Abstract
In the mammalian model of sex determination, embryos are considered to be sexually indifferent until the transient action of a sex-determining gene initiates gonadal differentiation. Although this model is thought to apply to all vertebrates, this has yet to be established. We have examined three lateral gynandromorph chickens with the aim of investigating the nature of the sex-determining mechanism in birds. These studies demonstrated that gynandromorph birds are genuine male:female chimeras, and suggested that male and female avian somatic cells may have an inherent sex identity. To test this hypothesis, we transplanted presumptive mesoderm between embryos of reciprocal sexes to generate embryos containing male:female chimeric gonads. In contrast to the outcome for mammalian mixed-sex chimeras, in chicken mixed-sex chimeras the donor cells were excluded from the functional structures of the host gonad. Most strikingly, in an instance where female tissue was transplanted into a male host, donor cells contributing to the developing testis retained a female identity and expressed a marker of female function. Our study demonstrates that avian somatic cells possess an inherent sex identity and that, in birds, sexual differentiation is substantively cell autonomous.
Sexual development in vertebrates is thought to be governed by general principles defined in the early to mid-twentieth century1,2. These principles state that the sexual phenotype of individuals is dependent on the gonad: male and female somatic cells and tissues are initially sexually indifferent and sexual dimorphism is imposed by the type of gonad that develops. Although these principles have been challenged, most notably by the work of Arnold and co-workers on songbird neural development3-6 and Renfree and co-workers on marsupial development7,8, these observations are generally considered as exceptions. In the currently accepted model, gonadal differentiation is triggered in sexually indifferent embryos by the transient action of a sex-determining gene. In mammals, the sex-determining gene is known to be the testis-determining Sry gene carried by the male-specific Y-chromosome9. Although all vertebrates are thought to conform to this general model, with the exception of Sry in mammals and Dmy in medaka 10,11, no other vertebrate sex-determining genes have been confirmed.
In terms of morphology, birds appear to conform to the mammalian pattern: male and female embryos are sexually indistinguishable until around days 5-6 of incubation (Hamburger & Hamilton12 (H&H) stage 28/29) when the action of a sex determining gene is thought to initiate testis or ovary development13. However, in birds, not only is the identity of the putative sex determining gene unknown, the nature of the sex-determining mechanism has not been established. Current theories of sex-determination in birds include the presence of an ovary-determining gene on the female-specific W-chromosome, and a dosage mechanism based on the number of Z-chromosomes (females have one Z and one W sex chromosome while males have two Z sex chromosomes)14. Currently, the best candidate for a testis-determining gene in birds is DMRT1 (doublesex and mab-3-related transcription factor 1). Expression of DMRT1 is restricted to the gonads and it has recently been shown that repressing levels of DMRT1 in male embryos has a ‘feminising’ effect on the developing testis15.
In an attempt to clarify the nature of the sex-determining mechanism in birds, we have investigated the composition of a number of gynandromorph chickens. These birds are rare, naturally occurring phenomena in which one side of the animal appears male and the other female16. We investigated these birds with the expectation that this condition resulted from a sex-chromosome aneuploidy on one side of the bird, and that our analysis would provide evidence regarding the nature of the avian sex-determining mechanism. Contrary to expectations, our analysis established that the gynandromorphs were in fact male:female chimeras, and that the gynandromorphic phenotype was due to ZZ (male) and ZW (female) somatic cells responding in different ways to the same profile of gonadal hormones. These observations led to a series of transcriptome screens and embryonic transplantation studies showing that male and female avian cells possessed an inherent sex identity. Our studies demonstrate that in chickens, gonadal development and the sexual phenotype are largely cell autonomous and not principally dependent on sex hormones.
