Abstract
It is accepted that pulmonary exposure of rodents to porcine pancreatic elastase (ELT) induces lesions that morphologically resemble human emphysema. Nonetheless, extensive analysis of this model has rarely been conducted. The present study was designed to extensively examine the effects of ELT on lung inflammation, cell damage, emphysematous change, and cholinergic reactivity in rats. Intratracheal administration of two doses of ELT induced 1) a proinflammatory response in the lung that was characterized by significant infiltration of macrophages and an increased level of interleukin-1β in lung homogenates, 2) lung cell damage as indicated by higher levels of total protein, lactate dehydrogenase, and alkaline phosphatase (ALP) in lung homogenates, 3) emphysema-related morphological changes including airspace enlargement and progressive destruction of alveolar wall structures, and 4) airway responsiveness to methacholine including an augmented Rn value. In addition, ELT at a high dose was more effective than that at a low dose. This is the novel study to extensively analyze ELT-induced lung emphysema, and the analysis might be applied to future investigations that evaluate new therapeutic agents or risk factors for pulmonary emphysema. In particular, ALP in lung homogenates might be a new biomarker for the disease progression/exacerbation.
Keywords: pulmonary emphysema, rat, airway hyperresponsiveness, ALP, LDH
Introduction
Chronic obstructive pulmonary disease (COPD) is currently the fourth leading cause of death in the United States, with up to 7 million patients diagnosed each year [1]. Recently, COPD is also paid to attention as a cause of death in Japan [2]. Furthermore, it is predicted by the World Health Organization that the prevalence of COPD will increase in the coming years to become the fifth most common cause of morbidity and the third most common chronic disease worldwide [3]. Patients with COPD have progressive airflow limitation leading to debilitating dyspnea and an inability to properly oxygenate the blood. COPD is most commonly seen in long-term smokers, and the development/progression increases by aging [1, 2]. However, little is still known about the pathogenesis of the disease.
Emphysema is a major component of COPD and is characterized by alveolar extracellular matrix destruction, resulting in airspace enlargement and a reduction in the alveolar capillary exchange area, which leads to partially reversible airflow obstruction, which is usually progressive and associated with an abnormal inflammatory response of the lung to noxious particles or gases. The proposed pathogenesis for emphysema development involves a combination of inflammation, elastase, a matrix metalloprotease imbalance, apoptosis, and oxidative stress [4]. It is accepted that pulmonary exposure to porcine pancreatic elastase (ELT), an enzyme that acts predominantly on elastin [5], elicits acute lung inflammation [6–8] followed by emphysematous change [9–13] and compromised lung function [14, 15], which resembles the human disease. However, the characteristics and appropriate pathobiological biomarkers of this model have not been fully identified. Furthermore, no extensive analysis of lung proinflammatory response/damage/histopathological change/functional alteration after several doses of ELT has ever been conducted, which hampers the development of an effective therapeutic strategy.
The present study was designed to conduct an extensive analysis of the effects of two doses of ELT on lung proinflammatory response, cell damage, emphysematous change, and functional alteration in rats. Simultaneously, we sought to explore alternate biomarkers for disease progression/exacerbation.
Materials and Methods
Animals
Nine-week-old SPF F344/DuCrlCrlj (Fischer) male rats (180–200 g body weight) were purchased from Charles River Japan (Osaka, Japan) and maintained in conventional conditions for 3 weeks. They were fed a commercial diet (CE-2; Japan Clea Co., Tokyo, Japan) and given water ad libitum. The rats were housed in an animal facility that was maintained at 22–26°C with 40–69% humidity and a 12-h light/dark cycle.
Induction of emphysema
The rats were randomly divided into three experimental groups and received an intratracheal administration of porcine pancreatic ELT (48.0 U/mg protein; CALBIOCHEM, EMD Biosciences Inc., CA) at 0, 20, or 160 U dissolved in 100 µl of Dulbecco’s calcium and magnesium-free phosphate-buffered saline (PBS; Takara Bio Inc., Shiga, Japan) under anesthesia with 4% halothane (Takeda Pharmaceutical Company, Ltd., Osaka, Japan). Twenty-one days after the intratracheal administration of ELT, we evaluated cellularity in bronchoalveolar lavage fluid (BALF), the concentrations of proinflammatory mediators and biochemical parameters in lung homogenates, lung histopathology, and lung function in the rats. The study protocol followed the National Institutes of Health guidelines for the experimental use of animals. All animal studies were approved by the Institutional Review Board of The National Institute for Environmental Studies.
