Abstract
A new liquid chromatography (LC)-negative ion electrospray ionization (ESI−)–tandem mass spectrometry (MS/MS) method with post-column addition of ammonia in methanol has been developed for the analysis of acid herbicides: 2,4-dichlorophenoxy acetic acid, 4-chloro-o-tolyloxyacetic acid, 2-(2-methyl-4-chlorophenoxy)butyric acid, mecoprop, dichlorprop, 4-(2,4-dichlorophenoxy) butyric acid, 2,4,5-trichlorophenoxy propionic acid, dicamba and bromoxynil, along with their degradation products: 4-chloro-2-methylphenol, 2,4-dichlorophenol, 2,4,5-trichlorophenol and 3,5-dibromo-4-hydroxybenzoic acid. The samples were extracted from the surface water matrix using solid-phase extraction (SPE) with a polymeric sorbent and analyzed with LC ESI− with selected reaction monitoring (SRM) using a three-point confirmation approach. Chromatography was performed on a Zorbax Eclipse XDB-C18 (50 × 4.6 mm i.d., 1.8 μm) with a gradient elution using water-methanol with 2 mM ammonium acetate mobile phase at a flow rate of 0.15 mL/min. Ammonia in methanol (0.8 M) was added post-column at a flow rate of 0.05 mL/min to enhance ionization of the degradation products in the MS source. One SRM transition was used for quantitative analysis while the second SRM along with the ratio of SRM1/SRM2 within the relative standard deviation determined by standards for each individual pesticide and retention time match were used for confirmation. The standard deviation of ratio of SRM1/SRM2 obtained from standards run on the day of analysis for different phenoxyacid herbicides ranged from 3.9 to 18.5%. Limits of detection (LOD) were between 1 and 15 ng L−1 and method detection limits (MDL) with strict criteria requiring <25% deviation of peak area from best-fit line for both SRM1 and SRM2 ranged from 5 to 10 ng L−1 for acid ingredients (except dicamba at 30 ng L−1) and from 2 to 30 ng L−1 for degradation products. The SPE-LC-ESI− MS/MS method permitted low nanogram-per-liter determination of pesticides and degradation products for surface water samples.
Keywords: liquid chromatography-tandem mass spectrometry, water analysis, pesticides, phenoxyacid herbicides, post-column reagent addition
Introduction
The phenoxyacid herbicides have widespread use for weed control for agricultural crops as well as residential lawns and are consequently of interest for environmental monitoring in surface and ground water. In Canada active ingredients of these herbicides formulations that have drinking water quality standards include 2,4-dichlorophenoxy acetic acid (2,4-D), dicamba, and bromoxynil with maximum acceptable concentrations of 100, 120, and 5 μg L−1, respectively. The degradation products 2,4-dichlorophenol and 2,4,5-trichlorophenol are also regulated at 900 and 5 μg L−1, respectively in drinking water.1 Canadian Water Quality Guidelines for the protection of aquatic life are more stringent with guidelines for total phenoxyacid herbicides (based on ester of 2,4-D) of 4.0 μg L−1, and guidelines for individual herbicides including dicamba (10 μg L−1), bromoxynil (5.0 μg L−1), 4-chloro-o-tolyloxyacetic acid (2.6 μg L−1) and the degradation products are grouped under total monochlorophenols (7 μg L−1), dichlorophenols (0.2 μg L−1), and trichlorophenols (18 μg L−1) in fresh-water systems.2 The degradation products can be more toxic and persistent than parent active ingredient. Mecoprop, 4-chloro-o-tolyloxyacetic acid, and 2-(2-methyl-4-chlorophenoxy)butyric acid degrade to 4-chloro-2-methylphenol; 2,4-dichlorophenoxy acetic acid, dichlorprop, and 4-(2,4-dichlorophenoxy) butyric acid degrade to 2,4-dichlorophenol; 2,4,5-trichlorophenoxy propionic acid to 2,4,5-trichlorophenol, and bromoxynil to 3,5-dibromo-4-hydroxybenzoic acid.3,4
Phenoxyacid herbicides have been analyzed using gas chromatography with either selective detectors or mass spectrometry but these methods are limited in that they do not include degradation products and require a time consuming derivatization step prior to GC due to the polarity and low volatility of these compounds.5 Chlorophenols have also been analyzed using GC with derivatization.6 More recently LC has been used for a few selected phenoxyacid herbicides, but existing methods are still limited by poor sensitivity for their degradation products with ultraviolet detection often preferred over mass spectrometry.7–12 Additional pre-concentration steps are included in the sample preparation to obtain better sensitivity. LC/MS ion sources that are most commonly used for acid and neutral herbicides include atmospheric pressure chemical ionization (APCI) and electrospray (ESI),11–14 with active ingredients showing greater sensitivity with ESI in negative ion mode.12–14 Instrumental detection limits using ESI−-SIM for phenoxyacid herbicides have been reported from 0.001–0.1 μg L−1,12,13 while GC or LC-MS methods for related methyl or chlorophenols has been reported 0.1 to 5 μg L−1.6,13 LC/MS methods lack confirmation ability as largely only the protonated molecular ion is observed. The acetate adduct [M+CH3OO]− can also be formed from acetate in the eluent.14 LC-tandem mass spectrometry often also reports only one fragment ion for each compound and it can be the same fragment ion for different phenoxyacids due to the same skeletal structure of this chemical class with the main differences existing in the substitution of the 2-position of the ring (methyl or chlorine) and in the β-position to the carboxylic function (e.g. hydrogen or methyl).13–15 Ionpairing LC has also been used for the separation of acidic pesticides to improve retention16 with addition of quaternary ammonium salts to the mobile phase and also as a keeper during sample concentration steps. Use of ion-pairing reagents or high salt concentrations however can lead to increased MS signal instability and maintenance for the mass spectrometer, and the mobile phases used for this separation also utilized acetonitrile as the organic modifier, which currently has higher cost and for many pesticides lower ionization capability than methanol mobile phases.17,18 Different bases including ammonia, trimethylamine, and 1,8-diazabicyclo-(5,4,0)undec-7-en have been used as post-column reagents to enhance ionization in MS sources.16,17
This new LC-ESI−-MS/MS method developed involved the use of a cross-linked polystyrene divinylbenzene (Chrom P) solid phase extraction (SPE) cartridge for extracting pesticides from (1 L) acidified water samples. Post-column reagent addition of ammonia in methanol prior to MS/MS detection is utilized to improve the sensitivity for degradation products of phenoxyacid herbicides. The goal of the present work was to develop a LC-tandem mass spectrometry method for simultaneous determination of phenoxyacid herbicides and their degradation products that provides the selectivity, confirmation ability, and detection limits required for analysis of complex aqueous matrices such as surface water samples taken from storm water ponds in the City of Regina. A three-point confirmation approach similar to our previous methods19–20 is used which includes retention time match, two SRM transitions for each compound with criteria that the response ratio of these two transitions must be within the standard deviation determined by standards.
