Abstract
The goal of this study was to determine material effects on cartilage regeneration for scaffolds with the same controlled architecture. The 3D polycaprolactone (PCL), poly (glycerol sebacate) (PGS), and poly (1,8 octanediol-co-citrate) (POC) scaffolds of the same design were physically characterized and tissue regeneration in terms of cell phenotype, cellular proliferation and differentiation, and matrix production were compared to find which material would be most optimal for cartilage regeneration in vitro. POC provided the best support for cartilage regeneration in terms of tissue ingrowth, matrix production, and relative mRNA expressions for chondrocyte differentiation (Col2/Col1). PGS was seen as the least favorable material for cartilage based on its relatively high de-differentiation (Col1), hypertrophic mRNA expression (Col10) and high matrix degradation (MMP13, MMP3) results. PCL still provided microenvironments suitable for cells to be active yet it seemed to cause de-differentiation (Col1) of chondrocytes inside the scaffold while many cells migrated out, growing cartilage outside the scaffold.
Keywords: Poly (1,8 Octanediol-co-Citrate) (POC); Poly (glycerol sebacate) (PGS); polycaprolactone (PCL); chondrogenesis; controlled scaffold tissue engineering
1. Introduction
The biomaterial and scaffold architecture design that best enhances chondrogenesis for Matrix Assisted Chondrocyte Implantation (MACI) remains an open yet critical question. A number of biomaterials with mechanical and surface properties attractive for cartilage regeneration have been put forth. However, there have been almost no head to head comparisons of these different materials as cartilage scaffolds. True material influences can only be ascertained if all materials are fabricated with the exact same architecture, as architecture itself can influence chondrogenesis.
In this study, we compare three biomaterials for cartilage tissue engineering: 1) Polycaprolactone (PCL), 2) Poly (Glycerol-co-Sebacate) (PGS), and 3) Poly (Octanediol-co-Citrate)(POC) in terms of mechanical properties, permeability properties, cartilage matrix production and cartilage-related gene expressions. All three materials were fabricated into the exact same architecture design which was previously found to facilitate matrix production and cellular differentiation of chondrocytes in vitro [1]. Thus, we eliminate scaffold architecture as a confounding variable to completely focus on material influences on chondrogenesis. One material, PCL, has a long history in tissue engineering while the other two, PGS and POC are relatively recently developed materials for tissue engineering. The rationale for selection of the three candidate materials was based on their mechanical stiffness (within or close to published ranges for articular cartilage), hydrophilicity, and potential use in the field of cartilage engineering. Furthemore, we wanted to be able to fabricate all chosen materials with the same architecture to remove architecture as a confounding influence on chondrogenesis. All three materials were seeded with primary chondrocytes in the same 3D scaffold design with spherical voids, which was found to enhance chondrogenesis in terms of matrix production and cellular differentiation of chondrocytes in vitro from a previous study in our laboratory [1].
Polycaprolactone (PCL) is one of the polyester polymers that have been most frequently used in the field of orthopedic tissue engineering. It is a biocompatible material that is FDA approved for cranial burr hole fillers and trapezoid joint spacers that is readily fabricated and biodegradable. Previous research has shown that PCL is a good candidate for cartilage tissue engineering in terms of cell attachment, proliferation, and matrix production [1–4]. Unlike PCL, PGS and POC are relatively new biomaterials in the field of tissue engineering and there are few published reports on their use for cartilage regeneration [5–7]. Both PGS and POC are rubber-like biodegradable polyester elastomers which are made by reacting an acid and alcohol monomers via condensation using high temperature and vacuum. Both are degraded by hydrolysis with non-toxic and natural metabolic intermediates degradation products. Due to these characteristics, both materials have been proposed as good scaffold candidates for soft tissue engineering (i.e. cartilage and blood vessels) [5,7–11].
Due to their recent development, and the lack of controlled 3D scaffold architectures, there has been no direct comparison of PGS and POC for cartilage scaffold materials. Such comparisons are critical to make informed design choices for cartilage tissue engineering matrices for use with autologous chondrocyte therapy or even with current cartilage resurfacing techniques like microfracture or mosaicplasty. However, rationale design decisions to determine optimal cartilage tissue engineering scaffolds will require studying material influences using the same architectures and then studying architectural influences using the same material. The goal of this study was to compare PCL, PGS, and POC material influences on chondrogenesis in terms of mechanical properties, cell activity, cartilage matrix production and gene expression utilizing scaffolds of the same fixed 3D designed architecture.
2. Materials and Methods
2.1. Synthesis of pre-Polymer
Poly (1, 8 Octanediol-co-Citrate) (POC)
All chemicals were purchased from Sigma-Aldrich (Milwaukee, WI). Poly (1, 8 Octanediol-co-Citrate) pre-polymer (pPOC) was synthesized as previously described by Yang J et al. [10, 12, 13] with some curing process modifications. Briefly, equimolar amounts of citric acid and 1,8-octanediol were added to a 500 ml three-neck round bottom flask fitted with an inlet and outlet adapter. The mixture was melted at 160–165 °C for 15–20 min under a flow of nitrogen gas while stirring. The temperature of the system was subsequently lowered to 140 °C for 45 min with constant stirring to create a pre-polymer.
Poly (glycerol sebacate) (PGS)
PGS pre-polymer (pPGS) was synthesized following methods described by Gao et al. [14]. Equimolar sebacic acid and glycerol were reacted under N2 at 120°C. After 24 hours, the N2 was removed and a vacuum of 50mTorr was pulled for an additional 48 hours, with a condenser attached.
