Abstract
Mineralocorticoid receptor (MR) plays a critical role in brain function. However, the regulatory mechanisms controlling neuronal MR expression that constitutes a key element of the hormonal response are currently unknown. Two alternative P1 and P2 promoters drive human MR gene transcription. To examine promoter activities and their regulation during neuronal differentiation and in mature neurons, we generated stably transfected recombinant murine embryonic stem (ES) cell lines, namely P1-GFP and P2-GFP, in which each promoter drove the expression of the reporter gene Green Fluorescent Protein (GFP). An optimized protocol, using embryoid bodies and retinoic acid, permitted to obtain a reproducible neuronal differentiation as revealed by the decrease in phosphatase alkaline activity, the concomitant appearance of morphological changes (neurites) and the increase in the expression of neuronal markers (nestin, β-tubulin III, MAP2) as demonstrated by immunocytochemistry and qPCR. Using these cell-based models, we showed that MR expression increased by 5-fold during neuronal differentiation, MR being preferentially if not exclusively expressed in mature neurons. Although the P2 promoter was always weaker than the P1 promoter during neuronal differentiation, their activities increased by 7- and 5-fold, respectively and correlated with MR expression. Finally, while progesterone and dexamethasone were ineffective, aldosterone stimulated both P1 and P2 activity and MR expression, an effect that was abrogated by knockdown of MR by siRNA. Concluding, we provide evidence for a tight transcriptional control of MR expression during neuronal differentiation. Given the neuroprotective and antiapoptotic role proposed for MR, the neuronal differentiation of ES cell lines opens potential therapeutic perspectives in neurological and psychiatric diseases.
Keywords: Aldosterone; pharmacology; Animals; Cell Differentiation; Cell Line; Embryonic Stem Cells; cytology; metabolism; Gene Expression Regulation, Developmental; drug effects; Green Fluorescent Proteins; genetics; metabolism; Humans; Immunohistochemistry; Mice; Microscopy, Confocal; Microscopy, Fluorescence; Neurons; cytology; metabolism; Promoter Regions, Genetic; genetics; RNA Interference; Receptors, Mineralocorticoid; genetics; Recombinant Fusion Proteins; genetics; metabolism; Reverse Transcriptase Polymerase Chain Reaction; Transfection
Keywords: Mineralocorticoid receptor, embryonic stem cell, neuronal differentiation, aldosterone, promoters, transcriptional regulation
INTRODUCTION
The mineralocorticoid receptor (MR), a transcription factor belonging to the nuclear receptor superfamilly, is expressed both in epithelial tissues, where it plays a critical role in mediating aldosterone-regulated transepithelial sodium transport, and in non-epithelial tissues such as the cardiovascular system and central nervous system where it controls important cardiovascular functions and behavioral processes (1). MR binds both aldosterone and glucocorticoid hormones with a similar affinity. In the brain, although aldosterone-selective MR is expressed in hypothalamic sites involved in the regulation of salt appetite (2), the highest MR expression is found in the limbic structures particularly in hippocampal neurons, where it is physiologically occupied by glucocorticoids.
In the central nervous system, MR is involved in the regulation of the Hypothalamic-Pituitary-Adrenocortical (HPA) axis activity under basal and stress conditions (3). MR activity, at low glucocorticoid levels, is associated with cell excitability and long-term potentiation, a cellular model for learning and memory formation (4). Animal models of MR overexpression or inactivation have also brought important information on MR function in the brain. Indeed, mice specifically overexpressing MR in the forebrain exhibited a reduction of anxiety-like behavior (5, 6), while mice lacking limbic MR had a normal anxiety-like behavior but improved in learning processes (7, 8). Moreover, studies on hippocampal neurons recently proposed an anti-apoptotic role for MR (9, 10), confirming studies using a MR knockout mouse model, which showed degeneration of hippocampal granule cells in adulthood (11), while reduced neuronal death was observed in MR overexpressing transgenic mice after transient cerebral global ischemia (6).
A key step in the mineralocorticoid signaling is the MR expression level. Differential brain MR capacities correlating with distinct responses of the HPA axis in two rat strains initially pointed out to the importance of MR expression level as a determinant factor of its action (12). This was further exemplified by knockout or overexpressing MR animals models (5–8). Even though MR expression levels constitute an essential element governing mineralocorticoid signaling in target tissues, very little is known concerning the regulatory mechanisms controlling neuronal MR abundance. It is well established that MR expression is regulated by transcriptional and post-transcriptional mechanisms (1). At the level of transcription, we have previously identified two functional promoters in the human MR gene, referred to as P1 and P2, corresponding to the 5′-flanking regions of the two first untranslated exons 1α and 1β (13). Functional characterization revealed that the P1 had a stronger basal transcriptional activity than the P2 promoter. While both promoters were stimulated by glucocorticoids, only P2 was activated by aldosterone. Moreover, as demonstrated in transgenic animals, P1 promoter was active in all MR-expressing tissues including the brain, whereas P2 promoter activity was weaker and appeared to be restricted during development (14).
To define the molecular mechanisms governing MR expression during neuronal differentiation, we have developed stably transfected recombinant murine embryonic stem (ES) cell lines, namely P1-GFP and P2-GFP, in which each promoter drives the expression of the Green Fluorescent Protein (GFP) used as a reporter gene. These models offer the possibility to investigate the regulation of MR expression and of the promoter activities during the early stages of neuronal differentiation and in mature neurons. We show that MR expression increases during neuronal differentiation which is associated with a parallel aldosterone-regulated control of P1 and P2 promoter activities.
MATERIALS AND METHODS
P1- and P2-GFP Constructs
P1- and P2-GFP constructs were obtained from the pGL3-Enhancer Vector (Promega, Charbonnieres, France), in which the luciferase gene was replaced by the GFP cDNA from pEGFP plasmid (Ozyme, Saint Quentin en Yveline, France). The HindIII-AvaII fragment of the hMR gene, referred to as P1, was amplified from the pGL2-HA plasmid (13) by PCR with GL1 and GL2 primers. This amplicon was thereafter ligated into the SmaI-HindIII sites of pGL3-Enhancer-GFP vector. The SspI-SspI fragment was extracted from the H31-P2 plasmid (15) by EcoRI and EcoRV digestion and was inserted into the unique SmaI site of pGL3-Enhancer-GFP plasmid after filling in all recessive ends with Klenow (Ozyme) treatment and dephosphorylation of pGL3-Enhancer by the Shrimp Alcaline Phosphatase (Promega). Transgenes have been subsequently sequenced to verify their integrity (Service de Génétique Moléculaire, Pharmacogénétique, Hormonologie, CHU Bicêtre, France).
Other constructs using the same HindIII-AvaII fragment and the same SspI-SspI fragment of hMR gene have been generated. They were obtained from H31-P1 and H31-P2 plasmids (15, 16), in which we have inserted the entire coding sequence of the GFP.
Transfection Procedures
For stable transfection, the 7241d embryonic stem (ES) cell line was used. This cell line has been derived in Cooney’s laboratory (Baylor College of Medicine, Houston Texas), from a blastocyst obtained from the breeding of LRH +/− heterozygote mice (17) and was genotyped, phenotyped and characterized as a standard wild type ES cell line (Le Menuet and Cooney, unpublished data). The undifferentiated cells 7241d were seeded in 100 mm-Petri dishes one day before transfection. Five μg of P1- or P2-GFP constructs were co-transfected with 1 μg of pcDNA3 plasmid, containing the neomycin gene (Invitrogen, Cergy-Pontoise, France), using 15 μl Lipofectamine 2000 reagent (Invitrogen). Forty-eight hours post-transfection, selection was initiated with 400 μg/ml of G418 (PAA, Les Mureaux, France). Neo-resistant clones were picked up after 5 days of G418 selection and propagated using the same medium. Two transfections, for each construct, were carried out, resulting in 11 neo-resistant clones having integrated the P1-GFP construct whereas 4 integrated the P2-GFP construct. In addition, the constructs obtained from H31-P1 and H31-P2 plasmids were stably transfected in undifferentiated ES cells.