Collection of BALF
The rats were anesthetized with sodium pentobarbital (Kyoritsu Seiyaku Co., Tokyo, Japan) via intraperitoneal injection (50 mg/kg) and exsanguinated from the abdominal aorta. A cannula was inserted into the trachea and secured with a suture. Their lungs were lavaged twice with 10 ml of sterile saline at 37°C, which was instilled bilaterally by syringe. The fluid was harvested by gentle aspiration. The collected fluid was cooled and centrifuged at 300 g for 10 min. Total cell counts were made using a Berker-Tulk counting chamber. Differential cell counts were assessed in cytologic preparations. Slides were prepared using an Autosmear (Sakura Seiki Co., Tokyo, Japan) and were stained with Diff-Quik (International reagents Co., Kobe, Japan). A total of 500 cells were counted under oil immersion microscopy (n = 6–8 in each group).
Quantitation of the levels of cytokines, proteins, and biochemical parameters in the lung tissue supernatants
Lung tissue supernatants were prepared as follows. After the collection of BALF, the lungs were removed and subsequently homogenized with 10 mM potassium phosphate buffer (pH 7.4) containing 0.1 mM ethylenediaminetetraacetic acid (Sigma, St Louis MO), 0.1 mM phenylmethanesulfonyl fluoride (Nacalai Tesque, Kyoto, Japan), 1 µM pepstatin A (Peptide Institute, Osaka, Japan), and 2 µM leupeptin (Peptide Institute) as described previously (14). The homogenates were then centrifuged at 105,000 g for 1 h at 4°C. Thereafter, the supernatants were stored at −80°C. Enzyme-linked immunosorbent assays (ELISA) for interleukin (IL)-1β (R&D systems, Minneapolis, MN), IL-4 (R&D systems), IL-6 (R&D systems), IL-13 (Biosource, Invitrogen, Carlsbad, CA), cytokine-induced neutrophil chemoattractant (CINC-1: R&D systems), and macrophage chemoattractant protein (MCP)-1 (Endogen, Pierce Biotechnology, Rockford, IL) were conducted in the lung tissue supernatants according to the manufacturer’s instructions. The detection limits of IL-1β, IL-4, IL-6, IL-13, CINC-1, and MCP-1 were 5 pg/ml, 5 pg/ml, 21 pg/ml, 1.5 pg/ml, 1.1 pg/ml, and 5 pg/ml, respectively. The total protein level in the lung tissue supernatants was measured by the Bradford method using a protein assay kit (BIO-RAD, Hercules, CA). Biochemical analyses of lactate dehydrogenase (LDH), γ-glutamyltranspeptidase (GTP), and alkaline phosphatase (ALP) in the lung tissue supernatants were performed using kits for LDH (Wako Pure Chemical Industries, Osaka, Japan), γ-GTP (Daiichi Pure Chemicals, Tokyo, Japan), and ALP (Wako Pure Chemical Industries).
Lung morphometry
In a separate series of experiments, the rats were anesthetized with sodium pentobarbital via intraperitoneal injection (50 mg/kg) and exsanguinated from the abdominal aorta. Both lungs were fixed with 10% buffered formalin infused through the trachea at a pressure of 20 cm H2O. After separation of the lobe, 2-mm thick blocks were taken for paraffin embedding. Three-micrometer thick sections were stained with haematoxylin and eosin to observe and evaluate the degree of infiltration of eosinophils, neutrophils, and mononuclear cells as well as lung structure (n = 6 in each group).
Analysis of lung function
Lung function was measured in open-chested, tracheostomized rats using a computer-controlled small-animal ventilator (flexiVent; Scireq, Montreal, Canada) as described previously [16–18]. In brief, the rats were anesthetized with sodium pentobarbital (40 mg/kg) and xylazine (15 mg/kg, Bayer HealthCare, Monheim, Germany) via intraperitoneal injection. The rats had their chests opened and were tracheostomized with a 5 mm section of metallic tubing and ventilated at 90 br/min with a tidal volume of 10 ml/kg and a positive end expiratory pressure of 3 cm H2O.
