Abstract
Non‐small cell lung carcinomas (NSCLC) overexpress the Her2/neu gene in approximately 59% of cases. Trastuzumab, a humanized monoclonal antibody, interferes with Her2 signaling and is approved for the treatment of Her2/neu overexpressing breast cancer. However, its therapeutic use in Her2/neu overexpressing NSCLC remains obscure. The present study aimed to determine the role of 64Cu‐labeled trastuzumab positron emission tomography (PET) for non‐invasive imaging of Her2/neu expression in NSCLC. Trastuzumab was conjugated with the bifunctional chelator 1, 4, 7, 10‐tetraazacyclododecane‐1, 4, 7, 10‐tetraacetic acid (DOTA) and radiolabeled with 64Cu. The molecular specificity of DOTA‐trastuzumab was determined in NSCLC cell lines with Her2/neu overexpression (NCI‐H2170) and negative expression (NCI‐H520). Imaging of Her2/neu expression was performed in NCI‐H2170 tumor‐bearing mice with 64Cu‐DOTA‐trastuzumab PET and 64Cu‐DOTA‐IgG. In vitro studies revealed specific binding of DOTA‐trastuzumab in the Her2/neu positive NCI‐H2170 cells, while no binding was seen in the Her2/neu negative NCI‐H520 cell line. Biodistribution and PET studies revealed a significantly high accumulation of 64Cu‐DOTA‐trastuzumab in the Her2/neu overexpressing NCI‐H2170 tumor at 24 and 48 h post‐injection (21.4 ± 1.4% and 23.2 ± 5.1% injection dose/gram (% ID/g), respectively). PET imaging of Her2/neu negative NCI‐H520 tumors showed much less uptake of 64Cu‐DOTA‐trastuzumab (4.0% ID/g). The NCI‐H2170 tumor uptake of 64Cu‐DOTA‐trastuzumab was significantly higher than that of 64Cu‐DOTA‐IgG (P < 0.0001). 64Cu‐DOTA‐trastuzumab showed a very clear image of a Her2/neu positive tumor and appeared to be effective as a PET tracer for imaging of Her2/neu gene expression in NSCLC, suggesting its potential clinical use for identifying patients that might benefit from trastuzumab‐based therapy.
(Cancer Sci 2010; 101: 1045–1050)
Lung cancer is one of the world’s leading causes of death, with a 5‐year survival rate of less than 10%.( 1 ) Activation of ras genes and human epidermal growth factor receptor 2 (Her2/neu) genes is encountered in subpopulations of non‐small cell lung carcinoma (NSCLC) patients and has been linked to shortened survival. Overexpression of the Her2/neu gene is closely associated with intrinsic multiple drug resistance in NSCLC cell lines.( 2 ) Her2/neu is a transmembrane tyrosine kinase belonging to the surface receptor family. It does not bind ligand but instead acts as a preferred heterodimerization partner for ligand‐activated sibling members to amplify mitotic signaling.( 3 ) Trastuzumab, a humanized monoclonal antibody that targets Her2/neu, inhibits neoplastic cell proliferation both in vitro and in vivo.( 4 ) In breast cancer, overexpression of Her2/neu is seen in 40% of cases, and its activation follows heterodimerization with a member of the EGFR family and triggers important biological effects such as proliferation, migration and differentiation.( 5 ) Trastuzumab significantly increases the survival of patients with advanced metastatic breast cancer.( 6 , 7 )
Overexpression of Her2/neu is reported in up to 59% of cases of NSCLC and the 2+/3+ overexpression rate is 5–20% in adenocarcinomas.( 8 , 9 , 10 ) As in breast cancer, studies have suggested that the overexpression of Her2/neu in NSCLC is associated with a worse prognosis than negative expression of Her2/neu.( 11 , 12 ) The role of trastuzumab targeting Her2/neu expression in the NSCLC has been largely marginalized. Even though NSCLC is usually chemoresistant, the synergistic effect between trastuzumab and chemotherapeutic agents was found to be greater in Her2/neu positive NSCLC than in breast cancer cell lines.( 13 , 14 ) NSCLC patients with Her2/neu overexpression (3+) in immunohistochemistry (IHC) had better survival when treated with trastuzumab‐based therapy than the overall population, but only a small percentage of patients benefited.( 10 ) The marked variation in functional anatomy and pathophysiology within human tumors and within individual patients may account for the high variability of responses observed in patients.
