Abstract

The enantioselective formation of chiral crystal of achiral nucleobase cytosine was achieved mediated by the crystal direction selective dehydration of crystal water in the achiral crystal of cytosine monohydrate (P21/c). Heat transfer from the enantiotopic face of the single crystal of cytosine monohydrate afforded the enantiomorphous crystal of anhydrous cytosine.
Crystallization of achiral compounds in enantiomorphs has been considered as one of the candidates for the origin of homochirality.1,2 However, such chiral crystallization(3) does not show any preference for the formation of one or the other enantiomorphs; that is, the produced crystalline chirality is spontaneously generated to follow the bimodal random distribution.(4) If it is possible to control the handedness of crystalline chirality formed from an achiral compound without using a chiral auxiliary, this should become another entry for the origin of chirality.(5)
 |
Here we report on the generation of crystal chirality by the thermal dehydration of the crystal water of cytosine(6) monohydrate 1·H2O, which belongs to achiral P21/c (eq 1). The thermal direction controls the crystal chirality of the resulting anhydrous crystal 1. That is, the dehydration of crystal water from the enantiotopic faces of an achiral crystal led to enantiomorphic crystals. Although there were reports on the chiral phenomena based on the enantiotopic faces of the achiral crystal,(7) to the best of our knowledge, there are no examples of crystal formation in which the enantiomorphs were efficiently controlled by the dehydration of crystal water.
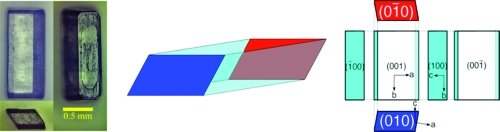
Although the cytosine crystal obtained from methanol belongs to the chiral space group P212121,8,9 crystallization in water affords the achiral monohydrate crystal belonging to the achiral P21/c.(10) Single crystals of 1·H2O with well-defined crystal faces can be obtained from water by slow evaporation at room temperature. The crystal morphology and Miller indices are shown in Figure 1. Each single crystal has parallelogram (010) and (01̅0) faces, which are vertical to the b-axis. These nonsuperimposable parallelogram faces are defined as blue colored b1- and red colored b2-faces (Figure 2). Then, the crystal was heated from these opposite faces by placing the respective faces on a hot plate (80−110 °C). After the whole crystal became cloudy, i.e., the crystal water had completely disappeared,(11) these solid white crystals were characterized by solid-state circular dichroism (CD) analysis(12) as KBr pellets. In the case of the resulting white solid heated from the b1-face, the negative Cotton effect was detected at approximately 310 nm ([CD(−)310KBr]-1). On the other hand, when the thermal direction was from the b2- to the b1-face, the positive Cotton effect was observed at approximately 310 nm ([CD(+)310KBr]-1).
Figure 1.
Single crystal of cytosine monohydrate 1·H2O.
Figure 2.
Generation of crystal chirality by the removal of crystal water of cytosine monohydrate 1·H2O.
To exclude any effect other than that of the shape of the crystal faces, a single crystal was cut into two pieces vertical to the b-axis, and the newly formed faces were placed on the hot plate to perform the dehydration step. In these experiments, the induction sense was the same as observed above without any exception, i.e., dehydration from the b1- and b2-face gave [CD(−)310KBr]- and [CD(+)310KBr]-1, respectively (Figure 2).
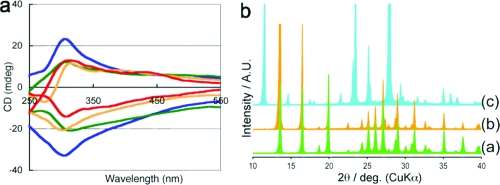
The relationship between the face images on the hot plate and the sign of the CD at 310 nm of anhydrous cytosine 1 is highly reproducible, as shown in Figure 3a. Although, the resulting white crystal is not a single crystal, the X-ray powder diffraction patterns of each crystal are shown in Figure 3b. The diffraction pattern of the dehydrated anhydrous cytosine 1 is essentially the same as the simulated pattern of the P212121 crystal. The pattern of 1·H2O is distinctly different from that of anhydrous 1; thus, the cytosine monohydrate 1·H2O is considered to be transferred to the crystal, similar to the case of the P212121 by the thermal dehydration of crystal water.
Figure 3.
