Abstract
We previously reported that tumor necrosis factor-α (TNF-α) and Fas receptor induce acute cellular injury, tissue damage, and motor and cognitive deficits after controlled cortical impact (CCI) in mice (Bermpohl et al. 2007); however, the TNF receptors (TNFR) involved are unknown. Using a CCI model and novel mutant mice deficient in TNFR1/Fas, TNFR2/Fas, or TNFR1/TNFR2/Fas, we tested the hypothesis that the combination of TNFR2/Fas is protective, whereas TNFR1/Fas is detrimental after CCI. Uninjured knockout (KO) mice showed no differences in baseline physiological variables or motor or cognitive function. Following CCI, mice deficient in TNFR2/Fas had worse post-injury motor and Morris water maze (MWM) performance than wild-type (WT) mice (p < 0.05 group effect for wire grip score and MWM performance by repeated measures ANOVA). No differences in motor or cognitive outcome were observed in TNFR1/Fas KO, or in TNFR2 or TNFR1 single KO mice, versus WT mice. Additionally, no differences in propidium iodide (PI)-positive cells (at 6 h) or lesion size (at 14 days) were observed between WT and TNFR1/Fas or TNFR2/Fas KO mice. Somewhat surprisingly, mice deficient in TNFR1/TNFR2/Fas also had PI-positive cells, lesion size, and motor and MWM deficits similar to those of WT mice. These data suggest a protective role for TNFR2/Fas in the pathogenesis of TBI. Further studies are needed to determine whether direct or indirect effects of TNFR1 deletion in TNFR2/Fas KO mice mediate improved functional outcome in TNFR1/TNFR2/Fas KO mice after CCI.
Key words: controlled cortical impact, gene knockout, inflammation, mice, tumor necrosis factor, traumatic brain injury
Introduction
Tumor necrosis factor-α (TNF) and Fas receptor are ligand/receptors of the tumor necrosis factor receptor superfamily (Locksley et al., 2001). TNF signal transduction occurs through two known cognate transmembrane glycoprotein receptors. Tumor necrosis factor receptor-1 (TNFR1), a death receptor by virtue of a cytosolic death domain sequence, is ubiquitously expressed and binds soluble as well as transmembrane TNF. TNF binding to TNFR1 may promote assembly of a TNF-TNFR1-TRADD death-inducing signaling complex (DISC), which initiates apoptotic cell death via activation of caspases, or programmed necrosis through activation of RIPK1 (Lin et al., 2004). On the other hand, TNFR1 signaling can be neuroprotective by a number of mechanisms, including NF-κB gene upregulation of proteins associated with calcium homeostasis (Cheng et al., 1994), free radical inhibition (Bruce-Keller et al., 1999), and apoptosis inhibitors, among others (Beg and Baltimore, 1996; Stehlik et al., 1998; Thompson et al., 2004). TNFR2 is expressed mainly on endothelium, microglia, and other immune cells, and a subset of neurons (McCoy et al., 2006). TNFR2 binds specifically to transmembrane TNF, activates downstream signaling pathways shared by TNFR1, and additionally modulates TNFR1 signaling at the receptor level (McCoy and Tansey, 2008). Although TNFR2 is not a death receptor, TNFR2 has important regulatory functions that may result in augmentation or suppression of TNFR1 signaling (Carpentier et al., 2004; Fotin-Mleczek et al., 2002).
A number of studies suggest that TNF/Fas signaling contributes to outcome after experimental stroke (Martin-Villalba et al., 2001), spinal cord injury (Kim et al., 2001), and traumatic brain injury (TBI; Longhi et al., 2008; Lotocki et al., 2004, 2006). We previously reported that mice lacking TNF and functional Fas receptor have decreased histopathology and functional deficits after controlled cortical impact (CCI; Bermpohl et al., 2007). That study showed that TNF and Fas mediate cell death and functional outcome in a redundant fashion; however, the exact TNFR combinations involved with Fas are unknown. Because TNF-α signals through TNFR1 and TNFR2, it is possible that either of these receptors (in combination with Fas) mediates some or all of the detrimental effects of TNF/Fas signaling after TBI. The goal of the current study was to identify TNFRs that contribute to functional outcome after CCI. To address this question we generated novel mutant mice deficient in TNFR1/Fas, TNFR2/Fas, and TNFR1/TNFR2/Fas, and examined the genotype/phenotype effects of each in a CCI model. Because TNFR1 and Fas are both death receptors, we hypothesized that the combination of TNFR1/Fas knockout would be protective, whereas TNFR2/Fas would have detrimental effects on histopathological and functional outcome after CCI. The data support the hypothesis for TNFR2/Fas, and support a possible detrimental role for TNFR1 in TNFR2/Fas KO mice.
Methods
In all studies, CCI was performed and data were obtained by experimenters blinded to study group.
Controlled cortical impact
The CCI model was used as previously described (Bermpohl et al., 2006). The trauma protocol was approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee. Mice (8–16 weeks old) were anesthetized with 4% isoflurane (Anaquest, Memphis, TN) in 70% nitrous oxide and the balance oxygen using a Fluotec 3 vaporizer (Colonial Medical, Amherst, NH) and positioned in a stereotaxic frame. Anesthesia was maintained using 2–3% isoflurane. A 5-mm craniotomy was made using a portable drill and trephine over the left parieto-temporal cortex, and the bone flap was removed. Mice were then subjected to CCI using a pneumatic cylinder with a 3-mm flat-tip impounder, velocity 6 m/sec, depth of 0.6 mm, and 100-msec impact duration. The bone flap was discarded and the scalp was sutured closed. The mice were returned to their cages to recover from anesthesia, and were generally able to ambulate within 15 min.
