Abstract
In addition to its endocytic function, the low density lipoprotein receptor-related protein 1 (LRP1) also contributes to cell signaling events. In the current study, the potential of LRP1 to modulate the platelet-derived growth factor (PDGF) signaling pathway was investigated. PDGF is a key regulator of cell migration and proliferation and mediates the tyrosine phosphorylation of LRP1 within its cytoplasmic domain. In WI-38 fibroblasts, PDGF-mediated LRP1 tyrosine phosphorylation occurred at 37 °C but not at 4 °C, where endocytosis is minimized. Furthermore, blockade of endocytosis with the dynamin inhibitor, dynasore, also prevented PDGF-mediated LRP1 tyrosine phosphorylation. Immunofluorescence studies revealed co-localization of LRP1 with the PDGF receptor after PDGF treatment within endosomal compartments, whereas surface biotinylation experiments confirmed that phosphorylated LRP1 primarily originates from intracellular compartments. Together, the data reveal the association of these two receptors in endosomal compartments where they form a signaling complex. To study the contribution of LRP1 to PDGF signaling, we used mouse embryonic fibroblasts genetically deficient in LRP1 and identified phenotypic changes in these cell lines in response to PDGF stimulation by performing phospho-site profiling. Of 38 phosphorylated proteins analyzed, 8 were significantly different in LRP1 deficient fibroblasts and were restored when LRP1 was expressed back in these cells. Importantly, the results revealed that LRP1 expression is necessary for PDGF-mediated activation of ERK. Overall, the studies reveal that LRP1 associates with the PDGF receptor in endosomal compartments and modulates its signaling properties affecting the MAPK and Akt/phosphatidylinositol 3-kinase pathways.
Keywords: Endocytosis, ERK, Growth Factors, Lipoprotein Receptor, MAP Kinases (MAPKs), ERK, Lipoprotein Receptors, MAPK, PDGF, Phosphoprotein
Introduction
The platelet-derived growth factor (PDGF)3 signaling pathway includes four ligands (PDGF A, B, C, and D) and two receptors (PDGFRα and PDGFRβ) that regulate cell migration, proliferation, and survival (1). The use of gene-deleted mice has identified unique roles for each component during development. For example, these studies have revealed an important contribution of PDGF-A to lung growth and the formation of alveoli (2) as well as the development of gastrointestinal villus cluster cells (3) and a critical role for PDGF-B in the development of microvascular pericytes (4).
In addition to its important contribution to development, the PDGF signaling pathway plays an important role in pathophysiological processes. Thus, enhanced activation of the PDGF signaling pathway is implicated in the development of atherosclerosis, vascular injury-induced restenosis, angiogenesis, and fibrosis (5). A significant contribution of PDGF to vascular remodeling was confirmed by studies demonstrating that neointimal smooth muscle cell accumulation was reduced by administration of either PDGF-B (6) or PDGFR neutralizing antibodies (7, 8) during vascular injury induced by balloon catheterization. Conversely, expression of PDGF-B in the vasculature leads to increased SMC proliferation and intima thickening (9).
A major consequence of the PDGF-signaling pathway is induction of cell proliferation. Studies have shown that the PDGF-mitogenic effect requires two distinct phases of signaling (10, 11). The first pulse of PDGF-mediated signaling moves the cell through the initial segment of the G0 to S interval and renders the cells responsive to a second pulse of growth factor. This first phase occurs within 30 min upon growth factor addition and requires activation of the ERK pathway and induction of the transcription factor c-Myc. The second phase of PDGF signaling, which occurs at prolonged exposure to PDGF, requires activation of protein kinase C that is necessary for progression into the S-phase.
Recent studies have found that the PDGF signaling pathway is modulated by the low density lipoprotein receptor-related protein 1 (LRP1) (12). LRP1 is a large endocytic receptor that binds numerous ligands and is involved in a variety of physiological processes (13, 14). Targeted deletion of the LRP1 gene in vascular smooth muscle cells causes PDGFR overexpression and excessive activation of this signaling pathway resulting in proliferation of vascular smooth muscle cells and increased susceptibility to the development of atherosclerosis (12). The mechanism by which LRP1 affects PDGF signaling pathway is poorly understood but may involve the ability of LRP1 to regulate the transforming growth factor-β signaling pathway (15–17).
LRP1 forms a complex with the PDGFR-β (12, 18), which mediates the tyrosine phosphorylation of the LRP1 cytoplasmic domain (19, 20). This in turn alters its ability to bind to adaptor proteins, such as Shc, which are involved in signaling pathways (19). LRP1 has been shown to be required for PDGF-stimulated migration of vascular smooth muscle cells (21). These observations raise the possibility that LRP1 may be an important component of signaling complexes assembled upon PDGF receptor activation and may provide a spatial and temporal resolution as well as specificity for the PDGF signaling pathway.
Studies in the current investigation were designed to test this possibility. Our results indicate that the recombinant PDGFR-β kinase domain directly catalyzes the tyrosine phosphorylation of LRP1 cytoplasmic domain and that this event occurs within endosomal compartments upon internalization of these two receptors. By employing a phosphoprotein scan, we discovered that PDGF stimulation of fibroblasts requires LRP1 to mediate ERK activation, revealing that LRP1 modulates the PDGF signaling pathway.
EXPERIMENTAL PROCEDURES
Cells and Antibodies
WI-38 fibroblasts were purchased from the ATCC. LRP1+/− (PEA-10), LRP1−/− (PEA-13), and B41 clones (LRP1−/− transfected with human LRP1) have been described (22). Rabbit anti-LRP1 IgG R2629 was prepared as described (18). Anti-LRP1 monoclonal 11H4 has been described (23).
Immunoblot Analysis
LRP1+/−, LRP1−/−, and B41 cells were plated ∼70% confluency in DMEM containing 10% fetal bovine serum. The media was then replaced with DMEM, and the cells were serum-starved overnight and then treated with various concentrations of PDGF-B (Cell Signaling Technology, Inc.) at 37 °C for the indicated times. The cells were lysed in the homogenization buffer supplemented with phosphatase and protease inhibitors (20 mm MOPS, 2 mm EGTA, 5 mm EDTA, 30 mm sodium fluoride, 60 mm β-glycerophosphate, pH 7.2, 20 mm sodium pyrophosphate, 1 mm sodium orthovanadate, 1% Nonidet P-40, 1 mm phenylmethylsulfonyl fluoride, 3 mm benzamidine, 5 μm pepstatin A, 10 μm leupeptin, 1 mm dithiothreitol). Whole cell lysates were separated by SDS-PAGE and then electrophoretically transferred to nitrocellulose membranes, which were first incubated for 1 h at room temperature in buffer containing 50 mm Tris, 150 mm NaCl, 0.1% Tween 20, and 5% nonfat dry milk. The membranes were then incubated overnight with specific antibodies and washed in buffer containing 50 mm Tris, 150 mm NaCl, and 0.1% Tween 20. Antibody binding to the immunoblots was detected by incubation with an appropriate IRDye® (LI-COR Biosciences)-conjugated secondary antibody. Immunoreactive bands were detected using the LI-COR Odyssey Infrared Imaging System.
