Abstract
Oxidative damage by reactive oxygen species is believed to be a contributor to the development of cancer and the physiological deterioration associated with aging. In this report, we describe the effect of reactive oxygen species exposure to a developing Caenorhabditis elegans organism containing a deletion in the homolog of BRCA1-associated protein 2 (BRAP-2). A mutant containing a deletion of brap-2 was highly sensitive to oxidizing conditions and demonstrated early larval arrest and lethality at low concentrations of the oxidative stress-inducing drug paraquat compared with the wild-type. This developmental arrest occurred early in the L1 stage and was dependent specifically on the function of the C. elegans ortholog of BRCA-1 tumor suppressor brc-1. We also show that developmental arrest in brap-2 mutants when exposed to oxidative stress was due to enhanced expression levels of the cell cycle inhibitor cki-1, and this increase in the expression levels of cki-1 requires brc-1 in brap-2 mutant animals. Our findings demonstrate that BRAP-2 is necessary for preventing an inappropriate response to elevated levels of reactive oxygen species by countering premature activation of BRC-1 and CKI-1.
Keywords: Cell Cycle, Development, Oxidative Stress, Reactive Oxygen Species (ROS), Signal Transduction, Tumor Suppressor
Introduction
Overproduction of reactive oxygen species (ROS)2 is known to lead to cytoplasmic and nuclear damage and has been implicated in many age-related diseases including Alzheimer, Parkinson, and cancer (1). Signaling pathways become activated by cells to limit the insult from ROS by generating antioxidant proteins (e.g. superoxide dismutase and glutathione S-transferase proteins), which remove high levels of ROS, along with other pathways that are activated to mend the damage by ROS that induce cell cycle arrest, activate DNA repair mechanisms, and promote ubiquitin-mediated proteolysis of modified proteins (2). If the cellular damage is too great to be repaired, apoptosis of the inflicted cell occurs (3).
A group of proteins that monitor the cell for excessive damage from genotoxic or oxidative stress are tumor suppressor genes, such as BRCA1, p53, and the RB tumor suppressor (4). BRCA1 has been studied extensively because mutations in the gene predispose women to breast and ovarian cancers and is important for DNA damage response and preventing tumor development (5). To maintain genomic integrity, BRCA1 also plays an important role in regulating cell cycle progression through a number of distinct pathways that include p21WAF1/CIP1, p53, and RB (6, 7).
Model organisms, such as Caenorhabditis elegans, have proven to be very useful in studying the function and signaling pathways of BRCA1. The BRCA1 ortholog (brc-1) is involved in DNA repair (8) and is required for double-stranded repair and responsible for E3-ubiquitin ligase activity at DNA damage sites following ionizing radiation (9, 10). BRC-1 carries out these functions with its heterodimeric partner, BRD-1 (8), a complex whose interaction is conserved between the mammalian homologs BRCA1 and BARD1 (9). Upon genotoxic stress, the BRC-1·BRD-1 complex interacts with chromatin and LET-70, the C. elegans homolog of the E2-conjugating enzyme Ubc5, and this interaction results in the transfer of ubiquitin from the E2-conjugating enzyme to the site of DNA damage for DNA repair (10).
Our interest is in the C. elegans homolog of Brap2, a protein associated with the tumor suppressor gene BRCA1 (11) and is also known as IMP (impedes mitogenic signal propagation) (12). Brap2 is a cytoplasmic protein capable of binding the nuclear localization signal motifs of BRCA1, preventing it from localizing into the nucleus (11), although the relevance of this interaction has not yet been demonstrated. Brap2 also binds the nuclear localization signal motifs found in the SV40 large T antigen, mitosin, and p21 during monocyte differentiation (11, 13, 14), regulates the assembly of the mitogenic signaling complex in response to Ras activation (12), and facilitates polyubiquitination of HsCdc14A (14).
