Abstract
The further conversion of an arachidonic acid hydroperoxide to a leukotriene A (LTA) type epoxide by specific lipoxygenase (LOX) enzymes constitutes a key step in inflammatory mediator biosynthesis. Whereas mammalian 5-LOX transforms its primary product (5S-hydroperoxyeicosatetraenoic acid; 5S-HPETE) almost exclusively to LTA4, the model enzyme, soybean LOX-1, normally produces no detectable leukotrienes and instead further oxygenates its primary product 15S-HPETE to 5,15- and 8,15-dihydroperoxides. Mammalian 15-LOX-1 displays both types of activity. We reasoned that availability of molecular oxygen within the LOX active site favors oxygenation, whereas lack of O2 promotes LTA epoxide synthesis. To test this, we reacted 15S-HPETE with soybean LOX-1 under anaerobic conditions and identified the products by high pressure liquid chromatography, UV, mass spectrometry, and NMR. Among the products, we identified a pair of 8,15-dihydroxy diastereomers with all-trans-conjugated trienes that incorporated 18O from H218O at C-8, indicative of the formation of 14,15-LTA4. A pair of 5,15-dihydroxy diastereomers containing two trans,trans-conjugated dienes (6E,8E,11E,13E) and that incorporated 18O from H218O at C-5 was deduced to arise from hydrolysis of a novel epoxide containing a cyclopropyl ring, 14,15-epoxy-[9,10,11-cyclopropyl]-eicosa-5Z,7E,13E-trienoic acid. Also identified was the δ-lactone of the 5,15-diol, a derivative that exhibited no 18O incorporation due to its formation by intramolecular reaction of the carboxyl anion with the proposed epoxide intermediate. Our results support a model in which access to molecular oxygen within the active site directs the outcome from competing pathways in the secondary reactions of lipoxygenases.
Keywords: Eicosanoid Structure, Eicosanoids Biosynthesis, Enzyme Mechanisms, Lipid Chemistry, Lipoxygenase Pathway
Introduction
Lipoxygenases (LOX)2 are named for their oxygenase activity with polyunsaturated fatty acids, yet they also catalyze other types of reaction. One of the most important in a biological context is leukocyte 5-LOX-catalyzed further conversion of the hydroperoxide product 5S-HPETE to leukotriene A4 (LTA4) (1). LTA4, an unstable epoxide, will then be subject to metabolism of downstream enzymes in the pathway to more stable and biologically active LTs, including the dihydroxy LTB4 and the peptidyl LTC4, LTD4, and LTE4 (2). Parallels of the LTA synthase activity of 5-LOX also exist in some other LOX enzymes. Mammalian 15-LOX-1, for example, can further convert 15S-HPETE to a structural analogue of LTA4, named 14,15-LTA4 based on the position of the epoxide group between C-14 and C-15 (3, 4). This 14,15-epoxide was recently given the name of eoxin A4 on account of its production by eosinophils and its further transformation, analogous to LTA4, to a series of biologically active peptidyl derivatives, 14,15-LTC4, -LTD4, and -LTE4 (eoxins C4, D4, and E4) (5).
Despite the general parallels between leukocyte 5-LOX and mammalian 15-LOX-1 within the physiological context, the two enzymes exhibit notable differences in their reactions toward 5S-HPETE and 15S-HPETE, respectively. Leukocyte 5-LOX gives almost exclusively LTA4 from 5S-HPETE, whereas the reaction of mammalian 15-LOX-1 with 15S-HPETE leads, in addition to 14,15-LTA4, to several other products, including two fatty acid dihydroperoxides, 8S,15S-diHPETE and 5S,15S-diHPETE (3). On the other end of the spectrum of reactivity compared with leukocyte 5-LOX is the prototypical plant lipoxygenase, soybean LOX-1. It converts 15S-HPETE exclusively to 8S,15S-diHPETE and 5S,15S-diHPETE (6). These diHPETEs are often referred to as “double oxygenation” products because they are formed, in essence, by oxygenation of arachidonic acid twice in succession at two different positions.
In the present study, we aimed to understand the mechanistic basis for the different preferences of LOX enzymes for LTA synthesis or double oxygenation. Some clues can be gleaned from the literature. Rabbit reticulocyte 15-LOX or porcine leukocyte 12-LOX (both being homologs of human 15-LOX-1), when incubated with 15S-HPETE under anaerobic reactions, gives a higher yield of 14,15-LTA4 at the expense of the double oxygenation products (3, 7). Conversely, under higher than normal oxygen pressures, potato 5-LOX can be forced to perform double oxygenation on 5S-HPETE (8). These studies imply that LTA synthesis and double oxygenation are in competition, and the concentration of molecular oxygen is a critical determinant of which process dominates. The latter point can be better understood when one considers that LTA synthesis does not involve molecular oxygen, whereas the double oxygenation reaction uses molecular oxygen as one of the two substrates. Because the catalysis should be influenced by oxygen concentrations in the enzyme active site, rather than in the atmosphere per se, we hypothesized that the different preferences of LOX isoenzymes for LTA synthesis or double oxygenation stem from different oxygen concentrations in their active sites.
With this hypothesis, we reasoned that soybean LOX-1, which catalyzes only double oxygenation with 15S-HPETE under usual aerobic conditions, might show 14,15-LTA4 synthase activity when molecular oxygen is excluded. In this paper, we have described such an anaerobic reaction of soybean LOX-1 with 15S-HPETE. As anticipated, we observed the formation of the hydrolysis products of the putative 14,15-LTA4. Moreover, as a mechanistically informative finding, we identified among the reaction products two novel 5,15-dihydroxy compounds and their δ-lactone derivatives. Based on their chemical structures, conditions of formation, and evidence from H218O incubations, we suggested that the 5,15-dihydroxy compounds and their δ-lactone derivatives result from nucleophilic attack by water (hydrolysis) and by the terminal carboxylate anion (lactonization), respectively, of a novel cyclopropyl epoxide formed via an LTA-synthase-related mechanism.
