Abstract
Bladder cancer, in its advanced stage, has very few therapeutic strategies with proven efficacy. Platinum-combination chemotherapy can be considered a standard for first-line therapy, but after progression there is no standard therapy, and the prognosis is very poor. The development of targeted therapies in the last few years has significantly changed the prognosis of a wide variety of tumors. In bladder cancer, there is no targeted therapy currently approved for its use in advanced disease. There is evidence that Her-2 amplification and/or overexpression is seen in bladder cancer, and may influence prognosis. Anti-Her-2 drugs, such as trastuzumab or lapatinib, are under investigation in urothelial neoplasms, but there is no phase III trial that has evaluated their use in bladder cancer. We review the published evidence about Her-2 determination, its influence on bladder carcinoma prognosis, the clinical development of anti-Her-2-targeted therapies, and the possible future research directions involving this pathway in bladder cancer.
Keywords: Bladder cancer, Her-2, Trastuzumab, Lapatinib
Introduction
Bladder cancer accounted for more than 70,000 cases in the last year in the United States, becoming the 4th most frequent cancer in men (7%) and the 9th leading cause of death among male patients with cancer (3%, more than 10,000 deaths; Jemal et al. 2009). There are limited therapeutic strategies with proven efficacy in advanced disease, with 5-year survival rates about 4–5%, so better knowledge of molecular biology of these tumors may lead to the discovery of new potential therapeutic targets for this illness.
Human epidermal growth factors are involved in oncogenesis through its action on several pathways leading to proliferation, angiogenesis, cell survival, and metastatic potential. The use of anti-Her-2 therapies has shown impressive activity in breast cancer, being trastuzumab, an antibody against Her-2/neu, part of the standard therapy in Her-2-positive breast cancer, either as adjuvant treatment or in metastatic disease (Dahabreh et al. 2008; Slamon et al. 2001). Recently, in the 2009 American Society of Clinical Oncology (ASCO) meeting, trastuzumab has also proven activity in gastric carcinoma (Van Cutsem et al. 2009), and this finding may help to spread the use of anti-Her-2 agents in other tumors apart from breast cancer.
Her-2 expression in bladder carcinoma is very variable between the different studies, ranging from 9 to 81% (Sato et al. 1992; Mellon et al. 1996; Wester et al. 2002; Gandour-Edwards et al. 2002; Chow et al. 2001). Although there are some interesting preclinical data, there are no phase III trials in bladder cancer exploring the use of anti-Her-2 therapies, and the clinical experience with these agents is very limited. In this paper, we will summarize the role of Her-2 pathway in the mechanisms of oncogenesis and progression in bladder cancer, and also review the clinical experience with anti-Her-2 therapies in this tumor type.
