Abstract
Individuals with spinal cord injuries above thoracic level 6 (T6) experience episodic bouts of life-threatening hypertension as part of a condition termed autonomic dysreflexia. The paroxysmal hypertension can be caused by a painful stimulus below the level of the injury. Targeted ablation of mesenteric projecting sympathetic neurons may reduce the severity of autonomic dysreflexia by reducing sympathetic activity. Therefore, cholera toxin B subunit (CTB) conjugated to saporin (SAP; a ribosomal inactivating protein that binds to and inactivates ribosomes) was injected into the celiac ganglion to test the hypothesis that targeted ablation of mesenteric projecting sympathetic neurons reduces the pressor response to pain in conscious, spinal cord-transected rats. Nine Sprague-Dawley male rats underwent a spinal cord transection between thoracic vertebrae 4 and 5. Following recovery (5 wk), all rats were instrumented with a radio telemetry device for recording arterial pressure and bilateral catheters in the gluteus maximus muscles for the infusion of hypertonic saline (hNa+Cl−). Subsequently, the hemodynamic responses to intramuscular injection of hNa+Cl− (100 μl and 250 μl, in random order) were determined. Following the experiments in the no celiac ganglia injected condition (NGI), rats received injections of CTB-SAP (n = 5) or CTB (n = 3) into the celiac ganglia. CTB-SAP rats, compared with NGI and CTB rats, had reduced pressor responses to hNa+Cl−. Furthermore, the number of stained neurons in the celiac ganglia and spinal cord (segments T6–T12), was reduced in CTB-SAP rats. Thus, CTB-SAP retrogradely transported from the celiac ganglia is effective at ablating mesenteric projecting sympathetic neurons and reducing the pressor response to pain in spinal cord-transected rats.
Keywords: autonomic dysreflexia, spinal cord injury, sympathectomy
autonomic regulation of the cardiovascular system is abnormal and unstable after midthoracic spinal cord injury (47). For example, spinal cord injuries above thoracic level 6 (T6) are associated with episodic bouts of life-threatening hypertension as part of a condition known as autonomic dysreflexia (AD). Lesions at or above T6 are necessary for the development of AD due to loss of supraspinal control to the critical splanchnic vascular bed and adrenal medulla. The adrenal medulla contains an important source of catecholamines for adrenergic receptors, while the splanchnic circulation is critical in regulating arterial blood pressure, because it receives ∼60% of the cardiac output and contains approximately one-third of the total blood volume (22). The splanchnic circulation is innervated by sympathetic nerves, which are vasoconstrictors through prevertebral sympathetic celiac and superior and inferior mesenteric ganglia, as well as spinal sensory nerves (dorsal root ganglia neurons), which are vasodilators via sensory neurotransmitters, such as calcitonin gene-related peptide (22).
The paroxysmal, life-threatening hypertension may be caused by pain, stimulation of the skin, distension of the urinary bladder or colon, and muscle spasms (11, 32). Physiologically, AD is caused by a massive sympathetic discharge triggered by the stimulus originating below the level of the spinal cord injury (20). If not treated promptly, the hypertension may produce cerebral and subarachnoid hemorrhage, seizures, and renal failure and may lead to death (34). The long-term consequence of repeated episodes of severe hypertension has yet to be determined.
Early interventions designed to prevent AD involved methodologies such as subarachnoid alcohol blocks, anterior rhizotomies, sacral extradural neurotomies, and cordectomies; however, these procedures often disrupt sexual, bladder, and bowel function (10, 38). Currently, chronic pharmacological blockade of components of the autonomic nervous system are employed to prevent AD; however, these interventions are associated with similar side effects (38). Furthermore, compliance for these agents is low due to the expense, forgetfulness, and episodic nature of the hypertension. Thus, additional interventions designed to attenuate the severity of AD have the potential to improve the quality of life for individuals and families with spinal cord injury.
Saporin (SAP) is a ribosomal-inactivating protein that binds to and inactivates ribosomes by disabling the cell's protein synthetic machinery, which causes the cell to die over a period of hours to days through apoptotic mechanisms (2, 39, 43). SAP can be linked to molecules that allow targeting of the toxin to a precisely defined population of cells (27). For example, SAP can be linked to retrogradely transported molecules like cholera toxin B subunit (CTB) that bind to specific membrane components that are differentially expressed on nerve cells. This provides a means for targeting SAP to a subset of neurons that could not previously be selectively ablated. For example, we recently documented that CTB-SAP retrogradely transported from the stellate ganglia is effective in ablating cardiac sympathetic neurons and reducing resting, exercise, and reflex sympathetic activity (30).
Therefore, we tested the hypothesis that celiac ganglia injection of SAP conjugated to CTB (CTB-SAP) would reduce the hemodynamic responses to a painful stimulus [intramuscular injection of hypertonic saline (hNa+Cl−)] in spinal cord-transected rats. We hypothesized that SAP would reduce the number of sympathetic postganglionic neurons in the celiac ganglia and virtually eliminate sympathetic preganglionic neurons (SPNs) of spinal cord segments T5–T12 without altering afferent function. We determined the size and number of sympathetic postganglionic neurons in the celiac ganglia; and since lesions at spinal segment T6 or above are required for the development of AD, we assessed spinal levels T6 and T7 for ablation of SPNs. To document that afferent function was not altered by SAP, we examined the dorsal root ganglia of spinal segments T5–T12. We studied conscious, chronically instrumented rats to negate the confounding effects of anesthetic agents and surgical trauma.
MATERIALS AND METHODS
Experimental procedures and protocols were reviewed and approved by the Animal Care and Use Committee of Wayne State University and adhered to the American Physiological Society's Guiding Principles in the Care and Use of Animals. Nine adult spinal cord-transected Sprague-Dawley male rats were studied before celiac ganglia injection [no celiac ganglia injection (NGI)]. Subsequently, the rats were randomly divided into two groups: 1) celiac ganglia injection of CTB (n = 3) and 2) celiac ganglia injection of CTB-SAP (n = 5). One rat in the NGI condition did not survive the celiac ganglion injection procedure.