Knockout mice
The mice were housed in a pathogen-free facility in a 12-h day/night cycle, and treated humanely in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice lacking both TNFR1 and Fas genes (R1/Fas KO) were generated on a C57BL/6 background from TNFR1-knockout mice (stock #002818; Jackson Laboratories, Bar Harbor, ME), and Fas knockout mice (stock #003233; Jackson Laboratories). Mice lacking both TNFR2 and functional Fas genes (R2/Fas KO) were generated on a C57BL/6 background from TNFR2-knockout mice (stock #002620; Jackson Laboratories), and Fas-knockout mice. TNFR1/TNFR2/Fas triple-knockout mice were generated by crossing R1/Fas KO- and R2/Fas-knockout mice. Knockout mice were genotyped using PCR primers and protocols recommended by Jackson Laboratories, and identified by the presence of the neomycin resistance cassette, and an absence of TNFR1 and/or TNFR2 and/or Fas wild-type alleles. Age-matched male mice were used for behavioral experiments. Since Jax C57BL/6 mice were used as controls, the high degree of inbreeding (≥10 backcrosses into C57BL/6) in each of the knockout strains ensures as uniform a genetic background as possible for all of the strains in the experiments.
Genotyping protocols
Primers oIMR0448, oIMR0449, and oIMR0450 were used to determine the presence of neomycin cassette (Neo) and TNFR1 wild-type (WT) allele. The sequences of the primers are oIMR0448: 5' TgT gAA AAg ggC ACC TTT ACg gC 3'; oIMR0449: 5' ggC TgC AgT CCA CgC ACT gg 3'; oIMR0745: 5' gCA CAg AAA gCA TgA TCC g 3'; and oIMR0450: 5' ATT CgC CAA TgA CAA gAC gCT gg 3'. oIMR0448 and oIMR0449 amplify a 300-bp DNA fragment from the Neo cassette, whereas oIMR0449 and oIMR0450 amplify a 470-bp DNA fragment from the TNFR1 WT allele.
The primers oIMR0338, oIMR0339, oIMR0340, and oIMR0341 were used to determine the presence of the Neo cassette and TNFR2 WT allele. The sequences of the primers are oIMR0338: 5' CCT CTC ATg CTg TCC Cgg AAT 3'; oIMR0339: 5' AgC TCC Agg CAC AAg ggC ggg 3'; oIMR0340: 5' Cgg TTC TTT TTg TCA AgA C 3'; and oIMR0341: 5' ATC CTC gCC gTC ggg CAT gC3'. oIMR0338 and oIMR0339 amplify a 200-bp DNA fragment from the TNFR1 WT allele, whereas oIMR0340 and oIMR0341 amplify a 400-bp DNA fragment from the Neo cassette. The primers oIMR1360, oIMR1361, oIMR158, and oIMR159 were used to identify the Fas WT allele and the Neo cassette, respectively. The sequences of the primers are oIMR1360: 5' TgTCCATCAAtggAAggTCT 3'; oIMR1361: 5' gCTTgAgTAAATACATCCCg 3'; oIMR158: 5' CTGAATGAACTGCAGGACGA 3'; and oIMR159: 5' ATACT TTCTCGGCAGGAGCA 3'. oIMR1360 and oIMR1361 amplify a 700-bp DNA product from the Fas WT allele, whereas oIMR158 and oIMR159 amplify a 172-bp DNA fragment on the Neo cassette.
Assessment of post-injury tissue damage
Morphometric image analysis (MCID Imaging Research Inc., St. Catherines, Ontario, Canada) was used to determine lesion volume and brain tissue loss after CCI. The mice were deeply anesthetized with isoflurane, decapitated, and the brains were removed and frozen in nitrogen vapor. Coronal brain sections (12 μm) were cut on a cryostat at 0.5-mm intervals from anterior to posterior and mounted on poly-l-lysine-coated slides. The sections were stained with hematoxylin and the area of the lesion was quantitated using image analysis according to the method of Cavalieri (Bermpohl et al., 2006). Lesion volume was expressed in cubic millimeters. Because lesion size in the CCI model can vary significantly over time, even in experiments performed within the same laboratory (Bermpohl et al., 2006; Clark et al., 2000), mutant mice were always subjected to CCI concomitantly with WT controls.
Evaluation of motor function
Vestibulomotor function was assessed using a wire-grip test (Bermpohl et al., 2006). The mice were placed on a metal wire (45 cm long) suspended 45 cm above protective padding and were allowed to traverse the wire for 60 sec. The latency that a mouse remained on the wire within a 60-sec interval was measured, and a wire-grip score was quantitated using a five-point scale. Testing was performed in triplicate and an average value was calculated for each mouse on each test day.
Assessment of spatial memory
The Morris water maze (MWM) was used to evaluate spatial memory performance as previously described (Bermpohl et al., 2006) with minor modifications. The MWM test was performed beginning at 8–10 days after CCI. The apparatus consisted of a white pool (90 cm in diameter and 60 cm deep) filled with water to a depth of 29 cm. Several highly visible cues were located on the walls of each of the four quadrants, as well as the walls of the room. The water temperature was maintained at 21–25°C. A clear acrylic glass goal platform 5 cm in diameter was positioned 0.5 cm below the water's surface approximately 15 cm from the southwest wall. Each mouse was subjected to a series of 1–2 trials per day. For each trial, the mice were randomized to one of four starting locations (north, south, east, and west), and placed in the pool facing the wall. The mice were given a maximum of 60 sec (for naïve animals) or 90 sec (for injured animals) to find the submerged platform. If the mouse failed to reach the platform in the allotted time, it was placed on the platform by the experimenter and allowed to remain there for 10 sec. The mice were placed in a warming chamber for at least 4 min between trials. To control for possible differences in visual acuity or sensorimotor function between groups, two trials were performed using a visible platform raised 0.5 cm above the surface of the water. Performance in the MWM was quantitated by latency to find the platform.
