Abstract
Investigators who study drug addiction are fortunate to have access to excellent animal models. Such models will be invaluable in the assessment of factors involved in the progression of drug addiction. The relevance of these findings, however, will depend on the general understanding of how each model is related to drug addiction. The present review focuses on several procedures that were designed to model the addiction process and questions whether these models are tapping into the same underlying process or whether each is addressing a unique feature. Furthermore, various factors (e.g., rate of drug onset, dose magnitude, early drug history, periods of abstinence) influencing the progression of these addiction-like changes in behavior are discussed.
Keywords: Cocaine, Escalation, Motivation, Sensitization, Tolerance, Self Administration
A better understanding of the neurobiology of drug addiction is beginning to emerge through the analysis of brain changes in individuals addicted to drugs. Recent advances in neuroimaging have shown activation of specific brain regions associated with drug craving (see reviews, [1,2]). Chronic drug abuse has also been shown to cause changes in brain structure and function (see reviews, [3,4,5]). Additional advancements in the application of molecular biology techniques has provided important post-mortem analyses of brain tissue from addicts (see reviews, [6,7]).
There are, however, many limitations and challenges associated with studying drug addiction in human subjects. Various confounding factors exist within samples of drug addicts that may be difficult to parse apart, such as differences in environment, genetics, poly-drug use and route of administration. Attrition is also a concern due to the general social instability associated with drug addicts. Moreover, various ethical dilemmas may restrict the experimental design of longitudinal studies and the administration of pharmacological treatments to illicit drug users.
Animal studies offer an opportunity to investigate specific aspects of the addiction process without the confounding factors associated with human studies. As this special issue of Drug Discovery Today attests, a variety of animal models have been developed which can be used to address fundamental issues related to drug abuse and dependence. Such models will be invaluable in the assessment of genetic influences, neurochemical changes, pharmacological variables and epigenetic influences involved in the addiction process. The relevance of these findings, however, will depend on the general understanding of how each model is related to drug addiction.
This review will focus on several procedures that have been developed to examine specific aspects of cocaine self-administration in rats. One fundamental question is whether each of the models is tapping into the same underlying process or whether each is addressing a unique feature. A continuing theme throughout this review will be the examination of whether the phenomena exposed by different procedures and models are correlated or dissociable.
Before describing the rat models, it is important to briefly consider the condition under study. A logical point of reference for those attempting to model addiction-like symptoms is the current Diagnostic and Statistical Manual [8]. Table 1 lists several criteria from the DSM-IV associated with substance dependence. Whereas some of these criteria (i.e., those listed above the dotted line in table 1) might be difficult to address using experimental animals, others have been successfully modeled. Note that this is a broad classification system which is used to diagnose individuals who are dependent on a variety of substances including alcohol, opiates and stimulants. Each criterion may not apply equally across all drug categories. For example, the degree of physical dependence associated with cocaine is an issue that has been debated over the years.
Table 1.
DSM IV criteria for substance dependence.
| DSM IV Criteria for Substance Dependence |
|---|
| Persistent desire, or unsuccessful attempts, to limit use |
| Important social, occupational, or recreational activities are reduced |
| Continued use despite knowledge of adverse health consequences |
| Increased Motivation: a great deal of time and energy expended to obtain substance |
| Increased Consumption: substance is taken in larger amounts of over longer periods of time |
| Withdrawal: substance is taken to relieve withdrawal symptoms |
| Tolerance: a need for increased amounts of substance to achieve desired effects; diminished effects with continued use |
Progressive Ratio Training and Final Ratio Escalation
Our lab has focused on modeling changes in the motivation to self-administer cocaine. A progressive ratio (PR) schedule has been developed for the study of cocaine self-administration in rats which essentially asks the question, “How hard is an animal willing to work to gain access to the next injection?” Figure 1A shows an example of the pattern of cocaine self-administration on a PR schedule. The first response of the session results in an intravenous cocaine infusion; thereafter the delivery of this reinforcer is contingent upon an increasing number of responses incremented through the following series: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603, etc. [9]. Note that the pattern of self-administration is characterized by evenly spaced post-infusion pauses followed by a period of relatively rapid responding for the requisite number of responses. Responding ceases at some point in the session, presumably when the response cost exceeds the reinforcing value of the drug. The dependent measure is the ‘break point’ or final ratio, defined as the response requirement corresponding to the final drug infusion. We have argued [10] that the final ratio can be considered as an index of an animal’s motivation to self-administer psychostimulant drugs.
Figure 1.

Final ratios or drug intake can escalate over time during cocaine self-administration. A). (14) Two cumulative records show responding on the first experimental day (left) and the final experimental day (right) of PR training. Note the escalation of responses expended (vertical lines) to receive a final injection (diagonal ticks) increased across sessions. B). (17) Speed of injection effects the escalation of final ratios. Animals show increased final ratios during PR training when a high cocaine dose (1.5 mg/kg/inf IV) is delivered over 5-s (circles) but not 25-s (triangles) or 50-s (squares). C). (38) LgA training results in an escalation in cocaine intake. LgA rats (open circles) show an increased rate of cocaine intake across daily 6-hr sessions; whereas ShA rats (filled circles) show stable responding across daily 2-hr sessions.