Co-immunoprecipitation of PDGFR-β with LRP1
∼80% confluent human primary fibroblasts (WI-38) were chilled to 4 °C for 1 h. Cells were incubated with or without PDGF-B for 1 h at 4 °C. Half of the PDGF-B treated cells were shifted to 37 °C for 7 min. Protein-protein interactions were stabilized by incubation with 3 mg/ml dimethyl dithiobispropionimidate (Thermoscientific) cross-linker for 2 h at 4 °C. All cells were lysed in radioimmune precipitation assay buffer (1% Nonidet P-40 in Tris-buffered saline, pH 7.4) supplemented with phosphatase and protease inhibitors, and LRP1 was immunoprecipitated by the anti-LRP1 polyclonal antibody (R2629). Immunocomplexes were pulled down with Dynabeads (Invitrogen) and eluted in 1% SDS, 24 mm dithiothreitol by boiling for 5 min. Elutants were alkylated by addition α-iodoacetamine to 0.1 m final concentration before immunoblot analysis.
PDGFR Kinase Assay
Purified GST-LRP1 intracellular domain (ICD) was incubated with recombinant active PDGFR-β kinase domain (PDGFR-β-kd Millipore) according to the manufacturer's protocol. Briefly, 20 μm GST or 20 μm GST-LRP1-ICD was incubated with or without PDGFR-β-kd in the presence of [γ-32P]ATP at 37 °C for 10 min. All reactions were stopped by the addition of 1× SDS sample buffer and resolved on 4–12% SDS gel. Gel was fixed and exposed to x-ray film.
Ligand Internalization Assay
To block dynamin-mediated endocytosis, cells were treated with a small molecule inhibitor of dynamin, dynasore (initially it was a generous gift from Dr. Kirchhausen, Immune Disease Institute, Boston, MA; later it was purchased from Santa Cruz Biotechnology, Inc.). Cells were preincubated with 200 μm dynasore at 37 °C for 1 h in serum-free conditions before the addition of PDGF-B. Human primary fibroblasts (WI-38 cells) were grown on 12-well plates at 1 × 105/well density in DMEM supplemented with 10% fetal bovine serum. Cells were serum-starved for 16 h before the experiment. Then they were incubated with or without 200 μm dynasore in DMEM supplemented with 20 mm HEPES for 1 h at 37 °C. All media were removed and replaced with DMEM supplemented with HEPES with or without dynasore in the presence of labeled ligands. Cells were then incubated with 10 ng/ml 125I-labeled PDGF-B (PerkinElmer Life Sciences) or 10 nm 125I-labeled RAP at 37 °C for 20 min. All media were removed; cells were washed twice with phosphate-buffered saline. Cells that were incubated with RAP were released from the plate by incubating with trypsin/EDTA solution containing 50 μg/ml proteinase K at 37 °C. Released cells were collected by centrifugation at 6000 rpm for 4 min. Supernatant was removed, and radioactivity was measured for surface bound ligand. The pellet was redissolved in 0.5 ml of 1 m NaOH, and radioactivity was measured representing internalized ligand. Cells incubated with PDGF-B were washed with 0.5 ml of 1 m glycine, pH 2.5, for 2 min and removed, and the radioactivity measured represented cell surface-bound ligand. Cells on the plate were lysed with 0.5 ml of 1 m NaOH, and lysate was measured for radioactivity representing internalized ligand.
Immunofluorescence Microscopy
Human primary fibroblasts (WI38 cells) were grown on gelatin-coated coverslips in DMEM supplemented with 10% fetal bovine serum until subconfluent. Cells were then serum-starved for 18 h before the experiment. To label LRP molecules undergoing endocytosis, monoclonal anti-LRP1 antibody 5A6 (60 nm) conjugated with Alexa-488 was added to the media and incubated with cells for 45 min at 37 °C. After washing to remove unbound antibodies, the cells were chilled on ice for 45 min. PDGF-B (40 nm) was then added to the media, and cells were incubated at 4 °C for 45 min followed by washing and raising the temperature to 37 °C for 12 min. The cells were then fixed in ice-cold methanol, permeabilized with ice-cold acetone, and immunostained with goat anti-hPDGFRβ (R&D, AF385). For the triple-label co-localization experiments, the following antibodies were used: affinity-purified rabbit anti-LRP1 antibody (R2629), goat anti-hPDGFR β (R&D, AF385), mouse monoclonal anti-EEA1, and anti-Rab11 (both BD Transduction Laboratories). Donkey Alexa-conjugated anti-rabbit, anti-mouse, and anti-goat secondary antibodies were used (all from Invitrogen). Stained coverslips were mounted onto glass slides using FluorSave Reagent (Calbiochem) and viewed with laser scanning system Radiance 2100 (Zeiss/Bio-Rad) equipped with the argon/krypton (488/568 nm) and red diode (638 nm) lasers. The images were acquired and stored in RAW format using LaserSharp2000 software (Zeiss, Inc.). The images were exported into TIFF format and processed by Adobe Photoshop software (Adobe Systems) for publication. Adjustments of brightness and contrast were made for the entire image. Identical adjustments were made to both “single channel” and “merged” images.
Phosphoprotein Analysis
Total cell lysates were prepared according protocols established by KinexusTM Multi-immunoblotting protocol. Briefly, cells were scraped in homogenization buffer described above, sonicated 4 times for 10 s each, and then centrifuged at 90,000 × g for 30 min at 4 °C in a Beckman tabletop TL-100 ultracentrifuge. Supernatant (detergent-solubilized lysate) protein concentration was quantified by BCA protein assay reagent (Thermo-Scientific). 500 μg of total cell lysate were subjected to protein phosphorylation analysis, which detects 38 cell signaling molecules using KinexusTM KCPS-1.3 phosphoprotein profiling screen (Kinexus Bioinformatics Corp. in Vancouver, British Columbia, Canada). The experiment was performed in duplicate.