Administration of propidium iodide and preparation of tissue sections for cell counting
Propidium iodide (PI; 10 mg/mL, Sigma-Aldrich, St. Louis, MO) diluted in PBS was administered by intraperitoneal injection (1 mg/kg) in a total volume of not more than 100 μL to the mice at 5 h after CCI. The mice were killed 1 h after PI injection (6 h after CCI), and the brains were frozen in nitrogen vapor. Cryostat brain sections (12 um) were cut at 150- to 200-μm intervals from the anterior to posterior hippocampus and placed on poly-L-lysine-coated slides, and stored at −80°C.
Assessment of propidium iodide–positive cell counts
We chose regions of interest for cell counts in contused cortex based on a previous report showing robust cell death and plasmalemma permeability of cells in perilesional cortex in our CCI model (Bermpohl et al., 2007). Cortical regions of interest were 200× microscopic fields (1100 × 1100 um) at the medial and lateral edges of the contusion. Brain sections were photographed using a Nikon Eclipse T300 fluorescence microscope (Tokyo, Japan), with excitation/emission filters of 568/585 nm. PI-positive cells were counted in a total of three brain sections located within the center of the contusion (bregma −1.90 to −2.70), and separated by at least 150 μm, for a total of nine 200× cortical fields assessed per animal. Cell count data for each mouse were the average of the nine cortical brain fields.
Statistical analysis
Data are mean ± SEM. Motor and MWM test data in each mutant mouse strain were compared to WT controls using two-factor repeated-measures analysis of variance (ANOVA; for group and time). PI-positive cell count and lesion volume data were analyzed by the rank-sum test. For all comparisons, p < 0.05 was regarded as significant.
Results
Generation and characterization of TNFR1/Fas, TNFR2/Fas, and TNFR1/TNFR2/Fas knockout mice
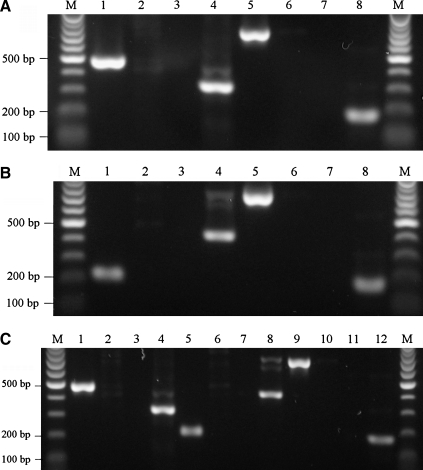
Mice deficient in TNFR1/Fas, TNFR2/Fas, and TNFR1/TNFR2/Fas were viable, fertile, and remained healthy for at least 6 months, by which time TNFR1/TNFR2/Fas KO mice developed abdominal tumors. Polymerase chain reaction analysis of the three lines of knockout mice yielded the expected results (Fig. 1), and the knockout mice lacked obvious brain anatomical or behavioral abnormalities. Figure 1A shows that in the TNFR1/Fas knockout mice, both TNFR1 and Fas wild-type alleles are absent. Figure 1B shows that in TNFR2/Fas knockout mice, both TNFR2 and Fas wild-type alleles are absent. Figure 1C shows that in TNFR1/TNFR2/Fas triple knockout mice, TNFR1, TNFR2, and Fas alleles are absent. At 3 min after the start of general anesthesia with body temperature maintained at 37°C, all knockout mice had baseline blood pressure and blood gases similar to WT mice (p = NS for all pairwise comparisons; Table 1).
FIG. 1.
Genotype analyses of knockout (KO) mice by polymerase chain reaction of tail DNA. Wild-type TNFR1 allele (470 bp) and neomycin resistance (Neo) cassette (300 bp) DNA sequences were amplified from tail DNA of mice using Jackson Laboratories protocol #002818. Wild-type TNFR2 allele (200 bp) and Neo cassette (400 bp) DNA sequences were amplified from tail DNA of mice using Jackson Laboratories protocol #002620. Wild-type Fas allele (700 bp) and Neo cassette (170 bp) DNA sequences were amplified from tail DNA of mice using Jackson Laboratories protocol #003233. (A) Wild-type versus R1/Fas KO mice (lanes 1, 3, 5, and 7: wild-type mice; lanes 2, 4, 6, and 8: R1/Fas KO mice). (B) Wild-type versus R2/Fas KO mice (lanes 1, 3, 5, and 7: wild-type mice; lanes 2, 4, 6, and 8: R2/Fas KO mice). (C) Wild-type versus triple KO mice (lanes 1, 3, 5, 7, 9, and 11: wild-type mice; lanes 2, 4, 6, 8, 10, and 12: triple KO mice; M, molecular-weight markers [100 bp]; TNFR, tumor necrosis factor receptor).
Table 1.
Baseline Physiological Measurements of Wild-Type and Mutant Mice
| MABP (mm Hg) | pH | Paco2 (mm Hg) | Pao2 (mm Hg) | |
|---|---|---|---|---|
| Wild-type (n = 3) | 98.8 ± 2.76 | 7.42 ± 0.01 | 35.7 ± 3.77 | 118.1 ± 6.28 |
| R1/Fas KO (n = 4) | 104.3 ± 4.94 | 7.43 ± 0.03 | 34.9 ± 1.42 | 116.7 ± 12.2 |
| R2/Fas KO (n = 4) | 92.7 ± 1.32 | 7.45 ± 0.02 | 35.0 ± 2.47 | 115.3 ± 13.16 |
| T KO (n = 3) | 98.2 ± 3.02 | 7.42 ± 0.03 | 31.7 ± 0.07 | 112.0 ± 10.02 |
MABP, mean arterial blood pressure; KO, knockout; Paco2, partial arterial carbon dioxide pressure; Pao2, partial arterial oxygen pressure.
