Abstract
A structure-activity relationship study was carried out on a series of thiohydantoins and their analogues 14 which led to the discovery of 92 (MDV3100) as the clinical candidate for the treatment of hormone refractory prostate cancer.
Introduction
Although prostate cancer can be initially treated with either castration or androgen receptor (AR) antagonists such as bicalutamide 1, nilutamide 2, and flutamide 3a (which is oxidized to the active metabolite hydroxyflutamide 3b), after a period of approximately 2–4 years, the cancer becomes resistant to such treatment (Scheme 1).1 Indeed in this castration resistant stage (formerly called hormone refractory or ‘androgen-independent’), former AR antagonists such as bicalutamide become partial agonists and their use in cancer treatment must be discontinued. Sawyers and coworkers showed that a 3–5 fold upregulation of the androgen receptor was the likely cause of the resistance to anti-androgens.2 They further demonstrated that castration resistant prostate cancer was still dependent on the ligand binding domain of AR for growth.2 Therefore we began a research program aimed at the identification of novel chemical structures which would be potent androgen receptor antagonists, especially in its upregulated state in castration resistant disease, without any significant agonist effect. We report here the results of our structure-activity relationship (SAR) study which led to the choice of 92 as the lead candidate for the treatment of castration-resistant prostate cancer (CRPC). This compound, named MDV3100, has completed phase 1–2 clinical trials and has now entered a phase 3 randomized trial for drug registration.3,4
Scheme 1.
We examined the literature on the binding of various compounds to the AR5 and the available crystal structures of the AR6 (there were only structures of the AR with compounds in an agonist binding mode)7 and binding calculations.8 We decided to begin with the structure of one of the strongest known binders to the AR, namely the non-steroidal AR agonist RU59063 4, the affinity of which for the AR is nearly equal to that of the well known steroidal agonist R1881 5, both of which are slightly higher than that of the natural ligand dihydrotestosterone 6 (DHT) (Scheme 2).9 Our plan was to vary systematically the structural units of this strong-binding agonist to see if we could obtain a reasonably strong-binding antagonist. We prepared several series of compounds in which each of the functional groups of this molecule were varied and measured the binding affinity and both the agonism and antagonism of each.
Scheme 2.
Synthesis
The syntheses of the compounds varied somewhat but usually involved three general routes. The first (Scheme 3) was a triply convergent process involving first a Strecker reaction of a substituted amine or aniline 7 with a ketone 8 and trimethylsilyl cyanide (or the preformed cyanohydrin 9) to generate the cyanoamine 10. The third component, the isothiocyanate 12, prepared usually in quantitative yield from the amine 11, was added to 10 to give the thiohydantoin-4-imine 13 (in which the group on the imine nitrogen could be either hydrogen of a thiocarbamoyl group derived from a second equivalent of the isothiocyanate). Hydrolysis of 13 afforded the desired thiohydantoins 14. A second general method of synthesis (Scheme 4) utilized an N1-unsubstituted thiohydantoin 15 (prepared from the ketone 8 with ammonium cyanide and hydrolysis) which was added to any of several 4-halo aromatic systems 16, e.g., X = F, Z = CN, NO2, etc., to give the 4-substituted phenyl thiohydantoins 17. Finally several additional analogues 19 could be prepared by diazotiz-ation of 4-aminophenyl thiohydantoins 18 and substitution with various groups, e.g., halogens, cyano, etc. (Scheme 5).
Scheme 3.
Scheme 4.
Scheme 5.
Testing Methods
Several systems were utilized to test the activity of the analogues. We used a prostate specific antigen (PSA) expression readout for normal LNCaP (hormone sensitive) cells and in LNCaP/AR cells, which were engineered (using viral infection with a cDNA encoding for the AR) to express 3–5 fold higher levels of the AR to mimic the clinical setting of CRPC.4 Tests in LNCaP cells were carried out in the presence of fetal bovine serum (FBS), whereas tests in LNCaP/AR cells were carried out in charcoal stripped serum to mimic the androgen depleted, castration resistant state. We also developed a luciferase reporter system utilizing ARR2PB-Luc, a piece of plasmid DNA that encodes firefly luciferin with AR binding sites in the natural promoter for probasin of rat prostate, which provides an easy quantitative assay for AR activity as a transcription factor.
Structure-Activity Relationship
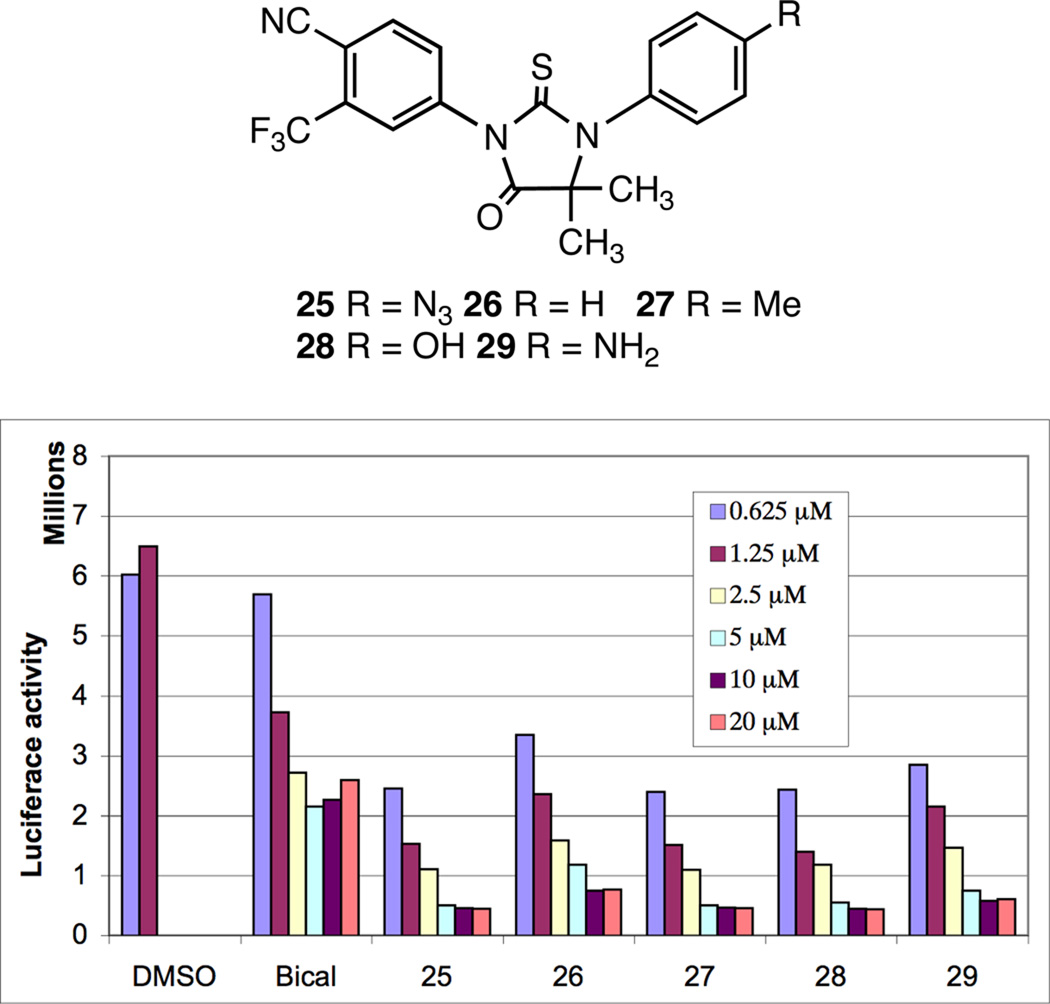
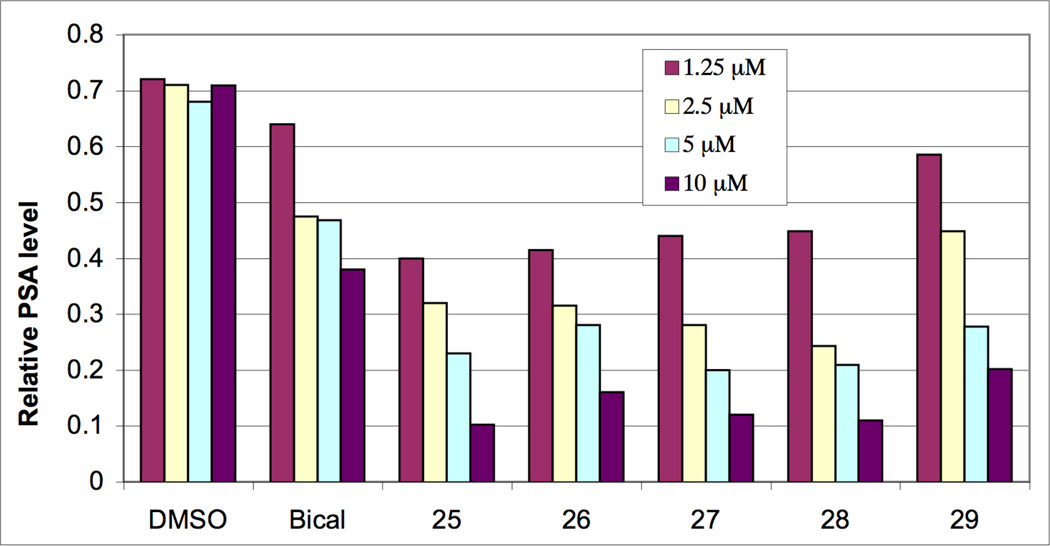
The first set of analogues prepared were analogues with azidoalkyl and azidoaryl groups at N1, 20–24 and 2510 with the hope that the small polar azide group might mimic the hydroxyl in 4 and give good binding. The activity vs normal LNCaP (hormone sensitive) cells was measured as relative prostate specific antigen (PSA) level vs. vehicle (DMSO) and using bicalutamide as a standard for antagonist activity in this androgen-dependent assay (Scheme 6). It can be seen that all six compounds had activity better than bicalutamide itself but that 25 (the 4-azidophenyl compound) was the best of this group. We next varied the group at the 4-position of the N1-phenyl ring and again all of the analogues, 25–29,11 were active (Scheme 7), both by the luciferase reporter assay and by relative PSA level. We then kept a methyl group as the substituent at the 4-position of the phenyl ring and varied the alkyl groups in the thiohydantoin ring from hydrogen to methyl, ethyl, and propyl. The activity was measured as relative luciferase activity vs. bicalutamide and 27 as standards (Scheme 8). All of these derivatives were much less active than the dimethyl compound 27, without significant differences between the hydrido analogues 30–33 and the dialkyl analogues, 34–35.11 Analogues with the alternative arrangement of the dialkyl substituents and the carbonyl (equivalent to switching the nitrogen substituents), 36–37,11 also had reduced activity relative to 27. However there was little difference in activity among the analogues. We next prepared a set of analogues (Scheme 9) featuring cycloalkyl substituents on the thiohydantoin ring, all of which were made from the corresponding ketones. Their activity was measured by the relative PSA expression levels vs bicalutamide and 27 as standards. All of these derivatives showed good activity with the cyclobutyl and cyclopentyl analogues, 38 and 39,11 being comparable to the dimethyl analogue 27. The 6-, 7-, and 8-membered rings, 40–4211 were slightly less active. The spiro N-methylpiperidine analogue 4311 was distinctly less active, which may be due to the fact that the nitrogen would likely be charged at cellular pH. We also tested several other aromatic rings and substitution patterns on the N1-aryl substituent (Scheme 10) using 27 as the standard. A compound with a methyl group at the 2-position (ortho to the thiohydantoin nitrogen), 44,11 was active while one with a charged carboxylic acid group at the 2-position, 45,11 was inactive. The compound with chloromethyl groups at C5 4611 had decreased activity while the analogue with a fluoromethyl group 4711 had good activity. Other functional groups were placed at the 4-position of the N1-phenyl substituent and their activity was assayed using a relative PSA readout vs standards (Scheme 11). Nearly all of the substituents showed good activity when compared to the best molecules in their series, e.g., the 4-phenyl and 4-hydroxy analogues, 48–49,11 were very similar in activity to 38. Similarly the 4-cyano and 4-nitro analogues, 50–51,11 were nearly as active as the methyl analogue 39, while the 4-trifluoromethyl analogue 5211 was slightly more active than the methyl analogue 27. Thus the 4-position of the 1-phenyl ring can bear many different substituents without losing activity. We also prepared and tested various imine and thione analogues of the active series using a relative PSA readout vs 27 and 38 as standards (Scheme 12). In all cases the thiohydantoins with the thiocarbonyl at C2 and the carbonyl at C4 were the most active although the imines, 53–54,11 were nearly as active as the parent compounds 27 and 38 while the dithiohydantoin and the hydantoin analogues, 55–56,11 were not as active as the parent thiohydantoin 28. Other imine derivatives, e.g., 57 and 60,11 were also somewhat active as shown in Scheme 13, using both relative PSA levels and the luciferase assay vs 21 and 22 as standards. The N-thiocarbamoyl analogues 58–6211 were prepared by using an excess of the isothiocyanate, e.g., 12, in the coupling reaction with the corresponding aniline. They were all active, but they are hydrolyzed to the corresponding thiohydantoins under cellular conditions. The condensation of the cyano-amine 10 with the isothiocyanate 12 gave a small amount of a new chemical entity, namely the thiazolidine-2,5-dimines, in which the 5-imino anion bears a thiocarbamoyl group. These compounds are presumably formed by attack of the sulfur atom, rather than the nitrogen atom, of the thioamide anion on the nitrile of the cyanoamine with subsequent trapping of that imine anion with another equivalent of the isothiocyanate. Two of these new analogues, 63–64,11 were tested for activity using both the relative PSA levels and the luciferase reporter assay (Scheme 14). Both of these new compounds showed excellent activity comparable to their parent thiohydantoin analogues 27 and 38. As far as we can tell, this is the first time any compounds with such a structure have been shown to have antiandrogen activity. Therefore these compounds represent a new structural class of antiandrogens. Since the activity of these two new compounds are extremely similar to their thiohydantoin counterparts in both the PSA and luciferase assay systems, it is interesting to speculate whether they are being converted, under the cellular testing conditions, into the thiohydantoins. That would require cleavage of the imino thiourea to the imine, opening of the ring via reformation of the nitrile with ejection of the thiolate anion, and then recyclization of the thioamide anion on to the nitrile via the nitrogen atom. Finally in our early set of compounds, we found several that were essentially devoid of activity (Scheme 15). Various derivatives of the nitrile, e.g., the amide 65,11 were inactive as were various analogues in which the ortho trifluoromethyl group was replaced by halogens, 66–69.11 Acyclic analogues, e.g., the chloromethylamide 70,11 were inactive as was the 4-oxooxazolidine-2-thione 71.11 Compounds with a spacer group between the ring nitrogen and the aryl group, e.g., the arylsulfonylamide 72 and the benzhydryl analogue 73,11 were inactive.
Scheme 6.
Scheme 7.
Scheme 8.
Scheme 9.
Scheme 10.
Scheme 11.
Scheme 12.
Scheme 13.
Scheme 14.
Scheme 15.
