Abstract
Results from clinical studies suggest that more than half of the 166 million dental restorations that were placed in the United States in 2005 were replacements for failed restorations. This emphasis on replacement therapy is expected to grow as dentists use composite as opposed to dental amalgam to restore moderate to large posterior lesions. Composite restorations have higher failure rates, more recurrent caries, and increased frequency of replacement as compared to amalgam. Penetration of bacterial enzymes, oral fluids, and bacteria into the crevices between the tooth and composite undermines the restoration and leads to recurrent decay and premature failure. Under in vivo conditions the bond formed at the adhesive/dentin interface can be the first defense against these noxious, damaging substances. The intent of this article is to review structural aspects of the clinical substrate that impact bond formation at the adhesive/dentin interface; to examine physico-chemical factors that affect the integrity and durability of the adhesive/dentin interfacial bond; and to explore how these factors act synergistically with mechanical forces to undermine the composite restoration. The article will examine the various avenues that have been pursued to address these problems and it will explore how alterations in material chemistry could address the detrimental impact of physico-chemical stresses on the bond formed at the adhesive/dentin interface.
Keywords: Adhesive/dentin interfacial bond, Structure, Physico-chemical stresses, Bonding durability, Adhesive development
This review article is focused on the adhesive/dentin interface and the role of this interface in the clinical performance of posterior composite restorations. The chemical composition of dentin adhesives has been reviewed recently,125 but there has been less attention devoted to a review of the structural and physicochemical factors that impact bond integrity and durability at the adhesive/dentin interface.
CLINICAL PERFORMANCE: COMPOSITE VS. DENTAL AMALGAM RESTORATIONS
In 2005, 166 million dental restorations were placed in the United States2 and clinical studies suggest that more than half were replacements for failed restorations. 74 Replacement of failed restorations accounts for nearly 70% of all restorative dentistry74 and the emphasis on replacement therapy is expected to increase as concern about mercury release from dental amalgam forces dentists to select alternative materials. Resin composite is the most commonly used alternative, 99 but moderate to large composite restorations have higher failure rates, more recurrent caries and increased frequency of replacement as compared to amalgam.3,11,63,72,74,99,102 For example, after 8 years the failure rate for posterior composite restorations was at least 50% greater than the high copper amalgam restorations.9 At 5 years, the need for additional treatment was 50% greater in children receiving composite restorations as compared to children treated with dental amalgam.11 Based on the review of dental records from 3,071 subjects, Simecek and colleagues reported in 2009 a significantly higher risk of replacement for posterior composite restorations as compared to amalgam.4 In a study of amalgam and composite restorations placed by 243 Norwegian dentists, the mean age of failed amalgam was ~11 years while the mean age for failed composite was statistically significantly lower at 6 years.72 Indeed, after nearly four decades of research the clinical lifetime of large to moderate posterior composite restorations continues to be approximately one-half that of dental amalgam. 127
The reduced clinical lifetime of moderate to large class II composite restorations can be very detrimental for our patients because removal of these restorations leads to loss of sound tooth structure. The removal of composite restorations produced significantly greater increases in cavity volume in comparison to the removal of amalgam.44 The increase in cavity volume and increased frequency of replacement means that significantly greater amounts of sound tooth structure will be lost with treatment and re-treatment of class II composite restorations.44 Over the lifetime of the patient, the additional loss of tooth structure will translate to the need for more complex restorations and eventually total tooth loss. The reduced longevity, increased frequency of replacement and the need for a more complex restoration means increased costs to the patient in terms of both time and money.124 (For clarification: class I restorations involve the biting surface only; class II restorations involve the biting surface plus one or both of the proximal surfaces.)
The primary factor in the premature failure of moderate to large composite restorations is secondary decay at the margins of the restorations.72 For example, in a study of radiographs from 459 adults, age 18–19 years, the investigators reported that among 650 interproximal restorations the failure rate as a result of secondary or recurrent decay was 43% for composite as compared to 8% for amalgam.63 In a separate study of amalgam and composite restorations placed in 8- to 12-year-old children, the primary reason for failure of both materials was secondary decay, but secondary decay was 3.5 times higher in composite restorations.3
In moderate to large class II composite restorations, secondary decay is most often localized gingivally (Fig. 1) and is linked to failure of the bond between the tooth and composite and increased levels of the cariogenic bacteria, Streptococcus mutans, at the perimeter of these materials.35,61,98 The composite is too viscous to bond directly to the tooth surface. To overcome this limitation, a low viscosity adhesive is used between the tooth and composite. Acid-etching provides effective mechanical bonding at the interface between enamel and adhesive, but bonding to dentin has been fraught with problems.
FIGURE 1.
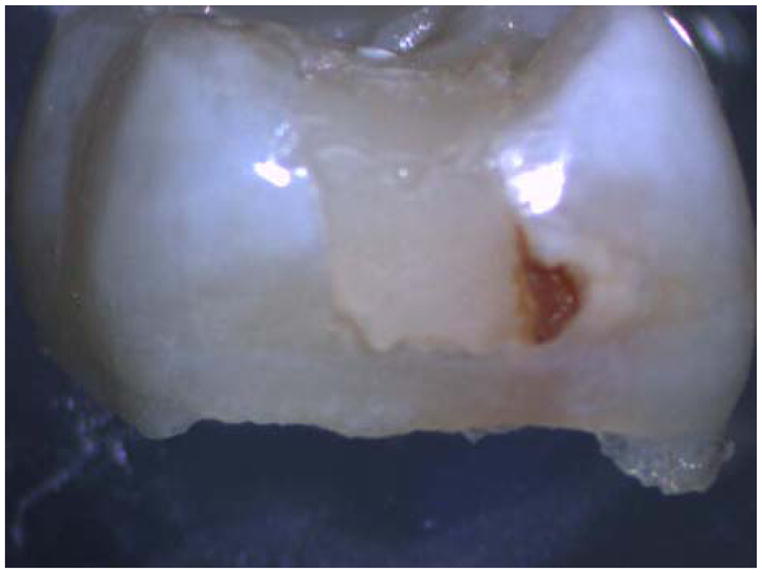
Exfoliated primary molar with Class II composite restoration. Note the stain indicating secondary decay at the gingival margin of the restoration.
Clinicians frequently find very little enamel available for bonding at the gingival margin of class II composite restorations and thus, the bond at the gingival margin depends on the integrity of the seal formed with dentin. Under clinical conditions one can frequently detect a separation between the composite material and the tooth surface at the gingival margin.94 These marginal gaps have been related to very technique sensitive and unreliable dentin bonding.55,94
A failed adhesive/dentin (a/d) bond means that there is a crevice that allows bacterial enzymes, oral fluids, and even bacteria to infiltrate the spaces between the tooth and composite. Penetration of these agents into crevices between the tooth and composite undermines the restoration and leads to recurrent decay, hypersensitivity and pulpal inflammation. 1,5,39,74,126 Clinical studies report poor marginal adaptation, marginal discoloration, and loss of retention of the composite restoration when the a/d interface is exposed to the oral cavity.6 Breakdown of the seal at the adhesive/dentin interface challenges the long-term viability of posterior composite restorations. 1,94,126 At the vulnerable gingival margin, the adhesive may be the primary barrier between the prepared tooth and the surrounding environment.
CHARACTERISTICS OF THE HEALTHY DENTIN BONDING SUBSTRATE
Dentin is the hydrated composite structure that constitutes the body of each tooth, providing both a protective covering for the pulp and serving as a support for the overlying enamel. It is composed of approximately 50% inorganic material, 30% organic material, and 20% water by volume.121 Dentin mineral is a carbonate rich, calcium deficient apatite.60,68 The organic component is predominantly type I collagen with minor contribution from other proteins that can be categorized as phosphoproteins, glycoproteins, and carboxyglutamate-containing proteins.8 The composition of dentinal fluid is reportedly similar to plasma.68
A unique feature of the dentin structure is the tubules that traverse the structure from the pulp cavity to the region just below the dentin–enamel junction (DEJ) or the dentin–cementum junction. The tubules, which could be modeled as narrow tunnels a few microns or less in diameter, represent the tracks taken by the odontoblastic cells from the pulp chamber to the respective junctions. Dentinal tubule diameter measures approximately 2.5 μm near the pulp and 0.9 μm near the DEJ.120 Tubule density and orientation vary from location-to-location; density is lowest at the DEJ and highest at the predentin surface at the junction to the pulp chamber. As an example, in young premolar and molar teeth the number of tubules ranges from 50,000 to 75,000 per square millimeter at the pulpal surface to approximately half as many per square millimeter in the proximity of the DEJ.121 The content of the tubules include fluid and odontoblast processes for all or part of their course. In contrast to root dentin, the tubules in coronal dentin are surrounded by a collar of highly mineralized peritubular dentin.129
The composition of the peritubular dentin is carbonate apatite with very small amounts of organic matrix whereas intertubular dentin, i.e., the dentin separating the tubules, is type I collagen matrix reinforced with apatite. Thus, the composition of intertubular dentin is primarily mineralized collagen fibrils; the fibrils are described as a composite of a collagen framework and thin plate-shaped carbonate apatite crystals whose c-axes are aligned with the collagen fibril axis.143 In healthy dentin, the majority of the mineralized collagen fibrils are perpendicular to the tubules.54
It is important to recognize that the composition of dentin is not static. It is influenced by the relative position of the dentin within the tooth, the age of the dentin, and the presence and/or absence of disease.68 Understanding the structure of dentin that is affected by age, disease, or trauma is fundamental to the development of improved dentin adhesives, but efforts in this area have been fraught with problems.108
DENTIN SMEAR LAYER
Another feature of the dentin surface that is a confounding variable in the development of a durable bond at the adhesive/dentin interface is the smear layer. Tooth surfaces that have been prepared with cutting or abrading instruments are characteristically covered with a 0.5–2 μm thick smear layer. The generation of frictional heat and plastic/elastic deformation during cutting and abrading are all factors in the formation of the smear layer. Smear layers formed on dentin cover the normal structural components, i.e., the intertubular dentin and penetrate several micrometers into the tubules to form smear plugs. Using scanning electron microscopy, Eick14 directly observed the tooth surface after cutting and reported that the dentin smear layer could be described as a disturbed film of organic and hydroxyapatite particles, generally less than 2 μm thick. Although it has been generally accepted that the composition of the dentin smear layer is a mixture of partly denatured collagen and mineral,14,17,84 definitive compositional, structural, and chemical reactivity data were not published until the last decade.109,130
Acids with a high reactivity rate with the mineral component of dentin have been considered a good reagent for removing the smear layer. These evaluations were based largely on scanning electron micrographs of acid-etched dentin, which portrayed a smooth dentin surface, fee of solid deposits, and open tubules. With few exceptions, these studies largely disregarded the collagen remnants of the smear or overlooked the inability of acid to act as a solvent for collagen.84,85 This oversight may be attributed in part to the microscopic nature of the smear layer and lack of resolution of many of the techniques used to study dentin demineralization.84 The conclusions that acid removed the smear layer are based primarily on morphologic evidence of open dentin tubules, but these observations largely reflect the effect of acid-etching on smear plugs overlying the tubules.
