Abstract
With rising obesity rates, nonalcoholic fatty liver disease is predicted to become the main cause of chronic liver disease in the next decades. Rising obesity prevalence is attributed to changes in dietary habits with increased consumption of palatable junk foods, but maternal malnutrition also contributes to obesity in progeny. This study examines whether a maternal junk food diet predisposes offspring to nonalcoholic fatty liver disease. The 144 rat offspring were fed either a balanced chow diet alone or with palatable junk foods rich in energy, fat, sugar, and/or salt during gestation, lactation, and/or after weaning up to the end of adolescence. Offspring fed junk food throughout the study exhibited exacerbated hepatic steatosis, hepatocyte ballooning, and oxidative stress response compared with offspring given free access to junk food after weaning only. These offspring also displayed sex differences in their hepatic molecular metabolic adaptation to diet-induced obesity with increased expression of genes associated with insulin sensitivity, de novo lipogenesis, lipid oxidation, and antiinflammatory properties in males, whereas the gene expression profile in females was indicative of hepatic insulin resistance. Hepatic inflammation and fibrosis were not detected indicating that offspring had not developed severe steatohepatitis by the end of adolescence. Hepatic steatosis and increased oxidative stress response also occurred in offspring born to junk food-fed mothers switched to a balanced chow diet from weaning, highlighting a degree of irreversibility. This study shows that a maternal junk food diet in pregnancy and lactation contributes to the development of nonalcoholic fatty liver disease in offspring.
With rising obesity rates, non-alcoholic fatty liver disease (NAFLD) requires the attention of endocrinologists; a maternal “junk food” diet is a factor contributing to the development of NAFLD in progeny by the end of adolescence.
Obesity is of widespread concern and associated with a range of disorders that affect multiple organs and endocrine systems. The liver is particularly affected, and epidemiological studies have shown that obesity, insulin resistance, and diabetes are associated with nonalcoholic fatty liver disease (NAFLD), although its etiology is not fully characterized (1,2). NAFLD is defined by excess hepatic fat accumulation in the absence of excess alcohol consumption (2). It can be benign but can also progress to nonalcoholic steatohepatitis (NASH) when excess hepatic fat promotes hepatocyte injury and fibrosis and can progress further to cirrhosis, liver failure, and cancer (2). The progression from simple steatosis to NASH involves a two-hit hypothesis (3) whereby excess hepatic fat (first hit) is accompanied by oxidative stress and inflammation (second hit), which promote fibrosis and cirrhosis (4,5). The prevalence of NASH is increasing and expected to become the first cause of chronic liver disease in the next 20 yr in the United States (6).
The root of obesity and related disorders is complex and involves multiple factors including genetics and lifestyle. Recent increase in obesity rates are, however, strongly linked to changes in dietary habits (7) with increased consumption of away-from-home foods (8); snacks (9); and junk foods that are dense in energy, fat, sugar, and/or sodium (10) but deprived of vitamins and micronutrients (11). Growing evidence, from both human and animal studies, suggests that maternal malnutrition in pregnancy and lactation also contributes to the development of obesity and associated disorders in offspring (12,13). Some studies have shown that the fatty acid composition and/or protein content of the maternal diet can affect hepatic lipid content and metabolic gene expression in progeny (14,15,16,17), whereas a maternal high-fat diet promotes steatosis and oxidative stress in fetal liver (18). Despite this evidence, the influence of a maternal diet rich in not only fat but also energy, sugar, and salt on the offspring’s liver is poorly characterized. We developed an animal model, based on the obesogenic supermarket (19) and cafeteria (20) diets to determine the effects of such a maternal diet in pregnancy and lactation and showed that it promoted overeating, obesity, and abdominal adiposity as well as hyperglycemia, hyperinsulinemia, and hyperlipidemia in progeny by the end of adolescence (21,22). The aim of the present study was to determine whether the same maternal junk food diet promotes hepatic steatosis and alters the expression of genes involved in glucose and lipid metabolism, oxidative stress response, inflammation, and fibrosis, thereby increasing the offspring’s susceptibility to NAFLD when compared with offspring born to mothers fed a balanced diet. The study focused on progeny given free access to junk food from weaning to model the easy accessibility to palatable foods in Western societies, but the maternal junk food diet’s irreversible effects were also examined in offspring fed a balanced diet from weaning.
Materials and Methods
Ethics
The animal work was carried out under Home Office license to comply with the U.K. Animals (Scientific Procedures) Act 1986 after approval by the Royal Veterinary College Ethics and Welfare Committee.
Animals
The animals used in this study were the same as those used in previous studies; therefore, protocols have been validated and published elsewhere (21,22). Briefly, 24 virgin female Wistar rats were mated (Charles River, Margate, Kent, UK). On the day a copulation plug was found, the dams were randomly assigned to one of four nutritional groups (see below). Litters were selected such that the number of pups in each litter was statistically the same across all nutritional groups to control for litter sizes during both gestation and lactation (22). Offspring survival rates and sex ratios were not affected by the diets. The pups were kept with their own mothers until weaning to reflect human conditions. At weaning (21 d postpartum), three male and three female offspring from each litter were selected to span the range of birth weights, thus taking into account intralitter variations for a more powerful statistical design. Male and female littermates were housed separately from weaning until culling at 10 wk postpartum, which corresponds to the end of adolescence in rats (23). A total of 144 offspring were analyzed, giving 36 offspring in each nutritional group (18 males plus 18 females), six litters per group and six animals per litter.
The rats were fed either a control (C) or junk food (J) diet during three critical growth phases, namely gestation, lactation, and postweaning. Four groups were analyzed, namely CCC, CCJ, JJC, and JJJ, with each letter corresponding to the diet given during gestation, lactation, and postweaning respectively (21). The control diet consisted of RM3 chow (SDS Ltd., Betchworth, Surrey, UK), whereas the junk food diet consisted of RM3 chow plus eight types of palatable industrially processed foods (biscuits, potato crisps, sweets, cheese, etc.) all supplied ad libitum. The junk food items were designed for human consumption and selected for their high energy, fat, sugar, and/or salt content to conform to the broad definition of junk food (10). Detailed information on ingredients, energy, and macronutrient intakes as well as their effects on body weights, serum glucose, insulin, triglyceride, and cholesterol have been published elsewhere (21,22). Gender-specific energy and macronutrient intakes relative to body weights are summarized in Supplemental Table 1 published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org. Food was removed 2 h before the animals were killed to stabilize blood metabolites, and the animals were culled by rising concentration of CO2.
Serum biochemistry
Blood samples were collected within minutes of death and sera stored at −80 C until subsequent liver function tests, namely the assay of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and γ-glutamyl transferase (GGT) by the Diagnostics Laboratory Services at the Royal Veterinary College (London, UK).
Sample processing
Livers were dissected out and weighed. Portions of the left and right lobes were either flash frozen in isopentane (Oil Red O) or fixed in buffered formalin [hematoxylin and eosin (H&E) and Masson’s trichrome] for histological analyses. The remaining liver samples were flash frozen in liquid nitrogen and stored at −80 C for subsequent RNA extraction and assays of triglyceride and cholesterol. The formalin fixed liver samples were wax embedded and processed for histological analysis as previously described (21).