EXPERIMENTAL PROCEDURES
Materials
Soybean LOX-1 (soybean P1, purified) and nordihydroguaiaretic acid (NDGA) were purchased from Cayman Chemical (Ann arbor, MI). We determined that the purified soybean LOX-1 employed in this work was ∼40% lower in specific activity than advertised (possibly due to two cycles of freeze-thawing), ∼20 μmol/min/mg using the Axelrod assay with sodium linoleate (9). Linoleic and arachidonic acids were purchased from NuChek Prep Inc. (Elysian, MN). [1-14C]Arachidonic acid was purchased from PerkinElmer Life Sciences. 15S-HPETE and 13S-HPODE were synthesized by reacting soybean LOX-1 with arachidonic acid at pH 9 under the normal aerobic conditions, followed by SP-HPLC purification (10). 5(RS)-HETE was chemically synthesized from arachidonic acid (11), and the enantiomers were resolved by chiral column HPLC as described (12). 5,15-diHETE was prepared similarly using 5S-HETE and/or 5R-HETE as substrate for soybean LOX-1, followed by reduction using NaBH4 and SP-HPLC purification. The Aldrich Atmosbag used for conducting the anaerobic incubations was purchased from Sigma-Aldrich.
Reaction Incubations and Extraction
The initial anaerobic reactions on an analytical scale were conducted inside an N2-inflated Aldrich Atmosbag. First, placed into the Aldrich Atmosbag were an open vial containing the enzyme solution (0.76 μm soybean LOX-1 in 1 ml of sodium phosphate, pH 7.5), an open vial containing the substrate solution (20–880 μm 15S-HPETE in 1 ml of sodium phosphate, pH 7.5), syringes, vial caps, and a bottle of methanol. The Atmosbag was then sealed and subjected to vacuuming and N2 inflation for three cycles. Twenty minutes was given for the enzyme and substrate solutions to reach equilibrium with the N2 atmosphere. Then the reaction was started by mixing the enzyme and the substrate. Reaction was stopped by the addition of an equal volume of methanol, followed by extraction using an Oasis HLB cartridge (Waters Corp.).
For the reactions in the presence of NDGA or 13S-HPODE, 80 μm NDGA or 13S-HPODE was included in the substrate solution. For structural analysis of the products, the incubations were scaled up to a volume of 20 ml using otherwise the same conditions. For incubations in 18O-enriched buffer, a volume of 0.5 ml was used, which is comprised of 0.4 ml of 96% H218O and 0.1 ml of 500 mm sodium phosphate, pH 7.5.
Kinetic Measurements
For kinetic measurements, the anaerobic reaction of soybean LOX-1 (usually 0.2 μm unless noted otherwise) with 15S-HPETE (50–500 μm) was monitored at 280 nm in an enclosed quartz cuvette using a PerkinElmer Lambda-35 spectrophotometer. N2-saturated sodium phosphate buffer (pH 7.5), glucose oxidase, glucose, and catalase were used to achieve the anaerobic conditions; subsequently, HPLC analyses verified that no oxygenation products were formed. The reaction volume is usually 1.1 ml. To avoid potential inhibition of alcohols on the enzyme activity, 15S-HPETE originally dissolved in methanol was first added to the quartz cuvette and then taken to dryness under a stream of N2. The cuvette was then placed at the bottom of a plastic bag filled with argon. 1 ml of N2-saturated buffer, 25 μl of glucose oxidase (stock concentration: 827 Sigma-units/ml), 1 μl of catalase (the stock solution was 5× diluted from the commercial solution of bovine liver catalase purchased from Sigma-Aldrich, C3155, ≥35,000 units/mg protein), and 50 μl of glucose (2 m stock, N2-saturated) was then added to the cuvette. The cuvette was then capped, taken out of the bag, shaken for a few seconds, and let standing for about 1 min. Reaction was started by the quick addition of 6 μl of soybean LOX-1 stock solution (final concentration 0.2 μm).
HPLC Analysis and Purification
The extracted products were analyzed by RP-HPLC and SP-HPLC using an Agilent 1100 HPLC system equipped with a diode array detector. The products from [1-14C]15S-HPETE were analyzed simultaneously by the diode array detector and by a Flo-One A-100 radioactive detector (Radiomatic Instruments and Chemical Co., Meriden, CT). The RP-HPLC analysis used a Waters Symmetry C18 column (5 μm, 0.46 × 25 cm), an isocratic solvent system of methanol/water/acetic acid (80:20:0.01 by volume), and a flow rate of 1 ml/min. The SP-HPLC analysis used a Beckman Ultrasphere® silica column (5 μm, 0.46 × 25 cm), an isocratic solvent system of hexane/isopropyl alcohol/acetic acid (100:2:0.1 by volume for less polar products followed by a strong solvent wash or 100:5:0.1 for polar products), and a flow rate of 1 ml/min. Products 2, 3, 4, and 5 (see Fig. 1) were purified by SP-HPLC. Products 1, 6, and 7 were purified by SP-HPLC followed by RP-HPLC. Separation of the two diastereomers of product 4 was achieved using a Chiralpak AD-RH column (5 μm, 0.46 × 15 cm), an isocratic solvent system of methanol/water/acetic acid (95:5:0.01 by volume), and a flow rate of 0.5 ml/min. For analysis of product 5, we used the same column, methanol/acetic acid (100:0.01 by volume), and a flow rate of 1 ml/min.
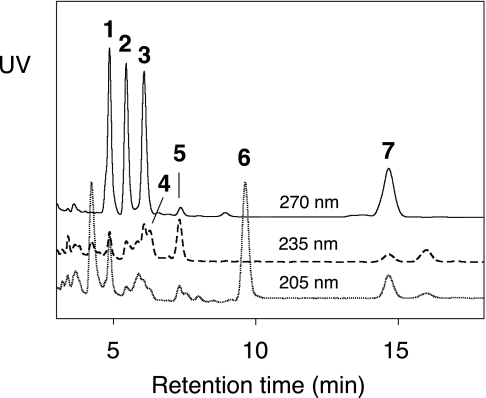
FIGURE 1.
RP-HPLC analysis of products from the anaerobic reaction of soybean LOX-1 (0.38 μm) with 15S-HPETE (40 μm). The products were eluted using a Waters Symmetry C18 column (0.46 × 25 cm), a solvent system of methanol/water/acetic acid (80:20:0.01 by volume), and a flow rate of 1 ml/min. Shown are the UV traces at 205, 235, and 270 nm monitored by an Agilent diode array detector. The products are designated 1–7 based on the order of elution.