A number of factors affect whether final ratios remain stable or escalate over time. When the PR schedule has been employed to evaluate the effects of lesions, hormonal manipulations or drug pretreatments on the reinforcing efficacy of cocaine [11,12,13], training procedures were used that produced very steady final ratios under baseline conditions. However, as our interest turned toward the addiction process, we sought to identify conditions which would produce an increase in final ratios over time – as might be expected if there were an addiction process affecting drug taking behavior. Figures 1A and 1B show an example of an increase in final ratios over time. The cumulative records in Figure 1A show final ratios increasing from an initial value of 118 on day 1 to a value of 328 on day 14. Clearly, the amount of time and energy devoted to drug taking increases in this animal over a relatively brief period. This effect occurs in the majority, albeit not all animals, and is only seen under specific conditions. The unit injection dose of cocaine is a critical factor affecting the escalation of final ratios over time. Morgan and colleagues [14] showed that final ratios were more likely to increase at higher unit doses, with 1.5 mg/kg/inj being optimal. Note that the 1.5 mg/kg/inj cocaine dose occurs at the top of the PR dose effect curve, as illustrated in figure 2, [15,16,14] and is selected over lower doses (0.75 mg/kg/inj) in choice experiments [15]. Thus the dose which is most likely to push the addiction process forward is also the most reinforcing dose and the one animals are most drawn toward if given a choice.
Figure 2.

PR training produces sensitization to the reinforcing effects of cocaine. (14) Two groups of rats were given access to 4 doses of cocaine under a PR schedule following different behavioral histories. One group of rats (filled squares) was tested after a self-administration history (see PR training) that results in an escalation of final ratios over 2 weeks. A second group of rats (open triangles) was tested after a history that produces stable final ratios. The augmentation of the reinforcing effects of cocaine following PR training was observed at all tested doses. Note the upward and leftward dose effect curve shift.
Liu and colleagues [17] demonstrated that the speed of drug infusion has an important influence on the escalation of final ratios. Three groups of rats were given access to the same unit dose of cocaine (1.5 mg/kg/inj) on a PR schedule; in one group the injections were delivered relatively quickly (i.e. 5 sec duration), while the remaining two groups received their injections over a longer period (25 and 50 sec). As illustrated in Figure 1B, no significant difference was observed between the three groups at the start of the two-week testing period. However, as testing continued the 5 sec group showed a significant increase in final ratios whereas the groups that received slower cocaine infusions (i.e., 25 or 50 sec) did not show this increase across sessions [17].
The escalation in final ratios represents a leftward and upward shift in the cocaine dose-response curve. The ascending limbs of two dose-response curves are shown in Figure 2. The data clearly show that higher unit injection doses of cocaine support higher final ratios. The lower curve is typical of many studies using training procedures which produce stable final ratios. The upper curve represents animals that were tested for 2 weeks using conditions that produced an escalation of final ratios; that is, they were given access to cocaine on a PR schedule reinforced with a high unit dose of cocaine (1.5 mg/kg/inj) delivered relatively rapidly (~5 sec). When other doses were subsequently tested, it was apparent that the escalating effects carried over to other doses causing an upward and leftward shift in the dose response curve [14].
An upward and leftward shift in a dose-response curve is referred to as ‘reverse tolerance’ or ‘sensitization’. Note that the term sensitization describes a change in a very specific drug response and is not a conclusion regarding a more global modification. That is, the data illustrated in Figure 2 only show that the behavioral response for self-administered cocaine is sensitized – not that the animal is sensitized. It is possible that other behavioral and neurochemical responses to cocaine might remain unchanged or even show changes in the opposite direction.
Behavioral Sensitization
How does sensitization to the reinforcing effects of cocaine relate to the literature on ‘behavioral and neurochemical sensitization’? It has long been known that a variety of specific behavioral responses to psychostimulant drugs can become augmented with repeated drug administrations [18,19,20]. This general phenomenon, commonly referred to as ‘behavioral’ sensitization, has been linked at a theoretical level with the addiction process [21,22,23]. Many detailed reviews have addressed specific issues, for example, the neurobiological [24] and external conditional factors [25] that influence the development of behavioral sensitization. This phenomenon is paralleled by the development of neurochemical sensitization (i.e., increased drug-induced extracellular dopamine in the ventral striatum), which is considered to be of fundamental importance in the expression of behavioral sensitization [23].
Behavioral and neurochemical sensitization have also been reported to occur in the human literature. Multiple investigators have reported that drug-associated behaviors in human subjects become sensitized following repeated amphetamine administrations [26,27], see review [28]. In addition, Boileau and colleagues [27] have demonstrated that amphetamine-induced neurochemical sensitization can be observed in the striatum of human subjects.
There is strong evidence linking behavioral and neurochemical sensitization with an augmentation of the reinforcing effects of psychostimulant drugs. Regimens of IP amphetamine administration that result in behavioral sensitization [29,30] and neurochemical sensitization [30,31] also result in increased final ratios on a PR schedule reinforced by IV amphetamine [30,31] or cocaine [32,33]. Although these data appear to strongly support the conclusion that behavioral and neurochemical sensitization is sufficient to produce an augmentation of the reinforcing effects of cocaine and amphetamine, the question remains whether behavioral and neurochemical sensitization is necessary to achieve this result. A conclusion suggesting that behavioral and neurochemical sensitization is sufficient but not necessary would imply that other important addiction processes exist.