Hepatic lipid assays
Hepatic lipids were extracted according to Folch et al. (24). Briefly, 200 mg of liver were homogenized in 12 ml chloroform/methanol [2:1 (vol/vol)], left to stand for 1 h, mixed with 5 ml of 0.88% KCl, and kept overnight at 4 C. Fractions of the organic phase were evaporated under nitrogen, resuspended in isopropanol, and assayed for total cholesterol and triglycerides using enzymatic-colorimetric methods (Sentinel Diagnostics, Milan, Italy).
RNA extraction, reverse transcription (RT), and real-time PCR
Detailed protocols for RNA extraction, RT, and real-time PCR have been published elsewhere (21). Briefly, whole-liver samples were homogenized in Tri-Reagent (Sigma, Dorset, UK) with subsequent chloroform extraction and ethanol precipitation followed by deoxyribonuclease treatment and further purification on RNeasy columns (QIAGEN, Crawley, UK). Concentration, purity, and integrity were measured and verified using the Nanodrop N-1000 system (Nanodrop Technologies, Wilmington, DE) and formaldehyde gel electrophoresis. RT was performed simultaneously in all samples using the same master mix of Quantitect RT kit from a fixed amount (1 μg) of total RNA (QIAGEN) following the manufacturer’s instructions. Real-time PCR primers were designed using the Primer-3 web software (Whitehead Institute for Biomedical Research, Cambridge, MA) and synthesized by MWG-Biotech (Ebersberg, Germany). Sequences and accession numbers are listed in Table 1. SyBR green fluorescence was measured after each real-time PCR cycle (Bio-Rad, Herts, UK). Relative target amplicon expression was assayed using the linear regression method (25) with R2 greater than 0.99 for each gene and expressed as arbitrary units.
Table 1.
Real-time PCR primers in the 5′ to 3′ direction
| Gene | Forward | Reverse | Product length | Access no. |
|---|---|---|---|---|
| ALT | agggtggagtatgcagttcg | ggcacggatgacctcagtaa | 105 | NM_031039 |
| AST | ttgctgggaaaaatttggac | cggaaatggtctgcacagtt | 141 | NM_013177 |
| ALP | ccttgaaaaatgccctgaaa | gcagctgtcactgtggagac | 103 | NM_013059 |
| GGT1 | gtgggggagttcctgcttat | gcaccagaaaccgattcttc | 107 | NM_053840 |
| Glut-2 | gacatcggtgtgatcaatgc | tgtcgtatgtgctggtgtga | 150 | NM_012879 |
| Glucokinase | ggatgcagaaggagatggac | agttggttcctcccaggtct | 144 | NM_012565 |
| Glycogen synthase 2 | tttcctgggaagtgaccaac | catgtttgttcatggcatcc | 198 | NM_013089 |
| Glycogen phosphorylase | tgctgcctgtgcatttctac | accagaggcgcatagtgttc | 144 | NM_022268 |
| FAS | gcacctgcagatcctttgat | gtcccggcattcagaatagt | 124 | NM_017332 |
| IR | atctcctgggattcatgctg | tactgggtccagggtttgag | 196 | M29014 |
| IGF-IR | catgcaggagtgtccatcag | ctcgccggatgttaataagc | 194 | NM_052807 |
| IGF-I | gcttgctcacctttaccag | aagtgtacttccttctgagtct | 300 | M17335 |
| IRS-1 | tcttggaatgtggaactgagg | tccagaaccttctatggcact | 162 | NM_012969 |
| IRS-2 | ccacacacctgtcctcattg | cagcaatggccaggacttat | 133 | XM_573948 |
| PPARα | gagaccctcggggatcttag | cgtcttgtgtcctgagcttg | 102 | NM_013196 |
| PPARδ | atgaggacaaacccacggta | gttccatgactgaccccact | 109 | NM_013196 |
| PPARγ | ccctggcaaagcatttgtat | actggcacccttgaaaaatg | 222 | AB011365 |
| LRH-1 | gcaccaggatcagagactcc | gaggcttccgtctccacttt | 143 | NM_021742 |
| CPT-1a | aaggtgctgctctcctacca | cgacctgagaggaccttgac | 101 | NM_031559.2 |
| UCP-2 | aagggacctctcccaatgtt | ccgaaggcagaagtgaagtg | 139 | NM_019354 |
| CYP2E1 | ccttgggaacatttttcagc | cacctccttgacagccttgt | 145 | NM_031543 |
| Gpx-1 | tgagaagtgcgaggtgaatg | cggggaccaaatgatgtact | 127 | NM_030826 |
| SOD-1 | ggagagcattccatcattgg | caatcacaccacaagccaag | 127 | NM_017050.1 |
| SOD-2 | ccgaggagaagtaccacgag | tagggctcaggtttgtccag | 129 | NM_017051 |
| Catalase | gtggttttcaccgacgagat | atcgtgggtgacctcaaagt | 108 | M11670 |
| TNF-α | gctccctctcatcagttcca | gcttggtggtttgctacgac | 102 | NM_012675 |
| IL-6 | accacccacaacagaccagt | acagtgcatcatcgctgttc | 136 | NM_012589 |
| Collagen 1α | tgttcagctttgtggacctc | ggtttccacgtctcaccatt | 136 | NM_053304 |
| Keratin 8 | cctggtggaggacttcaaga | aggcttcatccacatccttc | 101 | NM_199370 |
Statistical analyses
Statistical analyses were performed as previously reported (21,22) using the SPSS 14.0 for Windows software (SPSS Inc., Chicago, IL). Data were analyzed by hierarchical two-way ANOVA after log transformation whenever necessary to achieve normal distribution. The fixed factors were defined as dietary group and sex, whereas mother was defined as random factor. The models were set as group, sex, mother (group), and groupsex to take into account litter effects and intralitter variations. When the hierarchical ANOVA indicated groupsex interactions (P < 0.05), male and female offspring were analyzed separately by one-way ANOVA. Tukey honestly significantly different or Games-Howell post hoc tests were subsequently performed, depending on whether equal variances should be assumed. Correlations between some of the parameters measured were assessed by two-tailed Pearson’s tests. Results were considered statistically significant when P < 0.05.
Results
Liver mass and fat content
Hepatomegaly features in 75% of NAFLD patients (2). Table 2 shows that liver mass increased in offspring fed the J diet throughout the study (JJJ) compared with all other groups (P < 0.05 in all cases). This increase was approximately 20% for both JJJ male and female offspring compared with the CCC group. The liver mass of CCJ and JJC progeny did not differ from the CCC group. When liver mass was expressed relative (percentage) to body weight, there were no significant differences among all nutritional groups (P = 0.405); therefore, liver mass increased proportionally to body weight (Table 2).
Table 2.