Synthesis of HETEs and diHETE with trans,trans-Conjugated Dienes
Thiyl radical-induced isomerization (mercaptoethanol, UV light, room temperature (13, 14)) was used to convert cis-trans- to trans,trans-conjugated dienes. Reaction conditions had the starting material (2.5 mg, methyl ester of HETE or diHETE) in 0.5 ml of isopropyl alcohol containing 0.5 μl of mercaptoethanol in a quartz UV cuvette with Teflon cap. The cuvette was submerged in a solution of aqueous 1 m NaCl that served both to stabilize the temperature at <25 °C and absorb UV radiation below 220 nm. A low pressure immersion UV lamp rated with 3.5-watt output at 254 nm (part no. 12128-15, Ace Glass Inc. (Vineland, NJ)) (caution: harmful UV radiation) was placed about 15 cm above the cuvette. Aliquots of the reaction were analyzed at 30-min intervals by argentation HPLC using a cation exchange column (Sepralyte SCX 5-μm, 25 × 0.46 cm, originally purchased from Analytichem International (later owned by Varian)) in the silver form (15, 16) with a solvent of hexane/isopropyl alcohol/acetic acid in the proportions 70:30:0.1 (by volume) for mono-HETE methyl esters and 5,15-diHETE methyl ester, a flow rate of 1 ml/min, and with UV monitoring using an Agilent 1100 series diode array detector. 5-HETE and 15-HETE were isomerized in the conjugated diene, leading to accumulation of the trans,trans-HETEs (with two trans and two cis double bonds) with very minor peaks for the more highly isomerized derivatives. 5,15-diHETE was isomerized in one conjugated diene and, after 2–3 h of irradiation in both, giving the all-trans derivative as the major product.
Derivatization and GC-MS Analysis
Catalytic hydrogenations were performed in 100 μl of ethanol using about 1 mg of palladium on alumina and bubbling with hydrogen for 2 min at room temperature. The hydrogenated products were recovered by the addition of water and extraction with ethyl acetate. TMS ester TMS ether derivatives were prepared by treatment with bis(trimethylsilyl)-trifluoracetamide (10 μl) and pyridine (2 μl) at room temperature for 2 h. Subsequently, the reagents were evaporated under a stream of nitrogen, and the samples were dissolved in hexane for GC-MS.
Analysis of the methyl ester trimethylsilyl ether derivatives of the products was carried out by GC-MS in the positive ion electron impact mode (70 eV), using a Thermo Finnigan Trace DSQ ion trap instrument with the Xcalibur data system. Samples were injected at 150 °C, and after 1 min, the temperature was programmed to 300 °C at 20 °C/min. Spectra collected during elution of the GC peak (typically about 20 spectra) were averaged for calculation of the isotopic compositions.
ESI-LC-MS
ESI-LC-MS of purified product 4 and product 5 was performed using a Thermo Finnigan LC Quantum instrument. A Waters Symmetry C18 column (0.2 × 15 cm) was eluted with acetonitrile/water/ammonium acetate (50 mm, 50 mm, 10 mm) at 0.2 ml/min. The heated capillary ion lens was operated at 220 °C. Nitrogen was used as a nebulization and desolvation gas. The electrospray potential was held at 4 kV. Source-induced dissociation was set at −10 eV. Mass spectra were acquired over the mass range m/z 100–500 at 2 s/scan under the negative ion mode.
NMR
1H and 1H,1H COSY NMR spectra were recorded on a Bruker DXR 600-MHz spectrometer at 298 K. The ppm values are reported relative to residual non-deuterated solvent (δ = 7.16 ppm for C6H6).
RESULTS
An Anaerobic Reaction of Soybean LOX-1 with 15S-HPETE
To test the hypothesis that soybean LOX-1 is capable of catalyzing LTA synthesis in the absence of oxygen, initially we incubated the enzyme (0.38 μm) with various concentrations of 15S-HPETE (20–440 μm) at pH 7.5 under N2 for 2 h. Although analysis of reaction rates is complicated by the mixture of products formed (see below), subsequent analyses indicated that, for example, 100 μm 15S-HPETE is fully consumed within 10 min. Observed rates are presented in the supplemental material. Reactions were stopped by the addition of an equal volume of methanol, followed by extraction using an Oasis HLB cartridge. RP-HPLC analysis revealed that the substrate 15S-HPETE, at all concentrations tested, was completely converted to seven new compounds, designated 1–7 based on the order of elution (Fig. 1). We also examined the reaction carefully for the production of fatty acid dimers because their formation would be predicted if this reaction follows the same mechanism as the well studied anaerobic reaction of soybean LOX-1 with linoleic acid and 13S-HPODE (17–19). However, SP-HPLC and RP-HPLC analyses showed that this reaction gave no appreciable amounts of C–C linked dimers and confirmed that products 1–7 constitute the main products.
Contribution of the Fe(II) Enzyme to the Anaerobic Reaction with 15S-HPETE
Because soybean LOX-1 exists in two oxidation states, Fe(II) and Fe(III), we next questioned which enzyme form is responsible for the formation of the products. NDGA, as a redox-based LOX inhibitor, inhibits the oxygenase activity of LOX by reduction of Fe(III) LOX to Fe(II) LOX (20); therefore, in the presence of NDGA, soybean LOX-1 would be kept in the Fe(II) state, and only the products from the Fe(II) enzyme would be observed.
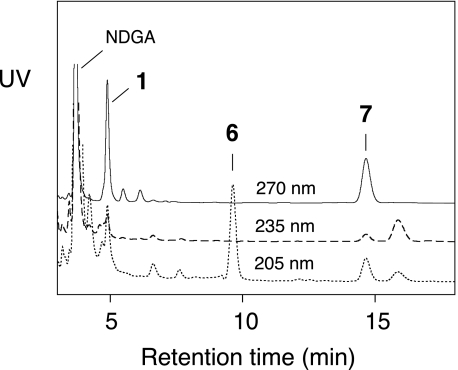
The anaerobic reaction of soybean LOX-1 (0.38 μm) with 15S-HPETE (40 μm) was performed in the presence of NDGA (80 μm) and subsequently analyzed by RP-HPLC. As shown in Fig. 2, only products 1, 6, and 7 were formed in the presence of NDGA, indicating that these are the products from the Fe(II) enzyme. In addition, we noted that NDGA significantly accelerated the rate of reaction, especially at substrate concentrations lower than 100 μm (e.g. see supplemental Fig. S1).
FIGURE 2.
RP-HPLC analysis of products from the anaerobic reaction of soybean LOX-1 (0.38 μm) with 15S-HPETE (40 μm) in the presence of NDGA (80 μm). As in Fig. 1, the products were eluted using a Waters Symmetry C18 column (0.46 × 25 cm), a solvent system of methanol/water/acetic acid (80:20:0.01 by volume), and a flow rate of 1 ml/min. Shown are the UV traces at 205, 235, and 270 nm monitored by an Agilent diode array detector.