Non‐invasive imaging of Her2/neu expression in NSCLC can help in many ways, particularly in patient stratification, before the use of trastuzumab‐based therapy in order to exclude patients that might not benefit. Monitoring of the anti‐Her2/neu treatment response can also be carried out more efficiently based on the non‐invasive quantification of Her2/neu expression and downregulation. The development of 111In‐labeled trastuzumab has shown specific uptake in human breast cancers and xenografts.
64Cu (half life 12.7 h, β+ 17.4%, β− 41%)‐labeled monoclonal antibody has been garnering interest in the field of targeted imaging due to its emission both β+ and β−, which allows a high tumor‐to‐background ratio for positron emission tomography (PET) imaging and targeted radiotherapy of cancer. The present study aimed to determine the potential role of 64Cu‐labeled trastuzumab positron emission tomography (PET) for non‐invasive imaging of Her2/neu expression in NSCLC.( 15 , 16 , 17 ) In this study we prepared 1, 4, 7, 10‐tetraazacyclododecane‐1, 4, 7, 10‐tetraacetic acid (DOTA)‐trastuzumab and radiolabeled it with 64Cu for PET imaging and biodistribution of Her2/neu overexpression in an NSCLC tumor model.
Materials and Methods
Cell line. The non‐small cell lung cancer cell lines NCI‐H2170 and NCI‐H520 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA), maintained in RPMI 1640 supplemented with 10% fetal bovine serum and 1% antibiotics (100 U/mL penicillin an 100 μg/mL streptomycin), and grown in 5% CO2 at 37°C in incubators with 100% humidity.
Chemicals. Trastuzumab (Herceptin®) was purchased from Chugai Pharmaceuticals (Tokyo, Japan). 64Cu was produced by the AVF cyclotron at the research facility Takasaki Ion Accelerators for Advanced Radiation Applications (TIARA) at the Japan Atomic Energy Association. Mono‐N‐hydoxysuccinimide ester DOTA(mDOTA) was purchased from Macrocyclics (Dallas, TX, USA).
Conjugation of trastuzumab with DOTA. To a solution of mDOTA (0.3 mg, 20 molar excess) in DMSO (5 μL), trastuzumab (4 mg/mL) in 500 μL borate buffered saline (0.1 M, pH 8.5) was added, and the mixture was incubated overnight at room temperature. The crude antibody was purified by using a Bio‐Spin 6 Tris column (Bio‐Rad, Foster City, CA, USA).
Immunocytochemistry. The NCI‐H2170 and NCI‐H520 cell lines were cultured in a 4‐well chamber. When the cells reached 80% confluence they were fixed with 4% paraformaldehyde for 45 min, washed with phosphate buffered saline (PBS) and incubated with DOTA‐trastuzumab (5 mg/mL, diluted at 1:2000) for 1 h at room temperature. After being thoroughly washed with PBS, they were incubated with anti‐human Alexa Fluor 488 (Molecular Probes, Eugene, CA, USA) at room temperature for 1 h. Mounting medium with DAPI (Vector, Burlingame, CA, USA) was used to fix and stain the cell nucleus. The cells were then observed under a fluorescence microscope (Olympus BX50; Olympus, Tokyo, Japan).
Radiolabeling. 64CuCl2 was provided in a dry state and was dissolved in sodium acetate buffer (0.25 M, pH 6.0). 30 μg of DOTA‐trastuzumab was added and the mixture was incubated at 40°C for 1.5 h. Then, 10 mm EDTA was added and the above solution was incubated for 15 min at 45°C. The labeling yield was checked with thin layer chromatography (TLC). The final purification was done using a Bio‐Spin 6 Tris column.
The average number of DOTA chelator molecules per trastuzumab molecule was determined using a non‐radioactive indium chloride (InCl3). To a known concentration of InCl3, DOTA‐trastuzumab in sodium acetate buffer (3 M, pH 6.0) was added and was radiolabeled with 111InCl3. The solution was incubated at 45°C for 90 min and the labeling yield was determined with TLC. The total number DOTA chelator molecules per trastuzumab molecule was calculated using the previously described equation with slight modification.( 16 ) The number of DOTA molecules per trastuzumab molecules = moles of InCl3 × labeling yield/moles (DOTA‐trastuzumab).