Experimental
Materials
Ethyl acetate and methanol were pesticide grade and supplied by Fisher Scientific. Deionized water was (<18 MΩcm resistivity) obtained from a Nanopure diamond™ system (Barnstead International, Dubuque, Iowa, USA). OmniPur ammonium acetate (>97%) and OmniTrace Ultra ammonia hydroxide (>99%) were obtained from EMD Biosciences, (Gibbstown, NJ, USA). The post-column solution of ammonia in methanol at 0.8 M was prepared from addition of 6.5 mL of OmniTrace Ultra ammonium hydroxide (21% w/w) to 93.5 mL methanol with the resulting solution having 6.5 v/v% water. The 2 mM ammonium acetate mobile phases were not pH adjusted and the % of methanol varied during the gradient between 65% to 90%. Based on flow rate, the percentage of water in the resulting solution after post-column reagent addition of 0.8 M ammonia in methanol changes only slightly to between 28% to 9.1% v/v% water. All mobile phase solvents were passed through 0.45 μm membrane filter from Nucleopore (Whatman, Florham Park, NJ, USA).
Individual herbicide or degradation product standards were prepared at 1.0 mg mL−1 from solids supplied Chem Service Inc. (West Chester, PA, USA) and included dicamba, bromoxynil, 4-chloro-o-tolyloxyacetic acid (MCPA), 2,4-dichlorophenoxy acetic acid (2,4-D), mecoprop, dichlorprop, 4-(2,4-dichlorophenoxy) butyric acid (2,4-DB), 2-(2-methyl-4-chlorophenoxy)butyric acid (MCPB), 2,4,5-trichlorophenoxy propionic acid (2,4,5-TP), 3,5-dibromo-4-hydroxybenzoic acid (DBHBA), 4-chloro-2-methylphenol (CMP), 2,4-dichlorophenol (DCP), 2,4,5-trichlorophenol (TCP). Deuterated internal standard, 2,4-dichlorophenoxy-3,5,6-d3-acetic-d2-acid (d5-2,4-D), was purchased as a solid from C/D/N Isotopes Inc. (Pointe-Claire, Quebec, ON, Canada) and 13C6-dilution and surrogate standards, 13C6-2,4, 5-trichlorophenoxyacetic acid (13C6-2,4,5-T) and 13C6-2,4-D, were purchased from Cambridge Isotopes Laboratories (CIL) Inc., at 100 μg mL−1. These solutions were further diluted to 1.0 μg mL−1 with pesticide grade methanol for standard solution preparation. It should be noted that 2,4,5-trichlorophenoxy-3,6-d2-acetic-d2-acid (d4-2,4,5-T) is now available from C/D/N isotopes and can be used to replace 13C6-2,4,5-T. Stock solutions were diluted to 1 μg mL−1 with pesticide grade methanol for use. Calibration standards ranged from 0.1 to 150 μg L−1 with internal standard at 100 μg L−1.
Filters used for water sample filtration included Whatman 934-AD, Whatman 41 ashless, and Whatman 0.45 μm nylon membrane filters (Canadawide Scientific, Ottawa, ON, Canada). This sequential filtration approach to smaller pore size filters was used to reduce plugging of filters and to speed up the filtration process. HPLC grade glacial acetic acid was obtained from EMD Biosciences (Gibbstown, NJ, USA) and used for pH adjustment of water samples.
Sample collection and site descriptions
A total of 86 surface water samples were collected from two stormwater ponds in the City of Regina weekly or during rainfall events from the beginning of May, 2007 to October, 2007. Windsor Park South storm water pond was established approximately 15 years ago with a surface area of 2.1 ha and operating depth of 1.8 m, while Windsor Park North located in a developing residential area with home construction was a natural wetland approach with a surface area of 0.9 ha and an operating depth varying between locations of 0.5 to 2.7 m. It is known that both parks and residential areas in the City of Regina use acid herbicides for lawn weed control with the most common formulation Killex (2,4-D 190 g/L, mecoprop 100 g/L, dicamba 18 g/L). The City of Regina uses 2,4-D, mecoprop, and dicamba within city limits. These acid herbicides as well as 2,4-DB, MCPA, MCPB, bromoxynil, dichlorprop are also used for control of broad-leaved weeds in cereal crops in Saskatchewan. Agricultural formulations of these acid herbicides include: bromoxynil + 2,4-D, bromoxynil + MCPA, dicamba + mecoprop + MCPA, or dichlorprop + 2,4-D + MCPA + MCPB.21
Sample preparation
Water samples were filtered sequentially through Whatman 934-AD glass microfibre filters, Whatman 41 ashless filters and Whatman 0.45 μm nylon membrane filters to remove particles prior to solid-phase extraction (SPE). Following filtration 10 mL of pesticide grade methanol was added to 1 L of filtered water to aid in the SPE flow. The pH of water samples was then adjusted to ~4.9 with acetic acid following filtration to facilitate adsorption of the analytes of interest onto the SPE columns.
SPE clean-up and concentration was accomplished on Supelco Superclean ENVI-Chrom-P, 1 g, 6 mL SPE tubes (Sigma-Aldrich, Oakville, ON, Canada). The SPE cartridges were preconditioned with 6 mL ethyl acetate, 6 mL of methanol, followed by 6 mL deionized water. The water sample was drawn through the SPE cartridge at a rate of 200 mL/hr by vacuum using a Supelco Visiprep DL SPE extraction manifold (Sigma-Aldrich). The SPE cartridges were dried with nitrogen for approximately 5 minutes until constant weight was achieved and then stored at −4 °C. Filtration and SPE steps described here were completed within 24 hours of sample collection to minimize potential for degradation. For elution of the pesticides from the SPE cartridges a wash step was first completed which involved addition of a surrogate spike, 13C6-2,4-D (20 μL of 10 μg mL−1) followed by 2 mL of 75/25 v/v% methanol/water. The eluted solvent was collected into fraction F0 and was shown to contain no pesticides of interest. The phenoxyacid herbicides and degrades were eluted into fraction F1 with 8 mL of 60/40 v/v% methanol/ethyl acetate giving 81–100% recoveries for the acid herbicides. The F1 extract was dried at 1 mL hr−1 to ~0.95 mL using the Visiprep DL SPE extraction manifold. This was followed by addition of dilution standard, 13C6-2,4,5-T, (50 μL of 1.0 μg mL−1) to give a total volume of 1 mL. SPE recoveries with 13C6-2,4-D surrogate were evaluated to be 90% ± 10%. Depending on the individual level of pesticides in the sample from 50 μL to 500 μL of 1 mL of the sample extract (F1) and addition of internal standard d5-2,4-D (50 μL of 1.0 μg mL−1) were diluted with methanol to a total volume of 1 mL for LC/MS/MS analysis. At these dilution factors no insoluble components in extracts were visible.