Under anaerobic conditions, linoleic acid also reduces Fe(III) soybean LOX-1 to Fe(II) soybean LOX-1, with itself being converted to a carbon-centered radical and then released from the enzyme active site (21). Accordingly, linoleic acid should have the same effect as NDGA does on the anaerobic reaction of soybean LOX-1 with 15S-HPETE. Indeed, in the presence of linoleic acid (150 μm), 15S-HPETE was converted to essentially the same set of products as in the presence of NDGA (data not shown).
Identification of the Fe(II) Enzyme Products 1, 6, and 7
As detailed in the supplemental material, products 1, 6, and 7 were identified by UV, LC-MS, and by NMR. Product 1 is the C15 aldehyde 15-oxopentadeca-5Z,8Z,11Z,13E-tetraenoic acid, product 6 is 13R-hydroxy-14S,15S-epoxyeicosa-5Z,8Z,11Z-trienoic acid, and product 7 is 15-oxoeicosa-5Z,8Z,11Z,13E-tetraenoic acid (15-KETE) (Scheme 1). Formation of the C15 aldehyde (product 1) and 15-KETE (product 7) is expected because they are structural analogues of the major C13 aldehyde and 13-KETE formed in the anaerobic reaction of the Fe(II) enzyme with 13S-HPODE (18). Product 7 is a direct analogue of threo-11-hydroxy-12,13S-trans-epoxyoctadec-9Z-enoic acid, another known compound derived from 13S-HPODE by soybean LOX-1 (22), and this threo-13-hydroxy-14,15-trans-epoxyalcohol was originally identified by Corey and Mehrotra in 1983 (23).
SCHEME 1.
Structures of products 1, 6, and 7.
Contribution of the Fe(III) Enzyme to the Anaerobic Reaction with 15S-HPETE
To examine the products from the Fe(III) enzyme, we took advantage of the fact that 13S-HPODE reacts with Fe(II) soybean LOX-1 stoichiometrically to give Fe(III) soybean LOX-1 (24). Thus, soybean LOX-1 (0.2 μm) was reacted with [1-14C]15S-HPETE (40 μm) in the presence of 13S-HPODE (80 μm). SP-HPLC with both UV and radioactivity detection revealed that 2, 3, 4, and 5 were the main products from the Fe(III) enzyme reaction (Fig. 3), the complementary set of products compared with those formed from reactions of the Fe(II) enzyme in the presence of NDGA or linoleic acid.
FIGURE 3.
SP-HPLC analysis of products from the anaerobic reaction of soybean LOX-1 (0.38 μm) with [1-14C]15S-HPETE (40 μm) in the presence of 13S-HPODE (80 μm). A, the 14C radiochromatogram recorded by a radioactive detector. B, the UV chromatogram at 235 and 270 nm recorded by a diode array detector. The products were eluted using a Beckman Ultrasphere® silica column (0.46 × 25 cm), a solvent system of hexane/isopropyl alcohol/acetic acid (100:5:0.1 by volume), and a flow rate of 1 ml/min.
Identification of the Fe(III) Enzyme Products 2 and 3
Products 2 and 3 were identified as 8R,15S-dihydroxyeicosa-5Z,9E, 11E,13E-tetraenoic acid (8R,15S-Z,E,E,E-diHETE) and 8S,15S-dihydroxyeicosa-5Z,9E,11E,13E-tetraenoic acid (8S,15S-Z,E,E, E-diHETE) respectively, based on the same UV, mass spectra (supplemental Figs. S3 and S4), and chromatographic properties they exhibited as the authentic standards. These 8,15-diols with an all-trans-conjugated triene are characteristic hydrolysis products of the unstable allylic epoxide, 14,15-LTA4.
Identification of the Fe(III) Enzyme Products 4 and 5
Products 4 and 5 display unusual UV spectra similar to yet distinguishable from that of the well known double oxygenation product 5,15-dihydroxyeicosa-6E,8Z,11Z,13E-tetraenoic acid (5,15-diHETE). In SP-HPLC solvents, the differences are the more striking (cf. Fig. 4, A and B). LC-ESI-MS (negative ion mode) showed that product 4 has the same relative molecular mass, 336, as 5,15-diHETE (whereas under the same conditions, product 5 was not detected; see below). GC-MS analysis of the TMS ester TMS ether derivative of hydrogenated product 4 established the 5,15-dihydroxy structure (supplemental Fig. S5); the prominent ions at m/z 401 and 261 arise from α-cleavage at the 5-hydroxyl, and m/z 173 and 489 come from α-cleavage at the 15-hydroxyl. Thus, product 4 is a geometric isomer of 5,15-diHETE. Unlike the well known 5,15-diHETE containing two cis-trans-conjugated diene chromophores (6), product 4 contains four double bonds, all in the trans configuration as indicated by the coupling constants (J ≈ 15 Hz) obtained from 1H NMR (Fig. 5A and supplemental Table S2) and 1H,1H COSY NMR analysis (supplemental Fig. S6).
FIGURE 4.
Comparison of the UV spectra of products 4 and 5 with that of 5,15-(E,Z,Z,E)-diHETE. A, the spectra in RP-HPLC solvent, methanol/water/acetic acid (80:20:0.01 by volume). B, the spectra in SP-HPLC solvent, hexane/isopropyl alcohol/acetic acid (100:5:0.1 by volume).
FIGURE 5.
1H NMR analysis of products 4 (A) and 5 (B) in d6-benzene. Shown is an expanded view of the region corresponding to olefinic protons. The splitting pattern of each olefinic proton is also illustrated. The proton signals are assigned with aid of 1H,1H COSY NMR (supplemental Figs. S6 and S8).
To confirm the structure of product 4, we synthesized all-trans-5,15-diHETE by thiyl radical-induced isomerization of the cis double bonds in 5,15-diHETE (see “Experimental Procedures”). The UV, chromatographic, and NMR properties of the synthetic compound were identical to those of product 4. In addition, comparison of the olefin regions of the NMR spectra of synthetic 6E,8E-5-HETE and 11E,13E-15-HETE helped with assignment of the individual olefin protons in all-trans-diHETE, in particular confirming that the H-6 signal is more upfield of H-14 and thus allowing assignment of the rest of the double bond proton signals from the COSY analysis (supplemental Fig. S6).
