution 24, 759–773 (doi:10.2307/2406556) [DOI] [PubMed] [Google Scholar]
- Wolf L. L., Stiles F. G., Hainsworth F. R.1976Ecological organization of a tropical, highland hummingbird community. J. Anim. Ecol. 45, 349–379 [Google Scholar]