Solid-state properties of cytosine crystals. a: Solid-state CD of anhydrous 1. Lines of the same color indicate the CD signals of the anhydrous crystals that arise from the identical single crystals of 1·H2O. b: X-ray powder diffraction patterns. (a) Cytosine 1 (P212121: simulation from single crystal data). (b) Dehydrated cytosine 1 (observed pattern). (c) Cytosine monohydrate 1·H2O (P21/c: simulation from single crystal data).
We assumed that the crystal chirality would emerge after reconstitution of the hydrogen bond (H-bond) network in the crystal. In the single crystal structure of anhydrous cytosine 1 (P212121), shown in Figure 4a, the H-bond between the carbonyl and primary amino group of neighboring molecules (indicated as the green lines) forms a helical arrangement (left-handed crystal was indicated).(13) A similar (but not helical) H-bond structure can be seen in the crystal of 1·H2O (recrystallized from water), viewed along the a-axis (Figure 4b). The green lines represent the H-bonds originally present in the crystal, which correspond to those of the P212121 crystal, and the orange lines indicate the newly formed H-bonds (A) and (B). The carbonyl and primary amino groups connected by H-bonds (A) and (B) are originally bonded by water mediated H-bonds. Thus, the removal of crystal water should lead to the creation of (A) and (B), which constitute the helical arrangement in the dehydrated crystal. It can also be seen that the order of formation between (A) and (B) determines the handedness of the resulting helical H-bond network. That is, the initial formation of H-bond (A) followed by H-bond (B) should induce the left-handed helicity, and vice versa. Because the direction-selective thermal transfer would control the order of the new H-bond formation, the enantiomorphous dehydrated crystal of cytosine 1 can therefore be obtained by heating from the enantiotopic (01̅0) and (010) faces of the achiral crystal of 1·H2O (Figure 4c).(14)
 |
Figure 4.
Formation of helical H-bond network by the thermal dehydration of crystal water.
In addition, the dehydrated 1 acted as the origin of chirality in asymmetric autocatalysis15,16 to afford enantioenriched products with the absolute configurations correlated to the sign of CD of resulting 1 as shown in eq 2.
In summary, we have demonstrated that the enantioselective formation of the chiral crystal of achiral cytosine can be obtained by the removal of crystal water. Thermal dehydration of the achiral crystal of cytosine monohydrate induces the chirality of the remaining anhydrous cytosine. The heat transfer from the enantiotopic (010) and (01̅0) faces of the achiral single crystal of 1·H2O affords the enantiomorphous anhydrous crystal of cytosine 1 with the chirality corresponding to that of the crystal face.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science.
Supporting Information Available
TG/DTA of cytosine monohydrate, asymmetric autocatalysis using dehydrated cytosine, comparison between the solid-state CD of anhydrous 1 using KBr and nujol mulls, and the determination of the absolute structure of P212121 crystal of cytosine. This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- a Eschenmoser A. Science 1999, 284, 2118. [DOI] [PubMed] [Google Scholar]; b Mislow K. Collect. Czech. Chem. Commun. 2003, 68, 849. [Google Scholar]; c Girard C.; Kagan H. B. Angew. Chem., Int. Ed. 1998, 37, 2923. [DOI] [PubMed] [Google Scholar]; d Green M. M.; Park J.-W.; Sato T.; Teramoto A.; Lifson S.; Selinger R. L. B.; Selinger J. V. Angew. Chem., Int. Ed. 1999, 38, 3139. [DOI] [PubMed] [Google Scholar]; e Feringa B. L.; Van Delden R. A. Angew. Chem., Int. Ed. 1999, 38, 3419. [DOI] [PubMed] [Google Scholar]; f Bonner W. A.; Rubenstein E. BioSystems 1987, 20, 99. [DOI] [PubMed] [Google Scholar]; g Kawasaki T.; Sato M.; Ishiguro S.; Saito T.; Morishita Y.; Sato I.; Nishino H.; Inoue Y.; Soai K. J. Am. Chem. Soc. 2005, 127, 3274. [DOI] [PubMed] [Google Scholar]; h Hazen R. M.; Sholl D. S. Nat. Mater. 2003, 2, 367. [DOI] [PubMed] [Google Scholar]