Unlike product 4, product 5 had eluded detection by ESI-MS, suggesting the lack of ionizable groups in the structure (e.g. as esters, including lactones). This was confirmed by GC-MS analysis of the TMS ether derivative of hydrogenated product 5, which gave a spectrum identical to that published for the TMS ether derivative of hydrogenated δ-lactone of 5,15-diHETE (25) (supplemental Fig. S7). Diagnostic ions include m/z 99, 173, and 327. 1H NMR and 1H,1H COSY NMR analysis of product 5 determined the configurations of the four double bonds to be all-trans (Fig. 5B and supplemental Fig. S8 and Table S2). Thus, product 4 is 5,15-dihydroxyeicosa-6E,8E,11E,13E-tetraenoic acid (5,15-all-trans-diHETE), and product 5 is the corresponding δ-lactone.
Products 4 and 5 Are Each a Mixture of Diastereomers
Although each appeared as a single peak on RP-HPLC and SP-HPLC, each could be further resolved using a chiral HPLC column into a 50:50 mixture of two peaks with identical UV spectra (supplemental Fig. S9). (Their NMR spectra are also identical because there were no indications of a mixture of two sets of signals in the spectra of product 4 or 5). Based on the reasonable assumption that the original S configuration of the substrate 15S-HPETE is retained in the 15-hydroxyl, we conclude that the peaks resolved on the chiral column are diastereomers rather than enantiomers, epimeric at C-5. This was confirmed using synthetic all-trans-diHETE prepared from a 2:1 mixture of 5S,15S- and 5R,15S-diHETE diastereomers. Similar to product 4, this synthetic all-trans-diHETE chromatographed as a single peak on RP- and SP-HPLC. On the Chiralpak AD-RH column, it resolved into the larger, earlier eluting peak of 5S,15S followed by the later eluting 5R,15S diastereomer. Mechanistic considerations that follow from the H218O experiments further support the conclusion that products 4 and 5 are each a mixture of diastereomers.
The Anaerobic Reaction in H218O
To understand the origin of the hydroxyls in products 2, 3, 4, and 5, we conducted the anaerobic reaction of soybean LOX-1 with 15S-HPETE in 18O-enriched water (80% H218O), followed by HPLC purification of the individual products, catalytic hydrogenation, TMS ether TMS ester derivatization, and GC-MS analysis.
In the mass spectrum of the derivatized pair of 8,15-diols with all-trans-conjugated trienes (products 2 and 3) from the H218O reaction, the ions containing the 8-hydroxyl were all shifted by +2 mass units (i.e. ions at m/z 303 to 305, 359–361, and 489–491), whereas the m/z 173 fragment containing the 15-hydroxyl remained unchanged (supplemental Figs. S10 and S11). Quantitative analysis of the isotopic ratios revealed that the 8-hydroxyl derives exclusively from water, whereas the 15-hydroxyl derives from the 15-hydroperoxyl of the substrate.
In accord with the results on the 8,15-diols and in sharp contrast to the known mechanisms of formation of 5,15-diHETE via double oxygenation, GC-MS analysis of derivatized hydrogenated all-trans-5,15-diHETE (product 4), established that the 5-hydroxyl comes from water and the 15-hydroxyl comes from the 15-hydroperoxyl group in the substrate (Fig. 6A). Ions containing the 5-hydroxyl were shifted by +2 mass units (to m/z 263, 313, and 491), whereas m/z 173, the characteristic fragment ion of the 15-hydroxyl, remained unchanged.
FIGURE 6.
GC-MS (electron ionization) analysis of TMS-derivatized hydrogenated products 4 and 5 from the anaerobic reaction in H218O. A, the mass spectrum of the TMS ether TMS ester derivative of hydrogenated product 4. B, the mass spectrum of the TMS ether derivative of hydrogenated product 5.
By contrast, GC-MS analysis of the derivatized hydrogenated product 5, the δ-lactone of all-trans-5,15-diHETE, indicated no 18O incorporation in the molecule (Fig. 6B). (The mass spectrum is identical to that of product 5 from H216O incubations.) The lack of 18O in the δ-lactone ring clearly demonstrated that product 5 was not formed by lactonization of product 4. Instead, we surmise that the C-5 oxygen in the δ-lactone ring of product 5 must derive from the free carboxylate group in the substrate.
A Cyclopropyl Epoxide as the Enzymatic Product
The formation of products 4 and 5 can be explained if the true enzymatic product is an allylic 14,15-epoxide with a cyclopropane ring (Fig. 7; see “Discussion”). This epoxide is opened up in one of two ways. Either water reacts at the end of the conjugated system to yield product 4 (with corresponding incorporation of 18O from H218O at C-5), or nucleophilic attack by the free carboxylate yields the δ-lactone derivative with no involvement of water in the transformation.
FIGURE 7.
The proposed mechanism of formation of products 4 and 5.
DISCUSSION
Background of This Study
Soybean LOX-1 was the first LOX to be discovered and has since received the most extensive mechanistic studies among the LOX enzymes (26, 27). Typically, the hallmarks of LOX catalysis, such as the antarafacial relationship between hydrogen abstraction and oxygen insertion, were first demonstrated for soybean LOX-1 and then recapitulated in studies on other LOX enzymes. One notable exception, however, is the LTA synthase type activity. From a historical point of view, because no such chemistry had been described for soybean LOX-1, it was not immediately recognized at the time of the discovery of LTA4 that the same enzyme, 5-LOX, catalyzes the formation of 5S-HPETE and LTA4. The recognition came a number of years later, when it was demonstrated that both 5-LOX and LTA4 synthase activities involve stereoselective hydrogen abstraction as the initial step, always co-elute during purification, and are inhibited to similar extents by LOX inhibitors (28, 29).
LTA Synthase Activity and LOX Dual Specificity
Both the primary LOX activity and the secondary LTA synthase activity start with hydrogen abstraction, yet at two different positions. Therefore, it has been suggested that LTA synthase activity is a consequence of dual specificity (i.e. the ability of the enzyme to abstract hydrogens from two different positions and thus make two distinct hydroperoxides) (29). Consistent with this suggestion, leukocyte 5-LOX gives the 8S-hydroperoxide from 8,11,14-eicosatrienoic acid (compared with the 5S-hydroperoxide from arachidonic acid) (30, 31). Similarly, mammalian 15-LOX-1 usually gives a mixture of 15S-HPETE and 12S-HPETE from arachidonic acid (32). However, dual specificity alone does not ensure LTA synthase type activity; for instance, the dual specificity of soybean LOX-1 is evidenced by the formation of 8S,15S-diHPETE and 5S,15S-diHPETE in its double oxygenation reaction with 15S-HPETE (6), yet no LTA synthase type activity has ever been detected for this enzyme under the usual aerobic conditions.