Surgical Procedures
All surgical procedures were performed using aseptic surgical techniques. Rats were anesthetized with pentobarbital sodium (50 mg/kg ip), atropinized (0.05 mg/kg ip), intubated, and prepared for aseptic surgery. Supplemental doses of pentobarbital sodium (10–20 mg/kg ip) were administered if the rat regained the blink reflex or responded during the surgical procedures.
Spinal cord transection.
After anesthesia was induced, the rats were intubated and positioned prone over a thoracic roll that slightly flexed the trunk. The fourth thoracic vertebra was exposed via a midline dorsal incision, and the spinous process and laminae were removed. Two ligatures (6-0 silk) were tightened around the underlying spinal cord between the T5 and T6 segments, and the spinal cord was completely transected by cutting between the ligatures with scissors (28). In this way, there was minimal bleeding. The completeness of the transection was confirmed by visual inspection of the lesion site. During the acute recovery period (∼10 days), all rats were handled at least six times daily. During these periods, visual inspections and physical manipulations were performed to detect and prevent pressure sores. In addition, the urinary bladder was voided by manual compression, and all animals were weighed. After this acute recovery period, rats required only daily inspection, and the bladders did not require manual compression. In addition, body weight was recorded at least every other day to determine the overall health of the animals. Specifically, body weight on the day of the spinal cord transection averaged 424 ± 11 g. At the completion of the studies, the body weights of the spinal cord-transected rats averaged 399 ± 11 g. It is important to note that spinal cord-transected rats eat unassisted and move themselves around the cage with their forelimbs. In fact, rats with identical spinal cord transections have been studied after dynamic exercise (7). Specifically, spinal cord-transected rats had their lower bodies secured onto a small cart and “ran” on a motor-driven treadmill. The spinal cord-transected rats propelled themselves with their upper bodies at 9–12 m/min for 40 min without the use of adversive stimuli (7).
At day 7 posttransection, the rats received a motor activity score using criteria described previously (52). The motor activity score was assessed by placing the animal on a paper-covered table and observing spontaneous motor activity for 1 min. Motor scores ranged from 0 to 5. A motor score of 5 indicates normal walking, whereas a score of 0 indicates no weight bearing or spontaneous voluntary movement in the hind limbs. All rats had a motor score of 0, which indicates no weight bearing. Upon completion of the studies, the site of the spinal transection was confirmed by autopsy. All rats were allowed to recover at least 5 wk.
Radiotelemetry implantation.
Subsequently, the rats were anesthetized as described above and a telemetry device (model no. TA11PA-C40; Data Sciences International) was implanted in all rats as previously described (6, 7) for chronic, untethered measurements of arterial pressure and heart rate, and a catheter was placed in the intraperitoneal space for the infusion of fluids and drugs. Specifically, the transmitter body and the intraperitoneal catheter were placed in the intraperitoneal space through a ventral abdominal approach. The intraperitoneal catheter was exteriorized on the dorsal aspect of the neck. The pressure sensor of the telemetry device, located within the tip of a catheter, was inserted into the descending aorta via the left carotid artery. In addition, bilateral catheters were placed in the gluteus maximus muscles for the infusion of hNa+Cl−. A minimum of 1 wk was allowed for recovery and for the animals to regain their presurgical weight. During the recovery period, the rats were handled, weighed, and acclimatized to the laboratory and investigators.
Experimental Procedures
Autonomic dysreflexia protocol.
After the recovery period, conscious, unrestrained rats were studied in their home cages (∼13,350 cm3) for all experiments. On the day of the experiment, rats were brought into the laboratory and allowed to adapt to the environment for approximately 1 h to ensure stable hemodynamic conditions (stabilization period). After the stabilization period, beat-by-beat, steady-state hemodynamic variables were recorded over 10 to 15 s. Subsequently, cardiac autonomic blockade was produced. Specifically, cardiac muscarinic-cholinergic receptor blockade was achieved by infusion of the nonspecific muscarinic-cholinergic receptor antagonist atropine methyl bromide (methylatropine 3 mg/kg), and cardiac β1-adrenergic receptor blockade was achieved by infusion of the specific β1-adrenergic receptor antagonist metoprolol (10 mg/kg) into the intraperitoneal catheter. This was done to eliminate the bradycardic response associated with AD since this response is not consistent (6, 7). The efficacy of cardiac autonomic blockade with intraperitoneal administration of these doses was previously determined (4). Subsequently, the heart rate and arterial pressure responses to 5% hNa+Cl− injected into the gluteus maximus muscle were determined (intervention period). Intramuscular injections of hNa+Cl− is an established model for examining pain mechanisms in both humans (49) and animals (3, 44, 45). The volumes of 100 and 250 μl (in random order with at least 20 min between injections) of hNa+Cl− were chosen because a volume of 250 μl injected into cat masseter muscle consistently yielded reliable results without producing muscle necrosis even after multiple injections (3, 44, 45). Intramuscular injection of hNa+Cl− produced robust pressor responses in spinal cord-transected rats (Fig. 1). The entire data collection took ∼2 h. At the end of the experiment, the rats were returned to their housing facilities.