Role of TNFR knockout in recovery of motor and cognitive function after CCI
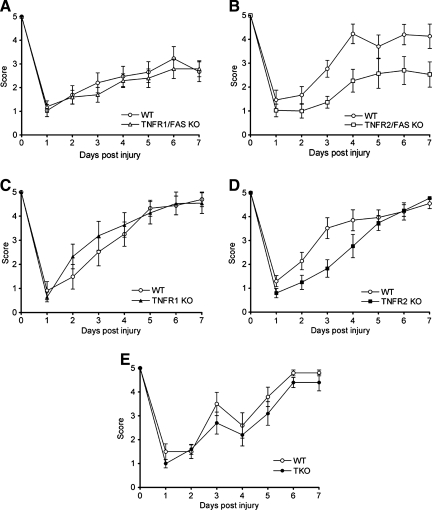
We previously published that mice deficient in functional Fas had pre- and post-injury motor performance similar to those of WT mice (Bermpohl et al., 2007). Thus we began by determining the effect of single (TNFR1 or TNFR2) or double (TNFR1/Fas and TNFR2/Fas) TNFR knockout on motor performance after CCI. Figure 2 shows the results of wire-grip tests in WT and mutant mice before and after CCI. Before CCI, there were no significant differences in motor function in any of the groups tested. Following CCI, wire-grip test performance was significantly worse in TNFR2/Fas KO than in WT mice (p < 0.05 for group effect); however, no differences in motor performance were observed between TNFR1 KO and WT, TNFR2 KO and WT, or TNFR1/Fas KO and WT mice. When TNFR1 signaling was eliminated from TNFR2/Fas KO in TNFR1/TNFR2/Fas (triple KO mice), we found no difference in motor function versus WT mice before and after CCI (Fig. 2).
FIG. 2.
Vestibulomotor function as assessed by the wire-grip test before and up to 7 days after controlled cortical impact (CCI). (A) Wild-type (WT, n = 16) and TNFR1/Fas KO (n = 17, p = ns). (B) WT (n = 10) and TNFR2/Fas KO (n = 10, p = 0.016 group effect by repeated measures ANOVA). (C) WT (n = 9) and TNFR1 KO (n = 10, p = ns). (D) WT (n = 10) and TNFR2 KO (n = 10, p = ns). (E) WT (n = 8) and triple KO (TNFR1/TNFR2/Fas KO, n = 6, p = ns; KO, knockout; ANOVA, analysis of variance; TNFR, tumor necrosis factor receptor).
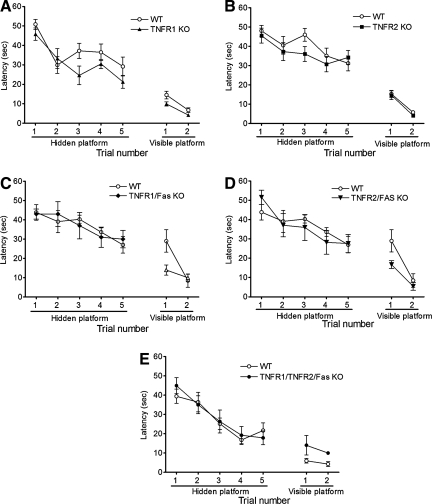
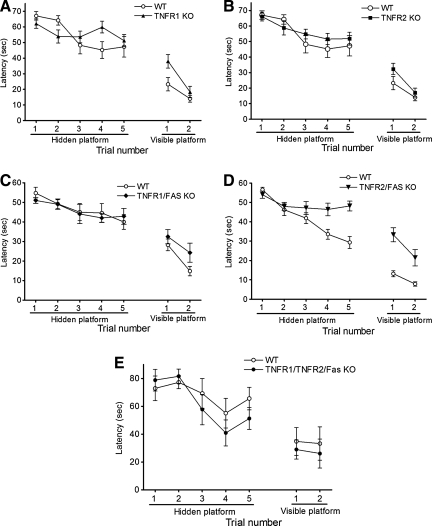
We next determined the effect of TNFR knockout on pre- and post-injury cognitive function using a Morris water maze paradigm. Figure 3 shows results of MWM testing in naïve (uninjured) WT and mutant mice. Latency to the hidden and visible platforms were similar among naïve TNFR1 KO, TNFR2 KO, TNFR1/Fas KO, TNFR2/Fas KO, and WT mice. Following CCI, mice deficient in TNFR1, TNFR2, or TNFR1/Fas performed similarly to WT mice; however, latency to the hidden platform in TNFR2/Fas KO mice was significantly worse than that of their respective WT controls (p < 0.05 group effect; Fig. 4). No difference in MWM performance was observed between naïve (Fig. 3) or injured (Fig. 4) TNFR1/TNFR2/Fas KO and WT mice in the hidden or visible platform trials.
FIG. 3.
Morris water maze performance in naïve animals. No difference in performance was observed between uninjured (naïve) wild-type (WT) mice and knockout (KO) mice in hidden or visible platform trials. (A) Wild-type (WT, n = 10) and TNFR1 KO (n = 10) mice. (B) WT (n = 10) and TNFR2 KO (n = 10) mice. (C) WT (n = 10) and TNFR1/Fas KO (n = 7) mice. (D) WT (n = 10) and TNFR2/Fas KO (n = 8) mice. (E) Naïve animals (n = 10 TKO and n = 4 WT; TNFR, tumor necrosis factor receptor; TKO, triple knockout).