LC/MS/MS
LC analyses were performed with a Waters LC system consisting of a 1525 μ binary pump, and column heater. A LEAP Technology autosampler (Carrboro, NC) was used for 5 μL injections at 10 μL/sec and three pre- and post- cleans with ethyl acetate followed by methanol to minimize sample carry-over. A guard column, Gemini C18, 4.0 mm × 2.0 mm i.d., (Phenomenex, Torrance, CA, USA) was connected to the analytical column, Zorbax Eclipse XDB-C18, 50 mm × 4.6 mm i.d., 1.8 μm, (Agilent, Mississauga, ON, Canada) which was placed in a column heater at 25 °C. Mobile phase was 2 mM CH3COONH4 at a flow rate of 0.15 mL min−1 with methanol at 65 v/v% for the first 15 minutes then a gradient from 65% to 90% over 10 minutes and held for 10 minutes. A solution containing ammonia in methanol (0.8 M, prepared fresh daily) was added post-column into mixing tee at a flow rate of 0.05 mL min−1 using a Shimadzu model LC-20AD pump (Man-Tech Associates, Guelph, ON, Canada). The LC system was connected to a Quattro Premier (Waters-Micromass, Milford, Massachusetts, USA) triple quadrupole, which had both electrospray ionization (ESI) and atmospheric chemical ionization (APCI). The temperature of the source was set to 120 °C, desolvation temperature 350 °C, desolvation gas 750 L/hr, and cone gas 150 L/hr. The optimized settings for ESI were: capillary voltage, 4.00 kV; extractor voltage, 3.0 V; RF lens 0 V. The collision gas used for SRM was argon (UHP) at 0.18 mL min−1 or 1.6 × 10−3 mbar. Cone voltage and collision energy were set-up in the MS method and shown in Table 1.
Table 1.
Liquid chromatography-tandem mass spectrometry (LC/MS/MS) retention time (tR), and selected reaction monitoring (SRM) transitions using electrospray ionization (ESI) in negative ion mode for phenoxyacid herbicides, their degradation products, and surrogate and internal standard.
| Analyte | tR (min) |
SRM m/z (collision energy (eV), cone voltage (V)) |
|---|---|---|
| Acid herbicides | ||
| 1. Dicamba | 6.85 | 218.8 > 174.9 (7, 18); 174.9 > 144.9 (6, 20) |
| 2. Bromoxynil | 7.82 | 275.5 > 81.0 (28, 28); 275.5 > 79.0 (20, 23) |
| 3. MCPA | 9.20 | 198.9 > 140.9 (16, 23); 140.9 > 105.2 (18, 30) |
| 4. 2,4-D | 9.34 | 219.0 > 160.9 (14, 21); 160.9 > 124.7 (18, 30) |
| 5. Mecoprop | 10.72 | 213.0 > 140.9 (13, 22); 140.9 > 105.2 (18, 30) |
| 6. Dichlorprop | 11.13 | 232.8 > 160.9 (14, 26); 160.9 > 124.7 (18, 30) |
| 7. 2,4-DB | 14.58 | 246.7 > 160.9 (14, 17); 160.9 > 124.7 (18, 30) |
| 8. MCPB | 14.72 | 226.9 > 140.9 (11, 20); 140.9 > 105.2 (18, 30) |
| 9. 2,4,5-TP | 15.55 | 266.7 > 194.5 (11, 18); 266.7 > 158.8 (26, 18) |
| Degradation products | ||
| 10. DBHBA | 6.16 | 295.0 > 250.3 (18, 23); 293.0 > 248.4 (20, 23) |
| 11. CMP | 23.20 | 140.9 > 105.2 (18, 30); 142.8 > 105.2 (18, 30) |
| 12. DCP | 24.99 | 160.9 > 124.7 (18, 30); 163.0 > 124.7 (18, 30) |
| 13. TCP | 32.45 | 194.6 > 158.9 (18, 30); 196.9 > 160.9 (20, 30) |
| Internal/Surrogate standards | ||
| 15. d5-2,4-D (Internal standard) |
9.20 | 224.4 > 164.3 (17,13); 226.4 > 166.2 (17, 17) |
| 16. 13C6-2,4-D (Surrogate standard) |
9.20 | 224.8 > 166.7 (13, 17); 166.7 > 130.6 (16, 38) |
| 17. 13C6-2,4,5-T (dilution standard) |
12.10 | 258.8 > 200.7 (12, 16); 258.8 > 165.0 (31, 16) |
First SRM transition (in bold): quantitative SRM, second SRM: qualitative SRM.