If sensitization of the dopamine system is the only process involved, then it would be predicted that animals showing an escalation in the reinforcing effects of cocaine in self-administration procedures should also show a sensitized dopamine response. Contrary to this hypothesis, Läck et al. [34] showed that the cocaine-induced psychomotor and neurochemical responses in fact showed tolerance in animals in which the reinforcing effects of cocaine were enhanced. Animals were provided access to cocaine under a PR schedule using conditions which produced an escalation in final ratios over 14 days. Upon the cessation of training, measurements for cocaine-induced psychomotor activation (Figure 3A) and extracellular dopamine in the ventral striatum (Figure 3B) were taken following either one-day (early withdrawal) or 14-days (late withdrawal) of forced abstinence. As illustrated in Figure 3A, it was found that the psychomotor activating effects of cocaine were reduced following a history of PR training in comparison to naïve animals. In addition, no apparent neurochemical sensitization to a cocaine challenge was observed in the ventral striatum. That is, cocaine-induced extracellular dopamine levels were shown to be identical between all groups in the nucleus accumbens core. In fact, as illustrated in Figure 3B, extracellular dopamine levels in the nucleus accumbens shell were significantly decreased following 1-day of withdrawal from PR training in comparison to naïve animals. However, this reduced dopamine effect was found to recover following 14 days of withdrawal. These data suggest that the sensitizing effects (i.e., increased final ratios) produced by PR training are dissociable from the development of behavioral and neurochemical sensitization. We conclude that neurochemical sensitization may be sufficient, but is not necessary for the development of an increased motivation to self-administer cocaine; thereby suggesting that more than one addiction process should be considered.
Figure 3.
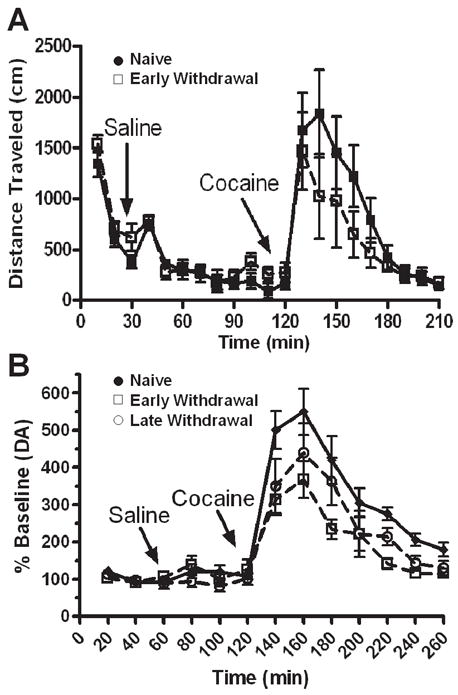
PR training does not produce behavioral and neurochemical sensitization. (37) A). PR training results in tolerance to the psychomotor activating effects of cocaine. PR trained rats (open squares) showed a tolerant psychomotor response to a cocaine challenge (15 mg/kg IP) in comparison to naïve rats (filled circles). B). PR trained animals show a tolerant cocaine-induced dopamine response in the nucleus accumbens shell. Administration of cocaine (15 mg/kg IP) resulted in decreased extracellular dopamine levels following both 1-day (open squares) and 14-days of withdrawal after PR training in comparison to naïve rats (filled circles).
Long-Access Training and Intake Escalation
Long-access training is another procedure that is used to model the addiction process. The phenomenon of long-access escalation (LgA), which models the increased drug intake observed in human addicts, has been clearly demonstrated by Ahmed and Koob [35]. These investigators have repeatedly shown that providing extended access (i.e., at least 6 hours) during daily Fixed-Ratio1 (FR1) self-administration sessions results in an increase, or ‘escalation,’ in the rate of drug intake over weeks [35,36]. As illustrated in figure 1C [35], the daily number of infusions that are self-administered by animals given short-access (i.e., 1 hour; ShA) to cocaine remained stable over 12 days (filled circles); whereas, when given long access (i.e., 6 hours) to cocaine the daily number of self-administered infusions drastically increased over 12 days (open circles). The observation of escalated rates of drug-intake after long-access training is a highly replicable phenomenon that has been documented to occur for several drugs of abuse including cocaine [35], methamphetamine [37] and heroin [38]. Many different laboratories are beginning to use this long-access training procedure in order to study the neurobiological [39,40,41] and behavioral [42,43] consequences of a history of escalated cocaine intake. Although this phenomenon is briefly reviewed here, more encompassing reviews exist that cover concepts such as the behavioral [44] and neurobiological [45] factors associated with long-access escalation.
