Abstract
Depressive symptoms are frequently elevated following breast cancer diagnosis. The stress generation hypothesis states that people with depression generate stressful events and these stressors lead to subsequent depression. This study tested the stress generation hypothesis over the first 5 years of cancer survivorship. Women with stage II or III breast cancer (N = 113) were accrued. Five mediation models were constructed, one for each year. Each model tested whether stressful events in each year mediated the relationship between depression at the beginning and end of that year. Stress generation was observed in the first 2 years following cancer diagnosis but not from 2 to 5 years after diagnosis. The relationship of depression to future stress in breast cancer patients may be moderated by phase of survivorship. Screening and treatment of depressive symptoms in cancer survivors may need to consider the generation of stressful events.
Keywords: Breast cancer, Survivorship, Stress generation, Depression
Introduction
Depression is often chronic (Maj et al. 1992; Mueller et al. 1999). Chronic depression can be particularly detrimental for cancer survivors. Depressive symptoms tend to be elevated following cancer diagnosis and depression may affect quality of life (Peters and Sellick 2006; Yen et al. 2006) and has been shown to covary with cancer death (Chida et al. 2008; Falagas et al. 2007; Reich et al. 2008). Data show depressive symptoms tend to peak at diagnosis, decline in the next year and stabilize thereafter (Burgess et al. 2005; Thornton et al. 2008), but a subset of patients may be at risk for continued depressive symptoms (Brothers and Andersen 2009; Golden-Kreutz and Andersen 2004; Stanton et al. 2005). Determining the factors sustaining depression in cancer populations is crucial for both psychological and health outcomes. The current study tests the stress generation hypothesis (Hammen 1991) as a factor in chronic depression among breast cancer survivors.
Hammen hypothesized that individuals with chronic depression may promote the occurrence of subsequent stressful events (Hammen 1991). The individual engages in behaviors such as excessively seeking reassurance, which may then lead to stressful events such as interpersonal conflict. The behavior of the individual is creating, albeit unintentionally, stressful events such as conflict with friends and family. These stressful events then worsen depressive symptoms. Hammen originally (1991) reported that women with a diagnosis of recurrent depression (at least two episodes including the current episode with the first episode occurring 8–16 years before the study) reported more stressful events over the subsequent 12 months than the comparison groups of women with bipolar disorder, a medical illness (diabetes or rheumatoid arthritis) or healthy controls. Since that demonstration, stress generation has been observed in adolescents (Cole et al. 2006; Daley et al. 1997; Rudolph et al. 2000; Wingate and Joiner 2004), and adults (Chun et al. 2004; Cui and Vaillant 1997; Davila et al. 1997; Holahan et al. 2005). Also, depressive symptoms generate stress in community samples as well as psychiatric samples (Chun et al. 2004). Research has also shown that depressive symptoms, not just a diagnosis of depression, generate stress (Davila et al. 1997; Jones et al. 2001; Uhrlass and Gibb 2007).
While depression can generate stress, whether depression in cancer patients continues to generate stress over time is unclear. The current study provides a unique opportunity to investigate stress generation during a lengthy stressful event, cancer diagnosis and treatment. This study also provides an opportunity to examine stress generation when most obvious stressors have concluded, i.e. after patients finish treatment. Cancer diagnosis is stressful (Andersen et al. 1989, 1998; Weisman and Worden 1976). Stress from cancer may have at least two potential effects relevant to stress generation. First, the stress from cancer may increase the likelihood of depressive symptoms (Golden-Kreutz and Andersen 2004). If this is the case, stress generation is most likely to occur during the most stressful time (shortly after diagnosis). Alternatively, the stress from cancer may overshadow any effect of elevated depressive symptoms, reducing a stress generation effect. In this alternative case, stress generation would only be present after the stress from cancer has subsided. The current study tests which of these two hypotheses characterize the breast cancer patients during their first 5 years of cancer survivorship. For clinicians helping cancer patients with elevated depressive symptoms, the results of the current study could determine when stress generation should be targeted for those cancer patients with comorbid depressive symptoms. If stress generation does not occur during a specific time in cancer survivorship (for instance, after treatment), treatment of depressive symptoms should not focus on stress generation. Conversely, if stress generation is present, psychological treatment should target stress-generating behaviors to prevent relapse.
We tested for stress generation in the 5 years following surgery for breast cancer. Patients were initially assessed after diagnosis and surgery but before adjuvant treatment. Follow-up assessments occurred at yearly intervals. For each year, depression at the start of the year (initial depression) was used as a predictor of depression at the end of the year (subsequent depression). Reported stressful events that occurred between initial depression and subsequent depression were tested as a mediator of the relationship between initial depression and subsequent depression. The first model tested the presence of stress generation in the year following cancer diagnosis. The second model tested for stress generation following cancer treatment and during recovery from treatment. The subsequent three models tested for stress generation there after.