Raman microspectroscopic characterization of the smear layer covering the intertubular dentin provides clear evidence that the collagen of the smear layer is not removed by acid-etching.109,130 The spectral results indicate that the disorganized collagen within the smear layer is denatured by a 15 s exposure to 35% phosphoric acid. Residual mineral is trapped in the denatured, gelatinized collagen. Ultimately, this gelatinous layer could inhibit the formation of an impervious seal at the adhesive/dentin interface; it could be one factor contributing to the weak link in the coupling of adhesive to dentin.
‘IDEAL’ HYBRID LAYER
The two fundamental processes involved in bonding an adhesive to dentin are: removal of the mineral phase from the dentin substrate without altering the collagen matrix and filling the voids left by the mineral with adhesive resin that undergoes complete in situ polymerization, i.e., the formation of a resin-reinforced or hybrid layer. The ideal hybrid layer would be characterized as a 3-dimensional polymer/collagen network that provides both a continuous and stable link between the bulk adhesive and dentin substrate. There is substantial evidence to suggest that this ideal objective is not achieved.7,38,41,97,104,106,110,131,134,136,137,140 Instead of serving as a stable connection between the bulk adhesive and subjacent intact dentin, the hybrid layer has been called the weakest link in the a/d bond.97,101
DENTIN BONDING AND THE HYBRID LAYER
Based on numerous morphologic investigations and bond strength studies,15,31,40,90,112 it is generally accepted that the primary factors critical in determining an adequate a/d bond are: wetting of the dentin substrate by components of the adhesive system15,18 and micromechanical interlocking via resin penetration and entanglement of exposed collagen fibrils in the demineralized dentin.37,104,134 Morphologic evidence of resin penetration of the exposed collagen fibrils was first reported by Nakabayashi75 and he called the distinct zone between the bulk adhesive and the non-demineralized dentin the ‘hybrid layer.’ Current adhesive systems that acid etch the dentin characteristically bond via hybridization.
The hybrid layer is formed when an adhesive resin penetrates a demineralized or acid-etched dentin surface and infiltrates the exposed collagen fibrils. During acid etching, the mineral phase is extracted from a zone that measures between 1 and ~10 μm of the dentin surface.16,132,138 The composition of the exposed substrate differs radically from mineralized dentin. For example, mineralized dentin is 50% mineral, 30% collagen, and 20% water by volume,69 whereas demineralized dentin is 30% collagen and 70% water.15,85 With removal of the mineral phase, the collagen fibers are suspended in water. If there is a substantial zone of demineralization and the water supporting the collagen network is removed either by air drying or the action of an air syringe the collagen will collapse.30,85 A collapsed collagen network reduces the porosity and inhibits resin penetration through the demineralized layer.85 It forms a barrier between the demineralized layer and the underlying intact or unreacted dentin surface.112,144 A collapsed collagen network severely compromises the a/d bond.15,30,112
WET BONDING
In the early 1990s, wet bonding was introduced to counteract the problems of collagen collapse. 27,29,32,38,48 Wet bonding means that the dentin is kept fully hydrated throughout the bonding procedure; the surface morphology of the demineralized layer does not change because the water supporting the collagen matrix is not removed.53 Bond strength results27,29,32,38,48 with “wet” bonding support these findings, that is, the higher bond strengths with this technique reflect the minimal collapse of “wet” vs. air-dried dentin collagen.85 It is speculated that moist dentin provides a more porous collagen network and that increased porosity means more space for adhesive infiltration.27–29,32,85,112
With wet bonding techniques, the channels between the demineralized dentin collagen fibrils are filled with water, solvent, conditioner, and/or oral fluids.85 The only mechanism available for adhesive resin infiltration is diffusion of the resin into whatever fluid is in the spaces of the substrate and along the collagen fibrils. Ideally, the solvent in combination with hydrophilic monomers, (e.g., hydroxyethyl methacrylate (HEMA)) conditions the collagen to remain expanded during adhesive infiltration. However, HEMA, a primary component in many single bottle commercial dentin adhesives, can dramatically reduce the evaporation of water.87 Hydrophobic monomers, such as 2,2-bis[4(2-hydroxy-3-methacryloyloxy-propyloxy)-phenyl] propane (BisGMA), would resist diffusing into these sites where there is residual water.26,105,106,110,134 Under in vivo conditions, there is little control over the amount of water left on the tooth. As a result, it is possible to leave the dentin surface so wet that the adhesive undergoes physical separation into hydrophobic and hydrophilic-rich phases.105
Results from our laboratory indicated that excess moisture prohibited the formation of an impervious, structurally integrated a/d bond at the gingival margin of Class II composite restorations.106,135 Clinicians must routinely attempt to bond to naturally wet substrates such as caries-affected dentin46 or deep dentin. 67,91,95,133 The water content of caries-affected dentin has been reported to be 2.7 times greater than that of normal dentin.46 In deep dentin, 22% of the surface area is exposed tubules while exposed tubules account for 1% of the surface area of dentin close to the DEJ.83 The large increase in surface area attributable to tubules means that in deep dentin, pulpal fluid will contribute additional moisture to that already present within the demineralized dentin matrix. Since our current adhesives are very sensitive to excess moisture, bonding to these clinically relevant substrates is a formidable challenge.108,135,139,141
EXTRINSIC AND INTRINSIC WATER ABSORPTION
Absorption of extrinsic water leads to plasticization of the adhesive and loss of interfacial a/d bond strength as a result of water attack. One example of the effect of water absorption on chemically cured poly-HEMA specimens is the dramatic decrease in physical properties after 24 h aqueous storage; the tensile properties were reduced to an almost gum-like quality.88 The mean values for tensile strength of dry and wet poly-HEMA specimens were ~18 and 1 MPa, respectively. This reduction was attributed to water sorption after polymerization and/or extraction of water-soluble unreacted monomers or oligomers. As a result of water uptake into the poly-HEMA specimens, the percent elongation increased from ~20% to 220%. The authors suggested that since there is no cross-linking in the poly-HEMA, the water allowed the linear chains to slide over one another thus, resulting in a 10-fold increase in percent elongation. Intrinsic water at concentrations >5 vol.% inhibited the light polymerization of HEMA, even with a 10-fold increase in the initiators camphoroquinone (CQ)/dimethylaminoethyl methacrylate (DMAEMA).
A study from our laboratory showed that at water concentrations ≥25 vol.%, BisGMA-based adhesive/water solutions mimicked oil and water mixtures in that they separated into distinct phases immediately following sonication.105 At 25 vol.% water the adhesive separates into particles made up primarily of BisGMA, the composition of the surrounding matrix material is primarily HEMA that exhibited limited monomer/polymer conversion. The limited conversion of the HEMA-rich phase suggests that either the photoinitator is localized to the hydrophobic phase or it is incompatible with the hydrophilic HEMA.142,145,148 The results from this investigation highlight the need for characterization of reactant mixtures prepared with and without water when these mixtures are proposed for applications in the wet, oral environment.
Related studies from our laboratory have shown spectral evidence of phase separation in a commercial total-etch BisGMA/HEMA adhesive bonded to wet, demineralized dentin matrices.103,134,138 Ethanol is the solvent in this commercial adhesive. The primary function of the solvent is to displace the water from the wet, demineralized dentin matrix, but the spectroscopic results indicate that there is enough water present to promote detrimental adhesive phase separation. In this study, the majority of the intertubular a/d interface was characterized by collagen fibrils from the demineralized dentin matrix with limited spectral contribution from the critical dimethacrylate component (BisGMA). Thus, the demineralized dentin matrix is primarily infiltrated by HEMA. HEMA has a low crosslink density and thus, it will tend to absorb extraneous water, leading to plasticization and breakdown of the adhesive. In this study, the HEMA exhibited limited monomer/polymer conversion and it is expected that the unreacted components would be released in the mouth.20
The sensitivity of our current adhesives to excess moisture is also reflected in the water-blisters that form in adhesives placed on over-wet surfaces.113–115 The optimum amount of wetness varies as a function of the adhesive system.119 Additionally, it is impossible to simultaneously achieve uniform wetness on all of the walls of the cavity preparation.118 Wet bonding is, in short, a very technique-sensitive procedure and optimum bonding with our current commercial adhesives occurs over a very narrow range of conditions, e.g., water content.95
One suggested approach to these problems is “ethanol-wet bonding.”6,77 One problem with this method is that in the clinical setting this solvent may be diluted because of repeated exposure of the material to the atmosphere or concentrated because of separation of the bonding liquids into layers within the bottle. Results from our lab have shown an inverse relationship between mechanical and thermal properties and the concentration of ethanol that is present during photo-polymerization of model BisGMA-based adhesives.148 In addition, the hybridization process is very sensitive to the ethanol content in the adhesive system.141 Although the effect of “ethanol-wet bonding” on durability is not known, results from our lab suggest that this approach will not overcome the clinical challenges associated with forming a durable bond at the adhesive/dentin interface.
SELF-ETCH ADHESIVES
Current strategies to promote bonding of the resinous materials to intrinsically wet substrates also include the incorporation of ionic and hydrophilic monomers into the adhesive.42 These adhesives etch and prime simultaneously, thus addressing the problems of collagen collapse and simplifying the bonding protocol. The original systems were two-step, self-etching systems, but in an effort to increase the efficiency of the procedure and reduce technique sensitivity, the manufacturers developed all-in-one single-step adhesives. The increased concentration of acidic resin monomers provided a system that etched the dentin and enamel simultaneously.