An escalation of final ratios on a PR schedule is fundamentally different from an escalation in intake on an FR1 schedule of reinforcement. Inspection of Figure 1 reveals a superficial similarity in time course for the two procedures. The escalation in final ratios shown in the PR group (Fig. 1B) shows approximately the same slope as the escalation in intake on an FR1 schedule shown by the LgA group (Fig 1C). It should be emphasized, however, that the two dependent measures are very different. Final ratios reflect some measure of the motivation to obtain drug, while rate of drug intake on an FR1 schedule of reinforcement reflects the blood or brain level at which an animal will titrate its intake [46,47,48,49] but see also [50]. These are very different concepts. In behavioral economic terms, one reflects “price” and the other “consumption”. It remains unclear whether self-administration histories that produce changes in consumption necessarily result in changes in the maximum price paid. A number of studies have reported that LgA training can result in an increase in final ratios for cocaine on a PR schedule [51,52,53] and faster runway times for cocaine [54] in comparison to ShA rats. However this does not appear to be a consistent result. Liu et al. [55] found that higher final ratios are not necessarily observed in LgA animals. Additionally, under certain conditions, escalating the rate of cocaine intake can suppress the development of final ratio escalation that occurs during PR training [14]. Therefore, it remains unclear whether LgA and PR training produce parallel escalations in both drug intake and the effort an animal is willing to expend to obtain cocaine.
Recently we have explored the use of a self-administration protocol which provides a measure of both price and consumption. The procedure, adapted from a study by Zittel-Lazarini, et al [56], uses a descending series of unit injection doses (237μg - 1 μg) to determine the threshold dose of cocaine. The term ‘threshold’ is defined as the lowest dose that maintains stable responding throughout the duration of the session. In their study, Zittell-Lazarini and colleagues [56] demonstrated that very high rates of responding can be maintained under an FR1 schedule of reinforcement by cocaine doses as low as 8 μg/inf. Due to the observations that the rate of cocaine intake maintained under an FR1 schedule of reinforcement is titrated around some blood or brain level [47,57,58] and maximal responding occurs at the threshold, these authors [56] first suggested that behavioral economics analyses could be applied to threshold data.
As shown in figure 4B and C, a relatively stable level of cocaine consumption is maintained as the available dose is decreased until a threshold dose is reached. Due to the observation that maximal responding occurs at this threshold dose, it can be inferred that the maintenance of drug intake at threshold is influenced by the price (i.e., effort) an animal expends to ‘defend’ a relative level of drug intake. An example of the high rates of responding that are engendered at threshold doses is illustrated in figure 4A. Here, responding at each individual animal’s threshold, which ranged from 23.7–2.4 μg/inf, occurred at a rate of 955 responses/hr ± 244 (SEM). In behavioral economics terms, the point at which maximal responding occurs in order to maintain a relative level of drug consumption is Pmax (i.e., maximal price; [59]). Although Pmax is normally assessed by increasing the response requirement necessary to receive a single injection of a constant drug dose [60,61], it is also valid to decrease the dose of a drug while holding the FR requirement constant [62,63,56]. Given that the threshold procedure apparently provides information about consumption in the high dose range in addition to a behavioral economics index of price, we applied this procedure to investigate the effects of LgA and PR training on the reinforcing effects of cocaine.
Figure 4.
PR and LgA training produce opposite effects in the threshold procedure. (64) Self-administration was assessed across a descending series of ten doses (237μg - 1 μg) under an FR1 schedule of reinforcement to measure consumption in the high dose range and thresholds in the low dose range. A) PR trained rats (filled circles) showed a higher response output (955 responses/hr ± 244 (SEM)) for remarkably low threshold doses (23.7−2.4 μg/inf) in comparison to control rats (open circles). B). PR trained rats (filled circles) did not consume more cocaine at high doses in comparison to control rats (open circles), but maintained consumption at lower thresholds. Note that considerable effort is expended to maintain consumption as these low doses. C). LgA trained rats (filled triangles) consumed more cocaine at high doses but reached threshold doses prior to ShA rats (open triangles).
LgA and PR training produced opposite behavioral effects on consumption and price in the threshold procedure. The effects of four different training histories on daily cocaine intake are shown in figure 4. As illustrated in figure 4B, it was found that PR trained animals did not maintain a higher level of cocaine consumption in comparison to a group of animals that were matched for intake and dose during training. The PR trained animals did, however, defend their rates of intake to lower unit doses in comparison to other groups. This maintenance of intake required a higher response output (955 responses/hr ± 244 SEM), the results of which are illustrated in figure 4A. Therefore, in behavioral economics terms, PR trained animals paid the highest behavioral price (Pmax) for cocaine in comparison to all other tested groups [64]. As expected, Figure 4C shows that LgA trained animals maintained a higher level of cocaine consumption at high doses in comparison to ShA animals. However, the threshold of the LgA animals was significantly higher than that of the ShA animals, suggesting the development of tolerance to the reinforcing effectiveness of cocaine. Therefore, LgA animals paid the lowest behavioral price (Pmax) for low doses of cocaine in comparison to all other tested groups [64]. In summary, LgA trained animals consumed more cocaine at high doses, but did not pay a high price to maintain consumption at low doses; by contrast, the PR trained animals did not consume more cocaine at high doses, but did expend a considerably higher behavioral price to maintain consumption at low doses. Overall, these findings suggest that LgA and PR training result in very unique behavioral phenotypes that exhibit distinct addiction-like behavioral manifestations. Again, these data suggest that more than one process may be involved in the development of addiction.