Surface Biotinylation Experiments
150-mm plates of subconfluent WI38 fibroblasts were serum-starved for 24 h before the experiment. Chilled cells were labeled with EZ-link biotin (Pierce) for 1 h, and unbound biotin was removed by washing cells with ice-cold Tris-buffered saline. Then the cells were incubated for 10 min in 37 °C DMEM with or without 40 nm PDGF-B. After incubation, cell lysates were prepared using radioimmune precipitation assay buffer (1% Nonidet P-40 in Tris-buffered saline, pH 7.4) supplemented with phosphatase and protease inhibitors, and LRP1 was immunoprecipitated by the anti-LRP1 polyclonal antibody (R2629) conjugated to Sepharose. LRP1 was then eluted from the immuno-Sepharose with 0.1 m glycine buffer, pH 2.5, and the biotinylated pool of LRP1 was precipitated by Neutravidin-Sepharose. LRP1 that was not bound to the Neutravidin-Sepharose was concentrated using StrataClean resin (Stratagene, Inc.). The biotin-labeled and unlabeled LRP1 pools were subjected to SDS-PAGE, transferred to nitrocellulose membrane, and probed with anti-phospho-Tyr (4G10-HRP) and monoclonal anti-LRP1 (11H4).
Proliferation Assay
LRP1+/−, LRP1−/−, and B41 mouse fibroblasts were seeded at 1.5 × 104/cm2 density on fibronectin-coated plates and were incubated overnight in DMEM media containing pyruvate, 25 mm HEPES, 1× ITS (insulin, transferring, sodium selenite; Sigma), and 0.05% fetal bovine serum. After treatment with 1 ng/ml PDGF for 24 h, the cells were trypsinized and counted.
RESULTS
The LRP1 Cytoplasmic Domain Is Directly Phosphorylated by the PDGFR-β Kinase Domain
To test whether or not the PDGFR-β can directly mediate the tyrosine phosphorylation of the LRP1 cytoplasmic domain, we incubated the purified GST-LRP1-ICD with the PDGFR-β kinase domain in the presence of [α-32P]ATP. The results, shown in Fig. 1A, reveal that the PDGFR-β kinase domain readily catalyzed the incorporation of 32P into the GST-LRP1-ICD (Fig. 1A, lane 3) but not into GST alone (Fig. 1A, lane 2). These results reveal that the PDGFR-β is capable of directly mediating the phosphorylation of the LRP1 cytoplasmic domain.
FIGURE 1.
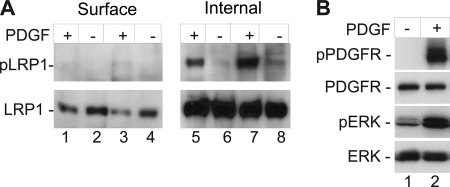
PDGF-mediated tyrosine phosphorylation of LRP1 preferentially occurs during endocytosis. A, purified recombinant PDGFR-β-kd was incubated with GST (lane 1) or GST-LRP1-ICD (lane 3) in the presence of [γ-32P]ATP at 37 °C for 10 min. GST (lane 2) and GST-LRP1-ICD (lane 4) were incubated with [γ-32P]ATP in the absence of PDGFR-β-kd as controls. The incorporation of 32P into the proteins was assessed by autoradiography after SDS-PAGE. Autophosphorylation of PDGFR-β-kd was detected at 65 kDa. B, WI-38 fibroblasts kept at either 4 or 37 °C were incubated with or without PDGF for 15 min. LRP1 was subjected to immunoprecipitation (IP) and then blotted with anti-phosphotyrosine IgG (top) or anti-LRP1 IgG (bottom). The control panel (left) shows immunoprecipitation with non-immune IgG (NI). C, shown is a time course for PDGF-induced LRP1 tyrosine phosphorylation at 4 °C. WI-38 fibroblasts were incubated for 10 min with PDGF at 37 °C (left) or for the indicated times at 4 °C (right). LRP1 tyrosine phosphorylation was detected after immunoprecipitation with anti-LRP1 IgG using anti-phosphotyrosine IgG. D, WI-38 fibroblasts were chilled to 4 °C and incubated with PDGF-B for 1 h. Cells were washed, and the temperature was raised to 37 °C to allow endocytosis. Levels of phospho-PDGFR (black circles) or phospho-LRP1 (gray circles) were measured by immunoblot analysis. The experiments was performed in duplicate. E, representative immunoblots from which the data in panel D were derived show the time course of PDGFR-β phosphorylation, LRP1 phosphorylation, and phospho-ERK generation.
PDGF-mediated Tyrosine Phosphorylation of LRP1 Preferentially Occurs at 37 °C during Endocytosis
In a prior study (19) we noted that the addition of PDGF-B to WI-38 fibroblasts cultured at 37 °C resulted in rapid tyrosine phosphorylation of the PDGFR-β. In contrast, LRP1 tyrosine phosphorylation peaked much later after the addition of PDGF (19), suggesting that PDGFR-β internalization may be a prerequisite for LRP1 phosphorylation. To identify the cellular compartments where PDGF-mediated phosphorylation of LRP1 can occur, we performed studies under conditions that allowed activation of the PDGFR-β but prevented receptor internalization. This was accomplished by incubating the cells at 4 °C, a temperature where endocytosis is minimized and does not occur (24). In our experiments WI-38 fibroblasts were incubated with PDGF at either 4 or 37 °C, and the extent of LRP1 tyrosine phosphorylation assessed by immunoprecipitation was followed by immunoblotting. The results (Fig. 1B) reveal that whereas PDGF readily mediated the phosphorylation of LRP1 at 37 °C, no tyrosine phosphorylation of LRP1 was detected at 4 °C, as equivalent amounts of LRP1 were loaded on the gels as assessed by anti-LRP1 immunoblotting (lower panel, Fig. 1B). Even when cells were incubated for extended time periods with PDGF at 4 °C, only trace amounts of LRP1-tyrosine phosphorylation was detected (Fig. 1C).
To measure the time course of PDGFR-β activation and LRP1-tyrosine phosphorylation, we first preincubated cells with PDGF-B at 4 °C and then washed the cells and added media prewarmed to 37 °C. The extent of tyrosine phosphorylation of both LRP1 and the PDGFR-β was measured while maintaining the temperature at 37 °C. The results (Fig. 1D) reveal that even at 4 °C, the PDGFRβ is fully phosphorylated, consistent with previous work revealing that autophosphorylation of the PDGFRβ occurs at this temperature (25). In contrast, no tyrosine phosphorylation of LRP1 was detected when the cells were maintained at 4 °C. Upon raising the temperature to 37 °C, however, we noted that LRP1 was tyrosine-phosphorylated in a time-dependent manner with peak tyrosine phosphorylation occurring around 15 min, a progression similar to that reported earlier (19).
Several PDGF-mediated events that occur upon interaction of this growth factor with its cellular receptor at 37 °C also occur when the temperature is reduced to 4 °C. These include receptor dimerization (26), autophosphorylation (25), and phospholipase Cγ association and phosphorylation (27, 28). To identify the PDGF-mediated signaling events that occur at 4 °C, we examined the extent of PDGFR-β autophosphorylation and components of various signaling pathways at 37 and 4 °C. The results confirm that the PDGFR-β is autophosphorylated at 4 °C. Furthermore, some but not all of the signaling pathways that are triggered by this receptor are activated at this low temperature. Thus, whereas we found that Shc is phosphorylated when cells are maintained at 4 °C (data not shown), we also noted that ERK is not activated at 4 °C and requires the cells to be at 37 °C for activation to occur (Fig. 1E).