Prompt L-Pop (3 M ESPE) was a system that represented this new generation of dentin adhesives.89 Acidic monomers in Prompt L-Popo consisted of methacrylated phosphoric acid mono- and diesters117 in which the phosphoric acid and methacrylate group were combined into one molecule. Initial results showed a system that effectively etched the dentin surface to create a uniform hybrid layer and long resin tags.131 However, these self-etching adhesive/dentin interface specimens quickly lost structural integrity during aqueous aging. Results from micro-Raman spectroscopic investigations suggested that water within the dentin tubules inhibited the polymerization of the acidic monomers. The poorly polymerized oligomers and unpolymerized acidic monomers continued to etch the surrounding dentin.131
The hydrophilic nature of components within the self-etch adhesive systems enhances water sorption and hydrolytic breakdown in the mouth.24,42,78,118,150 With these systems, the bonded interface lacks a nonsolvated hydrophobic resin coating and thus, the resultant hybrid layers behave as semi-permeable membranes permitting water movement throughout the bonded interface even after adhesive polymerization.6 The higher concentration of hydrophilic monomers in these systems is associated with decreased structural integrity at the a/d interface.6,92 In vivo aging studies have reported degradation of the a/d bond at 1-year even when the bonded dentin was protected by enamel from direct exposure to the oral environment.12 These results suggest that hydrophilicity and hydrolytic stability of resin monomers are generally antagonistic.118
DENTIN BONDING AT THE GINGIVAL MARGIN
In vitro studies have indicated that the gingival margin in class II composite restorations is the most common location of bonding failures.20 Purk and colleagues93 compared the microtensile dentin bond strength of gingival and proximal cavity walls of class II restorations. Their results showed that the dentin adhesive bond of resin-based composite to gingival walls was significantly weaker, and thus, at increased risk of failure compared to the bond to proximal walls.
Distinct differences in the depth of dentin demineralization and degree of adhesive penetration at the proximal and gingival margins of class II composite restorations have been reported.135 The spectroscopic results indicated a twofold difference in the depth of dentin demineralization at the gingival and proximal margins. The differences in demineralization depth may be due to the fact that dentin at the gingival margin is less mineralized than dentin at the proximal walls. For example, the mineral/matrix ratio in dentin at the proximal margin was more than twice the ratio at the gingival margin. Thus, acids are expected to etch dentin at the gingival margin faster and deeper than dentin at the proximal wall. In addition, the density and size of tubules at the gingival margin is greater than that at the proximal wall25; hence, acid etching is faster in dentin at the gingival margin.
There was also a distinct difference in adhesive infiltration with considerably less adhesive penetration at the gingival margin as compared to the proximal wall.135 It was suggested previously that resin could infiltrate dentin at the gingival margin more efficiently because of the increased number of tubules per unit area at the gingival margin.152 The authors suggested that the increased number of tubules/area would allow lateral diffusion of resin as well as vertical diffusion into the demineralized intertubular dentin. However, the water content is higher in dentin at the gingival margin, making it a difficult bonding substrate. This is not only because of the water already present within the demineralized dentin matrix, but also because patent tubules contribute to the contamination of the prepared surface with a great amount of dentinal fluid.82 The cumulative effect of the increased water led to reduced adhesive infiltration and lower monomer/polymer conversion of the adhesive at the gingival margin as compared to the proximal wall.135 Spectral results from the gingival margin of these Class II composite restorations indicated hydrolytic degradation of the adhesive and deterioration of the unprotected collagen after 3-months aqueous storage.106
BONDING TO ALTERED DENTIN
Much of our understanding of dentin bonding has been based on results from in vitro bond strength studies performed on sound, flat polished normal, healthy dentin. Although the results are of great value when comparing commercial bonding systems, sound healthy dentin is not the substrate most frequently encountered in clinical situations. Instead, clinicians usually must attempt to bond adhesives to caries-affected (c-a) dentin or abraded-sclerotic dentin.
Results from both morphologic and bond strength studies suggest that the characteristics of the substrate directly impact the bond formed at the adhesive/dentin interface.34,36,76 Using the same material on both healthy and affected (caries-affected or sclerotic) dentin previous authors have reported nearly a 30–40% drop in bond strength with both affected dentin substrates. 153,154 The hybrid layers formed on c-a dentin were thicker than those formed on healthy dentin. Presumably, the thicker hybrid layer may be due to the fact that c-a dentin is already partially demineralized and offers a more porous substrate for acid etching than healthy dentin.
FTIR microspectroscopy has been used to determine the relative composition, degree of cure, and inhomogeneities across the length and breadth of the adhesive/c-a dentin interface.108 Differences in mineral/matrix ratio, crystallinity, and collagen organization were noted in the comparison of healthy and c-a dentin. For example, features associated with the organic component showed a dramatic loss of structure and intensity in the spectra recorded from the c-a dentin. These results indicate disorganized and denatured collagen. There was also a dramatic reduction in monomer/polymer conversion when the adhesive was used on c-a dentin.108,141
In a separate but complementary study, morphologic investigation of adhesive bonding to c-a dentin from primary molars was noted to be sporadic and very irregular (Figs. 2 and 3).4 It is postulated that the lack of adhesive penetration in the caries-affected dentin is due to a phase transition in the collagen that has been disordered by caries.108 For example, the initial step in application of the adhesive used in this investigation is acid-etching. Acid-etching increases the porosity of the dentin by removing the mineral component to expose the collagen fibril network. Since caries-affected dentin may be characterized by zones of disorganized collagen, acid-etching could promote phase transition of this disorganized collagen to a gel. The gel could inhibit adhesive infiltration.
FIGURE 2.
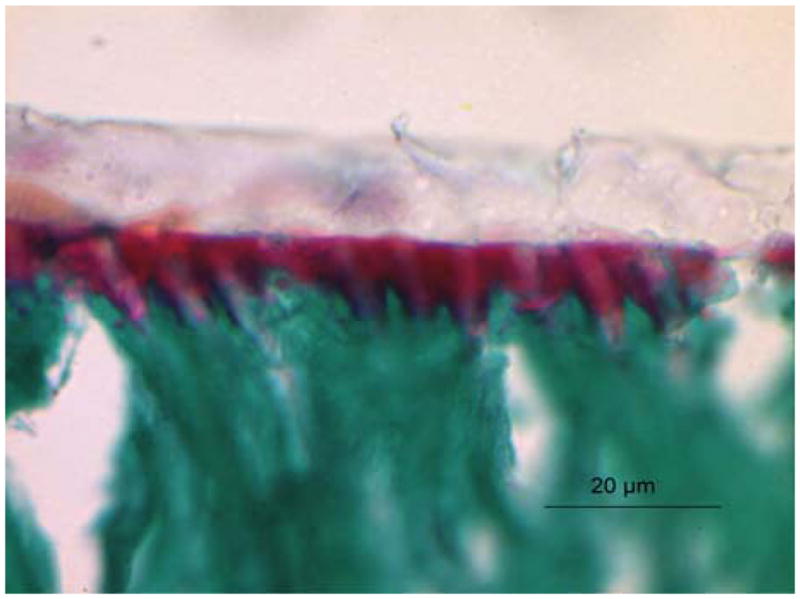
Light micrograph of adhesive/caries-free dentin interface stained with Goldner’s trichrome. As noted here and reported previously104,107,134 collagen that is not encased in adhesive is stained a distinct red, mineralized dentin stains green and the adhesive is a pale beige or clear. The distinct red zone at the adhesive/dentin interface indicated that the adhesive did not penetrate the full depth of the demineralized layer, i.e., it did not encapsulate the collagen fibrils throughout the width of the demineralized dentin.
FIGURE 3.
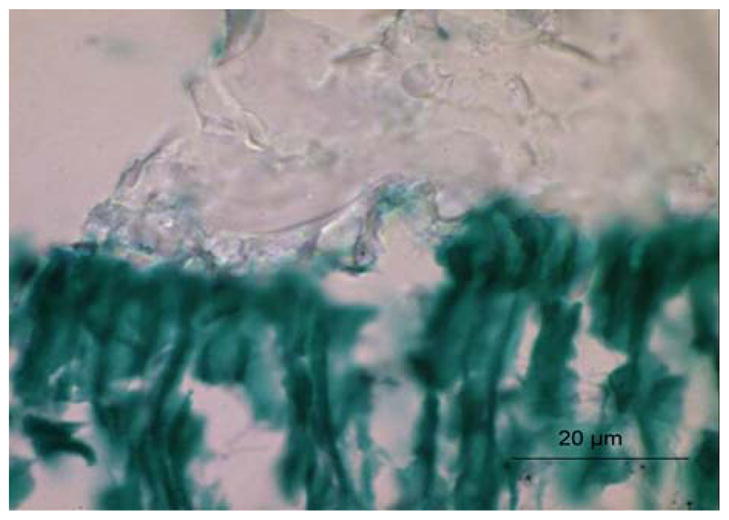
Light micrograph of adhesive/caries-affected dentin interface stained with Goldner’s trichrome. The bond between the adhesive and caries-affected dentin is very poor as evidenced by the irregular and highly porous pattern. The same commercial adhesive was utilized in both Figs. 2 and 3. All of the specimens shown in Figs. 1–3 were collected under an IRB approved protocol.
Adhesive infiltration of the wet, demineralized caries-affected dentin could also be impacted negatively by adhesive phase separation.107,131,134 As a result of phase separation, the majority of the intertubular a/d interface would be characterized by collagen fibrils with limited infiltration of the critical dimethacrylate component (BisGMA). The dimethacrylate BisGMA is the formulation component which contributes most to the crosslinked polymeric adhesive; the monomethacrylate (HEMA) cannot crosslink with itself and can only become part of a crosslinked system by copolymerizing with the BisGMA. Adhesive phase separation in combination with a (collagen) polymer-to-gel transition in the caries-affected dentin would translate to limited adhesive infiltration and poor bonding at the interface with this clinically relevant substrate.
These results, which describe distinct differences between bonding characteristics to healthy and caries-affected dentin, highlight the need for investigations on clinically relevant substrates as part of our efforts to develop adhesives that provide durable function under clinical conditions.66 Our understanding of adhesive bonding to clinically relevant substrates such as caries-affected and sclerotic dentin is very limited. The factors and mechanisms involved in the very low bond strengths to caries-affected dentin remain unclear.