Liver mass and lipid content
| Male offspring
|
Female offspring
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CCC | CCJ | JJC | JJJ | CCC | CCJ | JJC | JJJ | |
| Liver mass (g) | 22.24 ± 0.43a,b | 24.04 ± 0.80a | 20.25 ± 0.33b | 26.85 ± 1.14c | 12.06 ± 0.32a,b | 12.85 ± 0.35a | 10.83 ± 0.18b | 14.50 ± 0.66c |
| Liver mass/body weight100 | 5.74 ± 0.11 | 5.85 ± 0.13 | 5.50 ± 0.07 | 5.67 ± 0.13 | 5.13 ± 0.08 | 4.88 ± 0.10 | 4.90 ± 008 | 4.67 ± 0.08 |
| Liver triglycerides | ||||||||
| Liver (mg/g) | 8.71 ± 0.45a | 33.97 ± 2.74b | 13.43 ± 1.42c | 36.03 ± 2.52b | 9.22 ± 0.50a | 34.32 ± 4.53b | 14.67 ± 1.10c | 56.97 ± 3.86d |
| Milligrams per liver | 206.72 ± 17.22a | 821.79 ± 73.77b | 270.43 ± 26.75a | 982.59 ± 97.47c | 156.54 ± 19.58a | 512.71 ± 74.24b | 173.75 ± 18.27a | 847.21 ± 63.14c |
| Liver cholesterol | ||||||||
| Liver (mg/g) | 2.21 ± 0.07a | 3.00 ± 0.14b | 2.15 ± 0.70a | 2.81 ± 0.14b | 2.17 ± 0.12a | 2.65 ± 0.13b | 2.25 ± 0.06a | 2.72 ± 0.13b |
| Milligrams per liver | 49.31 ± 2.10a | 71.51 ± 2.34b | 43.65 ± 1.40a | 73.54 ± 4.47b | 26.29 ± 1.44a | 33.98 ± 1.74b | 24.39 ± 0.87a | 38.83 ± 2.03b |
Liver mass, triglyceride, and cholesterol content in 10-wk-old rat offspring fed either chow alone (C) or with a junk food diet (J) during gestation, lactation, and/or after weaning, respectively. Results indicate hepatomegaly and exacerbated steatosis in offspring fed junk food from fetal life (JJJ). Results are mean ± sem. Different superscript letters (a, b, c, d) refer to the post hoc analyses, with different letters indicating statistical significance (P < 0.05) between nutritional groups, whereas the absence of letters indicates nonsignificance (P > 0.05) from the hierarchical two-way ANOVA; n = 36 offspring (18 males and 18 females) in each nutritional group (see text for details on statistical analysis).
Hepatic triglycerides and total cholesterol were expressed either relative to 1 g of tissue (density) or for the whole liver (total). Table 2 shows increased hepatic triglyceride density in male and female offspring weaned on the J diet compared with those weaned on chow, with a greater increase in JJJ female but not male progeny compared with the CCJ group (P < 0.05 in all cases). Triglyceride density also increased in JJC progeny compared with CCC (P < 0.05 for both males and females); however, total liver triglyceride content was statistically similar between these two groups (Table 2). Total liver triglyceride was greater in JJJ offspring compared with all other groups (P < 0.05 for all), and it was higher in the CCJ group compared with CCC and JJC groups (P < 0.001 for all).
Liver cholesterol, whether expressed per gram of tissue or for the whole organ, was equally increased in male and female progeny weaned on the J diet compared with those weaned on chow (P < 0.001 for all) (Table 2).
Liver function tests
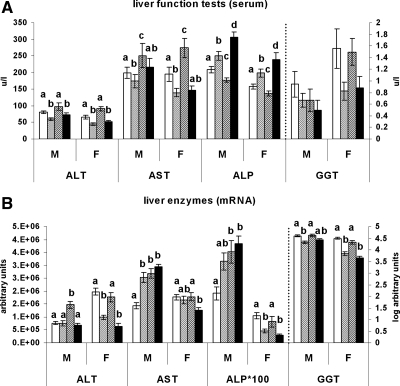
Serum ALT decreased in offspring weaned on the J diet compared with those weaned on chow (P < 0.05 in all cases), and the maternal diet did not affect those levels (Fig. 1A). Serum AST increased in the JJC group compared with all other groups (P < 0.05 for all) and decreased in the CCJ group (P = 0.041) but was unaffected in the JJJ group (P = 0.676) compared with the CCC group. Serum ALP was raised in the JJJ group compared with all other groups (P < 0.001) and in the CCJ group compared with both the CCC and JJC groups (P < 0.001 for both), whereas GGT was not affected by the dietary regimens (P = 0.402).
Figure 1.
Liver function tests and expression analysis. ALT, AST, ALP, and GGT levels in serum (A) and hepatic mRNA expression (B) in 10-wk-old rat offspring fed either chow alone (C) or with a junk food diet (J) during gestation, lactation, and postweaning, respectively. CCC, Open bars; CCJ, cross-hatching; JJC, diagonal striping; JJJ, solid black. Results are mean ± sem. Different letters (a, b, c, d) on the graphs indicate statistical differences between nutritional groups by hierarchical two-way ANOVA followed by post hoc analyses (P < 0.05), whereas the absence of letters (i.e. serum GGT) indicates nonsignificance (P > 0.05); n = 36 offspring (18 males and 18 females) in each nutritional group (see text for details on statistical analysis).
Pearson’s analyses showed a significant correlation between ALT mRNA expression (Fig. 1B) and serum levels in male and female offspring (P < 0.05 for both). In contrast, serum and hepatic mRNA for AST did not correlate (P = 0.482). There was a negative and positive correlation between serum and mRNA levels for ALP and GGT, respectively, in female offspring (P = 0.025 and P = 0.023) but not males (P = 0.089 and P = 0.099).
Histological examination
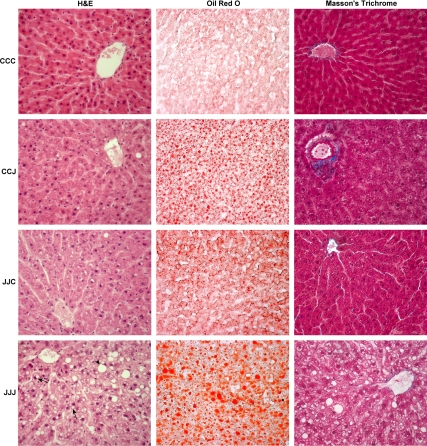
Figure 2 shows that JJJ offspring exhibited enlarged hepatocytes with both macro- and microvesicular steatosis (H&E). The exacerbated steatosis in the JJJ group was confirmed by Oil Red O staining, but there were no signs of inflammation or fibrosis (Masson’s trichrome). CCJ and JJC offspring did not show signs of ballooning degeneration, inflammation, or fibrosis. The histological analysis did not reveal major differences between male and female offspring within each nutritional group.
Figure 2.