Results and discussion
Optimization of SPE method
Conventional C18 silica based sorbent, graphitized carbon black, polystyrene divinyl benzene (PS-DVB), and poly(divinylbenzene co-N-vinylpyrrolidone) have been used previously for extraction of acid herbicides for soil extracts or water samples8,13,16,17 with the pH of the sample for SPE examined. We found similar to previous reports8,17 that ENVI-18 showed a stronger pH dependence with good recoveries at pH 4.0 (83%–98%) but when the pH was higher (7 or greater) or low (pH 2.0) recoveries for one or more acid herbicides in our study were poor (<60%). PS-DVB based sorbents have been shown to be less dependent on sample pH than conventional C-18,8 while other studies have shown that under acidic conditions some degradation products like TCP may exhibit poor recoveries.17 Chrom-P, a PS-DVB sorbent, showed good recoveries for all acid herbicides when the pH was ≤4.9 (see Table 2) with only dicamba having poor recoveries when pH was 6 or higher. Methanol, methanol/water, and methanol/ethylacetate solvent mixtures were tested for improving recoveries. Later experiments showed that the percentage of ethylacetate could be reduced from 60/40 v/v% to 40/60 v/v% with the same elution volume (8 mL). With this optimized procedure (8 mL of 60/40 v/v% methanol/ethyl acetate) the herbicides had recoveries between 81%–100% (Table 2). This SPE procedure was also tested for the four degradation products (DBHBA, TCP, DCP, CMP) and showed recoveries of 97, 72, 82, and 85%, respectively. Surrogate (13C6-2,4-D) recoveries were 90% ± 10% in samples analyzed. Significant loss of acid herbicides or their degradation products can occur during the drying step required to reduce the SPE extract volume. This was minimized by use of a slight vacuum and drying the sample to ~0.95 mL rather than to dryness. At this stage 50 μL of the dilution standard (13C6-2,4,5-T) is added such that the total volume of extract after drying should be 1 mL. The amount of 13C6-2,4,5-T was determined in each sample and used to correct the volume. This procedure does not require use of keeper reagents such as tetrabutylammonium chloride.16
Table 2.
Recoveries (%) obtained after solid-phase extraction with ChromP as a function of sample pH.
|
Recovery (%) |
|||||||
|---|---|---|---|---|---|---|---|
| Sample pH = 3.2 | Sample pH = 4.1 | Sample pH = 4.9 | Sample pH = 6.2 | Sample pH = 7.1 | Sample pH = 7.8 | Optimized SPE method sample pH = 4.9 | |
| Dicamba | 100 | 86 | 73 | 24 | 28 | 13 | 81 |
| 2,4-D | 88 | 80 | 79 | 86 | 79 | 85 | 93 |
| 2,4-DB | 92 | 78 | 79 | 77 | 75 | 76 | 85 |
| Dichlorprop | 89 | 79 | 82 | 82 | 72 | 82 | 100 |
| MCPA | 86 | 79 | 81 | 84 | 80 | 83 | 96 |
| 2,4,5-TP | 93 | 82 | 79 | 81 | 79 | 85 | 100 |
| Mecoprop | 89 | 79 | 79 | 85 | 78 | 88 | 100 |
| Bromoxynil | 78 | 71 | 69 | 79 | 69 | 71 | 96 |
| MCPB | 84 | 79 | 77 | 77 | 80 | 75 | 92 |
SPE conditions: 1 L of water spiked at 75 ng L−1 and eluted with 40/60 v/v% methanol/ethylacetate (F1 fraction); optimized SPE method conditions: 1 L of water spiked at 75 ng L−1 and eluted with 8 mL of 60/40 v/v% methanol/ethyl acetate (F1 fraction).
Optimization of MS-MS monitoring conditions
For optimization of MS/MS parameters, infusion experiments were conducted with both ESI and APCI (positive and negative ion mode) with direct infusion of each individual phenoxyacid herbicide or degradation product solution at 1 μg L−1 into the mass spectrometer with a syringe pump at 50 μL min−1 and mobile phase at 100 μL min−1. For all compounds APCI gave significantly poorer sensitivity than ESI, and ESI in negative ion mode (ESI−) provided the best sensitivity. Both methanol and acetonitrile were evaluated for the organic modifier of the mobile phase. Methanol gave approximately 2–5 times more intense signal than acetonitrile mobile phases as others have observed for a variety of pesticides.17,18 Formic acid was not added to the mobile phase as loss of sensitivity was observed. For most compounds, except bromoxynil and its degradate (DBHBA), the mass of the precursor ion selected for the first SRM transition corresponded to the deprotonated molecular ion [M-H]−of the target compound. Using daughter ion scans, two characteristic daughter ions with best sensitivity were selected for each compound. The general fragmentation pattern for chlorinated acid herbicides has been reported.14,15 A number of target compounds only observed one significant daughter ion from the deprotonated molecular ion with collision induced dissociation, however a second SRM transition could be obtained from further fragmentation of a daughter ion. Common second SRM transitions included 161 > 125, and 141 > 105 so these target compounds required complete LC separation prior to their identification (Table 1). Under our separation conditions, we found the SRM transitions from [M-H]− > 161 or [M-H]− > 10516 had poor sensitivity in comparison to 161 > 125 or 141 > 105. For a few compounds (bromoxynil, CMP, and DCP) the 37Cl or 81Br isotope peak of the precursor ion was chosen for the second SRM transition. In general the most intense SRM transition corresponded to a SRM transition from the deprotonated molecular ion, except from 2,4-DB. For 2,4-DB the SRM transition 246.7 > 160.9 was still chosen as the SRM transition for quantitative analysis although it was less sensitive than 160.9 > 124.7 as it was the fragmentation of the deprotonated molecular ion which was more unique than the 160.9 > 124.7 which was also observed for other pesticides (Table 1). The cone and collision energy for each of these daughter ions were optimized in order to achieve best sensitivity (Table 1). The most intense transition (first SRM transition (SRM1)) was in general selected for quantitative analysis, while the second most intense SRM transition (SRM2) along with the ratio of areas of SRM1/SRM2 (within specified standard deviation) and the retention time of the target compound are used for confirmation.
Optimization of post-column reagent addition of ammonia in methanol
Due to the large range of pKa’s (2–4.8 for phenoxyacid herbicides, 6.9–9.7 for degradation products) most of these compounds are ionized under typical reversed-phase LC separation conditions and often observe little retention or poor chromatographic resolution. The best chromatographic separation of this wider range of acid herbicides and their degradation products was achieved using a C18 column (4.6 mm × 50 mm, 1.8 μm, Zorbax) with 2 mM ammonium acetate in the mobile phase. Other methods have not included simultaneous determination of either this range of acid herbicides or inclusion of these degradation products.12,13,16,17 Adjustments of pH (between 6.5 to 9.8) of the mobile phase with ammonia resulted in loss of chromatographic resolution. It was not possible to make further adjustments in mobile phase composition for better MS sensitivity, required particularly for degradation products, without loss in resolution in the separation. Consequently post-column addition of ammonia in methanol was used to improve sensitivity for most of the degradation products. Methanol was also the organic modifier for the mobile phase (65 to 90 v/v% during gradient) and provided better sensitivity than other organic solvents. The resulting solution after post-column reagent addition has a low percentage of water (28 to 9.1% during the gradient step) with flow rate of mobile phase 0.15 mL min−1 and post-column reagent flow of 0.05 mL min−1 (6.5% water in the ammonia in methanol solution). Table 1 shows that DBHBA elutes during the initial part of the gradient (water content 28%), CMP and DCP elute near the end of the gradient step (water content ~12.5%–9.1%), and TCP elutes during the final hold of the gradient (9.1% water content after post-column reagent addition).