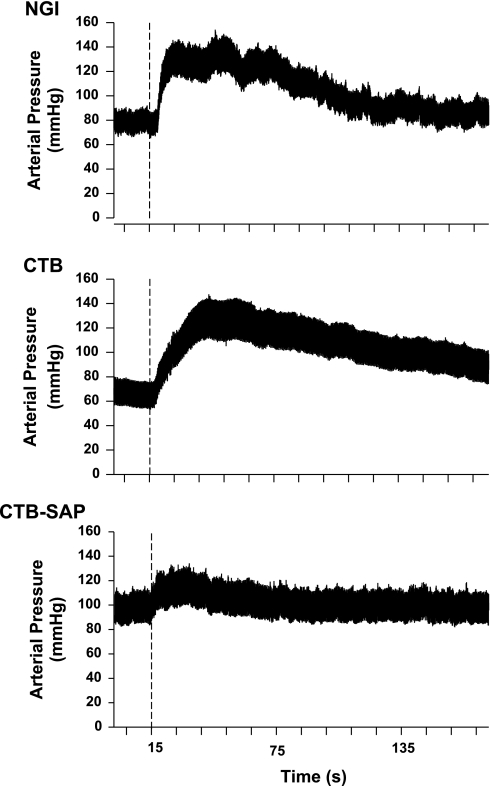
Fig. 1.
Analog recordings of arterial pressure before and during intramuscular injection of hypertonic saline in three spinal cord-transected rats with: 1) no celiac ganglia injection (NGI), 2) CTB injected into the celiac ganglia, and 3) CTB-SAP injected into the celiac ganglia. Dotted line indicates injection of hypertonic saline (250 μl). The pressor response to intramuscular injection of hypertonic saline was notably lower in the rat with celiac ganglion injection of CTB-SAP.
Autonomic dysreflexia protocol postceliac ganglia injections.
Following the NGI experiment, rats were randomly divided into two groups and received injections of CTB-SAP (n = 5) or CTB (n = 3) into the celiac ganglia. The animals were anesthetized as described above, and the celiac ganglion was approached via a ventral abdominal incision. The intestines were moved superiorly in the abdominal cavity, exposing the aorta, superior mesenteric artery, and celiac artery. The celiac ganglion was located on the descending aorta at the branch points between the celiac and superior mesenteric arteries. Nine microliters of CTB subunit (1 mg CTB dissolved in 200 μl of distilled water; cat. no. 103-B; List Biological Laboratories, Campbell, CA) or 9 μl of CTB conjugated with SAP (2.5 mg/ml; cat. no. IT-14; Advanced Targeting Systems, San Diego, CA) were mixed with 1 μl of 3% Evans blue dye. The purpose of the Evans blue dye was to visualize the injectate (CTB and SAP are colorless), assuring localization within the ganglia. All injections were confined within the celiac ganglia. The CTB/Evans blue dye or CTB-SAP/Evans blue dye solutions were pressure-injected with mineral oil into the ganglia using a glass micropipette. A minimum of 1 wk was allowed for recovery and for the animals to regain their presurgical weight. After the recovery period, the hemodynamic responses to intramuscular injection of hNa+Cl− were repeated as described above (Fig. 1).
Perfusion, tissue processing, and immunocytochemistry.
After completion of the studies (∼2 wk), an additional group of noninjected rats, as well as the CTB and CTB-SAP rats, were deeply anesthetized with pentobarbital sodium (100 mg/kg), injected with heparin (1,000 IU), and flushed transcardially with 500 ml of oxygenated tissue culture medium (cat. no. D-8900; Sigma, St. Louis, MO) followed by 1 liter of 4% formaldehyde in 0.1 M phosphate buffer, pH 7.4. Spinal cords and ganglia from all rats were removed and postfixed intact in formaldehyde for 3 days at room temperature on a shaker. After postfixation, the spinal cords were removed and divided into single segments from T5 to T12. The rostral edge of each dorsal root entry zone was taken as the rostral boundary for each segment. Thoracic segments T5–T12 were obtained because SPNs projecting to the celiac ganglia are concentrated in these segments (51). Spinal cords and ganglia were cryoprotected in increasing concentrations of sucrose (10, 20, and 30%), embedded in optimum cutting temperature compound and sectioned on a cryostat.
To determine the number and size of sympathetic postganglionic neurons and afferent neurons from the splanchnic region, the celiac ganglia and dorsal root ganglia (T5–T12), respectively, were sectioned at 10-μm intervals, mounted on SuperFrost Plus slides (Fisher), and stained with cresyl violet. The sympathetic postganglionic cell bodies, on every tenth section, were counted and measured with the aid of a ×20 objective and MicroBrightField Neurolucida software interfaced with a BH-2 Olympus microscope. Only neurons with distinct, prominent nucleoli were counted. Based on a study by Jones (17), no correction for split nucleoli is necessary in 10-μm sections.
Individual spinal cords segments (T5–T12) were sectioned horizontally at 25-μm intervals, and stored in 30/30% sucrose/ethanediol in 0.1 M phosphate buffer, pH 7.2, at −20°C until use. On the day of the analysis, the sections were rinsed (3 × 10 min each) in Tris PBS, pH 7.4 (TPBS) containing 0.03% Triton X-100 (TPBS-Triton) and were then incubated in 10% heat-inactivated normal horse serum (Invitrogen) in TPBS-Triton for at least 1 h. Sections were incubated in goat anti-CTB antiserum (1:400,000; List Biologicals) in TPBS-Triton containing 10% normal horse serum for 48 h at room temperature. After being rinsed (in TPBS, 3 × 10 min each), sections were incubated with biotinylated donkey anti-goat antiserum (1:500; Jackson Laboratories) in TPBS-Triton with 1% normal horse serum overnight at room temperature. Sections were rinsed again (in TPBS, 3 × 10 min each) and incubated 4–6 h in 1:1,500 ExtrAvidin peroxidase in TPBS-Triton (cat. no. E-2886; Sigma). Immunoreactive axons were revealed with the nickel-intensified diaminobenzidine reaction (26). Consistent with recent findings (30), injection of CTB into the celiac ganglia revealed many immunopositive neurons, whereas injection of CTB-SAP revealed no neurons (see Fig. 4).