Cell Culture
Mouse ES cells were grown on 0.1% gelatin-coated plates (Sigma-Aldrich, Lyon, France) and on feeder cells (STO Neomycin LIF, kindly provided by Dr Alan Bradley, The Wellcome Trust Sanger Institute, UK) treated with 15 μg/ml mitomycin C (Sigma-Aldrich) for 4 hours. Cells were cultured at 37°C in a humidified incubator gassed with 5% CO2.
Reagents
ES medium was composed of DMEM (PAA) containing 15% fetal calf serum (FCS specifically tested for ES culture (AbCys SA, Paris, France), 1X non-essential amino acids (PAA), 2 mM glutamine (PAA), 100 U/ml penicillin (PAA), 100 μg/ml streptomycin (PAA), 20 mM Hepes (PAA) and 100 μM β-mercaptoethanol (Sigma-Aldrich). Embryoid Bodies (EB) medium was similar to ES medium except that it contained 10% FCS without β-mercaptoethanol. Neuron medium was similar to EB medium but was supplemented with 5 μg/ml insulin (Sigma-Aldrich), 5 μg/ml transferrin (Sigma-Aldrich), and 29 nM sodium selenate (Sigma-Aldrich).
Differentiation of ES cells into Neuronal-like cells
ES cells were cultured on feeder cells for two days after thawing. Subsequently, they were cultured without feeder cells in ES medium with Leukemia Inhibitory Factor (LIF) 1,000 U/ml (AbCys) and differentiation was started after 2 days. To induce EB formation, ES cells were detached, dissociated into single cells with 0.25% trypsin (Invitrogen) and then 1.106 ES cells were plated onto non-adherent bacterial Petri dishes (Greiner Bio-one SAS, Courtaboeuf, France) in ES medium without LIF. EB medium with 1 μM all trans-retinoic acid (Sigma-Aldrich) was added after 2 days and was changed every other day. At day 7, EB were washed twice with PBS (PAA) and trypsinized for 10 min in a water bath at 37 °C. EB were then gently but thoroughly resuspended in 10 ml EB medium, then centrifuged for 3 min at 400 g at room temperature. The pellet was resuspended in neuron medium and the cell suspension was filtered through a 40-μm nylon cell strainer (BD Biosciences, USA). The cell suspension was immediately seeded at a density of 2.105 cells per cm2. Neuron medium was changed every 2 days.
Cell Treatment
After 24 h incubation in Dextran-Charcoal Coated (DCC) medium, aldosterone (Acros Organics, Halluin, France), dexamethasone or progesterone (Sigma-Aldrich), was added to the culture medium at day 13 of the neuronal differentiation. At day 14, total RNA was extracted by the Trizol reagent or cells were fixed.
Alkaline Phosphatase Assay
Cells were fixed for 2 min in 4% PBS-buffered paraformaldehyde (Sigma-Aldrich) and were rinsed once with PBS. During fixation, fresh staining solution was prepared by mixing Fast Red Violet, Naphtol AS-BI phosphate and water (2:1:1), according to the Quantitative Alkaline Phosphatase ES Characterization Kit protocol (Millipore, Saint Quentin en Yvelines, France). Fixed cells were incubated for 15 min with the staining solution at room temperature in the dark. Cells were rinsed with PBS and then photographed (microscope Motic AE21 and camera Olympus SP-51OUZ).
Immunocytochemistry
Cells were fixed with methanol for 10 min, rinsed with PBS-Tween 20 and incubated with 4% horse immunoglobulins for 20 min, prior to overnight incubation at 4°C, with the primary monoclonal antibodies diluted as follows: anti-tubulin beta III TU-20 (1/50) (AbCys), anti-nestin 4D4 (5 μg/ml) (AbCys), anti-APC CC1 (1/20) (Calbiochem, Nottingham, UK), and anti-GFAP G-A-5 (1/200) (Sigma-Aldrich). After endogenous peroxidase quenching with 3% H2O2 in PBS and washing, immunodetection was performed using the anti-mouse ImmPress reagent kit (Vector, Burlingame, CA) according to the manufacturer’s instructions. Negative mouse ascites and pre-immune mouse immunoglobulins of the same sub-class were used as negative controls.
Confocal Immunofluorescence Microscopy
Cells were fixed with methanol for 10 min, rinsed with PBS-Tween 20 and incubated with PBS 5%-BSA 0.1% casein block for 20 min. Cells were then incubated overnight at 4°C, with anti-MR 39N polyclonal antibody (4 μg/ml) followed by incubation with Alexa Fluor 555 goat anti-rabbit (1/1000) (Molecular Probes) for 1 h at room temperature. The cells were next rinsed in PBS, and incubated with the monoclonal antibodies anti-tubulin beta III TU-20 (1/100) (AbCys), or anti-nestin 4D4 (5 μg/ml) (AbCys), or anti-GFAP G-A-5 (1/200) (Sigma-Aldrich), or anti-GFP (1/30) (AbCys) for 2 h at room temperature followed by washing and incubation with Alexa Fluor 488 goat anti-mouse (1/1000) (Molecular Probe) for 1 h at room temperature. After washing with PBS and mounting with Fluorescence Mounting Medium (Dako, Trappes, France), cells were analyzed and imaged by confocal fluorescence microscopy (Zeiss HAL confocal microscope). The anti-MR antibody (39N) was generated in rabbits immunized with the human MR 1–18 peptide and purified by affinity chromatography (Double X/XP boosting antibody production program, Eurogentec, Seraing, Belgium). The specificity of the anti-MR antibody was previously characterized (18,19).
DNA extraction
DNA was extracted from cells with lysis buffer composed of 50 mM Tris pH8, 10 mM EDTA, 10% SDS, 400 mM NaCl, and supplemented with proteinase K (Euromedex, Souffelweyrsheim, France) 50 mg/ml, incubated overnight at 37°C, precipitated with isopropanol, rinsed with ethanol and dissolved in water.
RT-PCR
Total RNA was extracted with TRIZOL reagent (Invitrogen) according to the manufacturer’s recommendations and RNA were processed for RT-PCR analysis. Briefly, 2 μg of total RNA was treated using the DNase I Amplification Grade procedure (Invitrogen). RNA was then reverse-transcribed with 200 units of reverse transcriptase using the SuperscriptTM II kit (Invitrogen) according to the manufacturer’s recommendations using random hexamers (Promega). PCR were performed with a thermocycler (Stratagene, Paris, France) in a final volume of 25 μl, in 1X PCR buffer containing 1.5 mM MgCl2, 10 pmol of sense and antisense primers (see supplemental table), 200 μM dNTP, 1 unit of Taq polymerase, and 2 μl of the reverse transcription reaction. The PCR cycles were as followed: 95 °C for 5 min, specific hybridization temperature for 1 min, 72 °C for 1 min, during 1 cycle; 95 °C for 45 s, specific hybridization temperature for 45 s, 72 °C for 45 s, during 30 cycles; 72 °C for 7 min, during 1 cycle. Amplicons were thereafter separated onto agarose gels and visualized under UV excitation.
Quantitative Real Time PCR
Gene expression was quantified by real time PCR. Total RNA was processed for real time PCR carried out on an ABI 7300 Sequence Detector (Applied Biosystems, Courtaboeuf, France). Briefly, 1 μg of total RNA was treated using the DNase I Amplification Grade procedure (Invitrogen). RNA was then reverse-transcribed with 50 units of MultiScribe reverse transcriptase (Applied Biosystems). Samples were diluted 10-fold, and then 1/20 of the reverse transcription reaction was used for PCR using the Power SYBR® Green PCR master mix (Applied Biosystems). Final primer concentrations were 300 nM for each primer (see supplemental table). Reaction parameters were 50 °C for 2 min followed by 40 cycles at 95°C for 15 s, and 60 °C for 1 min. For preparation of standards, amplicons were purified from agarose gel and subcloned into pGEMT-easy plasmid (Promega), then sequenced to confirm the identity of each fragment. Standard curves were generated using serial dilutions of linearized standard plasmids, spanning 6 orders of magnitude and yielding correlation coefficients >0.98 and efficiencies of at least 0.95, in all experiments. Standard and sample values were determined in duplicate from independent experiments. Relative expression within a given sample was calculated as the ratio: attomol of specific gene/femtomol of 18S. Results are mean ± S.E.M and represent the relative expression compared with that obtained with control cells, which was arbitrary set at 1.