PDGF-mediated Tyrosine Phosphorylation of LRP1 Does Not Occur When Endocytosis Is Blocked
Dynamin functions in membrane fusion and is essential for clathrin-dependent endocytosis (29). Recently, a potent and highly specific non-competitive inhibitor of dynamin GTPase activity was identified that blocks dynamin-dependent endocytosis in cells (30, 31). We tested the ability of this inhibitor, termed dynasore, to prevent the internalization of LRP1. The results (Fig. 2A) show that dynasore completely blocked LRP1-mediated internalization of 125I-labeled RAP. The effect was reversible, and removing dynasore from the media allowed uptake of RAP (data not shown). The PDGFR-β undergoes ligand-stimulated endocytosis, and to determine if its internalization is also blocked by dynasore, we conducted experiments with 125I-labeled PDGF. The results (Fig. 2B) reveal that dynasore also blocked the internalization of 125I-labeled PDGF. Having confirmed that dynasore blocks the endocytosis of both LRP1 and the PDGFR-β, we determined if PDGF-mediated LRP1 tyrosine phosphorylation was also blocked by dynasore. The results (Fig. 2C) confirm that LRP1 tyrosine phosphorylation was completely blocked by dynasore. As expected, PDGF-mediated activation of the PDGFR-β was not affected by dynasore, but activation of ERK was inhibited (Fig. 2C).
FIGURE 2.
Blockade of endocytosis prevents PDGF-mediated tyrosine phosphorylation of LRP1. Cells treated with dynamin inhibitor fail to internalize LRP1 ligand RAP (A) and PDGF-B (B). WI-38 cells were serum-starved and incubated with or without dynasore (200 μm) at 37 °C for 1 h. 125I-RAP (10 nm) or 125I-labeled PDGF (10 ng/ml) were incubated with the cells at 37 °C for 20 min. Radioactivity released from the cell surface or associated with cell pellets was measured. C, WI-38 cells were serum-starved and incubated with or without dynasore (200 μm) at 37 °C for 1 h and were then incubated with or without PDGF-B for 15 min at 37 °C. Cells were lysed and analyzed by immunoprecipitation with anti-LRP1 polyclonal antibody (R2629). Both cell lysates (input) and immunoprecipitates (IP) were separated on 4–12% SDS gels and analyzed by immunoblotting. D, WI-38 cells were chilled to 4 °C for 1 h and either left untreated (lane 1) or treated with PDGF-β for 1 h at 4 °C. PDGF-β-treated cells were either left at 4 °C (lane 2) or shifted to 37 °C for 7 min (lane 3). All cells were incubated with dithiobispropionimidate cross-linker for 2 h at 4 °C and then lysed. Cell lysates and immunoprecipitates were analyzed as in panel C. E, shown are densitometry measurements of PDGFR-β co-immunoprecipitating with LRP1.
Functional LRP1 and PDGF-β Receptor Colocalize in Endosomal Compartments
To confirm that LRP1 co-localizes with the PDGFR-β after endocytosis, co-immunoprecipitation experiments were performed. In these experiments, fibroblasts were first chilled to 4 °C, and then LRP1 was immunoprecipitated, and the immunoprecipitate was subjected to immunoblot analysis with anti-PDGFR-β. These results revealed that very little PDGFR-β co-immunoprecipitated with LRP1 without prior PDGFR-β activation and when the cells were chilled to 4 °C to prevent endocytosis (Fig. 2D, lane 1). In a second experiment PDGF was added to the chilled cells, and then cell extracts were subjected to immunoprecipitation. Whereas this induced phosphorylation of the PDGF receptor, very little PDGF receptor co-immunoprecipitated with LRP1 (Fig. 2D, lane 2). Only when the PDGFR-β was first activated by PDGF at 4 °C, and then the temperature raised to 37 °C, did significant amounts of PDGFR-β co-immunoprecipitate with LRP1 (Fig. 2D, lane 3).
Prior experiments revealed prominent co-localization of the PDGFR-β and LRP1 in punctate structures presumed to represent endosomal compartments 15 min after PDGF addition to cells at 37 °C (18). To identify the cellular compartment where co-localization of these two receptors occurs, cells were first incubated with the LRP1 monoclonal antibody 5A6 at 37 °C to label the LRP1 involved in endocytosis. This antibody binds to the LRP1 light chain and does not dissociate during receptor recycling. The cells were then chilled to 4 °C and incubated with PDGF for 1 h at this temperature to activate PDGFR-β on the cell surface. Internalization of the PDGFR-β was initiated by raising the temperature to 37 °C for 12 min before fixing the cells and staining for PDGFR-β. The results of this experiment are shown in Fig. 3 and reveal extensive co-localization of the PDGFR-β and LRP1 in punctate structures. There was no co-localization of LRP1 with the PDGFR-β before stimulation. To identify the cellular compartment where these two receptors co-localize, cells were co-stained with early endosome antigen 1 (EEA1), a marker for early endosomes, and with Rab11, a marker for recycling endosomes. LRP1 and PDGFR-β were co-localized in both of these cellular compartments (Fig. 4).
FIGURE 3.
Functional LRP1 co-localizes with PDGFR-β after activation by PDGF. WI-38 fibroblasts were incubated with monoclonal antibody 5A6 to label the endocytic pool of LRP1. The cells were then chilled and incubated with PDGF (30 nm) for 1 h at 4 °C before raising the temperature to 37 °C for 12 min. Fixed and permeabilized cells were then stained for PDGFR-β. The position of the nucleus is outlined by dashed lines. The scale bar is 5 μm.
FIGURE 4.
Activated PDGFR-β and LRP1 co-localize in both early and late endosomal compartments. After incubation with PDGF-B for 15 min, WI38 fibroblasts were fixed and stained for LRP1 and PDGF-β as well as for early endosomes using anti-EEA1 and recycling endosomes using anti-Rab11. The position of the nucleus is outlined by dashed lines. The scale bar is 5 μm.