In vitro and in vivo stabilities. For the evaluation of in vitro stability, 64Cu‐DOTA‐trastuzumab was mixed with the murine serum, and the solution was incubated at 37°C. At 1, 24, and 48 h, aliquots of the radioactive sample were analyzed by size exclusion high performance liquid chromatography (SE‐HPLC). SE‐HPLC analysis was performed with a TSKgel Super SW3000 column (4.6 × 300 mm; Tosoh, Tokyo, Japan) eluted with 0.1 M phosphate buffer (pH 6.8). For evaluation of in vivo stability, blood was drawn from the hearts of mice at 1 and 48 h after the administration of 64Cu‐DOTA‐trastuzumab. Samples were centrifuged and passed through a polycarbonate membrane with a pore diameter of 0.45 μm. The radioactivity of the sample was analyzed by SE‐HPLC.
Small animal PET imaging and biodistribution. Animal experiments were conducted in accordance with our institutional guidelines and were approved by the Animal Care Committee. Six‐week‐old female Balb/c nude mice (CLEA, Tokyo, Japan) were maintained under specific pathogen‐free conditions and were provided with sterile food and water. Her2/neu overexpressing NCI‐H2170 or Her2/neu negative NCI‐H520 NSCLC cells (5 × 106 cells) were subcutaneously inoculated on the dorsal flank of the mice. When the tumor reached 5–8 mm in diameter, the mice were injected with about 8 MBq of 64Cu‐DOTA‐trastuzumab or 64Cu‐DOTA‐IgG via the tail vein. After anesthetizing with pentobarbital sodium, imaging was performed with a PET scanner (Inveon, Siemens, TN, USA). The 20–30 min static scans were obtained for every mouse. The images were reconstructed by using a 2‐dimensional ordered‐subsets expectation maximization algorithm. No correction was done for attenuation and scattering. Biodistribution was monitored after finishing the PET scan at 1, 24, and 48 h post‐injection. Mice were killed by decapitation and aliquots of blood were collected. The organs of interest were excised and weighed, and the radioactivity was determined with a γ‐counter (ARC‐7001; Aloka, Tokyo, Japan). The data were expressed as the % injected dose/gram (% ID/g).
Statistical analysis. Student’s t‐test was used to compare the difference of the variables. Data were expressed as the mean ± SD, and P‐values less than 0.05 were considered statistically significant.
Results
Radiolabeling of DOTA‐trastuzumab. The labeling efficiency of 64Cu‐DOTA‐trastuzumab was 92% without purification, and 99% after purification. The number of DOTA chelator molecules attached to the trastuzumab molecule was determined to be 5 to 6 per mole of trastuzumab.
Immunocytochemistry. Figure 1 shows the binding of DOTA‐trastuzumab on the membrane of Her2/neu positive NCI‐H2170 cells; no binding was seen in Her2/neu negative NCI‐H520 cells. Cells treated with human IgG also did not show any binding in Her2/neu positive NCI‐H2170 cells, confirming the binding specificity of DOTA‐trastuzumab to the Her2/neu.
Figure 1.
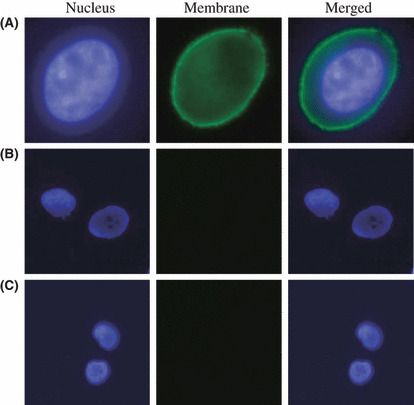
Fluorescence microscopy imaging of NSCLC cell lines. (A) Significant binding of DOTA‐trastuzumab is demonstrated in the membrane of Her2/neu positive NCI‐H2170 cells (magnification ×1200). (B) Her2/neu negative NCI‐H520 cells show no binding of DOTA‐trastuzumab (magnification ×800). (C) DOTA‐IgG did not show any binding to Her2/neu positive NCI‐H2170 cells (magnification ×800).
In vitro and in vivo stabilities. After incubation of 64Cu‐DOTA‐trastuzumab in murine serum for 48 h, 64Cu‐DOTA‐trastuzumab existed only in the intact form (retention time 19–20 min), and no transchelation to protein was seen (Fig. 2). Similarly, after administering 64Cu‐DOTA‐trastuzumab to mice, 64Cu‐DOTA‐trastuzumab was found only in the intact form and no transchelation was observed until 48 h which confirmed that 64Cu‐DOTA‐trastuzumab is a stable compound.
Figure 2.
