Abstract
Purpose
Tumor angiogenesis is an important factor for the continuous growth of human malignancies and can be used to predict the prognosis for patients. In the current study, we examined the expression of EGF-like domain 7 (EGFL7), an endothelial cell-derived secreted factor, in malignant gliomas and explored its clinical significance.
Methods
We determined the steady-state mRNA levels of EGFL7 from 36 fresh glioma samples by semi-quantitative RT–PCR and the protein levels from 45 paraffin-embedded glioma samples by immunohistochemistry, respectively. Normal brain tissues from 10 patients with brain trauma were used as control. We also analyzed the correlations between the expression levels of EGFL7 and various clinical parameters, including patient gender, age, tumor grade, tumor proliferation marker Ki-67, and microvessel density (MVD).
Results
We found that EGFL7 was not detectable in normal brain tissues, but was up-regulated in both tumor cells and vascular endothelial cells within malignant glioma. The expression level of EGFL7 in malignant glioma significantly correlated with the tumor grade, Ki-67 expression and MVD (P < 0.01).
Conclusions
Our data suggest that EGFL7 expression is a novel predictive factor for the clinical progression of malignant glioma, and may constitute a therapeutic target for anti-angiogenesis therapy in patients with the disease.
Keywords: Epidermal growth factor-like domain 7, Glioma, Angiogenesis, Microvessel density, Ki-67
Introduction
Malignant gliomas are the most common primary brain tumors in both children and adults, accounting for more than 70% of all brain tumors (Ohgaki 2009). Despite recent therapeutic advances, the survival of glioma patients is still poor, with a lower than 3% 5-year survival rate for patients with glioblastoma, the most common and most malignant subtype of glioma (Ohgaki et al. 2004). Gliomas share many common characteristics with other human solid tumors: limitless replicative potential, self-sufficiency in growth signals, insensitivity to anti-growth signals, evasion of apoptosis, sustained angiogenesis, and tumor invasion and metastasis, all of which are currently being examined for molecular targeted therapies (Hanahan and Weinberg 2000; Sathornsumetee et al. 2007). As an important regulator of continuous growth and subsequent metastasis of solid tumors, tumor angiogenesis was found to be an independent predictive factor for the growth and prognosis of gliomas (Bartels et al. 2006; Leon et al. 1996). Multiple angiogenic factors, such as basic fibroblast growth factor (bFGF), hepatocyte growth factor/scatter factor (HGF/SF), and vascular endothelial growth factor A (VEGF-A) have been suggested to modify the vasculature of malignant glioma. Pharmaceutical agents targeting these factors are being intensively tested in pre-clinical and clinical studies, with some offering promising results (Norden et al. 2008).
Recently, EGF-like domain 7 (EGFL7), a novel endothelial cell-derived secreted factor, was identified as an essential regulator of vascular tube formation (Parker et al. 2004). This novel factor was shown to have anti-apoptotic effects in endothelial cells challenged by hypoxia (Parker et al. 2004; Xu et al. 2008). However, few studies have looked into the correlation between EGFL7 expression and human diseases. In the present study, we examined the expression of EGFL7 and analyzed its correlation with various clinical parameters of malignant glioma.
Materials and methods
Human brain tissues
This study was approved by the Ethics Committee of Xiangya Hospital, Central South University, and all human samples were acquired with the patient’s consent. A total of 36 fresh tumor samples were surgically resected from treatment-naïve glioma patients (16 males and 20 females, average age 38.5 years, ranging from 2.5 to 70 years) admitted to our hospital between November 2006 and October 2007. Another 10 fresh normal brain tissue samples were isolated from patients undergoing internal decompression surgery following brain trauma. None of these patients had any evidence of cancer or obvious mydriasis. Four-μm thick tissue sections from both glioma and normal brain tissues were fixed in 10% formalin and sent for histological examinations. The remaining tissues were quickly frozen in liquid nitrogen until further use. By histological examination, all 36 samples were confirmed to be glioma. Based on the WHO Classification for Brain Tumors (2007), 21 of the tissue samples were classified as being Grade I-II, including 18 samples of astrocytoma, one of medulloblastoma, two of mixed glioma (oligoastrocytoma and ependymal astrocytoma) and one of ependymoma; 15 of the samples were classified as being Grade III–IV, including nine samples of anaplastic astrocytoma, two of anaplastic oligoastrocytoma and four of glioblastoma.