Gynandromorph birds are mixed sex chimeras
We obtained three adult lateral gynandromorph birds (designated G1, G2 and G3) which we maintained and observed over a period of 24 months. These birds occur naturally and it has been suggested that this condition results from the loss of a single sex chromosome at the 2-cell stage17. All three birds were ISA Brown commercial hybrids with sex-linked plumage colour. ISA Brown males are heterozygous for the dominant silver and recessive gold genes (Ss) and so have white plumage; females possess only the gold gene (s-) and have brown plumage. The birds displayed a striking bilateral asymmetry, where one side of the animal appeared phenotypically female and the other side phenotypically male (Supplementary Fig.1). Figure 1 shows a picture of G1 where the right side of the bird is female in colouration (brown) and has a small wattle and small leg spur. In contrast, the left side is male coloured (predominantly white), has a large wattle and a large leg spur, a heavier leg structure and an obviously greater mass of breast muscle, typical of a cockerel. Post mortem, whole tissues from both sides were weighed and measured and samples of all tissues were taken for later analysis. The measurements performed on individual tissues from both sides of all three animals supported the observation that these animals were, at least phenotypically, half male and half female. On the side that appeared male, tissues were larger and heavier and bones were longer and denser, than corresponding tissues and bones from the side with a female appearance (Supplementary Table 1). Fluorescent in situ hybridisation (FISH) analysis using Z and W chromosome probes was performed on preparations of blood cells from all three animals and on multiple preparations of cultured skin cells from both sides of birds G2 and G3. While an autosomal probe demonstrated a diploid chromosome constitution for G1 blood cells, the sex chromosome probes demonstrated that approximately half of gynandromorph G1 cells were female (ZW) and half were male (ZZ) (Fig. 2a). Similar FISH analyses of blood and primary fibroblast cultures from birds G2 and G3 demonstrated that all three animals were composed of a mixture of normal diploid male and female cells (Supplementary Fig.2 & Supplementary Table 2). While a recent analysis of a gynandromorph zebra-finch3 demonstrated that both Z-chromosome and W-chromosome containing cells were present, the possibility remained that such animals were composed of a mixture of ZW and Z0 cells. Here we show that gynandromorph birds are genuine male:female chimeras and provide an explanation for a phenomenon that has been debated for centuries18.
Figure 1.

Image of gynandromorph bird (G1)
ISA Brown bird where the right side has female characteristics and left side has male characteristics (white colour and larger wattle, breast musculature & spur).
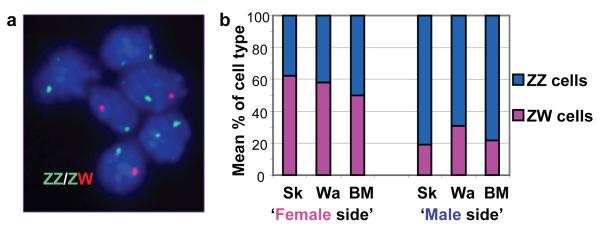
Figure 2.
Male and female cells in gynandromorph birds.
a. FISH analysis of sex chromosomes in gynandromorph blood cells.
Interphase nuclei prepared from cultured blood cells from gynandromorph G1 hybridised according to standard FISH procedures with probes specific to both the W and Z chromosome (XhoI repeat on W chromosome, and Z chromosome BAC containing the VLDL receptor gene identified by screening the HGMP chicken BAC library). Erythrocytes hybridised with probes for Z-chromosome (GREEN) and W-chromosome (RED). Cells contain either two Z-chromosomes or one Z and one W-chromosome. b. Mean relative proportions of ZZ and ZW cells in tissues from male and female sides of gynandromorph birds. Average percentage of ZW cells and ZZ cells (Supplementary Table 2) in three tissues from phenotypically female side and from phenotypically male side of three gynandromorph birds. Tissues from the sides that appear female contain more ZW (female) than ZZ (male) cells, while tissues from the sides that appear male are composed predominantly of ZZ cells. Sk=skin, Wa=wattle, BM=breast muscle.
We next investigated whether the apparent bilateral asymmetry reflected the distribution of ZZ and ZW cells by examining the cellular composition of a variety of tissues from both sides of the individual birds. Southern analysis using sex chromosome probes on genomic DNA extracted from multiple tissues, revealed that none of the tissues from either side were composed exclusively of either ZZ- or ZW-containing cells, i.e. all tissues examined comprised a mixture of both female and male cells (examples shown in Supplementary Fig. 3). Multiple Southern analyses were performed on separate DNA samples extracted from different regions of skin, from wattle and from breast muscle from both sides of all three birds, to quantify the relative proportions of male and female cells. Phosphorimager analyses comparing the hybridisation signal obtained from DNA from gynandromorphic tissues with that obtained from known amounts of male and female DNA, produced a measure of the relative proportion of male and female cells in each tissue. Figure 2b shows the mean proportion of female and male cells in skin, wattle and breast muscle from the ‘male’ side and ‘female’ side of all three birds. It is clear that tissues from the side that appeared female contained more ZW (female) than ZZ (male) cells, while tissues from the side that appeared male were composed predominantly of ZZ cells (Supplementary Table 2). Our data establishing the presence of both ZZ- and ZW-containing cells suggests that it is highly unlikely that these birds arise as a consequence of mutation at the two-cell stage of development, and would support the hypothesis that gynandromorphs arise as a result of failure of extrusion of a polar body during meiosis and subsequent fertilisation of both a Z- and W-bearing female pronucleus19.
