Abstract
The reaction of ram seminal vesicle (RSV) microsomes with arachidonic acid (AA) was examined using electron spin resonance (ESR), high performance liquid chromatography-electron spin resonance spectrometry (HPLC-ESR), and high performance liquid chromatography-electron spin resonance-mass spectrometry (HPLC-ESR-MS) combined use of spin trapping technique. A prominent ESR spectrum (αN = 1.58 mT and αHβ = 0.26 mT) was observed in the complete reaction mixture of ram seminal vesicle microsomes with arachidonic acid containing 2.0 mg protein/ml ram seminal vesicle (RSV) microsomal suspension, 0.8 mM arachidonic acid, 0.1 M 4-POBN, and 24 mM tris/HCl buffer (pH 7.4). The ESR spectrum was hardly observed for the complete reaction mixture without the RSV microsomes. The formation of the radical appears to be catalyzed by the microsomal components. In the absence of AA, the intensity of the ESR signal decreased to 16 ± 15% of the complete reaction mixture, suggesting that the radical is derived from AA. For the complete reaction mixture with boiled microsomes, the intensity of the ESR signal decreased to 49 ± 4% of the complete reaction mixture. The intensity of the ESR signal of the complete reaction mixture with indomethacin decreased to 74 ± 20% of the complete reaction mixture, suggesting that cyclooxygenese partly participates in the reaction. A peak was detected on the elution profile of HPLC-ESR analysis of the complete reaction mixture. To determine the structure of the peak, an HPLC-ESR-MS analysis was performed. The HPLC-ESR-MS analysis of the peak showed two prominent ions, m/z 266 and m/z 179, suggesting that the peak is a 4-POBN/pentyl radical adduct. An HPLC-ESR analysis of the authentic 4-POBN/pentyl radical adduct comfirmed the identification.
Keywords: radical, arachidonic acid, peroxidation, COX, ESR
Introduction
Cyclooxygenase (COX) catalyzes the primary step in the metabolism of unesterified arachidonic acid to bioactive prostanoids [1, 2]. The prostanoids participate in physiological processes, such as synaptic transmission, neurotransmitter release and cerebral blood flow regulation [3, 4]. The reaction of tissues to certain stresses such as inflammation or injuries is also caused by prostanoids. This metabolic pathway has been shown to play an important role in neurological and neurodegenerative diseases, such as stroke, epilepsy, and Alzheimer’s disease [3, 4].
The mechanism of prostaglandin synthesis predicts the formation of free radicals derived from the substrate fatty acid [5]. Indeed, Mason et al. observed a characteristic ESR spectrum for the reaction mixture of ram seminal vesicle microsomes with arachidonic acid in the presence of the spin trap, 2-methyl-2-nitrosopropane [6], suggesting that a carbon-centered free radical forms. Substitution of arachidonic acid by octadeuterated (5, 6, 8, 9, 11, 12, 14, 15)-arachidonic acid confirmed that the radical is derived from arachidonic acid. Furthermore, the ESR spectrum showed that the arachidonic acid-derived radical is bound to the spin trap at one of the eight deuterated positions [7].
Free radicals have been successfully detected and identified using ESR spectroscopy combined with spin trapping technique. High performance liquid chromatography-electron spin resonance spectrometry (HPLC-ESR) [8–11] and high performance liquid chromatography-electron spin resonance-mass spectrometry (HPLC-ESR-MS) [12] have also been employed for detection and identification of the radical adducts. To identify the radical formed in the reaction mixture of ram seminal vesicle microsomes with arachidonic acid, the HPLC-ESR and HPLC-ESR-MS were performed. In this paper, pentyl radical is detected and identified in the reaction mixtures of ram seminal vesicle microsomes with arachidonic acid using ESR, HPLC-ESR, and HPLC-ESR-MS combined use of spin trapping technique.
Materials and Methods
Chemicals
α-(4-Pyridyl-1-oxide)-N-tert-butylnitrone (4-POBN), a spin-trapping reagent was purchased from Tokyo Kasei Kogyo, Ltd. (Tokyo, Japan). Arachidonic acid and indomethacin were from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Pentylhydrazine oxalate was synthesized according to the method of Gever and Hayes [13]. All other chemicals used were of analytical grade.
Preparation of ram seminal vesicle microsomes
The ram seminal vesicles (obtained from a local slaughterhouse) were freed of extraneous tissue, cut into small pieces, and homogenized in 0.25 M sucrose (tissue to sucrose ratio of 1:9) using T25 digital ULTRA-TURRAX high-performance disperser (IKA-WERKE GMBH & CO. KG, Staufen, Germany). The homogenate was centrifuged at 16,000 g for 30 min at 4°C. The supernatant fraction was then centrifuged at 120,000 g for 30 min at 4°C. The pellet was suspended in 0.25 M sucrose. Protein concentration of the suspension was 4.8 mg/ml. It was kept at −80°C before use.