Another set of 45 glioma samples were obtained from the Human Brain Glioma Bank, Department of Pathology (Xiangya Hospital, Hunan, China) as paraffin-embedded sections. This set of samples was from 25 males and 20 females admitted to our hospital between July 2002 and April 2007. The age range for these patients was 2.5 years to 70 years (average 40.6 years old). Pathologically, these samples included 10 Grade I, 14 Grade II, 13 Grade III and 8 Grade IV gliomas.
Reverse transcription followed by semi-quantitative PCR
For the fresh brain tissue samples, total RNA was extracted using the Trizol reagent (Invitrogen, USA) following the manufacturer’s instructions. cDNA was then synthesized using oligo dT as the primer with AMV reverse transcriptase (Yoyobo, Japan). To determine the steady-state mRNA level of EGFL7 in each sample, PCR was performed with the following primers: EGFL7 forward primer 5′-TCT GGT GTT GGC AGT GGG C-3′, reverse primer 5′-TCG CAG GTG GTG AGG AAG G-3′, producing an amplicon of 135 bp. β-actin was used as a control, and its primer sequences were: forward primer 5′-CTG TCT GGC GGC ACC ACC AT-3′, reverse primer 5′-GCA ACT AAG TCA TAC TCC GC-3′, and produced a 254-bp amplicon. After 30 cycles of PCR, which was shown to fall in the linear range, we loaded the PCR products on 1.5% agarose gels and visualized the products by ethidium bromide staining. The optical density (OD) of PCR products was quantified using Electrophoresis Image Analyzer (Shanghai Tanon Science & Technology Co., Shanghai, China) and the relative EGFL7 mRNA level was presented as (ODEGFL7/ODβ-actin) ×100. A sample was defined as negative for EGFL7 mRNA if only the band for β-actin, but not for EGFL7 PCR, was visible. In contrast, a sample was considered positive if both bands were detectable.
Immunohistochemistry and immunocytochemistry
For immunohistochemistry, paraffin-embedded sections (4 μm) were de-paraffinized in xylene and rehydrated in a decreasing ethanol series diluted in distilled water. Antigen retrieval was performed in citric buffer (pH 6.0) at high temperature (92–98°C) for 15 to 20 min. Endogenous peroxidase activity was inactivated by 0.3% hydrogen peroxide in 0.01 M PBS for 30 min. Sections were blocked with 5% normal goat serum in 0.01 M PBS for 15 min at 37°C, followed by incubation with primary antibody diluted in the blocking solution overnight at 4°C in a humidified chamber. The primary antibodies (Santa Cruz, USA) and dilutions were as follows: EGFL7 at 1:1,000, Factor VIII at 1:500, and Ki-67 at 1:500. After incubation with the primary antibodies, the sections were washed and incubated with a biotinylated secondary antibody, followed by incubation with the ABC elite reagent, and allowed to develop a color reaction using the DAB substrate kit according to the manufacturer’s recommendations (Vector Laboratories, USA). After the color reaction, sections were counterstained with hemotoxylin (Vector Laboratories) and dehydrated through an ethanol series into xylene and mounted using Permount mounting media (Fisher Scientific). For immunocytochemistry analysis of EGFL7 expression in a human glioma cell line, U251 cells were fixed in 4% paraformaldehyde and stained with either the anti-EGFL7 antibody or an isotype control IgG (1:1,000, Santa Cruz, USA). The signal was developed with DAB substrate.
Quantification of immunohistochemical signals
To quantify the EGFL7 immunohistochemical signals, we followed the immunoreactive scoring (IRS) method as described previously (Eroglu and Sari 2007; Ko et al. 2003; Remmele and Stegner 1987). Briefly, five fields were randomly selected under high magnification (200×), and 200 tumor cells were counted from each field. IRS was calculated as staining intensity (SI) × percentage of positive cells (PP). The staining intensity was scored as follows: 0 for no staining, 1 for weak yellow staining, 2 for yellow staining and 3 for brown staining. The positive cells were defined as those with a clear border and yellow or brown staining distinct from the background. PP was scored as 0 for 0%, 1 for 1–10%, 2 for 11–50%, 3 for 51–80% and 4 for 80–100% positive cells. From the product of SI and PP, negative staining (−) was defined as an IRS value of 0, weak positive staining (+) as samples with an IRS value of 1–4, positive staining (++) as samples with a value of 5–8, and strong positive staining (+++) as samples with a value of 9–12.