MR knockdown by siRNA
Neurons were transiently transfected at day 12 with 100 nM siRNA (Eurogentec; sequences were reported in Supplemental Table), using Lipofectamine RNAiMAX (Invitrogen) in Opti-MEM Reduced Serum Medium (Invitrogen) according to the manufacturer’s recommendations. Six hours post-transfection, cells were incubated in DCC medium for 24 h followed by an additional 24h exposure to aldosterone. At day 14, total RNA was extracted and MR and GFP expression were measured by qPCR.
Statistical Analyses
Results represent mean ± SEM with at least 6 samples for each condition. Statistical analyses were performed using a non parametric Mann-Whitney test (Prism4, Graphpad Software, Inc., San Diego, CA).
RESULTS
Characterization of stably transfected embryonic stem cell lines P1-GFP and P2-GFP
P1 and P2-GFP ES cell lines have been generated to examine the two hMR promoter activities during neuronal differentiation. As shown in Figure 1A, the proximal P1 promoter contained the 5′-flanking region (969 bp) of the exon 1α and part of this first untranslated exon (239 bp). The P2 promoter was composed of the 5′-flanking region (1682 bp) of the untranslated exon 1β and the first 123 bp of this exon. These promoter regions were inserted into the pGL3-Enhancer Vector, which contains the SV40 enhancer, in order to enhance the basal activity of these promoters and thereby facilitate detection of the reporter gene GFP. After stable transfection of murine ES cells, neomycin-resistant clones were isolated and genomic integration of the constructs was determined by PCR (Figure 1B). Several recombinant ES cell lines, namely P1-GFP and P2-GFP, were established. We demonstrated the expression of GFP mRNA and endogenous MR mRNA by RT-PCR, in 7 days EB, thus confirming the functionality of the constructs in these ES cell lines (Figure 1C). The GFP protein was expressed in these EB, as visualized by fluorescence microscopy (Figure 1D). Similar results were obtained using two other independent stably transfected cell lines P1 and P2-GFP, generated with identical constructs except that they were devoid of enhancer, thereby validating our strategy (see Materials and Methods section, data not shown). Altogether, these cell-based systems offer the possibility to study MR promoter activities as well as the regulation of MR expression during the first steps of neuronal differentiation and in mature neurons.
Figure 1. Generation of hMR promoter-GFP expressing stable ES cell lines.
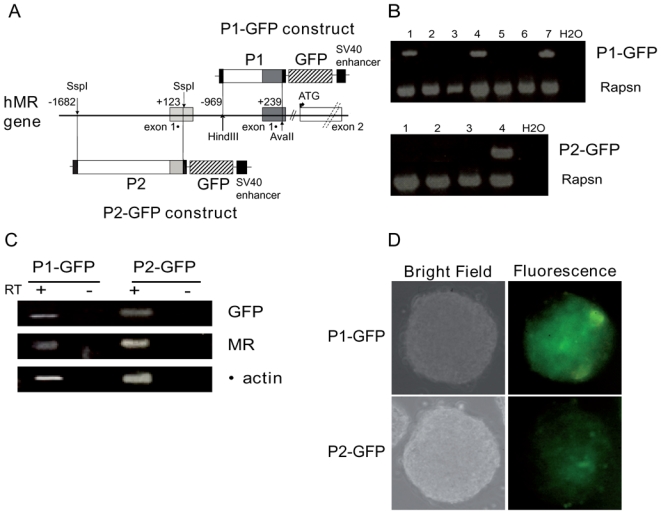
A) Schematic representation of the two P1-GFP and P2-GFP constructs. The 5′-flanking regions of hMR gene contains the two first untranslated exons 1α and 1β (solid boxes). The Hind III-Ava II (−969, +239) P1 fragment and SspI-SspI (−1682, +123) P2 fragment were used to generate P1-GFP and P2-GFP plasmids, respectively. The GFP (hatched box) was used as a reporter gene. As described in the “Materials and Methods” section the pGL3-Enhancer Vector contains the SV40 Enhancer (black box). B) PCR genotyping of undifferentiated resistant cell lines (numbered 1 to 7). Rapsn was used as internal genomic control. C) RT-PCR analyses of GFP, MR, β-actin (used as internal control) mRNA expression in 7 day embryoid bodies (from 1.106 cells in suspension in 15% FCS supplemented medium). D) GFP protein expression was observed in 7 day embryoid bodies (from 1.106 cells in suspension in 15% FCS supplemented medium). Original magnification: x20
Neuronal differentiation
The neuronal differentiation protocol used in the present study, essentially followed one previously described (20). Briefly, after withdrawal of LIF, ES-cells formed EB, which were incubated in non-adhesive bacterial dishes with retinoic acid (RA) for 5 days. EB were dissociated and incubated in the neuron specific medium, and after 3 days originated differentiated cells harbouring a neuron like phenotype. While a strong alkaline phosphatase activity was detected in undifferentiated ES cells, this activity dramatically decreased in RA-stimulated cells, thus confirming their commitment to neuronal differentiation (Figure 2A). After 5 days treatment with RA, the expression of the neuronal progenitor marker nestin and the mature neuronal marker Microtubule-Associated Protein-2 (MAP2) was already detected by quantitative real time PCR in EB. Incubation with neuron specific medium readily increased mRNA expression levels of these neuronal markers (Figure 2B–2C). The two other independent stably transfected cell lines P1 and P2-GFP, the parental line and the D3 ES cell line (ATTC number: CRL-11693), used as a wild type control, were differentiated into neurons according to the same protocol. No appreciable differences in the expression profile of neuronal markers were observed between the different cell lines. Therefore, the results represent the mean of all cell lines. Immunocytochemical studies, performed at the end of the neuronal differentiation procedure, showed that most cells expressed the neuronal markers nestin and βtubulin III (another marker of mature neurons) and displayed elongated neuritic processes. Of note, few cells, clustered in some areas, expressed the astrocyte marker GFAP (Glial Fibrillary Acidic Protein) or the oligodendrocyte marker APC (Adenomatous Polyposis Coli) (Figure 2D). These results conclusively demonstrate that this neuronal differentiation protocol allows to generate, in a reproducible manner, cultures mainly composed of cells expressing immature and mature neuronal markers, but also comprising a minority of cells expressing astroglial markers.
Figure 2. Neuronal differentiation of ES cell lines.
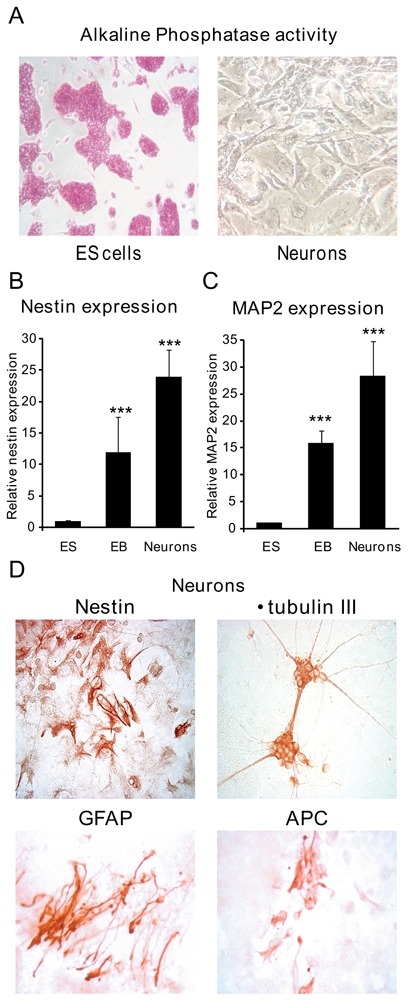
A) Alkaline Phosphatase activity of ES cells and neurons. Alkaline phosphatase activity was measured using the colorimetric Alkaline Phosphatase Detection assay kit. Original magnification: x10 B, C) Relative Nestin and MAP2 mRNA expression levels were determined using qPCR at different stages of differentiation: undifferentiated ES cells, embryoid bodies (EB) and neurons. Results are means ± SEM of six independent experiments of three samples performed in duplicate for each developmental stage, and represent the relative expression compared with basal levels of ES (arbitrarily set at 1). *** P<0.001. Mann Whitney test. Relative mRNA expression is normalized to 18S rRNA expression (see Materials and Methods section). D) Immunocytochemical detection of Nestin, β-tubulin III, GFAP and APC in neurons. Original magnification: x40.