The development of gonads in the gynandromorph birds was of obvious interest (Supplementary Fig. 4). The type of gonad present did not correspond to the external appearance but rather reflected the cellular composition of the individual organs. The gonads differed for each gynandromorph: G1 contained a testis-like gonad on the left side, G2 contained an ovary-like gonad on the left side, and G3 contained a swollen testis-like structure on the left side (in contrast to G1 and G2, G3 appeared female on the left side and male on the right). The G1 testis-like gonad was composed primarily of sperm-containing seminiferous tubules, while the G2 ovary-like gonad was composed predominantly of large and small follicles. The gonad from G3 comprised a mixture of empty tubules and small follicular-like structures (ovo-testis). Southern analyses demonstrated that the morphological appearance of the gonads conformed to the cellular composition in that the structures that appeared to be testis and ovary were composed principally of ZZ- and ZW-containing cells respectively, while the ‘ovo-testis’ comprised a mixture of ZZ and ZW-containing cells.
Although the findings from our gynandromorph analyses are uninformative in terms of elucidating the avian sex-determining mechanism, they do lead to the surprising conclusion that the classical dogma of sex differentiation, where the phenotype is mainly determined by gonadal hormonal secretions, does not apply to birds. These results strongly suggest that the avian phenotype is dependent on the nature of the cells comprising the individual tissue rather than being imposed by the type of gonad formed: both sides of these animals are exposed to an identical profile of gonadal products yet each side responds differently to these stimuli. For example, although it is well established that growth of the wattle is sensitive to testosterone20, it is clear from figure 1 that a major determinant in wattle size is the cellular composition of the tissue, and therefore cellular identity and gonadal hormones both play a significant role in establishing the sexual phenotype of this tissue. Our analyses led us to conclude that male and female chicken somatic cells may have a cell-autonomous sex identity.
Sex differences precede gonadal hormone influences
To investigate whether differences exist between male and female cells independently of any possible gonadal influences we compared the transcriptomes of male and female embryos at developmental stages prior to the formation of the gonads (data not shown). These analyses identified both mRNAs and miRNAs that were expressed in a sexually dimorphic fashion throughout the embryos at stages prior to the formation of the gonadal precursor (the genital ridge; H&H Stage 21) and well before the generally accepted point of sex determination in the chicken i.e. around day 5/6 of incubation. Screening for mRNAs expressed exclusively in male or in female embryos led to identification of an mRNA encoded by a W-chromosome gene that was expressed ubiquitously in females. This gene was designated Faf for Female Associated Factor and sequences deposited in the public databases (accession numbers AJ606294 – AJ606297 ). Whole mount in situ hybridisation analysis of embryos at stages prior to genital ridge formation showed that Faf mRNA is expressed throughout the female embryo as early as 18 hours of incubation (H&H stage 4) (Fig. 3a). We also identified a ubiquitously expressed miRNA that is present at levels approximately 10-fold higher in males than in females throughout development, including at stages prior to the expected point of sex determination (Figure 3b and Supplementary Fig 5). This miRNA is encoded on the Z-chromosome and the sequence has not previously been reported in any other species (gga-mir-2954, accession number AM691163). These observations are in agreement with other studies that have identified sexually dimorphic gene expression in the brain preceding morphological differentiation of the gonads, in both chicken and mouse21,22. Although the functions of these particular transcripts are unknown, these findings not only supported the concept that the tissue phenotype was not dependent on gonadal products, but also reinforced the suggestion that the phenotype was defined by inherent difference between the male and female cells.
Figure 3.
Sexually dimorphic expression in early chick embryos.
a. Expression of Faf in male and female embryos prior to development of genital ridge/gonads. Whole-mount ISH showing expression of Faf (purple colour) in embryos at 18hrs, 48hrs and 72hrs of development (H&H stages 4, 14 & 20). Faf is clearly expressed throughout the female embryos at all developmental stages and not expressed in male embryos. Faf is not expressed in extra-embryonic tissues of the female. The Faf transcript is encoded by the genomic DNA complementary to the intergenic regions between copies of the W-chromosome repeat gene Wpkci / HINTW 34, 35 and transcribed in the opposite orientation. m: male, f:female. b. Expression of novel chicken miRNA (gga-mir-2954). Expression in whole embryos at 48hrs (H&H 14) and 72 hrs (H&H 20) of development. This miRNA is clearly expressed in a sexually dimorphic fashion at stages prior to the sexual differentiation of the gonads. This miRNA matches sequence present in chicken Z-chromosome BAC clones AC192757 and AC187119. Loading Control= U6 RNA.