2.2. Scaffold Design & Fabrication
Previously developed image-based design processes and software were used to design 3D POC scaffold architectures [13, 15–18]. Porous polycaprolactone (PCL), PGS, and POC scaffolds (6.35mm diameter, 3.5mm height, 50% porosity, 900µm interconnected spherical pore shape with 310~320µm diameter of the windows between the pores) were designed using custom IDL programs (RSI, Boulder, CO) following previously reported methods [5,13,19]. In brief, wax molds with 3D-image based design architecture were built by a Solidscape Patternmaster™ machine and the wax molds were used directly to melt-cast PCL scaffolds in PTFE molds. PCL powder (43–50 kDa, Polysciences) packed into PTFE molds was melted at 115°C with −30 in.Hg vacuum for 2 hours and then wax molds were pushed into the warm PCL liquid. The wax molds were dissolved by ethanol after cool-down.
For PGS and POC scaffolds, inversely solid freeform fabricated hydroxyapatite (HA) molds were prepared before curing pPGS and pPOC into architecture scaffolds. As the wax molds melt at PGS and POC curing temperatures, the HA secondary molds were created from the wax molds as the HA easily withstands the pPGS and pPOC curing temperatures that reach over 100°C. pPGS or pPOC was poured into the wells of a Teflon mold and HA molds were embedded within each pre-polymer. The pPGS/HA/Teflon mold unit was post-polymerized at 150°C for 3 days. The pPOC/HA/Teflon mold unit was post-polymerized at 100°C for 1 day followed by curing at 100°C for 3 days more with vacuum (−20 in.Hg). The HA mold was removed using a decalcifying reagent (RDO, APEX Engineering Products Corp, Plainfield, IL) followed by incubation in water (Milli-Q water purification system, Billerica, Mass, USA) for 24 hours to obtain the final porous POC scaffolds.
2.3. Mechanical Tests
For scaffold unconfined compression tests, four to six porous scaffolds per each material were tested in compression (Alliance RT/30 electromechanical test frame, 50N load cell (POC, PGS) or 500N load cell (PCL) with 0.5% error range, MTS Systems Corp., MN) and TestWorks4 software (MTS Systems Corp., MN) was used to collect data during compression testing. MATLAB (The MathWorks Inc., MA) software was used to fit a nonlinear elasticity model, σ = A[eBε − 1] to experimental data. The sum of least square errors between the model stress and experimental stress was minimized using the LSQNONLIN minimization program in the MATLAB optimization toolbox. Tangent moduli were calculated at 10 and 30% strain from fit data and all residuals between model and experimental stress were below 1%.
2.4 Porosity and Permeability Measurements
Seven scaffolds per material were scanned in air using a MS-130 high resolution µCT scanner (GE Medical Systems, Toronto, CAN) at 19 µm voxel resolution, at 75 kV and 75 mA. The porosity of each specimen was calculated by defining a region of interest that encompassed the entire scaffold and an appropriate threshold level was applied to delineate the solid scaffold material using GEMS Microview software (GE Medical Systems, Toronto, CAN). All porosity scanning was performed before mechanical tests to avoid any artifacts due to compression. Scaffold permeability (N=6~7, each material) with and without composite Hyaluronic Acid (HyA)/collagen I (Col I – 6mg/ml) gel was measured using previously developed protocols on a permeability test set up [18,19]. Permeability of scaffolds with hydrogels was measured to mimic cell loading conditions in vitro or in vivo.
Chondrocytes were seeded into 3D scaffolds by first suspending the cells in media with composite HyA/Col I gels and then pushing the gel into the 3D scaffolds [17]. The gelation procedure is as follows: 625µL of Col I (stock concentration: 8.37mg/mL; BD Bioscience Discovery Labs, San Jose, CA) with 62.5 µL HyA (stock concentration: 3 mg/mL in 1.5M sodium chloride (NaCl), molecular weight 2.4~3 million Da; Hyalogic LLC, Edwardsville, KS) were well-mixed. The pH of the HyA/Col I suspension was increased with the addition of 9µL of 0.5N sodium hydroxide with 220 mg/mL sodium bicarbonate to initiate gelation. As soon as 0.5N sodium hydroxide is added to HyA/Col I gel mixture, gel contents were evenly re-suspended. Hydrogel mixtures are then dripped down onto pre-prepared sterile scaffolds until scaffolds were fully soaked and filled with gel to the top surface. This was followed by incubation at 37°C for 30 min to solidify gels further. 125µl of gel mixtures were used for each scaffold.
2.5 In Vitro Cell Culture & Histology
Porcine chondrocytes (pChon) were isolated from the full depth of metacarpophalangeal joints of domestic pigs and seeded onto scaffolds following methods previously published [17] with some modifications. In short, cells were re-suspended at a density of 20×106 cells/mL in 625µL of composite HyA/Col I (6mg/ml) with ~50µL of culture medium. Collagen gels are used as a cell carrier for all scaffolds to provide better cell distribution within scaffold pores. 5% hyaluronic acid was added to the collagen I gel to provide a favorable environment for chondrocyte differentiation/proliferation based on our previous work [20]. The remaining steps were the same as previously described (See 2.4). Before cell culture, PGS and POC scaffolds were sterilized by autoclave and PCL scaffolds were sterilized by incubation in 70% ethanol for 1 hour. After sterilization, all scaffolds were neutralized to physiological pH level by media incubation for 24~48 hours with brief PBS rinse prior to cell seeding. Scaffolds seeded with pChon were cultured with chondrogenic medium (basal medium (DMEM, 10% fetal bovine serum (FBS), 1% P/S, Gibco) supplemented with 50 mg/mL 2-phospho-L-ascorbic acid (Sigma)), 0.4mM proline (Sigma), 5 mg/mL insulin (Gibco), and 0.1mM non-essential amino acids (Gibco)) in 12-well plates. Chondrocytes were cultured for 0 (1d), or 4 weeks under gentle agitation on an orbital shaker and the media was changed every other day. Cell culture was maintained in a water-jacket incubator equilibrated with 5% CO2 at 37°C. For histology, constructs (N=3/material) at each time point were fixed in 10% buffered formalin overnight, dehydrated with a series of graded ethanol, and embedded in paraffin. Tissue sections were stained with safranin O/Fast green to assess cell distribution, morphology and sGAG production. Six to eight slides (4 sections/slide) were obtained from the center of each scaffold (top to bottom and left to right). Immunohistochemistry was used to detect collagen II following a previously established protocol [20]. Four slides (4 sections/slide) were obtained from the center of each material scaffold.