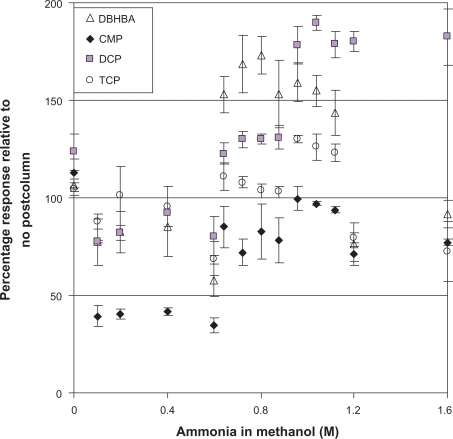
Figure 1 shows the influence of the concentration of the post-column reagent on sensitivity for the degradation products of the acid herbicides relative to when no post-column reagent addition is used. Three measurements of a 750 μg L−1 standard were taken at each concentration of ammonia in methanol and peak height was used to measure the response. The flow rate was also optimized to be 0.05 mL min−1, keeping total flow for the separation at 0.20 mL min−1. The addition of methanol alone (0 M ammonia in Fig. 1) can result in a slight improvement over no post-column system as observed for CMP and DCP. This effect may be due to either the increased total flow rate (0.15 mL/min to 0.20 mL/min) or a drop in the solvent water content. Since CMP and DCP elute in the latter portion of the LC gradient when methanol content is between 86–90 v/v%, the resulting drop in water content of effluent reaching the MS is small as the ammonia in methanol solution has 6.5% water. DBHBA which elutes during the initial part of the separation when the mobile phase has 65% methanol observes little change in response relative to no post-column and has the largest change in % water when the post-column reagent is added (35% to 28% water). The largest improvements in sensitivity with addition of ammonia in methanol were observed for DBHBA, DCP, and TCP with optimal ammonia concentration between 0.80–1.04 M (Fig. 1). In this range of ammonia concentration in methanol, CMP was similar or slightly lower in percentage response than with methanol alone. Subsequently further comparison was completed only at 0.80 and 1.04 M ammonia in methanol.
Figure 1.
Percentage response of degradation products relative to response when no post-column reagent system is used as a function of molarity of ammonia in methanol. Degradation Products: DBHBA measured at 295.0 > 250.3; DCP at 160.9 > 124.7; CMP at 140.9 > 105.2; and TCP at 194.6 > 158.9. Percentage response is response of post-column system/response no post-column system multiplied by 100. LC-ESI−-SRM response shown is average peak height of three replicates. Sample injection 5 μL degradation product at 750 μg L−1 with post-column flow of 50 μL min−1 and column flow of 150 μL min−1.
The optimized condition was determined by comparison of the method detection limit (MDL), defined herein as minimum standard concentration showing 25% deviation of peak area from the best-fit regression line of the calibration curves determined over 2–150 ng L−1. Table 3 shows that for both the quantitative and qualitative SRM transitions in general optimal conditions were obtained with 0.80 M ammonia in methanol. These conditions differ significantly from previous literature using an ion-pairing LC separation where ammonia was added post-column at 0.004 M with flow rate of 0.11 mL/min,16 however separation conditions as well as some sample preparation steps that could impact sensitivity were significantly different. This separation offers a typical reversed-phase separation using optimal MS column flow rates without the use of ion-pairing reagents, where high salt concentrations can lead to higher MS maintenance and it does not require acetonitrile in the mobile phase.
Table 3.
Correlation coefficient (R2) for Calibration Curves and Method Detection Limits (MDLs) of phenoxyacid herbicides and their degradation products at two different ammonia concentrations in methanol for the post-column reagent. Flow rate of post-column reagent held constant at 0.50 mL min−1, and mobile phase flow rate 0.15 mL min−1. Standards prepared at 1, 2, 5,10, 20, 30, 50, 70, 90, 110, 130, and 150 ng L−1.
| Analytes |
Post-column solution |
|||
|---|---|---|---|---|
|
0.80 M Ammonia in methanol |
1.04 M Ammonia in methanol |
|||
|
R2 SRM1/SRM2 |
MDL (ng L−1) SRM1/SRM2 |
R2 SRM1/SRM2 |
MDL (ng L−1) SRM1/SRM2 | |
| Phenoxyacid herbcides | ||||
| Dicamba | 0.978/0.904 | 15/30 | 0.976/0.816 | 70/70 |
| Bromoxynil | 0.985/0.985 | 5/2 | 0.988/0.994 | 10/10 |
| MCPA | 0.996/0.992 | 5/5 | 0.993/0.980 | 5/10 |
| 2,4-D | 0.994/0.993 | 10/5 | 0.995/0.985 | 10/15 |
| Mecoprop | 0.998/0.981 | 2/10 | 0.998/0.977 | 5/20 |
| Dichlorprop | 0.993/0.994 | 2/5 | 0.996/0.998 | 2/2 |
| 2,4-DB | 0.979/0.991 | 5/5 | 0.975/0.994 | 20/2 |
| MCPB | 0.995/0.991 | 2/10 | 0.996/0.976 | 5/30 |
| 2,4,5-TP | 0.995/0.993 | 10/10 | 0.998/0.997 | 5/10 |
| Degradation products | ||||
| DBHBA | 0.988/0.969 | 5/30 | 0.994/0.889 | 50/30 |
| CMP | 0.987/0.970 | 15/20 | 0.959/0.942 | 20/20 |
| DCP | 0.977/0.977 | 10/10 | 0.986/0.995 | 2/2 |
| TCP | 0.995/0.994 | 2/2 | 0.996/0.995 | 2/2 |
Note: Underlined are the best overall conditions for MDL.