FIG. 4.
Morris water maze performance in injured mice. (A) Wild-type (WT, n = 10) and TNFR1 KO (n = 17) mice. (B) WT (n = 10) and TNFR2 KO (n = 16) mice. (C) WT (n = 10) and TNFR1/Fas KO (n = 10) mice. (D) WT (n = 20) and TNFR2/Fas KO mice (n = 15, p = 0.003 group effect by repeated measures ANOVA). (E) TNFR1/TNFR2/Fas KO (n = 5) and WT (n = 6) mice (ANOVA, analysis of variance; KO, knockout; TNFR, tumor necrosis factor receptor).
Histopathology of controlled cortical impact in TNFR knockout mice
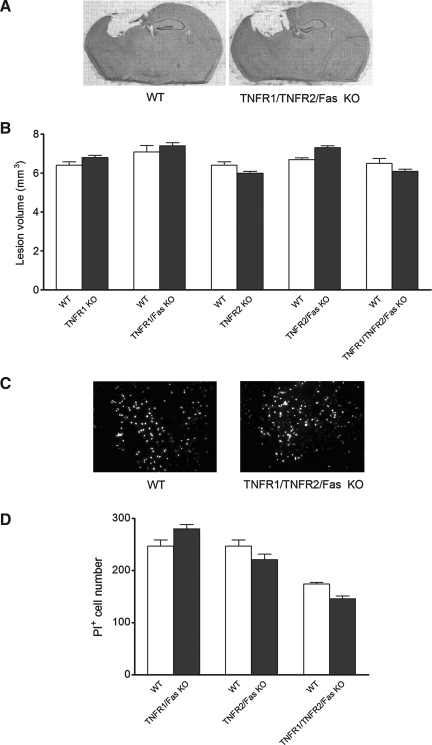
To examine for a possible relationship between histopathological damage and functional outcome in TNFR KO mice, we used in vivo propidium iodide labeling at 5–6 h to identify acutely injured cells with plasmalemma permeability, a marker of eventual cell death/loss in our CCI model (Whalen et al., 2008). We also assessed volume of the cavitary lesion at 2 weeks after CCI. PI-positive cell counts did not differ between any of the double or triple KO mice and their respective WT controls (Fig. 5). No differences in cavitary lesion size were observed between WT and any TNFR KO line at 2 weeks after CCI (Fig. 5).
FIG. 5.
Lesion volume and propidium iodide (PI)-positive cell counts of wild-type (WT) versus knockout mice after controlled cortical impact (CCI). (A) Representative images of brain sections at 14 days after CCI, showing cavitary lesions. (B) Lesion volume at 14 days after injury for WT (n = 10) versus TNFR1 KO (n = 18) mice, WT (n = 10) versus TNFR1/Fas KO (n = 10) mice, WT (n = 10) versus TNFR2 KO (n = 16) mice, WT (n = 20) versus TNFR2/Fas KO (n = 15) mice, and WT (n = 10) versus TNFR1/TNRF2/Fas KO (n = 9) mice. (C) Representative images of PI labeling in vivo. (D) PI-positive cell counts at 6 h after injury. The y axis is the number of PI-positive cells per 200 × field for WT (n = 4) versus TNFR1/Fas KO (n = 4) mice, WT (n = 4) versus TNFR2/Fas KO (n = 4) mice, and WT (n = 10) versus TNFR1/TNFR2/Fas KO (n = 8) mice (p = ns for all between-group comparisons; TNFR, tumor necrosis factor receptor; KO, knockout).
Discussion
To our knowledge this is the first study to report the effect of TNFR1/Fas, TNFR2/Fas, and TNFR1/TNFR2/Fas KO in an acute brain injury model. Contrary to our initial hypothesis that TNFR1 and Fas together play a prominent role in outcome after CCI, we found no functional or histopathological consequences of genetic inhibition of TNFR1 or TNFR1/Fas after CCI. Mice deficient in TNFR2/Fas, however, had worse motor and MWM outcome, whereas TNFR1/TNFR2/Fas KO mice had post-injury motor and MWM deficits similar to WT mice. Analysis of plasmalemma permeability to PI at 6 h and post-injury lesion size (2 weeks) showed no relationship between functional outcome and histopathology in TNFR KO mice. Taken together, these data suggest a beneficial role for the combination of TNFR2/Fas receptors in recovery of motor and cognitive function after TBI.
In general, few studies have addressed a role for TNFR in functional outcome after CNS injury (Longhi et al., 2008; Scherbel et al., 1999). Longhi and associates (2008) showed a positive effect of TNFR1 deletion and a negative effect of TNFR2 deletion on post-injury MWM performance following moderate CCI. In contrast, we and others (Sullivan et al., 1999) did not find differences in histopathological or functional outcome after CCI in mice lacking TNFR1 or TNFR2 alone. These conflicting data may be attributable to differences in the severity of the CCI models used, as well as anesthetic agent, differences in background strain genetics in TNFR KO mice, and other model-specific factors in the study by Longhi and associates (2008). Other studies have suggested a protective role for TNFR1 alone in seizure-induced cell death (Bruce et al., 1996), and cerebral ischemic and excitotoxic cell death (Gary et al., 1998), but a detrimental role in neuronal death following optic nerve crush injury (Tezel et al., 2004). Double knockout of TNFR1 and TNFR2 together (TNFR1/TNFR2 KO) increased brain cell death after TBI, seizures, and cerebral ischemia (Bruce et al., 1996; Sullivan et al., 1999), but reduced motoneuron cell death after facial axotomy (Raivich et al., 2002). Our data suggest a protective role for TNFR2/Fas in functional outcome after CCI.