Both the single-compartment model (using the snapshot method) and the constant-phase model (using the forced oscillation technique [FOT] method) of respiratory mechanics were applied to assess the airway responsiveness and lung function to methacholine (MCh). For the single-compartment model, total respiratory system resistance (R), elastance (E), and compliance (C) were determined. For the constant-phase model, Newtonian resistance (Rn), tissue damping (G), and tissue elastance (H) were determined. All data points were determined by the flexiVent software (version 5.0) by using multiple linear regressions to fit each data point to the single-compartment or the constant-phase model, as appropriate. The lung volume history of the mice was standardized prior to the measurement of lung mechanics using two deep inflations. After 5 min of regular mechanical ventilation, a sine wave perturbation was applied three times, and the mean was calculated to generate a baseline measurement. The respiratory system input impedance was measured during periods of apnea using a 3 s signal containing 19 mutually prime sinusoidal frequencies ranging from 0.25 to 19.625 Hz. These maneuvers generated data that were fitted to the single-compartment or the constant-phase compartment models, respectively. The means of these measurements for each mouse served as their baseline values. Following the acquisition of baseline data, airway responsiveness to aerosolized MCh (6.25 to 50 mg/mL; Tokyo Chemical Industry, Tokyo, Japan), delivered by an ultrasonic nebulizer, was assessed using both the snapshot and FOT methods. The aerosols were delivered for 10 s with ventilation at 90 br/min and a tidal volume of 3 ml/kg, after which the snapshot and FOT methods were applied consecutively every 6 s for 5 min. Peak responses during each 5-min period were determined, and only values with a coefficient of determination of 0.95 or greater were used (n = 10–12 in each group).
Statistical analysis
Data are shown as the mean ± SEM. The significance of differences among groups were determined using ANOVA with Dunnett’s test (Stat view version 5.0; Abacus Concepts Inc., Berkeley, CA). Values of p<0.05 were considered significant.
Results
The effects of ELT on airway inflammation
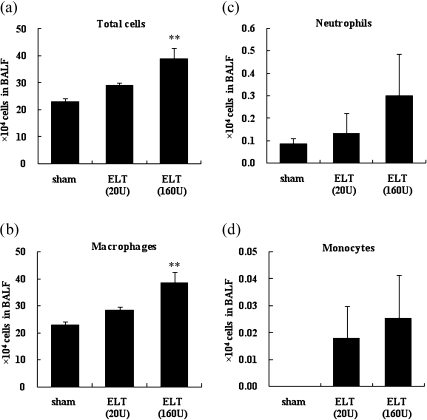
We examined the cellular profile in BALF 21 days after the intratracheal administration (Fig. 1). ELT increased the total number of cells and macrophages and tended to increase the numbers of neutrophils and monocytes as compared to the sham group (p<0.01 for total cells and macrophages in the ELT (160 U) group). ELT at a high dose was more effective than that at a low dose.
Fig. 1.
Cellularity in BALF after intratracheal challenge. BALF from each rat were collected 21 days after the intratracheal administration of ELT. Total cell counts were performed using a Berker-Tulk counting chamber. Differential cell counts were assessed in cytologic preparations stained with Diff-Quik. The results are means ± SEM (n = 6–8 in each group). (a) total cells, (b) macrophages, (c) neutrophils, (d) monocytes. **p<0.01 vs the sham group.
The effects of ELT on cytokine expression in the lung
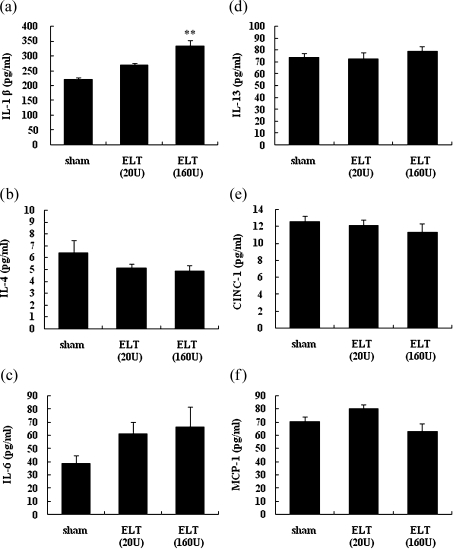
We examined the levels of proinflammatory cytokines and chemokines in lung tissue homogenates 21 days after the intratracheal administration of ELT (Fig. 2). ELT increased the level of IL-1β and tended to increase the level of IL-6 compared with the sham treatment (p<0.01 for IL-1β in ELT (160 U) group). ELT at a high dose was more effective than that at a low dose. The levels of the other cytokines were not altered by ELT treatment.