An escalation of cocaine intake is also dissociable from the development of behavioral and neurochemical sensitization. The literature suggests that the time-course of LgA training does not promote, and may actually suppress, the development of drug-induced behavioral sensitization. For example, multiple investigators have reported that LgA trained animals do not show behavioral sensitization in comparison to ShA trained animals [65,66,67,43]. In fact, Ben Shahar and colleagues [65,66] reported that LgA training resulted in rats that showed a tolerant cocaine-induced psychomotor response in comparison to ShA trained animals. These latter reports are consistent with our findings that LgA training can produce tolerance to cocaine-associated behavioral effects [64]. In addition, an important finding that appears to distinguish the effects of LgA training from neurochemical sensitization is that the dopamine response in the nucleus accumbens to a cocaine challenge is not increased in LgA trained rats [68]. Therefore, these data suggest that LgA and behavioral/neurochemical sensitization are distinct processes.
Sequence of Pharmacological Events and Abstinence
A sequence of pharmacological and behavioral events influences, in part, the emergence of observable tolerant or sensitized behavioral effects. The amount of drug exposure during initial training and the periods of abstinence following a history of drug administration greatly affect the expression of an escalation in the reinforcing effects of cocaine. Both of these considerations will be discussed independently and – as with the rate of drug injection and the magnitude of the available dose – will be shown to affect the development of addiction-like behavioral changes.
A history of high drug intake suppresses the development of an increased motivation to self-administer cocaine, but only transiently suppress increased final ratios later in the sequence. Morgan et al. [14] showed that final ratios generally increase as long as the quantity animals are permitted to self-administer during training is restricted. Figure 5 shows the expected escalation of final ratios with only a single day of training (filled circles). However, if animals are given access to high doses (1.5 mg/kg/inj) for several hours for 5 days, then the phenomenon of escalation is greatly attenuated (Figure 5, open circles). These data clearly demonstrate that greater drug access does not necessarily result in higher final ratios and in fact suggests that high cocaine intake retards the process which would result in an escalation of final ratios. It is important to emphasize that the sequence of events is critical. If higher levels of access are given after the final ratios have escalated, only transient suppression is seen. Figure 5 illustrates the effect of giving the escalated and non-escalated groups 5 days of high access (40 injections × 1.5 mg/kg/inj). The final ratios for the two groups temporarily converge, but surprisingly the two groups separate again after several days. We interpret these data to indicate that high access conditions, which can produce tolerance to the reinforcing effects of cocaine, prevent an escalation of final ratios. However, if animals are first exposed to a training procedure that fosters the escalation of final ratios, the presumed increased motivational state can survive periods of high intake.
Figure 5.
Early exposure to high levels of cocaine intake attenuates the escalation of final ratios. (14) The left pane shows the initial pharmacological history of two groups of animals. One group was given access to cocaine under an FR1 reinforcement schedule for 1 day (filled circles) and another for 5 days (open circles). The next pane shows the final ratios for both groups over 14 days. Note that only the group with a limited initial pharmacological history showed an increase in final ratios. The next pane illustrates that both groups then received access to cocaine for 5 days under an FR1 reinforcement schedule. Note that both groups showed an escalation in the rate of responding. The final pane illustrates that the effects of high cocaine intake only transiently suppressed final ratios in the group that originally showed an escalation in final ratios (filled circles).
Periods of abstinence can increase various drug-induced behavioral effects including final ratios, behavioral sensitization and cue-induced reinstatement responding. For example, we have repeatedly demonstrated that providing animals with extended access to cocaine (i.e., 4 trials/hr for 24 hrs/day) for at least 10 days followed by a deprivation period of 7 days produces animals that show increased final ratios when tested on a PR schedule [69,16]; see review [70]. As illustrated in figure 6A [16], final ratios were significantly increased following this procedure in comparison to baseline final ratios (i.e., those before extended access and deprivation). Note that the deprivation period is a necessary component of this phenomenon. That is, animals that are given access to cocaine on an FR1 reinforcement schedule instead of undergoing a deprivation period following extended access conditions do not increase final ratios [16]. These data clearly demonstrate that periods of abstinence can produce increases in the reinforcing effects of cocaine. The expression of behavioral sensitization is another good example of the effects of abstinence periods. As illustrated in figure 6B [71] the expression of behavioral sensitization is exacerbated by periods of forced abstinence. Here, Kalivas and Duffy [71] treated rats with cocaine (30 mg/kg i.p.) for five consecutive days before measuring drug (15 mg/kg cocaine i.p.) induced psychomotor activation following various abstinence periods. It was shown that the magnitude of the psychomotor response became augmented along the course of abstention. Likewise, these authors reported that the cocaine-induced dopamine response in the ventral striatum increased over the abstinence period [71].
Figure 6.

Periods of abstinence can increase final ratios, behavioral sensitization and cue-induced reinstatement. A). (70) Final ratios increase following abstinence periods. Seven days of abstinence following extended access (24-hr/day; 4 trials/hr; 10 days) conditions during cocaine self-administration results in rats that show increased final ratios (filled diamonds) in comparison to baseline final ratios (open circles). It should be noted that the abstinence period is necessary for the increase in final ratios. B). (72) Behavioral sensitization increases following abstinence periods. Cocaine-induced (15 mg/kg IP) psychomotor activation progressively increased over extended periods of abstinence following a sensitizing drug-treatment (30 mg/kg IP cocaine × 5 days). C). (73) Cue-reinforced responding increases, or incubates, over periods of abstinence. Following various periods of abstinence from cocaine self-administration animals were returned to operant chambers. Upon meeting extinction criteria (i.e., 15 responses/hr on a lever not paired with any cue), cue-induced reinstatement responding was assessed by reintroducing a conditioned tone-light cue in response to each lever press. The amount of tone-cue maintained responding increased following progressively longer periods of abstinence.