Gonadal chimeras confirm cell autonomous sexual differentiation
To test the hypothesis that the male and female cellular composition defines phenotype, we generated embryos containing chimeric gonads comprised of a mixture of male and female cells. Gonadal chimeras were generated by transplantation of sections of presumptive mesoderm from green fluorescent protein (GFP)-expressing embryos23 at developmental stage 12 (day 2) to replace the equivalent tissue of non-GFP embryos at the same stage of development (Fig 4a). Donor tissue was transplanted only to the left side of recipient embryos as only the left ovary develops fully in the chick. Transplanted embryos were returned to the incubator and allowed to develop until Stage 35 (day 9). By Stage 35, donor cells were incorporated into tissues on the left side of the embryo in the region between the fore and hind limbs (Supplementary Fig.6). In addition to the gonads, donor cells were observed in a variety of tissues including skin, muscle, mesonephros, Wolffian duct and Mullerian duct. A minimum of four donor:host chimeric gonads were generated for each of the four possible combinations; male:male, female:female, male:female and female:male. For each of these donor:host combinations, chimeras were generated with different levels of donor contribution, from examples where the contribution of donor cells was limited to isolated individual cells dispersed throughout the host gonad, to examples where areas of the host gonad were almost exclusively composed of donor cells. Gonad:mesonephros pairs were collected at Stage 35 and longitudinal frozen sections were prepared for confocal microscopy. GFP expression was used to estimate the extent of donor contribution to the individual chimeric gonads and immunohistochemistry (IHC) analysis was performed with antibodies for both anti-Mullerian hormone (AMH) and aromatase. AMH is a marker of functionally ‘male’ cells24(expressed by precursor Sertoli cells of the sex cords) while aromatase is a marker of functionally ‘female’ cells25 (expressed by cells in the female medullary region). At Stage 35 of development, the male gonad is composed of a thin layer of cortex tissue surrounding a medullary region which contains the developing sex cords (expressing AMH) separated by interstitial connective tissue. In contrast, the female left ovary comprises a greatly thickened cortex surrounding a smaller less-structured medullary region (expressing aromatase). Figure 4b shows the normal expression of AMH and aromatase in stage 35 male and female gonads respectively. It is clear that the testis is composed almost exclusively of medullary tissue and that AMH is expressed in distinct cord-like structures within this tissue. In contrast, the developing ovary comprises a definitive cortex enclosing a reduced medulla and aromatase is expressed in cells throughout the medulla. Examples of all four donor:host combinations of chimeric gonads are shown in Figure 4c and Supplementary Figure 7. It is clear that GFP expression did not affect the ability of donor cells to contribute to host tissues and to function normally: in each case of same-sex chimeras, either male or female donor cells were integrated into all somatic compartments of the respective host testis and ovary (cortex, sex cords and interstitial tissue). Moreover, when integrated into the appropriate ‘functional’ compartment, donor male cells expressed AMH and donor female cells expressed aromatase. In contrast, in mixed-sex chimeras the donor cells did not integrate into the ‘functional’ structures of the host gonad: female donor cells in host testis medulla were not recruited into the AMH-expressing sex cords and were restricted to the interstitial tissue, while male donor cells in host ovary were excluded from the aromatase-expressing structures. In mixed-sex chimeras the inability of donor cells to form functional host structures was evident regardless of the relative contribution of male and female cells (Supplementary Fig 7). The fact that female chicken cells in an environment and location that induces testicular development cannot be recruited into the functionally ‘male’ Sertoli cell compartment, and male cells in an ovary-inducing environment are excluded from a functionally ‘female’ compartment, strongly supports the suggestion that chicken somatic cells possess a cell-autonomous sexual identity.
Figure 4.
Expression of male and female markers in chimeric gonads.
a. Generation of chimeras. I) Schematic illustrating transplantation of presumptive mesoderm from GFP-expressing embryo to non-GFP embryo at day 2. II) Image of mesonephros and gonads from chimeric embryo at day 9 showing donor contribution to left gonad (g), mesonephros (m) and mullerian duct (md). b. Expression of female and male markers in embryonic gonads. Expression of aromatase (AROM) in ovary and anti-Mullerian hormone (AMH) in testis at day 9 is shown by IHC. c. Integration of GFP-donor cells into host gonads. Panels in first column show low magnification view of sections through host gonads and illustrate the extent of donor cell contribution. Remaining panels show higher magnification views of highlighted areas. Using IHC, donor cells are marked by GFP (green) while expression of AMH and aromatase are shown in red. Fourth column shows merged image of images from second and third columns. In same sex chimeras, GFP-expressing donor cells co-localise with AMH-expressing and aromatase-expressing cells in host testis and ovary respectively ( yellow/orange in fourth column). In mixed-sex chimeras GFP-expressing donor cells do not co-localise with AMH or aromatase. m: mesonephros, o: ovary, and t: testis. d. Retention of female donor phenotype in mixed-sex chimeras. IHC showing expression of AMH (red in top row of panels) and aromatase (red in bottom row of panels) in neighbouring sections from the gonad of a female:male (donor:host) chimera. Donor contribution is illustrated by GFP (green) expression. Third column shows merged image of images in first and second columns. Regions containing a significant host contribution (defined by bottom bracket in top row of panels) formed sex-cord like structures and expressed AMH. Female donor cells were not incorporated into AMH expressing sex cords, as shown by lack of GFP and AMH co-localisation. Regions composed primarily of female donor cells (defined by top bracket) behaved as ovarian-like tissue and expressed aromatase, as shown by co-localisation of GFP and aromatase (yellow/orange). Scale bars represent 100 μm. IHC was performed following standard procedures. Primary antibodies were (1:100) goat anti-human AMH (Santa Cruz Biotechnology), (1:200) mouse anti-human cytochrome P450 aromatase (AbD Serotec) and (1:250) rabbit anti-GFP conjugated to Alexa Fluor 488 (Invitrogen). Secondary antibodies were conjugated to Alexa Fluor 594 (Invitrogen).