In this study, we have characterized the C. elegans homolog of Brap2 called brap-2 and found that a deletion mutant of brap-2 is sensitive to oxidative stress during development, with exposure to hydrogen peroxide and paraquat leading to developmental arrest and lethality early in L1. Furthermore, developmental arrest was coordinated with increased expression of the cyclin kinase inhibitor cki-1 in seam cells. We also demonstrated that the growth arrest under oxidative stress conditions in the absence of brap-2 is dependent on the BRCA1 ortholog in C. elegans, brc-1. Finally, we demonstrated that the deletion of brc-1 decreases the levels of cki-1 expression, illustrating that the C. elegans homolog of BRCA1 is involved in cell cycle progression and that this function is conserved between nematodes and humans. Therefore, BRAP-2 is important for preventing an inappropriate response to elevated levels of reactive oxygen species by countering premature activation of BRC-1 and CKI-1.
EXPERIMENTAL PROCEDURES
C. elegans Strains
For normal and mutant strains, the worms are grown in agar plates containing a strain of Escherichia coli bacteria called OP50, as described by Brenner (15). The strain RB1346, which contains the brap-2(ok1492) deletion, was obtained from the Caenorhabditis Genetics Center and backcrossed three times to create the YF15 strain. Other strains used include N2, MT10430 (lin-35(n745)I), YF66 (lin-35(n745)I; brap-2(ok1492)II), CF1038 (daf-16(mu86)I), YF19 (daf-16(mu86)I; brap-2(ok1492)II), TJ1 (cep-1(gk138)I), YF63 (cep-1(gk138)I; brap-2(ok1492)II), DW102 (brc-1(tm1145)III), YF64 (brap-2(ok1492)II; brc-1(tm1145)III), VT825 (maIs113(cki-1::GFP)), YF69 (brap-2(ok1492)II; maIs113(cki-1::GFP)), PD4666 (ayIs6(hlh-8::GFP)) and YF73 (brap-2(ok1492)II; ayIs6(hlh-8::GFP)). All double mutants were created using standard C. elegans techniques and confirmed by single worm PCR to identify deletions when possible. Primers used for PCR are available upon request.
Determining the Site of Deletion in YF15 (brap-2 (okl492)) Strain
A worm from the YF15 (brap-2 (okl492)) was placed in 4 μl of 1× polymerase and 0.5 mg/ml proteinase K buffer and frozen at −80 °C for 1 h, incubated at 65 °C for 1 h, and incubated at 95 °C for 15 min to inactivate proteinase K. Single worm PCR was then completed, and the PCR product was purified using the GelElute PCR cleanup kit and sequenced.
Oxidative Stress Assay of F1 Progeny
Paraquat was purchased from Sigma (PS366) in powder form. To generate the concentration series of paraquat, a stock of 0.2 m paraquat was added to 250 μl of filtered H2O at the appropriate dilutions, added to 35 mm unseeded nematode growth plates, and allowed to dry overnight. The plates were seeded with OP50 the next day. The following day, adult worms of various genotypes were placed on the plates, allowed to lay eggs for 8 h, and removed. The progeny were then scored every day for a period of 7 days for developmental arrest, lethality, or as able to develop to an adult. Progeny that reached late L4 or the adult stage were removed to prevent them from laying eggs on the paraquat-containing plates.
Microscopy of Reporter Strains
Progeny of various strains carrying the reporter constructs maIs113(cki-1::GFP) and ayIs6(hlh-8::GFP) after oxidative stress treatment were mounted on 2% agarose pads in M9 buffer containing 2 mm levamisole and were visualized using an Olympus BX61 confocal microscope.
RESULTS
brap-2 Deletion Mutant Is Sensitive to Oxidative Stress and Arrest at Early L1 Stage
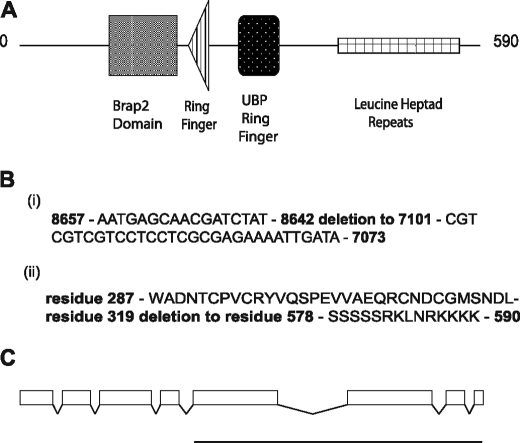
We set out to determine the developmental and signaling function of the Brap2 homolog in C. elegans. A homolog of Brap2 was identified and is the gene designated EEED8.16. Sequence analysis of the primary amino acid structure of EEED8.16 indicated that it is 36% identical to human Brap2, and it has the identical protein domains as Brap2 (Fig. 1A). The recognized domains in EEED8.16 from the N terminus are the Brap2 homology domain, an H2-RING finger domain, a UBP-zinc finger domain and a region consisting of the leucine heptad repeats. Attempts to find the Brap2 homology domain in another protein within the C. elegans genome failed. Based on the sequence identity between EEED8.16 and Brap2 and the similarity of their domain architecture, we conclude that EEED8.16 is the C. elegans homolog of Brap2 and named it brap-2.