The complete reaction mixture of ram seminal vesicle microsomes with arachidonic acid
The complete reaction mixture contained 0.1 M 4-POBN, 2.0 mg protein/ml ram seminal vesicle microsomes and 0.8 mM arachidonic acid in 24 mM tris buffer (pH 7.4). The reaction was started by adding ram seminal vesicle and performed for the ESR, HPLC-ESR, and HPLC-ESR-MS experiments for 60 min at 37°C.
Preparation of 4-POBN/pentyl radical adduct
The reaction mixture contained 0.1 M 4-POBN, 4 mg/ml pentylhydrazine oxalate, 0.2 mM CuCl2 and 45 mM carbonate buffer (pH 10.0) in a total volume of 2 ml. The reaction was started by adding CuCl2 after bubbling nitrogen gas. It was performed for 120 min under an air atmosphere. In order to purify 4-POBN/pentyl radical adduct, the reaction mixture was applied to the HPLC-ESR.
ESR measurements
The ESR spectra were obtained using a model JES-FR30 Free Radical Monitor (JEOL Ltd., Tokyo, Japan). Aqueous samples were aspirated into a Tefron tube centered in a microwave cavity. Operating conditions of the ESR spectrometer were: power, 4 mW; modulation width, 0.1 mT; sweep time, 4 min; sweep width, 10 mT; time constant, 0.3 s. Magnetic fields were calibrated by the splitting of MnO (ΔH3-4 = 8.69 mT).
HPLC-ESR chromatography
An HPLC used in the HPLC-ESR consisted of a model 7125 injector (Reodyne, Cotari, CA) and a model 655A-11 pump with a model L-5000 LC controller (Hitachi Ltd., Ibaragi, Japan). A semi-preparative column (300 mm long × 10 mm i.d.) packed with TSKgel ODS-120T (TOSOH Co., Tokyo, Japan) was used. Flow rate was 2.0 ml/min throughout the experiments. For the HPLC-ESR, two solvents were used: solvent A, 50 mM acetic acid; solvent B, 50 mM acetic acid/acetonitrile (20:80, v/v). A following combination of isocratic and linear gradient was used: 0–40 min, 100–20% A (linear gradient); 40–60 min, 80% B (isocratic). The eluent was introduced into a model JES-FR30 Free Radical Monitor. The ESR spectrometer was connected to the HPLC with a Teflon tube, which passed through the center of the ESR cavity. The operating conditions of the ESR spectrometer were: power, 4 mW; modulation width, 0.2 mT; time constant, 1 s. The magnetic field was fixed at the third peak in the doublet-triplet ESR spectrum (αN = 1.58 mT and αHβ = 0.26 mT) of the 4-POBN radical adduct.
HPLC-ESR-MS chromatography
HPLC and ESR conditions were as described in the HPLC-ESR. The operating conditions of the mass spectrometer were: nebulizer, 180°C; aperture 1, 120°C; N2 controller pressure, 2.0 kg/cm2 drift voltage, 70 V; multiplier voltage, 1800 V; needle voltage, 3000 V; polarity, positive; resolution, 48.
The mass spectra were obtained by introducing the eluent from the ESR detector into the LC-MS system just before the peaks were eluted. The flow rate was kept at 50 µl/min while the eluent was introducing into the mass spectrometer.
Results
ESR measurements of the reaction mixtures of ram seminal vesicle microsomes with arachidonic acid
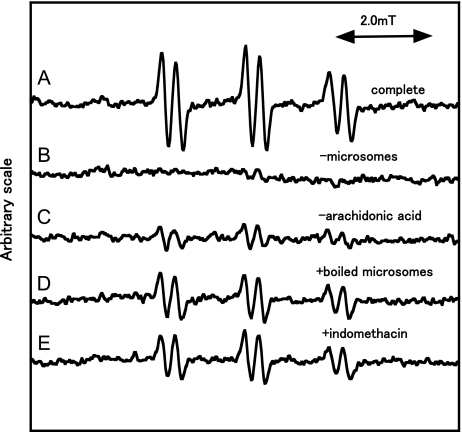
An ESR spectrum of the complete reaction mixture of ram seminal vesicle microsomes with arachidonic acid was measured (Fig. 1). A prominent ESR spectrum (αN = 1.58 mT and αHβ = 0.26 mT) was observed in the complete reaction mixture (Fig. 1A). The ESR spectrum was hardly observed for the complete reaction mixture without ram seminal vesicle microsomes (Fig. 1B, Table 1). In the absence of arachidonic acid, the intensity of the ESR signal decreased to 16 ± 15% (n = 3) of the complete reaction mixture (Fig. 1C, Table 1). For the complete reaction mixture with boiled microsomes, the ESR signal decreased to 49 ± 4% (n = 3) of the complete reaction mixture (Fig. 1D, Table 1). For the complete reaction mixture with indomethacin, the ESR signal decreased to 74 ± 20% (n = 3) of the complete reaction mixture (Fig. 1E, Table 1).