Regulation of MR expression during neuronal differentiation
To investigate the molecular mechanisms regulating MR expression, we first examined endogenous MR mRNA expression during neuronal differentiation by quantitative real-time PCR. We observed that MR expression rose approximately by 3-fold in EB and by 5-fold in mature neurons (Figure 3). As described above, a similar MR expression pattern was observed in all ES cell lines. Thus, we were able to define the temporal expression pattern of MR during ES differentiation and to demonstrate a progressive increase of MR abundance along with neuronal commitment. Double immunolabeling experiments using anti-MR (A) associated with anti-nestin (B) antibodies have been performed (Figure 4). The merged panel (C) shows the absence of MR and nestin colocalization, indicating that nestin-positive cells did not express the MR at a detectable level. On the contrary, double immunolabelling with anti-βtubulin III revealed a specific MR and βtubulin III colocalization (D–E–F). GFAP-positive cells did not express the MR (G–H–I), similarly to the nestin-positive cells. For each experiment, the negative control was performed using pre-immune mouse immunoglobulins of the same sub-class or non reactive mouse ascites (data not shown). Of note, MR localization is nucleocytoplasmic under these experimental conditions. Altogether, these data suggest that MR is preferentially if not exclusively expressed in mature neurons.
Figure 3. MR expression during neuronal differentiation.
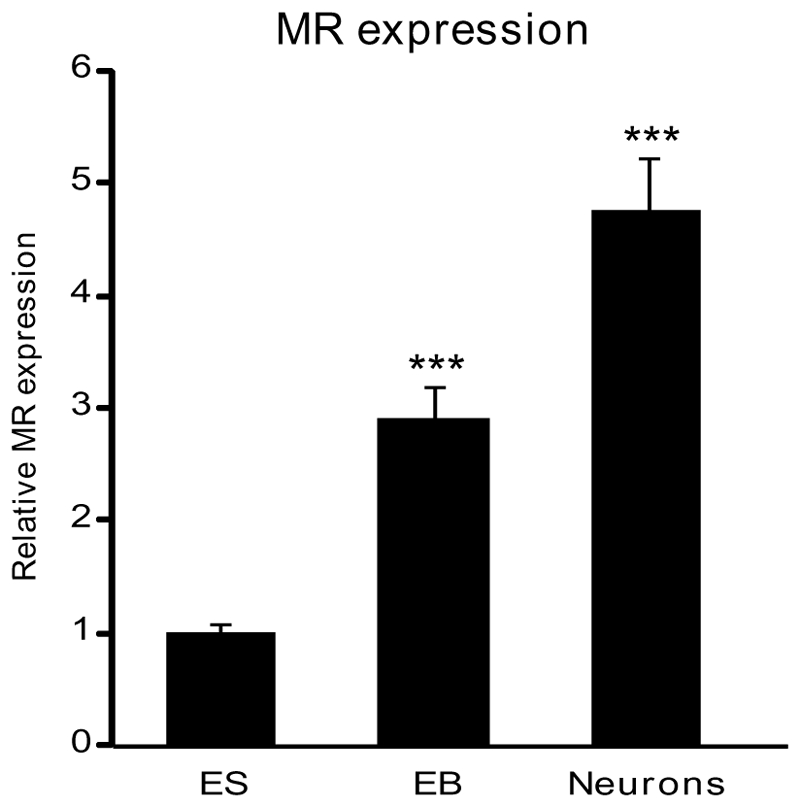
Relative MR mRNA expression levels were determined using qPCR at different stages of differentiation: undifferentiated ES cells, embryoid bodies (EB) and neurons. Results are means ± SEM of six independent experiments of three samples performed in duplicate for each developmental stage and represent the relative expression compared with basal levels of ES (arbitrarily set at 1). *** P<0.001. Mann Whitney test. Relative mRNA expression is normalized to 18S rRNA expression (see Materials and Methods section).
Figure 4. MR is exclusively expressed in neurons.
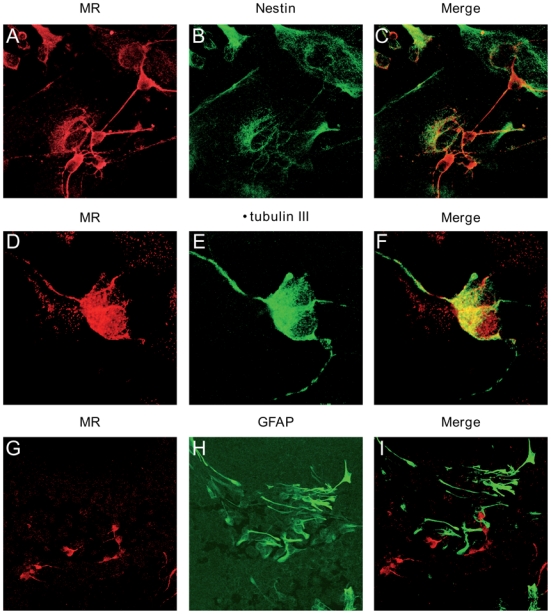
Double immunolabelling analyses of neurons with antibodies against MR (red) (left panel), Nestin (green), β-tubulin III (green), and GFAP (green) (middle panel), and merge (right panel). Original magnification: x40.
We next examined the variation of P1 and P2 promoter activities during neuronal differentiation of P1 and P2-GFP ES cell lines. The relative GFP expression was measured by quantitative real-time PCR, and was considered as a direct index of promoter activity. Figure 5A shows a 7-fold increase in GFP transcripts in P1-GFP derived differentiated neurons compared to that measured in the corresponding EB stage. Similarly, a 5-fold increase in GFP mRNA levels was observed in P2-GFP derived neurons, indicating that both P1 and P2 promoters were activated during neuronal differentiation. We also compared the relative GFP expression driven by either P1 or P2 promoter in undifferentiated ES cells as well as in neurons (Figure 5B). We thus demonstrated that the P1 activity was always higher than P2 activity by a 46-fold and a 6-fold factor in undifferentiated ES cells and in mature neurons, respectively. These findings were reproducibly obtained with the other recombinant ES cell lines. These results confirmed previous data obtained by transient transfection assays performed in fibroblast-like cells and in transgenic mice (13, 14) which indicated that the P1 promoter basal transcriptional activity is also stronger than that of P2 promoter, irrespective of the model studied. Double staining showed that GFP was present in P1-GFP MR-positive cells (Figure 5C), indicating that P1 promoter was transcriptionally active in neurons. In P2-GFP cells, GFP expression was most of the times below the immunodetection threshold, confirming that P2 activity is weaker than P1 activity. Altogether, our results clearly demonstrate that P1 and P2 promoters are both effective in driving MR expression throughout neuronal differentiation but with different strengths.
Figure 5. Comparison of P1 and P2 activity during neuronal differentiation.