Methods
Participants and procedures
Patients (N = 227) with an initial diagnosis of breast cancer, Stage II or III, were accrued following surgery but before adjuvant treatment for a randomized clinical trial (RCT) testing the effect of a biobehavioral intervention on stress and ultimately, disease progression (Andersen et al. 2004, 2008). The study was approved by the Ohio State University Institutional Review Board and informed consent was obtained from each participant. Following consent and accrual, women were randomized to either the intervention (n = 114; see Andersen et al. (2009) for description) or the assessment-only arm (n = 113). Women in the intervention arm of the RCT were excluded from the present study due to potential intervention effects and will not be described further. Only patients from the assessment- only arm of the RCT were included in the present study. See Table 1 for participant sociodemographics and disease characteristics. Participants were assessed at baseline and every 12 months for 5 years for a total of 6 data collection points. Trained interviewers conducted in-person assessments.
Table 1.
Descriptive statistics for demographic and disease characteristics for the study sample (n = 113) at the baseline assessment
| Variable | Mean (SD)/% |
|---|---|
| Sociodemographic | |
| Age (years) | 50.58 (10.61) |
| Race (% Caucasian) | 90.3% |
| Education (years) | 14.34 (2.57) |
| Marital Status (% partnered) | 72.6% |
| Family Income (1,000 $/year) | $65 ($84) |
| Prognostic | |
| Stage (II vs. III, % II) | 92.0% |
| Nodes (number positive) | 3.06 (5.27) |
| ER/PR status (% positive) | 68.1% |
| Treatment | |
| Surgery (lumpectomy vs. mastectomy, % mastectomy) | 57.5% |
| Radiation therapy (%; yes) | 52.2% |
| Chemotherapy (% yes) | 86.7% |
Means and standard deviations are reported for continuous variables. Percents are reported for dichotomous variables. ER/PR estrogen receptor/progesterone receptor
Measures
Depression
The 11-item Iowa short form of the Center for Epidemiological Studies Depression Scale (CES-D; Radloff 1977) was used to measure depressive symptoms. Each item is rated on a 3-point scale from 0 = hardly ever or never to 2 = much or most of the time over the previous week. Total scores range from 0 to 22. Higher scores reflect greater depressive symptoms. A score at or above 10 indicates clinically significant depressive symptoms (Andresen et al. 1994). Internal consistency was .73 in this sample, consistent with prior research (Himmelfarb and Murrell 1983; Kohout et al. 1993). Unlike other measures of depressive symptoms, the CES-D is relatively unaffected by physical symptoms and is commonly used in research with medical patients (Devins et al. 1988).
Stressful life events
The stress generation hypothesis specifically states that depression generates stressful events as opposed to perceived stress. The emphasis on stressful events as opposed to perceived stress is a strength of the stress generation hypothesis because perceived stress could be inflated by the depressed person’s negative views (Beck et al. 1979). Stressful life events were measured with the Life Events scale, adapted from the Women’s Health Initiative study (Matthews et al. 1997). Participants indicated if any of five stressful life events occurred during the previous year. The events were death/illness of family/friend, financial difficulty, divorce or break-up of family member or friend, major conflict with children or grandchildren, serious accident/robbery. The total reflects the number of different events reported (range 0–5) with higher scores indicate more frequent life events.
Analytic strategy
Depressive symptoms and stressful life events were examined prior to mediation analyses. The change over time in depressive symptoms and stressful life events was tested using one-way, repeated measures analysis of variance (ANOVA). Also, the percentage of women at or above the clinically significant cutoff of 10 for the CESDshort form (Andresen et al. 1994) was determined.
To test the indirect effect of initial depressive symptoms on subsequent depressive symptoms through generation of stress, mediation analyses were conducted. Mediation tests the indirect effect of one variable (the predictor) on the outcome through a hypothesized third variable (the mediator). The bootstrapping method of mediation analysis was used (Shrout and Bolger 2002). The indirect effect is calculated by multiplying the coefficients of the mediator regressed on the predictor and the outcome regressed on the mediator. To test whether the indirect effect is significantly different from zero, a confidence interval (CI) is constructed from the bootstrapped distribution using the specified alpha level (0.05). If the interval does not contain zero, the indirect effect is significant. Unlike alternative methods of testing mediation (Baron and Kenny 1986), this method tests mediation directly and does not necessarily indicate partial or complete mediation.
A total of five mediation models were constructed and analyzed using AMOS 16.0. Demographic or disease variable controls were specified a priori and were chosen based on the literature showing significant relationships with depression in cancer patients. The five controls used were age (Wenzel et al. 1999), income (Kim et al. 2008), education (Kim et al. 2008), marital status (Cuellar et al. 2003), and surgery type (Kim et al. 2008). In each model, depressive symptoms at baseline, 12, 24, 36 or 48 months were used as the predictor. Depressive symptoms 1 year later (12, 24, 36, 48 or 60 months, respectively) served as outcomes. Stressful events in the interim year (e.g. 12–24 months) were used as the mediator.