We found that elimination of TNFR1 in TNFR2/Fas KO mice (TNFR1/TNFR2/Fas KO) resulted in post-injury motor and MWM performance similar to WT mice. One explanation for this finding is that TNFR2 or Fas suppresses TNFR1 signaling. In this scenario, Fas signaling would be redundant, as inhibition of Fas alone (Bermpohl et al., 2007), or of TNFR1 or TNFR2 alone (the current study and Bruce et al., 1996), did not affect outcome after CCI. Thus TNFR1 signaling, unregulated by TNFR2/Fas, may exacerbate post-traumatic motor and cognitive deficits. Alternatively, other TNFR or TNFR-related pathways beneficial to outcome after TBI may be induced in TNFR1/TNFR2/Fas KO mice. It is known that cross-talk among TNFR family members may induce complex and unexpected phenotypes with multiple TNFR inhibition. For example, mice deficient in TNFR1/TNFR2 lack functional Fas signaling and are resistant to Fas-induced liver injury and death (Costelli et al., 2003). Why Fas receptor does not function normally in the absence of TNFR1 and TNFR2 is unknown, but it may be explained in part by developmental differences in the brain proteomes after multiple TNFR KO (Pejovic et al., 2004). Thus induction of compensatory beneficial signaling pathways when all three TNFR are inhibited together could allow triple KO mice to revert back to the WT post-injury motor and cognitive phenotypes.
Based on our previous findings in TNF/Fas KO mice (Bermpohl et al., 2007) and the fact that TNFR1 and TNFR2 are known cognate receptors for TNF, we anticipated protective effects of TNFR1/TNFR2/Fas KO on post-injury functional and histopathological outcome versus WT mice.
At least three factors may contribute to the observed lack of protection in TNFR1/TNFR2/Fas KO mice. First, TNF/Fas KO mice from our previous report (Bermpohl et al., 2007) had only modest improvement in post-injury motor function and MWM performance (they were different from WT mice only on the last hidden platform trial). Thus limited numbers of triple KO mice might not be expected to recapitulate the modest protective effects of TNF/Fas KO, given the inherent variability in functional outcome testing (Bermpohl et al., 2007; Gerlai, 2001). Second, knockout of three TNFR family members may have induced proteome changes or signaling mechanisms detrimental to outcome after TBI (Pejovic et al., 2004; Thompson et al., 2004). Finally, subtle differences in background genetics may play a role, as our current TNF/Fas KO line, which was rederived since the initial report by Bermpohl and colleagues (2007), has reduced PI-positive cells, but not reduced post-injury lesion size after CCI (M. Whalen, unpublished observations). In support of the latter explanation, treatment of TNF KO mice with anti-Fas ligand antibodies yielded a stronger beneficial effect on post-injury cognitive function than genetic inhibition of both TNF and Fas in TNF/Fas KO mice (Bermpohl et al., 2007). These observations underscore the limitations of using gene knockout approaches as the sole method to determine gene function in brain injury models. Although our data provide an important first look, further studies using alternative methods (e.g., RNA interference or blocking antibodies) are needed to confirm the protective role of TNFR2/Fas and the net results of multiple TNFR inhibition in TBI.
We used in vivo PI labeling to assess early fatal cellular injury (Bermpohl et al., 2007; Whalen et al., 2008), and assessed cavitary lesion size to determine whether functional outcome in TNFR KO mice could be attributed to increased histopathology. We found no differences in acute cell death or lesion size in any of the TNFR KO mice tested. These results are consistent with a general lack of correspondence between histopathological and functional outcome in animal TBI models. Thus TNFR signaling may influence functional outcome after TBI, independent of its effects on histopathology.
How TNFR signaling mediates functional recovery after TBI is unknown. TNFR may influence gene expression via NF-κB (Sullivan et al., 1999) and Jun–N-kinase (JNK) signaling (Ortolano et al., 2009), as well as necroptosis signaling through receptor interacting protein kinase-1 (Degterev et al., 2008, 2005) and −3 (Cho et al., 2009; Declercq et al., 2009). We and others have shown that RIPK1 and JNK inhibitors reduce cell death and improve motor and cognitive function after CCI in mice (Ortolano et al., 2009; You et al., 2008). Interaction between TNFR2 and glutamate induces prolonged activation of phosphatidylinositol-3-kinase–dependent NF-κB activation that enhances neuronal survival and modulates sensitivity to excitotoxic stress, such as that which occurs in TBI and contributes to motor and cognitive dysfunction (Marchetti et al., 2004). TNF-α increases TNFR2 expression in microglia, which directly or indirectly inhibits TNFR1 signaling and activates antioxidant mechanisms that protect microglia from TNF-induced injury (Dopp et al., 1997). Thus, dampening the response to excitotoxicity and increased neuroprotective gene expression are two possible mechanisms by which TNFR2 (and possibly Fas) may influence cell death and neurological dysfunction after TBI. The focus of the current study was to identify TNFR combinations that are involved in functional outcome measures after CCI; further work is needed to determine the relevant downstream mechanisms involved.
The finding that TNFR2/Fas KO mice had worse outcome after CCI has implications for the specific type of TNF signaling, as well as the cell types involved in post-traumatic motor and cognitive deficits. Both soluble and transmembrane TNF bind and activate TNFR1, but TNFR2 is activated only by transmembrane TNF (McCoy and Tansey, 2008). Thus, transmembrane TNF signaling through TNFR2 may serve to limit motor and cognitive dysfunction after TBI. Our data also implicate TNFR signaling through microglia and/or endothelial cells after CCI, since TNFR1 and TNFR2 are co-expressed in these cell types in brain, endothelium (Yager et al., 2008), and microglia (Hailer, 2008), and have been implicated in the pathogenesis of TBI.