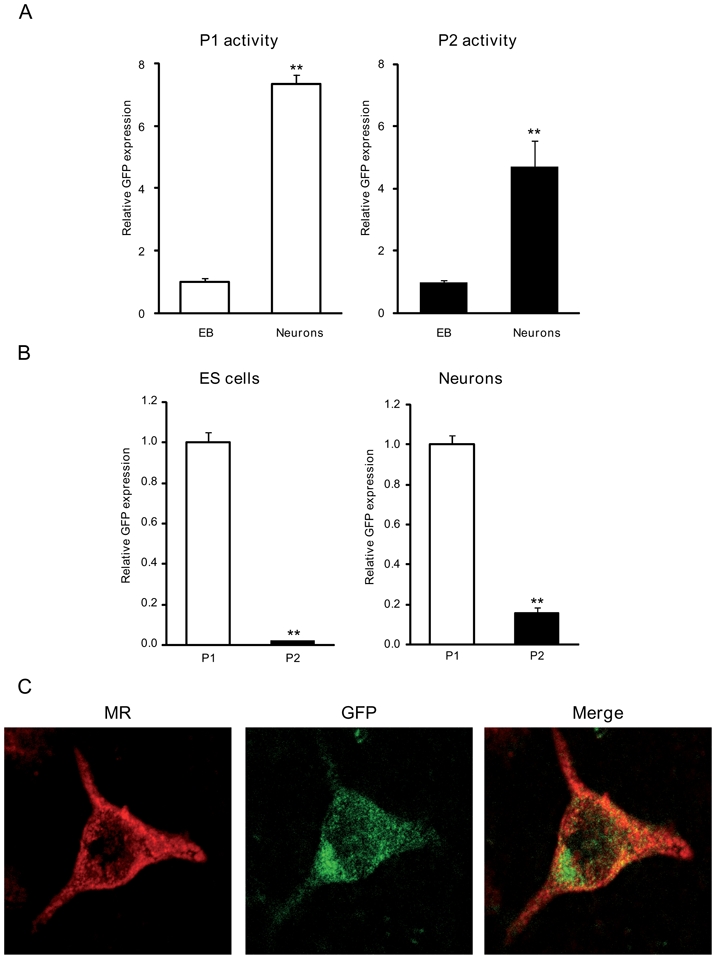
A) Relative GFP mRNA expression levels, which represent P1 and P2 activity, were determined using qPCR at different stages of differentiation: embryoid bodies (EB) and neurons. Results are means ± SEM of at least four independent experiments of three samples performed in duplicate and represent the relative expression compared with basal levels in embryoid bodies (arbitrarily set at 1). ** P<0.01. Mann Whitney test. Relative mRNA expression is normalized to 18S rRNA expression (see Materials and Methods section). B) Relative GFP mRNA expression levels were determined using qPCR at different stages of differentiation: ES cells and neurons. Results are means ± SEM of at least four independent experiments of three samples performed in duplicate and represent the relative P2 activity compared with basal levels of P1 activity (arbitrarily set at 1). ** P<0.01. Mann Whitney test. Relative mRNA expression is normalized to 18S rRNA expression (see Materials and Methods section). C) Double immunolabelling analyses of neurons with antibodies against MR (red) (left panel), and GFP (green) (middle panel), and merge (right panel). Original magnification: x40.
Hormonal regulation of P1 and P2 activity and MR expression in ES cell-derived neurons
Previously, in vitro characterization of the P1 and P2 promoters revealed that both promoters were activated by dexamethasone, via the glucocorticoid receptor (GR), whereas only P2 was activated by aldosterone, via MR (13). Moreover, it has been also demonstrated that progesterone, via the progesterone receptor (PR), increases MR mRNA levels in primary hippocampal neuron cultures (21). We thus decided to investigate the impact of these steroid hormones on MR expression in ES cell-derived neurons. First, we examined the expression pattern of GR and PR and demonstrated that their transcript levels also increased by 7-fold and 20-fold, respectively during neuronal differentiation (Supplemental figure). These results provide evidence that GR and PR are progressively and more intensively expressed during the first steps of neuronal differentiation, thus suggesting that they could represent key players in ES cell-derived mature neuron function.
We next exposed ES cell-derived neurons to aldosterone, dexamethasone or progesterone at day 13 of differentiation. Steroid-induced modification of P1 and P2 activity and MR mRNA expression were determined after 24 h treatment using quantitative real-time PCR. As shown in Figure 6A, 100 nM aldosterone increased GFP mRNA expression in P1-GFP neurons by 1.4-fold, whereas dexamethasone and progesterone failed to exert any significant effect. Dose-dependent curves demonstrated that the P1 promoter was activated only at the highest aldosterone concentration (Figure 6B). On the other hand, the P2 promoter was highly sensitive to aldosterone action since GFP mRNA levels already increased by a 1.5-fold factor in P2-GFP neurons at 1 nM aldosterone concentration (Figure 6C and 6D). Similarly, neuronal MR expression was significantly enhanced in the presence of 100 nM aldosterone, following the evolution of aldosterone-induced P1 promoter activity (Figure 6E and 6F). In sharp contrast, dexamethasone and progesterone had no significant effect on GFP and MR mRNA levels in these ES cell-derived neurons, suggesting that these steroid hormones did not regulate MR expression in neurons. In order to examine the involvement of MR in the regulation of its own promoters’ activity, we decided to knockdown MR expression by a siRNA strategy with two unrelated MR-specific siRNA in the P2-GFP ES-derived neurons since they exhibited the highest sensitivity to aldosterone action. As shown in Figure 7, compared with scrambled siRNA, a decrease in MR mRNA expression was obtained by each of two MR siRNA (Figure 7A) and was accompanied by the disappearance of aldosterone-stimulated GFP expression (Figure 7B). Altogether, these findings provide support for the involvement of MR, but not of GR, in the activation of both P1 and P2 promoters, pointing to the implication of the mineralocorticoid signaling pathway in the regulatory mechanisms governing MR expression in mature neurons.
Figure 6. Hormonal regulation of P1 and P2 activity and MR expression.
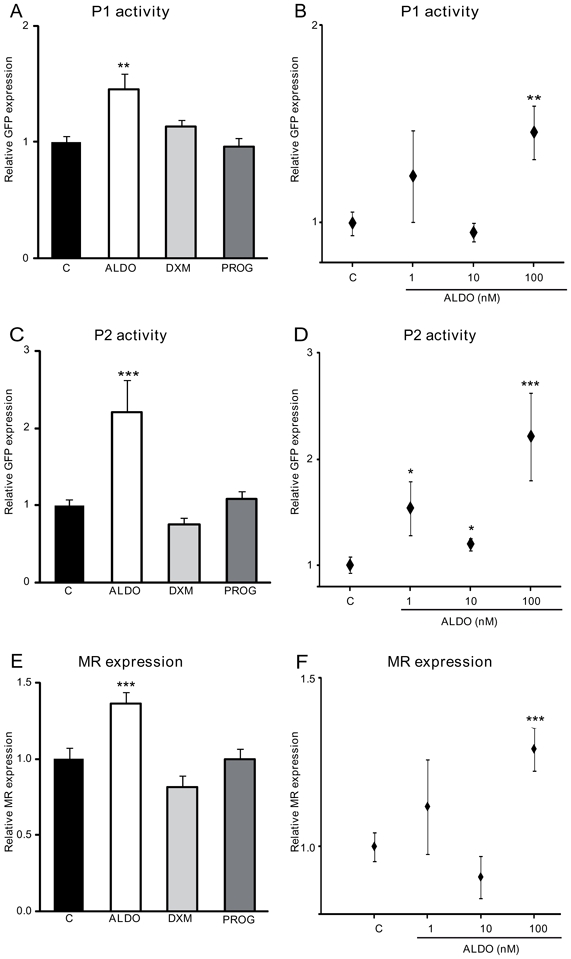
Neurons were exposed to 100 nM aldosterone (ALDO), dexamethasone (DXM) or progesterone (PROG) (A,C,E) or to increasing concentrations of aldosterone (1 to 100 nM) (B,D,F).