DEGRADATION OF THE HYBRID LAYER
It has been hypothesized that the in vivo degradation of the hybrid layer follows a cascade of events that begins when the dentin is acid-etched.96 Disruption of the tooth structure by drilling stimulates proteolytic enzymes such as matrix metalloproteinases (MMPs), which can degrade the exposed collagen component of the hybrid layer.86 This type of degradation is expected to be most important acutely in the period following adhesive application. The process proceeds with hydrolysis and consequent extraction of the adhesive resins that have infiltrated the demineralized dentin matrix.106,134 The extraction is facilitated by the ingress of water into the loosely cross-linked or hydrophilic domains of the adhesive. The hydrophilic domain exhibits limited monomer/polymer conversion because of adhesive phase separation105 and lack of compatibility between the photoinitiator and hydrophilic phase.142 The poorly polymerized hydrophilic phase degrades rapidly in the aqueous environment. Resin elution continues to occur through the nanoleakage channels146,147,149; water movement along the length and breadth of the hybrid layer becomes more rapid as transport pathways form relatively large water-filled channels.6,116,119 The previously resin-infiltrated collagen matrix is exposed and vulnerable to attack by proteolytic enzymes.10,86
DEGRADATION OF METHACRYLATE ADHESIVES
The structure of methacrylate adhesives suggests a general mechanism for their chemical and enzymatic degradation in oral fluids. On prolonged exposure of the restoration to oral fluids, water begins to penetrate the resin. As described above, water initially enters the matrix by diffusion into loosely cross-linked or hydrophilic domains or may be trapped within the matrix during photopolymerization in the moist environment of the mouth.45,151 Portions of the matrix may also be directly exposed to oral fluids, particularly at the gingival margin of Class II and V composite restorations. The presence of water promotes the chemical hydrolysis of ester bonds in methacrylate materials. This reaction is expected to be relatively slow at the neutral pH typical of saliva, but excursions in pH caused by foods or cariogenic bacteria may lead to transient acid or base catalysis. The carboxylate and alcohol degradation products of ester hydrolysis are more hydrophilic than the parent ester, further enhancing the local ingress of water. In addition, the carboxylate groups are anionic at normal salivary pH causing a degree of matrix swelling and strain due to charge repulsion. Over years of exposure to salivary fluids, local domains of the methacrylate network may become sufficiently degraded and/or hydrophilic to permit access by esterases, which greatly accelerate ester bond hydrolysis. Mechanical wear of the adhesive that may be exposed at the margins of the restorations may further accelerate matrix degradation by abrading the surface, increasing the surface area and allowing greater ingress of both water and enzymes.
Human saliva contains a variety of enzymes which may participate in the degradation of the adhesive as well as the composite.12,43,58,59,64,78,128 The susceptibility of acrylate dental materials to degradation by esterases is well established.21–23,59,73,155 The esterase-catalyzed degradation of monomethacrylates, dimethacrylates, and commercial dental resins has been documented in solution,21–23,155 in saliva samples,23,33,73 and in vivo.12 In vitro studies have typically used one or more of the following esterases: cholesterol esterase (CE; EC 3.1.1.13)21,23,155 acetylcholinesterase (ACHE; EC 3.1.1.7)21,155 and pseudocholinesterase (PCE, aka butyrylcholinesterase; EC 3.1.1.8).21,23,155 Human saliva samples have been shown to contain CE and PCE activity in sufficient quantity to degrade composite resins.23,47 In vitro degradation of dimethacrylates (BisGMA, TEGDMA) in the presence of PCE and CE was reduced by a specific esterase inhibitor, phenylmethylsulfonyl fluoride,23 supporting an esterase-catalyzed mechanism of degradation. Esterases in solution and in saliva catalyze the hydrolysis of both soluble methacrylate monomers and polymer particulates, 73,155 suggesting that solid dental restorations are directly susceptible to esterase attack. Monomers and polymers of the monomethacrylates (e.g., HEMA) have been shown to be more resistant to esterase digestion (by ACHE, CHE) than the dimethacrylates. 155 CE and PCE have been shown to act synergistically in degrading dimethacrylates (BisGMA, TEGDMA) in vitro.21 A modified dimethacrylate containing urethane segments (urethane-modified BisGMA) showed up to an 86-fold reduction in CE-catalyzed degradation relative to the unmodified control (BisGMA).22 This enhanced esterase resistance was attributed to the chemistry of the modified dimethacrylate, particularly the greater hydrophobicity and hydrogen-bonding capability of the urethane segments.22 Dimethacrylates containing aromatic functional groups or branched methacrylate linkages have also shown greater esterase resistance.155
Although many factors may contribute to the breakdown of methacrylate adhesives, their chemical “Achilles heel” may be the ester linkages. Indeed, the breaking of covalent bonds within the polymer by addition of water to ester bonds is considered one of the main reasons for resin degradation within the hybrid layer.118,119 When exposed to oral fluids, the ester bonds within the methacrylate matrix are vulnerable to two forms of hydrolytic attack: (i) chemical hydrolysis catalyzed by acids or bases, and (ii) enzymatic hydrolysis catalyzed by salivary enzymes, particularly esterases. Both require the presence of water in close association with the bond that will be hydrolyzed. Resin degradation is also directly related to water sorption and high water sorption has been reported for hydrophilic resin systems.45,65 These relationships highlight the challenges associated with the development of an adhesive that is resistant to hydrolytic attack, but also miscible with wet demineralized dentin matrices and compatible with our current dental composites.
MICROMECHANICS OF ADHESIVE/DENTIN INTERFACE USING FINITE ELEMENT ANALYSIS
Under function, dentin adhesives are subjected to both chemical and mechanical stresses and the interplay between these stresses can result in an alteration of the adhesive mechanical properties with time. The mechanical property change results from a variety of mechanisms including (i) proliferation of surface and subsurface flaws and (ii) change in the chemical nature of the polymer in the form of either strain hardening, embrittlement, crystallization, or plasticization. The change in adhesive properties has a significant affect upon the mechanical performance and durability of the a/d interface which is a complex construct of different material phases at the micro-scales. Based upon microscale structure–property measurements, our group has developed an idealized microstructural representation of the a/d interface70,71 that can be utilized to perform micromechanical finite element (μFE) analyses.
Mechanical loads under function are characterized by low amplitude relative to material strength and linear elasticity is a reasonable assumption for performing stress distribution analysis. The μFE analyses have shown that the different material phases at the a/d interface experience different stress amplitudes under functional load.70,71 As the mechanical properties of the hybrid layer, adhesive layer, and adhesive tags alter during function, stresses in the various components that comprise the a/d interface can redistribute. Under functional mechanical loads, those a/d interface components whose elastic modulus decreases at lower rates attract more stress.
Since different a/d interface components have different stress concentrations and may reach their failure strength at different stress-levels, the overall failure behavior is not necessarily determined by the weakest component. Instead, the component whose stress concentration is closest to its failure strength determines the failure. Therefore, under function, the overall time-dependent behavior of the interface is a complex function of the time-dependent behavior of individual material phases. We have utilized the μFE to calculate the stress distributions within the a/d interface at snap shots of time. The resultant stresses were analyzed to show the effect of stress concentrations on the mechanisms that govern the overall fatigue failure behavior of the a/d interface.
The stresses in the different phases of the a/d interface predicted by the μFE were used against S–N (stress-cycle) curves corresponding to each component of the a/d interface.100 Stress concentrations in the adhesive tags were predicted to vary from ~1.2 to 1.5 times the applied load depending upon the hybrid layer thickness and mechanical properties. The overall fatigue life of the a/d interface is governed by the fatigue life of the component with the shortest fatigue life at the local level. In addition, the endurance limit for the a/d interface is determined by the phase with the lowest endurance limit, while the strength is determined by the phase failing first under a statically applied load. We have utilized this principle, to predict SN curves for the a/d interfaces with a variety of hybrid layer thicknesses and mechanical properties. Figure 4 gives the predicted S–N curves for two cases: (1) with a well-infiltrated hybrid layer of uniform mechanical properties, and (2) with a poorly infiltrated hybrid layer of graded mechanical properties. The a/d interface thickness is 15 μm for both cases. As expected the interface with graded hybrid layer exhibits lower durability.
FIGURE 4.
Master SN curves for a/d interface under stress boundary conditions along with measured data.
The fatigue life prediction (Fig. 4) is based upon an isolated computational unit cell having periodic boundary conditions. Under actual clinical function, the unit cell for the a/d interface would have both strain and stress boundary conditions. Hence, it is important to consider two extreme cases of loading which can give upper and lower bounds in terms of fatigue life for the a/d interface. Measured SN data111 is also plotted to illustrate the predictive power of our methodology. The measured curve falls in the stress controlled region because the authors have performed the experiment on small rectangular beam samples which have a smaller number of unit cells as compared to the actual a/d interface. Fewer unit cells mean less constraint in the lateral direction. Hence, the unit cell in their study was likely experiencing stress boundary conditions with minimal constraint in the lateral direction, therefore the measured S–N curve falls in the region of stress controlled loading.
DEVELOPMENT OF WATER-COMPATIBLE, ESTERASE-RESISTANT ADHESIVES
Water is ubiquitous in the mouths of healthy patients and thus, it is imperative that we develop restorative materials that can function adequately in the presence of water. Forty years ago researchers were discussing the detrimental effect of water on bonding dental materials to the tooth; to date, this problem has not been resolved.57 One approach to the problem of bonding to wet dentin has been to increase the relative hydrophilicity of dentin adhesives with a goal of promoting increased wetting of the collagen. However, hydrophilic polymers absorb more water than more hydrophobic resins118; the consequence of this increased water sorption is lowered mechanical properties45 and increased degradation under wet conditions.12,78,128
There are several strategies for reducing hydrolytic degradation of methacrylate adhesives. One strategy involves selectively modifying methacrylate side chains so that they are both water compatible and esterase resistant.79–81 This can be accomplished by the use of bulky and/or branched functional groups that are poor esterase substrates but are sufficiently hydrophilic to be water compatible (e.g., by incorporating polar functional groups such as –OH). The published reports on the reduced esterase susceptibility of urethane-modified BisGMA22 and of acrylates with branched or aromatic side chains155 support this approach. Another strategy involves increasing matrix hydrophobicity following initial monomer penetration into the dentin layer. Secondary cross-linking of polar functional groups on methacrylate side chains could be employed to achieve this goal. Increasing the extent of conversion of methacrylate resins will reduce susceptibility to esterase hydrolysis by reducing the number of unreacted pendant groups.56,145 Clearly, any change in the chemical structure intended to increase esterase resistance and water compatibility will likely alter other chemical and physical properties of the adhesive.