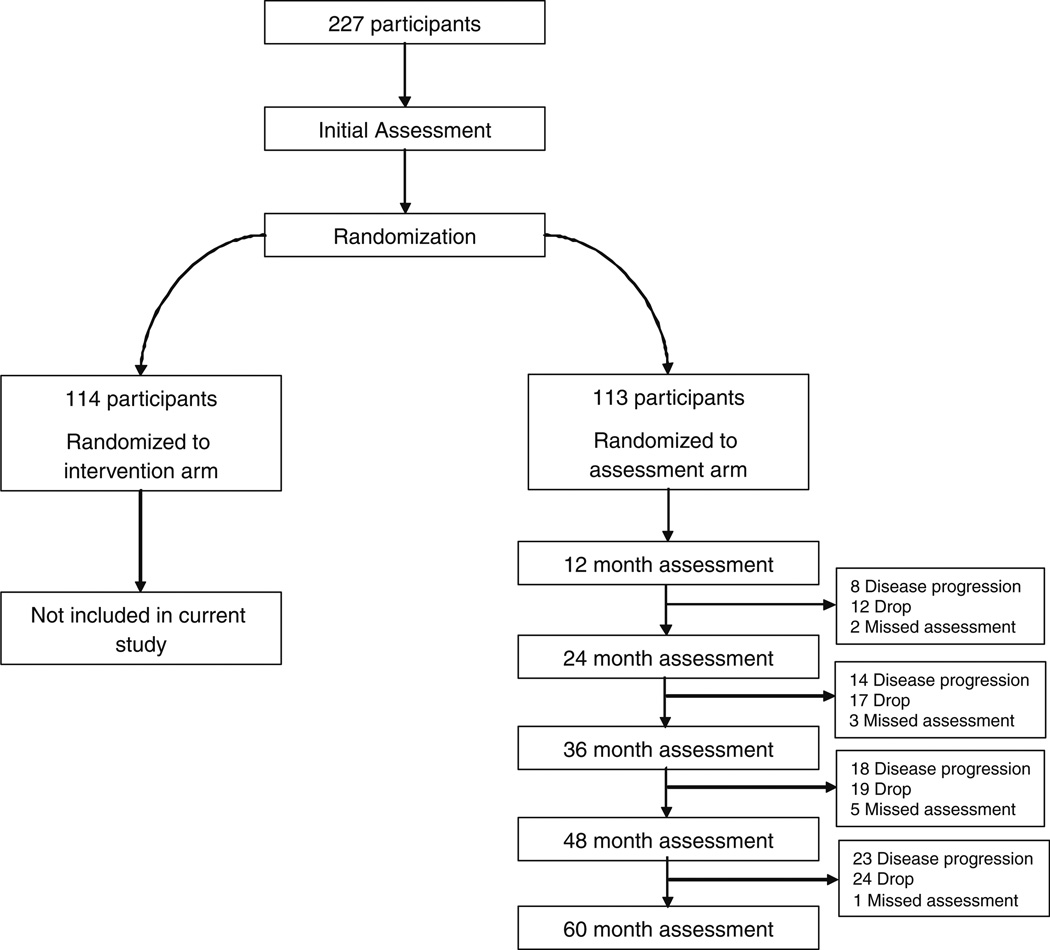
Women were included in analyses if they provided at least one of the following three data points for the particular analysis: initial depression; life events; and subsequent depression. Attrition resulted from three sources: disease progression (recurrence or death); discontinued study participation (drop); and a missed assessment but continued participation in the study. See Fig. 1 for study flow and attrition numbers by reason. All 113 women were included in the 0–12 month analysis. Twenty-two women (19% of total sample) were excluded from the 12–24 month analysis. Thirty-four women (30%) were excluded from the 24–36 month analysis. Forty-two (37%) were excluded from the 36–48 month analysis. Forty-eight (42%) were excluded from the 48–60 month analysis. Missing data were multiple imputed using Lisrel 8.51 in cases of missing one or two of the required data points. For each analysis, the following amount of data was imputed: 0–12 months, 16%; 12–24 months, 11%; 24–36 months, 8%; 36–48 months, 12%; and 48–60 months, 9%. We compared women who discontinued study participation to those continuing in the study on the following variables: age, marital status, income, education, surgery type, baseline depressive symptoms and baseline stressful events. Women who discontinued study participation by the second year had significantly higher baseline depressive symptoms [P < .05; mean for drops = 7.53 (SD = 3.18); mean for those continuing = 5.44 (SD = 3.37)] and fewer years of education [P < .05; mean for drops = 13.24 (SD = 2.97); mean for those continuing = 14.66 (SD = 2.47)] than those continuing participation. No other significant differences were noted.
Fig. 1.