A, B, C, D) Relative GFP mRNA expression levels were determined using qPCR. Results are means ± SEM of four independent experiments of six samples performed in duplicate and represent the relative expression compared with basal levels of control untreated cells (C) (arbitrarily set at 1). *** P<0.001 ** P<0.01 * P<0.05. Mann Whitney test. Relative mRNA expression is normalized to 18S rRNA expression (see Materials and Methods section). E, F) Relative MR mRNA expression levels were determined using qPCR. Results are means ± SEM of four independent experiments of six samples performed in duplicate and represent the relative expression compared with basal levels of control untreated cells (C) (arbitrarily set at 1). *** P<0.001. Mann Whitney test. Relative mRNA expression is normalized to 18S rRNA expression (see Materials and Methods section).
Figure 7. MR down-regulation inhibits aldosterone-induced P2 promoter activity.
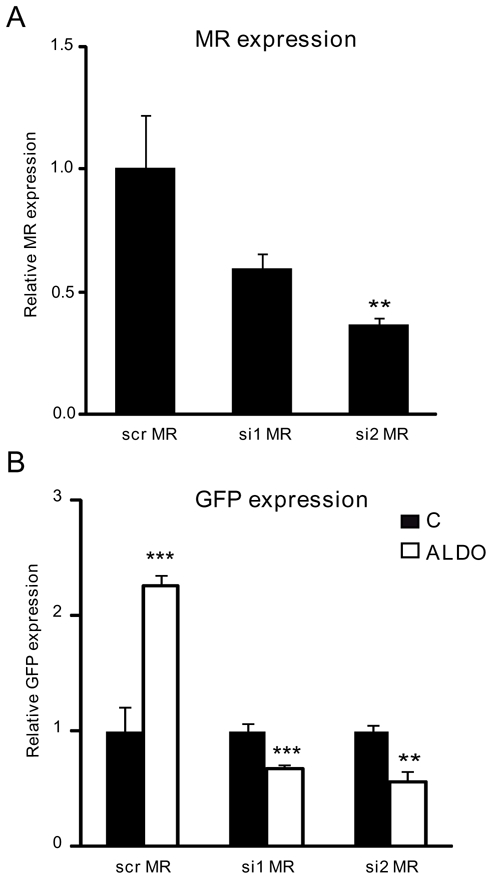
Neurons were transfected with either the control scrambled siRNA (scr MR) or by two unrelated MR siRNA (si1 MR, si2 MR). After transfection, neurons were incubated or not with 100 nM aldosterone (ALDO).
A) Relative MR mRNA expression levels were determined using qPCR. Results are means ± SEM of six samples performed in duplicate and represent the relative expression compared with basal levels of control scrambled siRNA transfected (scr MR) (arbitrarily set at 1). ** P<0.01. Mann Whitney test. Relative mRNA expression is normalized to 18S rRNA expression (see Materials and Methods section).
B) Relative GFP mRNA expression levels were determined using qPCR. Results are means ± SEM of six samples performed in duplicate and represent the relative expression compared with basal levels of control untreated cells (C) (arbitrarily set at 1). *** P<0.001 ** P<0.01. Mann Whitney test. Relative mRNA expression is normalized to 18S rRNA expression (see Materials and Methods section).
DISCUSSION
In the present study, we have established a novel ES cell model of neuronal differentiation to assess the molecular mechanisms involved in MR expression using stably transfected P1-GFP and P2-GFP ES cell lines. We found that MR expression gradually increases during neuronal differentiation and demonstrated that MR is preferentially, if not exclusively, expressed in mature neurons. This is concomitant with the increase of GR and PR expression, underlying the potential importance of these steroid receptors during neurogenesis and differentiation. We showed that both P1 and P2 promoter drive neuronal MR expression, the former being always stronger than the latter. Finally, we provided evidence that aldosterone treatment significantly induces both MR expression and P1 and P2 activity in mature neurons. These findings suggest the involvement of MR activation in the stimulation of P1 and P2 activities and on MR expression in mature neurons, a hypothesis supported by MR knockdown experiments. However, the precise molecular mechanisms responsible for the aldosterone-induced MR expression, and how P1 and P2 promoters are specifically activated by aldosterone remain to be determined.
The present work constitutes a detailed study characterizing MR expression during neuronal ES cell differentiation. Indeed, the ES cell model has been shown to be quite relevant in the neuronal field and widely used to decipher the activity or function of genes of interest and to better understand the pathophysiological mechanisms involved in some neurodegenerative diseases (22). A previous initial report demonstrated an increase of MR mRNA expression associated with a decrease of GR and PR expression during the first six days of spontaneous differentiation of human and murine ES cells (23). We provide evidence that the level of steroid receptors including MR, GR and PR increases during neuron commitment of murine ES cells in accordance with the increase of PR content demonstrated during differentiation of dopamine neurons derived from mouse ES cells (24).
It is well established that MR is expressed in the limbic system most notably in the hippocampus (25). Within the hippocampus, the highest density occurs in pyramidal cells of the CA1 region and in granule neurons of the dentate gyrus but in the adult hippocampus MR is not expressed in the neuronal stem cells (26). As opposed to GR, MR seems to be specifically expressed in mature neurons and is absent in glial cells (26), consistent with our findings which reveal that the majority of ES-derived mature neurons are MR-positive. In contrast, MR does not colocalize with GFAP-positive cells even though an increase of MR-expressing astrocytes was been reported in the striatum after ischemic conditions (27).
The question on the precise subcellular localization of neuronal MR is still debated, owing to the dual possibility of the classical nuclear tropism of MR acting as a hormone-dependent transcription factor (28, 29) and of the putative membrane MR mediating rapid, nongenomic effects via pre- as well as postsynaptic pathways (30). To further investigate this subcellular trafficking, we used ES-differentiated mature neurons which were submitted to aldosterone or glucocorticoid exposure. Under basal conditions, MR mostly resided in the cytoplasm of neurons with no obvious nuclear translocation after aldosterone treatment, supporting the possibility of a membrane-bound MR. In contrast, dexamethasone or corticosterone induced a clear nucleocytoplasmic redistribution (data not shown). Given the coexpression with neuronal GR, we formulate the hypothesis that GR plays an indispensable role in MR nuclear trafficking, via GR-MR heterocomplexes, in agreement with a recent study demonstrating the importance of the presence of GR in mediating MR-dependant transcription (31).
Another important issue, given the potential neuroprotective effect of MR (32, 33) is a better understanding of the regulatory mechanisms that control neuronal MR expression. Beside transcriptional control, the promoter activity during neuronal differentiation could also be regulated by epigenetic mechanisms. Indeed, some transcription factors (Oct4, Nanog, and Sox2) or histone modification and DNA methylation are coordinated to control the expression of pluripotent versus developmental genes in ES cells and during in vitro differentiation. A recent study shows that the promoters of a third of the genes expressed in undifferentiated ES cells are methylated and that these genes are implicated in development (34). Along this line, in silico analysis of the P2 promoter sequence revealed the presence of several GC-rich regions likely to be methylated, which could account for its weak activity. In a recent study, pyrosequencing of two CpG islands within rat MR gene promoter demonstrated a correlation between methylation and MR expression (35). Weaver et al showed that the methylation of CpG on the transcription factor NGFI-A (Nerve Growth Factor-Inductible protein A) response elements located in the GR promoter resulted in a transcriptional repression in hippocampal neurons (36). Interestingly, P2 promoter exhibited two NGFI-A response elements at position −834 bp and −857 bp, which could explain the modulation of this promoter activity observed during ES cells neuronal differentiation. Recently, a novel role for the β promoter of rat MR gene was defined (37). This promoter has a weak activity under basal conditions, but its activity increases under hypothermic anoxia in neonatal rat hippocampus. Given that the rat β promoter is the homologous of the human P2 promoter gene, this finding suggests a potential role for this otherwise weak promoter and opens interesting pathophysiological perspectives.