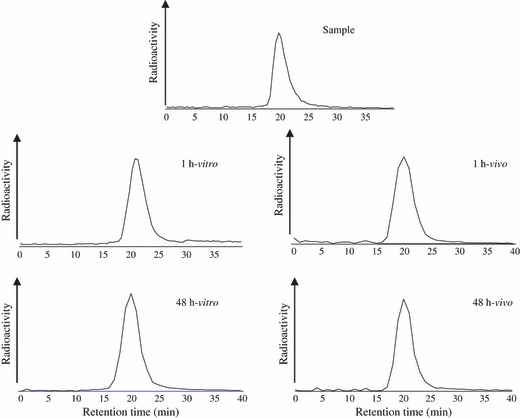
Radioactivity profiles of 64Cu‐DOTA‐tras‐tuzumab after incubation in murine serum (in vitro) for 1 and 48 h. The radioactivity of the sample was also analyzed. The right hand graph shows the radioactivity profiles in the blood of mice after administration of 64Cu‐DOTA‐trastuzumab at 1 and 48 h. The radioactivity was analyzed by SE‐HPLC.
Small animal PET imaging. Figure 3 shows the in vivo PET images of the Her2/neu positive NCI‐H2170 and Her2/neu negative NCI‐H520 xenografts obtained at several time points after injection of 64Cu‐DOTA‐trastuzumab or 64Cu‐DOTA‐IgG. Immediately after injection, radioactivity is present mainly in the well‐perfused organs. The accumulation in Her2/neu positive NCI‐H2170 tumors was clearly detected after 24 and 48 h of injection, while no significant accumulation was seen in the tumor region of Her2/neu negative NCI‐H520 xenogratfs after administration of 64Cu‐DOTA‐trastuzumab. Similarly, no significant accumulation was seen in the Her2/neu positive NCI‐H2170 tumors administered 64Cu‐DOTA‐IgG. The radioactivity of the liver was higher at 24 h owing to non‐specific uptake of the antibody via interaction with hepatic Fc receptors, but the radioactivity decreased at 48 h. The Her2/neu positive NCI‐H2170 tumors showed that the uptake of the tracer increased with the time and it was significantly higher than that of Her2/neu negative NCI‐H520 tumors (P < 0.001).
Figure 3.
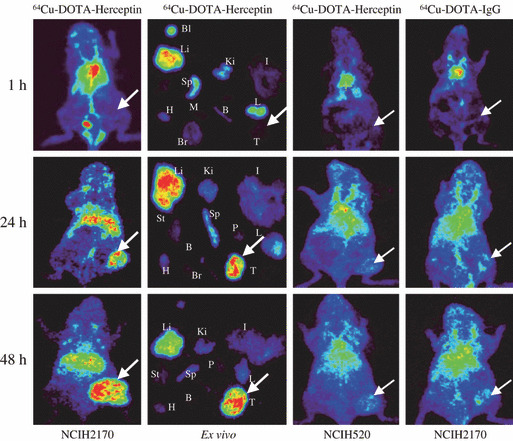
In vivo and ex vivo PET images of mice bearing Her2/neu negative NCI‐H520 and Her2/neu positive NCI‐H2170 tumors after injection of 64Cu‐DOTA‐trastuzumab or 64Cu‐DOTA‐IgG. In vivo and ex vivo studies showed no uptake of 64Cu‐DOTA‐trastuzumab in the Her2/neu positive NCI‐H2170 tumor after 1 h of administration. After 24 h of injection, significant accumulation was observed in the tumor and liver. At 48 h after the injection, the accumulation increased in the tumor but it decreased in the normal organs, including the liver. Negative tumor model NCI‐H520 xenografts injected with 64Cu‐DOTA‐trastuzumab showed no prominent accumulation. Similarly, Her2/neu positive NCI‐H2170 xenografts also did not show specific accumulation in the tumor when injected with 64Cu‐DOTA‐IgG. The arrow shows the tumor. Bl, blood; Li, liver; Ki, kidney; I, intestine; St, stomach; Sp, spleen; P, pancreas; H, heart; M, muscle; B, bone; L, lung; Br, brain; T, tumor.