Study flow diagram. 227 participants were randomized to the treatment or assessment arm (control group). Only women in the assessment arm were included in the current study. Reasons for exclusion from mediation analyses are provided
Results
Depressive symptoms were highest at baseline and declined following baseline (F(5,250) = 2.877, p = 0.015, partial η2 = 0.054). At baseline, 19.5% of women had clinically significant depressive symptoms using the CESD cutoff (Andresen et al. 1994). At each subsequent assessment (12, 24, 36, 48 and 60 months) 10.5, 11.5, 10.0, 11.9 and 11.9% of women had clinically significant depressive symptoms. The percentages of women in our sample with significant depressive symptoms was slightly lower than previous reports (23–29%) in other samples of cancer patients (Bower et al. 2000; Roscoe et al. 2005). The number of stressful events remained stable over the 5-year period with no significant change [F(5,250) = 0.511, P = 0.767, partial η2 = 0.010]. Participants tended to report at least 1 of the 5 stressful events each year (see Table 2 for means and standard deviations). The number of reported events is less than reported in other studies of cancer patients (Butler et al. 1999; Kornblith et al. 2001), however, this could have resulted from screening for only 5 events whereas other studies screen for 37–57 events. See Table 2 for correlations between depressive symptoms and stressful events and means of depressive symptoms and stressful events.
Table 2.
Means, standard deviations, and correlations between depressive symptoms and stressful events
| Mean (SD) | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CESD | |||||||||||||
| 1. Baseline | 5.98 (3.46) | 1.00 | |||||||||||
| 2. 12 Months | 4.09 (3.38) | .46** | 1.00 | ||||||||||
| 3. 24 Months | 4.22 (3.96) | .40** | .64** | 1.00 | |||||||||
| 4. 36 Months | 4.04 (4.09) | .41** | .51** | .61** | 1.00 | ||||||||
| 5. 48 Months | 4.03 (4.27) | .44** | .60** | .67** | .59** | 1.00 | |||||||
| 6. 60 Months | 4.04 (4.00) | .43** | .64** | .67** | .65** | .75** | 1.00 | ||||||
| SLE | |||||||||||||
| 7. Baseline | 1.27 (1.12) | .17* | .20** | .19* | .30** | .29** | .32** | 1.00 | |||||
| 8. 12 Months | 1.20 (0.99) | .24** | .45** | .34** | .42** | .26** | .41** | .36** | 1.00 | ||||
| 9. 24 Months | 1.38 (1.07) | .21** | .39** | .41** | .36** | .40** | .48** | .29** | .42** | 1.00 | |||
| 10. 36 Months | 1.29 (0.95) | −.05 | .07 | .16 | .28** | .15 | .17 | .21* | .26** | .36** | 1.00 | ||
| 11. 48 Months | 1.10 (0.98) | .25** | .20* | .23** | .24** | .31** | .35** | .22* | .30** | .37** | .24** | 1.00 | |
| 12. 60 Months | 1.36 (1.16) | .02 | .18 | .08 | .06 | .21* | .27** | .35** | .28** | .23** | .09 | .01 | 1.00 |
CESD center for epidemiological studies depression scale, SLE stressful life events
p<0.05,
p<.01
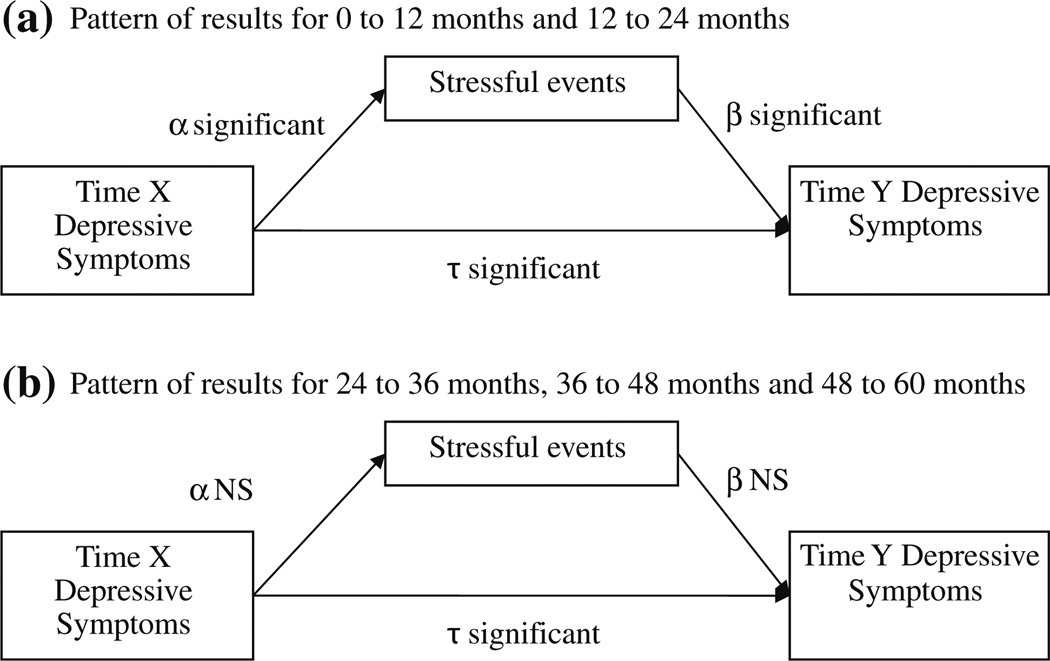
To test the consistency of the stress generation effect, five mediation models were constructed. Direct effects are reported in Table 3 and indirect effects (mediation effects) are reported in Table 4. Stressful events significantly mediated the effect of initial depressive symptoms on subsequent depressive symptoms for the first 2 years. From baseline to 12 months later, stressful events significantly mediated the effect of baseline depressive symptoms on 12-month depressive symptoms (see Table 4) supporting a stress generation effect. Stressful events from 12 to 24 months also significantly mediated the effect of 12-month depressive symptoms on 24-month depressive symptoms (see Table 4) also suggesting a stress generation effect. However, both direct effects from initial depressive symptoms to subsequent depressive symptoms were still significant (See Table 3 and Fig. 2). In Fig. 2, Time X refers to depressive symptoms at the beginning of the interval (baseline, 12, 24, 36, or 48 months) and Time Y refers to depressive symptoms at the end of the interval (12, 24, 36, 48 or 60 months).