Histological analysis. Representative H&E, Oil Red O, and Masson’s trichrome stains of livers from 10-wk-old rat offspring fed either chow alone (C) or with a junk food diet (J) during gestation, lactation, and postweaning, respectively (magnification, ×400). Male and female JJJ offspring exhibit hepatocyte ballooning (H&E) with macrovesicular (arrowhead) and microvesicular (arrows) steatosis and a greater degree of steatosis (Oil Red O) but absence of fibrosis (Masson’s) compared with all other groups. Gender differences were not evident.
Gene expression analysis
Glucose uptake and metabolic enzymes
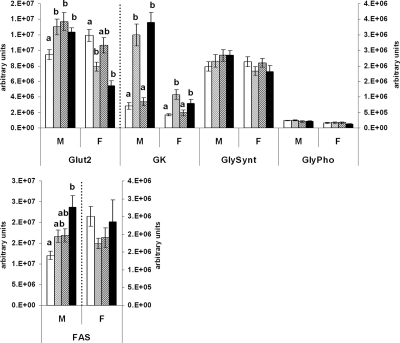
Glucose transporter (Glut)-2 mRNA was raised in all males fed the J diet at some stage in the study compared with the CCC group (P < 0.05 in all cases), and levels were not statistically different among CCJ, JJC, and JJJ groups. In females, Glut-2 mRNA was decreased in offspring weaned on the J diet (CCJ and JJJ groups) compared with the CCC group (P = 0.002 and P < 0.001, respectively), whereas levels in the JJC group were not different from any other group. Glucokinase mRNA was increased in offspring weaned on the J diet compared with those weaned on chow (P < 0.05 in all cases), and there were no differences between the CCC and JJC groups or between the CCJ and JJJ groups (Fig. 3).
Figure 3.
Glucose uptake and metabolic enzymes. Real-time PCR analysis of hepatic genes regulating glucose uptake, glycogenesis, glycogenolysis, and de novo lipogenesis in 10-wk-old rat offspring fed either chow alone (C) or with a junk food diet (J) during gestation, lactation, and postweaning, respectively. CCC, Open bars; CCJ, cross-hatching; JJC, diagonal striping; JJJ, solid black; GK, glucokinase; GlySynt, glycogen synthase; GlyPho, glycogen phosphorylase. Results are indicative of favored glucose uptake and de novo lipogenesis in JJJ males compared with the CCC group. Results are mean ± sem. Different letters (a, b) on the graphs indicate statistical differences between nutritional groups by hierarchical two-way ANOVA followed by post hoc analyses (P < 0.05), whereas the absence of letters indicates nonsignificance (P > 0.05); n = 36 offspring (18 males and 18 females) in each nutritional group (see text for details on statistical analysis).
The mRNA expressions of glycogen synthase and glycogen phosphorylase, which regulate glycogenesis and glycogenolysis, respectively, were not affected by the dietary regimens (P = 0.656 and P = 0.430, respectively) (Fig. 3).
In contrast, the mRNA expression of fatty acid synthase (FAS), which regulates the maximal capacity of de novo lipogenesis (26), was increased in JJJ male offspring compared with the CCC group (P = 0.003) but was not statistically different among CCJ, JJC, and JJJ groups (Fig. 3). In females, FAS expression was not affected (P = 0.421).
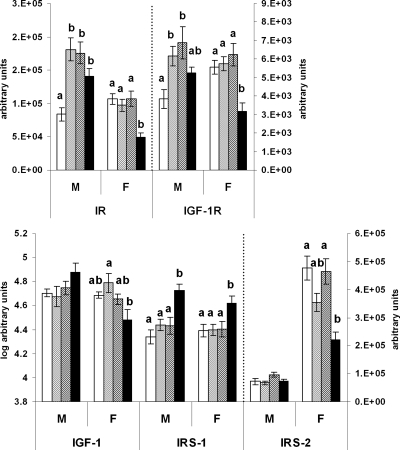
Insulin and IGF signal
Figure 4 shows that the insulin receptor (IR) mRNA increased in male offspring fed the J diet at some stage during the study (CCJ, JJC, and JJJ groups) compared with CCC offspring (P < 0.05 in all cases) and that levels were not different among CCJ, JJC, and JJJ groups. In contrast, JJJ females down-regulated their hepatic expression of IR compared with all other groups (P < 0.01), and expression did not differ among CCC, CCJ, and JJC groups. IGF-I receptor (IGF-IR) mRNA followed an expression profile similar to that of IR with a positive and significant Pearson’s correlation (P < 0.001 for males and females), but unlike IR, IGF-IR mRNA did not reach significance in JJJ males when compared with CCC, CCJ, and JJC groups (Fig. 4).
Figure 4.
Insulin and IGF signal. Real-time PCR analysis of hepatic genes regulating the insulin/IGF signal in 10-wk-old rat offspring fed either chow alone (C) or with a junk food diet (J) during gestation, lactation, and postweaning, respectively. CCC, Open bars; CCJ, cross-hatching; JJC, diagonal striping; JJJ, solid black. Results are suggestive of increased and decreased insulin/IGF signal in JJJ males and females, respectively, compared with the CCC group. Results are mean ± sem. Different letters (a, b) on the graphs indicate statistical differences between nutritional groups by hierarchical two-way ANOVA followed by post hoc analyses (P < 0.05), whereas the absence of letters indicates nonsignificance (P > 0.05); n = 36 offspring (18 males and 18 females) in each nutritional group (see text for details on statistical analysis).
Hepatic IGF-I mRNA expression was not affected in male progeny (P = 0.153) but was reduced in JJJ females compared with the CCJ group (P = 0.006). Insulin receptor substrate (IRS)-1 mRNA increased in both JJJ male and female offspring compared with all other groups (P < 0.01 in all cases) but was unchanged in CCC, CCJ, and JJC groups. IRS-2 mRNA expression profile was different from that of IRS-1 and was not significantly affected in male offspring (P = 0.088). In females, IRS-2 mRNA was reduced in the JJJ group compared with the CCC and JJC groups (P < 0.001 for both) but was not different from the CCJ group (P = 0.067). IRS-2 mRNA expression was similar in the CCC, CCJ, and JJC females.
Metabolic nuclear factors
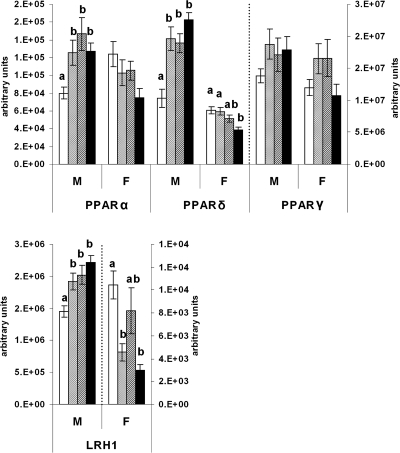
Pearson’s analyses revealed a positive correlation between peroxisome proliferator-activated receptor (PPAR)-α and PPARδ (P < 0.05 for males and females). Figure 5 shows that PPARα and PPARδ expressions were equally increased in male offspring fed the J diet at some stage (CCJ, JJC, and JJJ) compared with CCC offspring (P < 0.05, in all cases). In females, changes in PPARα expression did not reach statistical significance (P = 0.059), but PPARδ mRNA was reduced in the JJJ group compared with both the CCC and CCJ groups (P < 0.01 for both). In contrast, PPARγ mRNA expression was not statistically affected by the dietary regimens (P = 0.466).