Another related behavioral procedure used to model the addiction process has been termed ‘incubation of craving.’ This phenomenon of incubation refers to the observation that the amount of cue-induced responding observed during a reinstatement self-administration session increases in relation to the duration of the abstinence period [72,73]. As illustrated in figure 6C [72], the amount of cue-reinforced responding increases, or incubates, over periods of abstinence. In this study [72], animals self-administered cocaine under an FR1 schedule of reinforcement, and then underwent various periods of abstinence before being returned to operant chambers. Upon meeting extinction criteria (i.e., 15 responses/hr on a lever not paired with any cue), cue-induced reinstatement responding was assessed by reintroducing a conditioned tone-light cue in response to each lever press. The amount of tone-cue maintained responding increased following progressively longer periods of abstinence. The incubation of craving phenomenon has been reported to increase over the first three months of abstinence but wanes after six months [74]. Taken together, these studies suggest that periods of abstinence may contribute to the development of certain addiction-like behavioral effects.
Increased reinstatement responding can be dissociated from the development of behavioral sensitization. Many parallels linking behavioral sensitization to changes in drug-self-administration have been documented (see review, [75]). In fact, it has been hypothesized that the development of behavioral sensitization to the acute effects of drugs may facilitate the reinstatement of drug-seeking behavior [21] in addition to promoting drug-taking behavior [75]. In support of this hypothesis, it has been demonstrated that the expression of behavioral sensitization coincides with the expression of drug-induced reinstatement responding [76]. That is, following periods of abstinence, animals that show increased psychomotor sensitization also show increased reinstatement responding [76]. However, other observations suggest that an animal’s propensity to reinstate does not depend upon the development of behavioral sensitization. For example, LgA animals show increased reinstatement responding but do not exhibit increased behavioral sensitization in comparison to ShA animals [67,43]. Thus, sensitization may be sufficient, but is not necessary for the expression of increased reinstatement responding. This dissociation provides yet another example that independent processes exist.
How many addiction processes are there? If we are seeking the most parsimonious explanation we should start with as few assumptions as possible and only when confronted with a need should we add more complexity to the theory. The simplest idea is that there is a single process which is manifest in a variety of ways in the clinical population. If animal models demonstrate that fundamental behavioral characteristics are always correlated it would imply that a single process is involved. If, however, critical features can be dissociated then it would not only suggest multiple processes but also guide the identification of core aspects of the addiction process. Robust behavioral changes that develop over time and appear to be symptomatic of an addiction process have been modeled using distinct training procedures. In the present review we provide several examples showing that many of the changes resulting from these procedures are dissociable. While it may be difficult to determine whether each of these behavioral changes models an exact DSM criterion several clear associations have emerged. For example, the escalation in drug intake observed after LgA training clearly models increased consumption over time. Similarly the increased final ratios observed after PR training appears to address the DSM-IV criterion of increased time and energy expended to obtain cocaine. Because the phenomena discussed in this review are dissociable, they can be viewed as distinct components of drug addiction. This idea suggests the possibility that each of these behavioral phenomena have discrete neurobiological substrates. Overall, we conclude that drug addiction is too complex a disorder to be explained as a single process or alteration in the function of one particular transmitter; however, the identification of specific sub-processes may actually enhance our ability to isolate neural mechanisms that are unique to various animal models. The challenge for the field will be to identify and distinguish between a yet unidentified number of addiction processes. Progress will likely be made through further development of animal models that address DSM-IV symptomatology.