Fig. 4.
CTB-immunoreactive neurons of the T6–T7 spinal cord from rats that had CTB (A) or CTB-SAP (B) injected into the celiac ganglia. CTB-immunoreactive sympathetic preganglionic neurons (SPNs) were found on both sides of the column of the T6 and T7 spinal cord in CTB rats (A). However, virtually all of the CTB-immunoreactive SPNs were eliminated in the CTB-SAP group (B). Scale bar (bottom right) = 25 μm.
Data Analysis
All data are from nine rats before celiac ganglia injection (NGI): five rats in the CTB-SAP condition and three rats in the CTB condition (one CTB rat did not survive the final protocol). All recordings were sampled at 2 kHz, and the data were expressed as means ± SE. All data for the stabilization period were the average of every beat during the last 10–15 s of the period. For the intervention period, data were the average of every beat during the 10–15 s around the peak response. Two separate one-factor ANOVAs (1 before and 1 after cardiac autonomic blockade) were used to compare resting mean arterial pressure and heart rate (Table 1). Two separate two-factor ANOVAs with repeated measures (NGICTB vs. CTB and NGICTB-SAP vs. CTB-SAP) and the post hoc Holm-Sidak method were used to compare the pressor response to 100 and 250 μl hNa+Cl− in the NGI, CTB, and CTB-SAP groups (Fig. 2). Finally, a one-factor ANOVA was used to compare neuronal number and soma area between the NGI, CTB, and CTB-SAP groups (Fig. 3).
Table 1.
Resting mean arterial pressure (MAP) and heart rate (HR) before and after cardiac autonomic blockade in rats in the no celiac ganglia injected condition (NGI) and after cholera toxin B (CTB) or CTB conjugated to saporin (CTB-SAP) was injected into the celiac ganglia
| NGICTB | NGICTB-SAP | CTB | CTB-SAP | |
|---|---|---|---|---|
| Before Cardiac Autonomic Blockade | ||||
| MAP | 113 ± 7 | 102 ± 3 | 110 ± 5 | 104 ± 5 |
| HR | 487 ± 16 | 484 ± 18 | 480 ± 15 | 486 ± 14 |
| After Cardiac Autonomic Blockade | ||||
| MAP | 102 ± 7 | 94 ± 2 | 106 ± 6 | 99 ± 5 |
| HR | 367 ± 9 | 356 ± 6 | 354 ± 15 | 342 ± 4 |
Values are means ± SE. Control data for the CTB and CTB-SAP rats are presented separately. Specifically, the 3 animals that received CTB are matched with their response before injection (NGICTB). The 5 animals that received CTB-SAP are matched with their response before injection (NGICTB-SAP).
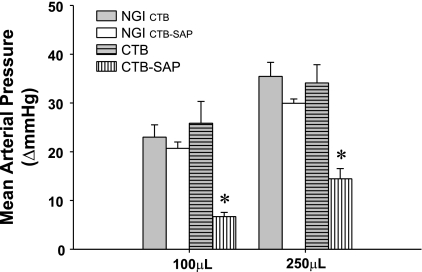
Fig. 2.
Change in mean arterial pressure in response to 100 μl and 250 μl of hNa+Cl− injected into the gluteus maximus muscle in the NGI group, CTB celiac-injected group, and CTB-SAP celiac-injected group. The control data for the CTB and CTB-SAP rats are presented separately. Specifically, the 3 animals that received CTB were matched with their response before injection (NGI CTB). The 5 animals that received CTB-SAP were matched with their response before injection (NGI CTB-SAP). There was no difference in the arterial pressure response to 100 μl or 250 μl of hNa+Cl− between the NGI and CTB groups. However, the arterial pressure response to both 100 μl and 250 μl of hNa+Cl− were significantly lower in the CTB-SAP group documenting an attenuated autonomic dysreflexia. *P < 0.05 CTB-SAP vs. NGI and CTB.
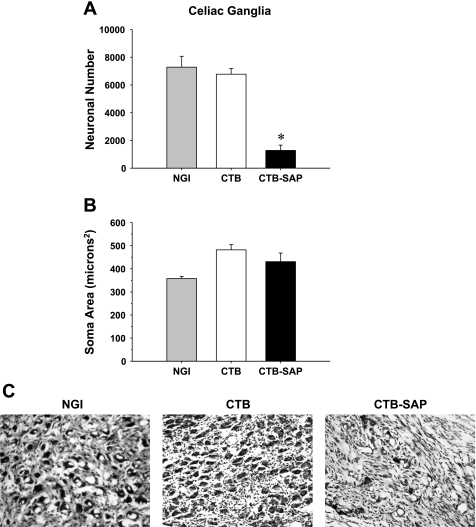
Fig. 3.
Neuronal number (A), average soma area per neuron (B), and cresyl violet-stained celiac neurons (C) from NGI rats, as well as rats that had CTB or CTB-SAP injected into the celiac ganglia. Counts of celiac neurons in the CTB-SAP group showed a significant reduction in the number of neurons compared with the NGI and CTB groups. These results are consistent with C showing many celiac neurons in the NGI and CTB groups and few neurons in the CTB-SAP group. However, of the 2,711 neurons analyzed, the soma area (B) was not different between the three groups. Scale bar (bottom right) = 25 μm. *P < 0.05 CTB vs. CTB-SAP.
RESULTS
Table 1 presents mean arterial pressure and heart rate at rest and before and after cardiac autonomic blockade in the three groups of rats: 1) NGI, 2) CTB injected into the celiac ganglia, and 3) CTB-SAP injected into the celiac ganglia. Resting mean arterial pressure and heart rate before and after blockade were not different between the three groups.