LTA Synthase Activity and Active Site Oxygen
We hypothesize that oxygen concentration in the enzyme active site is an additional factor that determines whether LTA synthase type activity will be present in a LOX or not, through influence on the rate of the competing process, double oxygenation. When oxygen concentration is low, as we would propose in the 5-LOX active site, double oxygenation barely occurs due to deprivation of oxygen co-substrate, so LTA synthase activity will occur without interference. By contrast, at high active site oxygen concentrations, as in soybean LOX-1, double oxygenation occurs so fast that LTA synthase type activity will be fully suppressed. To relieve this potential suppression of LTA synthase type activity imposed by molecular oxygen, here we performed the reaction of soybean LOX-1 with 15S-HPETE under anaerobic conditions. Consistent with our hypothesis, we observed under these conditions the formation of 8(RS),15S-Z,E,E,E-diHETEs, the expected hydrolysis products from 14,15-LTA4 (or the cis-epoxide isomer; see below). This result strongly supports our view that the soybean LOX-1 has all of the essential elements to fulfill the role of a LTA synthase, yet this potential has been obscured by the presence of high concentrations of oxygen in the active site, which favors the double oxygenation reaction.
An oxygen channel in the LOX protein might normally target O2 to the correct position on the reacting substrate (33, 34), or alternatively there could be an appropriately positioned oxygen pocket in the active site (35). Either way, oxygen could be well positioned for one substrate (e.g. arachidonic acid in mammalian 5-LOX) and out of place for another (5S-HPETE in 5-LOX) while adequately positioned for either arachidonic acid or 15S-HPETE in soybean LOX. It is expected that the Km(O2) for soybean LOX-1 using 15S-HPETE as substrate would be considerably lower than the Km(O2) for mammalian 15-LOX using the same substrate or the Km(O2) for 5-LOX using 5S-HPETE. These values for fatty acid hydroperoxide substrates remain to be determined. However, evidence does exist that suggests a high Km(O2) for LOX that normally exhibit LTA synthase activity; e.g. a large Km(O2) can be deduced from the observation that potato 5-LOX shows double oxygenation activity with 5S-HPETE only under high oxygen pressures (8). It would be of interest to test whether a similar switch occurs with mammalian 5-LOX or 15-LOX under hyperbaric conditions.
The Oxidation State of the Enzyme Involved in LTA Synthesis
Next, we questioned which enzyme form, Fe(III) or Fe(II) soybean LOX-1, is responsible for the formation of 8(RS),15S-Z,E,E,E-diHETEs. Although it is widely accepted that the Fe(III) LOX accounts for LTA synthesis, an alternative Fe(II) LOX pathway, originally proposed for LT formation in fatty acid autoxidation (36) and in hemoglobin-catalyzed transformation of fatty acid hydroperoxides (37, 38), is also feasible on purely chemical grounds (supplemental Fig. S12). The key difference of the two pathways is the order of the two chemical steps, hydrogen abstraction and peroxide bond cleavage; whereas the Fe(III) LOX pathway involves hydrogen abstraction followed by peroxide bond cleavage, the Fe(II) LOX pathway employs the same two steps in the reverse order. To clarify this issue in the present case, we dissected the anaerobic reaction of soybean LOX-1 with 15S-HPETE into two components: one catalyzed by the Fe(II) enzyme and the other by the Fe(III) enzyme, using NDGA and 13S-HPODE to keep the enzyme ferrous and ferric, respectively. We found that 8(RS),15S-Z,E,E,E-diHETEs are formed in the presence of 13S-HPODE but not in the presence of NDGA, thus determining unambiguously that the Fe(III) enzyme is the catalytic species in the soybean LOX-1-catalyzed formation of 14,15-LTA4 and the corresponding 8,15-diol hydrolysis products. Our result agrees well with the early studies using stereospecifically tritium-labeled arachidonic acid that established hydrogen abstraction as the first irreversible step in LT synthesis (28) and also agrees with the studies using LOX inhibitors that demonstrated the inhibitory effect of NDGA on LT formation from HPETEs, although at these early times the mechanism of inhibition of NDGA (3, 29) (i.e. by reduction of Fe(III) LOX to Fe(II) LOX) had yet to be clarified (20).
The Proposed Cyclopropyl Epoxide Intermediate Leading to 5(RS),15S-All-trans-diHETEs and the δ-Lactone Derivatives
The most intriguing finding in the present study is probably the detection of the novel compounds, 5(RS),15S-all-trans-diHETEs and the δ-lactone derivatives. Inspired by the structural similarities of 5(RS),15S-all-trans-diHETEs to 8(RS),15S-Z,E,E,E-diHETEs, the shared involvement of the Fe(III) enzyme in the formation of both, and similar incorporations of H218O, we propose that the 5(RS),15S-all-trans-diHETEs are also hydrolysis products of an unstable intermediate, formed in a mechanism with parallels to the synthesis of 14,15-LTA4. A feature of our proposal is involvement of first the ferric enzyme and then the ferrous species in activation at the two pentadiene units, followed by intramolecular coupling of two radicals to give an unstable epoxide, which, upon hydrolysis, gives the 5,15-diols containing four trans double bonds. Notably, a directly analogous mechanism can explain LTA4 or 14,15-LTA4 synthesis.
As illustrated in Fig. 7, similar to 14,15-LTA4 formation, hydrogen abstraction by the Fe(III) enzyme is the initial step, but it occurs at C-7 rather than C-10 to form a carbon-centered radical delocalized over the C-5–C-9 pentadiene. This initial step produces the Fe(II) enzyme, which then induces homolytic cleavage of the O–O bond of the 15-hydroperoxide group to give an alkoxyl radical at the C-11–C-15 pentadiene. At this stage, the reacting intermediate exists as an oxygen-centered and carbon-centered biradical. Due to its intrinsic instability, this biradical would rearrange, by reacting the oxygen-centered radical with the nearby double bonds to form an epoxide group and a second carbon- centered radical, followed by combination of the two carbon-centered radicals to form a new C–C bond, to give a covalently complete compound, an allylic 14,15-epoxide with a cyclopropane ring composed of C-9, C-10, and C-11, namely 14,15-epoxy-[9,10,11-cyclopropyl]-eicosa-5Z,7E,13E-trienoic acid.