Table 3.
Standardized direct effects. Arrows indicate the first variable predicting the second variable
| Path | Direct Effect | Bootstrap SE | p-value |
|---|---|---|---|
| Model I: Baseline to 12 months | |||
| α: Baseline CESD → SLE | 0.289* | 0.090 | 0.002 |
| β: SLE → 12 month CESD | 0.302* | 0.104 | 0.011 |
| τ: Baseline CESD → 12 month CESD | 0.396* | 0.082 | 0.002 |
| Model II: 12–24 months | |||
| α: 12 Month CESD → SLE | 0.363* | 0.108 | 0.002 |
| β: SLE → 24 month CESD | 0.218* | 0.104 | 0.040 |
| τ: 12 Month CESD → 24 month CESD | 0.635* | 0.083 | 0.002 |
| Model III: 24–36 months | |||
| α: 24 Month CESD → SLE | 0.122 | 0.117 | 0.269 |
| β: SLE → 36 month CESD | 0.064 | 0.101 | 0.465 |
| τ: 24 Month CESD → 36 month CESD | 0.577* | 0.080 | 0.002 |
| Model IV: 36–48 months | |||
| α: 36 Month CESD → SLE | 0.249 | 0.120 | 0.067 |
| β: SLE → 48 month CESD | 0.142 | 0.092 | 0.133 |
| τ: 36 Month CESD → 48 month CESD | 0.659* | 0.098 | 0.003 |
| Model V: 48–60 months | |||
| α: 48 Month CESD → SLE | 0.153 | 0.152 | 0.342 |
| β: SLE → 60 month CESD | 0.075 | 0.078 | 0.326 |
| τ: 48 Month CESD → 60 month CESD | 0.748* | 0.119 | 0.005 |
CESD center for epidemiological studies depression scale, SLE stressful life events. Controls in all models were age, income, education, marital status and surgery type.
p<0.05
Table 4.
Standardized indirect effects and confidence intervals
| Path | Indirect effect | Bias-corrected 95% CI |
|---|---|---|
| Baseline CESD→ SLE → 12 month CESD | 0.087* | 0.023, 0.215 |
| 12 Month CESD → SLE → 24 month CESD | 0.079* | 0.010, 0.217 |
| 24 Month CESD → SLE → 36 month CESD | 0.008 | −0.011, 0.097 |
| 36 Month CESD → SLE → 48 month CESD | 0.035 | −0.003, 0.134 |
| 48 Month CESD → SLE → 60 month CESD | 0.011 | −0.009, 0.089 |
Confidence intervals that do not include zero indicate a significant indirect effect
Significant effects indicate a stress generation effect. Arrows indicate the first variable predicting the second variable and the second variable predicting the third variable. CESD center for epidemiological studies depression scale, SLE stressful life events. Controls in all models were age, income, education, marital status and surgery type.
p<0.05
Fig. 2.
Summary of path diagrams of direct effects. α shows the pattern of significant direct effects for baseline through 24 months. β shows the pattern of significant direct effects for 24 months through 60 months. Time X refers to depressive symptoms at the initial time point. Time Y refers to the subsequent time point. NS Not significant. Controls in all models were age, income, education, marital status and surgery type
After the first 2 years, indirect effects were no longer significant (see Table 4), indicating that the stress generation effect was no longer evident after 24 months. The direct effects of initial depressive symptoms on stressful events were not significant, indicating that depressive symptoms did not predict more stressful events (See Table 3). The direct effects of stressful events on subsequent depressive symptoms were not significant, indicating that stressful events were not related to subsequent depressive symptoms (see Table 3). The direct effects of initial depressive symptoms on subsequent depressive symptoms were significant in all models (see Table 3 and Fig. 2).
Discussion
This study examined the stress generation hypothesis over 5 years and during different phases of the cancer experience. A stress generation effect was found in women with breast cancer, up to 24 months after surgery. After 24 months, the stress generation effect was no longer observed and stressful events did not mediate the relationship between initial depressive symptoms and subsequent depressive symptoms. Overall, this study supported the stress generation hypothesis by showing that when depressive symptoms were highest (closer to diagnosis), a stress generation effect was observed. That the effect was present only in the first 2 years after diagnosis suggests that stress generation may only be a factor in depressive symptoms during the first phases of cancer survivorship. The results supported our first hypothesis, suggesting that the added stress of a cancer diagnosis enhanced the stress generation effect.