Her-2 amplification and/or overexpression in bladder carcinoma
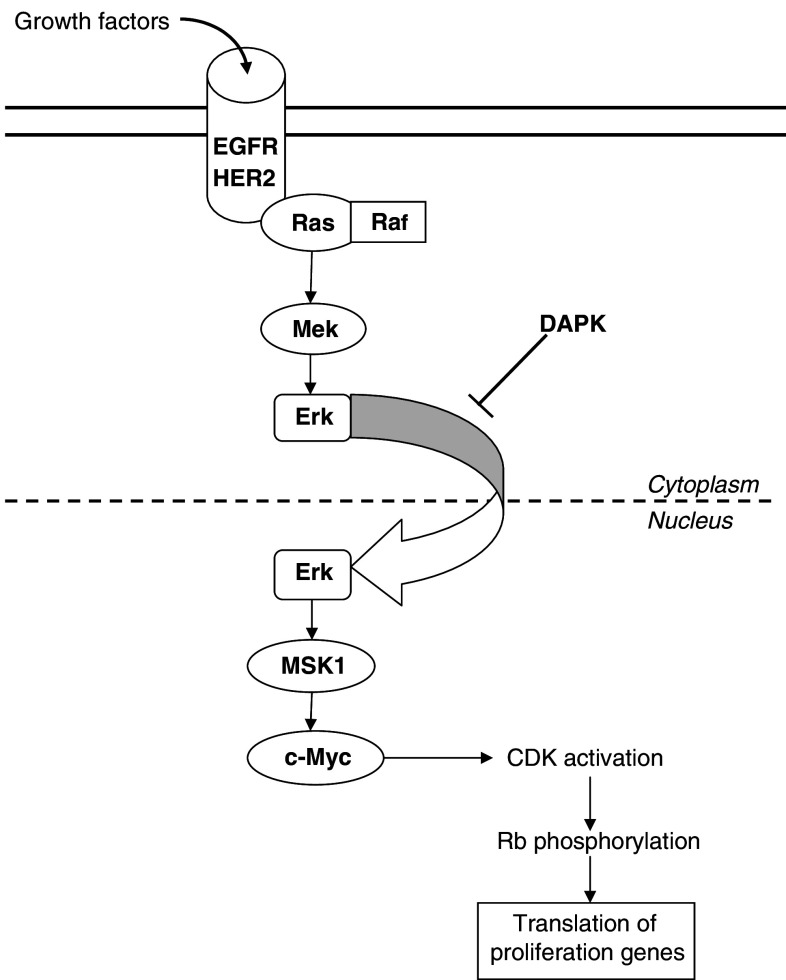
The Her-2/neu gene is located in chromosome 17q and encodes a transmembrane tyrosine kinase growth factor receptor, belonging to the family of epidermal growth factor receptors (EGFR; Bellmunt et al. 2003). Activation of this family of tyrosine kinase receptors leads to its dimerization and autophosphorylation. Through Ras activation, mitogenic signals are conducted by MAPK pathway, causing translocation of ERK into the nucleus and therefore activating the mitogen-activated and stress-activated protein kinase 1 (MSK1). This induces c-Myc and promotes cyclin-dependent kinases (CDKs), enhancing proliferation genes through Rb pathway (Mitra et al. 2006; Fig.1). This signal transduction can be inhibited in the cytoplasm by death-associated protein kinase (DAPK), an apoptosis promoter that blocks ERK translocation to the nucleus.
Fig. 1.
Summarized view of the EGFR-HER2 pathway
The true incidence of Her-2 amplification or overexpression is not well known and remains uncertain, ranging from 23 to 80% for overexpression and from 0 to 32% for amplification (Underwood et al. 1995; Jimenez et al. 2001; Latif et al. 2003; Coogan et al. 2004; Caner et al. 2008; Matsubara et al. 2008). This variability may be caused by heterogeneity in the laboratory tests, but also by the small number of most of these studies. ASCO has clearly defined a positive Her-2 status as immunohistochemistry (IHC) score of 3+, and a 2+ score must be disclosed by fluorescence in situ hybridization (FISH) to confirm Her-2 positivity (Wolff et al. 2007).
In 2009, a study published by Laé et al. (2009) assessed Her-2 gene amplification in a large cohort of 1005 patients with bladder carcinoma by standardized methods. This is the largest multicenter series investigating invasive urothelial bladder carcinomas by calibrated IHC and FISH methods. Her-2 overexpression was found in 9.2% of tumor samples, lower than reported in previous series. Variability in IHC assays may explain, at least in part, these discordant results, taking into account that FISH is a more objective technique than IHC. In this study, there was a complete concordance between IHC and FISH results in bladder carcinomas, as a quality control was performed to control internal and external factors that could influence these analyses.
Her-2 gene amplification was found in 5.1% of the tumor samples, also lower than reported before. Evidence from breast cancer suggest that benefit from anti-Her-2 therapy is restricted to cases with Her-2 amplification, so these cases could be candidates to this therapeutic approach with anti-Her-2 drugs, but this has not been clearly stated in bladder cancer. It must be noticed that about 35% of tumors with IHC 3+ showed important intratumoral heterogeneity that could make IHC difficult to interpret, and FISH is recommended in case of invasive urothelial carcinoma showing heterogeneous Her-2 IHC pattern.