Figure 5.
Metabolic nuclear factors. Real-time PCR analysis of hepatic nuclear factors regulating lipid homeostasis in 10-wk-old rat offspring fed either chow alone (C) or with a junk food diet (J) during gestation, lactation, and postweaning, respectively. CCC, Open bars; CCJ, cross-hatching; JJC, diagonal striping; JJJ, solid black. Results indicate sex differences in PPAR and LRH-1 expression in JJJ offspring. Results are mean ± sem. Different letters (a, b) on the graphs indicate statistical differences between nutritional groups by hierarchical two-way ANOVA followed by post hoc analyses (P < 0.05), whereas the absence of letters indicates nonsignificance (P > 0.05); n = 36 offspring (18 males and 18 females) in each nutritional group (see text for details on statistical analysis).
Liver receptor homolog (LRH)-1 mRNA expression was increased in all male offspring fed the J diet at some stage in the study compared with the CCC group (P < 0.05 in all cases), and levels did not differ among CCJ, JJC, and JJJ groups. In females, LRH-1 mRNA expression was reduced in those weaned on the J diet (CCJ and JJJ groups) compared with the CCC group (P ≤ 0.001 for both). Pearson’s analysis showed a strong correlation between LRH-1 and Glut-2 mRNAs (P < 0.001), but despite its characterized role in cholesterol transport (27), LRH-1 mRNA and hepatic cholesterol levels did not significantly correlate (P = 0.943 and P = 0.219 for cholesterol density in males and females; P = 0.860 and P = 0.100 for total cholesterol in males and females).
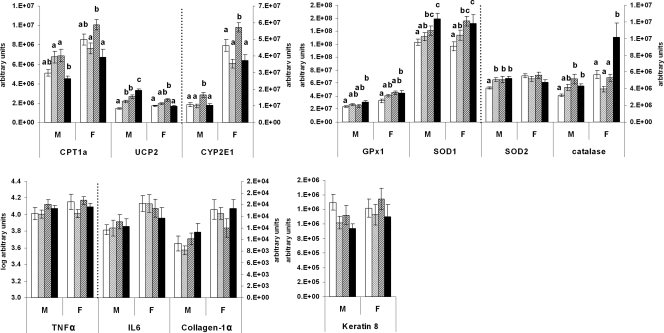
Lipid oxidation, oxidative stress response, and inflammation and fibrosis
The expression of carnitine palmitoyltransferase (CPT)- was similar to the CCC group in both JJJ male and female progeny (Fig. 6). The mitochondrial uncoupling protein (UCP)-2 mRNA was increased in male offspring from the JJJ group compared with all other groups. UCP-2 levels in JJJ females were similar to those in the CCC group. UCP-2 mRNA was also higher in the CCJ and JJC males as well as in CCJ females compared with the CCC group.
Figure 6.
Lipid oxidation, oxidative stress response, inflammation, and fibrosis. Real-time PCR analysis of genes involved in oxidative stress, inflammation, and fibrosis in 10-wk-old rat offspring fed either chow alone (C) or with a junk food diet (J) during gestation, lactation, and postweaning, respectively. CCC, Open bars; CCJ, cross-hatching; JJC, diagonal striping; JJJ, solid black. Results are suggestive of increased hepatic oxidative stress response in offspring born to junk food-fed mothers (JJC and JJJ groups) but no sign of inflammation or fibrosis. Results are mean ± sem. Different letters (a, b, c, d) on the graphs indicate statistical differences between nutritional groups by hierarchical two-way ANOVA followed by post hoc analyses (P < 0.05), whereas the absence of letters indicates nonsignificance (P > 0.05); n = 36 offspring (18 males and 18 females) in each nutritional group (see text for details on statistical analysis).
The expression of cytochrome P450 2E1 (CYP2E1) increased in JJC offspring compared with all other groups (P < 0.01 for all), whereas levels were similar among CCC, CCJ, and JJJ groups (Fig. 6).
An analysis of four antioxidant enzymes showed that JJJ offspring exhibited increased glutathione peroxidase (GPx)-1, superoxide dismutase (SOD)-1, and catalase mRNAs compared with the CCC group (P < 0.05 for all), whereas levels in the CCJ group were the same as in the CCC group (Fig. 6). In JJC males, SOD1 and catalase (P < 0.05 for both) but not GPx1 mRNAs were increased compared with the CCC group, and SOD1 was also increased in females. SOD2 was up-regulated in all male offspring fed the J diet at some stage in the study compared with the CCC group (P < 0.05 for all) but was not affected in females (Fig. 6).
Inflammation and fibrosis were assessed by measurements of the proinflammatory cytokines TNF-α and IL-6 (28,29) as well as collagen-1α and keratin 8 (30,31), respectively. Figure 6 shows that the expressions of TNFα (P = 0.071), IL-6 (P = 0.806), collagen-1α (P = 0.820), and keratin 8 (P = 0.165) were not affected.
Discussion
A maternal junk food diet promotes exacerbated steatosis and hepatocyte ballooning in offspring by the end of adolescence
Changes in dietary habits with increased intake of junk foods are believed to fuel the current obesity epidemic (8,10), with maternal malnutrition also contributing to obesity and related disorders in offspring (13). This study examines the role of a maternal junk food diet rich in energy, fat, sugar, and salt on the offspring’s susceptibility to NAFLD. The main finding is that although all offspring weaned on the J diet exhibited increased total liver triglyceride compared with offspring never fed junk food; this increase was exacerbated in offspring fed the J diet from fetal life. Interestingly, offspring born to junk food-fed mothers switched to an exclusive chow diet from weaning also exhibited increased triglyceride density compared with the CCC group at the end of adolescence. This coincides with a previous report in macaques showing that maternal high-fat feeding led to fetal liver steatosis, which persisted up to 6 months of age in offspring (18). Steatosis can be benign but can also induce hepatocyte ballooning injury, inflammation, and fibrosis (32). The exacerbated total hepatic triglyceride content in JJJ offspring was accompanied by vesicular steatosis and hepatocyte ballooning, whereas JJC and CCJ offspring did not show signs of injury, indicating more advanced liver damage in JJJ offspring. The histological analysis (Masson’s trichrome) did not however reveal signs of severe fibrosis and inflammation, which was confirmed by unchanged TNFα, IL-6, collagen-1α, and keratin 8 expressions. This suggests that although JJJ offspring showed signs of NAFLD, they did not appear to suffer from NASH.