To quantify the Ki-67 staining, we applied the Ki-67 labeling index (Ki-67 LI) (Takeuchi et al. 2003), where the values for cells were defined in the same way as EGFL7 staining. Five fields were randomly selected under high magnification (200×) and 200 cells were counted from each field. Ki-67 LI was then calculated as (number of positive tumor cells as defined by morphological method/total number of tumor cells counted) ×100.
Determination of microvessel density (MVD)
To determine the microvessel density, we followed the method proposed by Weidner et al. (1992). Briefly, we took the sections stained with vascular endothelial cell marker Factor VIII and scanned them under a light microscope at low magnification (100×) for fields with tumor infiltration and clearly stained, well contrasted (and most abundant) microvessels—neovascular hotspots. We then selected three neovascular hotspots from each section and counted the number of microvessels from three random high-magnification fields (200×) within each hotspot. Single or clustered endothelial cells with brown staining were counted as one microvessel, but the positive endothelial cells with a thick surrounding muscular layer or a lumen size greater than eight red blood cell diameters were not counted.
Statistical analysis
All data were analyzed using SPSS 13.0 software and presented as mean ± SD. Student’s t test was used to compare the mean values between two groups with equal variances and the Mann–Whitney U test was used for the data with unequal variances. One-way ANOVA was applied to test the differences among multiple groups. For groups with equal variances, pairwise comparison was performed with the Student–Newman–Keuls (SNK)-q test; for those with non-equal variances, the Kruskal–Wallis test was used. For correlation analysis, Pearson’s correlation coefficient was calculated for parametric variables, while Spearman’s correlation coefficient was used for non-parametric variables. The association between two categorical variables was determined using the χ2 test and the Fisher’s exact text when the expected value in one quadrant was less than 5. A P-value of less than 0.05 was considered statistically significant.
Results
The EGFL7 expression level significantly correlates with tumor grade
To examine the expression of EGFL7 in malignant gliomas, we collected two sets of human glioma samples, which included 36 fresh samples for mRNA analysis and 45 formalin-fixed and paraffin-embedded samples for protein detection. As a control, we acquired 10 normal brain tissue samples from patients with brain trauma but no brain tumors. We detected EGFL7 mRNA by RT–PCR analysis in some of the glioma samples, but not in any normal brain tissue samples. The average EGFL7 mRNA levels were significantly higher in Grade III–IV tumors than in Grade I–II tumors (Fig. 1a). The same trend was observed for EGFL7 protein levels when we used the IRS method to quantify the immunohistochemical signals (Fig. 1b).
Fig. 1.
The EGFL7 expression level significantly correlated with tumor grade. a The relative mRNA levels of EGFL7 (as a ratio to the β-actin level) in 36 glioma samples and 10 samples of normal brain tissue (control) were determined by semi-quantitative RT–PCR and compared between samples with different histological grades as defined by WHO standards. b The protein levels of EGFL7 from 45 brain glioma and 10 normal brain tissues (control) were examined by immunohistochemistry, quantified by the IRS method, and compared between samples with different histological grades. *P < 0.01 as compared to the control group; # P < 0.01 as compared to samples of Grade I–II
EGFL7 expression is significantly correlated with tumor grade, but not with patient gender or age
Given the observation that EGFL7 expression was not detectable in normal brain tissue, we next investigated whether there was any correlation between positive EGFL7 expression and other clinical parameters. As shown in Table 1, the positive rate for both EGFL7 mRNA and protein significantly correlated with tumor grade (P < 0.05), but not with patient gender or age (P > 0.05).
Table 1.