Optimization of separation and MS-MS monitoring conditions
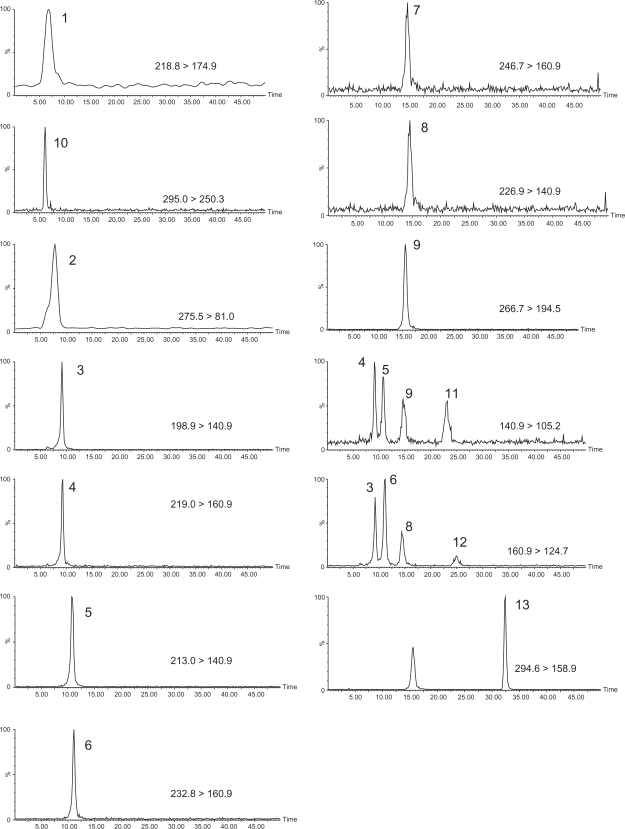
Figure 2 shows the SRM chromatograms obtained using the monitored SRM transitions for all target compounds. Co-eluting peaks include: 2,4-D and MCPA; mecoprop, dichlorprop, and 13C6-2,4,5-T; and 2,4-DB and MCPB. Chromatographic resolution problems are overcome by the added selectivity of tandem mass spectrometry with Table 1 showing that these co-eluting peaks have two unique SRM transitions. Figure 2 also shows for those compounds with the same SRM transition (generally SRM2 at 161 > 125 or 141 > 105) that chromatographic separation is achieved.
Figure 2.
Selected Reaction Monitoring (SRM) chromatograms of phenoxyacid herbicides and degradation products. Shown are quantitative SRMs with SRM transition labelled for each chromatogram. Note SRM at 140.9 > 105.2 and 160.9 > 124.7 are also the qualitative SRM transition for some analytes. Peak numbering for phenoxyacid herbicides are as follows: 1. dicamba; 2. bromoxynil; 3. MCPA; 4. 2,4-D; 5. mecoprop; 6. dichlorprop; 7. 2,4-DB; 8. MCPB; 9. 2,4,5-TP; and degradation products: 10. DBHBA; 11. CMP; 12. DCP; 13. TCP. See Table 1 for retention times. Standard 75 ng L−1.
Quantitative and confirmation analysis
As noted in the post-column reagent optimization linear calibration curves extended from MDL to 150 ng L−1. This is our normal working range but linearity has been observed to 500 ng L−1. MDL is defined by as the minimum concentration of a pesticide in a sample that can be quantified and is determined from the minimum concentration of standard with ≤25% deviation of peak area from the best-fit regression line of the calibration curve.19,20 Below these concentrations the standard deviation from the best-fit line quickly increases above 25%. As in tandem MS the noise level is very low, the instrument limit of detection (LOD) was determined from the Student’s t-value (98% confidence interval) multiplied by the standard deviation of the concentration of 10 replicate injections of a spiked standard near the estimated detection limit of most compounds (1 ng L−1 for 2,4-D, MCPA, mecoprop, dichlorprop, 2,4,5-TP, TCP; 5 ng L−1 for bromoxynil, 2,4-DB, and MCPB; 15 ng L−1 for DBHBA, CMP, DCP; and 20 ng L−1 for dicamba).19 Table 4 shows that the LODs are similar or slightly lower than the MDLs (Table 2, 0.80 M ammonia in methanol) at 1–7 ng L−1 in general (13 ng L−1 for dicamba) and are comparable or lower than those reported in the literature meeting the required criteria for pesticide analyses in Canada.
Table 4.
Limit of Detection (LOD), MDL and ratio of response of SRM1/SRM2 for phenoxyacid herbicides and their degradation products at optimal ammonia in Methanol Concentration. LOD and MDL highest reported level for both SRM1 and SRM2 (see MDL from Table 3); SRM1/SRM2 ± relative standard deviation (RSD) is a response of peak areas obtained on the day of analysis provided here for analysis of samples shown in Figure 1 and 2. Standard spike level is 1 ng L−1, except for bromoxynil and MCPB at 5 ng L−1, DBHBA, CMP, DCP at 15 ng L−1, and dicamba at 20 ng L−1. Ratios determined over linear dynamic range of MDL-100 ng L−1. Ammonia concentration of 0.80 M in methanol.
| LOD (ng L−1) | MDL (ng L−1) | Ratio of SRM1/SRM2 Areas ± %RSD | |
|---|---|---|---|
| Phenoxyacid herbicides | |||
| Dicamba | 13 | 30 | 3.76 ± 18.5% |
| Bromoxynil | 5 | 5 | 1.07 ± 6.7% |
| MCPA | 1 | 5 | 41.4 ± 6.2% |
| 2,4-D | 1 | 10 | 4.29 ± 5.3% |
| Mecoprop | 1 | 10 | 49.2 ± 8.7% |
| Dichlorprop | 1 | 5 | 1.69 ± 3.9% |
| 2,4-DB | 5 | 5 | 0.70 ± 8.2% |
| MCPB | 5 | 10 | 5.60 ± 10.1% |
| 2,4,5-TP | 1 | 10 | 7.59 ± 7.2% |
| Degradation products | |||
| DBHBA | 15 | 30 | 1.92 ± 9.1% |
| CMP | 15 | 20 | 3.00 ± 13.4% |
| DCP | 15 | 10 | 3.80 ± 12.9% |
| TCP | 1 | 2 | 1.90 ± 5.0% |
Table 4 also shows the ratio of areas of SRM1/SRM2 determined over the linear calibration range for both SRM transitions based on injection of calibration standards. The ratio varies for each component as well as the magnitude of the standard deviations that ranges from 3.9 to 18.5%. This tolerance defined by the standard deviation of the ratio can be significantly smaller than the 20% European Union (EU) guideline as we have also observed for other pesticides.19,20 The ratio should be determined daily from standards to account for shifts in instrument performance or slight changes in mobile phase composition.