Hepatic steatosis is sometimes associated with abnormal liver function tests with raised ALT and AST, which reflect cell damage and leakage into the bloodstream (2). In the present study, serum ALT and AST were not good indicators of hepatic steatosis as often occur in clinical situations (32). The surprising lower serum ALT levels in CCJ and JJJ offspring, which presented the greatest degree of steatosis correlated with mRNA levels, indicating that this may be due to reduced hepatic expression. ALT and AST catalyze the transfer of amino groups from alanine and aspartic acid, respectively, during gluconeogenesis (33). Although we did not measure specific dietary amino acid intake, we have reported that offspring weaned on the J diet consumed less protein than those weaned on chow (22). Therefore, the reduced ALT levels in CCJ and JJJ groups could be linked to a reduction in dietary alanine intake; however, this did not seem to apply to AST (aspartic acid), which is consistent with ALT’s greater hepatic specificity (33). The increased serum ALP in the JJJ group could reflect bile flow obstruction or cholestasis (33), consistent with exacerbated steatosis in this group but unchanged serum GGT, may imply absence of hepatobiliary disease in all offspring (33).
Sex differences in hepatic molecular metabolic adaptation to diet-induced obesity
Epidemiological studies suggest that men are more predisposed to NAFLD than women (34,35,36). This is reinforced by findings that hormone replacement therapy reduces the risk of NAFLD (37), whereas the estrogen antagonist tamoxifen promotes NASH (38).
In this study, it is not clear whether NAFLD was more severe in male than female progeny. Hepatic triglyceride density was higher in JJJ females, but this was not accompanied by greater histological hepatic damage, worse liver function tests, or increased inflammation and fibrosis. However, there were sex differences in the expression levels of metabolic genes, which could be attributed to sex differences in glycemia and insulinemia. We previously reported that JJJ male offspring were hyperinsulinemic with normal glycemia, whereas JJJ females were hyperglycemic with unaffected serum insulin. Both male and female JJJ progeny had raised serum triglyceride and cholesterol, but none of these parameters were statistically affected in the CCJ and JJC groups compared with the CCC group (21). Male offspring fed the J diet at some stage in the study exhibited a gene expression profile, which may be indicative of increased insulin signal characterized by raised IR, IGF-IR, and Glut-2 mRNAs. JJJ male offspring did not up-regulate any of these genes to a level greater than in the CCJ and JJC groups, but combined with increased circulating insulin (21), this may result in exacerbated hepatic insulin signal and glucose uptake in this group. This is further supported by elevated FAS expression in JJJ males, suggesting greater de novo lipogenesis, which may enhance their susceptibility to NASH because de novo lipogenesis markedly increases in hyperinsulinemic subjects with NASH (26,39).
A different expression profile was seen in female offspring. Those fed the J diet throughout the study exhibited a gene expression profile, which may be indicative of hepatic insulin resistance characterized by decreased IR and IGF-IR mRNAs compared with all other groups as well as decreased IRS-2 and Glut-2 mRNAs compared with the CCC group. Hepatic insulin resistance is closely associated with NAFLD and NASH (2); therefore, this gene expression profile could imply more advanced NAFLD in JJJ females. However, reduced hepatic glucose uptake may also protect JJJ females from further hepatic lipid accumulation via de novo lipogenesis, which is supported by unchanged FAS expression. Interestingly, we reported that the JJJ females used in this study exhibited a marked up-regulation of Glut-1, Glut-3, and lipoprotein lipase mRNAs in abdominal adipose tissue, accompanied by greater fat mass relative to body weight, which suggested greater uptake of systemic glucose and fatty acids by adipose tissue than in males (21). The possible hepatic insulin resistance despite raised blood glucose levels suggests that JJJ females may mobilize adipose tissue more actively to regulate glucose and lipid homeostasis (21). Given that adipose tissue is designed for the safe storage of excess nutrients (40), this may help protect females from hepatic de novo lipogenesis and thereby from NASH.
Sex differences also occurred in the expression profile of nuclear factors. PPARα and PPARδ promote the hepatic combustion of fatty acids via β-oxidation, and all three PPAR isoforms exhibit antiinflammatory properties, which protect from lipotoxicity and improve fibrosis (41,42). The elevation of both PPARα and PPARδ in male offspring fed the J diet at some stage in the study may indicate increased lipid β-oxidation, but the up-regulation was not greater in JJJ males over the CCJ and JJC groups despite greater liver triglyceride content in this group. Unlike males, female offspring fed the J diet did not up-regulate any of the PPAR isoforms, and PPARδ was decreased in JJJ females compared with both CCC and CCJ groups. This down-regulation could be linked to the predicted reduced insulin sensitivity in this group, and indeed, PPARδ has been shown to promote hepatic insulin sensitivity (43). Furthermore, PPARδ agonists suppress hepatic glucose output (43); therefore, the down-regulation of hepatic PPARδ in JJJ females coincides with the hyperglycemia previously reported in this group (21). The sex differences observed with regard to PPARα are not surprising because gender-specific expression has been reported (14). Males are also more susceptible to lipid accumulation and hypoglycemia in the absence of PPARα than females (44), which could be a greater incentive for PPARα up-regulation in junk food-fed males. LRH-1 is involved in hepatic cholesterol transport; it can also inhibit the hepatic expression of IL-6 (27), and its absence impairs intestinal lipid absorption (45). The LRH-1 up-regulation in CCJ, JJC, and JJJ males could confer some antiinflammatory protection, whereas its down-regulation in JJJ females could reduce intestinal lipid absorption (45) and protect against excess lipidemia. Taken together, the data support sex differences in the molecular metabolic adaptation to junk food feeding in JJJ progeny. It is unclear why these differences occurred and would require further investigation. Nutrition is unlikely to be the cause because data showed no gender-specific differences in food, energy, and macronutrient intakes within each nutritional group (Ref. 22 and Supplemental Table 1). However, at 10 wk postpartum, rats have undergone puberty (23); therefore, these differences could be attributed to sex hormones.
A maternal junk food diet in pregnancy and lactation induces a hepatic oxidative stress response in offspring
According to the two-hit hypothesis, excess hepatic lipid can affect mitochondrial respiratory activity and promote the formation of reactive oxygen species (ROS), leading to lipid peroxidation, inflammation, and fibrosis (3,4,46).
Mitochondrial β-oxidation is the main site for hepatic fatty acid metabolism and is highly controlled by CPT1a (47). During energy overconsumption, CPT1a activity is inhibited by malonyl-CoA (5) and its mRNA expression is reduced in NAFLD (48). β-Oxidation saturation can also lead to excessive acetyl-CoA entering the Krebs cycle, increasing electron delivery to the respiratory chain and potentially generating ROS (49). In the present study, the exacerbated steatosis in JJJ offspring was not accompanied by an equally exacerbated increase in CPT1a mRNA expression, which could be indicative of mitochondrial β-oxidation saturation and lead to further hepatic lipid accumulation (5,28,49).
It has also been proposed that when energy supplies exceed energy requirements, hepatic mitochondria may activate an alternative substrate oxidation pathway regulated by UCP-2, which reduces ROS formation but depletes ATP stores and increases susceptibility to NASH (46). UCP-2 is therefore believed to play a key role in NASH progression and its overexpression is a feature of NAFLD and NASH (46,48,49). In the present study, increased UCP-2 expression in JJJ males could increase their risk of NASH progression from the end of adolescence, although JJJ females did not show the same up-regulation. Microsomal lipid oxidation is another source of ROS and is partly regulated by CYP2E1, whose expression increases in NAFLD (49). Therefore, increased CYP2E1 expression in JJC offspring could increase their risk of NASH progression.