Under clinical function, dentin adhesives are subjected to both chemical and mechanical stresses. The interplay between the two forms of stress is expected to result in an alteration of the properties of the adhesive with time. The mechanical property change results from a variety of mechanisms including proliferation of surface and subsurface flaws due to combined effects of mechanical loads and exposure to salivary esterases and change in the chemical nature of the polymer. Our previous work has shown that the mechanical properties of the adhesive not only affect the overall bond or shear strength but have a profound influence on the load transfer mechanisms at the a/d interface.70,71 Therefore, the change in the mechanical property of the adhesive with time can result in a gradual loss of mechanical integrity.
The optimal adhesive will be produced by balancing the desired physical, chemical, and mechanical properties with the need for esterase resistance and water compatibility. We are balancing these factors by using an iterative combinatorial optimization (molecular design)/synthesis approach in combination with a/d interfacial multi-scale characterization 49,51,52,62,104,105,107,108,110,134,137–139,141 and modeling. 70,71,122 The combinatorial optimization allows the relative importance of each property to be varied, as an initial step in the process of predicting novel methacrylate structures for further evaluation.19 The in situ detection of the interfacial molecular structure and micro-mechanics of the bond formed between dentin and the newly formulated adhesives are used in 2-d and 3-d finite element (FE) models to determine stress distribution and concentration at the interface under simulated loading.50,70,71 Completing the FE modeling in parallel with our analytical analyses, has been critical to our development of a logical iterative process to streamline the number of adhesive formulations that must be fully analyzed.70,71 Results from the FE modeling are also used to further refine the computational molecular design. The interplay of the multiscale characterization with the modeling allows us to proceed in a logical and responsive fashion toward the development of a durable water-compatible, esterase-resistant dentin adhesive.
ADDRESSING THE WEAK LINK IN COMPOSITE RESTORATIONS
In summary, the a/d bond can be the first defense against substances that may penetrate and ultimately undermine the composite restoration in vivo. However, as indicated in a recent review of dental composite, the properties of the materials are one part of a complex problem.13 The success of clinical restorations depends on a variety of factors including proper technique, appropriate materials, and proper patient selection.13
In vitro and in vivo studies have suggested that several factors inhibit the formation of a durable a/d bond. These factors include: (1) water sorption and hydrolysis of the adhesive resin; (2) inadequate monomer/polymer conversion of the infiltrating adhesive; (3) incomplete resin infiltration; and (4) incomplete solvent evaporation.6,20,141,148 As described above, one strategy for reducing hydrolytic degradation involves selectively modifying methacrylate side chains so that they are both water compatible and esterase resistant.79–81 Inadequate monomer/polymer conversion may be addressed by including photoinitiators that are compatible with the hydrophilic components.145 Several strategies should be considered for reducing incomplete resin infiltration and inadequate solvent evaporation. These strategies include reduced demineralization depth, rubber dam isolation to limit the impact of water or saliva contamination, careful attention to handling, management, and storage of the adhesive to prevent solvent evaporation.
The failure of the a/d bond in concert with reports of increased levels of cariogenic bacteria at the perimeter of composite materials points to an interesting relationship between microbiology and adhesive degradation as key elements in the premature failure of moderate-to-large composite restorations. Adhesion of S. mutans to surfaces in the mouth creates an environment that supports the subsequent attachment and growth of other bacterial species, ultimately forming a micro-ecosystem known as a biofilm. Dental plaque biofilm cannot be eliminated.123 It may, however, be possible to reduce the pathogenic impact of the biofilm at the margin of the composite restoration by engineering novel, durable anti-cariogenic dentin adhesives.
Acknowledgments
The authors gratefully acknowledge research support from NIDCR grants DE14392 (PS) and K23DE/HD00468 (BSB).
References
- 1.Andersson-Wenckert IE, van Dijken JW, Kieri C. Durability of extensive Class II open-sandwich restorations with a resin-modified glass ionomer cement after 6 years. Am J Dent. 2004;17:43–50. [PubMed] [Google Scholar]
- 2.Beazoglou T, Eklund S, Heffley D, Meiers J, Brown LJ, Bailit H. Economic impact of regulating the use of amalgam restorations. Public Health Rep. 2007;122:657–663. doi: 10.1177/003335490712200513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardo M, Luis H, Martin MD, Leroux BG, Rue T, Leitao J, DeRouen TA. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J Am Dent Assoc. 2007;138:775–783. doi: 10.14219/jada.archive.2007.0265. [DOI] [PubMed] [Google Scholar]
- 4.Bohaty BS. PhD Dissertation. Kansas City: University of Missouri-Kansas City; 2010. [Google Scholar]
- 5.Brannstrom M. Communication between the oral cavity and the dental pulp associated with restorative treatment. Oper Dent. 1984;9:57–68. [PubMed] [Google Scholar]
- 6.Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008;24:90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Burrow MF, Satoh M, Tagami J. Dentin durability after three years using a dentin bonding agent with and without priming. Dent Mater. 1996;12:302–307. doi: 10.1016/s0109-5641(96)80038-8. [DOI] [PubMed] [Google Scholar]
- 8.Butler WT. Dentin extracellular matrix and dentinogenesis. Oper Dent. 1992;Supplement 5:18–23. [PubMed] [Google Scholar]
- 9.Collins CJ, Bryant RW, Hodge KLV. A clinical evaluation of posterior composite resin restorations: 8-year findings. J Dent. 1998;26:311–317. doi: 10.1016/s0300-5712(97)00019-5. [DOI] [PubMed] [Google Scholar]
- 10.De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, Van Meerbeek B. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res. 2005;84:118–132. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 11.DeRouen TA, Martin MD, Leroux BG, Townes BD, Woods JS, Leitao J, Castro-Caldes A, Luis H, Bernardo M, Rosenbaum G, Martins IP. Neurobehavioral effects of dental amalgam in children: a randomized clinical trial. JAMA. 2006;295:1784–1792. doi: 10.1001/jama.295.15.1784. [DOI] [PubMed] [Google Scholar]
- 12.Donmez N, Belli S, Pashley DH, Tay FR. Ultrastructural correlates of in vivo/in vitro bond degradation in self-etch adhesives. J Dent Res. 2005;84:355–359. doi: 10.1177/154405910508400412. [DOI] [PubMed] [Google Scholar]
- 13.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. J Dent Res. 2008;87:710–719. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eick JD. Smear layer—materials surface. Proc Finn Dent Soc. 1992;88(Suppl 1):225–242. [PubMed] [Google Scholar]
- 15.Eick JD, Cobb CM, Chappell RP, Spencer P, Robinson SJ. The dentinal surface: its influence on dentinal adhesion. Part III. Quint Int. 1993;24:571–582. [PubMed] [Google Scholar]
- 16.Eick JD, Gwinnet AJ, Pashley DH, Robinson SJ. Current concepts on adhesion to dentin. Crit Rev Oral Biol Med. 1997;8:306–335. doi: 10.1177/10454411970080030501. [DOI] [PubMed] [Google Scholar]
- 17.Eick JD, Wilko RA, Anderson CH, Sorenson S. Scanning electron microscopy and electron microprobe analysis of cut tooth surfaces. J Dent Res. 1970;49:1359–1368. doi: 10.1177/00220345700490063601. [DOI] [PubMed] [Google Scholar]
- 18.Erickson RL. Surface interactions of dentin adhesive materials. Oper Dent. 1992;Supplement 5:81–94. [PubMed] [Google Scholar]
- 19.Eslick J, Ye Q, Park J, Topp EM, Spencer P, Camarda KV. A computational molecular design framework for crosslinked polymer networks. Comput Chem Eng. 2009;33:954–963. doi: 10.1016/j.compchemeng.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater. 2006;22:211–222. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Finer Y, Jaffer F, Santerre JP. Mutual influence of cholesterol esterase and pseudocholinesterase on the biodegradation of dental composites. Biomaterials. 2004;25:1787–1793. doi: 10.1016/j.biomaterials.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Finer Y, Santerre JP. The influence of resin chemistry on a dental composite’s biodegradation. J Biomed Mater Res. 2004;69A:233–246. doi: 10.1002/jbm.a.30000. [DOI] [PubMed] [Google Scholar]
- 23.Finer Y, Santerre JP. Salivary esterase activity and its association with the biodegradation of dental composites. J Dent Res. 2004;83:22–26. doi: 10.1177/154405910408300105. [DOI] [PubMed] [Google Scholar]
- 24.Frankenberger R, Pashley DH, Reich SM, Lohbauer U, Petschelt A, Tay FR. Characterisation of resin-dentine interfaces by compressive cyclic loading. Biomaterials. 2005;26:2043–2052. doi: 10.1016/j.biomaterials.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Garberoglio P. The ratio of the densities of dentinal tubules on the cervical and axial wall in cavities. Quint Int. 1994;25:49–52. [PubMed] [Google Scholar]