Fig. 1.
ESR spectra of the reaction mixtures of ram seminal vesicle microsomes with arachidonic acid. The reaction and ESR conditions were as described in Materials and Methods. Total volume of the reaction mixtures was 200 µl. A, a complete reaction mixture of sam seminal vesicle microsomes with arachidonic acid; B, same as in A except that microsomes were omitted; C, same as in A except that arachidonic acid was omitted; D, a complete reaction mixture with boiled microsomes; E, same as in A except that indomethacin (2 mM) was added.
Table 1.
ESR peak heights of the reactions of ram seminal vesicles with arachidonic acid under several conditions
| Conditions | % control |
|---|---|
| −Microsomes | 12 ± 3 |
| −Arachidonic acid | 16 ± 15 |
| +Boiled microsomes | 49 ± 4 |
| +Indomethacin (2 mM) | 74 ± 20 |
Results are means ± SD of three experiments. ESR measurement and reaction were performed as described in Materials and Methods. The control is the complete reaction mixture.
HPLC-ESR and HPLC-ESR-MS analyses of the reaction mixture of ram seminal vesicle microsomes with arachidonic acid
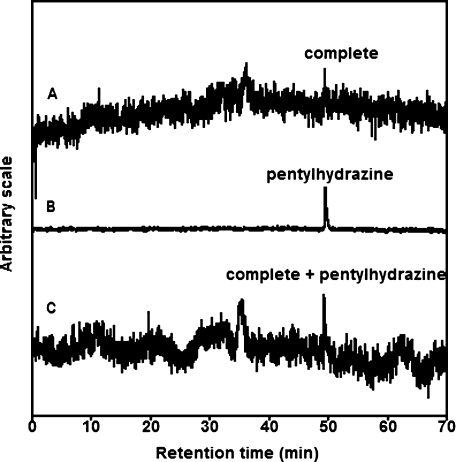
The HPLC-ESR analyses were performed for the complete reaction mixture of ram seminal vesicle microsomes with arachidonic acid (Fig. 2). A prominent peak was separated on the HPLC-ESR elution profile (Fig. 2A). The retention times of the peak was 49.4 min.
Fig. 2.
HPLC-ESR analyses of the reaction mixtures of ram seminal vesicle microsomes with arachidonic acid. The reaction and HPLC-ESR conditions were as described in Materials and Methods. Total volume of the complete reaction mixtures was 10 ml. A, 10 ml of complete reaction mixture; B, 10 µl of 4-POBN/pentyl radical adducts; C, 10 ml of complete reaction mixture mixed with 10 µl of 4-POBN/pentyl radical adducts diluted by one fifteenth.
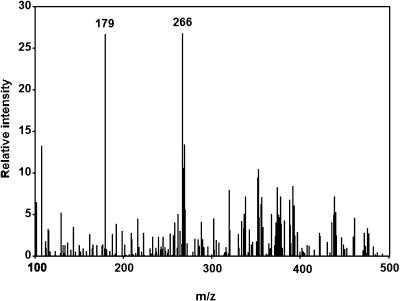
To determine the structure of the peak compound, an HPLC-ESR-MS analysis was performed. The HPLC-ESR-MS analysis of the peak compound gave ions at m/z 266 and m/z 179 (Fig. 3). The ion m/z 266 corresponds to the protonated molecule of 4-POBN/pentyl radical adduct, [M + H]+•. A fragment ion at m/z 179 corresponds to the loss of (CH3)3C(O)N from the protonated molecules.
Fig. 3.
HPLC-ESR-MS analysis. The reaction and HPLC-ESR-MS conditions were as described in Materials and Methods. Total volume of the reaction mixtures was 10 ml.