The rate of EGFL7 positivity significantly correlated with tumor grade, but not with patient gender or age
| Group | N | EGFL7 mRNA | N | EGFL7 protein | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| − | + | Positive rate (%) | − | + | ++ | +++ | Positive rate (%) | |||
| Male | 16 | 5 | 11 | 68.8 | 25 | 8 | 6 | 6 | 5 | 68.0 |
| Female | 20 | 7 | 13 | 65.0 | 20 | 6 | 4 | 7 | 3 | 70 |
| <40 years | 22 | 8 | 14 | 63.6 | 19 | 7 | 5 | 5 | 2 | 63.2 |
| ≥40 years | 14 | 4 | 10 | 71.4 | 26 | 7 | 5 | 8 | 6 | 73.1 |
| Grade I–II | 21 | 10 | 11 | 52.4 | 24 | 11 | 7 | 6 | 0 | 54.2 |
| Grade III–IV | 15 | 2 | 13 | 86.7* | 21 | 3 | 3 | 7 | 8 | 85.7* |
“−” no detectable expression of EGFL7 mRNA or protein; “+” detectable EGFL mRNA and weakly positive for EGFL7 protein; “++” positive for EGFL7 protein; “+++” strongly positive for EGFL7 protein
*P < 0.05 compared to Grade I–II samples
N number of patients or samples
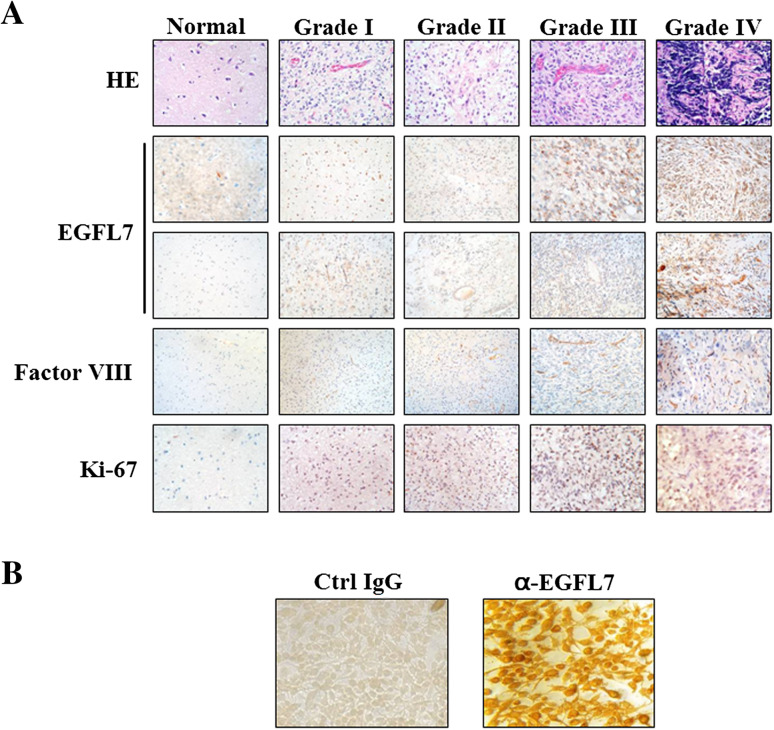
EGFL7 is expressed in both tumor cells and vascular endothelial cells in samples of malignant glioma
EGFL7 was identified as an endothelial cell-secreted factor that is expressed mainly in vascular endothelial cells in different organs (Schmidt et al. 2007). By immunohistochemical staining, we found that in tissue samples of malignant gliomas, EGFL7 was detectable in the cytoplasm of not only the vascular endothelial cells, but also the tumor cells. To verify the expression of EGFL7 in the tumor cells, we also examined the human glioma cell line U251 (Fig. 2b), which showed positive staining for EGFL7. In human glioma samples, the EGFL7 staining in both the tumor cells and vascular endothelial cells increased with tumor grade (Fig. 2a). The same trend was observed for Ki-67, a marker for tumor cell proliferation, and Factor VIII, a marker of vascular endothelial cells (Fig. 2a). Similar to the EGFL7 expression levels, both Ki-67 and Factor VIII levels, as quantified by Ki-67 LI and MVD respectively, dramatically increased with tumor grade (Fig. 3).
Fig. 2.