Ex vivo biodistribution study. Figure 4A shows the biodistribution of Her2/neu positive NCI‐H2170 tumor model mice at different time points. High retention of tracer was seen in blood (30.8 ± 2.1% ID/g) and blood rich organs such as the lungs (19.7 ± 2.8% ID/g) at 1 h but decreased at 24 h (14.0 ± 6.8, 7.6 ± 3.0% ID/g) and 48 h (9.5 ± 2.4, 6.2 ± 2.2% ID/g). The radioactivity in the liver increased with time (11.7 ± 2.6%ID/g at 1 h) and reached the peak at 24 h (26.9 ± 7.4% ID/g) then decreased at 48 h (16.9 ± 0.9% ID/g). The accumulation in the Her2/neu positive NCI‐H2170 tumors increased with time at 1, 24, and 48 h (0.6 ± 0.5, 21.3 ± 1.3, and 23.2 ± 5.1% ID/g), respectively. Figure 4B shows the biodistribution of 64Cu‐DOTA‐IgG in NCI‐H2170 xenografts at 24 and 48 h. The NCI‐H2170 tumor had a low level of tracer uptake (4.6 ± 1.2 at 24 h and 5.6 ± 1.0% ID/g at 48 h), possibly due to the enhanced permeability and retention effect of the tumor. Figure 5 shows the tumor‐to‐blood ratio at various time points. The ratio was greater than one at 24 h (1.5 ± 0.2) after intravenous injection with 64Cu‐DOTA‐trastuzumab and increased thereafter.
Figure 4.
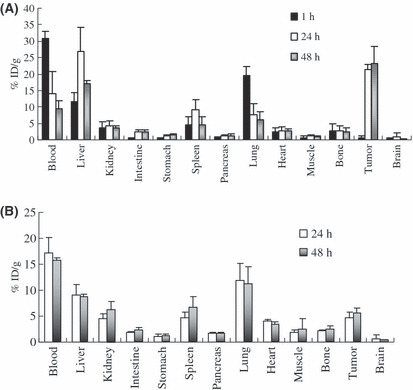
Biodistribution of the Her2/neu overexpressing NCI‐H2170 xenografts at different time points. (A) % ID/g of the organs after injection with 64Cu‐DOTA‐trastuzumab. (B) % ID/g in the organs after injection with 64Cu‐DOTA‐IgG. Tumor uptake was significantly higher in the mice injected with 64Cu‐DOTA‐trastuzumab (P < 0.0001).
Figure 5.
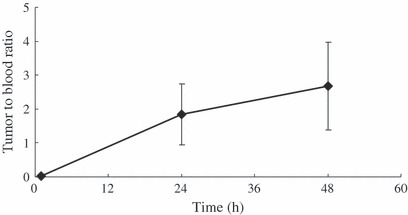
Tumor‐to‐blood ratio at various time points. The ratio was 1.52 ± 0.20 at 24 h post‐injection and it was 2.45 ± 1.29 at 48 h post‐injection. Each value represents the mean ± SD of three or four mice.
Discussion
The present study demonstrated that 64Cu‐DOTA‐trastuzumab is an excellent radiotracer for non‐invasive visualization of Her2/neu overexpressing NSCLC, as it showed specific accumulation in Her2/neu overexpressing NCI‐H2170 tumors in model mice, when compared to the Her2/neu negative NCI‐H520 tumor model. Similarly, the expression level was significantly lower in the NCI‐H2170 mice injected with 64Cu‐DOTA‐IgG. In vitro studies also confirmed the specific binding of DOTA‐trastuzumab to Her2/neu overexpressing NCI‐H2170 cells.
Trastuzumab has been clinically approved for the treatment of patients with Her2/neu expressing breast cancer. However, its role in Her2/neu positive NSCLC is largely marginalized. Given that only patients with Her2/neu 3+ in IHC are expected to benefit from trastuzumab‐based therapy in NSCLC, proper selection of patients is essential. Currently, IHC by Hercep test or gene amplification by fluorescence in situ hybridization (FISH) are the prevalent methods of screening patients overexpressing Her2/neu from among breast cancer patients;( 18 , 19 , 20 ) however, only 11–35% of the patients with Her2/neu overexpression in IHC or FISH responded to trastuzumab in phase II trials when it was given as single agent.( 21 ) The major drawbacks of these two methods are the need of tumor tissue and the fact that lung tumors are morphologically more heterogeneous than malignant breast tumors, and thus interpretation of Her2/neu positivity can be difficult.( 8 ) Therefore there is an urgent need for reliable markers that can noninvasively predict the right patients for the trastuzumab‐based therapy.
18F‐labeled fluorodeoxyglucose (18F‐FDG) PET, which measures differences in glucose utilization between tumors and the surrounding normal tissues, has shown significant result in management of NSCLC without the need of biopsy to stage the disease.( 22 , 23 ) Although this strategy is very useful for the diagnosis and staging of tumors, it does not provide any information on the degree of Her2/neu expression, which limits its use in predicting the patients that might benefit from trastuzumab‐based therapy. 64Cu‐DOTA‐trastuzumab provides this information by allowing a real‐time assay of overall tumor Her2/neu expression in patients, and thereby avoids the false‐negative results that arise from sampling errors and heterogeneity of expression in biopsied tumor samples. Further, it allows early detection and characterization of the tumor and help selecting the right patients for trastuzumab‐based therapy and monitoring of the treatment effects.