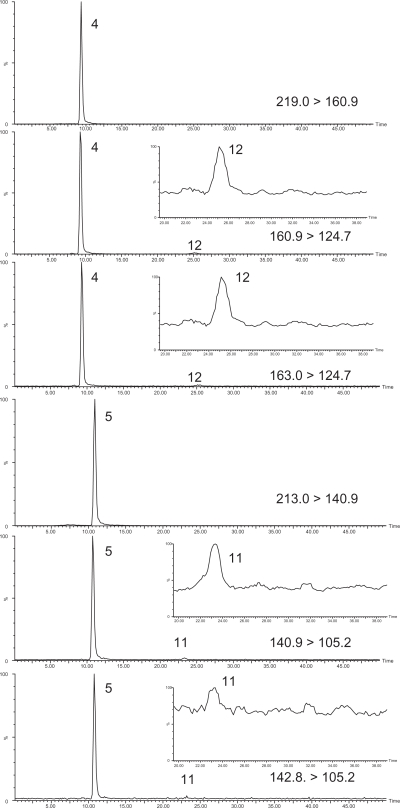
Figure 3 shows the SRM chromatogram for quantitative and qualitative SRM transitions (SRM1 and SRM2) for some of the degradation products (DCP and CMP) for a surface water sample (WP216). The ratios of SRM1/SRM2 obtained for DCP and CMP for sample WP216 were 3.97 and 3.03 and are within that obtained for standards (Table 3, DCP 3.80% ± 12.9%; CMP 3.00 ± 13.4%) with observed concentrations of 302 and 101 ng L−1. The main active ingredients in this sample were 2,4-D and mecoprop at much higher concentrations of 2755 and 2403 ng L−1, respectively with ratios of 4.52 and 49.0 within the tolerance of standards (Table 3). Table 5 shows that 2,4-D and mecoprop were detected in all 86 surface water samples and at the highest concentrations attributed to the prevalence of use of Killex formulations. A lower frequency of detection and lower average concentrations of MCPA and dichlorprop were observed. The distinct presence of dichlorprop indicates that a formulation more typical of agricultural applications may also be used within city limits. Only two degradation products, DCP and CMP, were detected in samples with levels ranging from 22 to 302 ng L−1 for DCP and 22 to 101 ng L−1 for CMP. As bromoxynil and 2,4,5-TP were not observed in any water samples, their degradation products, DBHBA and TCP, were also not detected. The lower levels of degradation products are attributed to the short residence time of the pesticides in the ponds between rain events and the fact that sampling took place largely on rain events when there were fresh releases from residential areas.
Figure 3.
Selected Monitoring Chromatograms (SRMs) for Sample WP216 containing 2,4-D and its degradation product DCP, and mecoprop and its degradation product CMP. Shown are both the quantitative and qualitative SRM transitions (SRM1 and SRM2) for analytes with expanded x-scale scale for the degradation products DCP and CMP. Quantitative and Qualitative SRM Transitions: 2,4-D are 219.0 > 174.9 and 174.9 > 144.9; DCP are 160.9 > 124.7 and 163.0 > 124.7; mecoprop are 213.0 > 140.9 and 140.9 > 105.2; and CMP are 140.9 > 105.2 and 142.8 > 105.2 (see Table 1). Analytes identified: 4. 2,4-D; 5. mecoprop; 11. CMP; and 12. DCP. Sample WP216 diluted at 1:2 with addition of IS for 5 μL injection.
Table 5.
Levels of phenoxyacid herbicides and their degradation products in surface water samples collected from two storm water ponds in the city of Regina. Average level determined for samples above detection limits. Shown only for pesticides detected. Total number of samples is 86.
| Compound | Average (ng L−1) | Maximum (ng L−1) | Minimum (ng L−1) | Number of Samples > MDL |
|---|---|---|---|---|
| MCPA | 82 | 379 | 11 | 61 |
| 2,4-D | 626 | 3267 | 36 | 86 |
| Mecoprop | 730 | 2403 | 15 | 86 |
| Dichlorprop | 261 | 1260 | 47 | 12 |
| CMP | 45 | 101 | 22 | 11 |
| DCP | 97 | 302 | 22 | 5 |
Conclusion
This paper has demonstrated a new LC-ESI− /MS/MS method with post-column addition of ammonia in methanol for simultaneous determination of phenoxyacid herbicides and their degradation products for the routine analysis of surface water samples. Other LC/MS methods do not offer this range of herbicides and do not simultaneously determine the degradation products or have the 3-point confirmation criteria. As these herbicides and degradation products have a wide range of pKa values the separation and MS detection needs have to be balanced particularly for this expanded list of compounds. The separation is achieved on a reversed-phase LC column with smaller particle size and column length (4.6 mm × 50 mm, 1.8 μm particle size) allowing for a flow rate (0.15 mL/min) suitable for direct flow without splitting into the mass spectrometer. No additional reagents either in the SPE procedures or in the mobile phase are required (such as surfactants or high salt concentrations) reducing maintenance requirements for the mass spectrometer. The use of post-column addition of ammonia in methanol improves the sensitivity of the degradation products (DBHBA, DCP, and TCP) allowing for their detection in storm water samples. The SPE procedure provides a 1000 fold concentration of pesticides using a polystyrene divinylbenzene sorbent with optimal pH of 4.9 and is suitable for the full range of analytes including degradation products. On-line SPE automated procedures have also been used for analysis of other pesticides where the Prospekt sample handling module and a column (10 mm × 2 mm i.d., PLRP-S copolymer) are utilized for on-line sample enrichment and do not require the time consuming drying step of extracts of off-line SPE procedures.22
The method involves filtration, acidification of filtered water, followed by SPE with Chrom P cartridge prior to LC/MS/MS determination. The LC/MS/MS method detection limits for most phenoxyacid herbicides were in the 5 to 10 ng L−1 range (except dicamba 30 ng L−1) and 2–30 ng L−1 for the degradation products. A more stringent 3-point confirmation approach is used. Method detection limits meet the required guidelines with minimum levels of different phenoxyacid herbicides or degradation products detected in samples ranging from 11–47 ng L−1.
Acknowledgments
This work was financially supported by the Natural Science and Engineering Council (NSERC) Discovery grant. The authors thank support from Canadian Foundation for Innovation (CFI) for the cleanroom laboratory and LC/MS/MS (Trace Analysis Facility, TAF). Authors thank staff from City of Regina and National Research Council-Centre for Sustainable Infrastructure Research (NRC-CSIR) for assistance in sample collection and K. Buehler and K. Starks (University of Regina) for assistance in sample preparation and sampling.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors report no conflicts of interest.