FIGURE 1.
Domain structure of brap-2 gene and characterization of ok1492 deletion allele. A, schematic representation of the domain structure of the C. elegans BRAP-2 protein, as predicted by the SMART modular architecture program. B, sequence the ok1492 allele. Four individual worms that were homozygous for the ok1492 allele underwent single worm PCR using the primers as stated under “Experimental Procedures.” The PCR products were purified using a standard PCR purification kit from Invitrogen and sequenced by The Centre for Applied Genomics situated at the Hospital for Sick Children in Toronto, Ontario, Canada. The sequence was compared with cosmid EEED8; the numbers refer to the nucleotides of this cosmid, and the deleted region is illustrated. The predicted protein sequence from expression of the ok1492 is also listed. The deletion results in the removal of 259 amino acids near the C-terminal portion of the BRAP-2 protein. C, genomic structure of the brap-2 locus. The boxes represent exons, and the introns are represented by the spaces between the exons. The region of brap-2 deleted in the ok1492 allele (exons 5–8) is represented by the thick line below the genomic structure.
We obtained a strain containing a deletion of brap-2 from the Caenorhabditis Genetics Center. This mutant (ok1492) contains a 1540-nucleotide deletion, removing exons 5–8 (Fig. 1C). The mutant BRAP-2 protein is predicted to be missing its final 256 amino acids, which consists of the UBP-zinc finger and leucine heptad repeat. The leucine heptad repeat region is usually necessary for homodimerization, and potentially both domains are involved in protein-protein interaction. Therefore, disruption of this region on BRAP-2 would probably severely impair the cellular function of the protein (16). However, the N terminus portion of the protein containing the Brap2 homology domain and the H2-RING finger may still be expressed, so, at the moment, we cannot rule out that expression of the truncated protein does not have an effect.
During the course of characterizing the brap-2 mutant, we tested its susceptibility to a number of stress-inducing conditions. One condition that produced a marked affect was the inclusion of H2O2 in the nematode growth medium/agar plates used for maintaining the animals. Our assay involved placing adult hermaphroditic worms on H2O2-containing plates and monitoring the development of the F1 generation over a period of 7 days. In both the presence of H2O2 and paraquat, brap-2 mutant nematodes demonstrated larval arrest and lethality, whereas wild-type N2 worms did not (Fig. 2, A and B). This effect was irreversible, as transfer of these arrested animals to non-paraquat-containing plates failed to develop. L1 arrest and lethality of brap-2(ok1492) was fully penetrant at the initial concentration tested (2 mm hydrogen peroxide), whereas N2 worms developed normally.
FIGURE 2.
Developmental arrest of brap-2(ok1492) arrest upon oxidative stress. A, DIC image (10× magnification) of a 2-day-old N2 worm grown on an NGM plate containing 2 mm t-butyl hydroperoxide. B, DIC image (20× magnification) of a 2-day-old brap-2(ok1492) mutant worm grown on a plate containing 2 mm t-butyl hydroperoxide. The N2 strain was able to grow to full adulthood, whereas the brap-2(ok1492) mutants arrested at the L1 larval stage. C, concentration dependence of brap-2(ok1492) to paraquat and transgenic rescue by cosmid containing brap-2. Adult N2 and brap-2(ok1492) nematodes were placed on plates containing different concentrations of paraquat, allowed to lay eggs and then were removed. Over 7 days, the progeny were scored for the proportion of animals dead (black), arrested in development (striped) or adult (gray). N2 and brap-2 mutant worms containing the transgene tqEx34 (containing EEED8 cosmid and pF25B3.3::GFP as co-injection marker) were scored at a concentration of 0.1 mm paraquat.