2.6 sGAG and DNA quantification
Before cell analysis, excessive out-layer tissues were removed from PCL scaffolds and analyzed separately for both sGAG/DNA quantification and mRNA gene expression analysis. Scaffolds (N=6) at both the 0 and 4 week time points were removed from culture, finely diced, and placed immediately into 1ml of pre-prepared papain solution (papain (10 units/mg: Sigma Aldrich #P4762), 1X PBS, 5mM cysteine HCL, 5mM EDTA, pH=6.0; mixed for 2h at 37°C then filtered). Scaffolds were digested in papain for 24 hours at 60°C then immediately stored at −20°C. The digested tissue-scaffold solution was analyzed by a dimethylmethylene blue (DMMB) assay following a previously established protocol [5]. Briefly, 10µl of sample was mixed with 200ul of DMMB reagent and absorbance was read on a plate reader (MultiSkan Spectrum, Thermo, Waltham, MA) at 525 nm. A standard curve was established from chondroitin 6-sulfate from shark (Sigma, C4384) to compare absorbance for samples [21], [22]. The total sGAG were normalized by DNA content which was measured using Hoechst dye 33258 methods (Sigma, #DNA-QF). In brief, 10ul digested sample was added to 200ul pre-prepared Hoechst solution and read with excitation at 355nm and emission at 460nm (Fluoroskan Ascent FL, Thermo, Waltham, MA) in a 96 well plate. Readings were compared to standard curves made from calf thymus DNA (Sigma, #DNA-QF) [1, 23].
2.7 Quantitative-PCR
Cartilage matrix specific genes (Type II collagen & aggrecan), chondrocyte de-differentiation marker genes (Type I & X collagen), matrix degradation indicator genes (matrix metalloproteinases 13 and 3 (MMP13, MMP3) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression were determined by quantitative PCR (qtPCR) using a Gene Amp 7700 sequence detection system (Applied Biosystems, Foster City, CA USA). Scaffolds (N=6/material) were removed from culture, briefly rinsed with PBS, cut into smaller pieces, and placed into RNAlater (Qiagen, Inc., Valencia, CA). Scaffolds immersed in RNAlater were kept at 4°C for 24 hours and stored at −20°C until analysis. Total RNA was extracted using a RNeasy Mini Kit (Qiagen, Inc., Valencia, CA) and reverse transcription was carried out using the SuperScript First-Strand synthesis kit (Invitrogen). A positive standard curve for each primer was obtained by qtPCR with serially-diluted cDNA sample mixtures. Samples were prepared using a Taqman universal PCR master mix (Applied Biosystems) and custom designed porcine primers [1]. The quantity of gene expressions were calculated with standard samples and normalized with GAPDH.
Statistical Analysis
Data are expressed as mean ± standard deviation. The statistical significance among different materials was calculated using linear regressions and one way ANOVA with post-hoc comparison (Tukey) or student t-test using SPSS software (SPSS for Windows, Rel 14.0. 2005 Chicago: SPSS Inc.). Data were taken to be significant, when a P-value of 0.05 or less was obtained.
3. Results
3.1 Scaffold design, fabrication, and characterization
In order to isolate design effects from material effects on chondrogenesis, scaffold designs were kept the same for all three materials in terms of pore shape, pore size, surface area, and porosity (Table 1, Figure 1). Due to material influences, PCL, PGS, and POC had different effective scaffold modulus and permeability (Table 1). Figure 1 shows example micro-CT images of scaffolds and a digital picture for the POC scaffold design (other scaffolds are similar in images thus not shown here). With no tissue ingrowth, the PCL scaffold tangent modulus was roughly two hundred times the PGS and POC effective scaffold tangent modulus at 10 % strain. However, with ingrowth of cartilaginous tissues at 4 weeks in vitro, the PCL effective scaffold tangent modulus was only twice that of the PGS and POC scaffolds at the same strain rate, which is likely due to the composite PCL/tissue tangent modulus being dominated by tissue growing on top of the PCL scaffolds. Even though scaffold designs were kept the same, effective scaffold permeability differed significantly between the different materials. Scaffold design permeability, a single physical design parameter that describes how multiple structural properties including pore size, pore shape, interconnectivity, porosity, and fenestration size affect mass transport, depends not only on scaffold design but also on scaffold material [1, 24]. Permeability without gel likely represents the permeability of scaffolds at 4 weeks without tissue ingrowth whereas permeability with gel represents the scaffolds at 0 wk with initial cell seeding. As most of collagen gels are degraded in a week during tissue formation, permeability is dynamically changing within the 4 week period. However it is likely that the trend of different permeability between the different materials remains. PCL, POC, and PGS are in order of increasing permeability without gel, 2.93 ± 0.73, 3.51 ± 0.95, and 8.31± 3.91 (10−7 m4/N·s). With gel, the same permeability rankings hold with PCL, POC, and PGS having 0.66 ± 0.24, 1.72 ± 0.45, and 3.0 ± 0.18 (10−7 m4/N·s) permeability, respectively (Table 1).
Table 1.