In order to confirm the chemical structure of the peak compound, HPLC-ESR analyses were performed for the reaction mixture of pentylhydrazine with Cu2+. It is well known that pentyl radical forms in the reaction mixture of pentylhydrazine with Cu2+ (11). An HPLC-ESR analysis of the reaction mixture of pentylhydrazine with Cu2+ gave a peak with almost the same retention time as the peak compound (Fig. 2B). When the peak fraction in the reaction mixture of pentylhydrazine with Cu2+ was mixed with the complete reaction mixture of ram seminal vesicle microsomes with arachidonic acid, the peak height of the peak compound increased (Fig. 2C), suggesting that the peak compound and 4-POBN/pentyl radical adduct are identical.
Discussion
In this study, the reaction of ram seminal vesicle microsomes with arachidonic acid was examined using ESR, HPLC-ESR and HPLC-ESR-MS combined use of spin trapping technique. A prominent ESR spectrum (αN = 1.58 mT and αHβ = 0.26 mT) was observed in the complete reaction mixture. The ESR spectrum was hardly observed for the complete reaction mixture without ram seminal vesicle microsomes. The ram seminal vesicle microsomes could be essential for the radical formation. The ESR spectrum was hardly observed in the absence of arachidonic acid, suggesting that the radical is derived from arachidonic acid.
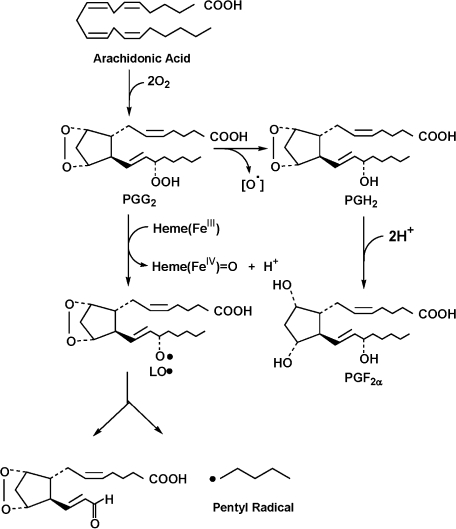
HPLC-ESR and HPLC-ESR-MS analyses showed that 4-POBN/pentyl radical adduct forms in a reaction mixture of ram seminal vesicle microsomes with arachidonic acid. A possible reaction path for the formation of the pentyl radical is shown (Scheme 1). The prostaglandin endoperoxide H synthases (PGHSs) exhibit two different but complementary enzymatic activities [1, 5]: (i) a COX (bis-oxygenase) which catalyzes the formation of prostaglandin G2 (PGG2) from arachidonic acid and two molecules of O2 and (ii) a peroxidase which facilitates the two-electron reduction of the 15-hydroperoxyl group of PGG2. Rota et al. reported the cytochrome-P450-catalyzed one electron reductive homolytic decomposition of LOOH [14]. The one electron reductive homolytic decomposition of LOOH is also catalyzed by the other compounds such as hemoglobin, myoglobin, and cytochrome c [15–17]. This reaction yields radical intermediates alkoxyl radical (LO•).
Scheme 1.
A possible reaction path for the formation of pentyl radical.
Heme(FeIII) + PGG2 → Heme(FeIV) = O + H+ + LO•…(1)
β-Scission of the LO• possibly forms the pentyl radical (Scheme 1). Mason et al. observed a characteristic ESR spectrum for the reaction mixture of ram seminal vesicle microsomes with arachidonic acid in the presence of the spin trap, 2-methyl-2-nitrosopropane [6], suggesting that a carbon-centered free radical forms. Substitution of arachidonic acid by octadeuterated (5, 6, 8, 9, 11, 12, 14, 15)-arachidonic acid confirmed that the radical is derived from arachidonic acid. Furthermore, the ESR spectrum showed that the arachidonic acid derived radical is bound to the spin trap at one of the eight octadeuterated positions [7]. The pentyl radicals detected in this paper is obviously different from the one detected by Mason et al. Different spin trap reagents seem to trap different radicals even in a similar reaction.
Recently, 8-iso-prostaglandin F2α (PGF2α), a major isoprostane generated through the non-enzymatic peroxidation of arachidonic acid, has been shown to be a reliable indicator of oxidative stresses in various clinical conditions [18]. Reports have shown that F2-isoprostanes are authentic biomarkers of lipid peroxidation and can be used as potential in vivo indicators of oxidant stress, as well as in evaluations of antioxidants or drugs for their free radical-scavenging properties [8]. The PGF2α is formed from PGG2 via prostaglandin endoperoxide H2 (PGH2). The pentyl radical detected in this paper is also form from PGG2. Thus, both pentyl radical and PGF2α are complementary biomarkers of lipid peroxidation.