The stress generation effect could have ceased after 24 months due to low depressive symptoms and different phases of cancer survivorship. First, consistent with other data (Burgess et al. 2005), depressive symptoms decreased during the first year after diagnosis and stabilized in later years. Some research suggests stress generation becomes stronger after depressive symptoms increase (Chun et al. 2004). By 24 months, depressive symptoms had declined and may not have been sufficient to generate stress. A possible explanation for a lack of stress generation after 24 months and the decrease in depressive symptoms could be changes occurring in cancer survivorship. Cancer survivorship has been divided into three phases (Mullan 1985; Stanton et al. 2005). The first phase, called acute survival, occurs during cancer diagnosis and treatment. The second, termed the re-entry phase (Stanton et al. 2005) or extended survival (Mullan 1985), begins at the end of treatment with the survivor returning to normal routines yet coping with fears of cancer recurrence. The third phase, permanent survival, is thought to occur there after, several years after diagnosis with a substantial reduction in recurrence fears (Mullan 1985). Patients in the current study had completed adjuvant treatment by 12 months, which constitutes acute survival. The second year (12–24 months) would constitute the re-entry phase, adding additional stress and making stress generation more likely to occur. This suggests a necessary amount of “background” stress may be required for stress generation. After 2 years, survivors may have been entering the third phase with lower cancer stress making stress generation less likely.
The study results have implications for the literature on stress generation. The majority of past studies testing the stress generation hypothesis only examined depressive symptoms at one time point and stressful events 1 year later (Chun et al. 2004; Daley et al. 1997; Davila et al. 1997; Wingate and Joiner 2004). These studies did not show whether depression continues to generate stress over multiple depressive episodes or across several years. Only one study of adolescents examined depressive symptoms and stressful events at multiple time points over 6 years (Cole et al. 2006). Future research should determine when changes in stress generation, similar to the results of the current study, occur in physically health populations or other samples with illness.
The results from the current study have potential clinical implications. First, patients may not mention and physicians often do not recognize depressive symptoms (Passik et al. 1998). Instead patients may mention new stressors. Screening for depressive symptoms in cancer patients may also need to consider new stressful events as a correlate of depressive symptoms. Second, in addition to screening concerns, clinicians treating cancer patients for elevated depressive symptoms may need to target patient behaviors that generate stress. However, targeting stress generation may only be important during the first 2 years after diagnosis and surgery, as this study suggests stress generation may cease thereafter.
The results should be considered within the assets and limitations of the data. The longitudinal design of this study was an asset. The assessments after surgery but before adjuvant treatment and the years following provided for the examination of the stress generation hypothesis across phases of cancer survivorship. However, the requirements of the trial may limit generalizability. First, we do not know if these results apply to psychiatric samples who have variable courses on depressive disorder. Also, a cancer diagnosis may differ from other chronic illnesses in ways that differentially affect stress generation. Second, the measure of life events may not have captured all stressful life events thereby limiting power. Third, women who discontinued participation by 24 months had significantly higher baseline depressive symptoms. This could have led to lower power and no stress generation effect after the second year.
Chronic depressive symptoms can be a serious problem for cancer survivors, affecting both psychological (Peters and Sellick 2006; Yen et al. 2006) and health outcomes (Chida et al. 2008; Falagas et al. 2007). The current study applied the stress generation hypothesis, a model from the psychopathology literature, to study chronic depressive symptoms in cancer patients. While future research is needed, results of this study have important implications for the screening and treatment of depressive symptoms in cancer patients. Interventions for stress generation may be needed. Future research should also determine whether psychological interventions can interrupt the stress generation process and thereby reduce chronic depressive symptoms.
Acknowledgments
The authors would like to thank the patients for their time and participation in the study. This research was supported by the National Cancer Institute (R01 CA92704; K05 CA098133); the American Cancer Society (RSGPB-03-248-01-PBP; PBR-89), US Army Medical Research and Development Command (DAMD17-94- J-4165; DAMD17-96-1-6294; DAMD17-97-1-7062), and the National Institutes of Health (M01-RR0034). The authors would also like to thank the Stress and Immunity Cancer Project team at the Ohio State University.
Contributor Information
Salene M. Wu, Email: wu.582@osu.edu.
Barbara L. Andersen, Email: salenewu@gmail.com.