Scaffold Descriptions (N=6~8)
| Material | PCL | PGS | POC |
|---|---|---|---|
| Porosity (%) | 48 ± 3.62 | 49 ± 2.36 | 50 ± 1.62 |
| Permeability without gel (10−7 m4/N·s) | 2.93 ± 0.73 | 8.31± 3.91 | 3.51 ± 0.95 |
| Permeability with gel (10−7 m4/N·s) | 0.66 ± 0.24 | 3.0 ± 0.18 | 1.72 ± 0.45 |
| Water contact angle (hydrophilicity) (°)* | 82.5 ± 1.0° | 32.0° | 15 ~ 53° |
| Surface Area (mm3) | 288 ± 38 | ||
| Pore Shape | Spherical | ||
| Designed Pore Size | 900µm | ||
| Designed Pore Strut Size | 315µm | ||
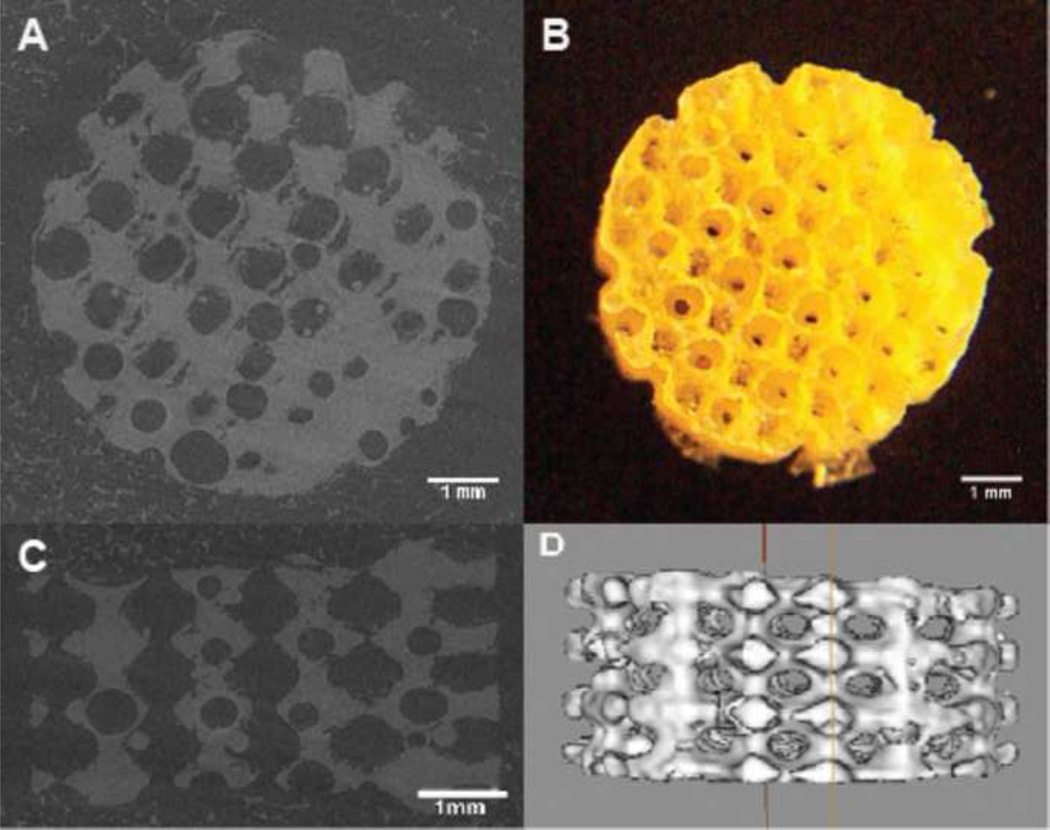
Figure 1.
(A) Top view of MicroCT image of a scaffold (B) a digital picture of a POC scaffold (C) Side view of MicroCT image of a scaffold (D) Isosurfaced 3D MicroCT image of a scaffold
Table 2 summarizes compressive tangent moduli for each material scaffold with or without tissues. PCL is more than 100 times stiffer than PGS and POC whereas PGS and POC have similar effective scaffold tangent moduli at 10% strain rate. After tissues were formed for 4 weeks, the tangent compressive moduli of both PGS and POC were increased ~ 400% compared to that of PGS and POC without tissues measured at the same strain rate. The effective scaffold tangent moduli of PCL at 4 week were only two times higher than that of PGS and POC at 10% strain. This result is probably due to excessive tissues outgrown on the top of PCL scaffolds. Thus effective scaffold tangent compressive moduli more likely reflected the properties of the formed tissues on the top of PCL scaffolds rather than the combined scaffold and tissue properties. This was not the case for PGS and POC as regenerated cartilaginous tissues were contained within the scaffolds (see Fig. 2B and 2C). At 30% strain, tangent moduli of all the scaffolds reflect better the entire tissue/scaffold construct (Table 2). The POC/tissue construct was the most compliant at 30% strain, followed by PGS (2× stiffer than POC) and PCL (10× stiffer than POC and 6× stiffer than PGS).
Table 2.
Mechanical Properties (N=4~6)
| Material | PCL | PGS | POC |
|---|---|---|---|
| Tangent modulus without tissues (0wk) (MPa)* | 21.8 ± 4.43 | 0.19 ± 0.06 | 0.13 ± 0.04 |
| Tangent modulus with tissues (4wk) (MPa)* | 1.43 ± 0.56 | 0.89 ± 0.19 | 0.73 ± 0.37 |
| Tangent modulus with tissues (4wk) (MPa)** | 13.49 ± 3.53 | 2.44 ± 0.69 | 1.26 ± 0.33 |
measured at 10% strain,
at 30% strain
Figure 2.
Digital pictures of three different material scaffolds with tissues grown for 4 weeks.