Acknowledgments
The authors would like to thank Keri Chiodo for helpful comments in the preparation of this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Gatley SJ, et al. PET imaging in clinical drug abuse research. Curr Pharm Des. 2005;11:3203–3219. doi: 10.2174/138161205774424717. [DOI] [PubMed] [Google Scholar]
- 2.Magalhaes AC. Functional magnetic resonance and spectroscopy in drug and substance abuse. Top Magn Reson Imaging. 2005;16:247–251. doi: 10.1097/01.rmr.0000194048.43739.d4. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein RZ, et al. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borne J, et al. Neuroimaging in drug and substance abuse part II: opioids and solvents. Top Magn Reson Imaging. 2005;16:239–245. doi: 10.1097/01.rmr.0000192154.34563.6b. [DOI] [PubMed] [Google Scholar]
- 5.Rojas R, et al. Neuroimaging in drug and substance abuse part I: cocaine, cannabis, and ecstasy. Top Magn Reson Imaging. 2005;16:231–238. doi: 10.1097/01.rmr.0000192156.46492.24. [DOI] [PubMed] [Google Scholar]
- 6.Bannon M, et al. Gene expression profiling in the brains of human cocaine abusers. Addict Biol. 2005;10:119–126. doi: 10.1080/13556210412331308921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemby SE. Assessment of genome and proteome profiles in cocaine abuse. Prog Brain Res. 2006;158:173–195. doi: 10.1016/S0079-6123(06)58009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 2000. [Google Scholar]
- 9.Richardson NR, et al. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 10.Arnold JM, et al. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- 11.Roberts DC, et al. Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacology. 1996;15:417–423. doi: 10.1016/0893-133X(96)00002-4. [DOI] [PubMed] [Google Scholar]
- 12.Roberts DC, et al. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- 13.Loh EA, et al. Break-points on a progressive ratio schedule reinforced by intravenous cocaine increase following depletion of forebrain serotonin. Psychopharmacology (Berl) 1990;101:262–266. doi: 10.1007/BF02244137. [DOI] [PubMed] [Google Scholar]
- 14.Morgan D, et al. Rapid and persistent sensitization to the reinforcing effects of cocaine. Neuropsychopharmacology. 2006;31:121–128. doi: 10.1038/sj.npp.1300773. [DOI] [PubMed] [Google Scholar]
- 15.Ward SJ, et al. Comparison of the reinforcing effects of cocaine and cocaine/heroin combinations under progressive ratio and choice schedules in rats. Neuropsychopharmacology. 2005;30:286–295. doi: 10.1038/sj.npp.1300560. [DOI] [PubMed] [Google Scholar]
- 16.Morgan D, et al. Binge self-administration and deprivation produces sensitization to the reinforcing effects of cocaine in rats. Psychopharmacology (Berl) 2005;178:309–316. doi: 10.1007/s00213-004-1992-6. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, et al. Sensitization of the reinforcing effects of self-administered cocaine in rats: effects of dose and intravenous injection speed. Eur J Neurosci. 2005;22:195–200. doi: 10.1111/j.1460-9568.2005.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tatum AL, et al. Experimental Cocaine Addiction. J Pharmacol Exp Ther. 1929;36:401–410. [Google Scholar]
- 19.Downs AW, et al. The effect of repeated doses of cocaine on the rat. J Pharmacol Exp Ther. 1932;46:199–200. [Google Scholar]
- 20.Segal DS, et al. Behavioral and neurochemical characteristics of stimulant-induced augmentation. Psychopharmacol Bull. 1987;23:417–424. [PubMed] [Google Scholar]
- 21.Robinson TE, et al. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 22.Robinson TE, et al. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 23.Robinson TE, et al. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 24.Vanderschuren LJ, et al. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 25.Robinson TE, et al. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev. 1998;22:347–354. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- 26.Strakowski SM, et al. Enhanced response to repeated d-amphetamine challenge: evidence for behavioral sensitization in humans. Biol Psychiatry. 1996;40:872–880. doi: 10.1016/0006-3223(95)00497-1. [DOI] [PubMed] [Google Scholar]
- 27.Boileau I, et al. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- 28.Sax KW, et al. Behavioral sensitization in humans. J Addict Dis. 2001;20:55–65. doi: 10.1300/J069v20n03_06. [DOI] [PubMed] [Google Scholar]
- 29.Vezina P, et al. The effect of previous exposure to amphetamine on drug-induced locomotion and self-administration of a low dose of the drug. Psychopharmacology (Berl) 1999;147:125–134. doi: 10.1007/s002130051152. [DOI] [PubMed] [Google Scholar]
- 30.Lorrain DS, et al. Previous exposure to amphetamine increases incentive to obtain the drug: long-lasting effects revealed by the progressive ratio schedule. Behav Brain Res. 2000;107:9–19. doi: 10.1016/s0166-4328(99)00109-6. [DOI] [PubMed] [Google Scholar]
- 31.Vezina P, et al. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci. 2002;22:4654–4662. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suto N, et al. Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in a D1 dopamine receptor dependent manner. Neuropsychopharmacology. 2002;27:970–979. doi: 10.1016/S0893-133X(02)00379-2. [DOI] [PubMed] [Google Scholar]
- 33.Suto N, et al. Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in an NMDA, AMPA/kainate, and metabotropic glutamate receptor-dependent manner. Neuropsychopharmacology. 2003;28:629–639. doi: 10.1038/sj.npp.1300075. [DOI] [PubMed] [Google Scholar]
- 34.Lack CM, et al. Increased breakpoints on a progressive ratio schedule reinforced by IV cocaine are associated with reduced locomotor activation and reduced dopamine efflux in nucleus accumbens shell in rats. Psychopharmacology (Berl) 2008;195:517–525. doi: 10.1007/s00213-007-0919-4. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed SH, et al. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed SH, et al. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- 37.Kitamura O, et al. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- 38.Lenoir M, et al. Supply of a nondrug substitute reduces escalated heroin consumption. Neuropsychopharmacology. 2008;33:2272–2282. doi: 10.1038/sj.npp.1301602. [DOI] [PubMed] [Google Scholar]