Subsequently, we determined the concentration dependence of brap-2 sensitivity to oxidative stress. For this assay, we seeded our plates with a solution containing the chemical paraquat, which generates superoxide radicals in vivo (17), prior to seeding the plates with OP50. Adult hermaphrodites were then placed on these plates and allowed to lay eggs, and the F1 generation was monitored for 7 days. The brap-2 mutant demonstrated a graded response to increasing concentration of paraquat and began to show susceptibility to oxidative stress at a concentration of 0.05 mm paraquat, with an increase in the number of L1 animals remaining on the plate after 7 days and resulted in complete arrest or death at a concentration of 0.2 mm (Fig. 2C). N2 worms, on the other hand, developed normally until 0.5 mm (Fig. 2C) and did not demonstrate complete arrest/death until 5 mm paraquat (data not shown).
The brap-2 gene is present on chromosome II and is part of an operon that includes the genes for an RNA-binding protein (EEED8.10) and the C. elegans homolog of the human PINK1 gene pink-1 (EEED8.9). To rescue the deletion strain for sensitivity to oxidative stress, we created transgenic worms containing the EEED8 cosmid (Fig. 2C). The transgene (tqEx34) was crossed into the brap-2 deletion strain, and animals were tested for their ability to develop in the presence of 0.1 mm paraquat. While in the absence of the transgene, brap-2 mutant worms displayed severe developmental arrest (only 3% reach the adult stage); in the presence of the transgene, >28% were able to develop completely through the larval stages, indicating that the EEED8 cosmid is capable of rescuing the oxidative sensitivity phenotype of the brap-2 deletion strain. Furthermore, we tested the transgene containing worms at higher concentrations of paraquat to determine whether the overexpressing brap-2 may lead to oxidative stress resistance, but it showed the same susceptibility to oxidative stress as N2 worms (data not shown).
We also determined the time of onset of L1 arrest by examining M-cell divisions (18). Wild-type worms under normal growth conditions hatch with a single M-cell that initially divides 5–6 h posthatching to form 16 cells by the end of L1 (19). M-cell divisions of developing larvae can be monitored using the integrated transgenic reporter construct hlh-8::gfp. For the majority of brap-2 worms developing on 0.2 mm paraquat plates (74%, n = 31), larval arrest occurred very early in L1, where between 1 and 4 M-cells were apparent from the presence of hlh-8::gfp expressing cells (Fig. 3C).
FIGURE 3.
brap-2(ok1492) arrest during the L1 stage. A, differentiation of the M-cell lineage during development (19). B, hlh-8::GFP expression of in two M-cells after 7 days of the brap-2(ok1492) L1-arrested nematode placed on a paraquat containing a plate. C, table of percentage of brap-2 mutant worms under oxidative stress conditions with specific number of cells from the M lineage.
Developmental Arrest in L1 Occurs Independently from the daf-16 Food-sensing Pathway
L1 diapause is a distinctive type of developmental option that occurs upon starvation (20, 21, 22). Because there is a similarity between starvation-inducing L1 arrest and brap-2 mutation exposed to oxidative stress, we set out to determine whether the two mechanisms affect common regulators of postembryonic growth and development. L1 diapause that results from nutrient deprivation requires the DAF-16/FoxO transcription factor that is repressed by the AGE-1/phosphoinositide 3-kinase pathway because worms lacking DAF-16 fail to arrest upon nutrient deprivation (20, 21). To determine whether the L1 developmental arrest of brap-2 worms upon exposure to oxidative stress required DAF-16, a double mutant of brap-2 and daf-16 was generated. We found that deleting daf-16 did not prevent L1 arrest caused by the brap-2 mutation, as 100% of brap-2 mutant were dead or arrested during development in the presence of paraquat versus 99% of the daf-16;brap-2 (Table 1). This indicates that the L1 arrest seen in brap-2 upon oxidative stress does not require daf-16, is not due to nutrient deprivation, and involves a distinct pathway.