Her-2 as a prognostic factor in bladder carcinoma
Acitvation of the EGFR pathway has been correlated with clinical outcome in bladder cancer, but its influence in prognosis has not been clearly stated yet. In one study conducted by Chakravarti et al. (2005), a positive EGFR inmunostaining was significantly associated with better overall survival (76 vs. 25% at 5 years, P = 0.044) and relapse-free survival (91 vs. 41% at 5 years, P = 0.042) in 73 patients with muscle-invasive bladder cancer treated with concurrent chemoradiation. This statistical significance was confirmed in the multivariate analysis. On the other hand, Her-2-positive IHC was associated with a reduced complete response rate after chemoradiation (50 vs. 81%, P = 0.03), but not with any survival parameters. This study suggests that Her-2 could be more related with resistance to chemoradiation approaches, and therefore may be more implied in local control of the disease, as well as EGFR expression seems to be a prognostic factor for distant relapse, but less related than Her-2 with response to chemoradiation.
Another study conducted by Lipponen et al. (1991) also showed a role of Her-2 in the prognosis of bladder carcinoma. In a group of 91 patients, 12% showed positive staining for Her-2, and 4% could be classified as 2+ or 3+ tumors. Moderate and heavy expression of Her-2 had a statistically significant correlation with higher grade, aneuploidy, and shorter overall survival over 14 years of follow-up. Due to the small size of the study, these findings must be taken with caution.
Finally, a study by Lonn et al. (1995) also proved a relation between Her-2 amplification in 23% of the patients (from a total of 178 cases) and grade, stage, and poorer survival. Taken together, these findings show a possible role of Her-2 as a prognostic factor in bladder carcinoma.
Nevertheless, another study published by Vollmer et al. (1998) showed that the role of an excess of Her-2 and EGFR was of minor importance, particularly in high-grade tumors; in these cases, presence of Her-2 and EGFR was related with a lesser probability of tumor invasion into the stroma of the bladder. Similar results were shown in a study by Mellon et al. (1996), where Her-2 had no apparent correlation with grade, tumor stage, EGFR expression or survival. Another study published by Rajjayabun et al. (2005) showed a significantly higher proportion of Her-2 amplification in T1 than in T2 or worse tumors, suggesting a role in the development of invasiveness in very initial tumors but without clear correlation with prognostic in invasive neoplasms. Finally, a more recent study published in 2008 by Liedberg et al. (2008) with 133 patients, analyzing tissue with immunohistochemistry and microarray technology, showed no clear correlation of Her-2 and prognosis, showing decreased expression of Her-2 in higher-grade tumors.
Therefore, although it is a very attractive therapeutic target, Her-2 doesn’t have a clearly defined role in oncogenesis and prognosis of bladder carcinoma. Although some studies correlate its overexpression and/or amplification with worse prognosis, some others find it as a possible initial step in the development of invasiveness, but not as a main prognostic factor.
Clinical development of anti-her-2 therapies
Maybe because of the difficult interpretation of the IHC results of Her-2 staining in bladder cancer, and also because of its uncertainty as a strong prognostic factor in this disease, there are very few clinical data regarding the use of anti-Her-2-targeted therapies.
One study published in 2005 (Peyromaure et al. 2005) proved the activity of trastuzumab, an anti-Her-2 monoclonal antibody, in six patients with metastatic urothelial tract carcinoma. All of these patients had a positive immunohistochemistry for Her-2. Four cases were treated with trastuzumab, paclitaxel, and carboplatin; one case with trastuzumab and paclitaxel; and the other case with trastuzumab monotherapy. The six patients achieved a partial response, ranging from 30 to 80% reduction in the size of the metastatic lesions. Interval between trastuzumab initiation and death varies from 8 to 22 months. This study showed a potential role for anti-Her-2 strategies in metastatic bladder carcinoma with Her-2 overexpression.