3.2 In vitro cell culture-proliferation, differentiation, and matrix production
Chondrocytes proliferated and produced cartilaginous matrix during the 4 week in vitro culture period (Figure. 2). Excessive outer tissues were grown on the top and bottom of the PCL scaffold whereas tissue were contained completely within the PGS and POC scaffolds (Fig. 2) Since we were interested in the amount of tissues formed inside the scaffold, we separated excessive outer tissues from PCL, denoting assays on tissue within the PCL scaffold as PCLin, assays on tissue outside the PCL scaffold as PCLouter, and assays on complete PCL tissue simply as PCL. POC produced the highest DNA content (531 ± 39 ng/ul) per construct, whereas PCLin produced the lowest content (54 ± 22 ng/ul) per construct\. PGS and PCLtotal showed similar DNA contents (363 ± 22 ng/ul and 346 ± 59 ng/ul respectively) per construct (Figure 3a). Over 4 weeks, POC showed highest proliferation rate, with an increase of DNA content from 322 ± 24 ng/ul to 531 ± 39 ng/ul per construct. PGS showed no significant difference in the amount of DNA over time. Note that PCLin showed a decrease from 269 ± 52 ng/ul to 54 ± 22 ng/ul per construct, likely reflecting the fact that more cells grew outside the PCL scaffold than inside (Figure 3b).
Figure 3.
(A) Amount of DNAs per construct at 4 weeks for different materials (PCLin: tissues inside PCL scaffolds only, PCL out: excessive outer layers removed from PCL scaffolds, PCL total = PCLin + PCLout) (Annotations ‘a’, ‘b’, ‘c’ shown in the graphs are statistically significant each other; PCLin, POC are significant to all other groups, PGS are significant to PCL in and POC only) (B) Changes in DNA content of chondrocytes for different materials over time is measured by amount of DNAs per scaffold suggesting some possible cell migration (especially for PCL) and exterior tissue growth. (Asterisk represents statistical significance. p≤0.05, N=6) (C) Matrix production per scaffold is quantified by amount of sGAG per construct for different materials. (PCLin, POC are significant to all other groups, PGS are significant to PCL in and POC, p≤0.05, N=6)
Sulfated-glycosaminoglycan (sGAG) content, measured through a DMMB assay, was used to quantify cartilaginous matrix production by chondrocytes (Figure 3(C)). This showed a similar trend to the DNA content. POC produced the most amount of sGAG per scaffold (1.88 ± 0.88 µg/µl) which was roughly eight times more than PCLin (0.22 ± 0.10 µg/µl) and two times more than PCLtotal (1.12 ± 0.53 µg/µl) and PGS (0.92 ± 0.32 µg/µl). However, PGS and PCLtotal were not significantly different from each other.
3.3 In vitro cell culture-gene expression
Quantitative-PCR was used to measure the messenger RNA expression for collagen by cells and for aggrecan and MMPs found in cartilage (Figure 4). Healthy articular cartilage is composed of a highly organized network of collagen and proteoglycans. Type II collagen is the major fibrillar collagen of articular cartilage, accounting for 90–95% of the overall collagen content and determining mechanical behavior [25]. When Type II collagen is destroyed, it is replaced with a type I collagen fibro-cartilage that does not have the same functional properties as type II collagen. The ratio of collagen 2 gene expression to collagen 1 gene expression, known as the “differentiation index”, attains a higher value with a more chondrocytic genotype, and a lower value with a more fibroblastic gene expression [26]. The differentiation index of POC was 4.31, significantly higher than that of PCLin, PGS, and PCLout (0.92, 1.31, 1.21 respectively), reflecting the sGAG quantification data. In contrast, the differentiation indexes of PCL and PGS were not significantly different from each other. PCL and PGS seemed to provide environments for cells to be active causing elevated expressions of both type II collagen and type I collagen genes. In contrast, POC was good for keeping type I collagen expression low while promoting type II collagen expression.
Figure 4.
Relative mRNA expression comparison for proteins among different materials. (PCL = PCL inner tissues only) (Annotations ‘a’, ‘b’, ‘c’ shown in the graphs are statistically significant each other. N=6, p≤0.05)
Aggrecan is the main proteoglycan found in cartilage, and is a typical marker of differentiated chondrocytes. The aggrecan expression of PGS and POCout were significantly higher than that of PCLin and POC. Type X collagen serves as a marker of the terminally differentiated (hypertrophic) chondrocyte phenotype, and detection of the type X collagen gene transcript and translation product are useful for studies of chondrocyte growth and differentiation [27,28]. The type X collagen expression also showed a similar trend as the type I collagen and aggrecan expressions among different materials with significance (p<0.1). PGS and PCLout showed the highest tendency to hypertrophy.
MMP-13 and MMP-3 play critical roles on extracellular matrix degradation. MMP-13 is a product of the chondrocytes that reside in the cartilage and MMP-3 is elevated in arthritis, which degrades non-collagen matrix components of the joints. In addition to collagen, MMP-13 also degrades the proteoglycan molecule, aggrecan, giving it a dual role in matrix destruction. [29–31]. When comparing the gene expressions of MMP-13 and MMP-3 for inner tissues, PGS showed the highest MMPs’ expressions which were five to ten times higher than PCLin and POC implying that degradation of collagens and aggrecan were actively taking place.
3.4 Histology and Immunohistochemistry
Safranin-O staining (Figure 5) supported the sGAG quantification data. POC showed the highest sGAG content with pores fully packed with sGAG stained tissues whereas PCL and PGS showed less tissues formed with sGAG stained. Also, even for outer layer tissues of POC, sGAG staining was darker than any other materials showing higher sGAG content. The safranin O staining of PCL confirmed that not much cartilaginous tissues formed inside the scaffold pore (less sGAG staining) and most of cartilage tissues were formed outside the PCL scaffold.