Fig. 2.
Cytokine and chemokine profiles in the lung after intratracheal challenge. Lung tissue samples of each rat were harvested 21 days after the intratracheal administration of ELT. The levels of IL-1β (a), IL-4 (b), IL-6 (c), IL-13 (d), CINC-1 (e), and MCP-1 (f) in the lung tissue supernatants were measured by ELISA. The results are means ± SEM (n = 6–8 in each group). **p<0.01 vs the sham group.
The effects of ELT on lung cell damage
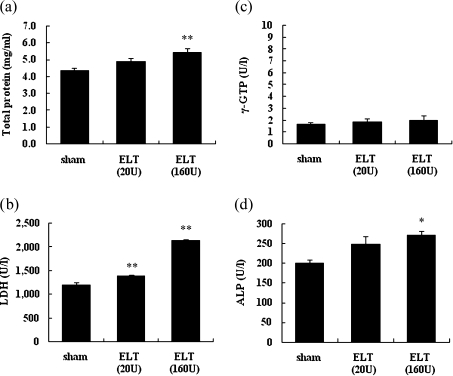
We examined the levels of total protein, LDH, γ-GTP, and ALP in the lung tissue homogenates 21 days after the intratracheal administration of ELT (Fig. 3). ELT increased the levels of total protein, ALP, and LDH compared with the sham treatment (p<0.01 for total protein in the ELT 160 U group and LDH in the ELT 20 U and 160 U groups; p<0.05 for ALP in the ELT 160 U group). ELT at a high dose was more effective than that at a low dose. The level of γ-GTP was low and was not altered by ELT treatment.
Fig. 3.
The levels of total protein, LDH, γ-GTP, and ALP in the lung after intratracheal challenge with ELT. The lung tissue samples of each rat were harvested 21 days after the intratracheal administration of ELT. The levels of total protein (a) LDH (b), γ-GTP (c), and ALP (d) in the lung tissue supernatants were measured. The results are means ± SEM (n = 6–8 in each group). *p<0.05 versus the sham group, **p<0.01 vs the sham group.
The effects of ELT on lung morphological changes
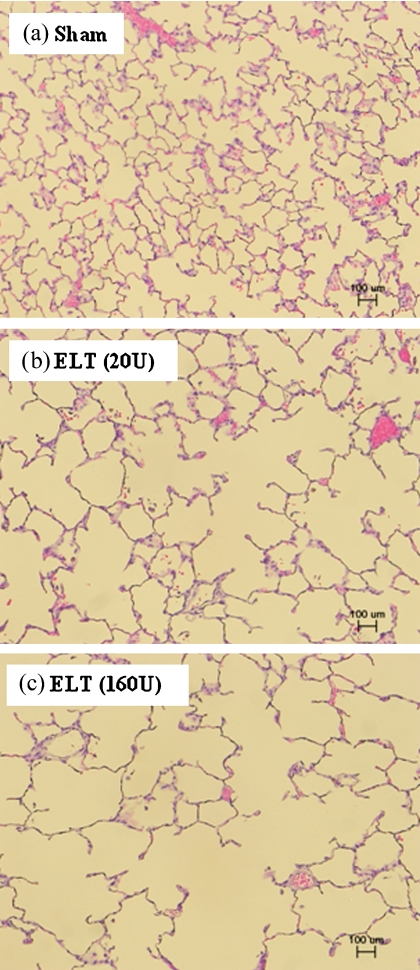
We evaluated lung specimens that had been stained with hematoxylin and eosin 21 days after the intratracheal administration of ELT (Fig. 4). No pathological changes were seen in the lung specimens obtained from the sham group. On the other hand, pathologic findings such as airspace enlargement and progressive destruction of alveolar wall structures were evident in the ELT-treated rats. ELT at a high dose was more effective than that at a low dose.