This is further supported by a striking example where the degree of the female donor contribution was sufficient to effectively generate an ‘ovo-testis’ in the host embryo (Fig. 4d). This mixed-sex chimera contained a gonad with an anterior portion composed almost exclusively of female cells. While the posterior portion contained testis-like medulla with AMH-expressing sex cords, the region composed of female cells did not form sex cords and did not express AMH. Most surprisingly, the female cells in this region expressed aromatase. This demonstrates that while female cells in a male embryo can correctly interpret gonadal location and differentiation signals, they respond in a cell-autonomous manner characteristic of a female genotype (and express aromatase). Our findings are in contrast with those from mammalian mixed-sex chimeras, where XX cells can become functional Sertoli cells and XY cells can become functional granulosa cells26,27.
These studies demonstrate that avian somatic cells possess a cell-autonomous sex identity. Our results support and extend the findings of Arnold and colleagues3 that showed that differences between male and female zebrafinch brains were a result of endogenous genetic differences in the brain cells themselves. Our analysis of lateral gynandromorph birds, showing that they are male:female chimeras, and our experimental generation of embryos with mixed sex chimeric gonads, together indicate that male and female somatic cells possess a sex identity. These observations suggest that there is a molecular mechanism functioning in every cell that confers a sex-specific identity that influences how individual cells respond to developmental and hormonal signals. We propose that cell-autonomous sex identity is dependent on sexually dimorphic gene expression which results from the ‘dosage compensation’ system which operates to equalize the phenotypic effects of characteristics determined by genes on the Z-chromosome. Recent evidence has shown that this system in birds is not chromosome-wide and results in a large number of gene expression differences between male and female cells 28-31. We have estimated that this system of dosage compensation would result in at least 300 non-compensated Z-chromosome genes31. Our identification of sexually dimorphic transcripts that are expressed ubiquitously from very early in development adds to these observations. On the basis of our findings, and from evidence of the dosage compensation system in birds, we propose that the overall mechanism of sex-determination in birds differs significantly from the mammalian model (Fig.5). Although sexually dimorphic differentiation of the gonads may be regulated independently from other somatic tissues, we propose that a male or female sex identity is imposed on the chicken soma early in development by sex chromosome transcription and it is this inherent molecular identity that triggers the appropriate testis or ovary gene cascade in the developing genital ridge (e.g. via DMRT115). While the gonads clearly have a significant influence on the adult phenotype they do not dictate somatic differences to the same extent as in mammals. It is also possible that elements of such a system are retained in certain mammals: Renfree and co-workers have shown that, in a marsupial mammal, the wallaby, formation of the mammary gland and scrotum is independent of gonadal hormones32 and rather than exhibiting transient localised expression, Sry shows widespread expression in multiple tissues well before the point of gonadal differentiation33. As Sry-type sex-determining mechanisms have not yet been established for all vertebrate species, it is possible that the model we propose where the phenotype of individual tissues is largely defined by an inherent sex identity of the somatic cells, is not restricted to birds.
Figure 5.
A novel mechanism of sex determination in the chicken.
A sexual identity is genetically imposed on the male and female chicken soma at fertilisation and is the major factor in determining the adult sexual phenotype. At the appropriate stage in development, the sexually-dimorphic transcripts underlying the male / female identity trigger expression in the genital ridge of the gene cascade responsible for testis / ovary development. The gonads have limited effects on the sexual phenotype. In contrast, in mammals, gonadal fate is dependent on transient expression of the testis-determining SRY gene in the indifferent early gonad. The mammalian gonads have a major influence on the sexual phenotype.