In 2007, a phase II trial conducted by Hussain et al. (2007) tested the combination of carboplatin, paclitaxel, gemcitabine, and trastuzumab in advanced urothelial carcinoma. A total number of 44 patients with Her-2-positive tumors were treated with this combination. Five of these patients (11%) achieved a complete response, 26 (59%) a partial response, 5 (11%) had stable disease, and 5 (11%) had no response assessment, with an overall response rate of 70%. Median time to progression was 9.3 months, and median survival was 14.1 months.
In this study, from a global of 109 patients, 57 were considered Her-2 positive at least by one method (either IHC or FISH), that is, a 52.3% of all the cases. From these 57 cases, 44 patients received the experimental therapy and were analyzed in this trial. As the authors reflect in the discussion, this high prevalence of Her-2-positive tumors should force the design of specific phase III trials for this population, but nowadays there is no such trial in Her-2-positive advanced bladder carcinoma.
Lapatinib, a tyrosine kinase inhibitor of EGFR and Her-2, has also been tested in this tumor, but with disappointing results. Preclinical data with this drug showed promising activity in transitional carcinoma cell lines (McHugh et al. 2007, 2009), enhancing the activity of concomitant chemotherapy in a dose-dependent fashion. A phase II trial by Willing et al. has been recently published (Wülfing et al. 2009). Lapatinib monotherapy was administered to 59 patients with transitional carcinoma who have progressed on a prior platinum-containing chemotherapy schedule. Of the 34 patients evaluable for response, only 1 patient achieved an objective response, and 18 had stable disease, with a median time to progression of 8.6 weeks and a median overall survival of 17.9 weeks. Although the global study was considered to be negative, further analysis showed a correlation between clinical benefit and EGFR and/or Her-2 overexpression, which warrants further investigation.
Therefore, up to now there is no phase III trial that has evaluated the role of any anti-Her-2 drug for bladder cancer. Some data seem promising, but they need to be confirmed to incorporate this strategy to the standard treatment of transitional cell tumors.
Discussion
Pathways involving Her-2 signaling have been related with prognostic and predictive features in a wide variety of neoplasms, but mainly in breast cancer, where trastuzumab has become a mainstay in the treatment of Her-2-positive tumors, whether as adjuvant or neoadjuvant therapy or in the metastatic setting. Recent data in Her-2-positive gastric carcinoma (Van Cutsem et al. 2009) suggest that anti-Her-2 therapies may have a role in the treatment of tumors apart from breast cancer.
Bladder carcinoma is a disease with very scarce therapeutic options in the advanced setting; in the same way, adjuvant therapies in localized high-risk patients have not clearly proven their efficacy, and neoadjuvant chemotherapy, although it has proven a small benefit, is not widely implemented.
The true incidence of Her-2 overexpression and/or amplification is not well known. Although data in previous studies range from 9 to 81%, a recent study published by Laé et al. (2009), including 1,005 tissue samples, and being currently the largest study analyzing this feature, has reported a rate of Her-2 gene amplification of only 5.1%. These variations can be explained, at least in part, by variability in IHC assays, regarding cut-off values, antibodies, kits, or protocols. This is why FISH seems to be a better approach, as it provides objective results. Therefore, standardized methods are needed to avoid this heterogeneity in Her-2 testing in bladder cancer, and FISH should be the preferred technique.