A Mutant PDGFR-β with Delayed Endocytic and Altered Trafficking Properties Shows Delayed Ability to Phosphorylate LRP1
Joly et al. (32) have described a PDGFR-β mutant that upon treatment with PDGF shows a decrease in internalization rate and fails to progress from early and/or recycling endosomes to later steps in the endocytic pathway and is, thus, not effectively degraded (33). The mutated receptor, termed the F5 mutant, has Tyr-740, -741, -771, -1009, and -1021 of the PDGFR-β all converted to Phe. In our experiments we used chimeric receptors designed by Fambrough et al. (34) to bypass endogenous PDGFR that are present in fibroblasts. These investigators developed a receptor containing the extracellular ligand binding domain of the human M-CSF receptor and the transmembrane and cytoplasmic domain of the human PDGFR-β. This chimeric receptor, termed ChiR(WT), has the signaling activity of the PDGFR-β but is activated by binding M-CSF. A mutant of this receptor, termed ChiR(F5), contains the five tyrosine mutations in the cytoplasmic domain. Our previous studies demonstrated that the chimeric receptor, ChiR(WT), readily promoted the tyrosine phosphorylation of LRP1 (18). We used the F5 mutant of this chimeric receptor, ChiR(F5), to determine if the tyrosine phosphorylation of LRP1 was delayed, as might be expected if tyrosine phosphorylation of LRP1 occurs upon PDGFR-β internalization. The results of this experiment, shown in Fig. 5, reveal that the addition of M-CSF to fibroblasts transfected with the ChiR(WT) receptor results in rapid tyrosine phosphorylation of the LRP1 cytoplasmic domain, and phosphorylated forms of LRP1 are detected within 2 min of M-CSF addition. In contrast, the addition of M-CSF to fibroblasts transfected with ChiR(F5) show a significant delay in the phosphorylation of LRP1. These results reveal that delaying the endocytosis of the PDGFR delays the tyrosine phosphorylation of LRP1 and is consistent with the notion that LRP1 tyrosine phosphorylation occurs upon receptor internalization.
FIGURE 5.
PDGF-mediated tyrosine phosphorylation of LRP1 by the F5 chimeric PDGFR/M-CSF receptor mutant is delayed. NIH 3T3 cells transfected with wild type chimeric PDGF/M-CSF receptor (ChiR(WT)) or the F5 mutant (ChiR(F5)) were serum-starved and then incubated with M-CSF. A, at the indicated times cell extracts were prepared, and levels of phosphorylated ChiR were detected by phosphotyrosine immunoblots after immunoprecipitation of the ChiR. B, LRP1 tyrosine phosphorylation was detected in the extracts after immunoprecipitation with anti-LRP1 IgG. C, phosphotyrosine LRP1 levels were quantified and are plotted for the wild type (WT, closed circles) and F5 mutant receptor (open circles).
Internal Pools of LRP1 Are Tyrosine-phosphorylated upon PDGFR-β Activation
Due to the rapid internalization rate of LRP1, most cellular LRP1 is located intracellularly within compartments of the endosomal pathway and is, therefore, not concentrated on the cell surface. We next performed experiments to determine if the PDGFR-β can mediate the phosphorylation of internal pools of LRP1. In these experiments WI-38 fibroblasts were first chilled to 4 °C and then incubated with the membrane-impermeable sulfo-NHS-LC biotin to label cell surface proteins. After labeling, the temperature was raised to 37 °C, and the cells were then incubated for 15 min with PDGF. Total cellular LRP1 was immunoprecipitated from cell extracts using an anti-LRP1 antibody, and the precipitate was then separated into biotinylated (surface) or non-biotinylated (intracellular) by a second precipitation with Avidin-Sepharose. The results of this experiment demonstrate that a significant amount of tyrosine-phosphorylated LRP1 is derived from the fraction that was not recognized by Avidin-Sepharose (Fig. 6A, lanes 5 and 7), and therefore, was not on the cell surface when tyrosine phosphorylation occurred. These results reveal that activation of the PDGFR-β is capable of mediating LRP1-tyrosine phosphorylation of intracellularly derived LRP1. This result is consistent with the fact that the majority of LRP1 is located within endosomal compartments due to its rapid internalization rate. Thus, only small amounts of LRP1 exist on the cell surface, which may account for the reduced amounts of surface-derived LRP1 that was tyrosine-phosphorylated (Fig. 6A, lanes 1 and 3). Alternately, the labeling procedure may not have labeled all surface LRP1, leading to an underestimate of the amount of surface LRP1 that is tyrosine-phosphorylated. The control experiments shown in Fig. 6B confirms that the PDGF signaling pathway was activated in this experiment by demonstrating PDGFR-β and ERK phosphorylation in response to the treatment.
FIGURE 6.
Internal pools of LRP1 are phosphorylated upon PDGF treatment. A, WI-38 fibroblasts were chilled to stop endocytosis, and cell surface proteins were biotinylated. Then cells were incubated without or with PDGF-B (40 nm) for 10 min at 37 °C, and LRP1 was immunoprecipitated with anti-LRP IgG. This was followed by Neutravidin-Sepharose precipitation i to separate biotinylated LRP1 from LRP1 that was not biotinylated. The biotin labeled (Surface) and unlabeled (Internal) LRP1 was subjected to PAGE and probed first with anti-phosphotyrosine antibody (top panel) and then with monoclonal anti-LRP1 antibody (bottom panel). B, control experiments confirm that the PDGFR-β was activated during this experiment. Immunoblot of cell extracts for phospho-PDGFR-β, total PDGFR-β, phospho-ERK, and total ERK.
LRP1 Regulates the Signaling Properties of the PDGF Receptor
To assess the potential of LRP1 to modulate the signaling properties of PDGFR-β, we used KinetworksTM phosphoprotein screen. This screen utilizes phosphorylation site-specific antibodies to quantify changes in the status of 38 known signaling proteins. Of the 38 phosphoproteins analyzed in this screen, we identified 5 whose degree of phosphorylation depends upon the expression of LRP1 (Fig. 7). Most striking was the observation that LRP1 increased the PDGF-mediated phosphorylation of ERK (Fig. 8A). LRP1 expression also modulated the phosphorylation levels of various proteins functioning in cell cycle regulation (Fig. 8B).
FIGURE 7.
KinetworksTM KPSS-1.3 phosphoprotein analysis of LRP1+/−, LRP1−/−, and B41 clones after PDGF-induced signaling. A, immunoblot analysis for LRP1 expression in cell extracts from LRP1−/+, LRP1−/−, and B41 clones using an antibody against LRP1-α subunit is shown. B–D, cells were serum-starved overnight then stimulated with 50 ng/ml PDGF-β for 15 min at 37 °C. Whole cell lysates were prepared and analyzed by phospho-site profiling where the expression and phosphorylation states of 38 signaling proteins were analyzed. Five phosphoproteins were significantly altered (circled) in MEFs expressing LRP1 (B) when compared with LRP1-deficient cell line (C). These proteins were restored in the B41 clones (D). The phosphoproteins are MEK1/2 (S18+S222) (1), Rb (S780) (2), Jun (S73) (3), Erk1 (T202+Y204) (4), and CDK1/2 (Y15) (5). The experiment was performed in duplicate.
FIGURE 8.