Online Methods
Embryo Transplantation
Generation of chimeric embryos:GFP embryos23 and ISA Brown embryos at H&H stage 11/12 (13-15 somites) were used as donor and host respectively. The blunt end of donor eggs was pierced to create an air hole and a ‘window’ cut on the midline. The embryos were removed and pinned on a 3% agarose surface containing 0.5% India ink. Embryos were kept moist by the addition of phosphate buffered saline (PBS) containing 100 μg/ml penicillin and 100 μg/ml streptomycin (PBS-pen/strep). A strip of lateral plate mesoderm flanking presumptive somites 21-23 was removed and stored in CO2-independent medium (Invitrogen) containing 10% FBS and pen/strep. Host eggs were windowed as above and kept moist by the addition of PBS- pen/strep. To help visualise somites, sterile India ink (20% in PBS-pen/strep) was injected under the host embryos. Using a microneedle, the vitelline membrane and a flap of ectoderm were folded back from the underlying mesoderm. A strip of lateral plate mesoderm was then removed from the host embryo taking care to leave the endoderm intact. The GFP-donor tissue was then inserted into the host site and the ectodermal flap replaced. Two ml of albumen was then withdrawn from the host eggs using a hypodermic syringe. Transplanted eggs were tightly sealed with tape and incubated at 37°C in a humidified incubator.
Immunostaining
Immunohistochemistry was carried out according to the method of Stern36. Briefly, tissues were fixed in 4% paraformaldehyde for 2 hrs at 4°C, equilibrated in 15% sucrose then embedded in 15% sucrose plus 7.5% gelatin in PBS, pH7.2. Sections, 15 μ thick, mounted on Superfrost slides (Menzel) were washed for 30 mins in PBS at 37°C and blocked in PBS containing 10% donkey serum, 1% BSA, 0.3% TritonX 100 and 0.05% Tween 20 for 2 hrs at room temperature. Incubation with primary antibodies was carried out overnight at 4°C, followed by washing in PBS containing 0.3% Triton X 100 and 0.05% Tween 20, and then incubation with secondary antibodies for 2 hrs at room temperature. After washing, the sections were treated with Hoechst solution (10μg/ml) for 5 mins to stain nuclei.
Fluorescent in situ hybridisation (FISH)
FISH analysis of metaphase or interphase preparations of chicken cells was performed by standard procedures37. Bacterial artificial chromosome (BAC) clones containing the VLDL receptor, aldolase B, CHRN or SCII genes were identified by screening the HGMP chicken BAC library and used to identify Z-chromosomes. A probe for the W-chromosome was prepared by PCR amplification of a portion of the Xho-I repeat region from the W chromosome38. Following gel purification, the probe was labelled by incorporation of either Biotin-16-dUTP (Roche) or digoxigenin-11-dUTP (Roche) during a further round of PCR. BAC DNA was prepared using Qiagen plasmid columns following recommendations for low-copy plasmid purification. Biotin 16–dUTP (Boehringer) and digoxigenin 11–dUTP (Boehringer) were incorporated into BAC DNA by nick translation and labelled probes were concentrated by precipitation in the presence of 5 μg of salmon sperm DNA as a carrier and 2 μg of sonicated chicken genomic DNA as competitor. The pellet was resuspended in 15 μl of hybridisation mix, denatured, and preannealed for 15 mins at 37°C to block repetitive sequences.
Whole mount in situ hybridisation
Chicken embryos and isolated gonads were fixed in 4% paraformaldehyde for 1 hour and whole mount in situ hybridisation was carried out according to the method of Henrique et al39. Digoxygenin-labelled probes were prepared from linearised plasmid clones using a Roche DIG RNA labelling kit to incorporate DIG-11-UTP by in vitro transcription with SP6 and T7 RNA polymerases.
RNA preparation
Total RNA was extracted from pools of male and female chick embryos and tissues using RNA-Bee (AMS Biotechnology (Europe) Ltd) according to the manufacturer’s instructions.
Differential Display
RNA expression profiles in male and female embryos were compared by Differential Display Reverse Transcription PCR (DDRT-PCR). Embryos were sexed 38 and pools of RNA from male and female embryos generated. DDRT-PCR was performed as described in Clinton et al40.
miRNA library construction
Low molecular weight RNAs (<40 nucleotides long) were isolated from total RNA by the use of a flashPAGE fractionator (Ambion). Micro-RNA libraries were constructed essentially as described by Lau et al 41 and Lee and Ambros 42.
microRNA Nothern analysis
Five μg of total RNA was separated by electrophoresis through a 15% TBE/Urea polyacrylamide gel (Bio-Rad) before transfer to Hybond-N+ membrane (GE Healthcare). Locked Nucleic Acid (LNA) oligonucleotides antisense to the mature miRNA were end-labelled (mirVana Probe and Marker kit, Ambion) with 32P-dATP (Perkin- Elmer) and hybridised to membranes containing miRNAs. Hybridisation was carried out in ULTRAhyb - oligo (Invitrogen) at 20-22°C below the estimated TM of the LNA.
Southern analysis
High molecular weight genomic DNA was extracted from tissues of embryonic and adult male and female chickens by standard phenol-chloroform procedures 43. DNA was digested with restriction endonucleases, subjected to electrophoresis on a 1% TBE gel and transferred to Hybond-N membrane. Probes labelled with 32P-dCTP were hybridised by standard procedures and signal recorded on high-sensitivity film (Kodak) and by phosphorimager analysis.