Figure 5.
Safranin-O/Fast-Green staining for sGAG. Dark crimson colored regions shown in the middle of PGS and POC sections are scaffold materials. (A: 4× magnification, B: 10× magnification)
Immunostaining of type II collagen (Figure 6) tracked relative mRNA expression data (Figure 4) such that PCL (inner and outer tissues combined) showed the strongest intensity of immunostaining for type II collagen and PGS and POC followed respectively. For cell morphology, PCL and POC showed more vivid chondrocytic phenotype with lacunae inside pore, between pores, and the most outer part of scaffolds, whereas more fibroblastic cells were found across the entire PGS scaffold, which also matched with type I collagen relative mRNA expression data (Figure 4) indicating higher de-differentiation. In general, outer tissues for all materials maintained a more chondrocytic cell morphology than the center part of the scaffold.
Figure 6.
Immunohistochemical analysis for Type II collagen (brown) with hematoxylin staining (purple) (A: tissues between pores: 10× magnification, B: tissues inside a pore: 20× magnification, C: Outer layer tissues: 20× magnification).
4. Discussion
This is the first time a true apples to apples comparison of scaffold material influence on chondrogenesis has been performed with the same designed 3D porous architecture. Many studies have reported how one or two materials affect chondrogenesis yet they cannot make a completely unfounded comparison of material effects due to lack of controlled scaffold design and the resultant differences in scaffold architecture [17, 32, 33]. Since scaffold design is also a critical factor affecting tissue regeneration within scaffold [1], we cannot isolate material effects on chondrogenesis unless we can test different scaffold materials fabricated with the same 3D architecture. Here, using an identical scaffold design for all three materials, we could make a direct comparison of scaffold material effect on chondrogenesis.
The most significant difference in terms of material effects, PCL, PGS, and POC scaffolds were seen in the permeability, hydrophilicity, and effective scaffold tangent moduli differences. Even though scaffold architectural permeability was kept the same for all materials, there were effective permeability differences among materials themselves. PGS was significantly more permeable than POC and PCL (Table 1) PCL showed significantly higher tangent moduli than other two materials (Table 2) with or without tissues. In general, the degradation rates of PGS and POC are much faster than that of PCL and the degradation rates and the rates of tissue formation are related to mechanical tangent moduli [8, 10, 34, 35].
PGS and POC tangent moduli increased significantly by 4 weeks, suggesting that tissue formation occurred faster than scaffold degradation. However, the tangent modulus for the PCL/tissue construct decreased greatly from 0 to 4 weeks of tissue culture. This was more likely because cartilage tissue grew over the scaffold, and thus dominated the compressive properties over the scaffold material. In terms of tangent modulus, PGS and POC were more similar to native cartilage tissues than PCL [5, 7], which may be more advantageous when applying these scaffolds in cartilage defects.
Cell proliferation measured by amount of DNA (live cells) per construct at 0 and 4 weeks clearly shows that POC provided the most favorable environment for cell proliferation in terms of overall or inner parts of scaffolds. When considering tissues formed in the inner parts of scaffolds only, PCL seemed to be least favorable material yet when comparing overall effects PGS and PCLtotal were not significantly different (Figure 3A). Proliferation over time (Figure 3B) also supports this contention since PGS and POC proliferated or at least kept the same number of cells whereas PCLin showed a significant decrease in cell numbers. This may be explained by low permeability, hydrophobicity and low wettability of PCL scaffolds compared to the other two materials.
As cells started to grow and tissues were formed, less nutrients from the media would flow in and out due to lower permeability for all materials. However, since PGS and POC may better retain fluid due to hydrophilicity, there would be more nutrients from media that are soaked into these materials available to cells inside of the scaffold. Excessive growth of tissue outside the PCL scaffolds may have further prevented nutrient diffusion within the PCL scaffold, leading to reduced cell proliferation.
It was reported that chondrocytes prefer decreased scaffold permeability within PCL scaffolds in terms of cartilaginous matrix production, promoting increases in aggrecan content and collagen 2: collagen 1 gene expression ratios [1]. This was true for POC which had a relatively lower permeability than PGS and showed the highest sGAG contents (Figure 3C and 5). Also, in terms of gene expression, its differentiation index (Col2/Col1) was the highest with relatively low type X collagen expression indicating a reduced tendency towards hypertrophy. However, even though PCL had permeability similar to POC, the results for PCL chondrogenesis were more similar to PGS in terms of matrix production and gene expression. When comparing inner tissues only, PCL showed the lowest cell number (Figure 3A), proliferation rate (Figure 3B), and thus lowest matrix production (Figure 3C). This could be again due to the presence of excessive outer tissues preventing sufficient nutrient supply to the innermost cells within the scaffold, causing these cells not to proliferate and to produce less sGAG. However tissues on the outside of the PCL scaffolds exposed to media still proliferated well and had high level of gene expressions, confirming a high level of cell activity.
PCLout and PGS showed similar pattern in terms of total amount of DNA, sGAG content, and relative mRNA expressions. Especially for relative mRNA expressions, PCL and PGS seemed to cause lower chondrocyte differentiation (shown by Col2/Col1 ratio) yet higher aggrecan production, higher rates of matrix degradation (shown by MMPs), and a higher tendency towards hypertrophy (shown by Col X). Even though POC did not show the highest expression of type II collagen and aggrecan, it did show a high differentiation index, lower hypertrophy tendency, and lower matrix degradation with the highest DNA and sGAG contents. Thus we could probably conclude that overall POC maybe the material that best enhances chondrogenesis out of the three materials examined. This is probably due to benefits delivered from combined effects of higher hydrophilicity and wettability retaining more media inside, and sufficiently low permeability to keep sGAG inside the scaffold while still allowing media flow.. PCL and PGS seemed to promote chondrocyte proliferation and activity in terms of gene expressions, however these chondrocytes may be more likely to proceed to hypertrophy as seen by the type X collagen expression and increased matrix degradation suggested by increased MMP3 and 13 gene expression.