Several studies have reported on the use of radiolabeled trastuzumab for imaging of Her2/neu overexpressing breast cancer.( 8 , 24 , 25 , 26 , 27 ) Behr et al. ( 8 ) reported that, out of 20 patients, 11 patients with intense uptake of 111In‐labeled trastuzumab responded well to the trastuzumab‐based therapy, whereas only one patient without uptake responded. Similarly, Perik et al. ( 25 ) showed that 111In‐labeled trastuzumab identified tumors in 13 out of 15 patients on the first SPECT scans, the overall detection rate of the tumors was 45%. Despite these promising results of radiolabeled trastuzumab for tumor imaging, SPECT has limitations, including the fact that it produces low‐resolution images prone to artifacts and attenuation. Some artifacts can easily be misidentified as perfusion defects.( 26 ) Even though the half‐life of PET radionuclides are shorter than those of SPECT, PET imaging has greater sensitivity, higher spatial resolution and the capability to perform quantitative measurements.( 28 ) Several positron emitters, such as 124I or 89Zr, which can be labeled with antibody, have been reported to have clear tumor accumulation in PET( 29 ). Their longer half‐life (4.2 days for 124I and 3.3 days for 89Zr) allows the imaging to be performed for a longer time. Production of 124I is too expensive to allow routine clinical application. After internalization, radioiodine‐labeled antibody is metabolized and the metabolite rapidly diffuses from the cell. 89Zr‐labeled cetuximab or 89Zr‐labeled bevacizumab have been reported to have a clear tumor accumulation and to be promising new PET tracers.( 30 , 31 ) 89Zr is a relatively new radionuclide, and further studies will be needed to better understand its usefulness. On the other hand 64Cu can be produced with high specific activity using a small biomedical cyclotron installed in hospitals or PET centers.( 32 , 33 ) 64Cu‐DOTA‐trastuzumab would be relatively easy to use in routine clinical practice.
Further, our studies showed that 64Cu‐DOTA‐trastuzumab is very stable in vitro and in vivo and remains in an intact form at least until 48 h, which allows enough time for the imaging. Overexpression of Her2/neu is a key component in promoting cell proliferation and whole body imaging with 64Cu‐DOTA‐trastuzumab PET can detect metastasis and recurrence earlier, as studies have reported that the uptake of metastatic tumors is higher than that of the primary tumor.( 25 ) Whole body imaging with 64Cu‐DOTA‐trastuzumab PET will have major role in detecting the involvement of hilar and mediastinal lymph nodes in NSCLC. The standard treatment for stage I NSCLC requires complete dissection of the hilar and mediastinal lymph nodes; however, the lymph node metastasis rate is only 20%, which means 80% of the patients undergo unnecessary invasion.( 34 ) Enhancing the accuracy of the detection of nodal involvement with 64Cu‐DOTA‐trastuzumab may provide opportunities to prevent the unnecessary invasion and guide the resection of malignant lymph nodes only.
The present study showed that the liver uptake of 64Cu‐DOTA‐trastuzumab was high, but comparable to that in previous studies.( 16 , 35 ) Multimodality imaging such as PET/CT provides detailed morphological information, and thus the accumulation in the non‐target regions is not particularly ciritical, but the high tumor accumulation is the most important property of the PET tracer.
For clinical use, imaging should be able to predict the degree of Her2/neu overexpression (+1, +2, +3) in NSCLC. A detailed study on the imaging of the differential expression of Her2/neu (+1, +2, +3) inoculated into a single model mouse is necessary. Despite these facts, the uptake of 64Cu‐DOTA‐trastuzumab in NCI‐H2170 tumors was very well detected (23.2 ± 5.1% ID/g), and had tumor‐to‐blood ratio of greater than one, suggesting that this tracer would allow effective noninvasive measurement of Her2/neu expression as well as the selection of appropriate patients for trastuzumab‐based therapy, which is a critically important distinction when the use of trastuzumab moves into an adjuvant setting for NSCLC. These results warrant the further examination of this radiopharmaceutical for imaging of Her2/neu in other lung cancer models using a greater number of time points.
In summary, 64Cu‐DOTA‐trastuzumab appears to be effective as a PET tracer for imaging Her2/neu in NSCLC, suggesting its potential for the clinical identification of patients that might benefit from trastuzumab‐based therapy. Since lung cancer is the leading cause of death throughout the world, it would be of great significance if even a small percentage of patients would respond to the trastuzumab‐based therapy.