EGFL7 was expressed in tumor (the 2nd row) and vascular endothelial cells (the 3rd row), and its expression level, as well as that for Ki-67 and Factor VIII, increased with tumor grade. a Normal brain tissue and gliomas were analyzed by Hemotoxylin and Eosin (HE) staining or immunohistochemistry using the indicated antibodies (yellow or brown signals). Brain tissue areas with abundant tumor cells (the 2nd row) or with enriched vascular endothelial cells (the 3rd row) were chosen to present the expressions of EGFL7 in these two compartments, respectively. For immunohistochemistry, the sections were counter-stained with hemotoxylin (blue signals). b The expression of EGFL7 was examined in human glioma cell line U251 by immunocytochemistry (right panel). The isotype control IgG was used as negative control (left panel)
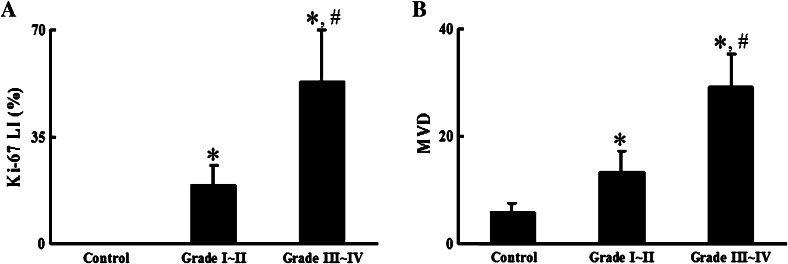
Fig. 3.
Ki-67 and microvessel density levels significantly correlated with tumor grade. Normal brain (control) and glioma tissue samples were stained with antibodies against either Ki-67 or Factor VIII and examined by immunohistochemistry. The levels of positive signals were quantified as Ki-67 labeling index (Ki-67 LI) (a) and microvessel density (MVD) (b) as detailed in the “Materials and methods” section. *P < 0.01 compared to the control group; # P < 0.01 compared to Grade I–II samples
There is a positive correlation between the expression levels of EGFL7 and Ki-67 and MVD
Since the levels of EGFL7, Ki-67 and MVD showed similar patterns of correlation with the tumor grade, we explored the correlations between these three factors. As shown in Fig. 4, all three factors were significantly and positively correlated with each other (P < 0.01).
Fig. 4.
EGFL7 expression, Ki-67 LI and MVD were positively and significantly correlated with each other. Based on the quantifications of immunohistochemistry signals from the 45 glioma samples, scatter plots were used to depict the correlations between Ki-67 LI and MVD (a) (r 2 = 0.787, P < 0.01), Ki-67 LI and EGFL7 (b) (r 2 = 0.793, P < 0.01) and MVD and EGFL7 (c) (r 2 = 0.684, P < 0. 01)
Discussion
Angiogenesis refers to the formation of new blood vessels from the pre-existing microvasculature. The process plays important roles under normal physiological conditions, such as during embryogenesis. However, under pathological conditions, the process can contribute to disease progression. For example, the continuous growth of tumors requires the angiogenic balance to be skewed towards pro-angiogenic factors in order to guarantee an adequate nutritional supply for the tumor. Angiogenesis is a complicated process, involving the positive and negative regulation of a diverse set of pro- and anti-angiogenic factors. EGFL7 is a novel pro-angiogenic factor essential for tubulogenesis during embryonic development that has anti-apoptotic effects in vascular endothelial cells (Parker et al. 2004; Xu et al. 2008). However, the expression status, biological functions and regulation of EGFL7 in pathological vasculogenesis and/or angiogenesis have not been fully explored.
In the present study, we examined the expression of EGFL7 in human malignant gliomas at both steady-state mRNA and protein levels. We found that at both levels, EGFL7 expression was not detectable in normal brain tissues, but was dramatically up-regulated in some samples of malignant glioma. In addition, the positivity rate and level of EGFL7 expression for both mRNA and protein significantly correlated with tumor grade, with the more malignant gliomas showing higher EGFL7 expression. This increase in both mRNA and protein in malignant gliomas indicates that the increase in EGFL7 occurs at or before transcription.
Although previous studies suggested that EGFL7 is secreted from vascular endothelial cells or their precursor cells, immunohistochemical staining indicated that in malignant gliomas, EGFL7 is expressed in both tumor cells and vascular endothelial cells. The expression of EGFL7 was also confirmed in human glioma cell line U251. This may imply that there is a previously un-identified function of EGFL7 in tumor cells, that there is a paracrine angiogenic function on the endothelial cells, or both. To understand how the expression of EGFL7 is turned on during the progression of glioma and what controls the location and expression of EGFL7 would benefit future development of anti-angiogenic therapy for malignant glioma and other diseases.