Fig. 4.
Microscopic findings in the lung after intratracheal challenge with ELT. Histologic changes in the lung of each rat were evaluated 21 days after the intratracheal administration of ELT. The sections were stained with hematoxylin and eosin, and histologic analyses were performed using a microscope (bar: 100 µm). (a) Sham group, (b) ELT 20 U group, (c) ELT 160 U group.
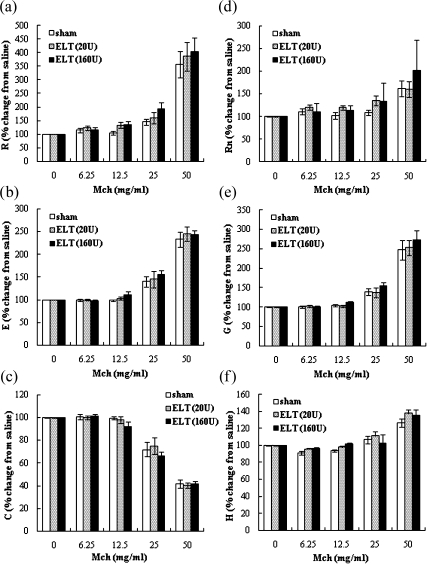
The effects of ELT on lung function
To evaluate the effects of ELT on lung function including responsiveness to Mch, we investigated R, E, C, Rn, G, and H 21 days after the intratracheal administration of ELT (Fig. 5). R, E, Rn, G, and H tended to be enhanced by ELT as compared to the sham treatment, although the enhancement did not reach statistical significance. ELT at a high dose was more effective than that at a low dose. C was not altered by ELT treatment.
Fig. 5.
Dose response curve of inhaled MCh after intratracheal challenge with ELT. Airway responsiveness/lung function to MCh, were measured by a flexiVent 21 days after the intratracheal administration of ELT. The parameters of R (a), E (b), and C (c) were measured by fitting the linear single-compartment model (using the snapshot method). The parameters of Rn (d), G (e), and H (f) were measured by fitting the constant phase model (using the FOT method). Data are shown as means ± SEM (n = 10–12 in each group).
Discussion
The present study has shown that intratracheal administration of ELT to rats induces/enhances 1) lung proinflammatory response, 2) lung cell damage, 3) emphysema–related lung morphological changes, and 4) airway responsiveness to MCh. This is the comprehensive study to extensively analyze an ELT-induced lung emphysema model.
Intratracheal administration of ELT reportedly induces acute lung inflammation [3, 6–8] followed by devastating emphysema [9–13] and compromised lung function [14, 15]. However, there have been few studies that have performed a comprehensive analysis of the above-mentioned parameters [3]. Thus, the first aim of this study was to characterize in detail the effects of ELT on proinflammatory response, damage, emphysematous change, and function including cholinergic hyperresponsiveness in the lung. As a result, we found an overall positive correlation among lung inflammatory response, emphysematous change, and cholinergic airway hyperresponsiveness. Previous reports have shown similar correlations [19, 20]. However, there are several differences between our study and the studies of others with respect to animal species/strains and ELT dose. Specifically, the mechanics and measuring methods for lung function were different. In the previous reports, lung function was only evaluated via the enhanced pause (Penh) index, which is measured using whole-body plethysmography. Penh does not measure lung mechanics directly. On the other hand, we evaluated lung function in open-chested rats that had received a tracheostomy on a flexiVent mechanical ventilator. Furthermore, Bellofiore’s and Scuri’s groups did not perform BAL or examine the lung expression of proinflammatory cytokines so as to strictly correlate lung inflammation with cholinergic airway hyperresponsiveness [19, 20]. Taken together, our study is novel in that performs a comprehensive analysis of ELT-induced emphysema in rats to examine/correlate lung inflammation/damage/structural change/function simultaneously. However, it is assumed that additional studies using larger number of rats are needed to ascertain which of these effects are significant.