ROS plays a central role in the progression from NAFLD to NASH (3,4). To assess the hepatic oxidative stress response, we examined the expression of four antioxidant enzymes, namely GPx1, SOD1, SOD2, and catalase. Their function is to neutralize ROS, but their induction and activity have been shown to increase with liver damage severity, oxidative stress, ROS production, and/or lipid peroxidation in various models of liver injury including NAFLD (48,50), viral hepatitis (51), and chronic ethanol administration (52). The present study showed that all four antioxidant enzymes examined increased in JJJ male offspring compared with the CCC group, whereas all but SOD2 increased in JJJ females. None of these enzymes were statistically increased in offspring given free access to junk food after weaning (CCJ) compared with the CCC group except for SOD2 in males. SOD1, SOD2, and catalase also increased in JJC male offspring, whereas SOD1 increased in JJC females. These results therefore indicate that a maternal junk food diet in pregnancy and lactation induced a hepatic oxidative stress response, which persisted up to the end of adolescence in offspring switched to a balanced diet from weaning. Increased oxidative stress could put offspring born to junk food-fed mothers at greater risk of NASH. Although no offspring exhibited histological signs of inflammation and fibrosis with unchanged expression of TNFα, IL6, collagen-1α, and keratin 8, the offspring used in this study were young, and given the exacerbated steatosis and oxidative stress response observed, those exposed to the junk food diet from fetal life may be expected to develop NASH early in adulthood.
Conclusions
This study identifies a maternal junk food diet in pregnancy and lactation as a factor contributing to the development of NAFLD in progeny. Offspring exposed to a diet rich in energy, fat, sugar, and salt from fetal life exhibited aggravated signs of NAFLD characterized by exacerbated hepatic steatosis, hepatocyte ballooning, and oxidative stress response by the end of adolescence compared with offspring given free access to junk food after weaning only. Increased steatosis and oxidative stress response also occurred in offspring born to junk food-fed mothers given a balanced chow diet from weaning, indicating irreversible damage. This study further supports the need to encourage healthy eating habits in pregnancy and lactation for the long-term health benefits of future generations.
Supplementary Material
Acknowledgments
We thank Tanya Hopcroft, Michael Avella, John Bredl, Steve Allen, Jennifer Yarin, Nwaamaka Ugwu Owoh, Samantha Farrington, and Biological Services Unit staff for their technical assistance.
Footnotes
This work was supported by the Wellcome Trust and the Society for Endocrinology.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 5, 2010
Abbreviations: ALP, Alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; C, control; CPT, carnitine palmitoyltransferase; CYP2E1, cytochrome P450 2E1; FAS, fatty acid synthase; GGT, γ-glutamyl transferase; Glut, glucose transporter; GPx, glutathione peroxidase; H&E, hematoxylin and eosin; IGF-IR, IGF-I receptor; IR, insulin receptor; IRS, insulin receptor substrate; J, junk food; LRH, liver receptor homolog; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PPAR, peroxisome proliferator-activated receptor; ROS, reactive oxygen species; RT, reverse transcription; SOD, superoxide dismutase; UCP, uncoupling protein.
References
- Adams LA, Lindor KD 2007 Nonalcoholic fatty liver disease. Ann Epidemiol 17:863–869 [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA 2005 Nonalcoholic steatohepatitis and the metabolic syndrome. Am J Med Sci 330:326–335 [DOI] [PubMed] [Google Scholar]
- Day CP, James OF 1998 Steatohepatitis: a tale of two “hits?” Gastroenterology 114:842–845 [DOI] [PubMed] [Google Scholar]
- Pérez-Carreras M, Del Hoyo P, Martín MA, Rubio JC, Martín A, Castellano G, Colina F, Arenas J, Solis-Herruzo JA 2003 Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology 38:999–1007 [DOI] [PubMed] [Google Scholar]
- Wei Y, Rector RS, Thyfault JP, Ibdah JA 2008 Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J Gastroenterol 14:193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lall CG, Aisen AM, Bansal N, Sandrasegaran K 2008 Nonalcoholic fatty liver disease. AJR Am J Roentgenol 190:993–1002 [DOI] [PubMed] [Google Scholar]
- Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O'Keefe JH, Brand-Miller J 2005 Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 81:341–354 [DOI] [PubMed] [Google Scholar]
- Guthrie JF, Lin BH, Frazao E 2002 Role of food prepared away from home in the American diet, 1977–78 versus 1994–96: changes and consequences. J Nutr Educ Behav 34:140–150 [DOI] [PubMed] [Google Scholar]
- Nielsen SJ, Siega-Riz AM, Popkin BM 2002 Trends in energy intake in U.S. between 1977 and 1996: similar shifts seen across age groups. Obes Res 10:370–378 [DOI] [PubMed] [Google Scholar]
- Anderson JW, Patterson K 2005 Snack foods: comparing nutrition values of excellent choices and “junk foods.” J Am Coll Nutr 24:155–156; discussion 156–157 [DOI] [PubMed] [Google Scholar]
- Maillot M, Darmon N, Vieux F, Drewnowski A 2007 Low energy density and high nutritional quality are each associated with higher diet costs in French adults. Am J Clin Nutr 86:690–696 [DOI] [PubMed] [Google Scholar]
- Law CM, Barker DJ, Osmond C, Fall CH, Simmonds SJ 1992 Early growth and abdominal fatness in adult life. J Epidemiol Commun Health 46:184–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage JA, Poston L, Taylor PD 2008 Developmental origins of obesity and the metabolic syndrome: the role of maternal obesity. Front Horm Res 36:73–84 [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang C, Terroni PL, Cagampang FR, Hanson M, Byrne CD 2005 High-unsaturated-fat, high-protein, and low-carbohydrate diet during pregnancy and lactation modulates hepatic lipid metabolism in female adult offspring. Am J Physiol Regul Integr Comp Physiol 288:R112–R118 [DOI] [PubMed] [Google Scholar]
- Hedin PA, Schultze MO 1955 Maternal diet and other factors affecting the lipid content of livers of very young rats. J Nutr 56:129–138 [DOI] [PubMed] [Google Scholar]
- Erhuma A, Salter AM, Sculley DV, Langley-Evans SC, Bennett AJ 2007 Prenatal exposure to a low-protein diet programs disordered regulation of lipid metabolism in the aging rat. Am J Physiol Endocrinol Metab 292:E1702–E1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosby AK, Maloney CA, Phuyal JL, Denyer GS, Bryson JM, Caterson ID 2003 Maternal protein restriction increases hepatic glycogen storage in young rats. Pediatr Res 54:413–418 [DOI] [PubMed] [Google Scholar]
- McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL 2009 Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 119:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Springer D 1976 Dietary obesity in adult rats: similarities to hypothalamic and human obesity syndromes. Physiol Behav 17:461–471 [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ 1978 A paradox in the control of energy intake in the rat. Nature 273:146–147 [DOI] [PubMed] [Google Scholar]
- Bayol SA, Simbi BH, Bertrand JA, Stickland NC 2008 Offspring from mothers fed a ‘junk food’ diet in pregnancy and lactation exhibit exacerbated adiposity that is more pronounced in females. J Physiol 586:3219–3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayol SA, Farrington SJ, Stickland NC 2007 A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br J Nutr 98:843–851 [DOI] [PubMed] [Google Scholar]
- Quinn R 2005 Comparing rat’s to human’s age: how old is my rat in people years? Nutrition 21:775–777 [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH 1957 A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509 [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RH, Moorman AF 2003 Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 339:62–66 [DOI] [PubMed] [Google Scholar]
- Postic C, Girard J 2008 The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab 34:643–648 [DOI] [PubMed] [Google Scholar]
- Venteclef N, Smith JC, Goodwin B, Delerive P 2006 Liver receptor homolog 1 is a negative regulator of the hepatic acute-phase response. Mol Cell Biol 26:6799–6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury J, Sanyal AJ 2005 Insulin resistance in NASH. Front Biosci 10:1520–1533 [DOI] [PubMed] [Google Scholar]
- Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE 2008 Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol 103:1372–1379 [DOI] [PubMed] [Google Scholar]
- Sahai A, Malladi P, Pan X, Paul R, Melin-Aldana H, Green RM, Whitington PF 2004 Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol 287:G1035–G1043 [DOI] [PubMed] [Google Scholar]
- Ku NO, Strnad P, Zhong BH, Tao GZ, Omary MB 2007 Keratins let liver live: mutations predispose to liver disease and crosslinking generates Mallory-Denk bodies. Hepatology 46:1639–1649 [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA 2007 Fatty liver and the metabolic syndrome. Curr Opin Gastroenterol 23:193–198 [DOI] [PubMed] [Google Scholar]
- Limdi JK, Hyde GM 2003 Evaluation of abnormal liver function tests. Postgrad Med J 79:307–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarapurkar D, Kamani P, Patel N, Gupte P, Kumar P, Agal S, Baijal R, Lala S, Chaudhary D, Deshpande A 2007 Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol 6:161–163 [PubMed] [Google Scholar]
- Kim HJ, Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, Lim SK, Kim KR, Lee HC, Huh KB, Cha BS 2004 Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med 164:2169–2175 [DOI] [PubMed] [Google Scholar]
- Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE 2005 Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics 115:e561–e565 [DOI] [PubMed] [Google Scholar]
- Bloomgarden ZT 2003 American Association of Clinical Endocrinologists (AACE) consensus conference on the insulin resistance syndrome: 25–26 August 2002, Washington, DC. Diabetes Care 26:933–939 [DOI] [PubMed] [Google Scholar]
- Bruno S, Maisonneuve P, Castellana P, Rotmensz N, Rossi S, Maggioni M, Persico M, Colombo A, Monasterolo F, Casadei-Giunchi D, Desiderio F, Stroffolini T, Sacchini V, Decensi A, Veronesi U 2005 Incidence and risk factors for non-alcoholic steatohepatitis: prospective study of 5408 women enrolled in Italian tamoxifen chemoprevention trial. BMJ 330:932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ 2005 Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115:1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J 2002 Fat in all the wrong places. Nature 415:268–269 [DOI] [PubMed] [Google Scholar]
- Andrews DB, Schwimmer JB, Lavine JE 2008 Fast break on the fat brake: mechanism of peroxisome proliferator-activated receptor-Δ regulation of lipid accumulation in hepatocytes. Hepatology 48:355–357 [DOI] [PubMed] [Google Scholar]
- Risérus U, Sprecher D, Johnson T, Olson E, Hirschberg S, Liu A, Fang Z, Hegde P, Richards D, Sarov-Blat L, Strum JC, Basu S, Cheeseman J, Fielding BA, Humphreys SM, Danoff T, Moore NR, Murgatroyd P, O'Rahilly S, Sutton P, Willson T, Hassall D, Frayn KN, Karpe F 2008 Activation of peroxisome proliferator-activated receptor (PPAR)Δ promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes 57:332–339 [DOI] [PubMed] [Google Scholar]
- Lee CH, Olson P, Hevener A, Mehl I, Chong LW, Olefsky JM, Gonzalez FJ, Ham J, Kang H, Peters JM, Evans RM 2006 PPARΔ regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci USA 103:3444–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouadi F, Weinheimer CJ, Saffitz JE, Pitchford C, Bastin J, Gonzalez FJ, Kelly DP 1998 A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor α-deficient mice. J Clin Invest 102:1083–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataki C, Magnier BC, Houten SM, Annicotte JS, Argmann C, Thomas C, Overmars H, Kulik W, Metzger D, Auwerx J, Schoonjans K 2007 Compromised intestinal lipid absorption in mice with a liver-specific deficiency of liver receptor homolog 1. Mol Cell Biol 27:8330–8339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serviddio G, Bellanti F, Tamborra R, Rollo T, Capitanio N, Romano AD, Sastre J, Vendemiale G, Altomare E 2008 Uncoupling protein-2 (UCP2) induces mitochondrial proton leak and increases susceptibility of non-alcoholic steatohepatitis (NASH) liver to ischaemia-reperfusion injury. Gut 57:957–965 [DOI] [PubMed] [Google Scholar]
- Eaton S 2002 Control of mitochondrial β-oxidation flux. Prog Lipid Res 41:197–239 [DOI] [PubMed] [Google Scholar]
- Kohjima M, Enjoji M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N, Takayanagi R, Nakamuta M 2007 Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med 20:351–358 [PubMed] [Google Scholar]
- Méndez-Sánchez N, Arrese M, Zamora-Valdés D, Uribe M 2007 Current concepts in the pathogenesis of nonalcoholic fatty liver disease. Liver Int 27:423–433 [DOI] [PubMed] [Google Scholar]
- Perlemuter G, Davit-Spraul A, Cosson C, Conti M, Bigorgne A, Paradis V, Corre MP, Prat L, Kuoch V, Basdevant A, Pelletier G, Oppert JM, Buffet C 2005 Increase in liver antioxidant enzyme activities in non-alcoholic fatty liver disease. Liver Int 25:946–953 [DOI] [PubMed] [Google Scholar]
- Severi T, Ying C, Vermeesch JR, Cassiman D, Cnops L, Verslype C, Fevery J, Arckens L, Neyts J, van Pelt JF 2006 Hepatitis B virus replication causes oxidative stress in HepAD38 liver cells. Mol Cell Biochem 290:79–85 [DOI] [PubMed] [Google Scholar]
- Nanji AA, Griniuviene B, Sadrzadeh SM, Levitsky S, McCully JD 1995 Effect of type of dietary fat and ethanol on antioxidant enzyme mRNA induction in rat liver. J Lipid Res 36:736–744 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.