While carrying out this SAR study using in vitro assays, we also decided to test the in vivo activity of lead compounds in animals to gauge their pharmacologic properties and ability to impair growth of castration-resistant prostate cancer xenograft models that are also resistant to bicalutamide. Therefore the ability of compounds 27 and 38 to decrease the growth of LAPC4/AR cells or LNCaP/AR cells grown as xenografts in castrate SCID mice was assayed. In a pilot experiment using the LAPC4/AR xenograft model (Figure 1),12 both compounds were more effective than bicalutamide with 38 being slightly superior, with an IC50 value of 124 nM for inhibition of PSA secretion. This thiohydantoin 38 also showed a good dose response in the castration-resistant xenograft assay (Figure 2). However, 38 had a short half-life with a very rapid clearance as shown in Figure 3. This was likely due to both a rapid metabolism (hydroxylation of the aromatic methyl group) and its relatively high c log P value of 4.20 (compared to 2.91 for bicalutamide). Therefore we decided to prepare additional analogues of 38 which would be more polar. In particular we decided to change the substituents on the aryl ring attached to N1, especially at the 4-position, to see if more polar and more stable analogues could be prepared. Therefore several simple analogues of 38 were prepared (Scheme 16), all of which showed good activity in the PSA secretion assay. The two benzylic alcohol analogues 74 and 75 as well as the aldehyde 76 exhibited IC50’s of 200–300 nM but were considerably more polar than 38. The extended amide and alcohol analogues 77–78 were even more active with IC50’s of 100–150 nM. A series of analogues with extended chains and heterocyclic units were prepared and their activity using PSA levels in the hormone refractory cell line LNCaP/AR was evaluated in vitro (Scheme 17). All the derivatives, with the exception of the (hydroxyethyl)amide and piperazine analogues 83 and 85, showed good activity, especially compared to bicalutamide which is inactive in this hormone-refractory assay. The most active compound was the N-methylbutyramide analogue 80, which was determined to have an IC50 of 92 nM with a c log P of 3.44. But as the data shows, several other analogues were also quite active with the N-methyl amides being generally more active than the amide themselves. The corresponding esters and acids were also prepared as well as the analogous phenylacetamide derivatives (two carbon chain) but the activity of all of these was weaker (data not shown). Although 80 showed excellent activity, its PK was also poor (Figure 4), although it was somewhat more available than the earlier analogue 38. Although hydrolysis of the N-methyl amide to the acid was seen, we postulated that one reason for the low serum concentration of both 38 and 80 was metabolism via oxidation of the electron-rich aromatic ring. To try to eliminate this problem, we decided to prepare analogues which had the electron-withdrawing group attached directly to the aromatic ring (Scheme 18), which yielded several very active compounds based on evaluation in the hormone refractory LNCaP/AR assay. Thus the sulfone 86, the ester 87, and the two amides 88–89 showed good activity as did the fluorophenol 90. However we found that the 3-fluoro amide analogue 91 (also called RD162)4,13 had not only excellent activity (Scheme 19) but also a superb pharmacokinetic (PK) profile. Its IC50 was 122 nM and it had a c log P of 3.20. However, the measure of its excellent PK profile was its steady state concentration as shown in Table 1. Compound 91 has almost the exact same exposure after a 10 mg/kg dose as bicalutamide, e.g., 9.9 mM vs 10 mM. And the IC50 of 91 is nearly 8 times lower than that of bicalutamide, 122 nM vs 1 mM. The concentration of 91 after iv and oral administration is shown in Figure 5. With this excellent PK profile, we decided to choose 91 as our lead drug candidate. Its activity on LNCaP/AR (HR) tumor size at 10 and 50 mg/kg once a day vs bicalutamide at the same dose (Figure 6) shows it to be very active. It is cytostatic at these doses. The dose response of 91 in LNCaP xenografts14 (Figure 7) shows that at least 1 mg/kg/day is required and that 10 mg/kg/day is optimal. We also assayed the activity of 91 on LNCaP xenografts over an extended period (Figure 8) which showed that it retains activity at 10 mg/kg/day for 31 days. Since 91 was initially screened in hormone-refractory models, we also looked at its effect in hormone sensitive cells 15 (Scheme 20) and found good activity vs LNCaP cells, albeit not as good as 80. But this relative liability is counterbalanced by its excellent PK properties. Thus it is possible that 91 might be able to be used for treatment of both types of prostate cancer, hormone sensitive as well as castration-resistant, but one must wait for data from clinical trials.
Figure 1.
Fold change in tumor volume of xenografts with bical, 27 and 38 (10 mg/kg)
Figure 2.
Dose response in change in tumor volume of xenografts with 38
Figure 3.
Serum concentration of 22 after IV injection n=3 mice
Scheme 16.
Scheme 17.
Figure 4.
PK curves of 38 and 80
Scheme 18.
Scheme 19.
Table 1.
| compd | IC50 (nM) | log P | Css 10 mpk (mM) |
|---|---|---|---|
| Bicalutamide (1) | 1000 | 2.91 | 10.0 |
| 38 | 124 | 4.20 | NA |
| 80 | 92 | 3.44 | 0.39 |
| 91 | 122 | 3.20 | 9.9 |
Figure 5.
Concentration of 91 after IV (blue) or oral (pink) administration
Figure 6.
Effect of bicalutamide and 91 on LNCaP/AR (HR) tumor size at 10 and 50 mg/kg once a day
Figure 7.
Dose response in tumor volume change of xenografts with 91 at 0.1, 1, and 10 mg/kg once a day
Figure 8.
Effect of change in tumor volume of xenografts with 91 at 10 mg/kg once a day
Scheme 20.
Effect of 38, 80, and 91 on hormone sensitive LNCaP cells
Several additional analogues of both 80 and 91 were prepared and tested (Scheme 21). We made both the dimethyl amide and the nitrile analogues of 80, 94–95,13 respectively, in order to try to identify a strong-binding analogue with better PK and, in particular, a longer half-life. Both of these compounds had quite good activity compared to earlier compounds. Two additional analogues of 91 were prepared and tested for their activity on castration resistant prostate cancer, namely the analogues with the cyclobutyl unit replaced by dimethyl 92 and cyclopentyl units 93.13 Both of these new analogues were very active in hormone-refractory LNCaP/AR with essentially the same activity as 91. A dose-response study (Figure 9) showed 92 to be a little more active than 91. Since the dimethyl analogue 92 offers the great advantage of an inexpensive starting material, acetone or its cyanohydrin, for its production, it was chosen as the drug candidate and subjected to metabolic stability, toxicology, and further animal studies. This compound 92 (also called RD162’) was licensed by Medivation, Inc. It has now entered phase 3 clinical trials for the treatment of castration-resistant prostate cancer.4 Further details on this compound will be reported in due course.
Scheme 21.
Figure 9.
Dose-response study of 91 and 92 (nM) on castration resistant LNCaP AR cells
Conclusion
We have described the structure-activity relationship study that led to the choice of 92 as a clinical candidate for the treatment of castration-resistant prostate cancer. Many analogous diarylthiohydantoins in this series showed good androgen receptor antagonism with essentially no agonism but the pharmacokinetic properties of 91 and its close analogues 92 and 93 led to the choice of 92 as the clinical candidate.
Experimental
General
All reactions were carried out under an argon atmosphere unless otherwise specified. Tetrahydrofuran (THF) and diethyl ether were distilled from benzoquinone ketyl radical under an argon atmosphere. Dichloromethane, toluene, benzene, pyridine, triethylamine, and diisopropylethylamine (DIPEA) were distilled from calcium hydride under an argon atmosphere. Dimethyl sulfoxide (DMSO) was distilled over calcium hydride and stored over 4 Å molecular sieves. All other solvents or reagents were purified according to literature procedures. 1H NMR and 13C NMR spectra were obtained on ARX-400, ARX-500 or Avance-500 spectrometers. The chemical shifts are reported in parts per million (ppm, δ). The coupling constants are reported in Hertz (Hz) and the resonance patterns are reported with notations as the following: br (broad), s (singlet), d (double), t (triplet), q (quartet) and m (multiplet). Infrared spectra were recorded on Nicolet 501 or Nicolet AVATAR 370 instruments using liquid films (neat) or in CDCl3 solution on NaCl plates and only the significant absorption bands are recorded (in cm−1). Thin-layer chromatography (TLC) was carried out using precoated silica gel sheets (Merck 60 F254). Visual detection was performed with ultraviolet light, p-anisaldehyde stain, potassium permanganate stain or iodine. Flash chromatography was performed using SilicaFlash™ P60 (60 Α, 40–63 µm) silica gel from SiliCycle Inc. with compressed air. HPLC was performed on a Waters HPLC using either a C18 reverse phase column or a normal silica gel column as appropriate. The purity of all final compounds was established to be at least 95% pure by a combination of TLC Rf values in several solvent systems and HPLC. Additionally the absence of any extraneous peaks in the proton NMR spectrum confirmed the high level of purity.
Synthesis of 20–24
4-Isothiocyanato-2-trifluoromethylbenzonitrile, 12a
4-Amino-2-trifluoromethylbenzonitrile (2.23 g, 12 mmol) was added portionwise over 15 min into a well-stirred heterogeneous mixture of thiophosgene (1 mL, 13 mmol) in water (22 mL) at 21 °C. Stirring was continued for an additional 1 h. The reaction medium was extracted with chloroform (3 × 15 mL). The combined organic phase was dried over MgSO4 and evaporated to dryness under reduced pressure to yield desired product as brownish solid and was used as such for the next step (2.72 g, 11.9 mmol, 99%).
1,4-Diazidobutane, 20a
To a mixture of 1,4-dibromobutane (21.6 g, 100 mmol) in DMF (100 mL) was added an aqueous solution of sodium azide (13.65 g, 210 mmol in 50 mL of water). The mixture was stirred and heated to 80 °C for 20 h, and then the medium was washed with brine (200 mL) extracted with hexane (3 × 300 mL). The combined organic layer was dried over MgSO4 and concentrated to yield 1,4-diazidobutane as a liquid (13.72 g, 9.8 mmol, 98%).
4-Azidobutylamine, 20b
To a mixture of 1,4-diazidobutane 20a (4.20 g, 30 mmol), aq. 1 M HCl (60 mL), diethyl ether (20 mL) and ethyl acetate (20 mL) cooled to 0 °C was added triphenylphos-phine portionwise during 1 h. The mixture was warmed to 21 °C and stirred for an additional 20 h and then the organic layer was separated from the aqueous layer. The aqueous phase was washed with ethyl ether (2 × 50 mL) to remove triphenylphosphine oxide residual. The aqueous phase was basified to a pH 13 by aq. NaOH and then was extracted with dichloromethane (3 × 100 mL). The combined dichloromethane layer was dried over MgSO4 and concentrated to yield 4-azidobutylamine (2.74 g, 24 mmol, 80%) as a liquid.
2-(4-Azidobutylamino)-2-methylpropionitrile, 20c
A mixture of 4-azidobutylamine 20b (0.57 g, 5 mmol), acetone cyanohydrin (0.425 g, 5 mmol) and Na2SO4 (0.2 g) was stirred at 21 °C for 12 h. The mixture was diluted with hexane and filtered off. The filtrate was concentrated to yield 2-(4-azidobutylamino)-2-methylpropionitrile (0.896 g, 4.95 mmol, 99%) as a liquid.
4-[3-(4-azidobutyl)-5-imino-4,4-dimethyl-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 20d
A mixture of isothiocyanate 12a (0.684 g, 3 mmol), 20c (0.543 g, 3 mmol) and triethylamine (0.04 g, 0.4 mmol) in THF (6 mL) was refluxed for 1 h. The medium was concentrated and chromatographed (dichloromethane:acetone, 6:1) to obtain 20d (0.834 g, 2.04 mmol, 68%) as an off-white solid.
4-[3-(4-Azidobutyl)-4,4-dimethyl-5-oxo-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 20
The mixture of 20d (0.818 g, 2.0 mmol), aq. 2 M HCl (5 mL) and methanol (20 mL) was heated to reflux for 1 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (25 mL) and extracted with dichloromethane (50 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane/acetone, 9:1) to yield 20 (0.803 g, 1.96 mmol, 98%) as an off-white solid. 1H NMR (400 MHz, CDCl3) δ 1.58 (s, 6H), 1.63–1.71 (m, 2H), 1.88–1.96 (m, 2H), 3.37 (t, J = 6.6 Hz, 2H), 3.71 (t, J = 8.1 Hz, 2H), 7.77 (dd, J = 8.2, 1.8 Hz, 1H), 7.89 (d, J = 1.8 Hz, 1H), 7.94 (d, J = 8.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 23.2, 25.5, 26.4, 43.7, 50.9, 65.1, 109.9, 114.9, 121.9 (q, J = 272.6 Hz), 127.0 (q, J = 4.9 Hz), 132.1, 133.4 (q, J = 33.0 Hz), 135.1, 137.1, 175.2, 178.4.
The same procedure was applied for the synthesis of 21–24.
4-[3-(3-Azidopropyl)-4,4-dimethyl-5-oxo-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 21
1H NMR (400 MHz, CDCl3) δ 1.51 (s, 6H), 2.01–2.08 (m, 2H), 3.38 (t, J = 6.4 Hz, 2H), 3.71 (t, J =7.8 Hz, 2H), 7.74 (dd, J = 8.2, 1.8 Hz, 1H), 7.87 (d, J = 1.8 Hz, 1H), 7.89 (d, J = 8.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 22.8, 27.4, 41.6, 49.0, 65.2, 109.6, 114.9, 120.0 (q, J = 272.4 Hz), 127.0 (q, J = 4.9 Hz), 132.3, 132.9 (q, J = 33.0 Hz), 135.2, 137.3, 175.1, 178.5.
4-[3-(5-Azidopentyl)-4,4-dimethyl-5-oxo-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 22
1H NMR (400 MHz, CDCl3) δ 1.44–1.50 (m, 2H), 1.56 (s, 6H), 1.61–1.67 (m, 2H), 1.80–1.86 (m, 2H), 3.27 (t, J = 6.7 Hz, 2H), 3.67 (t, J = 8.2 Hz, 2H), 7.77 (dd, J = 8.2, 1.8 Hz, 1H), 7.88 (d, J = 1.8 Hz, 1H), 7.92 (d, J = 8.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 23.1, 24.2, 27.6, 28.3, 44.0, 51.2, 65.1, 109.8, 114.9, 121.9 (q, J = 272.5 Hz), 127.0 (q, J = 4.9 Hz), 132.2, 133.2 (q, J = 33.0 Hz), 135.1, 137.2, 175.2, 178.3.
4-[3-(6-Azidohexyl)-4,4-dimethyl-5-oxo-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 23
1H NMR (400 MHz, CDCl3) δ 1.31–1.41 (m, 4H), 1.51 (s, 6H), 1.52–1.59 (m, 2H), 1.74–1.81 (m, 2H), 3.20 (t, J = 6.7 Hz, 2H), 3.62 (t, J = 8.2 Hz, 2H), 7.75 (dd, J = 8.2, 1.8 Hz, 1H), 7.88 (d, J = 1.8 Hz, 1H), 7.90 (d, J = 8.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 22.9, 26.1, 26.5, 27.8, 28.6, 44.1, 51.2, 65.1, 109.5, 114.9, 122.0 (q, J = 272.5 Hz), 127.0 (q, J = 4.9 Hz), 132.2, 133.2 (q, J = 33.0 Hz), 135.1, 137.3, 175.2, 178.1.
4-[3-(7-Azidoheptyl)-4,4-dimethyl-5-oxo-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 24
1H NMR (400 MHz, CDCl3) δ 1.30–1.42 (m, 6H), 1.53 (s, 6H), 1.54–1.59 (m, 2H), 1.74–1.81 (m, 2H), 3.21 (t, J = 6.7 Hz, 2H), 3.64 (t, J = 8.2 Hz, 2H), 7.76 (dd, J = 8.2, 1.8 Hz, 1H), 7.88 (d, J = 1.8 Hz, 1H), 7.91 (d, J = 8.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 23.0, 26.5, 26.6, 26.8, 27.9, 28.6, 44.2, 51.3, 65.1, 109.6, 114.9, 122.0 (q, J = 272.5 Hz), 127.0 (q, J = 4.9 Hz), 132.2, 133.2 (q, J = 33.0 Hz), 135.1, 137.3, 175.3, 178.1.
Synthesis of 25
2-Methyl-2-phenylaminopropanenitrile, 25a
A mixture of aniline (0.931 g, 10 mmol) and acetone cyanohydrin (2 mL) was heated to reflux and stirred for 20 h. After being cold to 21 °C, the reaction mixture was poured into ethyl acetate (40 mL) and washed with cold water (2 × 30 mL). The organic layer was dried over MgSO4, concentrated under vacuum to dryness to yield 6a (1.51g, 9.4 mmol, 94%) as a brown slurry liquid.
4-[3-Phenyl-4,4-dimethyl-5-oxo-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 6b, 25
A mixture of isothiocyanate 12a (0.274 g, 1.2 mmol) and 25a (0.160 g, 1 mmol) in DMF (0.2 mL) was stirred for 48 h. To this mixture were added methanol (10 mL) and 2 N HCl (3 mL). The second mixture was refluxed for 6 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (20 mL) and extracted with ethyl acetate (20 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane) to yield 6b (0.276 g, 0.71 mmol, 71%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.60 (s, 6H), 7.28–7.31 (m, 2H), 7.50–7.58 (m, 3H), 7.85 (dd, J = 8.3, 1.8 Hz, 1H), 7.96–7.99 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ 23.7, 66.4, 110.2, 114.8, 121.9 (q, J = 272.6 Hz), 127.1 (q, J = 4.7 Hz), 129.5, 129.8, 129.9, 132.2, 133.4 (q, J = 33.2 Hz), 135.1, 135.2, 137.2, 175.0, 179.9.
Synthesis of 27
2-Methyl-2-(4-methylphenyl)aminopropanenitrile, 27a
A mixture of p-toluidine (1.07 g, 10 mmol) and acetone cyanohydrin (10 mL) was heated to 80 °C and stirred for 4 h. The medium was concentrated and dried under vacuum to yield 27a (1.72 g, 9.9 mmol, 99%) as a brown solid.