Histological data (Figure 5) supported sGAG quantification data and type II collagen immunostaining supported type II collagen gene expression data. However, from Figure 6 we should note that while type II collagen immunostaining may give some useful qualitative information, it only gave partial information on chondrocytic differentiation. As Figure 4 shows, PCLtotal and PGS had high expression of type II collagen however they also had high expression of type I and X collagen as well compared to POC. It would not be possible to calculate a quantitative differentiation index using type I and type II immunostaining. Combining histological and immunohistological images with sGAG quantification and mRNA expression data likely gives the most complete picture of chondrogenesis.
The in vitro results presented here showed a significant dependence of chondrogenesis on scaffold material, eliminating pore architecture as a confounding variable by fabricating all scaffolds with the same architecture. However, these results must obviously be verified in in vivo cartilage defects, since mechanical loading, oxygen tension and host cells may affect chondrogenesis in this situation. For instance, engineered cartilage grown in scaffolds may cause chondrocytes to proceed to hypertrophy and matrix degradation such that they would end up promoting endochondral ossification before sufficient cartilage formation. In order to overcome these limitations, more in-vivo studies with small and large animals would be necessary for future clinical applications. Still, in vitro results are important since many in vivo studies will utilize in vitro culture periods to boost cartilage matrix production before transplantation.
Overall, this work confirms that scaffold material selection is an important factor that affects chondrogenic cellular differentiation and matrix production. It has been widely postulated yet never proven that the choice of material directly affects cell differentiation and chondrogenesis, since previous studies saw variation in both scaffold material and architecture, which confounds interpretation of experimental results. This work points to the capability to modulate and ultimately enhance chondrogenic potential with careful selections of material.
5. Conclusions
This study demonstrates that POC was more suitable for chondrocytes to form cartilaginous tissues in vitro compared to PCL and PGS when using the same scaffold design. POC showed the highest DNA and sGAG contents after 4 weeks of in vitro cell culture with highest differentiation index and the lowest hypertrophy and matrix degradation gene expression compared to PCL and PGS. Both PCL and PGS promoted chondrocytes to proliferate and express genes related to cartilage formation, but they promoted gene expression for cartilage destruction and ossification, which were not desired for cartilage regeneration. Since this was an in vitro study, results concerning material influence on cartilage regeneration should be further studied within in vivo cartilage defects.
Acknowledgements
This work was funded in part by a NIH grant R01 AR 053379. The authors thank Annie G. Mitsak and Carolyn Slope for help with PGS pre-polymer synthesis and scaffold fabrication, Huina Zhang for advices with data analysis, and Chris Strayhorn for assistance with histology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Kemppainen JM, Hollister SJ. Differential effects of designed scaffold permeability on chondrogenesis by chondrocytes and bone marrow stromal cells. Biomaterials. 2010 Jan;31(2):279–287. doi: 10.1016/j.biomaterials.2009.09.041. Epub 2009 Oct 8. [DOI] [PubMed] [Google Scholar]
- 2.Kim HJ, Lee JH, Im GI. Chondrogenesis using mesenchymal stem cells and PCL scaffolds. J Biomed Mater Res A. 2010 Feb;92(2):659–666. doi: 10.1002/jbm.a.32414. [DOI] [PubMed] [Google Scholar]
- 3.Izquierdo R, Garcia-Giralt N, Rodriguez MT, Caceres E, Garcia SJ, Gomez Ribelles JL, Monleon M, Monllau JC, Suay J. Biodegradable PCL scaffolds with an interconnected spherical pore network for tissue engineering. J Biomed Mater Res A. 2008;85:25–35. doi: 10.1002/jbm.a.31396. [DOI] [PubMed] [Google Scholar]
- 4.Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, Tuan RS. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Kemppainen JM, Hollister SJ. Tailoring the mechanical properties of 3D-designed poly(glycerol sebacate) scaffolds for cartilage applications. J Biomed Mater Res A. 2010 Jan 20; doi: 10.1002/jbm.a.32653. [DOI] [PubMed] [Google Scholar]
- 6.Hollister SJ, Liao EE, Moffitt EN, Jeong CG, Kemppainen JM. In: Defining design targets for tissue engineering scaffolds. Fundamentals of tissue engineering and regenerative medicine. Meyer U, editor. Berlin: Springer Verlag; [Google Scholar]
- 7.Kang Y, Yang J, Khan S, Anissian L, Ameer GA. A new biodegradable polyester elastomer for cartilage tissue engineering. Wiley Periodicals, Inc.; 2006. published online ( www.interscience.wiley.com). [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat Biotechnol. 2002;20:602–606. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 9.Nijst CL, Bruggeman JP, Karp JM, Ferreira L, Zumbuehl A, Bettinger CJ, Langer R. Synthesis and characterization of photocurable elastomers from poly(glycerol-co-sebacate) Biomacromolecules. 2007;8:3067–3073. doi: 10.1021/bm070423u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Webb AR, Pickerill SJ, Hageman G, Ameer GA. Synthesis and evaluation of poly(diol citrate) biodegradable elastomers. Biomaterials. 2006;27:1889–1898. doi: 10.1016/j.biomaterials.2005.05.106. [DOI] [PubMed] [Google Scholar]