It has been proposed that neuronal MR expression is subjected to a tight regulatory control by different stimuli. For instance, MR expression increases in primary hippocampus neuron cultures after serotonin exposure (38) or after antidepressant treatment in adrenalectomized male mice (39). Dexamethasone and progesterone also increase rat MR gene transcription in primary hippocampal neurons (21, 40). Using ES-derived mature neurons, we demonstrated that aldosterone but not glucocorticoid or progesterone significantly increases both neuronal MR expression as well as P1 and P2 activity. Moreover, we showed that down-regulation of MR expression by siRNA in ES-derived neurons leads to a disappearance of aldosterone-stimulated GFP expression, providing evidence that MR is at least in part implicated in the regulation of its own promoter activity. This does not exclude that other mechanisms as recently proposed (41) might be involved in the aldosterone-regulated P1 and P2 promoter activities. However, the mechanisms by which aldosterone-activated MR controls MR promoter activity and whether this occurs through direct or indirect interaction with others transcription factors remain to be established. Collectively, these findings suggest a pivotal role of neuronal MR abundance and of its subcellular localization which are likely important players involved in the complex developmental regulatory pattern (42, 43), the corticosteroid-regulated synaptic plasticity (30) and neuronal excitability (4). The importance of MR function in the brain is largely demonstrated by animal models of MR overexpression or inactivation. No major morphological alteration of the CNS was detected in adult MR−/− mice, suggesting that MR is mainly involved in the maintenance of cellular and structural integrity of neurons rather that in CNS development itself. This is in accordance with the neuroprotective and antiapoptotic role proposed for MR (10, 32). Taken together, these experimental data emphasize the physiological and pathophysiological importance of MR in the limbic brain. In this context, neuronal differentiation of ES cell lines proves to be a useful model to investigate novel signaling pathways involved in modulation of neuronal MR expression, opening some potential therapeutic perspectives in neurological and psychiatric diseases.
Acknowledgments
Grant supports: This work was supported by funds from Inserm, University Paris-Sud 11 and the Programme National de Recherche en Reproduction et Endocrinologie (PNRRE). MM was a recipient of a fellowship from the Ministère de l’Enseignement Supérieur et de la Recherche and from the Société Française d’Endocrinologie.
Footnotes
Disclosure information: M. M., G.M, .S.V., P.L, D.L., M.L. have nothing to disclose.
References
- 1.Viengchareun S, Le Menuet D, Martinerie L, Munier M, Pascual-Le Tallec L, Lombes M. The mineralocorticoid receptor: insights into its molecular and (patho)physiological biology. Nucl Recept Signal. 2007;5:e012. doi: 10.1621/nrs.05012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geerling JC, Loewy AD. Aldosterone-sensitive neurons in the nucleus of the solitary: efferent projections. J Comp Neurol. 2006;498:223–250. [PubMed] [Google Scholar]
- 3.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 4.Joels M. Functional actions of corticosteroids in the hippocampus. Eur J Pharmacol. 2008;583:312–321. doi: 10.1016/j.ejphar.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 5.Rozeboom AM, Akil H, Seasholtz AF. Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc Natl Acad Sci U S A. 2007;104:4688–4693. doi: 10.1073/pnas.0606067104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai M, Horsburgh K, Bae SE, Carter RN, Stenvers DJ, Fowler JH, Yau JL, Gomez-Sanchez CE, Holmes MC, Kenyon CJ, Seckl JR, Macleod MR. Forebrain mineralocorticoid receptor overexpression enhances memory, reduces anxiety and attenuates neuronal loss in cerebral ischaemia. Eur J Neurosci. 2007;25:1832–1842. doi: 10.1111/j.1460-9568.2007.05427.x. [DOI] [PubMed] [Google Scholar]
- 7.Berger S, Wolfer DP, Selbach O, Alter H, Erdmann G, Reichardt HM, Chepkova AN, Welzl H, Haas HL, Lipp HP, Schutz G. Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc Natl Acad Sci U S A. 2006;103:195–200. doi: 10.1073/pnas.0503878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinks V, Berger S, Gass P, de Kloet ER, Oitzl MS. Mineralocorticoid receptors in control of emotional arousal and fear memory. Horm Behav. 2009 doi: 10.1016/j.yhbeh.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Almeida OF, Conde GL, Crochemore C, Demeneix BA, Fischer D, Hassan AH, Meyer M, Holsboer F, Michaelidis TM. Subtle shifts in the ratio between pro- and antiapoptotic molecules after activation of corticosteroid receptors decide neuronal fate. FASEB J. 2000;14:779–790. doi: 10.1096/fasebj.14.5.779. [DOI] [PubMed] [Google Scholar]
- 10.Crochemore C, Lu J, Wu Y, Liposits Z, Sousa N, Holsboer F, Almeida OF. Direct targeting of hippocampal neurons for apoptosis by glucocorticoids is reversible by mineralocorticoid receptor activation. Mol Psychiatry. 2005;10:790–798. doi: 10.1038/sj.mp.4001679. [DOI] [PubMed] [Google Scholar]
- 11.Gass P, Kretz O, Wolfer DP, Berger S, Tronche F, Reichardt HM, Kellendonk C, Lipp HP, Schmid W, Schutz G. Genetic disruption of mineralocorticoid receptor leads to impaired neurogenesis and granule cell degeneration in the hippocampus of adult mice. EMBO Rep. 2000;1:447–451. doi: 10.1093/embo-reports/kvd088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oitzl MS, van Haarst AD, Sutanto W, de Kloet ER. Corticosterone, brain mineralocorticoid receptors (MRs) and the activity of the hypothalamic-pituitary-adrenal (HPA) axis: the Lewis rat as an example of increased central MR capacity and a hyporesponsive HPA axis. Psychoneuroendocrinology. 1995;20:655–675. doi: 10.1016/0306-4530(95)00003-7. [DOI] [PubMed] [Google Scholar]
- 13.Zennaro MC, Le Menuet D, Lombes M. Characterization of the human mineralocorticoid receptor gene 5′-regulatory region: evidence for differential hormonal regulation of two alternative promoters via nonclassical mechanisms. Mol Endocrinol. 1996;10:1549–1560. doi: 10.1210/mend.10.12.8961265. [DOI] [PubMed] [Google Scholar]
- 14.Le Menuet D, Viengchareun S, Penfornis P, Walker F, Zennaro MC, Lombes M. Targeted oncogenesis reveals a distinct tissue-specific utilization of alternative promoters of the human mineralocorticoid receptor gene in transgenic mice. J Biol Chem. 2000;275:7878–7886. doi: 10.1074/jbc.275.11.7878. [DOI] [PubMed] [Google Scholar]
- 15.Le Menuet D, Isnard R, Bichara M, Viengchareun S, Muffat-Joly M, Walker F, Zennaro MC, Lombes M. Alteration of cardiac and renal functions in transgenic mice overexpressing human mineralocorticoid receptor. J Biol Chem. 2001;276:38911–38920. doi: 10.1074/jbc.M103984200. [DOI] [PubMed] [Google Scholar]
- 16.Le Menuet D, Zennaro MC, Viengchareun S, Lombes M. Transgenic mouse models to study human mineralocorticoid receptor function in vivo. Kidney Int. 2000;57:1299–1306. doi: 10.1046/j.1523-1755.2000.00966.x. [DOI] [PubMed] [Google Scholar]