TABLE 1.
Larval arrest and lethality of brap-2 mutants do not require daf-16
Adult worms from N2 (wild-type), brap-2(ok1492), daf-16(mu86) and daf-16(mu86);brap-2(ok1492) strains were allowed to lay eggs on plates containing OP50 and 0.1 mm paraquat for 8 h and removed. The progeny were analyzed over 7 days and scored for larval lethality, larval arrest, and full development to the adult stage. This experiment was done three times, and the cumulative totals were pooled. Total numbers of worms scored are in parentheses.
| Genotype | Larval lethality | Larval arrest | Adult |
|---|---|---|---|
| % | |||
| Wild-type (177) | 0 | 0 | 100 |
| brap-2 (105) | 99 | 1 | 0 |
| daf-16 (159) | 1 | 0 | 99 |
| daf-16;brap-2 (132) | 91 | 8 | 1 |
Developmental Arrest of brap-2 Requires brc-1
Mammalian Brap2 was originally identified as an associated protein of BRCA1 (11) capable of binding BRCA1 nuclear localization signal by yeast two-hybrid, although the biological significance of this interaction has not been demonstrated. C. elegans contains a BRCA1 homolog, called BRC-1, which is required for DNA repair upon UV radiation (8). Therefore, to further investigate the biological role of brc-1 and to determine whether the developmental arrest seen in brap-2(ok1492) animals under oxidative stress requires a functional brc-1 gene, we created a double brap-2;brc-1 mutant, placed the adult hermaphrodites on 0.1 mm paraquat containing plates and monitored the F1 worms for 7 days. Unlike the brap-2(ok1492) worms, a significant number (39% for the double mutant compared with 3% for brap-2) of the brap-2;brc-1 animals were capable of developing to adult stage (Table 2).
TABLE 2.
Larval arrest and lethality of brap-2 mutants require brc-1
Adult worms from N2 (wild-type), brap-2(ok1492), brc-1(tm1145), brap-2(ok1492);brc-1(tm1145), lin-35(n745), lin-35(n745);brap-2(ok1492), cep-1(gk138), and cep-1(gk138);brap-2(ok1492) strains were allowed to lay eggs on plates containing OP50 and 0.1 mm paraquat for 8 h and removed. The progeny were analyzed over 7 days and scored for larval lethality, larval arrest, and full development to adult stage. This experiment was done three times, and the cumulative totals were pooled. Total numbers of worms scored are in parentheses.
| Genotype | Larval lethality | Larval arrest | Adult |
|---|---|---|---|
| % | |||
| Wild-type (295) | 0 | 0 | 100 |
| brap-2 (142) | 99 | 1 | 0 |
| brc-1 (200) | 0 | 0 | 100 |
| brap-2;brc-1 (106) | 9 | 52 | 39 |
| lin-35 (109) | 0 | 23 | 77 |
| lin-35;brap-2 (82) | 100 | 0 | 0 |
| cep-1 (161) | 8 | 70 | 22 |
| cep-1;brap-2 (120) | 61 | 37 | 2 |
Mammalian BRCA1 cell cycle arrest activity has been shown to be dependent on p53 and Rb (23, 24). Therefore, we tested the Rb and p53 homologs in C. elegans (lin-35 and cep-1) to determine whether the developmental arrest of the brap-2 mutant in the presence of oxidative stress required the function of these two genes. Double mutants were synthesized carrying the brap-2(ok1492) with lin-35(n745) and brap-2(ok1492) with cep-1(gk138) and tested for their ability to develop in the presence of paraquat. It was apparent that lin-35 was completely unable to rescue the oxidative stress-inducing larval arrest phenotype of brap-2 minus worms, indicating that lin-35 was not required (Table 2). However, removal of cep-1 function moderately prevented larval lethality (61% versus 91% lethality for brap-2 alone; Table 2) and further development through the L3 or L4 stages (data not shown), but the double mutant strain was not able to develop completely to the adult stage. Therefore, developmental arrest and lethality in brap-2 worms exposed to oxidative stress appears to require brc-1 specifically and is independent of lin-35 and cep-1.