4-[3-(4-Methylphenyl)-4,4-dimethyl-5-oxo-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 27
A mixture of isothiocyanate 12a (0.547 g, 2.4 mmol) and 27a (0.348 g, 2 mmol) in DMF (0.6 mL) was stirred for 36 h. To this mixture were added methanol (20 mL) and 2 N HCl (5 mL). The second mixture was refluxed for 6 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (30 mL) and extracted with ethyl acetate (40 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane) to yield 27 (0.596 g, 1.48 mmol, 74%) as a white powder. 1H NMR (CDCl3, 400 MHz)δ 1.61 (s, 6H), 2.44 (s, 3H), 7.17–7.20 (m, 2H), 7.33–7.36 (m, 2H), 7.86 (dd, J = 8.3, 1.8 Hz, 1H), 7.96–7.98 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ 21.3, 23.6, 66.4, 110.0, 114.9, 121.9 (q, J = 272.6 Hz), 127.1 (q, J = 4.7 Hz), 129.2, 130.6, 132.2, 132.3, 133.4 (q, J = 33.2 Hz), 135.2, 137.2, 140.1, 175.1, 179.9.
Synthesis of 28
2-(4-Hydroxyphenylamino)-2-methylpropanenitrile, 28a
A mixture of 4-aminophenol (1.09 g, 10 mmol), acetone cyanohydrin (10 mL) and MgSO4 (2 g) was heated to 80 °C and stirred for 4 h. After concentration of the reaction medium under vacuum, compound 28a was crystallized from water (20 mL). The solid was filtered and dried to yield 28a (1.69 g, 9.6 mmol, 96%).
4-[3-(4-Hydroxyphenyl)-5-imino-4,4-dimethyl-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 28b
Triethylamine (0.101 g, 1 mmol) was added to a solution of isothiocyanate 12a (0.456 g, 2 mmol) and 28a (0.352 g, 2 mmol) in THF (5 mL). The reaction mixture was stirred at 0 °C for 48 h and then concentrated to yield a dark residue which was subjected to flash chromatography (dichloromethane/acetone, 85:15) to afford 28b (0.274 g, 0.68 mmol, 34%).
4-[3-(4-Hydroxyphenyl)-4,4-dimethyl-5-oxo-2-thioxoimidazolidin-1-yl]-2-trifluoromethyl-benzonitrile, 28
A mixture of 28b (0.202 g, 0.5 mmol) in aq. 2 N HCl (2 mL) and methanol (5 mL) was heated to reflux for 2 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (10 mL) and extracted with ethyl acetate (10 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane/acetone, 9:1) to yield 28 (0.198 g, 0.49 mmol, 98%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.57 (s, 6H), 6.26 (s, OH), 6.90–6.93 (m, 2H), 7.11–7.14 (m, 2H), 7.84 (dd, J = 8.3, 1.8 Hz, 1H), 7.95–7.98 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ 23.6, 66.5, 109.9, 114.9, 115.7, 116.8, 121.9 (q, J = 272.7 Hz), 127.2 (q, J = 4.7 Hz), 130.6, 132.3, 133.5 (q, J = 33.2 Hz), 135.3, 137.2, 157.0, 175.3, 180.2.
Synthesis of 29
4-Aminophenyl)carbamic acid tert-butyl ester, 29a
An aqueous solution of potassium carbonate (1.52 g, 11 mmol in 5 mL of water) was added to a solution of 1,4-diaminobenzene (3.24 g, 30 mmol) in a mixture of THF (30 mL) and DMF (10 mL). To this mixture was added di-tert-butyl pyrocarbonate, Boc2O (2.18 g, 10 mmol), dropwise over 0.5 h. The reaction mixture was stirred for an additional 4 h at 21 °C. The mixture was then poured into cold water (40 mL) and extracted with chloroform (3 × 50 mL). The combined organic phase was dried over MgSO4 and concentrated to yield a brown residue which was subjected to flash chromatography (dichloromethane/acetone, 4:1) to afford 29a as a yellow solid (1.98 g, 9.5 mmol, 95%) (yield based on Boc2O).
{4-[(1-Cyano-1-methylethyl)amino]phenyl}carbamic acid tert-butyl ester, 29b
The mixture of 29a (0.83 g, 4 mmol), acetone cyanohydrin (4 mL) and MgSO4 (2 g) was heated to 80 °C and stirred over 2.5 h. After cooling down to 21 °C, compound 29b was crystallized from water (30 mL). The solid was filtered and dried to yield 29b (1.08 g, 3.9 mmol, 98%).
{4-[3-(4-cyano-3-trifluoromethylphenyl)-4-imino-5,5-dimethyl-2-thioxo-imidazolidin-1-yl]-phenyl}carbamic acid tert-butyl ester, 29c
Triethylamine (0.202 g, 2 mmol) was added to a solution of isothiocyanate 12a (0.456 g, 2 mmol) and 29b (0.57 g, 2 mmol) in dry THF (5 mL). The reaction mixture was stirred at 21 °C for 15 h and then concentrated to yield a dark residue which was subjected to flash chromatography (ethyl ether/acetone, 97:3) to afford 29c (0.15 g, 0.3 mmol, 15%).
4-[3-(4-aminophenyl)-4,4-dimethyl-5-oxo-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 29
The mixture of 29c (0.15 g, 0.3 mmol) in aq. 3N HCl (1 mL) and methanol (4 mL) was heated to reflux for 2 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (5 mL) and extracted with dichloromethane (8 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane/acetone, 9:1) to yield 29 (0.118 g, 0.29 mmol, 97%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ 1.54 (s, 6H), 6.73–6.75 (m, 2H), 7.00–7.03 (m, 2H), 8.02 (dd, J1 = 8.2 Hz, J2 = 1.8 Hz, 1H), 8.16 (d, J = 1.8 Hz, 1H), 8.20 (d, J = 8.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 22.7, 66.2, 109.1, 114.3, 114.9, 120.4, 122.0 (q, J = 272.5 Hz), 127.0 (q, J = 4.9 Hz), 130.4, 132.5 (q, J = 33.0 Hz), 133.4, 135.6, 138.5, 149.2, 175.3, 180.4.
Synthesis of 25
4-[3-(4-Azidophenyl)-4,4-dimethyl-5-oxo-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 25
An aqueous solution of sulfuric acid (25% wt, 1 mL) was added to a solution of 29 (0.10 g, 0.25 mmol) in acetone (1 mL) cooled to −5 °C. An aqueous solution of NaNO2 (0.024 g, 0.35 mmol, in 0.5 mL of water) was added slowly the above mixture over 0.1 h. The reaction mixture was allowed to stir at −5 °C for an additional 1 h and then an aqueous solution of NaN3 (0.02 g, 0.3 mmol in 0.3 mL of water) was added dropwise. Upon completion of the addition, the reaction medium was warmed to 21 °C and stirred for an additional 3 h. The product was extracted with dichloromethane (3 × 5 mL). The combined organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane) to yield 25 (0.08 g, 0.18 mmol, 72%) as a yellowish solid. 1H NMR (400 MHz, CDCl3) δ 1.54 (s, 6H), 7.17–7.20 (m, 2H), 7.27–7.30 (m, 2H), 7.84 (dd, J1 = 8.3, 1.8 Hz, 1H), 7.96 (d, J = 1.8 Hz, 1H), 7.97 (d, J = 8.3 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 23.7, 66.4, 110.1, 114.8, 120.4, 122.1 (q, J = 272.5 Hz), 127.0 (q, J = 4.7 Hz), 131.1, 131.5, 132.3, 133.3 (q, J = 33.0 Hz), 135.3, 137.1, 141.7, 174.8, 180.1. MS for C19H13F3N6OS, calculated 430.4, found 430.1.
Synthesis of 30–37
N-(4-Cyano-3-trifluoromethylphenyl)-N’-(4-methylphenyl)thiourea, 30a
Addition of a solution of 4-methylaniline in DMF to a solution of the isothiocyanate 12a in DMF solution at 21 °C for 1 h afforded the desired unsymmetrical thiourea 30a in 99% yield.
4-[3-(4-Methylphenyl)-5-oxo-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 30
Treatment of a solution of the thiourea 30a in THF at 0 °C with a solution of chloroacetyl chloride in THF for 1 h gave a 95% yield of a 1:1 mixture of the two thiohydantoins, the 4-oxo and the 5-oxo regioisomers, 30 and 30b. These were easily separated by column chromatography on silica gel (dichloromethane) to give pure 30.
4-[3-(4-Methylphenyl)-4-methyl-5-oxo-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 31
To a suspension of NaH in THF cooled to 0 °C was added the unsubstituted thiohydantoin 30 and the mixture was stirred for 30 min. A solution of methyl iodide in THF was added and the mixture stirred for 5 h. Normal aqueous workup and extraction afforded the crude product which was purified by column by column chromatography on silica gel (dichloromethane) to give in 5% yield the pure 4-methylthiohydantoin 31.
4-[3-(4-Methylphenyl)-4-ethyl-5-oxo-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 32
To a suspension of NaH in THF cooled to 0 °C was added the unsubstituted thiohydantoin 30 and the mixture was stirred for 30 min. A solution of ethyl iodide in THF was added and the mixture stirred for 5 h. Normal aqueous workup and extraction afforded the crude product which was purified by column by column chromatography on silica gel (dichloromethane) to give in 5% yield the pure 4-ethylthiohydantoin 32.
4-[3-(4-Methylphenyl)-5-oxo-4-propyl-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 33
To a suspension of NaH in THF cooled to 0 °C was added the unsubstituted thiohydantoin 30 and the mixture was stirred for 30 min. A solution of propyl iodide in THF was added and the mixture stirred for 5 h. Normal aqueous workup and extraction afforded the crude product which was purified by column by column chromatography on silica gel (dichloromethane) to give in 5% yield the pure 4-propylthiohydantoin 33.
4-[3-(4-Methylphenyl)-4,4-diethyl-5-oxo-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 34
To a suspension of NaH in THF cooled to 0 °C was added the 4-ethylthiohydantoin 32 and the mixture was stirred for 30 min. A solution of ethyl iodide in THF was added and the mixture stirred for 5 h. Normal aqueous workup and extraction afforded the crude product which was purified by column by column chromatography on silica gel (dichloromethane) to give in 5% yield the pure 4,4-diethylthiohydantoin 34.
4-[3-(4-Methylphenyl)-4-ethyl-4-methyl-5-oxo-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 35
To a suspension of NaH in THF cooled to 0 °C was added the 4-methylthiohydantoin 31 and the mixture was stirred for 30 min. A solution of ethyl iodide in THF was added and the mixture stirred for 5 h. Normal aqueous workup and extraction afforded the crude product which was purified by column by column chromatography on silica gel (dichloromethane) to give in 5% yield the pure 4,4-diethylthiohydantoin 35.
4-[3-(4-Methylphenyl)-5-ethyl-5-methyl-4-oxo-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 36
To a suspension of NaH in THF cooled to 0 °C was added the unsubstituted 4-oxothiohydantoin 21b and the mixture was stirred for 30 min. A solution of methyl iodide in THF was added and the mixture stirred for 5 h. Normal aqueous workup and extraction afforded the crude product which was purified by column by column chromatography on silica gel (dichloromethane) to give in 5% yield the 5-methyl-4-oxothiohydantoin 36a. To a suspension of NaH in THF cooled to 0 °C was added the monomethyl 4-oxothiohydantoin 36a and the mixture was stirred for 30 min. A solution of ethyl iodide in THF was added and the mixture stirred for 5 h. Normal aqueous workup and extraction afforded the crude product which was purified by column by column chromatography on silica gel (dichloromethane) to give in 5% yield the 5- ethyl-5-methyl-4-oxothiohydantoin 36.
4-[3-(4-Methylphenyl)-5,5-diethyl-4-oxo-2-thioxoimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 37
To a suspension of NaH in THF cooled to 0 °C was added the unsubstit-uted 4-oxothiohydantoin 21b and the mixture was stirred for 30 min. A solution of ethyl iodide in THF was added and the mixture stirred for 5 h. Normal aqueous workup and extraction afforded the crude product which was purified by column by column chromatography on silica gel (dichloromethane) to give in 5% yield the 5-ethyl-4-oxothiohydantoin 37a. To a suspension of NaH in THF cooled to 0 °C was added the monoethyl 4-oxothiohydantoin 37a and the mixture was stirred for 30 min. A solution of ethyl iodide in THF was added and the mixture stirred for 5 h. Normal aqueous workup and extraction afforded the crude product which was purified by column by column chromatography on silica gel (dichloromethane) to give in 5% yield the 5,5-diethyl-4-oxothiohydantoin 37.
Synthesis of 39
1-(4-Methylphenyl)aminocyclopentanecarbonitrile, 39a
Trimethylsilyl cyanide (0.865 mL, 7 mmol) was added dropwise to a mixture of p-toluidine (0.535 g, 5 mmol) and cyclopentanone (0.589 g, 7 mmol). The reaction mixture was stirred at 21 °C for 6 h and then concentrated under vacuum to obtain a brown liquid which was subjected to chromatography (dichloromethane) to yield 35a (0.981 g, 4.9 mmol, 98%) as a yellowish solid.
4-(4-Oxo-2-thioxo-1-(4-methylphenyl)-1,3-diazaspiro[4.4]non-3-yl)-2-trifluoromethylbenzonitrile, 39
A mixture of isothiocyanate 12a (0.296 g, 1.3 mmol) and 39a (0.2 g, 1 mmol) in DMF (0.2 mL) was stirred for 48 h. To this mixture were added methanol (10 mL) and aq. 2 N HCl (3 mL). The second mixture was refluxed for 6 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (20 mL) and extracted with ethyl acetate (30 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane) to yield 39 (0.3 g, 0.7 mmol, 70%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.47–1.57 (m, 2H), 1.81–1.92 (m, 2H), 2.20–2.24 (m, 2H), 2.27–2.34 (m, 2H), 2.43 (s, 3H), 7.18–7.22 (m, 2H), 7.33–7.36 (m, 2H), 7.86 (dd, J = 8.2, 1.8 Hz, 1H), 7.96 (d, J = 8.2 Hz, 1H), 7.98 (d, J = 1.8 Hz, 1H); 13C NMR (CDCl3, 100 MHz) δ 21.3, 25.2, 36.3, 75.1, 110.0, 114.9, 121.9 (q, J = 272.5 Hz), 127.1 (q, J = 4.7 Hz), 129.5, 130.7, 123.2, 133.0, 133.4 (q, J = 33.2 Hz), 135.1, 137.4, 140.0, 176.3, 180.2.
Synthesis of 38
1-(4-Methylphenyl)aminocyclobutanecarbonitrile, 38a
Trimethylsilyl cyanide (0.93 mL, 7 mmol) was added dropwise to a mixture of p-toluidine (0.535 g, 5 mmol) and cyclobutanone (0.42 g, 6 mmol). The reaction mixture was stirred at 21 °C for 6 h and then concentrated under vacuum to obtain a brown liquid which was subjected to chromatography (dichloromethane) to yield 37a (0.912 g, 4.9 mmol, 98%) as a yellowish solid.
4-(8-Oxo-6-thioxo-5-(4-methylphenyl)-5,7-diazaspiro[3.4]oct-7-yl)-2-trifluoromethylbenzonitrile, 38
A mixture of isothiocyanate 12a (0.912 g, 4 mmol) and 38a (0.558 g, 3 mmol) in DMF (0.5 mL) was stirred at 21 °C for 24 h. To this mixture were added methanol (30 mL) and aq. 2 N HCl (6 mL). The second mixture was refluxed for 6 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (50 mL) and extracted with ethyl acetate (60 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane) to yield 38 (0.959 g, 2.31 mmol, 77%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.62–1.69 (m, 1H), 2.16–2.22 (m, 1H), 2.46 (s, 3H), 2.55–2.66 (m, 4H), 7.19–7.26 (m, 2H), 7.36–7.42 (m, 2H), 7.86 (dd, J = 8.3, 1.8 Hz, 1H), 7.96 (d, J = 8.3 Hz, 1H), 7.99 (d, J = 1.8 Hz, 1H); 13C NMR (CDCl3, 100 MHz) δ 13.7, 21.3, 31.4, 67.4, 109.9, 114.9, 121.9 (q, J = 272.6 Hz), 127.1 (q, J = 4.7 Hz), 129.5, 130.8, 132.2, 132.4, 133.3 (q, J = 33.2 Hz), 135.2, 137.3, 140.1, 175.0, 180.0.