As commented before, although data are conflicting, Her-2 seems to have a role as a prognostic factor in this neoplasm. It has been involved in reduced response to chemoradiation (Chakravarti et al. 2005), higher grade and stage, and shorter survival (Lipponen et al. 1991; Lonn et al. 1995). The ability of Her-2 to activate pro-survival pathways with anti-apoptotic effects is well known, and it seems logical that Her-2 has a role in invasiveness and progression. Nevertheless, some other studies have not confirmed this data, hypothesizing that Her-2 overexpression is of minor importance in the prognosis of urothelial carcinomas (Vollmer et al. 1998; Mellon et al. 1996; Rajjayabun et al. 2005; Liedberg et al. 2008). Added to heterogeneity in Her-2 testing, the inclusion of superficial carcinomas as well as muscle-invasive disease may have introduced a conflicting factor in these analyses, making it difficult to obtain solid conclusions about the prognostic role of Her-2 in muscle-invasive and advanced disease. Also, there is considerable intratumoral heterogeneity in Her-2 overexpression, up to 35% in bladder carcinomas with IHC 3+ (Laé et al. 2009), which may also alter the results, this is another reason to prefer FISH for Her-2 testing, as it can be useful in IHC heterogeneous pattern samples.
Finally, the clinical development of anti-Her-2 therapies, mainly trastuzumab, has shown us mostly the same problems. Although some studies with chemotherapy combined with trastuzumab achieve high response rates in advanced disease (Hussain et al. 2007), no current phase III trial with this schedule to detect response rates and survival differences is ongoing. Derived from what we know from basic and translational research, maybe the patient selection has not been appropriated in the few trials with anti-Her-2 agents in urothelial carcinoma. Standardized Her-2 testing by FISH should be warranted in future trials, and only cases with Her-2 amplification should be included, as these seem to be the most benefited. Surely, this would make hard to recruit patients, but small trials with well-selected population may offer us more information than bigger trials with unselected population, that anyway are difficult to carry in bladder cancer, as previous efforts have shown us.
Future research directions
Currently, there is an ongoing phase I/II trial with paclitaxel and radiation with or without trastuzumab in patients who have undergone prior transurethral bladder resection for muscle-invasive transitional cell carcinoma of the bladder, and are not eligible for radical cistectomy. This is a non-randomized trial, and patients receive trastuzumab along with paclitaxel and radiotherapy depending on the Her-2 status.
A phase II/III trial is recruiting patients to evaluate the role of lapatinib in EGFR or Her-2 overexpressing locally advanced or metastatic breast cancer, as maintenance therapy after first-line chemotherapy, compared with placebo. Patients must have achieved objective response or at least stable disease after 4–8 cycles of chemotherapy. This is a randomized, multicenter trial, and first data are expected for 2011.
Another study with lapatinib that is currently recruiting patients is a phase I trial, conducted by the European organisation for research and treatment of cancer (EORTC). This trial evaluates the combination of lapatinib, cisplatin, and gemcitabine as first-line therapy of metastatic urothelial tract carcinoma. Patients must have EGFR and/or Her-2 overexpression. For other anti-Her-2 drugs, such as pertuzumab or neratinib, there is no ongoing trial focused on bladder cancer nowadays, although they are being tested in phase I trials with advanced solid tumors.
Apart from the clinical development of new targeted therapies, we need further basic and translational research to better define patients that may benefit from this strategy. It is not clear whether amplification of Her-2 is needed to derive some benefit of these therapies, or Her-2 overexpression patients without amplification may also be considered suitable for anti-Her-2 therapy. Also, the prognostic role of Her-2 amplification and/or overexpression has not clearly been proved, although everything points to a role in oncogenesis and invasiveness that may provide a worse prognosis for this group of patients. Deeper knowledge of the molecular biology of Her-2 pathway and its implications in bladder cancer will help us to achieve a better patient selection and to identify new potential therapeutic targets.
Conclusions
Bladder cancer is an aggressive neoplasm, with very dismal prognosis and few therapeutic possibilities in advanced stages. The development of targeted therapies is crucial to better select patients who can derive the most benefit from these drugs, and to spare toxicities from unspecific approaches. Anti-Her-2 therapies have proved its efficacy mainly in breast cancer, but research is currently broadening the spectrum of tumors that can obtain benefit from this strategy. In bladder cancer, although Her-2 amplification and/or overexpression seem to be infrequent, there can be a group of patients candidate to this treatment. Identifying these particular cases and developing the ideal way to deliver these therapies must be warranted in future research, to optimize the prognosis of this tumor.