BRC-1 Increases the Expression of cki-1 in brap-2 Mutants during Oxidative Stress
The cyclin-dependent kinase inhibitor cki-1 is required for developmental cell-cycle arrest including embryonic cell cycle exit and timing of postembryonic cell divisions (22, 25). cki-1 is highly expressed in the V lineage, also known as seam/blast cells, which divide asymmetrically during early larva development and contribute to the lateral epidermal seam as the worm body elongates. To test whether cki-1 expression levels were altered during development of brap-2 mutants in the presence of oxidative stress, transgenic worms brap-2 deletion mutants that contain the cki-1::GFP transcriptional reporter construct were exposed to paraquat and showed high expression within the seam cells (compare Fig. 4, A and B) indicating that these blast cells have arrested cell cycle progression at lower concentrations of paraquat than do wild-type nematodes.
FIGURE 4.
brc-1-dependent brap-2 expression of cki-1 upon oxidative stress induces developmental arrest. Worms carrying an integrated cki-1::GFP reporter gene were crossed into N2 (A), brap-2(ok1492) (B) and brap-2(ok1492) (C); brc-1(tm1145) worms and allowed to hatch in the presence of 0.1 mm paraquat. One-day-old larvae were then removed and placed under 60× magnification. Expression of cki-1::GFP is present in N2 and mutant animals in some neurons in the head and tail, whereas strong expression is visible in the blast cells of brap-2(ok1492) L1s (white arrows), but this up-regulation is reduced in the brap-2;brc-1 double mutant.
In mammals, BRCA-1 has been demonstrated to induce p21 in cultured human cancer cells to inhibit progression of the cell cycle (6). To test the possibility that cki-1 expression levels is affected by the brc-1 deletion, we created transgenic worms that contained the cki-1::GFP transcriptional reporter in the brap-2;brc-1 double mutants. When exposed to paraquat plates, these worms failed to show elevated levels of cki-1 gene expression (Fig. 4C), indicating that the requirement of brc-1 to induce developmental arrest of brap-2 mutants under oxidative stress is due to the induction of cki-1 gene expression.
DISCUSSION
C. elegans has demonstrated to be a useful organism for studying oxidative stress response, with numerous studies showing mutants with sensitivity to the paraquat during adulthood. In this study, we investigated the effect of superoxide radicals in the developing organism. At high concentrations, wild-type worms exhibit larval arrest and lethality during chronic exposure, whereas at much lower concentrations, a mutant of the Brap2 homolog (brap-2) in C. elegans is hypersensitive to paraquat during development. This hypersensitivity was shown to involve an increase in gene expression of the cell cycle inhibitor cki-1 in blast/seam cells, suggesting that cell cycle arrest is occurring during larval development of brap-2 worms under these conditions. Furthermore, we found that a genetic interaction between BRCA1 and Brap2 is functionally relevant as the larval arrest of brap-2 mutant animals to oxidative stress requires a functional brc-1 allele, which is responsible for an increase in the expression levels of the cell cycle inhibitor cki-1.
BRCA1 is necessary for DNA damage repair and maintaining genomic integrity, a function that is conserved with the BRC-1 homolog in C. elegans. In mammals, genomic instability due to the absence of BRCA1 function blocks cell proliferation and induces apoptosis. There are a number of distinct pathways that influence the cell cycle that involves BRCA1-interacting proteins such as ATM, BARD1, Rb, p53, and p21WAF1/CIP1. In C. elegans, we show here that BRC-1 does play a role in cell cycle progression by regulating the p21 ortholog cki-1, at least in the context of oxidative stress response during development, although in this case, the pathway does not require the Rb and p53 homologs lin-35 and cep-1.