Figure 2 presents the change in mean arterial pressure in response to 100 μl and 250 μl of hNa+Cl− injected into the gluteus maximus muscle in the NGI, CTB, and CTB-SAP conditions. The control data for the CTB and CTB-SAP, rats are presented separately. Specifically, the three animals that received CTB were matched with their response before injection (NGICTB). The five animals that received CTB-SAP were matched with their response before injection (NGICTB-SAP). The change in mean arterial pressure in response to 100 μl or 250 μl of hNa+Cl− was not different between the NGI and CTB groups (F = 0.154, df = 1). However, the pressor response to 250 μl was significantly greater than the response to 100 μl across groups (F = 37.896, df = 1). In sharp contrast, the arterial pressure response to both 100 μl and 250 μl of hNa+Cl− were significantly lower in the CTB-SAP group documenting an attenuated AD (F = 171.4, df = 1). Furthermore, the pressor response to 250 μl was significantly greater than the response to 100 μl across groups (F = 52.7, df = 1).
Figure 3 presents neuronal number (A), average soma area per neuron (B), and cresyl violet-stained celiac neurons (C) from rats that had NGI or CTB or CTB-SAP injected into the celiac ganglia. Counts of celiac neurons in the CTB-SAP group showed a significant reduction in the number of neurons compared with both the NGI and CTB groups (1,275 ± 382 vs. 7,284 ± 784 and 6,775 ± 414, respectively). These results are consistent with Fig. 3C showing many celiac neurons in the NGI and CTB groups with few neurons in the CTB-SAP group. However, of the 2,711 neurons analyzed, the soma area (Fig. 3B) was not different between the three groups (NGI: 358 ± 9, CTB: 482 ± 22, and CTB-SAP: 430 ± 37 microns2).
Figure 4 presents CTB-immunoreactive neurons of the T6–T7 spinal cord from rats that had CTB or CTB-SAP injected into the celiac ganglia. CTB-immunoreactive SPNs were found on both sides of the column of the T6–T7 spinal cord in CTB rats (Fig. 4A). However, virtually all of the CTB-immunoreactive SPNs were eliminated in the CTB-SAP group (Fig. 4B). Control slides (without primary antibody) did not have labeled cells.
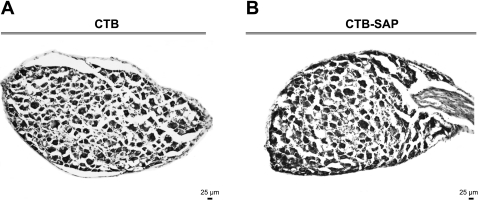
Figure 5 presents cresyl violet-stained dorsal root ganglia neurons from rats that had CTB or CTB-SAP injected into the celiac ganglia. Rats with celiac ganglion injections of CTB-SAP did not have afferents disrupted since neurons within the dorsal root ganglia were not affected, suggesting that afferent pathways remained functional.
Fig. 5.
Cresyl violet-stained neurons of the dorsal root ganglia from rats that had CTB (A) or CTB-SAP (B) injected into the celiac ganglia. It is clear that CTB-SAP did not alter neurons within the dorsal root ganglia. Scale bar (bottom right) = 25 μm.
DISCUSSION
In this study, we tested the hypothesis that celiac ganglion injection of CTB-SAP would reduce the hemodynamic responses to pain (AD) in spinal cord-transected rats. The major findings of this study include: 1) CTB-SAP rats had a reduced blood pressure response to intramuscular injection of hNa+Cl− (Figs. 1 and 2), and 2) associated with the reduced pressor response to hNa+Cl− was a reduction in the number and/or area of celiac postganglionic neurons and celiac projecting SPNs (Figs. 3 and 4) without affecting afferent neurons (Fig. 5). Since AD is caused by a massive sympathetic discharge triggered by the stimulus originating below the level of the spinal cord injury (20), these data suggest that CTB-SAP reduced the sympathetic response to a painful stimulus below the level of the lesion in spinal cord-transected rats.
Autonomoc dysreflexia occurs in as many as 85% of individuals with spinal cord injuries above T6 and is characterized by severe hypertension, sweating, dizziness, nausea, and severe headaches. AD may be caused by stimulation of the skin, distension of the urinary bladder or colon, pain, and muscle spasms. The afferent stimulation below the level of the injury results in massive sympathetic activity that causes vasoconstriction of most vascular beds below the injury and baroreflex-mediated bradycardia due to parasympathetic activation and sympathetic withdrawal above the lesion with resultant vasodilatation that mediates headaches and skin flushing. To prevent the baroreflex-mediated bradycardia, which has been documented to be inconsistent (6, 7), we performed cardiac autonomic blockade. We were interested in blocking the massive sympathetic activity to the mesenteric vasculature and cardiac autonomic blockade has no effect on this response.
Reductions in sympathetic responses are clinically important because excessive sympathetic responses are responsible for, and/or contribute to, the morbidity and mortality associated with many cardiovascular disorders. For example, the mechanisms mediating several forms of life-threatening hypertension involve excess sympathetic activity. Sympathetic activity increases cardiac output (heart rate and stroke volume) and vascular resistance as well as the release of vasoactive substances leading to elevations in blood pressure. Accordingly, reducing sympathetic activity and/or blocking the sympathetic responses to stimuli are the first-line therapy for many hypertensive disorders. For example, β-adrenergic receptor antagonists are the first-line therapy for hypertension. However, despite their favorable effects, adverse complications (due to generalized sympathoinhibition; e.g., fatigue, impotence) limit compliance to these treatments. For example, the Medical Research Council trial (35) on hypertensive patients documented that for every myocardial infarction or stroke prevented by β-adrenergic receptor antagonists, three patients withdrew from the study secondary to impotence and another seven withdrew because of fatigue (37). Targeted, celiac ganglion injection of CTB-SAP may overcome these issues because celiac ganglion injection of CTB-SAP selectively reduces sympathetic activity to the mesenteric vasculature, avoiding generalized sympathoinhibition. Thus, this procedure should avoid limitations of medical therapy, such as incomplete compliance due to medical complications.