References
- Andersen BL, Anderson B, DeProsse C. Controlled prospective longitudinal study of women with cancer: Ii. Psychological outcomes. Journal of Consulting and Clinical Psychology. 1989;57(6):692–697. doi: 10.1037//0022-006x.57.6.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Farrar WB, Golden-Kreutz DM, Glaser R, Emery CF, Crespin TR, et al. Psychological, behavioral, and immune changes after a psychological intervention: A clinical trial. Journal of Clinical Oncology. 2004;22(17):3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Farrar WB, Golden-Kreutz D, Kutz LA, MacCallum R, Courtney ME, et al. Stress and immune responses after surgical treatment for regional breast cancer. Journal of the National Cancer Institute. 1998;90(1):30–36. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Golden-Kreutz DM, Emery CF, Thiel DL. Biobehavioral intervention for cancer stress: Conceptualization, components, and intervention strategies. Cognitive and Behavioral Practice. 2009 Available online 4/3/09. [Google Scholar]
- Andersen BL, Yang H-C, Farrar WB, Golden-Kreutz DM, Emery CF, Thornton LM, et al. Psychologic intervention improves survival for breast cancer patients: A randomized clinical trial. Cancer. 2008;113:3450–3458. doi: 10.1002/cncr.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen EM, Carter WB, Malmgren JA, Patrick DL. Screening for depression in well older adults: Evaluation of a short form of the ces-d. American Journal of Preventive Medicine. 1994;10(2):77–84. [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guildford Press; 1979. [Google Scholar]
- Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. Journal of Clinical Oncology. 2000;18(4):743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- Brothers BM, Andersen BL. Hopelessness as a predictor of depressive symptoms for cancer patients coping with recurrence. Psycho-Oncology. 2009;18:267–275. doi: 10.1002/pon.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: Five year observational cohort study. BMJ. 2005;330(7493):702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler LD, Koopman C, Classen C, Spiegel D. Traumatic stress, life events, and emotional support in women with metastatic breast cancer: Cancer-related traumatic stress symptoms associated with past and current stressors. Health Psychology. 1999;18(6):555–560. doi: 10.1037//0278-6133.18.6.555. [DOI] [PubMed] [Google Scholar]
- Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nature Clinical Practice Oncology. 2008;5(8):466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- Chun C-A, Cronkite RC, Moos RH. Stress generation in depressed patients and community controls. Journal of Social & Clinical Psychology. 2004;23(3):390–412. [Google Scholar]
- Cole DA, Nolen-Hoeksema S, Girgus J, Paul G. Stress exposure and stress generation in child and adolescent depression: A latent trait-state-error approach to longitudinal analyses. Journal of Abnormal Psychology. 2006;115(1):40–51. doi: 10.1037/0021-843X.115.1.40. [DOI] [PubMed] [Google Scholar]
- Cuellar N, Chochran S, Ladner C, Mercier B, Townsend A, Harbaugh B, et al. Depression and the use of conventional and nonconventional interventions by rural patients. Journal of the American Psychiatric Nurses Association. 2003;9:151–158. [Google Scholar]
- Cui X-J, Vaillant GE. Does depression generate negative life events? Journal of Nervous and Mental Disease. 1997;185(3):145–150. doi: 10.1097/00005053-199703000-00003. [DOI] [PubMed] [Google Scholar]
- Daley SE, Hammen C, Burge D, Davila J, Paley B, Lindberg N, et al. Predictors of the generation of episodic stress: A longitudinal study of late adolescent women. Journal of Abnormal Psychology. 1997;106(2):251–259. doi: 10.1037//0021-843x.106.2.251. [DOI] [PubMed] [Google Scholar]
- Davila J, Bradbury TN, Cohan CL, Tochluk S. Marital functioning and depressive symptoms: Evidence for a stress generation model. Journal of Personality and Social Psychology. 1997;73(4):849–861. doi: 10.1037//0022-3514.73.4.849. [DOI] [PubMed] [Google Scholar]
- Devins GM, Orme CM, Costello CG, Binik YM, et al. Measuring depressive symptoms in illness populations: Psychometric properties of the center for epidemiologic studies depression (ces-d) scale. Psychology & Health. 1988;2(2):139–156. [Google Scholar]
- Falagas ME, Zarkadoulia EA, Ioannidou EN, Peppas G, Christodoulou C, Rafailidis PI. The effect of psychosocial factors on breast cancer outcome: A systematic review. Breast Cancer Research. 2007;9(4):R44. doi: 10.1186/bcr1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden-Kreutz DM, Andersen BL. Depressive symptoms after breast cancer surgery: Relationships with global, cancer-related, and life event stress. Psycho-oncology. 2004;13(3):211–220. doi: 10.1002/pon.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology. 1991;100(4):555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- Himmelfarb S, Murrell SA. Reliability and validity of five mental health scales in older persons. Journal of Gerontology. 1983;38(3):333–339. doi: 10.1093/geronj/38.3.333. [DOI] [PubMed] [Google Scholar]