Acknowledgments
This work was supported by the 21st century COE program from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of Interest
None declared by all authors.
Correction added on 7 September 2023, after initial online publication. A duplicate of this article was published under the DOI 10.1111/j.1347-9032.2009.01480.x. This duplicate has now been deleted and its DOI redirected to this version of the article.
References
- 1. Micheli A, Mugno E, Krogh V et al. Cancer prevalence in European registry areas. Ann Oncol 2002; 13: 840–65. [DOI] [PubMed] [Google Scholar]
- 2. Tsai CM, Chang KT, Perng RP et al. Correlation of intrinsic chemoresistance of non‐small cell lung cancer cell lines with HER‐2/neu gene expression but not with ras gene mutations. J Natl Cancer Inst 1993; 85: 897–901. [DOI] [PubMed] [Google Scholar]
- 3. Garrett TP, McKern NM, Lou M et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell 2003; 11: 495–505. [DOI] [PubMed] [Google Scholar]
- 4. Baselga J, Norton L, Albanell J et al. Recombinant humanized anti‐HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 1998; 58: 2825–31. [PubMed] [Google Scholar]
- 5. Tubbs RR, Pettay JD, Roche PC, Stoler MH, Jenkins RB, Grogan TM. Discrepancies in clinical laboratory testing of eligibility for trastuzumab therapy: apparent immunohistochemical false‐positives do not get the message. J Clin Oncol 2001; 19: 2714–21. [DOI] [PubMed] [Google Scholar]
- 6. Behr TM, Béhé M, Wörmann B. Trastuzumab and breast cancer. N Engl J Med 2001; 345: 995–6. [DOI] [PubMed] [Google Scholar]
- 7. Slamon DJ, Leyland‐Jones B, Shak S et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344: 783–92. [DOI] [PubMed] [Google Scholar]
- 8. Hirsch FR, Franklin WA, Veve R et al. HER2/neu expression in malignant lung tumors. Semin Oncol 2002; 29: 51–8. [DOI] [PubMed] [Google Scholar]
- 9. Hirsch FR, Varella‐Garcia M, Franklin WA et al. Evaluation of HER‐2/neu gene amplification and protein expression in non‐small cell lung carcinomas. Br J Cancer 2002; 86: 1449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gatzemeier U, Groth G, Butts C et al. Randomized phase II trial of gemcitabine‐cisplatin with or without trastuzumab in HER2‐positive non‐small‐cell lung cancer. Ann Oncol 2004; 15: 19–27. [DOI] [PubMed] [Google Scholar]
- 11. Tateishi M, Ishida T, Mitsudomi T et al. Prognostic implication of transforming growth factor alpha in adenocarcinoma of the lung‐an immunohistochemical study. Br J Cancer 1991; 63: 130–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kern JA, Slebos RJ, Top B et al. C‐erbB‐2 expression and codon 12 K‐ras mutations both predict shortened survival for patients with pulmonary adenocarcinomas. J Clin Invest 1994; 93: 516–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bunn PA Jr, Helfrich B, Soriano AF et al. Expression of Her‐2/neu in human lung cancer cell lines by immunohistochemistry and fluorescence in situ hybridization and its relationship to in vitro cytotoxicity by trastuzumab and chemotherapeutic agents. Clin Cancer Res 2001; 10: 3239–50. [PubMed] [Google Scholar]
- 14. Pegram MD, Lopez A, Konecny G et al. Trastuzumab and chemotherapeutics: drug interactions and synergies. Semin Oncol 2000; 27: 21–5. [PubMed] [Google Scholar]
- 15. Anderson CJ, Connett JM, Schwarz SW et al. Copper‐64‐labeled antibodies for PET imaging. J Nucl Med 1992; 33: 1685–91. [PubMed] [Google Scholar]
- 16. Cai W, Chen K, Mohamedali KA et al. PET of vascular endothelial growth factor receptor expression. J Nucl Med 2006; 47: 2048–56. [PubMed] [Google Scholar]
- 17. Philpott GW, Schwarz SW, Anderson CJ et al. RadioimmunoPET: detection of colorectal carcinoma with positron‐emitting copper‐64‐labeled monoclonal antibody. J Nucl Med 1995; 36: 1818–24. [PubMed] [Google Scholar]