Synthesis of 40
1-(4-Methylphenyl)aminocyclohexanecarbonitrile, 40a
Sodium cyanide (0.147g, 3 mmol) was added to a mixture of p-toluidine (0.214 g, 2 mmol) and cyclohexanone (0.294 g, 3 mmol) in 90% acetic acid (3 mL). The reaction mixture was stirred at 21 °C for 12 h and then 20 mL of ethyl acetate was added. The organic layer was washed with water (3 × 10 mL), dried over magnesium sulfate and concentrated under vacuum to dryness to yield 40a (0.398 g, 1.86 mmol, 93%) as a brown solid.
4-(4-Imino-2-thioxo-1-(4-methylphenyl)-1,3-diazaspiro[4.5]dec-3-yl)-2-trifluoromethylbenzonitrile, 40b
Triethylamine (0.05 g, 0.5 mmol) was added to a solution of isothiocyanate 12a (0.228 g, 1 mmol) and 40a (0.214 g, 1 mmol) in THF (2 mL). The reaction mixture was stirred at 21 °C for 2 d and then concentrated to yield a dark residue which was subjected to flash chromatography (dichloromethane/acetone, 95:5) to afford 40b (0.035 g, 0.08 mmol, 8%).
4-(4-Oxo-2-thioxo-1-(4-methylphenyl)-1,3-diazaspiro[4.5]dec-3-yl)-2-trifluoromethylbenzonitrile, 40
A mixture of 40b (0.035 g, 0.08 mmol) in aq. 2 N HCl (1 mL) and methanol (3 mL) was heated to reflux for 2 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (5 mL) and extracted with ethyl acetate (6 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane) to yield 40 (0.034 g, 0.076 mmol, 95%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.02–1.05 (m, 1H), 1.64–1.76 (m, 4H), 2.03–2.12 (m, 5H), 2.44 (s, 3H), 7.12–7.15 (m, 2H), 7.33–7.36 (m, 2H), 7.85 (dd, J = 8.2, 1.8 Hz, 1H), 7.96 (d, J = 8.3 Hz, 1H), 7.97 (d, J = 1.8 Hz, 1H); 13C NMR (CDCl3, 100 MHz) δ 20.7, 21.3, 24.0, 32.6, 67.4, 109.9, 114.9, 122.0 (q, J = 272.5 Hz), 127.3 (q, J = 4.6 Hz), 130.0, 130.5, 132.0, 132.5, 133.3 (q, J = 33.2 Hz), 135.2, 137.3, 140.1, 174.1, 180.1.
Synthesis of 41
1-(4-Methylphenyl)aminocycloheptanecarbonitrile, 41a
Sodium cyanide (0.147g, 3 mmol) was added to a mixture of p-toluidine (0.214 g, 2 mmol) and cycloheptanone (0.337 g, 3 mmol) in acetic acid 90% (3 mL). The reaction mixture was stirred at 21 °C for 12 h and then 20 mL of ethyl acetate was added. The organic layer was washed with water (3 × 10 mL), dried over magnesium sulfate and concentrated under vacuum to dryness to yield 41a (0.438 g, 1.92 mmol, 96%) as a brown solid.
4-(4-Imino-2-thioxo-1-(4-methylphenyl)-1,3-diazaspiro[4.6]undec-3-yl)-2-trifluoromethylbenzonitrile, 41b
Triethylamine (0.05 g, 0.5 mmol) was added to a solution of isothiocyanate 12a (0.228 g, 1 mmol) and 41a (0.228 g, 1 mmol) in THF (2 mL). The reaction mixture was stirred at 21 °C for 2 d and then concentrated to yield a dark residue which was subjected to flash chromatography (dichloromethane/acetone, 95:5) to afford 41b (0.036 g, 0.08 mmol, 8%).
4-(4-Oxo-2-thioxo-1-(4-methylphenyl)-1,3-diazaspiro[4.6]undec-3-yl)-2-trifluoromethylbenzonitrile, 41
A mixture of 41b (0.036 g, 0.08 mmol) in aq. 2 N HCl (1 mL) and metha-nol (3 mL) was heated to reflux for 2 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (5 mL) and extracted with ethyl acetate (6 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane) to yield 41 (0.034 g, 0.075 mmol, 94%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.24–134 (m, 2H), 1.37–1.43 (m, 2H), 1.53–1.60 (m, 2H), 1.74–1.82 (m, 2H), 2.19–2.25 (m, 4H), 2.44 (s, 3H), 7.16–7.19 (m, 2H), 7.32–7.35 (m, 2H), 7.83 (dd, J = 8.2, 1.8 Hz, 1H), 7.95–7.97 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ 21.4, 22.2, 30.9, 36.3, 71.1, 110.0, 114.9, 121.9 (q, J = 272.5 Hz), 127.2 (q, J = 4.6 Hz), 129.6, 130.5, 132.3, 133.0, 133.2 (q, J = 33.2 Hz), 135.1, 137.4, 140.0, 175.9, 179.7.
Synthesis of 43
1-Methyl-4-(4-methylphenylamino)piperidine-4-carbonitrile, 43a
Sodium cyanide (0.318 g, 6.5 mmol) was added to a mixture of p-toluidine (0.535 g, 5 mmol) and 1-methyl-4-piperidinone (0.678 g, 6 mmol) in acetic acid 90% (5 mL). The reaction mixture was stirred at 21 °C for 6 h and then 100 mL of dichloromethane was added. The organic layer was washed with aq 2 N NaOH (2 ×5 0 mL), dried over magnesium sulfate, concentrated and chromatographed (dichloromethane and then acetone) to obtain 43a (0.722 g, 3.15 mmol, 63%).
4-(4-Imino-8-methyl-2-thioxo-1-(4-methylphenyl)-1,3,8-triazaspiro[4.5]dec-3-yl)-2-trifluoromethylbenzonitrile, 43b
Triethylamine (0.02, 0.2 mmol) was added to a solution of isothiocyanate 12a (0.228 g, 1 mmol) and 43a (0.114 g, 0.5 mmol) in THF (2 mL). The reaction mixture was stirred at 21 °C for 20 h and then concentrated to yield a dark residue which was subjected to flash chromatography (dichloromethane/acetone, 90:10, and then acetone) to afford 43b (0.059 g, 0.13 mmol, 26%).
4-(8-Methyl-4-oxo-2-thioxo-1-(4-methylphenyl)-1,3,8-triazaspiro[4.5]dec-3-yl)-2-trifluoromethylbenzonitrile, 43
A mixture of 43b (0.059 g, 0.13 mmol) in aq. 2 N HCl (1 mL) and methanol (3 mL) was heated to reflux for 2 h. After being cooled to 21 °C, the reaction mix-ture was poured into cold water (5 mL) and extracted with ethyl acetate (10 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane:acetone, 60:40) to yield 43 (0.055 g, 0.012 mmol, 92%) as a white powder. 1H NMR (acetone-d6, 400 MHz) δ 1.93–1.99 (m, 1H), 2.00–2.04 (m, 1H), 2.18 (s, 3H), 2.24–2.28 (m, 2H), 2.38 (s, 3H), 2.61–2.72 (m, 4H), 7.18–7-20 (m, 2H), 7.32–7.35 (m, 2H), 8.03 (dd, J1 = 8.2, 1.8 Hz, 1H), 8.16 (d, J = 1.8 Hz, 1H), 8.22 (d, J = 8.2 Hz, 1H); 13C NMR (acetone-d6, 100 MHz) δ 20.3, 31.4, 45.1, 49.8, 65.1, 109.1, 114.8, 122.4 (q, J = 275.1 Hz), 127.7 (q, J = 4.8 Hz), 130.0, 130.5, 131.9 (q, J = 32.6 Hz), 132.6, 133.5, 135.6, 138.3, 139.4, 174.0, 180.6.
Synthesis of 49
1-(4-Hydroxyphenyl)aminocyclobutanecarbonitrile, 49a
Trimethylsilyl cyanide (0.93 mL, 7 mmol) was added dropwise to a mixture of 4-hydroxyaniline (0.545 g, 5 mmol) and cyclobutanone (0.42 g, 6 mmol). The reaction mixture was stirred at 21 °C for 6 h and then concentrated under vacuum to obtain a brown liquid which was subjected to chromatography (dichloromethane:acetone, 98:2) to yield 49a (0.903 g, 4.8 mmol, 96%) as a yellowish solid.
4-(8-Oxo-6-thioxo-5-(4-hydroxyphenyl)-5,7-diazaspiro[3.4]oct-7-yl)-2-trifluoromethylbenzonitrile, 49
A mixture of isothiocyanate 12a (0.57 g, 2.5 mmol) and 49a (0.376 g, 2 mmol) in DMF (0.5 mL) was stirred at 21 °C for 40 h. To this mixture were added methanol (30 mL) and aq. 2 N HCl (5 mL). The second mixture was refluxed for 6 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (40 mL) and extracted with ethyl acetate (50 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane: acetone, 98:2) to yield 49 (0.659 g, 1.58 mmol, 79%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.55–1.63 (m, 1H), 2.01–2.09 (m, 1H), 2.50–2.65 (m, 4H), 6.97–7.01 (m, 2H), 7.20–7.24 (m, 2H), 8.02 (dd, J = 8.3, 1.8 Hz, 1H), 8.14 (d, J = 1.8 Hz, 1H), 8.21 (d, J = 8.3 Hz, 1H); 13C NMR (acetone-d6, 100 MHz) δ 13.4, 31.3, 67.5, 108.9, 114.8, 116.1, 123.5 (q, J = 271.5 Hz), 127.4 (q, J = 4.9 Hz), 131.3, 131.8 (q, J = 32.7 Hz), 133.3, 135.5, 136.2, 138.5, 158.1, 175.1, 180.7.
Synthesis of 50–51
1-Aminocyclopentanecarbonitrile, 50a
Ammonia anhydrous was bubbled into a mixture of cyclopentanone (0.452 g) and trimethylsilyl cyanide (0.66 mL, 5 mmol). The ammonia was refluxed with a Dry Ice-acetone condenser. After 1 h of reflux, the ammonia was allowed to evaporate from the medium and then the remaining mixture was concentrated under vacuum to yield 50a (0.522 g, 4.75 mmol, 95%) as a colorless liquid.
4-(4-Imino-2-thioxo-1,3-diazaspiro[4.4]non-3-yl)-2-trifluoromethylbenzonitrile, 50b
Triethylamine (0.101 g, 0.1 mmol) was added to a solution of isothiocyanate 12a (0.684 g, 3 mmol) and 50a (0.33 g, 3 mmol) in THF (5 mL). The reaction mixture was stirred at 21 °C for 5 h and then concentrated to yield a brown residue which was subjected to flash chromatography (dichloromethane/acetone, 93:7) to afford 50b (0.741 g, 2.19 mmol, 73%).
4-(4-Oxo-2-thioxo-1,3-diazaspiro[4.4]non-3-yl)-2-trifluoromethylbenzonitrile, 50c
A mixture of 50b (0.741 g, 2.19 mmol) in aq. 2 N HCl (4 mL) and methanol (20 mL) was heated to reflux for 1 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (20 mL) and extracted with ethyl acetate (40 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane) to yield 50c (0.72 g, 2.12 mmol, 97%) as a white powder.
4-[1-(4-Cyanophenyl)-4-oxo-2-thioxo-1,3-diazaspiro[4.4]non-3-yl]-2-trifluoromethylbenzonitrile, 50
A mixture of 50c (0.0678 g, 0.2 mmol), 1,8-diazabicyclo[5.4.0]undec-7-ene (0.061 g, 0.4 mmol) and 4-fluorocyanobenzene (0.048 g, 0.4 mmol) in dimethylformamide (0.5 mL) was placed in a sealed tube under argon and heated to 140 °C for 5 d. The reaction mixture was poured into ethyl acetate (5 mL) and washed with water (2 × 10 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane) to yield 50 (0.023 g, 0.052 mmol, 26%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.51–1.55 (m, 2H), 1.90–1.93 (m, 2H), 2.12–2.16 (m, 2H), 2.33–2.38 (m, 2H), 7.47–7.50 (m, 2H), 7.81–7.87 (m, 3H), 7.95–7.99 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ 25.2, 36.5, 75.3, 110.3, 113.9, 114.7, 117.5, 121.8 (q, J = 272.6 Hz), 127.0 (q, J = 4.8 Hz), 131.2, 132.1, 133.6 (q, J = 34.3 Hz), 133.8, 135.3, 136.9, 140.0, 175.6, 180.1.
4-[1-(4-Nitrophenyl)-4-oxo-2-thioxo-1,3-diazaspiro[4.4]non-3-yl]-2-trifluoromethylbenzonitrile, 51
A mixture of 50c (0.0678 g, 0.2 mmol), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, 0.05 g, 0.33 mmol) and 4-fluoronitrobenzene (0.056 g, 0.4 mmol) in dimethylformamide (0.5 mL) was placed in a sealed tube under argon and heated to 130 °C for 40 h. The reaction mixture was poured into ethyl acetate (5 mL) and washed with water (2 × 10 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane) to yield 51 (0.038 g, 0.084 mmol, 42%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.53–1.56 (m, 2H), 1.90–1.93 (m, 2H), 2.14–2.18 (m, 2H), 2.37–2.40 (m, 2H), 7.54–7.57 (m, 2H), 7.85 (dd, J = 8.2, 1.8 Hz, 1H), 7.97 (d, J = 1.8 Hz, 1H), 7.98 (d, J = 8.2 Hz, 1H), 8.39–8.43 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ 25.2, 36.5, 75.3, 110.3, 114.8, 121.8 (q, J = 272.6 Hz), 125.2, 127.0 (q, J = 4.7 Hz), 131.4, 132.1, 133.6 (q, J = 34.3 Hz), 135.3, 136.9, 141.7, 148.1, 175.6, 180.2.
Synthesis of 52
2-Methyl-2-(4-trifluoromethylphenyl)aminopropanenitrile, 52a
A mixture of 4-trifluoromethylaniline (1.61 g, 10 mmol), acetone cyanohydrin (5 mL) and magnesium sulfate (2 g) was heated to 80 °C and stirred for 12 h. To the medium was added ethyl acetate (50 mL) and then washed with water (3 × 30 mL). The organic layer was dried over MgSO4 and concentrated under vacuum to dryness to yield 52a (2.166 g, 9.5 mmol, 95%) as brown solid.
4-(4,4-Dimethyl-5-oxo-2-thioxo-3-(4-trifluoromethylphenyl)imidazolidin-1-yl)-2-trifluoromethylbenzonitrile, 52
A mixture of isothiocyanate 12a (0.114 g, 0.5 mmol) and 52a (0.092 g, 0.4 mmol) in DMF (0.3 mL) was stirred at 21 °C for 48 h. To this mixture were added methanol (10 mL) and aq. 2 N HCl (3 mL). The second mixture was refluxed for 6 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (20 mL) and extracted with ethyl acetate (20 mL). The organic layer was dried over MgSO4, concentrated and chromato-graphed (dichloromethane) to yield 52 (0.117 g, 0.256 mmol, 64%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.61 (s, 6H), 7.45–7.49 (m, 2H), 7.80–7.83 (m, 2H), 7.85 (dd, J = 8.3, 1.8 Hz, 1H), 7.97 (d, J = 1.8 Hz, 1H), 7.99 (d, J = 8.2 Hz, 1H); 13C NMR (CDCl3, 100 MHz) δ 23.8, 66.6, 110.3, 114.8, 121.8 (q, J = 272.6 Hz), 123.5 (q, J = 271.1 Hz), 127.0 (q, J = 4.6 Hz), 127.1 (q, J = 4.7 Hz), 130.3, 131.9 (q, J = 32.9 Hz), 132.2, 133.5 (q, J = 33.3 Hz), 135.3, 136.9, 138.4, 174.6, 179.9.
Synthesis of 74–76
1-(4-Hydroxymethylphenylamino)cyclobutanecarbonitrile, 74a
Trimethylsilyl cyanide (0.66 mL, 5 mmol) was added dropwise to a mixture of 4-aminobenzyl alcohol (0.492 g, 4 mmol) and cyclobutanone (0.35 g, 5 mmol) in dichloromethane (10 mL). The reaction mixture was stirred at 21 °C for 6 h and then concentrated under vacuum to obtain a brown liquid which was subjected to chromatography (dichloromethane) to yield 74a (0.677 g, 3.36 mmol, 84%) as a brown solid.