- 39.Ferrario CR, et al. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 40.Ben Shahar O, et al. Changes in levels of D1, D2, or NMDA receptors during withdrawal from brief or extended daily access to IV cocaine. Brain Res. 2007;1131:220–228. doi: 10.1016/j.brainres.2006.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Briand LA, et al. Persistent Alterations in Cognitive Function and Prefrontal Dopamine D2 Receptors Following Extended, but Not Limited, Access to Self-Administered Cocaine. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanderschuren LJ, et al. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 43.Knackstedt LA, et al. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther. 2007;322:1103–1109. doi: 10.1124/jpet.107.122861. [DOI] [PubMed] [Google Scholar]
- 44.Ahmed SH. Imbalance between drug and non-drug reward availability: a major risk factor for addiction. Eur J Pharmacol. 2005;526:9–20. doi: 10.1016/j.ejphar.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 45.Koob GF, et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Lynch WJ, et al. Regulation of drug intake. Exp Clin Psychopharmacol. 2001;9:131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- 47.Oleson EB, et al. Neuropsychopharmacology. 2008. Dopamine uptake changes associated with cocaine self-administration. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmed SH, et al. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology (Berl) 2005;180:473–490. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- 49.Tsibulsky VL, et al. Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Res. 1999;839:85–93. doi: 10.1016/s0006-8993(99)01717-5. [DOI] [PubMed] [Google Scholar]
- 50.Panlilio LV, et al. Variability of drug self-administration in rats. Psychopharmacology (Berl) 2003;167:9–19. doi: 10.1007/s00213-002-1366-x. [DOI] [PubMed] [Google Scholar]
- 51.Paterson NE, et al. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- 52.Allen RM, et al. Continuous intracerebroventricular infusion of the competitive NMDA receptor antagonist, LY235959, facilitates escalation of cocaine self-administration and increases break point for cocaine in Sprague-Dawley rats. Pharmacol Biochem Behav. 2007;88:82–88. doi: 10.1016/j.pbb.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wee S, et al. Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol. 2008;18:303–311. doi: 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ben Shahar O, et al. Heightened drug-seeking motivation following extended daily access to self-administered cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:863–869. doi: 10.1016/j.pnpbp.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y, et al. Effects of extended-access self-administration and deprivation on breakpoints maintained by cocaine in rats. Psychopharmacology (Berl) 2005;179:644–651. doi: 10.1007/s00213-004-2089-y. [DOI] [PubMed] [Google Scholar]
- 56.Zittel-Lazarini A, et al. A critical transition in cocaine self-administration: behavioral and neurobiological implications. Psychopharmacology (Berl) 2007;192:337–346. doi: 10.1007/s00213-007-0724-0. [DOI] [PubMed] [Google Scholar]
- 57.Lynch WJ, et al. Regulation of drug intake. Exp Clin Psychopharmacol. 2001;9:131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- 58.Pettit HO, et al. Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol Biochem Behav. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- 59.Hursh SR. Behavioral economics of drug self-administration and drug abuse policy. J Exp Anal Behav. 1991;56:377–393. doi: 10.1901/jeab.1991.56-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cosgrove KP, et al. Effects of bremazocine on self-administration of smoked cocaine base and orally delivered ethanol, phencyclidine, saccharin, and food in rhesus monkeys: a behavioral economic analysis. J Pharmacol Exp Ther. 2002;301:993–1002. doi: 10.1124/jpet.301.3.993. [DOI] [PubMed] [Google Scholar]
- 61.Wade-Galuska T, et al. A behavioral economic analysis of cocaine and remifentanil self-administration in rhesus monkeys. Psychopharmacology (Berl) 2007;194:563–572. doi: 10.1007/s00213-007-0858-0. [DOI] [PubMed] [Google Scholar]
- 62.Bickel WK, et al. Behavioral economics of drug self-administration. I Functional equivalence of response requirement and drug dose. Life Sci. 1990;47:1501–1510. doi: 10.1016/0024-3205(90)90178-t. [DOI] [PubMed] [Google Scholar]
- 63.Bickel WK, et al. Behavioral economics: a novel experimental approach to the study of drug dependence. Drug Alcohol Depend. 1993;33:173–192. doi: 10.1016/0376-8716(93)90059-y. [DOI] [PubMed] [Google Scholar]
- 64.Oleson EB, et al. Behavioral economic assessment of price and cocaine consumption following self-administration histories which produce escalation of either final ratios or intake. Neuropsychopharmacology. 2008;34:796–804. doi: 10.1038/npp.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ben Shahar O, et al. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 66.Ben Shahar O, et al. Prolonged daily exposure to i. v cocaine results in tolerance to its stimulant effects. Pharmacol Biochem Behav. 2005;82:411–416. doi: 10.1016/j.pbb.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 67.Ahmed SH, et al. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- 68.Ahmed SH, et al. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology (Berl) 2005;180:473–490. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- 69.Morgan D, et al. Increases in the reinforcing efficacy of cocaine after particular histories of reinforcement. Behav Pharmacol. 2002;13:389–396. doi: 10.1097/00008877-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 70.Morgan D, et al. Sensitization to the reinforcing effects of cocaine following binge-abstinent self-administration. Neurosci Biobehav Rev. 2004;27:803–812. doi: 10.1016/j.neubiorev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Kalivas PW, et al. Time course of extracellular dopamine and behavioral sensitization to cocaine. II Dopamine perikarya. J Neurosci. 1993;13:276–284. doi: 10.1523/JNEUROSCI.13-01-00276.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grimm JW, et al. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grimm JW, et al. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu L, et al. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology (Berl) 2004;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- 75.Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 76.De Vries TJ, et al. Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci. 1998;10:3565–3571. doi: 10.1046/j.1460-9568.1998.00368.x. [DOI] [PubMed] [Google Scholar]