References
- 1.Smith W.L., Murphy R.C. In: The eicosanoids: cyclooxygenase, lipoxygenase, and epoxygenase pathways, in Biochemistry of Lipids, Lipoproteins and Membranes, 5th edition. Vance D.E., Vance J.E., editors. Elsevier; Amsterdam: 2008. pp. 331–362. [Google Scholar]
- 2.Smith W.L., DeWitt D.L., Garavito R.M. Cyclooxygenase: structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 3.Kam P.C., See A.U. Cyclo-oxygenase isoenzymes: physiological and pharmacological role. Anaesthesia. 2000;55:442–449. doi: 10.1046/j.1365-2044.2000.01271.x. [DOI] [PubMed] [Google Scholar]
- 4.Katori M., Majima M. Cyclooxygenase-2: its rich diversity of roles and possible application of its selective inhibitors. Inflamm. Res. 2000;49:367–392. doi: 10.1007/s000110050605. [DOI] [PubMed] [Google Scholar]
- 5.Rouzer C.A., Marnett L.J. Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases. Chem. Rev. 2003;103:2239–2304. doi: 10.1021/cr000068x. [DOI] [PubMed] [Google Scholar]
- 6.Mason R.P., Kalyanaraman B., Tainer B.E., Eling T.E. A carbon-centered free radical intermediate in the prostaglandin synthetase oxidation of arachidonic acid. J. Biol. Chem. 1980;255:5019–5022. [PubMed] [Google Scholar]
- 7.Schreiber J., Eling T.E., Mason R.P. The oxidation of arachidonic acid by the cyclooxygenase activity of purified prostaglandin H synthase: spin trapping of a carbon-centered free radical intermediate. Arch. Biochem. Biophys. 1986;249:126–136. doi: 10.1016/0003-9861(86)90567-9. [DOI] [PubMed] [Google Scholar]
- 8.Rokushika S., Taniguchi H., Hatano H. Flow ESR detector for liquid chromatography of radicals. Anal. Lett. 1975;8:205–213. [Google Scholar]
- 9.Makino K., Hatano H. Separation and characterization of short-lived radicals in DL-methionine aqueous solution by high speed liquid chromatograph equipped with ESR spectrometer. Chem. Lett. 1979:119–122. [Google Scholar]
- 10.Iwahashi H., Ikeda A., Negoro Y., Kido R. Detection of radical species in haematin-catalysed retinoic acid 5,6-epoxidation by using h.p.l.c.-e.p.r. spectrometry. Biochem. J. 1986;236:509–514. doi: 10.1042/bj2360509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwahashi H., Albro P.W., McGown S.R., Tomer K.B., Mason R.P. Isolation and identification of α-(4-pyridyl-1-oxide)-N-tert-butylnitrone radical adducts formed by the decomposition of the hydroperoxides of linoleic acid, linolenic acid, and arachidonic acid by soybean lipoxygenase. Arch. Biochem. Biophys. 1991;285:172–180. doi: 10.1016/0003-9861(91)90346-k. [DOI] [PubMed] [Google Scholar]
- 12.Iwahashi H., Parker C.E., Mason R.P., Tomer K.B. Combined liquid chromatography/electron paramagnetic resonance spectrometry/electrospray ionization mass spectrometry for radical identification. Anal. Chem. 1992;64:2244–2252. doi: 10.1021/ac00043a011. [DOI] [PubMed] [Google Scholar]
- 13.Gever G., Hayes K. Alkylhydrazines. J. Org. Chem. 1949;14:813–818. [Google Scholar]
- 14.Rota C., Barr D.P., Martin M.V., Guengerich F.P., Tomasi A., Mason R.P. Detection of free radicals produced from the reaction of cytochrome P-450 with linoleic acid hydroperoxide. Biochem. J. 1997;328:565–571. doi: 10.1042/bj3280565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwahashi H. Some polyphenols inhibit the formation of pentyl radical and octanoic acid radical in the reaction mixture of linoleic acid hydroperoxide with ferrous ions. Biochem. J. 2000;346:265–273. [PMC free article] [PubMed] [Google Scholar]
- 16.Iwahashi H., Nishizaki K., Takagi I. Cytochrome c catalyses the formation of pentyl radical and octanoic acid radical from linoleic acid hydroperoxide. Biochem. J. 2002;361:57–66. doi: 10.1042/0264-6021:3610057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwahashi H., Kumamoto K., Hirai T. The formation of the 7-carboxyheptyl radical from 13-hydroperoxy-9,11-octadecadienoic acid catalyzed by hemoglobin and myoglobin under anaerobic conditions. J. Biochem. 2003;133:679–685. doi: 10.1093/jb/mvg087. [DOI] [PubMed] [Google Scholar]