In conclusion, TNFR2/Fas KO mice had significant motor and MWM deficits compared to WT mice following CCI. These deficits were partially improved by further elimination of TNFR1 in TNFR2/Fas KO mice (TNFR1/TNFR2/Fas KO mice). Further work is needed to elucidate the relevant mechanisms associated with these findings. It is hoped that elucidation of the TNFR combinations associated with altered outcome after TBI can be used to identify relevant downstream signaling pathways, and provide new therapeutic targets for patients with TBI (You et al., 2008).
Acknowledgment
This work was supported by NIH/National Institute of Neurological Diseases and Stroke grant RO1NS47447 to M.J.W.
Author Disclosure Statement
No competing financial interests exist.
References
- Beg A.A. Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- Bermpohl D. You Z. Korsmeyer S.J. Moskowitz M.A. Whalen M.J. Traumatic brain injury in mice deficient in Bid: effects on histopathology and functional outcome. J. Cereb. Blood Flow Metab. 2006;26:625–633. doi: 10.1038/sj.jcbfm.9600258. [DOI] [PubMed] [Google Scholar]
- Bermpohl D. You Z. Lo E.H. Kim H.H. abd Whalen M.J. TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice. J. Cereb. Blood Flow Metab. 2007;27:1806–1818. doi: 10.1038/sj.jcbfm.9600487. [DOI] [PubMed] [Google Scholar]
- Bruce A.J. Boling W. Kindy M.S. Peschon J. Kraemer P.J. Carpenter M.K. Holtsberg F.W. Mattson M.P. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat. Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller A.J. Geddes J.W. Knapp P.E. McFall R.W. Keller J.N. Holtsberg F.W. Parthasarathy S. Steiner S.M. Mattson M.P. Anti-death properties of TNF against metabolic poisoning: mitochondrial stabilization by MnSOD. J. Neuroimmunol. 1999;93:53–71. doi: 10.1016/s0165-5728(98)00190-8. [DOI] [PubMed] [Google Scholar]
- Carpentier I. Coornaert B. Beyaert R. Function and regulation of tumor necrosis factor type 2. Curr. Med. Chem. 2004;11:2205–2212. doi: 10.2174/0929867043364694. [DOI] [PubMed] [Google Scholar]
- Cheng B. Christakos S. Mattson M.P. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994;12:139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Cho Y.S. Challa S. Moquin D. Genga R. Ray T.D. Guildford M. Chan F.K. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R.S. Kochanek P.M. Watkins S.C. Chen M. Dixon C.E. Seidberg N.A. Melick J. Loeffert J.E. Nathaniel P.D. Jin K.L. Graham S.H. Caspase-3 mediated neuronal death after traumatic brain injury in rats. J. Neurochem. 2000;74:740–753. doi: 10.1046/j.1471-4159.2000.740740.x. [DOI] [PubMed] [Google Scholar]
- Costelli P. Aoki P. Zingaro B. Carbo N. Reffo P. Lopez-Soriano F.J. Bonelli G. Argiles J.M. Baccino F.M. Mice lacking TNFalpha receptors 1 and 2 are resistant to death and fulminant liver injury induced by agonistic anti-Fas antibody. Cell Death Differ. 2003;10:997–1004. doi: 10.1038/sj.cdd.4401281. [DOI] [PubMed] [Google Scholar]
- Declercq W. Vanden Berghe T. Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138:229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Degterev A. Hitomi J. Germscheid M. Ch'en I.L. Korkina O. Teng X. Abbott D. Cuny G.D. Yuan C. Wagner G. Hedrick S.M. Gerber S.A. Lugovskoy A. Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A. Huang Z. Boyce M. Li Y. Jagtap P. Mizushima N. Cuny G.D. Mitchison T.J. Moskowitz M.A. Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- Dopp J.M. Mackenzie-Graham A. Otero G.C. Merrill J.E. Differential expression, cytokine modulation, and specific functions of type-1 and type-2 tumor necrosis factor receptors in rat glia. J. Neuroimmunol. 1997;75:104–112. doi: 10.1016/s0165-5728(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Fotin-Mleczek M. Henkler F. Samel D. Reichwein M. Hausser A. Parmryd I. Scheurich P. Schmid J.A. Wajant H. Apoptotic crosstalk of TNF receptors: TNF-R2-induces depletion of TRAF2 and IAP proteins and accelerates TNF-R1-dependent activation of caspase-8. J. Cell Sci. 2002;115:2757–2770. doi: 10.1242/jcs.115.13.2757. [DOI] [PubMed] [Google Scholar]
- Gary D.S. Bruce-Keller A.J. Kindy M.S. Mattson M.P. Ischemic and excitotoxic brain injury is enhanced in mice lacking the p55 tumor necrosis factor receptor. J. Cereb. Blood Flow Metab. 1998;18:1283–1287. doi: 10.1097/00004647-199812000-00001. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Behavioral tests of hippocampal function: simple paradigms complex problems. Behav. Brain Res. 2001;125:269–277. doi: 10.1016/s0166-4328(01)00296-0. [DOI] [PubMed] [Google Scholar]
- Hailer N.P. Immunosuppression after traumatic or ischemic CNS damage: it is neuroprotective and illuminates the role of microglial cells. Prog. Neurobiol. 2008;84:211–233. doi: 10.1016/j.pneurobio.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Kim G.M. Xu J. Song S.K. Yan P. Ku G. Xu X.M. Hsu C.Y. Tumor necrosis factor receptor deletion reduces nuclear factor-kappaB activation, cellular inhibitor of apoptosis protein 2 expression, and functional recovery after traumatic spinal cord injury. J. Neurosci. 2001;21:6617–6625. doi: 10.1523/JNEUROSCI.21-17-06617.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. Choksi S. Shen H.M. Yang Q.F. Hur G.M. Kim Y.S. Tran J.H. Nedospasov S.A. Liu Z.G. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J. Biol. Chem. 2004;279:10822–10828. doi: 10.1074/jbc.M313141200. [DOI] [PubMed] [Google Scholar]