4-[8-(4-Hydroxymethylphenyl)-5-oxo-7-thioxo-6-azaspiro[3.4]oct-6-yl]-2-trifluoromethylbenzonitrile, 74
A mixture of isothiocyanate 12a (0.342 g, 1.5 mmol) and 74a (0.21 g, 1 mmol) in dry DMF (0.5 mL) was stirred at 21 °C for 24 h. To this mixture were added methanol (20 mL) and HCl aq. 2 N (5 mL). The second mixture was refluxed for 6 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (40 mL) and extracted with ethyl acetate (60 mL). The organic layer was dried over MgSO4, concentrated and chroma-tographed (dichloromethane:acetone, 90:10) to yield 74 (0.296 g, 0.69 mmol, 69%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.63–1.68 (m, 1H), 2.17–2.26 (m, 1H), 2.52–2.68 (m, 4H), 4.75 (s, 2H), 7.30 (d, J = 8.1 Hz, 2H), 7.58 (d, J = 8.1 Hz, 2H), 7.88 (dd, J = 8.3, 1.8 Hz, 1H), 7.95–7.98 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ 13.7, 31.5, 64.4, 67.5, 109.9, 114.9, 121.9 (q, J = 272.6 Hz), 127.1 (q, J = 4.7 Hz), 128.3, 130.0, 132.2, 133.3, 133.4 (q, J = 33.2 Hz), 134.2, 137.2, 142.9, 174.9, 179.9.
4-[5-(4-Formylphenyl)-8-oxo-6-thioxo-5,7-diazaspiro[3.4]oct-7-yl]-2-trifluoromethylbenzonitrile, 75
To a mixture of 74 (0.303 g, 0.7 mmol) and the Dess-Martin periodinane (0.417g, 1 mmol) in dichloromethane (5 mL) was added pyridine (1.01 g, 1 mmol). The mixture was stirred for 2 h at 21 °C and then ethyl ether (10 mL) was added to precipitate the by-product of the reaction. After filtration and concentration under reduced pressure, the mixture was chromatographed (dichloromethane:acetone, 95:5) to yield 75 (0.24 g, 0.56 mmol, 80%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.62–1.73 (m, 1H), 2.24–2.30 (m, 1H), 2.50–2.58 (m, 2H), 2.69–2.75 (m, 2H), 7.53 (d, J = 8.1 Hz, 2H), 7.85 (dd, J = 8.3, 1.8 Hz, 1H), 7.97–7.99 (m, 2H), 8.11 (d, J = 8.1 Hz, 2H), 10.12 (s, 1H); 13C NMR (CDCl3, 100 MHz) δ 13.7, 31.7, 67.5, 110.2, 114.8, 121.9 (q, J = 272.6 Hz), 127.0 (q, J = 4.7 Hz), 129.1, 131.0, 131.2, 132.2, 133.3 (q, J = 33.2 Hz), 135.3, 136.9, 140.5, 174.5, 179.8, 190.8.
4-{5-[4-(1-Hydroxyethyl)phenyl]-8-oxo-6-thioxo-5,7-diazaspiro[3.4]oct-7-yl}-2-trifluoromethylbenzonitrile, 76
A mixture of 75 (0.043 g, 0.1 mmol) and THF (1 mL) in a flamed-dried flask was placed under argon and cooled to −78 °C. Methylmagnesium iodide (1.1 mL, 0.1 M) was added. The mixture was stirred at −78 °C for 30 min and warmed slowly to 21 °C. The medium was washed with water (3 mL) and extracted with ethyl acetate (10 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane:ace-tone, 95:5) to yield 76 (0.037 g, 0.082 mmol, 82%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.57 (d, J = 6.5 Hz, 3H), 1.61–1.71 (m, 1H), 2.09 (d, J = 3.2 Hz, OH), 2.16–2.28 (m, 1H), 2.52–2.60 (m, 2H), 2.63–2.69 (m, 2H), 5.00 (qd, J = 6.5, 3.1 Hz, 1H), 7.29 (d, J = 8.3 Hz, 2H), 7.60 (d, J = 8.2 Hz, 2H), 7.85 (dd, J = 8.3, 1.8 Hz, 1H), 7.95–7.98 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ 13.7, 25.3, 31.5, 67.4, 69.8, 110.0, 114.9, 121.9 (q, J = 272.6 Hz), 127.0 (q, J = 4.7 Hz), 127.1, 129.9, 132.2, 133.4 (q, J = 33.2 Hz), 134.1, 135.2, 137.1, 147.6, 174.9, 179.9.
Synthesis of 77–78
(E)-3-{4-[7-(4-Cyano-3-trifluoromethylphenyl)-8-oxo-6-thioxo-5,7-diazaspiro[3.4]oct-5-yl]-phenyl}acrylic acid ethyl ester, 77a
A mixture of 75 (0.043 g, 0.1 mmol) and (carbethoxyethylidene) triphenylphosphorane (0.039 g, 0.12 mmol) in dichloromethane (2 mL) was stirred at 21 °C for 10 h. The mixture was concentrated and chromatographed (dichloromethane) to yield 77a (0.048 g, 0.096 mmol, 96%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.35 (t, J = 7.1 Hz, 3H), 1.66–1.70 (m, 1H), 2.19–2.65 (m, 1H), 2.51–2.69 (m, 2H), 2.66–2.72 (m, 2H), 4.28 (q, J = 7.1 Hz, 2H), 6.51 (d, J = 16.1 Hz, 1H), 7.35 (d, J = 8.3 Hz, 2H), 7.72 (d, J = 8.3 Hz, 2H), 7.73 (d, J = 16.1 Hz, 1H), 7.85 (dd, J = 8.3, 1.8 Hz, 1H), 7.96–7.98 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ 13.7, 14.3, 31.6, 60.8, 67.5, 110.0, 114.9, 120.5, 121.8 (q, J = 272.6 Hz), 127.0 (q, J = 4.7 Hz), 129.5, 130.5, 132.2, 133.4 (q, J = 33.2 Hz), 135.2, 136.0, 136.5, 137.0, 142.7, 166.5, 174.7, 179.8.
(E)-3-{4-[7-(4-Cyano-3-trifluoromethylphenyl)-8-oxo-6-thioxo-5,7-diaza-spiro[3.4]oct-5-yl]phenyl}acrylic acid, 77b
A mixture of 77a (0.025 g, 0.05 mmol) and a solution of sodium hydroxide (2 mL, 2 M) in methanol (2 mL) was stirred at 21 °C for 5 h. The methanol was evaporated. The residue was adjusted to pH 5 by aq. 2 N HCl and then extracted with ethyl acetate (3 × 50 mL). The organic layer was dried over MgSO4 and concentrated to dryness to obtain 119b (0.02 g, 0.042 mmol, 85%).
(E)-3-{4-[7-(4-cyano-3-trifluoromethylphenyl)-8-oxo-6-thioxo-5,7-diazaspiro[3.4]oct-5-yl]-phenyl}-2-propanamide, 77
To a suspension of 77b (0.02 g, 0.042 mmol) in THF (1 mL) cooled to −5 °C was added thionyl chloride (0.007 mL, 0.1 mmol). The medium was stirred at −5 °C for 1 h. Then ammonia was bubbled into the mixture. The excess of ammonia was condensed by a reflux condenser cooled at −78 °C for 30 min and then was allowed to evaporate. The medium was filtered and the filtrate was concentrated and chromatographed (dichloromethane: acetone, 70:30) to yield 77 (0.014 g, 0.03 mmol, 71%) as an off-white powder. 1H NMR (DMSO-d6, 400 MHz) δ 1.49–1.52 (m, 1H), 1.88–1.93 (m, 1H), 2.37–2.46 (m, 2H), 2.57–2.62 (m, 2H), 6.66 (d, J = 15.9 Hz, 1H), 7.16 (bs, 1H), 7.43 (d, J = 8.3 Hz, 2H), 7.47 (d, J = 15.9 Hz, 1H), 7.58 (bs, 1H), 7.78 (d, J = 8.3 Hz, 2H), 8.03 (dd, J = 8.3, 1.8 Hz, 1H), 8.23 (d, J = 1.8 Hz, 1H), 8.34 (d, J = 8.3 Hz, 1H).
(E)-4-{5-[4-(3-Hydroxy-1-propenyl)phenyl]-8-oxo-6-thioxo-5,7-diazaspiro[3.4]oct-7-yl}-2-trifluoromethylbenzonitrile, 78
To a mixture of 77a (0.05 g, 0.1 mmol) in dichloromethane (2 mL) at −78 °C was added a solution of diisobutylaluminum hydride in THF (0.11 mL, 1 M, 0.11 mmol). The mixture was stirred at −78 °C for 3 h. After being warmed to 21 °C, the mixture was washed with an aqueous solution of sodium thiosulfate and extracted with ethyl acetate. The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane:acetone, 95:5) to yield 78 (0.040 g, 0.089 mmol, 89%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.57–1.68 (m, 1H), 2.17–2.39 (m, 1H), 2.55–2.61 (m, 2H), 2.61–2.67 (m, 2H), 4.39 (d, J = 4.7 Hz, 2H), 6.47 (dt, J1 = 16.0, 5.3 Hz, 1H), 6.70 (d, J = 16.0 Hz, 1H), 7.29 (d, J = 8.3 Hz, 2H), 7.59 (d, J = 8.3 Hz, 2H), 7.85 (dd, J = 8.3, 1.8 Hz, 1H), 7.96–7.98 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ 13.7, 31.5, 63.4, 67.4, 110.0, 114.8, 120.5, 121.8 (q, J = 272.6 Hz), 127.0 (q, J = 4.7 Hz), 127.9, 129.2, 130.1, 131.1, 132.1, 133.4 (q, J = 33.2 Hz), 135.2, 137.1, 138.4, 174.8, 179.9.
Synthesis of 79–80
4-[4-(1-Cyanocyclobutylamino)phenyl]butanoic acid, 79a
Trimethylsilyl cyanide (0.50 g, 5 mmol) was added dropwise to a mixture of 4-(4-aminophenyl)butyric acid (0.537 g, 3 mmol), cyclobutanone (0.35 g, 5 mmol) and sodium sulfate (1 g) in 1,4-dioxane (10 mL). The mixture was stirred for 15 h. After filtration to remove the sodium sulfate, the mixture was concentrated under vacuum to obtain a brown liquid which was subjected to chromatography (dichloromethane: acetone, 50:50) to yield 79a (0.665 g, 2.58 mmol, 86%) as a yellowish solid.
4-{4-[7-(4-cyano-3-trifluoromethylphenyl)-8-oxo-6-thioxo-5,7-diazaspiro[3.4]oct-5-yl]phenyl} butanoic acid methyl ester, 79b
A mixture of isothiocyanate 12a (0.547 g, 2.4 mmol) and 79a (0.342 g, 1.5 mmol) in DMF (2 mL) was stirred at 21 °C for 15 h. To this mixture were added methanol (10 mL) and aq. 2 N HCl (5 mL). The second mixture was refluxed for 3 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (10 mL) and extracted with ethyl acetate (3 × 30 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane) to yield 79b (0.594 g, 1.18 mmol, 79%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.60–1.70 (m, 1H), 1.98–2.07 (m, 2H), 2.14–2.26 (m, 1H), 2.40 (t, J = 7.4 Hz, 2H), 2.52–2.60 (m, 2H), 2.62–2.68 (m, 2H), 2.74 (t, J = 7.4 Hz, 2H), 3.68 (s, 3H), 7.22 (d, J = 8.2 Hz, 2H), 7.38 (d, J = 8.2 Hz, 2H), 7.86 (dd, J = 8.3, 1.8 Hz, 1H), 7.95 (d, J = 8.3 Hz, 1H), 7.98 (d, J = 1.8 Hz, 1H); 13C NMR (CDCl3, 100 MHz) δ 13.7, 26.1, 31.4, 33.5, 34.8, 51.7, 67.5, 109.9, 114.9, 121.9 (q, J = 272.7 Hz), 127.1 (q, J = 4.7 Hz), 129.7, 130.1, 132.3, 133.0, 133.3 (q, J = 33.2 Hz), 135.2, 137.2, 143.5, 173.8, 175.0, 179.9.
4-{4-[7-(4-Cyano-3-trifluoromethylphenyl)-8-oxo-6-thioxo-5,7-diaza-spiro[3.4]oct-5-yl]phenyl} butanoic acid, 79c
A mixture of 79b (0.501 g, 1 mmol) and a solution of sodium hydroxide (10 mL, 2 M) in methanol (10 mL) was stirred at 21 °C for 5 h. The methanol was evaporated. The residue was adjusted to pH 5 by aq. 2 M HCl and then the mixture was extracted with ethyl acetate (3 × 50 mL). The organic layer was dried over MgSO4 and concentrated to dryness to obtain 79c (0.482 g, 0.99 mmol, 99%). 1H NMR (CDCl3, 400 MHz) δ 1.60–1.70 (m, 1H), 1.98–2.07 (m, 2H), 2.14–2.26 (m, 1H), 2.45 (t, J = 7.3 Hz, 2H), 2.51–2.59 (m, 2H), 2.62–2.68 (m, 2H), 2.77 (t, J = 7.3 Hz, 2H), 7.23 (d, J = 8.1 Hz, 2H), 7.40 (d, J = 8.1 Hz, 2H), 7.85 (dd, J = 8.3, 1.8 Hz, 1H), 7.95 (d, J = 8.3 Hz, 1H), 7.97 (d, J = 1.8 Hz, 1H); 13C NMR (CDCl3, 100 MHz) δ 13.7, 25.9, 31.4, 33.4, 34.7, 67.5, 109.9, 114.9, 121.9 (q, J = 272.6 Hz), 127.1 (q, J = 4.7 Hz), 129.8, 130.1, 132.3, 133.0, 133.4 (q, J = 33.1 Hz), 135.2, 137.2, 143.3, 174.9, 178.9, 179.9.
4-{4-[7-(4-Cyano-3-trifluoromethylphenyl)-8-oxo-6-thioxo-5,7-diaza-spiro[3.4]oct-5-yl]phenyl} butanamide, 79
To a suspension of 79c (0.097 g, 0.2 mmol) in THF (10 mL) cooled to −5 °C was added thionyl chloride (0.019 mL, 0.26 mmol). The mixture was stirred at −5 °C for 1 h. Then ammonia was bubbled into the mixture. The excess ammonia was condensed by a reflux condenser cooled to −78 °C for 30 min and then was allowed to evaporate. The mixture was filtered. The filtrate was concentrated and chromatographed (dichloromethane:acetone, 70:30) to yield 79 (0.093 g, 0.19 mmol, 95%) as an off-white powder. 1H NMR (CDCl3, 400 MHz) δ 1.57–1.70 (m, 1H), 2.00–2.08 (m, 2H), 2.16–2.25 (m, 1H), 2.31 (t, J = 7.3 Hz, 2H), 2.51–2.59 (m, 2H), 2.62–2.68 (m, 2H), 2.77 (t, J = 7.3 Hz, 2H), 5.56 (bs, 1H), 5.65 (bs, 1H), 7.22 (d, J = 8.2 Hz, 2H), 7.39 (d, J = 8.2 Hz, 2H), 7.85 (dd, J = 8.3, 1.8 Hz, 1H), 7.95 (d, J = 8.3 Hz, 1H), 7.97 (d, J = 1.8 Hz, 1H); 13C NMR (CDCl3, 100 MHz) δ 13.7, 26.5, 31.4, 34.8, 35.0, 67.5, 109.9, 114.9, 121.9 (q, J = 272.7 Hz), 127.1 (q, J = 4.7 Hz), 129.8, 130.1, 132.2, 133.0, 133.3 (q, J = 33.2 Hz), 135.2, 137.2, 143.5, 173.8, 174.9, 179.9.
N-Methyl-4-{4-[7-(4-cyano-3-trifluoromethylphenyl)-8-oxo-6-thioxo-5,7-diaza-spiro[3.4]-oct-5-yl]phenyl}butanamide, 80
To a suspension of 79c (0.097 g, 0.2 mmol) in THF (10 mL) cooled to −5 °C was added thionyl chloride (0.019 mL, 0.26 mmol). The mixture was stirred at −5 °C for 1 h. Then methylamine was bubbled into the mixture at −5 °C for 30 min. The mixture was filtered. The filtrate was concentrated and chromatographed (dichloro-methane:acetone, 75:25) to yield 80 (0.095 g, 0.19 mmol, 95%) as an off-white powder. 1H NMR (CDCl3, 400 MHz) δ 1.52–1.64 (m, 1H), 1.94–2.01 (m, 2H), 2.10–2.17 (m, 1H), 2.20 (t, J = 7.3 Hz, 2H), 2.46–2.62 (m, 4H), 2.69 (t, J = 7.3 Hz, 2H), 2.73 (d, J = 4.7 Hz, 3H), 6.09 (bs, 1H), 7.16 (d, J = 8.2 Hz, 2H), 7.33 (d, J = 8.2 Hz, 2H), 7.82 (dd, J = 8.3, 1.8 Hz, 1H), 7.91 (d, J = 8.3 Hz, 1H), 7.94 (d, J = 1.8 Hz, 1H); 13C NMR (CDCl3, 100 MHz) δ 13.7, 26.2, 26.8, 31.4, 35.0, 35.7, 67.5, 109.7, 114.9, 121.9 (q, J = 272.7 Hz), 127.1 (q, J = 4.7 Hz), 129.7, 130.0, 132.3, 133.3 (q, J = 33.2 Hz), 133.8, 135.2, 137.3, 143.7, 173.3, 174.9, 179.8.