The mechanisms by which ELT dose-dependently tends to enhance airway responsiveness to MCh remains unclear. By using the same emphysema model, Scuri et al. have shown that ELT causes bronchoconstriction possibly via a bradykinin-dependent pathway [20]. On the other hand, neutrophil elastase can induce airway smooth muscle cell proliferation in vitro [21]. In addition, IL-1β reportedly affects bronchoconstriction in asthmatic subjects [22, 23]. In the present study, IL-1β was increased in lung tissue homogenates by ELT and was linked to pathological structural changes and augmented cholinergic airway hyperresponsiveness. Furthermore, the increase was more effective at a high dose of ELT than at a low dose. Therefore, it can be proposed that ELT tended to enhance cholinergic airway reactivity through 1) bradykinin-dependent pathway, 2) promoted smooth muscle cell proliferation, 3) IL-1β-mediated pathway, and so on. Nevertheless, it should be clarified by additional research whether ELT significantly enhances airway reactivity against MCh and which mechanisms underlie the phenomenon.
Another aim of the current study was to explore a new biomarker for disease progression/exacerbation in the model. Pauluhn et al. [24, 25] implicated escaped enzymes in BALF in early pulmonary responses to chemical (polymeric diphenylmethane-4,4'-diisocyanate)-induced toxicity. Additionally, Cobben et al. [26] suggested that LDH activity in BALF is a diagnostic tool for infectious lung diseases. In the respiratory system, ALP is a marker for assessing the degree of pneumocyte type II cell stimulation, differentiation, and damage [25–27], and LDH is a marker of general cytotoxicity [25], and its elevation is associated with several pulmonary disorders [28]. However, as far as we have searched in Medline database, there are no reports that have measured these enzymes in an in vivo emphysema model. In the present study, we first demonstrated that activity of these two enzymes is elevated in lung homogenates by ELT. The elevation was more effective at a high dose of ELT than that at a low dose, which parallels the lung proinflammatory response and emphysematous change. Furthermore, previous studies [24, 25, 29] examined these levels only in BALF. Taken together, we suggest that LDH and ALP in lung tissue homogenates are candidates for new biomarkers of disease progression/deterioration in pulmonary emphysema. Moreover, the present results raise the possibility that this assay could be applied to other pathological pulmonary conditions in which epithelial damage is a central feature of the pathogenesis, e.g. acute lung injury, pulmonary fibrosis, viral pneumonia, particulate matters-induced lung toxicity, and so on.
In summary, the present extensive analysis has shown that intratracheal administration of ELT induces/enhances lung proinflammatory response, lung cell damage, emphysema-related lung morphological changes, and airway responsiveness to MCh. In addition, ELT is more effective at a higher dose. This is the novel study to examine ELT-induced pulmonary emphysema in a extensive manner in order to correlate these parameters, and this analysis might be applied to future evaluations of new therapeutic agents or risk factors, although additional research is required. In particular, ALP in lung homogenates might be an alternative biomarker for disease progression/exacerbation.
Acknowledgments
This study was partly supported by grants of National Institute for Environmental Studies. The authors thank to Miho Sakurai, Satomi Abe and Naoko Ueki for their technical assistance.
Abbreviations
- COPD
chronic obstructive pulmonary disease
- ELT
elastase
- BALF
bronchoalveolar lavage fluid
- ELISA
enzyme-linked immunosorbent assays
- IL
interleukin
- CINC