Similar to other allylic epoxides, at non-alkaline pH values, this cyclopropyl epoxide may react with even the weakest nucleophiles, such as water in aqueous solutions, to give 5(RS),15S-all trans-diHETEs or with the carboxylate group in the molecule itself to give the corresponding δ-lactones. Because water is involved in the former but not the latter process, only the 5(RS),15S-diols and not the δ-lactones incorporate water at C-5, exactly what we found using H218O. Finally, as a subtle point of the proposed mechanism, the cyclopropane ring is formed only in the covalently complete final product, as opposed to in any radical intermediates, because such radical intermediates containing a cyclopropane ring and an adjacent radical would be highly energetically unfavorable (39).
The stereochemistry of the epoxide group in the putative 14,15-LTA4 and the cyclopropyl epoxide intermediates cannot be determined based on the analysis of the hydrolysis products in the present study. Although perhaps not widely recognized, LOX-catalyzed formation of the cis-epoxy isomer of allylic epoxides has been documented, and the cis-epoxy isomer would hydrolyze to the same set of products as the trans-epoxide isomer, with only a subtle difference in the product ratios (8).
Conclusions
In summary, our study demonstrates the competition between dioxygenation, which requires the presence of appropriately positioned molecular oxygen in the LOX active site, and leukotriene biosynthesis, which proceeds anaerobically. For an enzyme such as soybean LOX-1 in which O2 is readily available for dioxygenation reactions, leukotriene biosynthesis is not observed until the enzyme is placed in an anaerobic environment. Conversely, we surmise that in LOX enzymes in which leukotriene synthesis occurs under aerobic conditions, molecular oxygen is less readily available in the active site. In the case of soybean LOX-1, the dioxygenase reactions on 15S-HPETE are initiated via hydrogen abstractions at C-7 and C-10, giving 5S,15S-diHETE and 8S,15S-diHETE respectively, and the LTA synthase type reactions are initiated in the same way under anaerobic conditions (Fig. 8). The hydrogen abstraction at C-7 leads to formation of the novel cyclopropyl-containing epoxide, which in turn gives the 5,15-diols with all-trans double bonds and the corresponding δ-lactones.
FIGURE 8.
Comparison of the aerobic and anaerobic reactions of soybean LOX-1 with 15S-HPETE. Aerobic reaction is initiated by the H-7 and H-10 hydrogen abstractions (pro-R hydrogens, designated as in arachidonic acid rather than 15S-HPETE) with 15S-HPETE in the reversed orientation, antarafacial oxygenation giving the 5S,15S-diHPETE and 8S,15S-diHPETE products, respectively (6, 44, 45). Anerobically, analogous hydrogen abstractions can account for formation of the cyclopropyl epoxide and 14,15-LTA4, respectively, followed by non-enzymatic hydrolysis to give the stable diol end products.
In addition to driving the reaction initiated by the Fe(III) enzyme down the LT synthase pathway, a lack of active site oxygen may play a role in other non-canonical reactions of lipoxygenases. An example from the present study is formation by the Fe(II) enzyme of the threo-13-hydroxy-14,15-trans-epoxyalcohol, the 15-ketone, and C15 aldehyde. These reactions strongly resemble those of two unusual lipoxygenases that appear to be structurally intact and yet catalyze no detectable formation of fatty acid hydroperoxides with any natural polyunsaturated fatty acid. One of these, a specific LOX enzyme in maize (ZmLOX6) acts as a hydroperoxide lyase (40), forming aldehydes of the type found with the anaerobic soybean LOX-1. The other is the epidermal LOX-3 of mammalian skin, known from genetic studies to be required for proper differentiation in the epidermis and which displays hydroperoxide isomerase activity (41, 42), forming epoxyalcohols and ketones, again similarly to the anaerobic soybean LOX-1. In both cases, a lack of available oxygen within the active site may contribute to both the lack of activity with natural fatty acids and the acquisition of new activities with fatty acid hydroperoxides. The concept of a limited access to oxygen within the lipoxygenase active site as a basis for new activities might also be extended to include the plant “type-II” LOX enzymes, such as soybean LOX-3, that catalyze mainly nonspecific peroxidation of fatty acids and their esters and further conversion of the hydroperoxide products to ketones (9, 43). In their case, it appears that hydrogen abstraction is followed by release of the fatty acid radical and that reaction with oxygen occurs outside the LOX active site. The enzyme, being left in the ferrous state, would in turn react with fatty acid hydroperoxides to give alkoxyl radicals that lead eventually to ketones. A lack of access to oxygen within the active site may prompt this outcome.
Finally, we note the implications for the mechanism of leukotriene A4 synthesis based on the proposed mechanism of formation of the epoxy-cyclopropyl fatty acid. The involvement of both the ferric and ferrous iron and the production of a fleeting biradical species in both types of reaction are strongly implied.
Supplementary Material
Acknowledgments
We thank Harold W. Gardner and Ned. A. Porter for valuable discussions and William E. Boeglin for chemical synthesis of all-trans-5,15-diHETE and trans,trans-HETEs.
This work was supported, in whole or in part, by National Institutes of Health Grant AR-051968 (to A. R. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S15, Tables S1–S3, and additional references.
- LOX
- lipoxygenase
- LT
- leukotriene
- HPODE
- hydroperoxyoctadecadienoic acid
- HPETE
- hydroperoxyeicosatetraenoic acid
- HETE
- hydroxyeicosatetraenoic acid
- diH(P)ETE
- dihydro(pero)xyeicosatetraenoic acid
- HPLC
- high pressure liquid chromatography
- RP
- reversed phase
- SP
- straight phase
- GC
- gas chromatography
- MS
- mass spectrometry
- LC
- liquid chromatography
- ESI
- electrospray ionization
- NDGA
- nordihydroguaiaretic acid
- TMS
- trimethylsilyl
- KETE
- ketoeicosatetraenoic acid.