LRP1 expression influences activation of the MAPK pathway. Quantification of the results from Fig. 7 in LRP1+/−, LRP1−/−, and B41 clones is shown. Several signaling pathways affected by LRP1 expression are phosphorylation states of MAPK pathway-related proteins (A) and cell cycle-related proteins (B). *, mean values significantly larger than that of LRP1+/− or B41 cells (p < 0.05); **, mean values significantly smaller than that of LRP1+/− or B41 cells (p < 0.05).
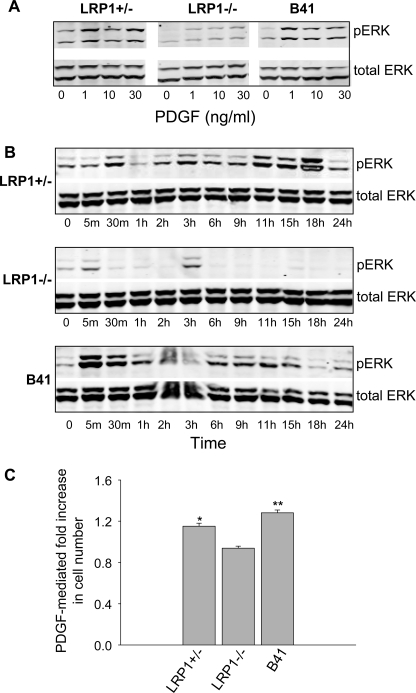
Because De Donatis et al. (35) found that cell proliferation was only induced at higher PDGF concentrations, we examined the effect of PDGF concentration on ERK phosphorylation. Even at high PDGF concentrations (30 ng/ml), ERK phosphorylation was suppressed in the LRP1−/− fibroblasts (Fig. 9A). Continuous stimulation of fibroblasts with PDGF results in a time-dependent ERK phosphorylation in LRP1+/− and B41 clones that peaks between 5 and 30 min. A second phase of ERK phosphorylation was noted between 9 and 18 h in both cell lines expressing LRP1 (Fig. 9B). In contrast, the magnitude of ERK phosphorylation was suppressed at all times tested in LRP1−/− cells (Fig. 9B). Because ERK activation is associated with the mitogenic effect of PDGF receptor activation, we examined the effect of PDGF on MEF growth. The results reveal that PDGF treatment of MEFs resulted in an enhancement of cell growth in cells expressing LRP1 while having no effect on the growth of LRP1-deficient cells (Fig. 9C).
FIGURE 9.
PDGF-mediated ERK phosphorylation and cell proliferation requires LRP1 in mouse fibroblasts. A, differential ERK phosphorylation after PDGF stimulation in LRP1+/−, LRP1−/−, and B41 MEFs is shown. All cells were serum-starved overnight then stimulated with PDGF at the indicated concentrations for 15 min at 37 °C. Total cell lysates with phospho-specific ERK IgG (rabbit) are shown. ERK phosphorylation levels were compared with the total ERK protein using specific mouse IgG on the same membrane by using multiplexed infrared detection (Odyssey System). B, shown is the time course of differential ERK phosphorylation during continuous PDGF stimulation. After overnight starvation, cells were induced with 30 ng/ml PDGF-β at 37 °C. At the indicated times, cell lysates of each cell line were prepared and analyzed for phosphor-ERK and total ERK as in panel A. C, LRP1+/−, LRP1−/−, and B41 MEFs were tested for PDGF-stimulated cell proliferation. Serum-starved quiescent cells were stimulated with 30 ng/ml PDGF-β at 37 °C. After 24 h cells were counted with hemacytometer. Experiment was performed in triplicate. *, mean of LRP1+/− significantly larger than that of LRP1−/− (p < 0.01); **, mean of B41 significantly larger than that of LRP1−/− (p < 0.001).
DISCUSSION
The PDGF signaling pathway makes important contributions to both developmental processes as well as to wound repair mechanisms. This pathway is carefully regulated, as excess PDGF signaling activity results in the development of fibrosis, vascular injury-induced restenosis, angiogenesis, and atherosclerosis (5). PDGF is a potent mitogen for fibroblasts and smooth muscle cells, and recent studies employing a mouse model of cervical carcinogenesis provide compelling evidence that targeting PDGF signaling in carcinoma-associated fibroblasts can slow the progression of disease by impairing the growth of the invading carcinoma (36). The PDGF signaling pathway is also thought to contribute to pulmonary fibrosis by facilitating the recruitment of fibroblasts resulting in the formation of fibroblastic foci that are hallmarks of this disease (37). Infusion of PDGF-B into rats after carotid injury (38) or the expression of recombinant PDGF-B in porcine arteries (9) caused a significant increase in thickening of the vessel wall due to smooth muscle cell proliferation and matrix deposition by these cells (39).
In vivo studies in mice have discovered that LRP1 in vascular smooth muscle cells functions to suppress the PDGF signaling pathway by reducing PDGFR-β levels and function (12) by mechanisms that are not fully understood. PDGF-mediated activation of the PDGFR-β is known to result in the tyrosine phosphorylation of LRP1 at the second NPXY motif within its cytoplasmic domain (18–20). This results in increased association of LRP1 with adaptor proteins, including Shc (19), which are involved in signaling pathways. Cell fractionation experiments suggested that LRP1 is tyrosine-phosphorylated in caveolae (20), a cellular compartment where the PDGFR is thought to reside (40). On the other hand, immunofluorescence and immunogold electron microscopy reveal prominent co-localization of activated forms of the PDGFR-β and LRP1 in internalized compartments that appear to be endosomes (18). Furthermore, these latter studies observed very little, if any, co-localization of these receptors in resting cells (18).
To resolve these apparent discrepancies, the first objective of experiments in this study was to identify the cellular compartment(s) where LRP1 can be phosphorylated. Whereas our data confirm that LRP1 can be phosphorylated on the cell surface upon PDGFR-β activation, several lines of data that when combined provide convincing evidence that the majority of LRP1 tyrosine phosphorylation mediated by PDGF occurs within endosomal compartments after internalization of activated PDGFR-β. First, we demonstrate that the kinase domain of PDGFR-β can directly catalyze the phosphorylation of LRP1-ICD. Second, the addition of PDGF to cells that are chilled to 4 °C, a temperature where endocytosis is prevented, results in activation of the PDGFR-β, with only trace amounts of LRP1 tyrosine phosphorylation detected. Third, blocking endocytosis of LRP1 as well as the PDGFR-β with a potent dynamin inhibitor prevents PDGF-mediated LRP1 tyrosine phosphorylation. Fourth, a mutant PDGF receptor with delayed ability to undergo endocytosis showed a corresponding delay in LRP1-tyrosine phosphorylation. Fifth, surface biotinylation experiments revealed that most phosphorylated LRP1 originates from intracellular compartments. Finally, immunofluorescence experiments confirm that LRP1 and the PDGFR-β co-localize within both early and recycling endosomal compartments. Together, these data reveal that the majority of tyrosine-phosphorylated LRP1 originates within endosomal compartments.