I certify that there is no actual or potential conflict of interest in relation to this article.
Conflict of interest statement
None.
References
- Bellmunt J, Hussain M, Dinney CP (2003) Novel approaches with targeted therapies in bladder cancer. Therapy of bladder cancer by blockade of the epidermal growth factor receptor family. Crit Rev Oncol Hematol 46:S85–S104 [DOI] [PubMed] [Google Scholar]
- Caner V, Turk NS, Duzcan F et al (2008) No strong association between Her-2/neu protein overexpression and gene amplification in high-grade invasive urothelial carcinomas. Pathol Oncol Res 14:261–266 [DOI] [PubMed] [Google Scholar]
- Chakravarti A, Winter K, Wu CL et al (2005) Expression of the epidermal growth factor receptor and Her-2 are predictors of favorable outcome and reduced complete response rates, respectively, in patients with muscle-invading bladder cancers treated by concurrent radiation and cisplatin-based chemotherapy: a report from the radiation therapy oncology group. Int J Radiat Oncol Biol Phys 62(2):309–317 [DOI] [PubMed] [Google Scholar]
- Chow NH, Chan SH, Tzai TS et al (2001) Expression profiles of ErbB family receptors and prognosis in primary transitional cell carcinoma of the urinary bladder. Clin Cancer Res 7:1957–1962 [PubMed] [Google Scholar]
- Coogan CL, Estrada CR, Kapur S, Bloom KJ (2004) Her-2/neu protein overexpression and gene amplification in human transitional cell carcinoma of the bladder. Urology 63:786–790 [DOI] [PubMed] [Google Scholar]
- Dahabreh IJ, Linardou H, Siannis F et al (2008) Trastuzumab in the adjuvant treatment of early-stage breast cancer: a systematic review and meta-analysis of randomized controlled trials. Oncologist 13:620–630 [DOI] [PubMed] [Google Scholar]
- Gandour-Edwards R, Lara PN Jr, Folkins AK et al (2002) Does HER2/neu expression provide prognostic information in patients with advanced urothelial carcinoma? Cancer 95:1009–1015 [DOI] [PubMed] [Google Scholar]
- Hussain MHA, MacVicar GR, Petrylak DP et al (2007) Trastuzumab, paclitaxel, carboplatin and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase II National Cancer Institute trial. J Clin Oncol 25:2218–2224 [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E et al (2009) Cancer statistics, 2009. CA Cancer J Clin 59(4):225–249 [DOI] [PubMed] [Google Scholar]
- Jimenez RE, Hussain M, Blanco FJ et al (2001) Her-2/neu overexpression in muscle-invasive urothelial carcinoma of the bladder: prognostic significance and comparative analysis in primary and metastatic tumors. Clin Cancer Res 7:2440–2447 [PubMed] [Google Scholar]
- Laé M, Couturier J, Oudard S et al. (2009) Assessing Her-2 gene amplification as a potential target for therapy in invasive urothelial bladder cancer with a standardized methodology: results in 1005 patients. Ann Oncol. doi:10.1093/annonc/mdp488 [DOI] [PMC free article] [PubMed]
- Latif Z, Walters AD, Dunn I et al (2003) Her2/neu overexpression in the development of muscle-invasive transitional cell carcinoma of the bladder. Br J Cancer 83:1305–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedberg F, Anderson H, Chebil G et al (2008) Tissue microarray based analysis of prognostic markers in invasive bladder cancer: much effort to no avail? Urol Oncol Semin Ori 26:17–24 [DOI] [PubMed] [Google Scholar]
- Lipponen P, Eskelinen M, Syrjanen S et al (1991) Use of inmunohistochemically demonstrated c-erb B-2 oncoprotein expression as a prognostic factor in transitional cell carcinoma of the urinary bladder. Eur Urol 20:238–242 [DOI] [PubMed] [Google Scholar]