Supplementary Material
Acknowledgements
This work was supported by DEFRA and BBSRC (BB/E015425 / 1). We thank G. Robertson, S. Wilson, A. Sherman, M. Hutchison, F.Thomson and Rhona Mitchell for technical support and for provision of fertilised eggs and embryos.
Special thanks go to T. Cannon of Colchester for donation of gynandromorph bird G1.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Information Reprints and permissions information is available at www.nature.com/reprints.
References
- 1.Lillie FR. Sex-determination and sex-differentiation in mammals. Proc. Natl. Acad. Sci. USA. 1917;3:464–470. doi: 10.1073/pnas.3.7.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jost A. Hormonal factors in the sex differentiation of the mammalian foetus. Phil. Trans. R. Soc. Lond. B. 1970;259:119–130. doi: 10.1098/rstb.1970.0052. [DOI] [PubMed] [Google Scholar]
- 3.Agate RJ, et al. Neural, not gonadal, origin of brain sex differences in a gynandromorph finch. Proc. Natl. Acad. Sci. USA. 2003;100:4873–4878. doi: 10.1073/pnas.0636925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wade J, Arnold AP. Functional testicular tissue does not masculinize development of the zebra finch song system. Proc. Natl. Acad. Sci. USA. 1996;93:5264–5268. doi: 10.1073/pnas.93.11.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold AP. Sexual differentiation of the zebra finch song system: positive evidence, negative evidence, null hypothesis, and a paradigm shift. J. Neurobiol. 1997;33:572–584. [PubMed] [Google Scholar]
- 6.Wade J, Arnold AP. Sexual differentiation of the zebra finch song system. Ann. N.Y. Acad. Sci. 2004;1016:540–559. doi: 10.1196/annals.1298.015. [DOI] [PubMed] [Google Scholar]
- 7.Renfree MB, Short RV. Sex determination in marsupials: evidence for a marsupial-eutherian dichotomy. Phil. Trans. R. Soc. Lond. B. 1988;322:41–53. doi: 10.1098/rstb.1988.0112. [DOI] [PubMed] [Google Scholar]
- 8.Glickman SE, Short RV, Renfree MB. Sexual differentiation in three unconventional mammals: spotted hyenas, elephants and tammar wallabies. Horm. Behav. 2005;48:403–417. doi: 10.1016/j.yhbeh.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Sekido R, Lovell-Badge R. Sex determination and SRY: down to a wink and a nudge? Trends Genet. 2009;25:19–29. doi: 10.1016/j.tig.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda M, et al. DMY gene induces male development in genetically female (XX) fish. Proc. Natl. Acad. Sci. USA. 2007;104:3865–3870. doi: 10.1073/pnas.0611707104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volff J-N, Kondo M, Schartl M. Medaka dmy/dmrt1Y is not the universal primary sex-determing gene in fish. Trends. Genet. 2003;19:196–199. doi: 10.1016/S0168-9525(03)00051-9. [DOI] [PubMed] [Google Scholar]
- 12.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 13.Smith CA, Sinclair AH. Sex determination in the chicken embryo. J. Exp. Zool. 2001;290:691–699. doi: 10.1002/jez.1119. [DOI] [PubMed] [Google Scholar]
- 14.Clinton M. Sex determination & gonadal development: a bird’s eye view. J. Exp. Zool. 1998;281:457–465. doi: 10.1002/(sici)1097-010x(19980801)281:5<457::aid-jez10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Smith CA, et al. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature. 2009;461:267–271. doi: 10.1038/nature08298. [DOI] [PubMed] [Google Scholar]
- 16.Hutt FB. Genetics of the Fowl. McGraw-Hill Book Company Inc.; New York: 1949. [Google Scholar]
- 17.Cock AG. Half-and-half mosaics in the fowl. J. Genet. 1954;53:49–80. [Google Scholar]
- 18.Birkhead T. The Wisdom of Birds. An Illustrated History of Ornithology. Bloomsbury Publishing Plc.; London: 2008. [Google Scholar]
- 19.Hollander WF. Sectorial mosaics in the domestic pigeon:25 more years. J. Hered. 1975;66:197–202. [PubMed] [Google Scholar]
- 20.Owens IPF, Short RV. Hormonal basis of sexual dimorphism in birds:implications for new theories of sexual selection. TREE. 1995;10:44–47. doi: 10.1016/s0169-5347(00)88967-3. [DOI] [PubMed] [Google Scholar]
- 21.Scholz B, et al. Sex-dependent gene expression in early brain development of chicken embryos. BMC Neurosci. 2006;7:12. doi: 10.1186/1471-2202-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewing P, Shi T, Horvath S, Vilain E. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Mol. Brain Res. 2003;118:82–90. doi: 10.1016/s0169-328x(03)00339-5. [DOI] [PubMed] [Google Scholar]