It is becoming clear that signaling events prompted by activation of receptor-tyrosine kinases often occur within endosomal compartments. Early indications of this potential came from fractionation studies that demonstrated a complex of activated epidermal growth factor receptor, tyrosine-phosphorylated Shc, GRB2, and mSOS in endosomal fractions (41). Additional evidence that endosomal receptors can form signaling complexes was obtained by developing a system whereby the PDGFR or epidermal growth factor receptor were reversibly inhibited and internalized in a manner that did not require their activation. Removal of the inhibitor in endosomes resulted in the assembly of a signaling complex for each receptor (42, 43). Interestingly, use of RNAi to mediate silencing of clathrin revealed that endocytosis of the vascular endothelial growth factor receptor is required for full activation of ERK (44). Results in the current study revealed that activation of the PDGFR in cells chilled to 4 °C to prevent endocytosis also prevents ERK phosphorylation, consistent with the notion that endocytosis of the PDGFR may be required for full activation of ERK as well.
The fact that LRP1 is a substrate for PDGF signaling pathway raised the possibility that LRP1 may directly influence this pathway, and testing this hypothesis was a second objective of this study. Our results reveal that in mouse embryonic fibroblasts LRP1 does indeed modulate the degree of phosphorylation of a number of molecules. Most striking is its effect on the MAPK pathway, where LRP1 expression appears to be required for effective phosphorylation of ERK. Our studies observed higher levels of activated MEK1/2, an upstream kinase of the MAPK pathway, in LRP1-expressing cells, indicating that the mechanism by which LRP1 influences PDGF-mediated ERK phosphorylation does not involve inhibition of the MEK1/2 activation. We explored the possibility that LRP1 regulates the MAPK pathway by modulating the activity of phosphatases like MKP3 and MKP1–2, which dephosphorylate ERK. However, we did not observe any LRP1 dependence on the levels of phosphorylated active forms of these phosphatases in our cells (data not shown). Nonetheless, LRP1 may regulate MAPK signaling by sequestering phosphatases to the pathway or by enabling activated MAPK binding to the respective docking proteins. Recent work identified ERK as a substrate for tumor suppressor density-enhanced phosphatase (DEP-1) (45). DEP-1 is a transmembrane protein containing eight fibronectin type III repeats within its ectodomain and a cytosolic catalytic domain. At this time it is not known if LRP1 influences the function or activity of this phosphatase.
ERK signaling is known to have important consequences in cell cycle regulation and cell migration, and indeed, deregulated ERK kinase activity was observed in a large portion of human tumors, underscoring its importance in cell cycle regulation (46). Li et al. (21) discovered an essential requirement for LRP1 expression for vascular smooth muscle cells to migrate effectively in response to PDGF. Furthermore, because ERK activation along with c-Myc expression is a required first step for the mitogenic effect of PDGF (11), LRP1 is likely to alter PDGF-stimulated cell growth in these cells. Indeed, we found that PDGF treatment of MEFs resulted in an enhancement of cell growth while having no effect on the growth of LRP1-deficient cells. Recently, LRP1 was shown to be required for the growth inhibitor properties of transforming growth factor-β in mink lung epithelial cells (Mv1Lu cells) (15) and Chinese hamster ovary cells (16), suggesting that LRP1 has the potential to modulate multiple signaling pathways affecting cellular growth. Further investigations are required to reveal if LRP1 provides cross-talk with other signaling pathways to fine-tune highly regulated cell proliferation, migration, and survival.
In addition to its effect on cell cycle regulation, recent studies have shown that ERK signaling initiated via LRP1 association with ligands has important consequences on the production of proteinases (47). Thus, the serpin protease nexin 1 (PN-1) in complex with target proteases binds LRP1 and activates ERK signaling, which in turn is required for induction of MMP-9 expression (47).
In summary, results from the current investigation reveal that activation of the PDGF signaling pathway results in tyrosine phosphorylation of the LRP1 cytoplasmic domain within endosomal compartments. The two receptors form a signaling complex in endosomes that appears essential for ERK phosphorylation. This in turn has the potential to effect cell cycle regulation as well as stimulation of a variety of molecules, such as proteinases, which can alter the interaction of cells with the extracellular matrix.
This work was supported by National Institutes of Health Grants PO1 HL54710 and HL50784 (both to D. K. S.).
- PDGF
- platelet-derived growth factor
- PDGF-B
- PDGF BB
- PDGFR-β
- PDGF receptor β
- LRP1
- low density lipoprotein receptor-related protein 1
- RAP
- receptor, associated protein
- ERK
- extracellular signal regulated kinase
- kd
- kinase domain
- EEA1
- endosome antigen 1
- DMEM
- Dulbecco's modified Eagle's medium
- MOPS
- 4-morpholinepropanesulfonic acid
- ICD
- intracellular domain
- GST
- glutathione S-transferase
- M-CSF
- macrophage colony-stimulating factor
- MEF
- mouse embryonic fibroblast
- MEK
- mitogen-activated protein kinase/extracellular signal-regulated kinase kinase.