cytokine-induced neutrophil chemoattractant
- MCP
macrophage chemoattractant protein
- LDH
lactate dehydrogenase
- GTP
glutamyltranspeptidase
- ALP
alkaline phosphatase
- FOT
forced oscillation technique
- MCh
methacholine
- R
resistance
- E
elastance
- C
compliance
- Rn
Newtonian resistance
- G
tissue damping
- H
tissue elastance
- Penh
enhanced pause
References
- 1.Croxton T.L., Weinmann G.G., Senior R.M., Hoidal J.R. Future research directions in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002;165:838–844. doi: 10.1164/ajrccm.165.6.2108036. [DOI] [PubMed] [Google Scholar]
- 2.Mannino D.M., Buist A.S. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 3.Birrell M.A., Wong S., Hele D.J., McCluskie K., Hardaker E., Belvisi M.G. Steroid-resistant inflammation in a rat model of chronic obstructive pulmonary disease is associated with a lack of nuclear factor-kappaB pathway activation. Am. J. Respir. Crit. Care Med. 2005;172:74–84. doi: 10.1164/rccm.200409-1257OC. [DOI] [PubMed] [Google Scholar]
- 4.Groneberg D.A., Chung K.F. Models of chronic obstructive pulmonary disease. Respir. Res. 2004;5:18. doi: 10.1186/1465-9921-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandl I., Darnule T.V., Fierer J.A., Keller S., Turino G.M. Elastin degradation in human and experimental emphysema. Adv. Exp. Med. Biol. 1977;79:221–231. doi: 10.1007/978-1-4684-9093-0_19. [DOI] [PubMed] [Google Scholar]
- 6.Dubaybo B.A., Crowell L.A., Thet L.A. Changes in tissue fibronectin in elastase induced lung injury. Cell Biol. Int. Rep. 1991;15:675–686. doi: 10.1016/0309-1651(91)90068-t. [DOI] [PubMed] [Google Scholar]
- 7.Lucey E.C., Keane J., Kuang P.P., Snider G.L., Goldstein R.H. Severity of elastase-induced emphysema is decreased in tumor necrosis factor-alpha and interleukin-1beta receptor-deficient mice. Lab. Invest. 2002;82:79–85. doi: 10.1038/labinvest.3780397. [DOI] [PubMed] [Google Scholar]
- 8.Hayes J.A., Korthy A., Snider G.L. The pathology of elastase-induced panacinar emphysema in hamsters. J. Pathol. 1975;117:1–14. doi: 10.1002/path.1711170102. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn C. 3rd, Tavassoli F. The scanning electron microscopy of elastase-induced emphysema. A comparison with emphysema in man. Lab. Invest. 1976;34:2–9. [PubMed] [Google Scholar]
- 10.Kaplan P.D., Kuhn C., Pierce J.A. The induction of emphysema with elastase. I. The evolution of the lesion and the influence of serum. J. Lab. Clin. Med. 1973;82:349–356. [PubMed] [Google Scholar]
- 11.Snider G.L., Lucey E.C., Stone P.J. Animal models of emphysema. Am. Rev. Respir. Dis. 1986;133:149–169. doi: 10.1164/arrd.1986.133.1.149. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn C., Yu S.Y., Chraplyvy M., Linder H.E., Senior R.M. The induction of emphysema with elastase. II. Changes in connective tissue. Lab. Invest. 1976;34:372–380. [PubMed] [Google Scholar]
- 13.Miyazaki N., Takamoto M., Kinjo M., Ishibashi T. Ultrastructural studies of elastase-induced experimental emphysema. Jpn. J. Exp. Med. 1979;49:241–250. [PubMed] [Google Scholar]
- 14.Tepper J., Pfeiffer J., Aldrich M., Tumas D., Kern J., Hoffman E., McLennan G., Hyde D. Can retinoic acid ameliorate the physiologic and morphologic effects of elastase instillation in the rat? Chest. 2000;117:242S–244S. doi: 10.1378/chest.117.5_suppl_1.242s. [DOI] [PubMed] [Google Scholar]
- 15.Mao J.T., Goldin J.G., Dermand J., Ibrahim G., Brown M.S., Emerick A., McNitt-Gray M.F., Gjertson D.W., Estrada F., Tashkin D.P., Roth M.D. A pilot study of all-trans-retinoic acid for the treatment of human emphysema. Am. J. Respir. Crit. Care Med. 2002;165:718–723. doi: 10.1164/ajrccm.165.5.2106123. [DOI] [PubMed] [Google Scholar]