Malignant gliomas are highly vascularized tumors (Tuettenberg et al. 2006), as supported by our finding in this study that the MVD in glioma samples was significantly higher than in normal brain, and that there was a tumor grade-dependent increase in expression. Other studies have revealed an association between MVD and the invasiveness of glioma, demonstrating the role of MVD as an independent factor for predicting patient prognosis (Leon et al. 1996). In this study, we found that there was a significant and positive correlation between EGFL7 expression and MVD, implying that EGFL7 may play a role in promoting angiogenesis. Previous studies have indicated that EGFL7 is involved in tubulogenesis (De Maziere et al. 2008; Parker et al. 2004). Besides, it has been recently demonstrated that EGFL7 is over-expressed in hepatocellular carcinoma (HCC), predominantly in HCC cells rather than mesenchymal cells, the expression level of which significantly correlated with poor prognosis of HCC (Wu et al. 2009). Further mechanistic study revealed that EGFL7 contributed to the migration and metastasis of HCC cells through the activation of focal adhesion kinase (FAK) (Wu et al. 2009). In human non-small cell lung cancers (NSCLCs), however, EGFL7 has been suggested to stimulate the proliferation of cancer cells (Sun et al. 2009). Considering the plethora of functions exerted by EGFL7 during tumorigenesis, more mechanistic studies on its role in glioma should be carried out in the future.
Another important factor for predicting the prognosis of glioma is Ki-67, a non-histone nuclear protein expressed specifically in proliferating cells (Kogiku et al. 2008). Compared to other markers for cell proliferation, such as proliferating cell nuclear antigen (PCNA) and DNA polymerase, Ki-67 has shorter half-life and is immediately degraded once the cells stop proliferating, which allows for a more accurate reflection of cell proliferation. Several studies have established the utility of the Ki-67 labeling index (Ki-67 LI) as an indicator of the proliferation status of tumors and as a prognostic indicator for glioma (Berny et al. 2004; Faria et al. 2006; Johannessen and Torp 2006). Consistent with these findings, in the current study, we found that Ki-67 LI and MVD significantly and positively correlated with each other and with tumor grade, further supporting that the proliferative activity of glioma depends on the level of angiogenesis. Furthermore, the expression of EGFL7 also positively correlated with Ki-67 LI, suggesting that the up-regulation of EGFL7 is closely associated with the proliferation and invasion of glioma.
In summary, to our best knowledge, this is the first study to examine the expression of EGFL7 in malignant glioma. We found that there was a tumor grade-dependent up-regulation of EGFL7 at both mRNA and protein levels. In addition, there were positive correlations between the expression of EGFL7 and Ki-67 LI and MVD, suggesting the importance of EGFL7 in the angiogenesis, proliferation, and progression of malignant glioma. We conclude that EGFL7 may be used as a predictive marker for glioma prognosis and as a potential therapeutic target for malignant glioma.
Acknowledgments
This work was supported by grants from National Natural Sciences Foundation of China (Project number: 30500558) and from Hunan Science and Technology Department Fund Project (Project number: 2009JT3049).
Conflict of interest statement
All authors state there is no conflict of interest.