Synthesis of 81–83
3-[4-(1-cyanocyclobutylamino)phenyl]propanoic acid, 81a
Trimethylsilyl cyanide (0.4 g, 4 mmol) was added dropwise to a mixture of 3-(4-aminophenyl)propionic acid (0.33 g, 2 mmol), cyclobutanone (0.35 g, 5 mmol) and sodium sulfate (1 g) in 1,4-dioxane (5 mL). The mixture was stirred for 15 h. After filtration to remove sodium sulfate, the mixture was concentrated under vacuum to obtain a brown liquid which was subjected to chromatography (dichloromethane: acetone, 50:50) to yield 81a (0.472 g, 1.93 mmol, 97%) as a yellowish solid.
3-{4-[7-(4-Cyano-3-trifluoromethylphenyl)-8-oxo-6-thioxo-5,7-diazaspiro[3.4]oct-5-yl]phenyl} propanoic acid methyl ester, 81b
A mixture of isothiocyanate 12a (0.661 g, 2.9 mmol) and 81a (0.472 g, 1.93 mmol) in DMF (2 mL) was stirred at 21 °C for 15 h. To this mixture were added methanol (10 mL) and aq. 2 N HCl (5 mL, 2 M). The second mixture was refluxed for 3 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (10 mL) and extracted with ethyl acetate (3 × 30 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane) to yield 81b (0.582 g, 1.19 mmol, 62%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.60–1.70 (m, 1H), 2.14–2.26 (m, 1H), 2.51–2.56 (m, 2H), 2.58–2.67 (m, 2H), 2.71 (t, J = 7.8 Hz, 2H), 3.05 (t, J = 7.8 Hz, 2H), 3.69 (s, 3H), 7.23 (d, J = 8.2 Hz, 2H), 7.41 (d, J = 8.2 Hz, 2H), 7.85 (dd, J = 8.3, 1.8 Hz, 1H), 7.95 (d, J = 8.3 Hz, 1H), 7.98 (d, J = 1.8 Hz, 1H); 13C NMR (CDCl3, 100 MHz) δ 13.7, 30.5, 31.4, 35.1, 51.8, 67.5, 109.9, 114.9, 121.9 (q, J = 272.7 Hz), 127.1 (q, J = 4.7 Hz), 129.9, 130.0, 132.3, 133.2, 133.3 (q, J = 33.2 Hz), 135.7, 137.2, 142.5, 173.1, 174.9, 179.9.
3-{4-[7-(4-Cyano-3-trifluoromethylphenyl)-8-oxo-6-thioxo-5,7-diaza-spiro[3.4]oct-5-yl]phenyl} propanoic acid, 81c
A mixture of 81b (0.487 g, 1 mmol) in methanol (10 mL) and solution of sodium hydroxide (10 mL, 2 M) was stirred at 21°C for 5 h. The methanol was evaporated. The residue was adjusted to pH 5 by aq. 2 N HCl and was then extracted with ethyl acetate (3×50 mL). The organic layer was dried over MgSO4 and concentrated to dryness to obtain 81c (0.472 g, 0.99 mmol, 99%).
3-{4-[7-(4-Cyano-3-trifluoromethylphenyl)-8-oxo-6-thioxo-5,7-diaza-spiro[3.4]oct-5-yl]phenyl} propanamide, 81
To a suspension of 81c (0.094 g, 0.2 mmol) in THF (10 mL) cooled at −5 °C was added thionyl chloride (0.019 mL, 0.26 mmol). The medium was stirred at −5 °C for 1 h. Then ammonia was bubbled into the mixture. The excess ammonia was condensed by a reflux condenser cooled to −78 °C for 30 min and then was allowed to evaporate. The mixture was filtered. The filtrate was concentrated and chromatographed (dichloromethane:acetone, 70:30) to yield 81 (0.09 g, 0.19 mmol, 95%) as an off-white powder. 1H NMR (acetone-d6, 400 MHz) δ 1.52–160 (m, 1H), 2.01–2.09 (m, 1H), 2.49–2.58 (m, 4H), 2.61–2.67 (m, 2H), 2.98 (t, J = 7.5 Hz, 2H), 6.20 (bs, 1H), 6.78 (bs, 1H), 7.31 (d, J = 8.2 Hz, 2H), 7.44 (d, J = 8.2 Hz, 2H), 8.03 (dd, J = 8.3 Hz, 1.8 Hz, 1H), 8.15 (d, J = 1.8 Hz, 1H), 8.22 (d, J = 8.3 Hz, 1H); 13C NMR (acetone-d6, 100 MHz) δ 13.4, 30.7, 31.2, 36.4, 67.5, 109.0, 114.8, 122.5 (q, J = 271.5 Hz), 127.5 (q, J = 4.7 Hz), 129.5, 130.0, 131.8 (q, J = 32.5 Hz), 133.3, 133.8, 135.6, 138.4, 143.2, 171.6, 174.9, 178.0.
3-{4-[7-(4-Cyano-3-trifluoromethylphenyl)-8-oxo-6-thioxo-5,7-diaza-spiro[3.4]oct-5-yl]phenyl}-N-methylpropanamide, 82
To a suspension of 81c (0.094 g, 0.2 mmol) in THF (10 mL) cooled to −5 °C was added thionyl chloride (0.019 mL, 0.26 mmol). The mixture was stirred at −5 °C for 1 h. Then methylamine was bubbled into the mixture at −5 °C for 30 min. The medium was filtered. The filtrate was concentrated and chromatographed (dichloromethane:ace-tone, 75:25) to yield 82 (0.092 g, 0.19 mmol, 95%) as an off-white powder. 1H NMR (acetone-d6, 400 MHz) δ 1.51–1.60 (m, 1H), 2.01–2.11 (m, 1H), 2.48–2.58 (m, 4H), 2.61–2.67 (m, 2H), 2.77 (d, J = 4.6 Hz, 3H), 2.98 (t, J = 7.5 Hz, 2H), 7.03 (bs, NH), 7.33 (d, J = 8.2 Hz, 2H), 7.42 (d, J = 8.2 Hz, 2H), 8.01 (dd, J = 8.3, 1.8 Hz, 1H), 8.13 (d, J = 1.8 Hz, 1H), 8.20 (d, J = 8.3 Hz, 1H); 13C NMR (acetone-d6, 100 MHz) δ 13.4, 25.3, 30.0, 31.2, 37.0, 67.6, 109.0, 114.8, 122.5 (q, J = 271.5 Hz), 127.4 (q, J = 4.7 Hz), 129.5, 130.0, 131.9 (q, J = 32.5 Hz), 133.3, 133.8, 135.6, 138.4, 143.1, 171.7, 175.0, 178.0.
3-{4-[7-(4-Cyano-3-trifluoromethylphenyl)-8-oxo-6-thioxo-5,7-diaza-spiro[3.4]oct-5-yl]-phenyl}-N-(2-hydroxyethyl)propanamide, 83
To a suspension of 81c (0.094 g, 0.2 mmol) in THF (10 mL) cooled to −5 °C was added thionyl chloride (0.019 mL, 0.26 mmol). The mixture was stirred at −5 °C for 1 h. Then 2-aminoethanol (0.0183 g, 0.03 mmol) was added into the mixture at −5 °C. After stirring for an additional 30 min, the mixture was filtered. The filtrate was concentrated and chromatographed (dichloromethane:acetone, 50:50) to yield 83 (0.093 g, 0.18 mmol, 90%) as an off-white powder. 1H NMR (acetone-d6, 400 MHz) δ 1.51–161 (m, 1H), 2.01–2.11 (m, 1H), 2.49–2.66 (m, 6H), 2.99 (t, J = 7.5 Hz, 2H), 3.27 (dd, J = 11.2, 5.6 Hz, 2H), 3.51 (dd, J = 11.2, 5.6 Hz, 2H), 3.87 (bs, OH), 7.20 (bs, NH), 7.33 (d, J = 8.2 Hz, 2H), 7.43 (d, J = 8.2 Hz, 2H), 8.02 (dd, J = 8.3, 1.8 Hz, 1H), 8.14 (d, J = 1.8 Hz, 1H), 8.22 (d, J = 8.3 Hz, 1H); 13C NMR (acetone-d6, 100 MHz) δ 13.4, 31.0, 31.2, 37.1, 42.0, 61.2, 67.6, 109.0, 114.8, 122.5 (q, J = 271.5 Hz), 127.4 (q, J = 4.7 Hz), 129.6, 130.0, 131.9 (q, J = 32.5 Hz), 133.3, 133.8, 135.6, 138.4, 143.0, 171.9, 175.0, 178.1.
Synthesis of 84–85
4-(4-Aminophenyl)piperazine-1-carboxylic acid tert-butyl ester, 84a
A mixture of 4-iodoaniline (0.654 g, 3 mmol), piperazine-1-carboxylic acid tert-butyl ester (0.67 g, 3.6 mmol), potassium phosphate (1.272 g, 6 mmol), ethylene glycol (0.33 mL) and copper iodide (0.03 g, 0.15 mmol) in 2-propanol (3 mL) was placed in a sealed tube and heated under argon atmosphere to 80 °C for 30 h. After being cooled to 21 °C, the mixture was washed with water (50 mL) and extracted with ethyl acetate (100 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane:acetone, 70:30) to yield 84a (0.36 g, 1.3 mmol, 43%) as a yellow powder.
4-[4-(1-Cyanocyclobutylamino)phenyl]piperazine-1-carboxylic acid tert-butyl ester, 84b
Trimethylsilyl cyanide (0.3 g, 3 mmol) was added dropwise to a mixture of 84a (0.415 g, 1.5 mmol), cyclobutanone (0.21 g, 3 mmol) and sodium sulfate (1 g) in dichloromethane (5 mL). The mixture was stirred for 15 h. After filtration to remove the sodium sulfate, the mixture was concentrated under vacuum to obtain a brown liquid which was subjected to chromatography (dichloromethane:acetone, 75:25) to yield 84b (0.448 g, 1.26 mmol, 84%) as a yellow solid.
4-[8-Oxo-5-(4-piperazin-1-ylphenyl)-6-thioxo-5,7-diazaspiro[3.4]oct-7-yl]-2-trifluoromethylbenzonitrile, 84
A mixture of isothiocyanate 12a (0.228 g, 1 mmol) and 84b (0.472 g, 0.63 mmol) in DMF (1 mL) was stirred at 21 °C for 20 h. The mixture was concentrated and chromatographed (dichloromethane:acetone, 90:10) to yield the crude thiohydantoin imine 84c (0.173 g, 0.296 mmol, 47%) as an off-white powder. A mixture of this crude imine 84c (0.117 g, 0.2 mmol), methanol (5 mL) and aq. 2 N HCl (2 mL) was refluxed for 2 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (10 mL) and extracted with ethyl acetate (3 × 30 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane:acetone, 50:50 and then methanol:acetone, 50:50) to yield 84 (0.089 g, 0.184 mmol, 92%) as a white powder. 1H NMR (CD3OD, 400 MHz) δ 1.51–1.61 (m, 1H), 2.01–2.11 (m, 1H), 2.48–2.59 (m, 4H), 2.95 (t, J = 4.6 Hz, 4H), 3.19 (t, J = 4.7 Hz, 4H), 7.03 (d, J = 8.9 Hz, 2H), 7.16 (d, J = 8.9 Hz, 2H), 7.86 (dd, J = 8.3, 1.8 Hz, 1H), 8.02 (d, J = 8.3 Hz, 1H), 8.07 (d, J = 1.8 Hz, 1H); 13C NMR (CD3OD, 100 MHz) δ 13.2, 30.9, 45.1, 48.9, 67.5, 108.9, 114.8, 115.9, 122.3 (q, J = 271.7 Hz), 126.4, 127.3 (q, J = 4.7 Hz), 130.4, 132.2 (q, J = 33.2 Hz), 133.0, 135.4, 138.1, 152.1, 175.4, 180.4.
4-{5-[4-(4-Methanesulfonylpiperazin-1-yl)phenyl]-8-oxo-6-thioxo-5,7-diazaspiro[3.4]oct-7-yl}-2-trifluoromethylbenzonitrile, 85
A mixture of 84 (0.049g, 0.1 mmol), met-hanesulfonyl chloride (0.012 mL, 0.15 mmol) and triethylamine (0.15 mL) in dichloromethane was stirred at 21 °C for 5 h. The mixture was filtered and the filtrate was concentrated and chro-matographed (dichloromethane: acetone, 95:5) to yield 85 (0.042 g, 0.074 mmol, 74%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.62–1.70 (m, 1H), 2.14–2.23 (m, 1H), 2.51–2.58 (m, 2H), 2.61–2.67 (m, 2H), 2.84 (s, 3H), 3.39 (s, 8H), 7.05 (d, J = 8.9 Hz, 2H), 7.20 (d, J = 8.9 Hz, 2H), 7.84 (dd, J = 8.3, 1.8 Hz, 1H), 7.95 (d, J = 8.3 Hz, 1H), 7.97 (d, J = 1.8 Hz, 1H); 13C NMR (CDCl3, 100 MHz) δ 13.7, 31.4, 34.6, 45.7, 48.4, 67.5, 109.8, 114.9, 117.0, 121.9 (q, J = 272.7 Hz), 126.8, 127.1 (q, J = 4.7 Hz), 130.7, 132.3, 133.4 (q, J = 33.2 Hz), 135.2, 137.3, 151.1, 175.0, 180.2.
Synthesis of 86
1-(4-Methanesulfonylphenylamino)cyclobutanecarbonitrile, 86a
Trimethylsilyl cyanide (0.4 g, 4 mmol) was added dropwise to a mixture of 4-methanesulfonylphenylamine hydrochloride (0.415 g, 2 mmol), cyclobutanone (0.28 g, 4 mmol) and sodium sulfate (1 g) in DMF (3 mL). The mixture was stirred for 15 h at 120 °C. After filtration to remove the sodium sulfate, the filtrate was washed with brine and extracted with ethyl acetate. The organic layer was concentrated and chromatographed (dichloromethane:acetone, 90:10) to yield 86a (0.116 g, 0.44 mmol, 22%) as a yellowish solid. 4-Methanesulfonylphenylamine (0.201g, 1.17 mmol, 59%) was also recovered.
4-[5-(4-Methanesulfonylphenyl)-8-oxo-6-thioxo-5,7-diazaspiro[3.4]oct-7-yl]-2-trifluoromethylbenzonitrile, 86
A mixture of isothiocyanate 12a (0.0.141 g, 0.62 mmol) and 86a (0.11 g, 0.42 mmol) in dry DMF (2 mL) was stirred at 21 °C for 3 d. To this mixture were added methanol (10 mL) and aq. 2 N HCl (5 mL). The second mixture was refluxed for 3 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (10 mL) and extracted with ethyl acetate (3 × 30 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane:acetone, 97:3) to yield 86 (0.031 g, 0.065 mmol, 15%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.63–1.72 (m, 1H), 2.21–2.28 (m, 1H), 2.46–2.54 (m, 2H), 2.68.2.74 (m, 2H), 3.16 (s, 3H), 7.57 (d, J = 8.3 Hz, 2H), 7.85 (dd, J = 8.3, 1.8 Hz, 1H), 7.97 (d, J = 1.8 Hz, 1H), 7.98 (d, J = 8.3 Hz, 1H), 8.17 (d, J = 8.3 Hz, 2H); 13C NMR (CDCl3, 100 MHz) δ 13.6, 31.8, 44.4, 67.5, 110.2, 114.8, 122.4 (q, J = 271.5 Hz), 127.0 (q, J = 4.9 Hz), 129.4, 131.4, 132.1, 133.6 (q, J = 33.3 Hz), 135.3, 136.8, 140.3, 141.8, 174.4, 179.9.
Synthesis of 87–88
4-Aminobenzoic acid methyl ester, 87a
Concentrated sulfuric acid was slowly added to a mixture of 4-aminobenzoic acid (4 g, 29.2 mmol) in methanol cooled to 0 °C. After the addition, the mixture was stirred at 21 °C for 5 h. The mixture was washed with a saturated solution of sodium bicarbonate and extracted with ethyl acetate. The organic layer was dried over MgSO4 and concentrated under vacuum to obtain 87a (4.22 g, 27.9 mmol, 96%) as an off-white solid.