References
- 1.Health Canada Guidelines for Canadian drinking water quality summary table, prepared for Federal-Provincial-Territorial Committee on drinking water of the Federal-Provincial-Territorial Committee on Health and the Environment, May 2008.
- 2.Summary of Canadian Water Quality Guidelines for the Protection of Aquatic Life, Canadian Council of Ministers of the Environment, 2006.
- 3.Dąbrowska D, Kot-Wasik A, Namieśnik J. Stability Studies of Selected Phenoxyacid herbicides in water samples and determination of their transformation products. Bull Environ Contam Toxicol. 2006;77:245–51. doi: 10.1007/s00128-006-1056-1. [DOI] [PubMed] [Google Scholar]
- 4.Fukumori F, Hausinger RP. Purification and characterization of 2,4-dichlorophenoxyacetate/α-ketoglutarate dioxygenase. J Biol Chem. 1993;268:24311–7. [PubMed] [Google Scholar]
- 5.Waite DT, Cessna AJ, Grover R, Kerr LA, Snihura AD. Environmental concentrations of agricultural herbicides: 2,4-D and triallate. J Environ Qual. 2002;31:129–44. doi: 10.2134/jeq2002.1290. [DOI] [PubMed] [Google Scholar]
- 6.Fattahi N, Samadi S, Assadi Y, Hosseini MRM. Solid-phase extraction combined with dispersive liquid-liquid microextraction-ultra preconcentration of chlorophenols in aqueous samples. J Chromatogr A. 2007;1169:63–9. doi: 10.1016/j.chroma.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Puig D, Silgoner I, Grasserbauer M, Barceló D. Part-per-trillion level determination of priority methyl-, nitro-, and chlorophenols in river water samples by automated on-line liquid/solid extraction followed by Liquid chromatography/mass spectrometry using atmospheric pressure chemical ionization and ion spray interfaces. Anal Chem. 1997;69:2756–61. [Google Scholar]
- 8.Moret S, Sánchez JM, Salvadó V, Hidalgo M. The evaluation of different sorbents for the preconcentration of phenoxyacetic acid herbicides and their metabolites from soils. J Chromatogr A. 2005;1099:55–63. doi: 10.1016/j.chroma.2005.08.078. [DOI] [PubMed] [Google Scholar]
- 9.Lin CY, Huang SD. Application of liquid-liquid-liquid microextraction and ion-pair liquid chromatography coupled with photodiode array detection for determination of chlorophenols in water. J Chromatogr A. 2008;1193:79–84. doi: 10.1016/j.chroma.2008.03.082. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Zhao X, Shi Y, et al. Mixed hemimicelles solid-phase extraction based on cetyltrimethylammonium bromide-coated nano-magnets Fe3O4 for the determination of chlorophenols in environmental water samples coupled with liquid chromatography/spectrophotometry detection. J Chromatogr A. 2008;1180:24–31. doi: 10.1016/j.chroma.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Santos TCR, Rocha JC, Barceló D. Determination of rice herbicides, their transformation products and clofibric acid using on-line solid-phase extraction followed by liquid chromatography with diode array and atmospheric pressure chemical ionization mass spectrometric detection. J Chromatogr A. 2000;879:3–12. doi: 10.1016/s0021-9673(00)00100-x. [DOI] [PubMed] [Google Scholar]
- 12.Crescenzi C, Di Corcia A, Marchese S, Sameri R. Determination of acidic pesticides in water by a benchtop electrospray liquid chromatography mass spectrometer. Anal Chem. 1995;67:1968–75. [Google Scholar]
- 13.Køppen B, Spliid NH. Determination of acidic herbicides using liquid chromatography with pneumatically assisted electrospray ionization mass spectrometry and tandem mass spectrometry. J Chromatogr A. 1998;803:157–68. doi: 10.1016/s0021-9673(97)01219-3. [DOI] [PubMed] [Google Scholar]
- 14.Baglio D, Kotzia D, Larsen BR. Atmospheric pressure ionization multiple mass spectrometric analysis of pesticides. J Chromatogr A. 1999;854:207–20. doi: 10.1016/s0021-9673(99)00740-2. [DOI] [PubMed] [Google Scholar]
- 15.Niessen WMA. Group-specific fragmentation of pesticides and related compounds in liquid chromatography-tandem mass spectrometry. J Chromatogr A. doi:10.1016/j.chroma.2009.09.058. [DOI] [PubMed]
- 16.Marchese S, Perret D, Gentili A, et al. Determination of phenoxyacid herbicides and their phenolic metabolites in surface and drinking water. Rapid Commun Mass Spectrom. 2002;16:134–41. doi: 10.1002/rcm.557. [DOI] [PubMed] [Google Scholar]
- 17.Carabias-Martinez R, Rodríguez-Gonzalo R, Revilla-Ruiz P. Determination of weakly acidic endocrine-disrupting compounds by liquid chromatographymass spectrometry with post-column base addition. J Chromatogr A. 2004;1056:131–8. doi: 10.1016/j.chroma.2004.06.121. [DOI] [PubMed] [Google Scholar]
- 18.Steen RJCA, Hogenboom AC, Leonards PEG, et al. Ultra-trace-level determination of polar pesticides and their transformation products in surface and estuarine water samples using column liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A. 1999;857:157–99. doi: 10.1016/s0021-9673(99)00772-4. [DOI] [PubMed] [Google Scholar]
- 19.Raina R, Sun L. Trace level determination of selected organophosphorus pesticides and their degradation products in environmental air samples by liquid chromatography-positive ion electrospray tandem mass spectrometry. J Environ Sci and Health Part B. 2008;43:323–32. doi: 10.1080/03601230801941667. [DOI] [PubMed] [Google Scholar]
- 20.Raina R, Hall P. Comparison of gas chromatography-mass spectrometry and gas chromatography-tandem mass spectrometry with electron ionization and negative-ion chemical ionization for analyses of pesticides at trace levels in atmospheric samples. Anal Chem Insights. 2008;3:111–25. doi: 10.4137/aci.s1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guide to Crop Protection, Saskatchewan Ministry of Agriculture, Regina, Saskatchewan, 2009
- 22.Hogenboom AC, Niessen WMA, Brinkman UATh. On-line solid-phase extraction-short column liquid chromatography combined with various tandem mass spectrometric scanning strategies for the rapid study of transformation of pesticides in surface water. J Chromatogr A. 1999;841:33–44. doi: 10.1016/s0021-9673(99)00268-x. [DOI] [PubMed] [Google Scholar]