Footnotes
C. Huang and X. Li contributed equally to this work.
References
- Bartels U, Hawkins C, Jing M, Ho M, Dirks P, Rutka J, Stephens D, Bouffet E (2006) Vascularity and angiogenesis as predictors of growth in optic pathway/hypothalamic gliomas. J Neurosurg 104:314–320 [DOI] [PubMed] [Google Scholar]
- Berny W, Weiser A, Markowska-Woyciechowska A, Jarmundowicz W, Zub W, Zaluski R (2004) Analysis of PCNA, Ki67, AgNOR and p53 expression in brain glial tumors. Neurol Neurochir Pol 38:457–463 [PubMed] [Google Scholar]
- De Maziere A, Parker L, Van Dijk S, Ye W, Klumperman J (2008) Egfl7 knockdown causes defects in the extension and junctional arrangements of endothelial cells during zebrafish vasculogenesis. Dev Dyn 237:580–591 [DOI] [PubMed] [Google Scholar]
- Eroglu A, Sari A (2007) Expression of c-kit proto-oncogene product in breast cancer tissues. Med Oncol 24:169–174 [DOI] [PubMed] [Google Scholar]
- Faria MH, Goncalves BP, do Patrocinio RM, de Moraes-Filho MO, Rabenhorst SH (2006) Expression of Ki-67, topoisomerase IIalpha and c-MYC in astrocytic tumors: correlation with the histopathological grade and proliferative status. Neuropathology 26:519–527 [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70 [DOI] [PubMed] [Google Scholar]
- Johannessen AL, Torp SH (2006) The clinical value of Ki-67/MIB-1 labeling index in human astrocytomas. Pathol Oncol Res 12:143–147 [DOI] [PubMed] [Google Scholar]
- Ko CD, Kim JS, Ko BG, Son BH, Kang HJ, Yoon HS, Cho EY, Gong G, Ahn SH (2003) The meaning of the c-kit proto-oncogene product in malignant transformation in human mammary epithelium. Clin Exp Metastasis 20:593–597 [DOI] [PubMed] [Google Scholar]
- Kogiku M, Ohsawa I, Matsumoto K, Sugisaki Y, Takahashi H, Teramoto A, Ohta S (2008) Prognosis of glioma patients by combined immunostaining for survivin, Ki-67 and epidermal growth factor receptor. J Clin Neurosci 15:1198–1203 [DOI] [PubMed] [Google Scholar]
- Leon SP, Folkerth RD, Black PM (1996) Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer 77:362–372 [DOI] [PubMed] [Google Scholar]
- Norden AD, Drappatz J, Wen PY (2008) Novel anti-angiogenic therapies for malignant gliomas. Lancet Neurol 7:1152–1160 [DOI] [PubMed] [Google Scholar]
- Ohgaki H (2009) Epidemiology of brain tumors. Methods Mol Biol 472:323–342 [DOI] [PubMed] [Google Scholar]
- Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, Burkhard C, Schuler D, Probst-Hensch NM, Maiorka PC et al (2004) Genetic pathways to glioblastoma: a population-based study. Cancer Res 64:6892–6899 [DOI] [PubMed] [Google Scholar]
- Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier DY et al (2004) The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature 428:754–758 [DOI] [PubMed] [Google Scholar]
- Remmele W, Stegner HE (1987) Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 8:138–140 [PubMed] [Google Scholar]
- Sathornsumetee S, Reardon DA, Desjardins A, Quinn JA, Vredenburgh JJ, Rich JN (2007) Molecularly targeted therapy for malignant glioma. Cancer 110:13–24 [DOI] [PubMed] [Google Scholar]
- Schmidt M, De Maziere A, Smyczek T, Gray A, Parker L, Filvaroff E, French D, van Dijk S, Klumperman J, Ye W (2007) The role of Egfl7 in vascular morphogenesis. Novartis Found Symp 283:18–28 (Discussion 28–36, 238–241) [DOI] [PubMed] [Google Scholar]
- Sun Y, Bai Y, Zhang F, Wang Y, Guo Y, Guo L (2009) MiR-126 inhibits non-small cell lung cancer cells proliferation by targeting EGFL7. Biochem Biophys Res Commun [DOI] [PubMed]
- Takeuchi H, Ozawa S, Ando N, Kitagawa Y, Ueda M, Kitajima M (2003) Cell-cycle regulators and the Ki-67 labeling index can predict the response to chemoradiotherapy and the survival of patients with locally advanced squamous cell carcinoma of the esophagus. Ann Surg Oncol 10:792–800 [DOI] [PubMed] [Google Scholar]
- Tuettenberg J, Friedel C, Vajkoczy P (2006) Angiogenesis in malignant glioma–a target for antitumor therapy? Crit Rev Oncol Hematol 59:181–193 [DOI] [PubMed] [Google Scholar]
- Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G (1992) Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst 84:1875–1887 [DOI] [PubMed] [Google Scholar]
- Wu F, Yang LY, Li YF, Ou DP, Chen DP, Fan C (2009) Novel role for epidermal growth factor-like domain 7 in metastasis of human hepatocellular carcinoma. Hepatology 50:1839–1850 [DOI] [PubMed] [Google Scholar]
- Xu D, Perez RE, Ekekezie II, Navarro A, Truog WE (2008) Epidermal growth factor-like domain 7 protects endothelial cells from hyperoxia-induced cell death. Am J Physiol Lung Cell Mol Physiol 294:L17–L23 [DOI] [PubMed] [Google Scholar]