REFERENCES
- 1.Andrae J., Gallini R., Betsholtz C. (2008) Genes Dev. 22, 1276–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boström H., Willetts K., Pekny M., Levéen P., Lindahl P., Hedstrand H., Pekna M., Hellström M., Gebre-Medhin S., Schalling M., Nilsson M., Kurland S., Törnell J., Heath J. K., Betsholtz C. (1996) Cell 85, 863–873 [DOI] [PubMed] [Google Scholar]
- 3.Karlsson L., Lindahl P., Heath J. K., Betsholtz C. (2000) Development 127, 3457–3466 [DOI] [PubMed] [Google Scholar]
- 4.Lindahl P., Johansson B. R., Levéen P., Betsholtz C. (1997) Science 277, 242–245 [DOI] [PubMed] [Google Scholar]
- 5.Levitzki A. (2004) Cytokine Growth Factor Rev. 15, 229–235 [DOI] [PubMed] [Google Scholar]
- 6.Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. (1991) Science 253, 1129–1132 [DOI] [PubMed] [Google Scholar]
- 7.Giese N. A., Marijianowski M. M., McCook O., Hancock A., Ramakrishnan V., Fretto L. J., Chen C., Kelly A. B., Koziol J. A., Wilcox J. N., Hanson S. R. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 900–909 [DOI] [PubMed] [Google Scholar]
- 8.Hart C. E., Kraiss L. W., Vergel S., Gilbertson D., Kenagy R., Kirkman T., Crandall D. L., Tickle S., Finney H., Yarranton G., Clowes A. W. (1999) Circulation 99, 564–569 [DOI] [PubMed] [Google Scholar]
- 9.Nabel E. G., Yang Z., Liptay S., San H., Gordon D., Haudenschild C. C., Nabel G. J. (1993) J. Clin. Invest. 91, 1822–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balciunaite E., Jones S., Toker A., Kazlauskas A. (2000) Curr. Biol. 10, 261–267 [DOI] [PubMed] [Google Scholar]
- 11.Jones S. M., Kazlauskas A. (2001) Nat. Cell Biol. 3, 165–172 [DOI] [PubMed] [Google Scholar]
- 12.Boucher P., Gotthardt M., Li W. P., Anderson R. G., Herz J. (2003) Science 300, 329–332 [DOI] [PubMed] [Google Scholar]
- 13.Herz J., Strickland D. K. (2001) J. Clin. Invest. 108, 779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lillis A. P., Van Duyn L. B., Murphy-Ullrich J. E., Strickland D. K. (2008) Physiol. Rev. 88, 887–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang S. S., Ling T. Y., Tseng W. F., Huang Y. H., Tang F. M., Leal S. M., Huang J. S. (2003) FASEB J. 17, 2068–2081 [DOI] [PubMed] [Google Scholar]
- 16.Tseng W. F., Huang S. S., Huang J. S. (2004) FEBS Lett. 562, 71–78 [DOI] [PubMed] [Google Scholar]
- 17.Boucher P., Li W. P., Matz R. L., Takayama Y., Auwerx J., Anderson R. G., Herz J. (2007) PLoS ONE 2, e448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton C. S., Loukinova E., Mikhailenko I., Ranganathan S., Gao Y., Haudenschild C., Strickland D. K. (2005) J. Biol. Chem. 280, 27872–27878 [DOI] [PubMed] [Google Scholar]
- 19.Loukinova E., Ranganathan S., Kuznetsov S., Gorlatova N., Migliorini M. M., Loukinov D., Ulery P. G., Mikhailenko I., Lawrence D. A., Strickland D. K. (2002) J. Biol. Chem. 277, 15499–15506 [DOI] [PubMed] [Google Scholar]
- 20.Boucher P., Liu P., Gotthardt M., Hiesberger T., Anderson R. G., Herz J. (2002) J. Biol. Chem. 277, 15507–15513 [DOI] [PubMed] [Google Scholar]
- 21.Li Y., Lu W., Bu G. (2003) FEBS Lett. 555, 346–350 [DOI] [PubMed] [Google Scholar]
- 22.Salicioni A. M., Mizelle K. S., Loukinova E., Mikhailenko I., Strickland D. K., Gonias S. L. (2002) J. Biol. Chem. 277, 16160–16166 [DOI] [PubMed] [Google Scholar]
- 23.Kowal R. C., Herz J., Goldstein J. L., Esser V., Brown M. S. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 5810–5814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silverstein S. C., Steinman R. M., Cohn Z. A. (1977) Annu. Rev. Biochem. 46, 669–722 [DOI] [PubMed] [Google Scholar]
- 25.Frackelton A. R., Jr., Tremble P. M., Williams L. T. (1984) J. Biol. Chem. 259, 7909–7915 [PubMed] [Google Scholar]
- 26.Bishayee S., Majumdar S., Khire J., Das M. (1989) J. Biol. Chem. 264, 11699–11705 [PubMed] [Google Scholar]
- 27.Kumjian D. A., Wahl M. I., Rhee S. G., Daniel T. O. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 8232–8236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wahl M. I., Olashaw N. E., Nishibe S., Rhee S. G., Pledger W. J., Carpenter G. (1989) Mol. Cell. Biol. 9, 2934–2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mettlen M., Pucadyil T., Ramachandran R., Schmid S. L. (2009) Biochem. Soc. Trans. 37, 1022–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macia E., Ehrlich M., Massol R., Boucrot E., Brunner C., Kirchhausen T. (2006) Dev. Cell 10, 839–850 [DOI] [PubMed] [Google Scholar]
- 31.Newton A. J., Kirchhausen T., Murthy V. N. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 17955–17960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joly M., Kazlauskas A., Fay F. S., Corvera S. (1994) Science 263, 684–687 [DOI] [PubMed] [Google Scholar]
- 33.Joly M., Kazlauskas A., Corvera S. (1995) J. Biol. Chem. 270, 13225–13230 [DOI] [PubMed] [Google Scholar]
- 34.Fambrough D., McClure K., Kazlauskas A., Lander E. S. (1999) Cell 97, 727–741 [DOI] [PubMed] [Google Scholar]
- 35.De Donatis A., Comito G., Buricchi F., Vinci M. C., Parenti A., Caselli A., Camici G., Manao G., Ramponi G., Cirri P. (2008) J. Biol. Chem. 283, 19948–19956 [DOI] [PubMed] [Google Scholar]
- 36.Jain R. K., Lahdenranta J., Fukumura D. (2008) PLoS Med. 5, e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scotton C. J., Chambers R. C. (2007) Chest 132, 1311–1321 [DOI] [PubMed] [Google Scholar]
- 38.Jawien A., Bowen-Pope D. F., Lindner V., Schwartz S. M., Clowes A. W. (1992) J. Clin. Invest. 89, 507–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pompili V. J., Gordon D., San H., Yang Z., Muller D. W., Nabel G. J., Nabel E. G. (1995) Arterioscler. Thromb. Vasc. Biol. 15, 2254–2264 [DOI] [PubMed] [Google Scholar]
- 40.Liu P., Ying Y., Ko Y. G., Anderson R. G. (1996) J. Biol. Chem. 271, 10299–10303 [DOI] [PubMed] [Google Scholar]
- 41.Di Guglielmo G. M., Baass P. C., Ou W. J., Posner B. I., Bergeron J. J. (1994) EMBO J. 13, 4269–4277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y., Pennock S. D., Chen X., Kazlauskas A., Wang Z. (2004) J. Biol. Chem. 279, 8038–8046 [DOI] [PubMed] [Google Scholar]
- 43.Pennock S., Wang Z. (2003) Mol. Cell. Biol. 23, 5803–5815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lampugnani M. G., Orsenigo F., Gagliani M. C., Tacchetti C., Dejana E. (2006) J. Cell Biol. 174, 593–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sacco F., Tinti M., Palma A., Ferrari E., Nardozza A. P., Hooft van Huijsduijnen R., Takahashi T., Castagnoli L., Cesareni G. (2009) J. Biol. Chem. 284, 22048–22058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoshino R., Chatani Y., Yamori T., Tsuruo T., Oka H., Yoshida O., Shimada Y., Ari-i S., Wada H., Fujimoto J., Kohno M. (1999) Oncogene 18, 813–822 [DOI] [PubMed] [Google Scholar]
- 47.Fayard B., Bianchi F., Dey J., Moreno E., Djaffer S., Hynes N. E., Monard D. (2009) Cancer Res. 69, 5690–5698 [DOI] [PubMed] [Google Scholar]