- Holahan CJ, Moos RH, Holahan CK, Brennan PL, Schutte KK. Stress generation, avoidance coping, and depressive symptoms: A 10-year model. Journal of Consulting and Clinical Psychology. 2005;73(4):658–666. doi: 10.1037/0022-006X.73.4.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DJ, Beach SRH, Forehand R. Stress generation in intact community families: Depressive symptoms, perceived family relationship stress, and implications for adolescent adjustment. Journal of Social and Personal Relationships. 2001;18(4):443–462. [Google Scholar]
- Kim SH, Son BH, Hwang SY, Han W, Yang JH, Lee S, et al. Fatigue and depression in disease-free breast cancer survivors: Prevalence, correlates, and association with quality of life. Journal of Pain and Symptom Management. 2008;35(6):644–655. doi: 10.1016/j.jpainsymman.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the ces-d depression symptoms index. Journal of Aging and Health. 1993;5(2):179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- Kornblith AB, Herndon JE, II, Zuckerman E, Viscoli CM, Horwitz RI, Cooper MR, et al. Social support as a buffer to the psychological impact of stressful life events in women with breast cancer. Cancer. 2001;91(2):443–454. doi: 10.1002/1097-0142(20010115)91:2<443::aid-cncr1020>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Maj M, Veltro F, Pirozzi R, Lobrace S, et al. Pattern of recurrence of illness after recovery from an episode of major depression: A prospective study. American Journal of Psychiatry. 1992;149(6):795–800. doi: 10.1176/ajp.149.6.795. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Shumaker SA, Bowen DJ, Langer RD, Hunt JR, Kaplan RM, et al. Women’s health initiative. Why now? What is it? What’s new? American Psychologist. 1997;52(2):101–116. doi: 10.1037//0003-066x.52.2.101. [DOI] [PubMed] [Google Scholar]
- Mueller TI, Leon AC, Keller MB, Solomon DA, Endicott J, Coryell W, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. American Journal of Psychiatry. 1999;156(7):1000–1006. doi: 10.1176/ajp.156.7.1000. [DOI] [PubMed] [Google Scholar]
- Mullan F. Seasons of survival: Reflections of a physician with cancer. New England Journal of Medicine. 1985;313(4):270–273. doi: 10.1056/NEJM198507253130421. [DOI] [PubMed] [Google Scholar]
- Passik SD, Dugan W, McDonald MV, Rosenfeld B, Theobald DE, Edgerton S. Oncologists’ recognition of depression in their patients with cancer. Journal of Clinical Oncology. 1998;16(4):1594–1600. doi: 10.1200/JCO.1998.16.4.1594. [DOI] [PubMed] [Google Scholar]
- Peters L, Sellick K. Quality of life of cancer patients receiving inpatient and home-based palliative care. Journal of Advanced Nursing. 2006;53(5):524–533. doi: 10.1111/j.1365-2648.2006.03754.x. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The ces-d scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Reich M, Lesur A, Perdrizet-Chevallier C. Depression, quality of life and breast cancer: A review of the literature. Breast Cancer Research and Treatment. 2008;110(1):9–17. doi: 10.1007/s10549-007-9706-5. [DOI] [PubMed] [Google Scholar]
- Roscoe JA, Morrow GR, Hickok JT, Mustian KM, Griggs JJ, Matteson SE, et al. Effect of paroxetine hydrocholoride (paxil) on fatigue and depression in breaset cancer patients receiving chemotherapy. Breast Cancer Research and Treatment. 2005;89(3):243–249. doi: 10.1007/s10549-004-2175-1. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C, Burge D, Lindberg N, Herzberg D, Daley SE. Toward an interpersonal life-stress model of depression: The developmental context of stress generation. Development and Psychopathology. 2000;12(2):215–234. doi: 10.1017/s0954579400002066. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychol Methods. 2002;7(4):422–445. [PubMed] [Google Scholar]
- Stanton AL, Ganz PA, Rowland JH, Meyerowitz BE, Krupnick JL, Sears SR. Promoting adjustment after treatment for cancer. Cancer. 2005;104(11 Suppl):2608–2613. doi: 10.1002/cncr.21246. [DOI] [PubMed] [Google Scholar]
- Thornton LM, Carson WE, I II, Shapiro CL, Farrar WB, Andersen BL. Delayed emotional recovery after taxane-based chemotherapy. Cancer. 2008;113(3):638–647. doi: 10.1002/cncr.23589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrlass DJ, Gibb BE. Childhood emotional maltreatment and the stress generation model of depression. Journal of Social & Clinical Psychology. 2007;26(1):119–130. [Google Scholar]
- Weisman A, Worden J. The existential plight in cancer: Significance of the first 100 days. International Journal of Psychiatry in Medicine. 1976;7(1):1–15. doi: 10.2190/uq2g-ugv1-3ppc-6387. [DOI] [PubMed] [Google Scholar]
- Wenzel LB, Fairclough DL, Brady MJ, Cella D, Garrett KM, Kluhsman BC, et al. Age-related differences in the quality of life of breast carcinoma patients after treatment. Cancer. 1999;86(9):1768–1774. [PubMed] [Google Scholar]
- Wingate LR, Joiner TE. Depression-related stress generation: A longitudinal study of black adolescents. Behavior Therapy. 2004;35(2):247–261. [Google Scholar]
- Yen J-Y, Ko C-H, Yen C-F, Yang M-J, Wu C-Y, Juan C-H, et al. Quality of life, depression, and stress in breast cancer women outpatients receiving active therapy in Taiwan. Psychiatry and Clinical Neurosciences. 2006;60(2):147–153. doi: 10.1111/j.1440-1819.2006.01479.x. [DOI] [PubMed] [Google Scholar]