- Locksley R.M. Killeen N. and Lenardo M.J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Longhi L. Ortolano F. Zanier E.R. Perego C. Stocchetti N. De Simoni M.G. Effect of traumatic brain injury on cognitive function in mice lacking p55 and p75 tumor necrosis factor receptors. Acta Neurochir. Suppl. 2008;102:409–413. doi: 10.1007/978-3-211-85578-2_80. [DOI] [PubMed] [Google Scholar]
- Lotocki G. Alonso O.F. Dietrich W.D. Keane R.W. Tumor necrosis factor receptor 1 and its signaling intermediates are recruited to lipid rafts in the traumatized brain. J. Neurosci. 2004;24:11010–11016. doi: 10.1523/JNEUROSCI.3823-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotocki G. de Rivero Vaccari J.P. Perez E.R. Alonso O.F. Curbelo K. Keane R.W. Dietrich W.D. Therapeutic hypothermia modulates TNFR1 signaling in the traumatized brain via early transient activation of the JNK pathway and suppression of XIAP cleavage. Eur. J. Neurosci. 2006;24:2283–2290. doi: 10.1111/j.1460-9568.2006.05123.x. [DOI] [PubMed] [Google Scholar]
- Marchetti L. Klein M. Schlett K. Pfeizenmaier K. Eisel U.L. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J. Biol. Chem. 2004;279:32869–32881. doi: 10.1074/jbc.M311766200. [DOI] [PubMed] [Google Scholar]
- Martin-Villalba A. Hahne M. Kleber S. Vogel J. Falk W. Schenkel J. Krammer P.H. Therapeutic neutralization of CD95-ligand and TNF attenuates brain damage in stroke. Cell Death Differ. 2001;8:679–686. doi: 10.1038/sj.cdd.4400882. [DOI] [PubMed] [Google Scholar]
- McCoy M.K. Martinez T.N. Ruhn K.A. Szymkowski D.E. Smith C.G. Botterman B.R. Tansey K.E. Tansey M.G. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease. J. Neurosci. 2006;26:9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy M.K. Tansey M.G. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J. Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortolano F. Colombo A. Zanier E.R. Sclip A. Longhi L. Perego C. Stocchetti N. Borsello T. De Simoni M.G. c-Jun N-terminal kinase pathway activation in human and experimental cerebral contusion. J. Neuropathol. Exp. Neurol. 2009;68:964–971. doi: 10.1097/NEN.0b013e3181b20670. [DOI] [PubMed] [Google Scholar]
- Pejovic V. Soskic V. Pan W. Kastin A.J. Brain proteome of mice lacking the receptors for tumor necrosis factor alpha. Proteomics. 2004;4:1461–1464. doi: 10.1002/pmic.200300687. [DOI] [PubMed] [Google Scholar]
- Raivich G. Liu Z.Q. Kloss C.U. Labow M. Bluethmann H. Bohatschek M. Cytotoxic potential of proinflammatory cytokines: Combined deletion of TNF receptors TNFR1 and TNFR2 prevents motoneuron cell death after facial axotomy in adult mouse. Exp. Neurol. 2002;178:186–193. doi: 10.1006/exnr.2002.8024. [DOI] [PubMed] [Google Scholar]
- Scherbel U. Raghupathi R. Nakamura M. Saatman K.E. Trojanowski J.Q. Neugebauer E. Marino M.W. McIntosh T.K. Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proc. Natl. Acad. Sci. U.S.A. 1999;96:8721–8726. doi: 10.1073/pnas.96.15.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlik C. de Martin R. Kumabashiri I. Schmid J.A. Binder B.R. Lipp J. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J. Exp. Med. 1998;188:211–216. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan P.G. Bruce-Keller A.J. Rabchevsky A.G. Christakos S. Clair D.K. Mattson M.P. Scheff S.W. Exacerbation of damage and altered NF-kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J. Neurosci. 1999;19:6248–6256. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G. Yang X. Yang J. Wax M.B. Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res. 2004;996:202–212. doi: 10.1016/j.brainres.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Thompson C. Gary D. Mattson M. Mackenzie A. Robertson G.S. Kainic acid-induced naip expression in the hippocampus is blocked in mice lacking TNF receptors. Brain Res. Mol. Brain Res. 2004;123:126–131. doi: 10.1016/j.molbrainres.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Whalen M.J. Dalkara T. You Z. Qiu J. Bermpohl D. Mehta N. Suter B. Bhide P.G. Lo E.H. Ericsson M. Moskowitz M.A. Acute plasmalemma permeability and protracted clearance of injured cells after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 2008;28:490–505. doi: 10.1038/sj.jcbfm.9600544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager P.H. You Z. Qin T. Kim H.H. Takahashi K. Ezekowitz A.B. Stahl G.L. Carroll M.C. Whalen M.J. Mannose binding lectin gene deficiency increases susceptibility to traumatic brain injury in mice. J. Cereb. Blood Flow Metab. 2008;28:1030–1039. doi: 10.1038/sj.jcbfm.9600605. [DOI] [PubMed] [Google Scholar]
- You Z. Savitz S.I. Yang J. Degterev A. Yuan J. Cuny G.D. Moskowitz M.A. Whalen M.J. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 2008;28:1564–1573. doi: 10.1038/jcbfm.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]