It is important to note that sympathectomy (ganglion ablation) is markedly different from targeted ablation of celiac sympathetic pre- and postganglionic neurons. Sympathectomy ablates fibers passing through the ganglia, disrupting afferent signals as well as efferent fibers ascending and descending within the sympathetic chain. In this context, rats with celiac ganglion injections of CTB-SAP did not have afferents disrupted, since neurons within the dorsal root ganglia were not affected (Fig. 5), suggesting that afferent pathways remained functional. This is important because many biological functions, for example the defecation reflex, are initiated by afferent stimuli and are mediated, in part, by spinal reflexes. Individuals with spinal injuries can take advantage of this reflex loop by using digital rectal stimulation to assist with defecation and other biological functions. However, additional studies will be required to further characterize this new procedure.
In addition, sympathectomy largely prevents norepinephrine release (possibly leading to denervation supersensitivity). Thus, there is no way to grade the level of sympathetic denervation. In sharp contrast, with celiac injections of CTB-SAP, it may be possible to adjust the dosage to partially denervate targets to desirable levels. Finally, this new method provides an additional technique to selectively denervate sympathetic control to the vasculature providing options for susceptible individuals.
Clinical Implications
Prior to World War II, 80% of individuals with spinal cord injury died within 3 yr of the injury. However, with the advent of antibiotic drugs and advancements in acute care and rehabilitation, the life expectancy of individuals with spinal cord injury has increased to near that for able-bodied individuals. Importantly, cardiovascular disease is now a leading cause of death and morbidity for individuals with spinal cord injuries (12). Autonomic dysreflexia may be a contributing factor since the long-term consequence of repeated episodes of severe hypertension has yet to be determined. However, it is well known that increased arterial pressure variability is a significant cardiovascular disease risk factor (9). Thus, interventions designed to reduce episodic bouts of hypertension may prevent end-organ damage, cerebral and subarachnoid hemorrhage, seizures, and renal failure and may prevent death.
It is important to note that the hypertension associated with AD is markedly underrecognized (10). This is a serious concern, because a continuous, strong, graded, independent, and etiologically significant relationship between elevated blood pressure and cardiovascular and cerebrovascular risk has been described (50). Thus, early intervention for all individuals with hypertension is recommended based on studies documenting that antihypertensive therapy reduces mortality and ameliorates symptoms of hypertension in individuals with accelerated hypertension as well as individuals with so-called benign hypertension. These results suggest that reducing the hemodynamic response to AD may have long-term beneficial consequences in individuals with spinal cord injury.
In addition, individuals with spinal cord injuries have distinct hemodynamic responses to a variety of activities of daily living. For example, it is well established that individuals with midthoracic cord injury have elevated heart rates and lower stroke volumes at rest and during activity than able-bodied individuals (16). Similarly, rats with midthoracic spinal cord injury have elevated heart rates (Table 1 and Refs. 21, 31, and 33), as well as increased cardiac sympathetic tonus (28, 46). Numerous studies have documented that an elevated heart rate is a strong risk factor for the development of cardiovascular disease (18, 42). Furthermore, elevated cardiac sympathetic activity is responsible for, and/or contributes to, the morbidity and mortality associated with many cardiovascular disorders. Thus, reducing cardiac sympathetic tonus with stellate ganglia injection of CTB-SAP may reduce the incidence of cardiovascular disorders in the spinal cord injury population (8, 28, 29, 48). This hypothesis merits future investigation.
Limitations
Although some investigators have raised questions with preparations where a common carotid artery (CCA) is ligated due to theoretical concerns about central ischemia and/or loss of a baroreceptor station, we have used this procedure for many years and do not believe it is a concern.
Specifically, in an extensive evaluation of rodent models of cerebral ischemia, Ginsberg and Busto (15) concluded that unilateral CCA ligation in rats leaves the cerebral energy state unchanged. Although Levine and Payan (23) first observed that ∼20% of Mongolian gerbils (Meriones unguiculatus) subjected to unilateral CCA ligation developed severe neurologic signs and died within 2 days; subsequent histologic studies revealed an absence of the expected posterior communicating arteries that, in rats, connect the carotid and vertebrobasilar arterial systems (24). Thus, concerns regarding central ischemia are unwarranted.
Regarding baroreceptor denervation, blockade of the left carotid sinus did not alter the reflex control of the circulation, which led the authors to suggest the existence of a central communication between the two carotid sinuses (1). Furthermore, Ciriello et al. (5) have shown that the afferents from the carotid and aortic baroreceptors converge to the same areas in the central nervous system. It is possible that because of the lack of information from one carotid baroreceptor, the responses to changes in pressure at the aortic baroreceptors increase. Finally, over 4–7 days after bilateral carotid sinus denervation, the strength of control of both peripheral resistance and atria rate, as well as mean arterial pressure, returned to the levels observed before denervation (40). Considering previous studies, as well as our experience with this procedure over several years, we do not believe that ligation of a single CCA affects the neural control of the circulation.
In conclusion, the results of this study show that CTB-SAP retrogradely transported from the celiac ganglia is effective at ablating sympathetic neurons innervating the mesenteric vasculature and reducing the hemodynamic response to intramuscular hNa+Cl−. This new procedure may become an option for individuals experiencing AD especially when compliance with pharmacological management is low due to the expense, forgetfulness, and the episodic nature of the hypertension.