- 18. Zinner RG, Glisson BS, Fossella FV et al. Trastuzumab in combination with cisplatin and gemcitabine in patients with Her2‐overexpressing, untreated, advanced non‐small cell lung cancer: report of a phase II trial and findings regarding optimal identification of patients with Her2‐overexpressing disease. Lung Cancer 2004; 44: 99–110. [DOI] [PubMed] [Google Scholar]
- 19. Rosell R. Toward customized trastuzumab in HER‐2/neu‐overexpressing non‐small‐cell lung cancers. J Clin Oncol 2004; 22: 1171–3. [DOI] [PubMed] [Google Scholar]
- 20. Pellegrini C, Falleni M, Marchetti A et al. HER‐2/Neu alterations in non‐small cell lung cancer: a comprehensive evaluation by real time reverse transcription‐PCR, fluorescence in situ hybridization, and immunohistochemistry. Clin Cancer Res 2003; 9: 3645–52. [PubMed] [Google Scholar]
- 21. Tokunaga E, Oki E, Nishida K et al. Trastuzumab and breast cancer: developments and current status. Int J Clin Oncol 2006; 11: 199–208. [DOI] [PubMed] [Google Scholar]
- 22. Sampath L, Kwon S, Ke S et al. Dual‐labeled trastuzumab‐based imaging agent for the detection of human epidermal growth factor receptor 2 overexpression in breast cancer. J Nucl Med 2007; 48: 1501–10. [DOI] [PubMed] [Google Scholar]
- 23. Perik PJ, Lub‐De Hooge MN, Gietema JA et al. Indium‐111‐labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2‐positive metastatic breast cancer. J Clin Oncol 2006; 24: 2276–82. [DOI] [PubMed] [Google Scholar]
- 24. Tang Y, Wang J, Scollard DA et al. Imaging of HER2/neu‐positive BT‐474 human breast cancer xenografts in athymic mice using (111) In‐trastuzumab (Herceptin) Fab fragments. Nucl Med Biol 2005; 32: 51–8. [DOI] [PubMed] [Google Scholar]
- 25. Lub‐de Hooge MN, Kosterink JG, Perik PJ et al. Preclinical characterisation of 111In‐DTPA‐trastuzumab. Br J Pharmacol 2004; 143: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rahmim A, Zaidi H. PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun 2008; 29: 193–207. [DOI] [PubMed] [Google Scholar]
- 27. Pentlow KS, Graham MC, Lambrecht RM, Cheung NK, Larson SM. Quantitative imaging of I‐124 using positron emission tomography with applications to radioimmunodiagnosis and radioimmunotherapy. Med Phys 1991; 18: 357–66. [DOI] [PubMed] [Google Scholar]
- 28. Nagengast WB, de Vries EG, Hospers GA et al. In vivo VEGF imaging with radiolabeled bevacizumab in a human ovarian tumor xenograft. J Nucl Med 2007; 48: 1313–9. [DOI] [PubMed] [Google Scholar]
- 29. Perk LR, Visser GW, Vosjan MJ et al. (89)Zr as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals (90)Y and (177)Lu in tumor‐bearing nude mice after coupling to the internalizing antibody cetuximab. J Nucl Med 2005; 46: 1898–906. [PubMed] [Google Scholar]
- 30. Line BR, White CS. Positron emission tomography scanning for the diagnosis and management of lung cancer. Curr Treat Options Oncol 2004; 5: 63–73. [DOI] [PubMed] [Google Scholar]
- 31. Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology 2004; 231: 305–32. [DOI] [PubMed] [Google Scholar]
- 32. McCarthy DW, Shefer RE, Klinkowstein RE et al. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl Med Biol 1997; 24: 35–43. [DOI] [PubMed] [Google Scholar]
- 33. Obata A, Kasamatsu S, McCarthy DW et al. Production of therapeutic quantities of (64)Cu using a 12 MeV cyclotron. Nucl Med Biol 2003; 30: 535–9. [DOI] [PubMed] [Google Scholar]
- 34. Asamura H, Nakayama H, Kondo H et al. Lymph node involvement, recurrence, and prognosis in resected small, peripheral, non‐small‐cell lung carcinomas: are these carcinomas candidates for video‐assisted lobectomy? J Thorac Cardiovasc Surg 1996; 111: 1125–34. [DOI] [PubMed] [Google Scholar]
- 35. Eiblmaier M, Meyer LA, Watson MA et al. Correlating EGFR expression with receptor‐binding properties and internalization of 64Cu‐DOTA‐cetuximab in 5 cervical cancer cell lines. J Nucl Med 2008; 49: 1472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