REFERENCES
- 1.Rådmark O., Werz O., Steinhilber D., Samuelsson B. (2007) Trends Biochem. Sci. 32, 332–341 [DOI] [PubMed] [Google Scholar]
- 2.Funk C. D. (2001) Science 294, 1871–1875 [DOI] [PubMed] [Google Scholar]
- 3.Bryant R. W., Schewe T., Rapoport S. M., Bailey J. M. (1985) J. Biol. Chem. 260, 3548–3555 [PubMed] [Google Scholar]
- 4.Maas R. L., Brash A. R., Oates J. A. (1981) Proc. Natl. Acad. Sci. U.S.A. 78, 5523–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feltenmark S., Gautam N., Brunnström A., Griffiths W., Backman L., Edenius C., Lindbom L., Björkholm M., Claesson H. E. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 680–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Os C. P., Rijke-Schilder G. P., Van Halbeek H., Verhagen J., Vliegenthart J. F. (1981) Biochim. Biophys. Acta 663, 177–193 [DOI] [PubMed] [Google Scholar]
- 7.Yokoyama C., Shinjo F., Yoshimoto T., Yamamoto S., Oates J. A., Brash A. R. (1986) J. Biol. Chem. 261, 16714–16721 [PubMed] [Google Scholar]
- 8.Corey E. J., Wright S. W., Matsuda S. P. (1989) J. Am. Chem. Soc. 111, 1452–1455 [Google Scholar]
- 9.Axelrod B., Cheesbrough T. M., Laakso S. (1981) Methods Enzymol. 71, 441–451 [Google Scholar]
- 10.Brash A. R., Song W. (1996) Methods Enzymol. 272, 250–259 [DOI] [PubMed] [Google Scholar]
- 11.Corey E. J., Albright J. O., Barton A. E., Hashimoto S. I. (1980) J. Am. Chem. Soc. 102, 1435–1436 [Google Scholar]
- 12.Schneider C., Boeglin W. E., Brash A. R. (2000) Anal. Biochem. 287, 186–189 [DOI] [PubMed] [Google Scholar]
- 13.Ferreri C., Samadi A., Sassatelli F., Landi L., Chatgilialoglu C. (2004) J. Am. Chem. Soc. 126, 1063–1072 [DOI] [PubMed] [Google Scholar]
- 14.Moussebois C., Dale J. (1966) J. Chem. Soc. C 260–264 [Google Scholar]
- 15.Powell W. S. (1982) Methods Enzymol. 86, 530–543 [DOI] [PubMed] [Google Scholar]
- 16.Christie W. W., Breckenridge G. H. (1989) J. Chromatogr. A 469, 261–269 [Google Scholar]
- 17.de Groot J. J., Veldink G. A., Vliegenthart J. F., Boldingh J., Wever R., van Gelder B. F. (1975) Biochim. Biophys. Acta 377, 71–79 [DOI] [PubMed] [Google Scholar]
- 18.Garssen G. J., Vliegenthart J. F., Boldingh J. (1971) Biochem. J. 122, 327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garssen G. J., Vliegenthart J. F., Boldingh J. (1972) Biochem. J. 130, 435–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemal C., Louis-Flamberg P., Krupinski-Olsen R., Shorter A. L. (1987) Biochemistry 26, 7064–7072 [DOI] [PubMed] [Google Scholar]
- 21.de Groot J. J., Garssen G. J., Vliegenthart J. F., Boldingh J. (1973) Biochim. Biophys. Acta 326, 279–284 [DOI] [PubMed] [Google Scholar]
- 22.Garssen G. J., Veldink G. A., Vliegenthart J. F., Boldingh J. (1976) Eur. J. Biochem. 62, 33–36 [DOI] [PubMed] [Google Scholar]
- 23.Corey E. J., Mehrotra M. M. (1983) Tetrahedron Lett. 24, 4921–4922 [Google Scholar]
- 24.de Groot J. J., Garssen G. J., Veldink G. A., Vliegenthart J. F., Boldingh J. (1975) FEBS Lett. 56, 50–54 [DOI] [PubMed] [Google Scholar]
- 25.Maas R. L., Turk J., Oates J. A., Brash A. R. (1982) J. Biol. Chem. 257, 7056–7067 [PubMed] [Google Scholar]
- 26.Gardner H. W. (1991) Biochim. Biophys. Acta 1084, 221–239 [DOI] [PubMed] [Google Scholar]
- 27.Vliegenthart J. F. G., Veldink G. A. (1982) Free Radicals in Biology (Pryor W. ed) Vol. 5, pp. 29–64, Academic Press, New York [Google Scholar]
- 28.Maas R. L., Brash A. R. (1983) Proc. Natl. Acad. Sci. U.S.A. 80, 2884–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu T., Rådmark O., Samuelsson B. (1984) Proc. Natl. Acad. Sci. U.S.A. 81, 689–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borgeat P., Hamberg M., Samuelsson B. (1976) J. Biol. Chem. 251, 7816–7820 [PubMed] [Google Scholar]
- 31.Shimizu T., Izumi T., Seyama Y., Tadokoro K., Rådmark O., Samuelsson B. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 4175–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bryant R. W., Bailey J. M., Schewe T., Rapoport S. M. (1982) J. Biol. Chem. 257, 6050–6055 [PubMed] [Google Scholar]
- 33.Knapp M. J., Klinman J. P. (2003) Biochemistry 42, 11466–11475 [DOI] [PubMed] [Google Scholar]
- 34.Saam J., Ivanov I., Walther M., Holzhütter H. G., Kuhn H. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 13319–13324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider C., Pratt D. A., Porter N. A., Brash A. R. (2007) Chem. Biol. 14, 473–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamberg M. (1983) Biochim. Biophys. Acta 752, 353–356 [DOI] [PubMed] [Google Scholar]
- 37.Hamberg M. (1983) Biochim. Biophys. Acta 752, 191–197 [DOI] [PubMed] [Google Scholar]
- 38.Sok D. E., Chung T., Sih C. J. (1983) Biochem. Biophys. Res. Commun. 110, 273–279 [DOI] [PubMed] [Google Scholar]
- 39.Griller D., Ingold K. U. (1980) Acc. Chem. Res. 13, 317–323 [Google Scholar]
- 40.Gao X., Stumpe M., Feussner I., Kolomiets M. (2008) Planta 227, 491–503 [DOI] [PubMed] [Google Scholar]
- 41.Brash A. R., Yu Z., Boeglin W. E., Schneider C. (2007) FEBS J. 274, 3494–3502 [DOI] [PubMed] [Google Scholar]
- 42.Yu Z., Schneider C., Boeglin W. E., Marnett L. J., Brash A. R. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 9162–9167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukushige H., Wang C., Simpson T. D., Gardner H. W., Hildebrand D. F. (2005) J. Agric. Food. Chem. 53, 5691–5694 [DOI] [PubMed] [Google Scholar]
- 44.Brash A. R. (1999) J. Biol. Chem. 274, 23679–23682 [DOI] [PubMed] [Google Scholar]
- 45.Maas R. L., Ingram C. D., Porter A. T., Oates J. A., Taber D. F., Brash A. R. (1985) J. Biol. Chem. 260, 4217–4228 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.