- a Green B. S.; Lahav M.; Rabinovich D. Acc. Chem. Res. 1979, 12, 191. [Google Scholar]; b Lennartson A.; Olsson S.; Sundberg J.; Håkansson M. Angew. Chem., Int. Ed. 2009, 48, 3137. [DOI] [PubMed] [Google Scholar]
- a Matsuura T.; Koshima H. J. Photochem. Photobiol., C 2005, 6, 7. [Google Scholar]; b Sakamoto M. Chem.—Eur. J. 1997, 3, 684. [Google Scholar]
- a Kondepudi D. K.; Kaufman R.; Singh N. Science 1990, 250, 975. [DOI] [PubMed] [Google Scholar]; b McBride J. M.; Carter R. L. Angew. Chem., Int. Ed. 1991, 30, 293. [Google Scholar]
- a Claborn K.; Isborn C.; Kaminsky W.; Kahr B. Angew. Chem., Int. Ed. 2008, 47, 5706. [DOI] [PubMed] [Google Scholar]; b Chenchaiah P. C.; Holland H. L.; Richardson M. F. Chem. Commun. 1982, 436. [Google Scholar]; c Kuhn A.; Fischer P. Angew. Chem., Int. Ed. 2009, 48, 6857. [DOI] [PubMed] [Google Scholar]; d Vaida M.; Shimon L. J. W.; Weisinger-Lewin Y.; Frolow F.; Lahav M.; Leiserowitz L.; McMullan R. K. Science 1988, 241, 1475. [DOI] [PubMed] [Google Scholar]; e Viedma C. Phys. Rev. Lett. 2005, 94, 065504. [DOI] [PubMed] [Google Scholar]; f Ribo J. M.; Crusats J.; Sagues F.; Claret J.; Rubires R. Science 2001, 292, 2063. [DOI] [PubMed] [Google Scholar]; g Breslow R.; Levine M. S. Proc. Natl. Acad. Sci. U.S.A. 2004, 103, 12979. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Hayashi Y.; Matsuzawa M.; Yamaguchi J.; Yonehara S.; Matsumoto Y.; Shoji M.; Hashizume D.; Koshino H. Angew. Chem., Int. Ed. 2006, 45, 4593. [DOI] [PubMed] [Google Scholar]; i Klussmann M.; Iwamura H.; Mathew S. P.; Wells D. H.; Pandya U.; Armstrong A.; Blackmond D. G. Nature 2006, 441, 621. [DOI] [PubMed] [Google Scholar]
- Robertson M. P.; Miller L. Nature 1995, 375, 772. [DOI] [PubMed] [Google Scholar]
- a Addadi L.; Berkovitch-Yellin Z.; Weissbuch I.; Lahav M.; Leiserowitz L. Top. Stereochem. 1986, 16, 1. [Google Scholar]; b Weissbuch I.; Addadi L.; Leiserowitz L.; Lahav M. J. Am. Chem. Soc. 1988, 110, 561. [Google Scholar]; c Gunn E.; Sours R.; Kaminsky W.; Kahr B. J. Am. Chem. Soc. 2006, 128, 14234. [DOI] [PubMed] [Google Scholar]
- Barker D. L.; Marsh R. E. Acta Crystallogr. 1964, 17, 1581. [Google Scholar]
- Kawasaki T.; Suzuki K.; Hakoda Y.; Soai K. Angew. Chem., Int. Ed. 2008, 47, 496. [DOI] [PubMed] [Google Scholar]
- Jeffrey G. A.; Kinoshita Y. Acta Crystallogr. 1963, 16, 20. [Google Scholar]
- TG/DTA of powdered 1·H2O shows that the first ca. 14% of weight loss occurs, and is completed, in the temperature range 60−80 °C, which corresponds to the release of crystal water. See also Figure S1.
- Kuroda R. Mol. Supramol. Photochem. 2004, 11, 385. [Google Scholar]
- The relationship between the sign of solid-state CD and helicity of cytosine in the crystal lattice was assigned as shown in Figure S4.
- cf.Nery J. D.; Eliash R.; Bolbach G.; Weissbuch I.; Lahav M. Chirality 2007, 19, 612. [DOI] [PubMed] [Google Scholar]
- a Soai K.; Shibata T.; Morioka H.; Choji K. Nature 1995, 378, 767. [Google Scholar]; b Kawasaki T.; Matsumura Y.; Tsutsumi T.; Suzuki K.; Ito M.; Soai K. Science 2009, 324, 492. See also refs (1g) and (9). [DOI] [PubMed] [Google Scholar]
- Reviews:; a Soai K.; Shibata T.; Sato I. Acc. Chem. Res. 2000, 33, 382. [DOI] [PubMed] [Google Scholar]; b Soai K.; Kawasaki T. Top. Curr. Chem. 2008, 284, 1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.