4-[(Cyanodimethylmethyl)-amino]-benzoic acid methyl ester, 87b
A mixture of 4-aminobenzoic acid methyl ester (0.32 g, 2.12 mmol), acetone cyanohydrin (3mL) and sodium sulfate (1 g) was refluxed for 15 h. After filtration to remove the sodium sulfate, the filtrate was washed with brine and extracted with ethyl acetate. The organic layer was concentrated and chromatographed (dichloromethane:acetone, 60:40) to yield 87b (0.398 g, 1.95 mmol, 92%) as a white solid.
4-[3-(4-Cyano-3-trifluoromethylphenyl)-5,5-dimethyl-4-oxo-2-thioxo-imidazolidin-1-yl]benzoic acid methyl ester, 87
A mixture of isothiocyanate 12a (0.228 g, 1 mmol) and 87b (0.14 g, 0.64 mmol) in DMF (2 mL) was heated under microwave irradiation at 60 °C for 12 h. To this mixture were added methanol (6 mL) and aq. 2 N HCl (2 mL). The second mixture was refluxed for 4 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (10 mL) and extracted with ethyl acetate (3 × 30 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane; dichloromethane:acetone, 75:25) to yield 87 (0.18 g, 0.4 mmol, 63%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.60 (s, 6H), 3.95 (s, 3H), 7.40 (d, J = 8.6 Hz, 2H), 7.84 (dd, J = 8.2, 1.9 Hz, 1H), 7.96 (d, J = 1.2 Hz, 1H), 7.97 (d, J = 8.2 Hz, 1H), 8.21 (d, J = 8.6 Hz, 2H); 13C NMR (CDCl3, 100 MHz) δ 23.8, 52.6, 66.6, 110.3, 114.8, 121.9 (q, J = 272.7 Hz), 127.1 (q, J = 4.7 Hz), 129.8, 131.2, 131.4, 132.2, 133.5 (q, J = 32.3 Hz), 135.3, 137.0, 139.2, 165.9, 174.7, 179.7.
4-[3-(4-Cyano-3-trifluoromethylphenyl)-5,5-dimethyl-4-oxo-2-thioxo-imidazolidin-1-yl]-N-methylbenzamide, 88
A mixture of 87 (0.02 g, 0.0435 mmol) and methylamine (2 mL distilled from its 40% aqueous solution) was kept at −20 °C for 15 h. After evaporation of the methylamine, the mixture was chromatographed (dichloromethane:acetone, 80:20) to yield 88 (0.01 g, 0.0224 mmol, 51%). The ester 87 (0.08 g, 0.0179 mmol, 41%) was also recovered. 1H NMR (Acetone-d6, 400 MHz) δ 1.60 (s, 6H), 2.90 (d, J = 4.6 Hz, 3H), 7.48 (d, J = 8.6 Hz, 2H), 7.80 (bs, 1H), 7.99 (d, J = 8.6 Hz, 2H), 8.06 (dd, J = 8.2, 1.8 Hz, 1H), 8.18 (d, J = 1.8 Hz, 1H), 8.25 (d, J = 8.2 Hz, 1H); 13C NMR (Acetone-d6, 100 MHz) δ 23.8, 54.0, 66.5, 110.3, 114.8, 121.9 (q, J = 272.7 Hz), 127.1 (q, J = 4.7 Hz), 128.2, 129.9, 133.5 (q, J = 32.3 Hz), 135.7, 135.8, 138.2, 138.3, 139.2, 166.0, 174.9, 179.7.
Synthesis of 89
4-(1-Cyanocyclobutylamino)benzoic acid, 89a
Sodium cyanide (0.245 g, 5 mmol) was added to a mixture of 4-aminobenzoic acid (0.274 g, 2 mmol) and cyclobutanone (0.21 g, 3 mmol) in 90% acetic acid (4.5 mL). The reaction mixture was stirred at 21 °C for 15 h. The mixture was washed with aqueous HCl (pH 2) and extracted with ethyl acetate. The organic layer was dried over magnesium sulfate and concentrated to dryness under vacuum to yield 89a (0.426 g, 1.97 mmol, 99%) as a white solid.
4-[7-(4-Cyano-3-trifluoromethylphenyl)-8-oxo-6-thioxo-5,7-diazaspiro[3.4]oct-5-yl]-benzoic acid methyl ester, 89b
A mixture of isothiocyanate 12a (0.51 g, 2.22 mmol) and 89a (0.343 g, 1.59 mmol) in DMF (2 mL) was heated under microwave irradiation at 60 °C and stir-red for 16 h. To this mixture were added methanol (10 mL) and aq. 2 M HCl (5 mL). The second mixture was refluxed for 12 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (20 mL) and extracted with ethyl acetate (3 × 30 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane:acetone, 95:5) to yield 89b (0.09 g, 0.196 mmol, 12%) as a white powder. 1H NMR (CDCl3, 400 MHz) δ 1.67–1.71 (m, 1H), 2.20–2.26 (m, 1H), 2.49–2.57 (m, 2H), 2.66–2.73 (m, 2H), 3.96 (s, 3H), 7.42 (d, J = 8.4 Hz, 2H), 7.85 (dd, J = 8.3, 1.7 Hz, 1H), 7.97 (d, J = 8.3 Hz, 1H), 7.98 (d, J = 1.7 Hz, 1H), 8.26 (d, J = 8.3 Hz, 2H); 13C NMR (CDCl3, 100 MHz) δ 13.7, 31.6, 52.6, 67.5, 110.1, 114.8, 121.8 (q, J = 272.7 Hz), 127.0 (q, J = 4.7 Hz), 130.2, 131.4, 131.5, 132.2, 133.4 (q, J = 33.2 Hz), 135.2, 137.0, 139.2, 165.9, 174.6, 179.7.
N-methyl-4-[7-(4-cyano-3-trifluoromethylphenyl)-8-oxo-6-thioxo-5,7-diazaspiro-[3.4]oct-5-yl]benzamide, 89
A mixture of 89b (0.046 g, 0.1 mmol) and methylamine (1 mL distilled from its 40% aqueous solution) was kept at −20 °C for 15 h. After evaporation of the methylamine, the mixture was chromatographed (dichloromethane:acetone, 80:20) to yield 89 (0.041 g, 0.085, 84%). 1H NMR (CDCl3, 400 MHz) δ 1.63–1.70 (m, 1H), 2.18–2.26 (m, 1H), 2.48–2.56 (m, 2H), 2.65–2.71 (m, 2H), 3.05 (d, J = 4.8 Hz, 3H), 6.32 (bs, 1H), 7.39 (d, J = 8.3 Hz, 2H), 7.84 (dd, J = 8.3, 1.7 Hz, 1H), 7.95–7.98 (m, 4H); 13C NMR (CDCl3, 100 MHz) δ 13.6, 27.0, 31.6, 67.4, 110.3, 114.8, 121.8 (q, J = 272.7 Hz), 127.0 (q, J = 4.7 Hz), 128.7, 130.3, 132.1, 133.3 (q, J = 33.2 Hz), 135.2, 136.3, 137.0, 137.8, 167.2, 174.6, 179.8.
Synthesis of 91
N-Methyl-2-fluoro- 4-nitrobenzamide, 91a
Thionyl chloride (2.38 g, 20 mmol) was added slowly to a solution of 2-fluoro-4-nitrobenzoic acid (2.97 g, 16 mmol) in DMF (50 mL) cooled at −5 °C. The mixture was stirred for an additional 1 h at −5 °C. Methylamine (0.62 g, 20 mmol; freshly distilled from its 40%aqueous solution) was added to the reaction medium. The second mixture was stirred for an additional 1 h. Ethyl acetate (300 mL) was added to the mixture, which was washed with brine (3 × 150 mL). The organic layer was dried over MgSO4, and concentrated to yield 91a (2.89 g, 14.6 mmol, 91%) as a yellow solid. 1H NMR (acetone-d6, 400 MHz) δ 3.05 (d, J = 4.3 Hz, 3H), 6.31 (dd, J = 13.5, 2.1 Hz, 1H), 6.40 (dd, J = 8.5, 2.1 Hz, 1H), 7.64 (dd, J = 8.6, 8.6 Hz, 1H).
N-Methyl-2-fluoro-4-aminobenzamide, 91b
A mixture of 91a (2.89 g, 14.6 mmol) and iron (5.04 g, 90 mmol) in ethyl acetate (40 mL) and acetic acid (40 mL) was refluxed for 1 h. The solid particles were filtered off. The filtrate was washed with water and extracted with ethyl acetate. The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane:acetone, 95:5) to yield 91b (2.3 g, 13.7 mmol, 94%) as an off-white solid. 1H NMR (acetone-d6, 400 MHz) δ 2.86 (d, J = 4.3 Hz, 3H), 5.50 (bs, 2H), 6.37 (dd, J1 = 14.7, 2.1 Hz, 1H), 6.50 (dd, J = 8.5, 2.1 Hz, 1H), 7.06 (bs, 1H), 7.68 (dd, J = 8.8, 8.8 Hz, 1H); 13C NMR (acetone-d6, 100 MHz) δ 25.8, 99.6 (d, J = 13.8 Hz), 109.2 (d, J = 12.8 Hz), 110.0 (d, J = 1.6 Hz), 132.5 (d, J = 4.8 Hz), 153.5 (d, J = 12.6 Hz), 162.2 (d, J = 242.5 Hz), 164.0 (d, J = 3.1 Hz).
N-Methyl-4-(1-cyanocyclobutylamino)-2-fluorobenzamide, 91c
Sodium cyanide (1.47 g, 30 mmol) was added to a mixture of 91b (1.68 g, 10 mmol) and cyclobutanone (1.4 g, 20 mmol) in 90% acetic acid (20 mL). The reaction mixture was stirred at 80 °C for 24 h. The mixture was washed with water and extracted with ethyl acetate. The organic layer was dried over magnesium sulfate and concentrated to dryness under vacuum. The solid was washed with a 50:50 mixture of ethyl ether and hexane (10 mL) to remove cyclobutanone cyanohydrin to afford after filtration 91c (2.19 g, 8.87 mmol, 89%). 1H NMR (CDCl3, 400 MHz) δ 1.87–1.95 (m, 1H), 2.16–2.27 (m, 1H), 2.35–2.41 (m, 2H), 2.76–2.83 (m, 2H), 2.97 (d, J = 4.4 Hz, 3H), 4.68 (bs, 1H), 6.29 (dd, J = 14.3, 1.8 Hz, 1H), 6.48 (dd, J = 8.3, 1.8 Hz, 1H), 6.75 (q, J = 4.4 Hz, 1H), 7.90 (dd, J = 8.3, 8.3 Hz, 1H); 13C NMR (CDCl3, 100 MHz) δ 15.7, 26.7, 33.9, 49.4, 100.2 (d, J = 29.5 Hz), 110.6, 111.0 (d, J = 11.8 Hz), 120.8, 133.1 (d, J = 4.2 Hz), 148.4 (d, J = 12.0 Hz), 162.0 (d, J = 244.1 Hz), 164.4 (d, J = 3.6 Hz).
N-Methyl-4-[7-(4-cyano-3-trifluoromethylphenyl)-8-oxo-6-thioxo-5,7-diazaspiro-[3.4]oct-5-yl]-2-fluorobenzamide, 91
A mixture of isothiocyanate 12a (2.16 g, 9.47 mmol) and 91c (1.303 g, 5.27 mmol) in DMF (20 mL) was heated under microwave irradiation at 80 °C for 16 h. To this mixture was added methanol (50 mL) and aq. 2 N HCl (20 mL). The second mixture was refluxed for 3 h. After being cooled to 21 °C, the reaction mixture was poured into cold water (100 mL) and extracted with ethyl acetate (150 mL). The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane:acetone, 95:5) to yield 91 (1.43 g, 3.0 mmol, 57%) as a yellow powder. 1H NMR (CDCl3, 400 MHz) δ 1.65–1.75 (m, 1H), 2.18–2.30 (m, 1H), 2.49–2.57 (m, 2H), 2.67–2.73 (m, 2H), 3.07 (d, J = 4.4 Hz, 3H), 6.75 (q, J = 4.6 Hz, 1H), 7.17 (dd, J = 11.5, 1.9 Hz, 1H), 7.26 (dd, J = 8.3, 1.9 Hz, 1H), 7.83 (dd, J = 8.2, 2.0 Hz, 1H), 7.95 (d, J = 1.8 Hz, 1H), 7.97 (d, J = 8.3 Hz, 1H) 8.30 (dd, J = 8.3, 8.3 Hz, 1H); 13C NMR (CDCl3, 100 MHz) δ 13.6, 27.0, 31.7, 67.4, 110.3, 114.8, 118.2, 118.5, 121.9 (q, J = 272.7 Hz), 126.6, 127.0 (q, J = 4.8 Hz), 132.1, 133.3 (q, J = 33.2 Hz), 133.8, 135.3, 136.8, 139.1 (d, J = 10.9 Hz), 160.5 (d, J = 249.1 Hz), 162.7 (d, J = 3.3 Hz), 174.3, 179.8; 19F NMR (CDCl3, 100 MHz) δ −111.13, −62.58.
Synthesis of 92
2-Fluoro-4-nitrobenzoic acid, 92a
Periodic acid (1.69 g, 7.41 mmol) was dissolved in acetonitrile (25 mL) by vigorous stirring, and then chromium trioxide (0.16 g, 1.60 mmol) was dissolved into the solution. 2-fluoro-4-nitrotoluene (0.33 g, 2.13 mmol) was added to the above solution with stirring. A white precipitate formed immediately with exothermic reaction. After 1 h of stirring, the supernatant liquid of the reaction mixture was decanted to a flask, and the solvent was removed by evaporation. The residues were extracted with dichloromethane (2×30 mL) and water (2×30 mL). The organic layer was dried over MgSO4, and concentrated to give 92a (0.32 mg, 81%) as a white solid. 1H NMR (CDCl3, 400 MHz) δ 8.06 (ddd, 1H, J = 9.9, 2.2, 0.3 Hz), 8.13 (ddd, 1H, J = 8.6, 2.2, 0.9 Hz), 8.25 (ddd, 1H, J = 8.6, 7.0, 0.3 Hz).
N-Methyl-2-fluoro-4-(1,1-dimethylcyanomethyl)aminobenzamide, 92b
A mixture of 91b (96 mg, 0.57 mmol), acetone cyanohydrin (0.3 mL, 3.14 mmol) and magnesium sulfate (50 mg) was heated to 80 °C and stirred for 12 h. To the medium was added ethyl acetate (25 mL) and then washed with water (2 × 25 mL). The organic layer was dried over MgSO4 and concentrated and the residue was purified with SiO2 column chromatography (dichloromethane:acetone, 95:5) to give 92b (101 mg, 75%) as a white solid. 1H NMR (CDCl3, 400 MHz) δ 1.74 (s, 6H), 2.98 (dd, 3H, J = 4.8, 1.1 Hz), 6.58 (dd, 1H, J = 14.6, 2.3 Hz), 6.63 (dd, 1H, J = 8.7, 2.3 Hz), 6.66 (br s, 1H), 7.94 (dd, 1H, J = 8.7, 8.7 Hz).
N-Methyl-4-[3-(4-cyano-3-trifluoromethylphenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl]-2-fluorobenzamide, 92
A mixture of 92b (30 mg, 0.13 mmol) and 12a (58 mg, 0.26 mmol) in DMF (1 mL) was heated under microwave irradiation at 100 °C for 11 h. To this mixture was added methanol (20 mL) and aq. 1 N HCl (5 mL). The second mixture was refluxed for 1.5 h. After being cooled to room temperature, the reaction mixture was poured into cold water (50 mL) and extracted with ethyl acetate (50 mL). The organic layer was dried over MgSO4, concentrated and the residue was purified with SiO2 column chromatography (dichloromethane:acetone, 95:5) to give 92 (30 mg, 51%) as colorless crystals. 1H NMR (CDCl3, 400 MHz) δ 1.61 (s, 6H), 3.07 (d, 3H, J = 4.1 Hz), 6.71 (m, 1 H), 7.15 (dd, 1H, J = 11.7, 2.0 Hz), 7.24 (dd, 1H, J = 8.4, 2.0 Hz), 7.83 (dd, 1H, J = 8.2, 2.1 Hz), 7.95 (d, 1H, J = 2.1 Hz), 7.99 (d, 1H, J = 8.2 Hz), 8.28 (dd, 1H, J = 8.4, 8.4 Hz). 13C NMR (CDCl3, 125 MHz) δ 23.8, 26.9, 66.5, 110.3, 114.6, 117.7, 117.9, 121.7 (q, J = 272.3 Hz), 126.1, 126.9 (q, J = 4.6 Hz), 132.0, 133.3, 133.6 (q, J = 33.4 Hz), 135.2, 136.7, 138.9 (d, J = 10.8 Hz), 160.3 (d, J = 248.6 Hz), 162.6 (d, J = 3.3 Hz), 174.3, 179.6. 19F NMR (CDCl3, 100 MHz) δ −111.13, −62.58. HRMS: found 465.1023 [M+H]+, calculated for [C21H16F4N4O2S+H]+ 465.1003.