- 17.Gu P, Goodwin B, Chung AC, Xu X, Wheeler DA, Price RR, Galardi C, Peng L, Latour AM, Koller BH, Gossen J, Kliewer SA, Cooney AJ. Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol Cell Biol. 2005;25:3492–3505. doi: 10.1128/MCB.25.9.3492-3505.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinerie L, Viengchareun S, Delezoide AL, Jaubert F, Sinico M, Prevot S, Boileau P, Meduri G, Lombes M. Low renal mineralocorticoid receptor expression at birth contributes to partial aldosterone resistance in neonates. Endocrinology. 2009;150:4414–4424. doi: 10.1210/en.2008-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viengchareun S, Kamenicky P, Teixeira M, Butlen D, Meduri G, Blanchard-Gutton N, Kurschat C, Lanel A, Martinerie L, Sztal-Mazer S, Blot-Chabaud M, Ferrary E, Cherradi N, Lombes M. Osmotic stress regulates mineralocorticoid receptor expression in a novel aldosterone-sensitive cortical collecting duct cell line. Mol Endocrinol. 2009;23:1948–1962. doi: 10.1210/me.2009-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bibel M, Richter J, Lacroix E, Barde YA. Generation of a defined and uniform population of CNS progenitors and neurons from mouse embryonic stem cells. Nat Protoc. 2007;2:1034–1043. doi: 10.1038/nprot.2007.147. [DOI] [PubMed] [Google Scholar]
- 21.Castren M, Patchev VK, Almeida OF, Holsboer F, Trapp T, Castren E. Regulation of rat mineralocorticoid receptor expression in neurons by progesterone. Endocrinology. 1995;136:3800–3806. doi: 10.1210/endo.136.9.7649087. [DOI] [PubMed] [Google Scholar]
- 22.Crews L, Mizuno H, Desplats P, Rockenstein E, Adame A, Patrick C, Winner B, Winkler J, Masliah E. Alpha-synuclein alters Notch-1 expression and neurogenesis in mouse embryonic stem cells and in the hippocampus of transgenic mice. J Neurosci. 2008;28:4250–4260. doi: 10.1523/JNEUROSCI.0066-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie CQ, Jeong Y, Fu M, Bookout AL, Garcia-Barrio MT, Sun T, Kim BH, Xie Y, Root S, Zhang J, Xu RH, Chen YE, Mangelsdorf DJ. Expression profiling of nuclear receptors in human and mouse embryonic stem cells. Mol Endocrinol. 2009;23:724–733. doi: 10.1210/me.2008-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz NF, Guerra-Arraiza C, Diaz-Martinez NE, Salazar P, Molina-Hernandez A, Camacho-Arroyo I, Velasco I. Changes in the content of estrogen alpha and progesterone receptors during differentiation of mouse embryonic stem cells to dopamine neurons. Brain Res Bull. 2007;73:75–80. doi: 10.1016/j.brainresbull.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 26.Garcia A, Steiner B, Kronenberg G, Bick-Sander A, Kempermann G. Age-dependent expression of glucocorticoid- and mineralocorticoid receptors on neural precursor cell populations in the adult murine hippocampus. Aging Cell. 2004;3:363–371. doi: 10.1111/j.1474-9728.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- 27.Oyamada N, Sone M, Miyashita K, Park K, Taura D, Inuzuka M, Sonoyama T, Tsujimoto H, Fukunaga Y, Tamura N, Itoh H, Nakao K. The role of mineralocorticoid receptor expression in brain remodeling after cerebral ischemia. Endocrinology. 2008;149:3764–3777. doi: 10.1210/en.2007-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishi M, Ogawa H, Ito T, Matsuda KI, Kawata M. Dynamic changes in subcellular localization of mineralocorticoid receptor in living cells: in comparison with glucocorticoid receptor using dual-color labeling with green fluorescent protein spectral variants. Mol Endocrinol. 2001;15:1077–1092. doi: 10.1210/mend.15.7.0659. [DOI] [PubMed] [Google Scholar]
- 29.Nishi M, Kawata M. Dynamics of glucocorticoid receptor and mineralocorticoid receptor: implications from live cell imaging studies. Neuroendocrinology. 2007;85:186–192. doi: 10.1159/000101917. [DOI] [PubMed] [Google Scholar]
- 30.Olijslagers JE, de Kloet ER, Elgersma Y, van Woerden GM, Joels M, Karst H. Rapid changes in hippocampal CA1 pyramidal cell function via pre- as well as postsynaptic membrane mineralocorticoid receptors. Eur J Neurosci. 2008;27:2542–2550. doi: 10.1111/j.1460-9568.2008.06220.x. [DOI] [PubMed] [Google Scholar]
- 31.Tsugita M, Iwasaki Y, Nishiyama M, Taguchi T, Shinahara M, Taniguchi Y, Kambayashi M, Nishiyama A, Gomez-Sanchez CE, Terada Y, Hashimoto K. Glucocorticoid receptor plays an indispensable role in mineralocorticoid receptor-dependent transcription in GR-deficient BE(2)C and T84 cells in vitro. Mol Cell Endocrinol. 2009;302:18–25. doi: 10.1016/j.mce.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Fujioka A, Fujioka T, Ishida Y, Maekawa T, Nakamura S. Differential effects of prenatal stress on the morphological maturation of hippocampal neurons. Neuroscience. 2006;141:907–915. doi: 10.1016/j.neuroscience.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 33.Macleod MR, Johansson IM, Soderstrom I, Lai M, Gido G, Wieloch T, Seckl JR, Olsson T. Mineralocorticoid receptor expression and increased survival following neuronal injury. Eur J Neurosci. 2003;17:1549–1555. doi: 10.1046/j.1460-9568.2003.02587.x. [DOI] [PubMed] [Google Scholar]
- 34.Fouse SD, Shen Y, Pellegrini M, Cole S, Meissner A, Van Neste L, Jaenisch R, Fan G. Promoter CpG methylation contributes to ES cell gene regulation in parallel with Oct4/Nanog, PcG complex, and histone H3 K4/K27 trimethylation. Cell Stem Cell. 2008;2:160–169. doi: 10.1016/j.stem.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Arguelles DB, Culty M, Zirkin BR, Papadopoulos V. In utero exposure to di-(2-ethylhexyl) phthalate decreases mineralocorticoid receptor expression in the adult testis. Endocrinology. 2009;150:5575–5585. doi: 10.1210/en.2009-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weaver IC, D’Alessio AC, Brown SE, Hellstrom IC, Dymov S, Sharma S, Szyf M, Meaney MJ. The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J Neurosci. 2007;27:1756–1768. doi: 10.1523/JNEUROSCI.4164-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang P, Rogalska J, Walker CA, Burke M, Seckl JR, Macleod MR, Lai M. Injury-induced mineralocorticoid receptor expression involves differential promoter usage: a novel role for the rat MRbeta variant. Mol Cell Endocrinol. 2009;305:56–62. doi: 10.1016/j.mce.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Lai M, McCormick JA, Chapman KE, Kelly PA, Seckl JR, Yau JL. Differential regulation of corticosteroid receptors by monoamine neurotransmitters and antidepressant drugs in primary hippocampal culture. Neuroscience. 2003;118:975–984. doi: 10.1016/s0306-4522(03)00038-1. [DOI] [PubMed] [Google Scholar]
- 39.Heydendael W, Jacobson L. Differential effects of imipramine and phenelzine on corticosteroid receptor gene expression in mouse brain: potential relevance to antidepressant response. Brain Res. 2008;1238:93–107. doi: 10.1016/j.brainres.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Castren M, Trapp T, Berninger B, Castren E, Holsboer F. Transcriptional induction of rat mineralocorticoid receptor gene in neurones by corticosteroids. J Mol Endocrinol. 1995;14:285–293. doi: 10.1677/jme.0.0140285. [DOI] [PubMed] [Google Scholar]
- 41.Bunda S, Wang Y, Mitts TF, Liu P, Arab S, Arabkhari M, Hinek A. Aldosterone stimulates elastogenesis in cardiac fibroblasts via mineralocorticoid receptor-independent action involving the consecutive activation of Galpha13, c-Src, the insulin-like growth factor-I receptor, and phosphatidylinositol 3-kinase/Akt. J Biol Chem. 2009;284:16633–16647. doi: 10.1074/jbc.M109.008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kretz O, Schmid W, Berger S, Gass P. The mineralocorticoid receptor expression in the mouse CNS is conserved during development. Neuroreport. 2001;12:1133–1137. doi: 10.1097/00001756-200105080-00017. [DOI] [PubMed] [Google Scholar]
- 43.Vazquez DM, Lopez JF, Morano MI, Kwak SP, Watson SJ, Akil H. Alpha, beta, and gamma mineralocorticoid receptor messenger ribonucleic acid splice variants: differential expression and rapid regulation in the developing hippocampus. Endocrinology. 1998;139:3165–3177. doi: 10.1210/endo.139.7.6095. [DOI] [PubMed] [Google Scholar]