- 26.Guo X, Spencer P, Wang Y, Ye Q, Yao X, Williams K. Effects of a solubility enhancer on penetration of hydrophobic component in model adhesives into wet demineralized dentin. Dent Mater. 2007;23:1473–1481. doi: 10.1016/j.dental.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gwinnett AJ. Altered tissue contribution to interfacial bond strength with acid conditioned dentin. Am J Dent. 1994;7:243–246. [PubMed] [Google Scholar]
- 28.Gwinnett AJ. Chemically conditioned dentin: a comparison of conventional and environmental scanning electron microscopy findings. Dent Mater. 1994;10:150–155. doi: 10.1016/0109-5641(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 29.Gwinnett AJ. Dentin bond strength after air drying and rewetting. Am J Dent. 1994;7:144–148. [PubMed] [Google Scholar]
- 30.Gwinnett AJ. Quantitative contribution of resin infiltration/hybridization to dentin bonding. Am J Dent. 1993;6:7–9. [PubMed] [Google Scholar]
- 31.Gwinnett AJ, Tay FR, Pang KM, Wei SHY. Quantitative contribution of the collagen network in dentin hybridization. Am J Dent. 1996;9:140–144. [PubMed] [Google Scholar]
- 32.Gwinnett AJ, Yu S. Effect of long-term water storage on dentin bonding. Am J Dent. 1995;8:109–111. [PubMed] [Google Scholar]
- 33.Hagio M, Kawaguchi M, Motokawa W, Mizayaki K. Degradation of methacrylate monomers in human saliva. Dent Mater J. 2006;25:241–246. doi: 10.4012/dmj.25.241. [DOI] [PubMed] [Google Scholar]
- 34.Haj-Ali R, Walker MP, Williams K, Wang Y, Spencer P. Histomorphologic characterization of non-carious and caries-affected dentin/adhesive interfaces. J Prosthodontics. 2006;15:82–88. doi: 10.1111/j.1532-849X.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- 35.Hansel C, Leyhausen G, Mai UE, Geurtsen W. Effects of various resin composite (co)monomers and extracts on two caries-associated micro-organisms in vitro. J Dent Res. 1998;77:60–67. doi: 10.1177/00220345980770010601. [DOI] [PubMed] [Google Scholar]
- 36.Harnirattisai C, Inokoshi S, Hosoda H, Shimade Y. Interfacial morphology of an adhesive composite resin and etched caries-affected dentin. Oper Dent. 1992;17:222–228. [PubMed] [Google Scholar]
- 37.Hashimoto M, Ohno H, Endo K, Kaga M, Sano H, Oguchi H. The effect of hybrid layer thickness on bond strength: demineralized dentin zone of the hybrid layer. Dent Mater. 2000;16:406–411. doi: 10.1016/s0109-5641(00)00035-x. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto M, Ohno H, Kaga M, Endo K, Sano H, Oguchi H. In vivo degradation of resin-dentin bonds in humans over 1 to 3 years. J Dent Res. 2000;79:1385–1391. doi: 10.1177/00220345000790060601. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto M, Ohno H, Kaga M, Endo K, Sano H, Oguchi H. Resin-tooth adhesive interfaces after long-term function. Am J Dent. 2001;12:211–215. [PubMed] [Google Scholar]
- 40.Hashimoto M, Ohno H, Sano H, Kaga M, Oguchi H. In vitro degradation of resin-dentin bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomaterials. 2003;24:3795–3803. doi: 10.1016/s0142-9612(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto M, Ohno H, Sano H, Tay FR, Kaga M, Kudou Y, Oguchi H, Araki Y, Kubota M. Micromorphological changes in resin-dentin bonds after 1 year of water storage. J Biomed Mater Res Appl Biomater. 2002;63:306–311. doi: 10.1002/jbm.10208. [DOI] [PubMed] [Google Scholar]
- 42.Hebling J, Pashley DH, Tjaderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;84:741–746. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 43.Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. J Prosthet Dent. 2001;85:162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 44.Hunter AR, Treasue ET, Hunter AJ. Increases in cavity volume associated with the removal of class 2 amalgam and composite restorations. Operat Dent. 1995;20:2–6. [PubMed] [Google Scholar]
- 45.Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, et al. Effect of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials. 2005;26:6449–6459. doi: 10.1016/j.biomaterials.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 46.Ito S, Saito T, Tay FR, Carvalho RM, Yoshiyama M, Pashley DH. Water content and apparent stiffness of non-caries versus caries-affected human dentin. J Biomed Mater Res B Appl Biomater. 2005;72:109–116. doi: 10.1002/jbm.b.30130. [DOI] [PubMed] [Google Scholar]
- 47.Jaffer F, Finer Y, Santerre JP. Interactions between resin monomers and commercial composite resins with human saliva derived esterases. Biomaterials. 2002;23:1707–1719. doi: 10.1016/s0142-9612(01)00298-8. [DOI] [PubMed] [Google Scholar]
- 48.Kanca J. Improved bond strength through acid etching of dentin and bonding to wet dentin surfaces. J Am Dent Assoc. 1992;123:235–243. doi: 10.14219/jada.archive.1992.0248. [DOI] [PubMed] [Google Scholar]
- 49.Katz JL, Bumrerraj S, Dreyfuss J, Wang Y, Spencer P. Micromechanics of the dentin/adhesive interface. J Biomed Mater Res (Appl Biomater) 2001;58:366–371. doi: 10.1002/jbm.1030. [DOI] [PubMed] [Google Scholar]
- 50.Katz JL, Misra A, Spencer P, Wang Y, Bumrerraj S, Nomura T, Eppell SJ, Tabib-Azar M. Multiscale mechanics of hierarchical structure/property relationships in calcified tissues and tissue/material interfaces. Mater Sci Eng C. 2007;27:450–468. doi: 10.1016/j.msec.2006.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katz JL, Misra A, Spencer P, Wang Y, Bumrerraj S, Nomura T, Eppell SJ, Tabib-Azar M. Multiscale mechanics of hierarchical structure/property relationships in calcified tissues and tissue/material interfaces. J Mater Sci Eng C. 2007;27:450–468. doi: 10.1016/j.msec.2006.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katz JL, Spencer P, Nomura T, Wagh A, Wang Y. Micromechanical properties of demineralized dentin collagen with and without adhesive infiltration. J Biomed Mater Res. 2003;66A:120–128. doi: 10.1002/jbm.a.10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kinney JH, Balooch M, Marshall SJ, Marshall GW. Atomic force microscope study of dimensional changes in dentine during drying. Arch Oral Biol. 1993;38:1003–1007. doi: 10.1016/0003-9969(93)90114-2. [DOI] [PubMed] [Google Scholar]
- 54.Kinney JH, Pople JA, Marshall GW, Marshall SJ. Collagen orientation and crystallite size in human dentin: a small angle X-ray scattering study. Calcif Tissues Int. 2001;69:31–37. doi: 10.1007/s00223-001-0006-5. [DOI] [PubMed] [Google Scholar]
- 55.Kleverlaan CJ, Feilzer AJ. Polymerization shrinkage and contraction stress of dental resin composites. Dent Mater. 2005;21:1150–1157. doi: 10.1016/j.dental.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Kostoryz EL, Dharmala K, Ye Q, Wang Y, Huber J, Park JG, Snider G, Katz JL, Spencer P. Enzymatic biodegradation of HEMA/bisGMA adhesives formulated with different water content. J Biomed Mater Res B. 2009;88:394–401. doi: 10.1002/jbm.b.31095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kugel G, Ferrari M. The Science of Bonding: from first to sixth generation. JADA. 2000;131:20s–25s. doi: 10.14219/jada.archive.2000.0398. [DOI] [PubMed] [Google Scholar]
- 58.Labow RS, Duguay DG, Santerre JP. The enzymatic hydrolysis of a synthetic biomembrane: a new substrate for cholesterol and carboxyl esterases. J Biomater Sci Polym Ed. 1994;6:169–179. doi: 10.1163/156856294x00293. [DOI] [PubMed] [Google Scholar]
- 59.Lee YK, Powers JM. Influence of salivary organic substances on the discoloration of esthetic dental materials—a review. J Biomed Mater Res. 2006;76:397–402. doi: 10.1002/jbm.b.30380. [DOI] [PubMed] [Google Scholar]
- 60.LeGeros RZ. Calcium phosphates in oral biology and medicine. In: Meyers HM, editor. Monographs in Oral Science. Basel: Karger; 1991. p. 121. [PubMed] [Google Scholar]
- 61.Leinfelder KF. Do restorations made of amalgam out-last those made of resin-based composite? J Am Dent Assoc. 2000;131:1186–1187. doi: 10.14219/jada.archive.2000.0355. [DOI] [PubMed] [Google Scholar]
- 62.Lemor RM, Kruger MB, Wieliczka DM, Swafford JR, Spencer P. Spectroscopic and morphologic characterization of the dentin/adhesive interface. J Biomed Optics. 1999;4:22–27. doi: 10.1117/1.429917. [DOI] [PubMed] [Google Scholar]
- 63.Levin L, Coval M, Geiger SB. Cross-sectional radiographic survey of amalgam and resin-based composite posterior restorations. Quint Int. 2007;38:511–514. [PubMed] [Google Scholar]
- 64.Levine MJ. Salivary macromolecules. A structure/function synopsis. Ann N Y Acad Sci. 1993;694:11–16. doi: 10.1111/j.1749-6632.1993.tb18337.x. [DOI] [PubMed] [Google Scholar]
- 65.Malacarne J, Carvalho RM, de Goes MF, Svizero N, Pashley DH, Tay FR, Yiu CK, Carrilho MR. Water sorption/solubility of dental adhesive resins. Dent Mater. 2006;22:973–980. doi: 10.1016/j.dental.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 66.Marangos O, Misra A, Spencer P, Bohaty B, Katz JL. Physico-mechanical properties determination using microscale homotopic measurements: application to sound and caries-affected primary tooth dentin. Acta Biomater. 2009;5:1338–1348. doi: 10.1016/j.actbio.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marshall GW, Inai N, Magidi ICW, Balooch M, Kinney JH, Tagami J, Marshall SJ. Dentin demineralization: effects of dentin depth, pH and different acids. Dent Mater. 1997;13:338–343. doi: 10.1016/s0109-5641(97)80104-2. [DOI] [PubMed] [Google Scholar]
- 68.Marshall GW, Marshall SJ, Kinney JH, Balooch M. The dentin substrate: structure and properties related to bonding. J Dent. 1997;25:441–458. doi: 10.1016/s0300-5712(96)00065-6. [DOI] [PubMed] [Google Scholar]
- 69.Marshall J, Dentin GW. Microstructure and characterization. Quint Int. 1993;24:606–617. [PubMed] [Google Scholar]