Mammalian Brap2 is postulated to function as a cytoplasmic retention protein for BRCA1, p21, and Cdc14 (11, 13, 14) and also may target the Ras signaling scaffold protein KSR to the triton insoluble fraction of the cell (12). It contains a H2-RING finger and functions as an E3 ligase capable of ubiquitinating itself, allowing for redistribution of associated proteins to other locations within the cell, such as the nucleus, upon the proteasomal degradation of Brap2 (12, 26, 27). The C. elegans BRAP-2 contains all of the currently recognizable domains of the mammalian form and would appear likely to fulfill the same cellular function as a cytoplasmic retention protein for BRC-1 and/or CKI-1 and prevent its inappropriate activation in the presence of lower levels of reactive oxygen species. However, during our studies, we were unable to detect any direct interaction between BRAP-2 and BRC-1 or CKI-1. With respect to the mammalian BRCA1, the site of Brap2 interaction to BRCA1 was mapped to a region found in exon 11 (11), which is present in a specific splicing variant of BRCA1. The brc-1 gene in C. elegans has no region that demonstrates homology with this exon (8). Therefore, although BRC-1 and BRAP-2 in C. elegans function genetically in a pathway responsible for oxidative stress response, the direct physical interaction between these two proteins does not appear to be conserved. Furthermore, the increase in cki-1 activity in brap-2 mutants appears to occur at the transcriptional level and not due to a direct physical interaction between these two proteins.
We cannot rule out that other BRAP-2-interacting proteins may be required for the sensitivity to oxidative stress displayed by brap-2 minus worms. The mammalian Brap2 has been shown to interact with other cell cycle-regulating proteins, such as Cdc14. Interaction of BRAP-2 with the C. elegans homologs of Cdc14 has not yet been demonstrated, but it is possible that there is a coordinated response to enhanced oxidative species controlled by BRAP-2 to prevent progression of cell cycle than just the activation of BRC-1 to increase CKI-1 levels.
Because Brap2 has also been postulated to be a negative regulator of the Ras signaling pathway, it is possible that Brap2 mediates Ras signaling to BRCA1. Ras has been shown to be important in ROS-signaling pathways through the RTK adaptor Shc (28). There are three Ras homologs in C. elegans; the most well known gene is let-60, which has a well defined role in cell fate decisions of the vulva precursor cells (29). We tested brap-2 to determine whether it is involved in the let-60 signaling pathway by creating a double mutant with brap-2 deleted in the let-60(gf) background but saw no increase in the number of multivulva animals. We also tested let-60(gf) for oxidative sensitivity by incubating these animals on paraquat plates but only a slight sensitivity toward oxidative stress during development was apparent. These results suggest that brap-2 is neither a negative regulator of the LET-60 signaling pathway, nor is LET-60 essential in oxidative stress response. However, we have yet to test the other two Ras homologs in C. elegans (ras-1 and ras-2) for oxidative stress sensitivity, so a role of BRAP-2 in Ras signaling cannot be dismissed.
In conclusion, we found that the C. elegans Brap2 homolog is hypersensitive to paraquat during development that leads to an increase in gene expression of the cyclin-dependent kinase inhibitor cki-1 in blast/seam cells. The larval arrest of brap-2 mutant animals to oxidative stress specifically requires a wild-type brc-1 allele, which is responsible for the increase in the expression levels of cki-1. Therefore, BRAP-2 is necessary to prevent the aberrant induction of the BRC-1/CKI-1 signaling network caused by excessive ROS and indicates that BRAP-2 is required for cells to tolerate enhanced amounts of ROS. If this function is conserved in mammals, Brap2 may prevent cell cycle arrest and cell death due to excessive production of ROS.
Acknowledgment
We thank Dr. Peter St. George-Hyslop (Centre forResearch in Neurodegenerative Diseases, Toronto, Canada) for initial discussions regarding BRAP-2.
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada.
- ROS
- reactive oxygen species.