Synthesis of 93
N-Methyl-4-(1-cyanocyclopentylamino)-2-fluorobenzamide, 93a
A mixture of 91b (62 mg, 0.37 mmol), cyclopentanone (0.07 mL, 0.74 mmol) and TMSCN (0.1 mL, 0.74 mmol) was heated to 80 °C and stirred for 13 h. To the medium was added ethyl acetate (2 × 20 mL) and then washed with water (2 × 20 mL). The organic layer was dried over MgSO4 and concentrated and the residue was purified with SiO2 column chromatography (dichloromethane:acetone, 95:5) to give 93a (61 mg, 63%) as a white solid. 1H NMR (CDCl3, 400 MHz) δ 1.82–1.95 (m, 4H), 2.10–2.18 (m, 2H), 2.36–2.45 (m, 2H), 2.99 (dd, 3H, J = 4.8, 1.1 Hz), 4.60 (br s, 1H), 6.50 (dd, 1H, J = 14.6, 2.3 Hz), 6.59 (dd, 1H, J = 8.8, 2.3 Hz), 6.65 (br s, 1H), 7.95 (dd, 1H, J = 8.8, 8.8 Hz).
N-Methyl-4-[3-(4-cyano-3-trifluoromethylphenyl)-4-oxo-2-thioxo-1,3-diazaspiro[4.4]nonan-1-yl]-2-fluorobenzamide, 93
A mixture of 193a (57 mg, 0.22 mmol) and 12a (0.15 g, 0.65 mmol) in DMF (3 mL) was heated under microwave irradiation at 130 °C for 12 h. To this mixture was added methanol (20 mL) and aq. 1 N HCl (5 mL). The second mixture was refluxed for 1.5 h. After being cooled to room temperature, the reaction mixture was poured into cold water (50 mL) and extracted with ethyl acetate (50 mL). The organic layer was dried over MgSO4, concentrated and the residue was purified with SiO2 column chromatography (dichloromethane:acetone, 95:5) to give 93 (56 mg, 48%) as a pale yellowish solid. 1H NMR (CDCl3, 400 MHz) δ 1.49–1.59 (m, 2H), 1.85–1.96 (m, 2H), 2.13–2.21 (m, 2H), 2.32–2.41 (m, 2H), 3.07 (d, 3H, J = 4.3 Hz), 6.67–6.77 (m, 1H), 7.17 (dd, 1H, J = 11.7, 1.8 Hz), 7.27 (dd, 1H, J = 8.4, 1.8 Hz), 7.84 (dd, 1 H, J = 8.3, 1.8 Hz), 7.96 (d, 1H, J = 1.8 Hz), 7.98 (d, 1H, J = 8.3 Hz), 8.28 (dd, 1H, J = 8.4, 8.4 Hz). 13C NMR (CDCl3, 125 MHz) δ 25.1, 26.9, 36.3, 75.2, 110.2, 114.6, 118.1, 118.3, 121.7 (q, J = 275.9 Hz), 126.4, 127.0 (q, J = 3.8 Hz), 132.0, 133.3, 133.5 (q, J = 33.4 Hz), 135.1, 136.8, 139.6 (d, J = 10.1 Hz), 160.3 (d, J = 245.4 Hz), 162.5 (d, J = 3.3 Hz), 175.5, 179.9. 19F NMR (CDCl3, 100 MHz) δ −111.23, −62.57. HRMS: found 491.1156 [M+H]+, calculated for [C23H18F4N4O2S+H]+ 491.1159.
Synthesis of 94
4-[4-(2,2,2-Trifluoroacetylamino)phenyl]butyric acid, 94a
Trifluoroacetic anhydride (0.85 mL, 6.14 mmol) was added to a solution of 4-(4-aminophenyl)butyric acid (0.5 g, 2.79 mmol) in chloroform (10 mL) at 0 °C. The mixture was warmed to room temperature and stirred for 3 h. The mixture was partitioned with chloroform (20 mL) and water (20 mL). The organic layer was dried over MgSO4, concentrated and the residue was purified with SiO2 column chromatography (dichloromethane:acetone, 9:1) to give 94a (0.53 g, 69%). 1H NMR (CDCl3, 400 MHz) δ 1.96 (p, 2H, J = 7.5 Hz), 2.38 (t, 2H, J = 7.5 Hz), 2.68 (t, 2H, J = 7.5 Hz), 7.22 (d, 2H, J = 8.5 Hz), 7.48 (d, 2H, J = 8.5 Hz), 7.81 (br s, 1H).
N,N-Dimethyl-4-[4-(2,2,2-Trifluoroacetylamino)phenyl]butyramide, 94b
Thionyl chloride (71 mg, 0.60 mmol) was added slowly to a solution of 94a (0.15 g, 0.55 mmol) in DMF (5 mL) cooled at −5 °C. The mixture was stirred for an additional 1 h at −5 °C. Excess dimethylamine (freshly distilled from its 40% aqueous solution) was added to the reaction medium. The second mixture was stirred for an additional 1 h. Ethyl acetate (50 mL) was added to the mixture, which was washed with brine (2 × 50 ml). The organic layer was dried over MgSO4, and concentrated to yield 94b (0.17 g, quant.) as a yellowish solid. 1H NMR (CDCl3, 400 MHz) δ 1.89 (p, 2H, J = 7.7 Hz), 2.27 (t, 2H, J = 7.7 Hz), 2.60 (t, 2H, J = 7.7 Hz), 2.89 (s, 3H), 2.91 (s, 3H), 7.11 (d, 2H, J = 8.6 Hz), 7.55 (d, 2H, J = 8.6 Hz), 9.70 (br s, 1H).
N,N-Dimethyl-4-(4-aminophenyl)butyramide, 94c
A 1 N NaOH solution (3 mL) was added to a solution of 94b (0.17 g, 0.55 mmol) in MeOH (2 mL) at room temperature. The mixture was stirred for 14 h. The mixture was partitioned with chloroform (25 mL) and water (25 mL). The organic layer was dried over MgSO4, and concentrated and the residue was purified with SiO2 column chromatography (dichloromethane:acetone, 9:1) to give 94c (74 mg, 66%) as a white solid. 1H NMR (CDCl3, 400 MHz) δ 1.91 (p, 2H, J = 7.7 Hz), 2.28 (t, 2H, J = 7.7 Hz), 2.56 (t, 2H, J = 7.7 Hz), 2.92 (s, 6H), 3.56 (br s, 2H), 6.61 (d, 2H, J = 8.3 Hz), 6.97 (d, 2H, J = 8.3 Hz).
N,N-Dimethyl-4-[4-(1-cyanocyclobutylamino)phenyl]butyramide, 94d
A mixture of 94c (74 mg, 0.36 mmol), cyclobutanone (54 mg, 0.78 mmol) and TMSCN (77 mg, 0.78 mmol) was heated to 80 °C and stirred for 15 h. To the medium was added ethyl acetate (2 × 20 mL) and then washed with water (2 × 20 mL). The organic layer was dried over MgSO4 and concentrated and the residue was purified with SiO2 column chromatography (dichloromethane:acetone, 9:1) to give 94d (58 mg, 57%) as a white solid. 1H NMR (CDCl3, 400 MHz) δ 1.93 (p, 2H, J = 7.6 Hz), 2.11–2.28 (m, 2H), 2.30 (t, 2H, J = 7.6 Hz), 2.33–2.42 (m, 2H), 2.60 (t, 2H, J = 7.6 Hz), 2.75–2.83 (m, 2H), 2.93 (s, 3H), 2.94 (s, 3H), 3.94 (br s, 1H), 6.59 (d, 2H, J = 8.5 Hz), 7.07 (d, 2H, J = 8.5 Hz).
N,N-dimethyl-4-[4-(7-(4-cyano-3-trifluoromethylphenyl)-8-oxo-6-thioxo-5,7-diazaspiro-[3.4]octan-5-yl)phenyl]butanamide, 94
A mixture of 94d (58 mg, 0.20 mmol) and 12a (74 mg, 0.32 mmol) in DMF (3 mL) was heated under reflux for 2 h. To this mixture was added methanol (20 mL) and aq. 1 N HCl (5 mL). The second mixture was refluxed for 1.5 h. After being cooled to room temperature, the reaction mixture was poured into cold water (50 mL) and extracted with ethyl acetate (50 mL). The organic layer was dried over MgSO4, concen-trated and the residue was purified with SiO2 column chromatography (dichloromethane:acetone, 95:5) to give 94 (44 mg, 42%) as a pale yellowish solid. 1H NMR (CDCl3, 400 MHz) δ 1.62–1.73 (m, 1H), 2.04 (p, 2H, J = 7.5 Hz), 2.15–2.30 (m, 1H), 2.40 (t, 2H, J = 7.5 Hz), 2.52–2.63 (m, 2 H), 2.62–2.70 (m, 2H), 2.78 (t, 2H, J = 7.5 Hz), 2.96 (s, 3H), 2.99 (s, 3H), 7.22 (d, 2H, J = 8.3 Hz), 7.42 (d, 2H, J = 8.3 Hz), 7.86 (d, 1H, J = 8.2 Hz), 7.97 (d, 1H, J = 8.2 Hz), 7.98 (s, 1H).
Synthesis of 95
4-[5-(4-(3-Cyanopropyl)phenyl)-8-oxo-6-thioxo-5,7-diazaspiro[3.4]octan-7-yl]-2-(trifluoromethyl) benzonitrile, 95
A solution of DMSO (0.01 mL, 0.12 mmol) in dry dichloromethane (1 mL) was added to a stirred solution of oxalyl chloride (0.01 mL, 0.09 mmol) in dry dichloromethane (2 mL) at −78 °C. After 15 min, a dichloromethane solution of 79 (35 mg, 0.07 mmol) was added to the reaction mixture. Stirring was continued for 20 min at -78 °C, and then triethylamine (0.03 mL, 0.22 mmol) was added. After 30 min at −78 °C, the reaction mixture was warmed to room temperature and then reaction was quenched with saturated aq. NH4Cl solution. The reaction mixture was diluted with dichloromethane, and extracted with dichloromethane. The organic layer was dried over MgSO4, concentrated and chromatographed (dichloromethane:acetone, 95:5) to yield 95 (29 mg, 87%) as a viscous oil. 1H NMR (CDCl3, 400 MHz) δ 1.63–1.73 (m, 1H), 2.07 (p, 2H, J = 7.3 Hz), 2.18–2.30 (m, 1H), 2.42 (t, 2H, J = 7.3 Hz), 2.52–2.62 (m, 2H), 2.63–2.73 (m, 2H), 2.90 (t, 2H, J = 7.3 Hz), 7.27 (d, 2H, J = 8.4), 7.43 (d, 2H, J = 8.4 Hz), 7.86 (dd, 1H, J = 8.3, 1.8 Hz), 7.98 (d, 1H, J = 8.3 Hz), 7.98 (d, 1 H, J = 1.8).
Acknowledgment
We thank CaPCURE and the Prostate Cancer Foundation for generous financial support. SO also acknowledges support from an NIH SPORE grant (5P50CA92131). CLS is an Investigator of the Howard Hughes Medical Institute and a Doris Duke Distinguished Clinical Scientist.
Abbreviations
- AR
androgen receptor
- CRPC
castration-resistant prostate cancer
- DHT
dihydrotestosterone
- DMSO
dimethyl sulfoxide
- FBS
fetal bovine serum
- HR
hormone refractory
- PK
pharmacokinetic
- PSA
prostate specific antigen
- SAR
structure-activity relationship
- SCID
severe combined immunodeficient
References
- 1.a) Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]; b) Gelmann EP. Molecular biology of the androgen receptor. J. Clin. Oncol. 2002;20:3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]; c) Balk SP. Androgen receptor as a target in androgen-independent prostate cancer. Urology. 2002;60:132–138. doi: 10.1016/s0090-4295(02)01593-5. [DOI] [PubMed] [Google Scholar]
- 2.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nature Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 3.Information on the clinical trials of this compound can be obtained at www.clinicaltrials.gov
- 4.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen C, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teutsch G, Goubet F, Battmann T, Bonfils A, Bouchoux F, Cerede E, Gofflo D, Gaillard-Kelly M, Philibert D. Non-steroidal antiandrogens: Synthesis and biological profile of high-affinity ligands for the androgen receptor. J. Steroid Biochem. Molec. Biol. 1994;48:111–119. doi: 10.1016/0960-0760(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 6.a) Matias PM, Donner P, Coelho R, Thomaz M, Peixoto C, Macedo S, Otto N, Joschko S, Scholz P, Wegg A, Bäsler S, Schäfer M, Egner U, Carrondo MA. Structural evidence for ligand specificity in the binding domain of the human Androgen receptor: implications for pathogenic gene mutations. J. Biol. Chem. 2000;275:26164–26171. doi: 10.1074/jbc.M004571200. [DOI] [PubMed] [Google Scholar]; b) Sack JS, Kish KF, Wang C, Attar RM, Kiefer SE, An Y, Wu GY, Scheffler JE, Salvati ME, Krystek SR, Jr, Weinmann R, Einspahr HM. Crystallographic structures of the ligand-binding domains of the androgen receptor and its T877A mutant complexed with the natural agonist dihydrotestosterone. Proc. Natl. Acad. Sci. 2001;98:4904–4909. doi: 10.1073/pnas.081565498. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ostrowski J, Kuhns JE, Lupisella JA, Manfredi MC, Beehler BC, Krystek SR, Jr, Bi Y, Sun C, Seethala R, Golla R, Sleph PG, Fura A, An Y, Kish KF, Sack JS, Mookhtiar KA, Grover GJ, Hamann LG. Pharmacological and x-ray structural characterization of a novel selective androgen receptor modulator: Potent hyperanabolic stimulation of skeletal muscle with hypostimulation of prostate in rats. Endocrinology. 2006;148:4–12. doi: 10.1210/en.2006-0843. [DOI] [PubMed] [Google Scholar]
- 7.Recently the x-ray crystal structure of the mutant W741L AR ligand-binding domain bound to R-bicalutamide was solved. This mutation results in agonist activity. Bohl CE, Gao W, Miller DD, Bell CE, Dalton JT. Structural basis for antagonism and resistance of bicalutamide in prostate cancer. Proc. Natl. Acad. Sci. USA. 2005;102:6201–6206. doi: 10.1073/pnas.0500381102.
- 8.Marhefka CA, Moore BM, II, Bishop TC, Kirkovsky L, Mukherjee A, Dalton JT, Miller DD. Homology Modeling Using Multiple Molecular Dynamics Simulations and Docking Studies of the Human Androgen Receptor Ligand Binding Domain Bound to Testosterone and Nonsteroidal Ligands. J. Med. Chem. 2001;44:1729–1740. doi: 10.1021/jm0005353. [DOI] [PubMed] [Google Scholar]
- 9.Schuurmans ALG, Bolt J, Voorhorst MM, Blankenstein RA, Mulder E. Regulation of growth and epidermal growth factor receptor levels of LNCaP prostate tumor cells by different steroids. Int. J. Cancer. 1988;42:917–922. doi: 10.1002/ijc.2910420622. [DOI] [PubMed] [Google Scholar]
- 10.Jung ME, Ouk S, Sawyers CL, Chen CD, Welsbie D. “Methods and Materials for Assessing Prostate Cancer Therapies and Compounds.”. 10/590,445 U.S. Patent Appl.
- 11.Sawyers CL, Jung ME, Chen CD, Ouk S, Welsbie D. “Novel Androgen Receptor Inhibitors with Minimal Agonistic Activities.”. 60/680,835 U.S. Patent Appl.
- 12.The fold changes in the tumors were measured once the tumors were palpable.
- 13.Jung ME, Sawyers CL, Ouk S, Tran C, Wongvipat J. “Selected Diarylthiohydantoin Compounds.”. 60/756.552 U.S. Patent Appl.
- 14.AR overexpressing LNCaP cells were injected in the flanks of castrated SCID mice, subcutaneously. When tumors reach about 100–200 cubic mm, they are randomized into five groups. Each group has six animals. After they reach this tumor volume, they are given orally either vehicle or 91 at 0.1, 1, and 10 mg/kg everyday. The tumors are measured three-dimensionally, width, length and depth, using a caliper.
- 15.LNCaP in 10% FBS, split on day 1, add drugs on day 5, harvest on day 6 and day 10 for PSA.