- 16.Inoue K., Takano H., Yanagisawa R., Sakurai M., Abe S., Yoshino S., Yamaki K., Yoshikawa T. Effects of nanoparticles on lung physiology in the presence or absence of antigen. Int. J. Immunopathol. Pharmacol. 2007;20:737–744. doi: 10.1177/039463200702000409. [DOI] [PubMed] [Google Scholar]
- 17.Inoue K., Takano H., Ichinose T., Tomura S., Yanagisawa R., Sakurai M., Sumi D., Cho A.K., Hiyoshi K., Kumagai Y. Effects of naphthoquinone on airway responsiveness in the presence or absence of antigen in mice. Arch. Toxicol. 2007;81:575–581. doi: 10.1007/s00204-007-0186-5. [DOI] [PubMed] [Google Scholar]
- 18.Inoue K., Takano H., Yanagisawa R., Sakurai M., Abe S., Yoshino S., Yamaki K., Yoshikawa T. Effects of components derived from diesel exhaust particles on lung physiology related to antigen. Immunopharmacol. Immunotoxicol. 2007;29:403–412. doi: 10.1080/08923970701675002. [DOI] [PubMed] [Google Scholar]
- 19.Bellofiore S., Eidelman D.H., Macklem P.T., Martin J.G. Effects of elastase-induced emphysema on airway responsiveness to methacholine in rats. J. Appl. Physiol. 1989;66:606–612. doi: 10.1152/jappl.1989.66.2.606. [DOI] [PubMed] [Google Scholar]
- 20.Scuri M., Forteza R., Lauredo I., Sabater J.R., Botvinnikova Y., Allegra L., Abraham W.M. Inhaled porcine pancreatic elastase causes bronchoconstriction via a bradykinin-mediated mechanism. J. Appl. Physiol. 2000;89:1397–1402. doi: 10.1152/jappl.2000.89.4.1397. [DOI] [PubMed] [Google Scholar]
- 21.Huang C.D., Chen H.H., Wang C.H., Chou C.L., Lin S.M., Lin H.C., Kuo H.P. Human neutrophil-derived elastase induces airway smooth muscle cell proliferation. Life Sci. 2004;74:2479–2492. doi: 10.1016/j.lfs.2003.07.059. [DOI] [PubMed] [Google Scholar]
- 22.Matera M.G., Calzetta L., Peli A., Scagliarini A., Matera C., Cazzola M. Immune sensitization of equine bronchus: glutathione, IL-1beta expression and tissue responsiveness. Respir. Res. 2005;6:104. doi: 10.1186/1465-9921-6-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hakonarson H., Herrick D.J., Serrano P.G., Grunstein M.M. Autocrine role of interleukin 1beta in altered responsiveness of atopic asthmatic sensitized airway smooth muscle. J. Clin. Invest. 1997;99:117–124. doi: 10.1172/JCI119122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pauluhn J. Acute inhalation toxicity of polymeric diphenyl-methane 4,4'-diisocyanate in rats: time course of changes in bronchoalveolar lavage. Arch. Toxicol. 2000;74:257–269. doi: 10.1007/s002040000114. [DOI] [PubMed] [Google Scholar]
- 25.Pauluhn J., Emura M., Mohr U., Popp A., Rosenbruch M. Two-week inhalation toxicity of polymeric diphenylmethane-4,4'-diisocyanate (PMDI) in rats: analysis of biochemical and morphological markers of early pulmonary response. Inhal. Toxicol. 1999;11:1143–1163. doi: 10.1080/089583799196637. [DOI] [PubMed] [Google Scholar]
- 26.Edelson J.D., Shannon J.M., Mason R.J. Alkaline phosphatase: a marker of alveolar type II cell differentiation. Am. Rev. Respir. Dis. 1988;138:1268–1275. doi: 10.1164/ajrccm/138.5.1268. [DOI] [PubMed] [Google Scholar]
- 27.Henderson R.F., Scott G.G., Waide J.J. Source of alkaline phosphatase activity in epithelial lining fluid of normal and injured F344 rat lungs. Toxicol. Appl. Pharmacol. 1995;134:170–174. doi: 10.1006/taap.1995.1181. [DOI] [PubMed] [Google Scholar]
- 28.Drent M., Cobben N.A., Henderson R.F., Jacobs J.A., Wouters E.F., van Dieijen-Visser M.P. BAL fluid LDH activity and LDH isoenzyme pattern in lipoid pneumonia caused by an intravenous injection of lamp oil. Eur. Respir. J. 1996;9:2416–2418. doi: 10.1183/09031936.96.09112416. [DOI] [PubMed] [Google Scholar]
- 29.Cobben N.A., Jacobs J.A., van Dieijen-Visser M.P., Mulder P.G., Wouters E.F., Drent M. Diagnostic value of BAL fluid cellular profile and enzymes in infectious pulmonary disorders. Eur. Respir. J. 1999;14:496–502. doi: 10.1034/j.1399-3003.1999.14c04.x. [DOI] [PubMed] [Google Scholar]