- 70.Misra A, Spencer P, Marangos O, Wang Y, Katz JL. Micromechanical analysis of dentin/adhesive interface using finite element method. J Biomed Mater Res. 2004;70B:56–65. doi: 10.1002/jbm.b.30012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Misra A, Spencer P, Marangos O, Wang Y, Katz JL. Parametric study of the effect of phase anisotropy on the micromechanical behavior of dentin/adhesive interfaces. J R Soc Interface. 2005;2:145–157. doi: 10.1098/rsif.2005.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mjor IA, Dahl JE, Moorhead JE. Age of restorations at replacement in permanent teeth in general dental practice. Acta Odontol Scand. 2000;58:97–101. doi: 10.1080/000163500429208. [DOI] [PubMed] [Google Scholar]
- 73.Munksgaard EC, Freund M. Enzymatic hydrolysis of (di)methacrylates and their polymers. Scand J Dent Res. 1990;98:261–267. doi: 10.1111/j.1600-0722.1990.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 74.Murray PE, Windsor LJ, Smyth TW, Hafez AA, Cox CF. Analysis of pulpal reactions to restorative procedures, materials, pulp capping, and future therapies. Crit Rev Oral Biol Med. 2002;13:509–520. doi: 10.1177/154411130201300607. [DOI] [PubMed] [Google Scholar]
- 75.Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16:265–273. doi: 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- 76.Nakajima M, Sano H, Burrow MF, Tagami J, Yoshiyama M, Ebisu S, Ciucchi B, Russell CM, Pashley DH. Tensile bond strength and SEM evaluation of caries-affected dentin using dentin adhesives. J Dent Res. 1995;74:1679–1688. doi: 10.1177/00220345950740100901. [DOI] [PubMed] [Google Scholar]
- 77.Nishitani Y, Yoshiyama M, Donnelly AM, Agee KA, Sword J, Tay FR, Pashley DH. Effects of resin hydrophilicity on dentin bond strength. J Dent Res. 2006;85:1016–1021. doi: 10.1177/154405910608501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okuda M, Pereira PN, Nakajima M, Tagami J, Pashley DH. Long-term durability of resin dentin interface: nanoleakage vs. microtensile bond strength. Oper Dent. 2002;27:289–296. [PubMed] [Google Scholar]
- 79.Park JG, Ye Q, Topp EM, Kostoryz EL, Wang Y, Kieweg SL, Spencer P. Preparation and properties of novel dentin adhesives with esterase resistance. J Appl Polym Sci. 2008;107:3588–3597. doi: 10.1002/app.27512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park JG, Ye Q, Topp EM, Lee CH, Kostoryz EL, Misra A, Spencer P. Dynamic mechanical analysis and esterase degradation of dentin adhesives containing a branched methacrylate. J Biomed Mater Res B. 2009;91:61–70. doi: 10.1002/jbm.b.31374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park JG, Ye Q, Topp EM, Spencer P. Enzyme-catalyzed hydrolysis of dentin adhesives containing a new urethane-based trimethacrylate monomer. J Biomed Mater Res B. 2009;91:562–571. doi: 10.1002/jbm.b.31430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pashley DH. Clinical correlations of dentin structure and function. J Prosthet Dent. 1991;66:777–781. doi: 10.1016/0022-3913(91)90414-r. [DOI] [PubMed] [Google Scholar]
- 83.Pashley DH. Dentin: a dynamic substrate in dentistry. Scanning Microsc. 1989;3:161–176. [PubMed] [Google Scholar]
- 84.Pashley DH. Smear layer: overview of structure and function. Proc Finn Dent Soc. 1992;88(Suppl 1):215–224. [PubMed] [Google Scholar]
- 85.Pashley DH, Ciucchi B, Sano H, Horner JA. Permeability of dentin to adhesive agents. Quint Int. 1993;24:618–631. [PubMed] [Google Scholar]
- 86.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–221. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 87.Pashley EL, Zhang Y, Lockwood PE, Rueggeberg FA, Pashley DH. Effects of HEMA on water evaporation from water-HEMA mixtures. Dent Mater. 1998;14:6–10. doi: 10.1016/s0109-5641(98)00003-7. [DOI] [PubMed] [Google Scholar]
- 88.Paul SJ, Leach M, Rueggeberg FA, Pashley DH. Effect of water content on the physical properties of model dentine primer and bonding resins. J Dent. 1999;27:209–214. doi: 10.1016/s0300-5712(98)00042-6. [DOI] [PubMed] [Google Scholar]
- 89.Perdigao J, Frankenberger R, Rosa BT, Breschi L. New trends in dentin/enamel adhesion. Am J Dent. 2000;13:25D–30D. [PubMed] [Google Scholar]
- 90.Perdigao J, Swift EJ, Denehy GE, Wefel JS, Donly KJ. In vitro bond strengths and SEM evaluation of dentin bonding systems to different dentin substrates. J Dent Res. 1994;73:44–55. doi: 10.1177/00220345940730010601. [DOI] [PubMed] [Google Scholar]
- 91.Pereira PNR, Okuda M, Sano H, Yoshikawa T, Burrow MF, Tagami J. Effect of intrinsic wetness and regional difference on dentin bond strength. Dent Mater. 1999;15:46–53. doi: 10.1016/s0109-5641(99)00013-5. [DOI] [PubMed] [Google Scholar]
- 92.Peumans M, Kanumilli P, De Munck J, Van Landuyt K, Lambrechts P, Van Meerbeek B. Clinical effectiveness of contemporary adhesives: a systematic review of current clinical trials. Dent Mater. 2005;21:864–881. doi: 10.1016/j.dental.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 93.Purk JH, Dusevich V, Glaros AG, Spencer P, Eick JD. In vivo versus in vitro microtensile bond strength of axial versus gingival cavity preparation walls in Class II resin-based composite restorations. JADA. 2004;135:185–193. doi: 10.14219/jada.archive.2004.0150. [DOI] [PubMed] [Google Scholar]
- 94.Roulet JF. Benefits and disadvantages of tooth-coloured alternatives to amalgam. J Dent. 1997;25:459–473. doi: 10.1016/s0300-5712(96)00066-8. [DOI] [PubMed] [Google Scholar]
- 95.Roulet JF, Degrange M, editors. Adhesion: The Silent Revolution in Dentistry. Quintessence Publishing Co., Inc; 1999. p. 263. [Google Scholar]
- 96.Sano H. Microtensile testing, nanoleakage, and biodegradation of resin-dentin bonds. J Dent Res. 2006;85:11–14. doi: 10.1177/154405910608500102. [DOI] [PubMed] [Google Scholar]
- 97.Sano H, Yoshikawa T, Pereira PNR, Kanemura N, Morigami M, Tagami J, Pashley DH. Long-term durability of dentin bonds made with a self-etching primer, in vivo. J Dent Res. 1999;78:906–911. doi: 10.1177/00220345990780041101. [DOI] [PubMed] [Google Scholar]
- 98.Santerre JP, Shajii L, Leung BW. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit Rev Oral Biol Med. 2001;12:136–151. doi: 10.1177/10454411010120020401. [DOI] [PubMed] [Google Scholar]
- 99.Simecek JW, Diefenderfer KE, Cohen ME. An evaluation of replacement rates for posterior resin-based composite and amalgam restorations in US Navy and Marine Corps recruits. J Am Dent Assoc. 2009;140:200–209. doi: 10.14219/jada.archive.2009.0134. [DOI] [PubMed] [Google Scholar]
- 100.Singh V. Mechanical Engineering. Lawrence: University of Kansas; 2009. Viscoelastic and fatigue properties of dental adhesives and their impact on dentin-adhesive interface durability. M.S. Thesis. [Google Scholar]
- 101.Soappman MJ, Nazari A, Porter JA, Arola D. A comparison of fatigue crack growth in resin composite, dentin and the interface. Dent Mater. 2007;23:608–614. doi: 10.1016/j.dental.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 102.Soncini JA, Maserejian NN, Trachtenberg F, Tavares M, Hayes C. The longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth: findings From the New England Children’s Amalgam Trial. J Am Dent Assoc. 2007;138:763–772. doi: 10.14219/jada.archive.2007.0264. [DOI] [PubMed] [Google Scholar]
- 103.Spencer P, Katz JL, Tabib-Azar M, Wang Y, Wagh A, Nomura T. Hyperspectral analysis of collagen infused with BisGMA-based polymeric adhesive. In: Yaszemski MJ, Trantolo DJ, Lewandrowski KU, Hasirci V, Altobelli DE, Wise DL, editors. Tissue Engineering and Novel Delivery Systems. New York: Marcel Decker; 2003. pp. 599–632. [Google Scholar]
- 104.Spencer P, Swafford JR. Unprotected protein at the dentin-adhesive interface. Quint Int. 1999;30:501–507. [PubMed] [Google Scholar]
- 105.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res. 2002;62:447–456. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 106.Spencer P, Wang Y, Bohaty B. Interfacial chemistry of moisture-aged class II composite restorations. J Biomed Mater Res B. 2006;77:234–240. doi: 10.1002/jbm.b.30434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Spencer P, Wang Y, Katz JL. Identification of collagen encapsulation at the dentin/adhesive interface. J Adhesive Dent. 2004;6:91–95. [PubMed] [Google Scholar]
- 108.Spencer P, Wang Y, Katz JL, Misra A. Physicochemical interactions at the dentin/adhesive interface using FTIR chemical imaging. J Biomed Optics. 2005;10:031104. doi: 10.1117/1.1914844. [DOI] [PubMed] [Google Scholar]
- 109.Spencer P, Wang Y, Walker MP, Swafford JR. Molecular structure of acid-etched dentin smear layers-in situ study. J Dent Res. 2001;80:1802–1807. doi: 10.1177/00220345010800090601. [DOI] [PubMed] [Google Scholar]
- 110.Spencer P, Wang Y, Walker MP, Wieliczka DM, Swafford JR. Interfacial chemistry of the dentin/adhesive bond. J Dent Res. 2000;79:1458–1463. doi: 10.1177/00220345000790070501. [DOI] [PubMed] [Google Scholar]
- 111.Staninec M, Kim P, Marshall GW, Ritchie RO, Marshall SJ. Fatigue of dentin-composite interfaces with four-point bend. Dent Mater. 2008;24:799–803. doi: 10.1016/j.dental.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tam LE, Pilliar RM. Fracture surface characterization of dentin-bonded interfacial fracture toughness specimens. J Dent Res. 1994;73:607–619. doi: 10.1177/00220345940730030601. [DOI] [PubMed] [Google Scholar]
- 113.Tay FR, Gwinnett AJ, Pang KM, Wei SHY. An optical, micromorphological study of surface moisture in the total etched resin-dentin interface. Am J Dent. 1996;9:43–48. [PubMed] [Google Scholar]