Perspectives and Significance
Since 1914, members of the medical community have used celiac plexus blockade with a combination of steroid and anesthetic solutions to temporarily interrupt nociception stimuli from the pancreas in individuals with abdominal pain associated with chronic pancreatitis and advanced pancreatic cancer (19). Subsequently, permanent destruction of celiac ganglia neurons using a phenol- or alcohol-based solution was introduced for individuals with severe pain from intra-abdominal malignancies. These procedures are typically performed by anesthesiologists, interventional radiologists, and surgeons using a variety of percutaneous or intraoperative techniques (13, 25, 36, 53). Currently, with the development of the linear array echo endoscope, endosonographers easily localize and directly deliver anesthetic agents to the celiac plexus (14). Since it has become easy to localize and directly deliver agents into the celiac plexus (41), celiac ganglia injections of CTB-SAP may be effective for conditions associated with excess sympathetic activity. However, additional studies are required to further characterize the physiological responses to this procedure and determine whether this new approach is safe and efficacious for the treatment of conditions associated with excess sympathetic activity. For example, the optimal dose along with correlations between the amount of neuronal destruction with CTB-SAP and the reduction in the pressor response to painful stimuli merits investigation. Careful analysis of potential gastrointestinal or urogenital effect must be conducted as well as the determination of whether targeted ablation of mesenteric projecting sympathetic neurons causes any further functional deficit.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-88615.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Behnia R, Koushanpour E, Sinclair DM. Pressure-heart rate relationship in intact and after stepwise elimination of three major baroreflex loops in dogs. Anesth Analg 83: 965–974, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Bolognesi A, Tazzari PL, Olivieri F, Polito L, Falini B, Stirpe F. Induction of apoptosis by ribosome-inactivating proteins and related immunotoxins. Int J Cancer 68: 349–355, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Capra NF, Ro JY. Experimental muscle pain produces central modulation of proprioceptive signals arising from jaw muscle spindles. Pain 86: 151–162, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Chandler MP, DiCarlo SE. Acute exercise attenuates cardiac autonomic regulation in hypertensive rats. Hypertension 26: 676–683, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Ciriello J, Hrycyshyn AW, Calaresu FR. Horseradish peroxidase study of brain stem projections of carotid sinus and aortic depressor nerves in the cat. J Auton Nerv Syst 4: 43–61, 1981 [DOI] [PubMed] [Google Scholar]
- 6.Collins HL, DiCarlo SE. TENS attenuates response to colon distension in paraplegic and quadriplegic rats. Am J Physiol Heart Circ Physiol 283: H1734–H1739, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Collins HL, DiCarlo SE. Acute exercise reduces the response to colon distension in T5 spinal rats. Am J Physiol Heart Circ Physiol 282: H1566–H1570, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Collins HL, Rodenbaugh DW, DiCarlo SE. Paraplegia alters cardiac electrophysiology and increases the susceptibility to ventricular arrhythmias. In: Progress in Brain Research, edited by Weaver L, Polosa C. New York: Elsevier Science, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Collins HL, Rodenbaugh DW, DiCarlo SE. Daily exercise attenuates the development of arterial blood pressure related cardiovascular risk factors in hypertensive rats. Clinc Exp Hypertens 22: 193–202, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Comarr AE, Eltorai I. Autonomic dysreflexia/hyperreflexia. J Spinal Cord Med 20: 345–354, 1997 [PubMed] [Google Scholar]
- 11.Corbett JL, Debarge O, Frankel HL, Mathias C. Cardiovascular responses in tetraplegic man to muscle spasm, bladder percussion and head-up tilt. Clin Exp Pharmacol Physiol Suppl 2: 189–193, 1975 [PubMed] [Google Scholar]
- 12.DeVivo MJ. Causes and costs of spinal cord injury in the United States. Spinal Cord 35: 809–813, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg 80: 290–295, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Faigel DO, Veloso KM, Long WB, Kochman ML. Endosonography-guided celiac plexus injection for abdominal pain due to chronic pancreatitis. Am J Gastroenterol 91: 1675, 1996. [PubMed] [Google Scholar]
- 15.Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke 20: 1627–1642, 1989 [DOI] [PubMed] [Google Scholar]
- 16.Jacobs PL, Mahoney ET, Robbins A, Nash M. Hypokinetic circulation in persons with paraplegia. Med Sci Sports Exerc 34: 1401–1407, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Jones R. Split nucleoli as a source of error in nerve cell counts. Stain Technol 19: 91–95, 1937 [Google Scholar]
- 18.Julius S, Palantini P, Nesbitt SD. Tachycardia: an important determinant of coronary risk in hypertension. J Hypertens Suppl 16: S9–S15, 1998 [PubMed] [Google Scholar]
- 19.Kappis M. Erfahrungen mit localanesthesie bie bauchoperationen. Verh Dtsch Ges Chir 43: 87–89, 1914 [Google Scholar]
- 20.Krassioukov A, Claydon VE. The clinical problems in cardiovascular control following spinal cord injury: an overview. Prog Brain Res 152: 223–229, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Krassioukov AV, Weaver LC. Episodic hypertension due to autonomic dysreflexia in acute and chronic spinal cord-injured rats. Am J Physiol Heart Circ Physiol 268: H2077–H2083, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Kreulen DL. Properties of the venous and arterial innervation in the mesentery. J Smooth Muscle Res 39: 269–279, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Levine S, Payan H. Effects of ischemia and other procedures on the brain and retina of the gerbil (Meriones unguiculatus). Exp Neurol 16: 255–262, 1966 [DOI] [PubMed] [Google Scholar]
- 24.Levine S, Sohn D. Cerebral ischemia in infant and adult gerbils. Relation to incomplete circle of Willis. Arch Pathol 87: 315–317, 1969 [PubMed] [Google Scholar]