- 11.Motlagh D, Yang J, Lui KY, Webb AR, Ameer GA. Hemocompatibility evaluation of poly(glycerol-sebacate) in vitro for vascular tissue engineering. Biomaterials. 2006;27:4315–4324. doi: 10.1016/j.biomaterials.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Webb AR, Ameer GA. Novel Citric Acid-Based Biodegradable Elastomers for Tissue Engineering. Adv Mater. 2004;16:511–516. [Google Scholar]
- 13.Kim K, Jeong CG, Hollister SJ. Non-invasive monitoring of tissue scaffold degradation using ultrasound elasticity imaging. Acta Biomater. 2008;4:783–790. doi: 10.1016/j.actbio.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao J, Crapo PM, Wang Y. Macroporous elastomeric scaffolds with extensive micropores for soft tissue engineering. Tissue Eng. 2006;12:917–925. doi: 10.1089/ten.2006.12.917. [DOI] [PubMed] [Google Scholar]
- 15.Hollister SJ, Maddox RD, Taboas JM. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials. 2002;23:4095–4103. doi: 10.1016/s0142-9612(02)00148-5. [DOI] [PubMed] [Google Scholar]
- 16.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 17.Liao E, Yaszemski M, Krebsbach P, Hollister S. Tissue-engineered cartilage constructs using composite hyaluronic acid/collagen I hydrogels and designed poly(propylene fumarate) scaffolds. Tissue Eng. 2007;13:537–550. doi: 10.1089/ten.2006.0117. [DOI] [PubMed] [Google Scholar]
- 18.Jeong CG, Hollister SJ. Mechanical, permeability, and degradation properties of 3D designed poly(1,8 octanediol-co-citrate)(POC) scaffolds for soft tissue engineering. J Biomed Mater Res B Appl Biomater. 2010 Jan 20; doi: 10.1002/jbm.b.31568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemppainen JM. PhD thesis. Ann Arbor, MI, U.S.A.: The University of Michigan; 2008. Mechanically stable solid freeform fabricated scaffolds with permeability optimized for cartilage tissue engineering. [Google Scholar]
- 20.Liao E, Yaszemski M, Krebsbach P, Hollister S. Tissue-engineered cartilage constructs using composite hyaluronic acid/collagen I hydrogels and designed poly(propylene fumarate) scaffolds. Tissue Eng. 2007;13:537–550. doi: 10.1089/ten.2006.0117. [DOI] [PubMed] [Google Scholar]
- 21.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochem Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 22.Chandrasekhar S, Esterman MA, Hoffman HA. Microdetermination of proteoglycans and glycosaminoglycans in the presence of guanidine hydrochloride. Anal Biochem. 1987;161:103–108. doi: 10.1016/0003-2697(87)90658-0. [DOI] [PubMed] [Google Scholar]
- 23.Kim YJ, Sah RL, Doong JY, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168–176. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 24.Li SH, de Wijn JR, Layrolle P, de Groot K. Accurate geometric characterization of macroporous scaffold of tissue engineering. Bioceramics. 2003;240–2:541–545. [Google Scholar]
- 25.Bruckner P, van der Rest M. Structure and function of cartilage collagens. Microsc Res Tech. 1994;28:378–384. doi: 10.1002/jemt.1070280504. [DOI] [PubMed] [Google Scholar]
- 26.Martin I, Jakob M, Schafer D, Dick W, Spagnoli G, Heberer M. Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthritis Cartilage. 2001;9:112–118. doi: 10.1053/joca.2000.0366. [DOI] [PubMed] [Google Scholar]
- 27.Bohme K, Conscience-Egli M, Tschan T, Winterhalter KH, Bruckner P. Induction of proliferation or hypertrophy of chondrocytes in serum-free culture: the role of insulin-like growth factor-I, insulin, or thyroxine. J Cell Biol. 1992;116:1035–1042. doi: 10.1083/jcb.116.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen G. The role of type X collagen in facilitating and regulating endochondral ossification of articular cartilage. Orthod Craniofac Res. 2005;8:11–17. doi: 10.1111/j.1601-6343.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- 29.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 30.Malemud CJ. Matrix metalloproteinases: role in skeletal development and growth plate disorders. Front Biosci. 2006;11:1702–1715. doi: 10.2741/1916. [DOI] [PubMed] [Google Scholar]
- 31.Cawston TE, Wilson AJ. Understanding the role of tissue degrading enzymes and their inhibitors in development and disease. Best Pract Res Clin Rheumatol. 2006;20:983–1002. doi: 10.1016/j.berh.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Banu N, Banu Y, Sakai M, Mashino T, Tsuchiya T. Biodegradable polymers in chondrogenesis of human articular chondrocytes. J Artif Organs. 2005;8:184–191. doi: 10.1007/s10047-005-0302-3. [DOI] [PubMed] [Google Scholar]
- 33.Gong Y, Ma Z, Zhou Q, Li J, Gao C, Shen J. Poly (lactic acid) scaffold fabricated by gelatin particle leaching has good biocompatibility for chondrogenesis. J Biomater Sci Polym Ed. 2008;19:207–221. doi: 10.1163/156856208783432453. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Kim YM, Langer R. In vivo degradation characteristics of poly (glycerol sebacate) J Biomed Mater Res A. 2003;66:192–197. doi: 10.1002/jbm.a.10534. [DOI] [PubMed] [Google Scholar]
- 35.Lam CX, Hutmacher DW, Schantz JT, Woodruff MA, Teoh SH. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J Biomed Mater Res A. 2009;90:906–919. doi: 10.1002/jbm.a.32052. [DOI] [PubMed] [Google Scholar]
- 36.Tan PS, Teoh SH. Effect of stiffness of polycaprolactone (PCL) membrane on cell proliferation. Materials Science and Engineering: C. 2006;27:304–308. [Google Scholar]
- 37.Wang Y, Sheppard BJ, Langer R. Poly (glycerol sebacate) ― a novel biodegradable elastomer for tissue engineering. Mat Res Soc Symp Proc. 2002;724:N11.1.1. [Google Scholar]