- 114.Tay FR, Gwinnett AJ, Wei SHY. Micromophological sepctrum from overdrying to overwetting acid-conditioned dentin in water-free, acetone-based, single-bottle primer/adhesives. Dent Mater. 1996;12:236–244. doi: 10.1016/s0109-5641(96)80029-7. [DOI] [PubMed] [Google Scholar]
- 115.Tay FR, Gwinnett AJ, Wei SHY. The overwet phenomenon: a transmission electron microscopic study of surface moisture in the acid-conditioned, resin-dentin interface. Am J Dent. 1996;9:161–166. [PubMed] [Google Scholar]
- 116.Tay FR, Hashimoto M, Pashley DH, Peters MC, Lai SC, Yiu CK, Cheong C. Aging affects two modes of nanoleakage expression in bonded dentin. J Dent Res. 2003;82:537–541. doi: 10.1177/154405910308200710. [DOI] [PubMed] [Google Scholar]
- 117.Tay FR, Pashley DH. Aggressiveness of contemporary self-etching systems. I: depth of penetratio beyond dentin smear layers. Dent Mater. 2001;17:296–308. doi: 10.1016/s0109-5641(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 118.Tay FR, Pashley DH. Have dentin adhesives become too hydrophilic? J Can Dent Assoc. 2003;69:726–731. [PubMed] [Google Scholar]
- 119.Tay FR, Pashley DH. Water treeing—a potential mechanism for degradation of dentin adhesives. Am J Dent. 2003;16:6–12. [PubMed] [Google Scholar]
- 120.Ten Cate AR. Oral Histology. St. Louis: Mosby; 1994. p. 174. [Google Scholar]
- 121.Ten Cate AR. Repair and regeneration of dental tissue. In: Ten Cate AR, editor. Oral Histology Development, Structure, and Function. St. Louis: Mosby; 1994. pp. 456–468. [Google Scholar]
- 122.Thiagarajan G, Deshmukh K, Wang Y, Misra A, Katz JL, Spencer P. Nano finite element modeling of the mechanical behavior of biocomposites using multi-scale (virtual internal bond) material models. J Biomed Mater Res A. 2007;83:332–344. doi: 10.1002/jbm.a.31241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thomas JG, Nakaishi LA. Managing the complexity of a dynamic biofilm. J Am Dent Assoc. 2006;137:10S–15S. doi: 10.14219/jada.archive.2006.0409. [DOI] [PubMed] [Google Scholar]
- 124.Tobi H, Kreulen CM, Vondeling H, van Amerongen WE. Cost-effectiveness of composite resins and amalgam in the replacement of amalgam class II restorations. Commun Dent Oral Epidemiol. 1999;27:137–143. doi: 10.1111/j.1600-0528.1999.tb02003.x. [DOI] [PubMed] [Google Scholar]
- 125.Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, Coutinho E, Suzuki K, Lambrechts P, Van Meerbeek B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757–3785. doi: 10.1016/j.biomaterials.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 126.Van Meerbeek B, Van Landuyt K, De Munck J, Hashimoto M, Peumans M, Lambrechts P, Yoshida Y, Inoue S, Suzuki K. Technique-sensitivity of contemporary adhesives. Dent Mater J. 2005;24:1–13. doi: 10.4012/dmj.24.1. [DOI] [PubMed] [Google Scholar]
- 127.Van Nieuwenhuysen JP, D’Hoore W, Carvalho J, Qvist V. Long-term evaluation of extensive restorations in permanent teeth. J Dent. 2003;31:395–405. doi: 10.1016/s0300-5712(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 128.Wadgaonkar B, Ito S, Svizero N, Elrod D, Foulger S, Rodgers R, Oshida Y, Kirkland K, Sword J, Rueggeberg F, Tay F, Pashley D. Evaluation of the effect of water-uptake on the impedance of dental resins. Biomaterials. 2006;27:3287–3294. doi: 10.1016/j.biomaterials.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 129.Wang R, Weiner S. Human root dentin: structure anistropy and vickers microhardness isotropy. Connect Tissue Res. 1998;39:269–279. doi: 10.3109/03008209809021502. [DOI] [PubMed] [Google Scholar]
- 130.Wang Y, Spencer P. Analysis of acid-treated dentin smear debris and smear layers using confocal Raman microspectroscopy. J Biomed Mater Res. 2002;60:300–308. doi: 10.1002/jbm.10108. [DOI] [PubMed] [Google Scholar]
- 131.Wang Y, Spencer P. Continuing etching of an all-in-one adhesive in wet dentin tubules. J Dent Res. 2005;84:350–354. doi: 10.1177/154405910508400411. [DOI] [PubMed] [Google Scholar]
- 132.Wang Y, Spencer P. Effect of acid etching time and techniques on interfacial characteristics of the adhesive-dentin bond using differential staining. Eur J Oral Sci. 2004;112:293–299. doi: 10.1111/j.1600-0722.2004.00127.x. [DOI] [PubMed] [Google Scholar]
- 133.Wang Y, Spencer P. Evaluation of the interface between one-bottle adhesive systems and dentin by Goldner’s trichrome stain. Am J Dent. 2005;18:66–72. [PubMed] [Google Scholar]
- 134.Wang Y, Spencer P. Hybridization efficiency of the adhesive dentin interface with wet bonding. J Dent Res. 2003;82:141–145. doi: 10.1177/154405910308200213. [DOI] [PubMed] [Google Scholar]
- 135.Wang Y, Spencer P. Interfacial chemistry of Class II composite restoration: structure analysis. J Biomed Mater Res. 2005;75A:580–587. doi: 10.1002/jbm.a.30451. [DOI] [PubMed] [Google Scholar]
- 136.Wang Y, Spencer P. Overestimating hybrid layer quality in polished adhesive/dentin interfaces. J Biomed Mater Res. 2004;68A:735–746. doi: 10.1002/jbm.a.20105. [DOI] [PubMed] [Google Scholar]
- 137.Wang Y, Spencer P. Physicochemical interactions at the interfaces between self-etch adhesive systems and dentin. J Dent. 2004;32:567–579. doi: 10.1016/j.jdent.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 138.Wang Y, Spencer P. Quantifying adhesive penetration in adhesive/dentin interface using confocal Raman microspectroscopy. J Biomed Mater Res. 2002;59:46–55. doi: 10.1002/jbm.1215. [DOI] [PubMed] [Google Scholar]
- 139.Wang Y, Spencer P, Hager C, Bohaty B. Comparison of interfacial characteristics of adhesive bonding to superficial versus deep dentin using SEM and staining techniques. J Dent. 2006;34:26–34. doi: 10.1016/j.jdent.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 140.Wang Y, Spencer P, Yao X. Micro-Raman imaging analysis of monomer/mineral distribution in intertubular region of adhesive/dentin interfaces. J Biomed Optics. 2006;11:024005-024001–024005-024007. doi: 10.1117/1.2187992. [DOI] [PubMed] [Google Scholar]
- 141.Wang Y, Spencer P, Yao X, Brenda B. Effect of solvent content on resin hybridization in wet dentin bonding. J Biomed Mater Res A. 2007;82:975–983. doi: 10.1002/jbm.a.31232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang Y, Spencer P, Yao X, Ye Q. Effect of co-initiator and water on the photoreactivity and photopolymerization of HEMA/camphoroquinone-based reactant mixtures. J Biomed Mater Res. 2006;78A:721–728. doi: 10.1002/jbm.a.30733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weiner S, Veis A, Beniash E, Arad T, Dillon JW, Sabsay B, Siddiqui F. Peritubular dentin formation: crystal organization and the macromolecular constituents in human teeth. J Struct Biol. 1999;126:27–41. doi: 10.1006/jsbi.1999.4096. [DOI] [PubMed] [Google Scholar]
- 144.Wieliczka DM, Kruger MB, Spencer P. Raman imaging of dental adhesive diffusion. Appl Spectrosc. 1997;51:1593–1596. [Google Scholar]
- 145.Ye Q, Park J, Topp E, Spencer P. Effect of photoinitiators on the in vitro performance of a dentin adhesive exposed to simulated oral environment. Dent Mater. 2009;25:452–458. doi: 10.1016/j.dental.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ye Q, Park JG, Topp E, Wang Y, Misra A, Spencer P. In vitro performance of nano-heterogeneous dentin adhesive. J Dent Res. 2008;87:829–833. doi: 10.1177/154405910808700911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ye Q, Spencer P, Wang Y. Nanoscale patterning in crosslinked methacrylate copolymer networks: an atomic force microscopy study. J Appl Polym Sci. 2007;106:3843–3851. doi: 10.1002/app.27044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ye Q, Spencer P, Wang Y, Misra A. Relationship of solvent to the photopolymerization process, properties, and structure in model dentin adhesives. J Biomed Mater Res A. 2007;80:342–350. doi: 10.1002/jbm.a.30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ye Q, Wang Y, Spencer P. Nanophase separation of polymers exposed to simulated bonding conditions. J Biomed Mater Res B. 2009;88:339–348. doi: 10.1002/jbm.b.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yiu CK, King NM, Pashley DH, Suh BI, Carvalho RM, Carrilho MR, Tay FR. Effect of resin hydrophilicity and water storage on resin strength. Biomaterials. 2004;25:5789–5796. doi: 10.1016/j.biomaterials.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 151.Yoshida E, Uno S, Nodasaka Y, Kaga M, Hirano S. Relationship between water status in dentin and interfacial morphology in all-in-one adhesives. Dent Mater. 2007;23:556–560. doi: 10.1016/j.dental.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 152.Yoshiyama M, Carvalho R, Sano H, Horner J, Brewer PD, Pashley DH. Interfacial morphology and strength of bonds made to superficial versus deep dentin. Am J Dent. 1995;8:297–302. [PubMed] [Google Scholar]
- 153.Yoshiyama M, Carvalho RM, Sano H, Horner JA, Brewer PD, Pashley DH. Regional bond strengths of resins to human root dentine. J Dent. 1996;24:435–442. doi: 10.1016/0300-5712(95)00102-6. [DOI] [PubMed] [Google Scholar]
- 154.Yoshiyama M, Urayama A, Kimochi T, Matsuo T, Pashley DH. Comparison of conventional vs. self-etching adhesive bonds to caries-affected dentin. Oper Dent. 2000;25:163–169. [PubMed] [Google Scholar]
- 155.Yourtee DM, Smith RE, Russo KA, Burmaster S, Cannon JM, Eick JD, Kostoryz EL. The stability of methacrylate biomaterials when enzyme challenged: Kinetic and systematic evaluations. J Biomed Mater Res. 2001;57:523–531. doi: 10.1002/1097-4636(20011215)57:4<522::aid-jbm1198>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]