- 25.Lillemoe KD, Cameron JL, Kaufman HS, Yeo CJ, Pitt HA, Sauter PK. Chemical splanchnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trial. Ann Surg 217: 447–455, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Llewellyn-Smith IJ, DiCarlo SE, Collins HL, Keast JR. Enkephalin-immunoreactive interneurons extensively innervate sympathetic preganglionic neurons regulating the pelvic viscera. J Comp Neurol 488: 278–289, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Llewellyn-Smith IJ, Martin CL, Arnolda LF, Minson JB. Retrogradely transported CTB-saporin kills sympathetic preganglionic neurons. Neuroreport 10: 307–312, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Lujan HL, Chen Y, DiCarlo SE. Paraplegia increased cardiac NGF content, sympathetic tonus and the susceptibility to ischemia-induced ventricular tachycardia in conscious rats. Am J Physiol Heart Circ Physiol 296: H1364–H1372, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lujan HL, DiCarlo SE. T5 spinal cord transection increases susceptibility to reperfusion-induced ventricular tachycardia by enhancing sympathetic activity in conscious rats. Am J Physiol Heart Circ Physiol 293: H3333–H3339, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Lujan HL, Palani G, Chen Y, Peduzzi-Nelson J, DiCarlo SE. Targeted ablation of cardiac sympathetic neurons reduces resting, reflex and exercise-induced sympathetic activation in conscious rats. Am J Physiol Heart Circ Physiol 296: H1305–H1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maiorov DN, Weaver LC, Krassioukov AV. Relationship between sympathetic activity and arterial pressure in conscious spinal rats. Am J Physiol Heart Circ Physiol 272: H625–H631, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Mathias CJ, Frankel HL. Clinical manifestations of malfunctioning sympathetic mechanisms in tetraplegia. J Auton Nerv Syst 7: 303–312, 1983 [DOI] [PubMed] [Google Scholar]
- 33.Mayorov DN, Adams MA, Krassioukov AV. Telemetric blood pressure monitoring in conscious rats before and after compression injury of spinal cord. J Neurotrauma 18: 727–736, 2001 [DOI] [PubMed] [Google Scholar]
- 34.McGuire TJ, Kumar VN. Autonomic dysreflexia in the spinal cord injured: what the physician should know about this medical emergency. Postgrad Med 80: 81–89, 1986 [DOI] [PubMed] [Google Scholar]
- 35.Medical Research Council Medical Research Council trial of treatment of hypertension in older adults: principal results MRC Working Party. BMJ 304: 405–412, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mercadante S. Celiac plexus block versus analgesics in pancreatic cancer pain. Pain 52: 187–192, 1993 [DOI] [PubMed] [Google Scholar]
- 37.Messerli FH, Grossman E. Beta-blocker therapy and depression. JAMA 288: 1845–1846, 2002 [PubMed] [Google Scholar]
- 38.Naftchi NE. Mechanism of autonomic dysreflexia: contributions of catecholamine and peptide neurotransmitters. Ann NY Acad Sci 579: 133–148, 1990 [DOI] [PubMed] [Google Scholar]
- 39.Nayate A, Moore SA, Weiss RM, Taktakishvili O, Lin LH, Talman WT. Cardiac damage after lesions of the nucleus tractus solitarii. Am J Physiol Regul Integr Comp Physiol 296: R272–R279, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Leary DS, Scher AM. Time course of recovery of arterial pressure control after carotid denervation. Am J Physiol Heart Circ Physiol 258: H73–H79, 1990 [DOI] [PubMed] [Google Scholar]
- 41.O'Toole TM, Schmulewitz N. Complication rates of EUS-guided celiac plexus blockade and neurolysis: results of a large case series. Endoscopy 41: 593–597, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Palantini P, Julius S. Heart rate and cardiovascular risk. J Hypertens 15: 3–17, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Potts JT, Fong AY, Anguelov PI, Lee S, McGovern D, Grias I. Targeted deletion of neurokinin-1 receptor expressing nucleus tractus solitarii neurons precludes somatosensory depression of arterial baroreceptor-heart rate reflex. Neuroscience 145: 1168–1181, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ro JY, Capra NF. Evidence for subnucleus interpolaris in craniofacial muscle pain mechanisms demonstrated by intramuscular injections with hypertonic saline. Brain Res 842: 166–183, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Ro JY, Capra NF. Modulation of jaw muscle spindle afferent activity following intramuscular injections with hypertonic saline. Pain 92: 117–127, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Rodenbaugh DW, Collins HL, DiCarlo SE. Increased susceptibility to ventricular arrhythmias in hypertensive paraplegic rats. Clin Exp Hypertens 25: 349–358, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Rodenbaugh DW, Collins HL, DiCarlo SE. Paraplegia differentially increases arterial blood pressure related cardiovascular disease risk factors in normotensive and hypertensive rats. Brain Res 980: 242–248, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Rodenbaugh DW, Collins HL, Nowacek DG, DiCarlo SE. Increased susceptibility to ventricular arrhythmias is associated with changes in Ca2+ regulatory proteins in paraplegic rats. Am J Physiol Heart Circ Physiol 285: H2605–H2613, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Rubin TK, Gandevia SC, Henderson LA, Macefield VG. Effects of intramuscular anesthesia on the expression of primary and referred pain induced by intramuscular injection of hypertonic saline. J Pain 10: 829–835, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. Arch Intern Med 153: 598–615, 1993 [DOI] [PubMed] [Google Scholar]
- 51.Strack AM, Sawyer WB, Marubio LM, Loewy AD. Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res 455: 187–191, 1988 [DOI] [PubMed] [Google Scholar]
- 52.Von Euler M, Akesson E, Samuelsson EB, Seiger A, Sundstrom E. Motor performance score: a new algorithm for accurate behavioral testing of spinal cord injury in rats. Exp Neurol 137: 242–254, 1996 [DOI] [PubMed] [Google Scholar]
- 53.Wang PJ, Shang MY, Qian Z, Shao CW, Wang JH, Zhao XH. CT-guided percutaneous neurolytic celiac plexus block technique. Abdom Imaging 31: 710–718, 2006 [DOI] [PubMed] [Google Scholar]