- 23.McGrew M, et al. Localised axial progenitor cell populations in the avian tail bud are not committed to a posterior Hox identity. Development. 2008;135:2289–2299. doi: 10.1242/dev.022020. [DOI] [PubMed] [Google Scholar]
- 24.Nishikimi H, et al. Sex differentiation and mRNA expression of P450c17, P450arom and AMH in gonads of the chicken. Mol. Reprod. Dev. 2000;55:20–30. doi: 10.1002/(SICI)1098-2795(200001)55:1<20::AID-MRD4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 25.Nomura O, Nakabayashi O, Nishimori K, Yasue H, Mizuno S. Expression of five steroidogenic genes including aromatase gene at early developmental stages of chicken male and female embryos. J. Steroid Biochem. Mol. Biol. 1999;71:103–109. doi: 10.1016/s0960-0760(99)00127-2. [DOI] [PubMed] [Google Scholar]
- 26.Patek CE, et al. Sex chimaerism, fertility and sex determination in the mouse. Development. 1991;113:311–325. doi: 10.1242/dev.113.1.311. [DOI] [PubMed] [Google Scholar]
- 27.Burgoynne PS, Buehr M, McLaren A. XY follicle cells in ovaries of XX-XY female mouse chimaeras. Development. 1988;104:683–688. doi: 10.1242/dev.104.4.683. [DOI] [PubMed] [Google Scholar]
- 28.McQueen HA, et al. Dosage compensation in birds. Curr. Biol. 2001;11:253–257. doi: 10.1016/s0960-9822(01)00070-7. [DOI] [PubMed] [Google Scholar]
- 29.Melamed E, Arnold AP. Regional differences in dosage compensation on the chicken Z-chromosome. Genome Biol. 2007;8:R202. doi: 10.1186/gb-2007-8-9-r202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellegren H, et al. Faced with inequality: chicken do not have a general dosage compensation of sex-linked genes. BMC Biol. 2007;5:40. doi: 10.1186/1741-7007-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McQueen HA, Clinton M. Avian sex chromosomes: dosage compensation matters. Chrom. Res. 2009;17:687–697. doi: 10.1007/s10577-009-9056-8. [DOI] [PubMed] [Google Scholar]
- 32.O WS, Short RV, Renfree MB, Shaw G. Primary genetic control of somatic sexual differentiation in a mammal. Nature. 1988;331:716–717. doi: 10.1038/331716a0. [DOI] [PubMed] [Google Scholar]
- 33.Harry JL, Koopman P, Brennan FE, Graves JA, Renfree MB. Widespread expression of the testis-determining gene SRY in a marsupial. Nat. Genet. 1995;11:347–349. doi: 10.1038/ng1195-347. [DOI] [PubMed] [Google Scholar]
- 34.Hori T, Asakawa S, Itoh Y, Shimizu N, Mizuno S. Wpkci, encoding an altered form of PKCI, is conserved widely on the avian W chromosome and expressed in early female embryos: implications of its role in female sex determination. Mol. Biol. Cell. 2000;11:3645–3660. doi: 10.1091/mbc.11.10.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith CA, Roeszler KN, Sinclair AH. Genetic evidence against a role for W-linked histidine triad nucleotide binding protein (HINTW) in avian sex determination. Int. J. Dev. Biol. 2009;53:59–67. doi: 10.1387/ijdb.082742cs. [DOI] [PubMed] [Google Scholar]
Methods References
- 36.Stern CD. Immunocytochemistry of embryonic material. In: Stern CD, Holland PWH, editors. Essential Developmental Biology: A Practical Approach. IRL Press; Oxford, UK: 1993. pp. 193–212. [Google Scholar]
- 37.McQueen HA, et al. CpG islands of chicken are concentrated on microchromosomes. Nature Genetics. 1996;12:321–324. doi: 10.1038/ng0396-321. [DOI] [PubMed] [Google Scholar]
- 38.Clinton M, Haines L, Belloir B, Mcbride D. Sexing chick embryos: a rapid and simple protocol. Br. Poultry Sci. 2001;42:134–138. doi: 10.1080/713655025. [DOI] [PubMed] [Google Scholar]
- 39.Henrique D, et al. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- 40.Clinton M, Miele G, Nandi S, McBride D. Identification of disease markers by differential display: prion disease. In: Liang P, Meade JD, Pardee AB, editors. Differential Display Methods and Protocols. 2nd edition. Humana Press; Totowa, NJ: 2006. pp. 157–178. [DOI] [PubMed] [Google Scholar]
- 41.Lau NC, Lim LP, Weinstein EG, Bartel GP, et al. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 42.Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294:862–864. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook J, Russell D. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.