REFERENCES
- 1.Weinberg F., Chandel N. S. (2009) Cell. Mol. Life Sci. 66, 3663–3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burhans W. C., Heintz N. H. (2009) Free Radic. Biol. Med. 47, 1282–1293 [DOI] [PubMed] [Google Scholar]
- 3.Martindale J. L., Holbrook N. J. (2002) J. Cell. Physiol. 192, 1–15 [DOI] [PubMed] [Google Scholar]
- 4.Steele R. J., Thompson A. M., Hall P. A., Lane D. P. (1998) Brit. J. Surgery 85, 1460–1467 [DOI] [PubMed] [Google Scholar]
- 5.Thompson D., Easton D. F.the Breast Cancer Linkage Consortium (2002) J. Natl. Cancer Inst. 94, 1358–1365 [DOI] [PubMed] [Google Scholar]
- 6.Somasundaram K., Zhang H., Zeng Y. X., Houvras Y., Peng Y., Zhang H., Wu G. S., Licht J. D., Weber B. L., El-Deiry W. S. (1997) Nature 389, 187–190 [DOI] [PubMed] [Google Scholar]
- 7.Deng C. X. (2006) Nucleic Acids Res. 34, 1416–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulton S. J., Martin J. S., Polanowska J., Hill D. E., Gartner A., Vidal M. (2004) Curr. Biol. 14, 33–39 [DOI] [PubMed] [Google Scholar]
- 9.Adamo A., Montemauri P., Silva N., Ward J. D., Boulton S. J., La Volpe A. (2008) EMBO Rep. 9, 287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polanowska J., Martin J. S., Garcia-Muse T., Petalcorin M. I., Boulton S. J. (2006) EMBO J. 25, 2178–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S., Ku C. Y., Farmer A. A., Cong Y. S., Chen C. F., Lee W. H. (1998) J. Biol. Chem. 273, 6183–6189 [DOI] [PubMed] [Google Scholar]
- 12.Matheny S. A., Chen C., Kortum R. L., Razidlo G. L., Lewis R. E., White M. A. (2004) Nature 427, 256–260 [DOI] [PubMed] [Google Scholar]
- 13.Asada M., Ohmi K., Delia D., Enosawa S., Suzuki S., Yuo A., Suzuki H., Mizutani S. (2004) Mol. Cell Biol. 24, 8236–8243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J. S., Hu H. Y., Zhang S., He M., Hu R. M. (2009) Biotechnol. Lett. 31, 615–621 [DOI] [PubMed] [Google Scholar]
- 15.Brenner S. (1974) Genetics 77, 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaya P., Premkumar M., Varman Thampan R. (2001) Biochim. Biophys. Acta 1499, 171–179 [DOI] [PubMed] [Google Scholar]
- 17.Tawe W. N., Eschbach M. L., Walter R. D., Henkle-Dührsen K. (1998) Nucleic Acids Res. 26, 1621–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kniazeva M., Euler T., Han M. (2008) Genes Dev. 22, 2102–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sulston J. E., Horvitz H. R. (1977) Dev. Biol. 56, 110–156 [DOI] [PubMed] [Google Scholar]
- 20.Baugh L. R., Sternberg P. W. (2006) Curr. Biol. 16, 780–785 [DOI] [PubMed] [Google Scholar]
- 21.Henderson S. T., Bonafe M., Johnson T. E. (2006) J. Gerontol. 61, 444–460 [DOI] [PubMed] [Google Scholar]
- 22.Fukuyama M., Gendreau S. B., Derry W. B., Rothman J. H. (2003) Dev. Biol. 260, 273–286 [DOI] [PubMed] [Google Scholar]
- 23.Aprelikova O. N., Fang B. S., Meissner E. G., Cotter S., Campbell M., Kuthiala A., Bessho M., Jensen R. A., Liu E. T. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 11866–11871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacLachlan T. K., Takimoto R., El-Deiry W. S. (2002) Mol. Cell. Biol. 22, 4280–4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong Y., Roy R., Ambros V. (1998) Development 125, 3585–3597 [DOI] [PubMed] [Google Scholar]
- 26.Kipreos E. T. (2005) WormBook (The C. elegans Research Community, ed.) http://www.wormbook.org, 10.1895/wormbook.1.36.1 [DOI]
- 27.Moore R., Boyd L. (2004) Genesis 38, 1–12 [DOI] [PubMed] [Google Scholar]
- 28.Migliaccio E., Giorgio M., Mele S., Pelicci G., Reboldi P., Pandolfi P. P., Lanfrancone L., Pelicci P. G. (1999) Nature 402, 309–313 [DOI] [PubMed] [Google Scholar]
- 29.Sundaram M. V. (2006) WormBook (The C. elegans Research Community, ed.) http://www.wormbook.org, 10.1895/wormbook.1.80.1 [DOI]