- Lonn U, Lonn S, Friberg S et al (1995) Prognostic value of the amplification of c-erb-B2 in bladder carcinoma. Clin Cancer Res 1:1189–1194 [PubMed] [Google Scholar]
- Matsubara H, Yamada Y, Naruse K et al (2008) Potential for Her-2/neu molecular targeted therapy for invasive bladder carcinoma: comparative study of immunohistochemistry and fluorescence in situ hybridization. Oncol Rep 19:57–63 [PubMed] [Google Scholar]
- McHugh LA, Kriajevska M, Mellon JK, Griffiths TR (2007) Combined treatment of bladder cancer cell lines with lapatinib and varying chemotherapy regimens–evidence of scheduled-dependent synergy. Urology 69(2):390–394 [DOI] [PubMed] [Google Scholar]
- McHugh LA, Sayan AE, Mejlvang J et al (2009) Lapatinib, a dual inhibitor of ErbB-1/-2 receptors, enhances effects of combination chemotherapy in bladder cancer cells. Int J Oncol 34(4):1155–1163 [DOI] [PubMed] [Google Scholar]
- Mellon JK, Lunec J, Wright C et al (1996a) C-erbB-2 in bladder cancer: molecular biology, correlation with epidermal growth factor receptors and prognostic value. J Urol 155:321–326 [DOI] [PubMed] [Google Scholar]
- Mellon JK, Lunec J, Wright C et al (1996b) C-Erb-B2 in bladder cancer: molecular biology, correlation with epidermal growth factor receptors and prognostic value. J Urol 155(1):321–326 [DOI] [PubMed] [Google Scholar]
- Mitra AP, Datar RH, Cote RJ (2006) Molecular pathways in invasive bladder cancer: new insights into mechanisms, progression, and target identification. J Clin Oncol 24:5552–5564 [DOI] [PubMed] [Google Scholar]
- Peyromaure M, Scotte F, Amsellen-Ouzana D, Vieillefond A, Oudard S, Beuzeboc P (2005) Trastuzumab in metastatic transitional cell carcinoma of the urinary tract: report on six patients. Eur Urol 48:771–778 [DOI] [PubMed] [Google Scholar]
- Rajjayabun PH, Keegan PE, Lunec J, Mellon JK (2005) ErbB receptor expression patterns in human bladder cancer. Urology 66(1):196–200 [DOI] [PubMed] [Google Scholar]
- Sato K, Moriyama M, Mori S et al (1992) An immunohistologic evaluation of C-erb-B-2 gene product in patients with urinary bladder carcinoma. Cancer 70:2493–2498 [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jones B, Shak S et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that over-expresses HER2. N Engl J Med 344:783–792 [DOI] [PubMed] [Google Scholar]
- Underwood M, Bartlett J, Reeves J et al (1995) C-erbB-2 gene amplification: a molecular marker in recurrent bladder tumors? Cancer Res 55:2422–2430 [PubMed] [Google Scholar]
- Van Cutsem E, Kang Y, Chung H et al. (2009) Efficacy results from the ToGA trial: a phase III study of trastuzumab added to standard chemotherapy (CT) in first-line human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer (GC). J Clin Oncol 27:18 s (suppl; abstr LBA4509) [Google Scholar]
- Vollmer RT, Humphrey PA, Swanson PE et al (1998) Invasion of the bladder by transitional cell carcinoma: its relation to histologic grade and expression of p53, MIB-1, c-Erb-B2, epidermal growth factor receptor and bcl-2. Cancer 82:715–723 [DOI] [PubMed] [Google Scholar]
- Wester K, Sjostrom A, de la Torre M et al (2002) HER-2: a posible target for therapy of metastatic urinary bladder carcinoma. Acta Oncol 41:282–288 [DOI] [PubMed] [Google Scholar]
- Wolff AC, Hammond ME, Schwartz JN et al (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145 [DOI] [PubMed] [Google Scholar]
- Wülfing C, Machiels JH, Richel DJ et al (2009) A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer 115(13):2881–2890 [DOI] [PubMed] [Google Scholar]