Abstract
Induced pluripotent stem cell (iPS) technology has launched a new platform in regenerative medicine aimed at deriving unlimited replacement tissue from autologous sources through somatic cell reprogramming using stemness factor sets. In this way, authentic cardiomyocytes have been obtained from iPS and recently demonstrated in proof-of-principle studies to repair infarcted heart. Optimizing the cardiogenic potential of iPS progeny would ensure a maximized yield of bioengineered cardiac tissue. Here, we reprogrammed fibroblasts in the presence or absence of c-MYC to determine if the acquired cardiogenicity is sensitive to the method of nuclear reprogramming. Using lentiviral constructs that expressed stemness factors SOX2, OCT4, and KLF4 with or without c-MYC, iPS clones generated through fibroblast reprogramming demonstrated indistinguishable characteristics for 5 days of differentiation with similar cell morphology, growth rates, and chimeric embryo integration. However, 4-factor c-MYC dependent nuclear reprogramming produced iPS progeny that consistently prolonged the expression of pluripotent Oct-4 and Fgf4 genes and repressed cardiac differentiation. In contrast, 3-factor c-MYC-less iPS clones efficiently up-regulated pre-cardiac (CXCR4, Flk-1, and Mesp1/2) and cardiac (Nkx2.5, Mef2c, and Myocardin) gene expression patterns. In fact, 3-factor iPS progeny demonstrated early and robust cardiogenesis during in vitro differentiation with consistent beating activity, sarcomere maturation, and rhythmical intracellular calcium dynamics. Thus, nuclear reprogramming independent of c-MYC enhances production of pluripotent stem cells with innate cardiogenic potential.
INTRODUCTION
Induced pluripotent stem cell (iPS) technology offers a unique approach to produce autologous stem cells from somatic tissue sources [1,2]. Forced expression of stemness factors, such as OCT3/4, SOX2, KLF4, and c-MYC, has proven adequate to reprogram ordinary cells, inducing a pluripotent ground state without the need of an embryonic source [3–6]. By unmasking the reversibility of cell fate through coerced reprogramming of gene expression, this robust platform has exposed the atavistic potential [7–9] and regenerative capability of reprogrammed fibroblasts [10–13]. Bioengineered iPS that acquire an embryonic stem cell-like phenotype can produce diverse cytotypes, including authentic cardiac myocytes [13–18].
iPS-derived progeny that differentiate into tissue-specific lineages creates an opportunity for the development of patient specific discovery, diagnostic, and therapeutic strategies [19–22]. To optimize diverse personalized applications, iPS technology must reproducibly generate functional cardiomyocytes [23]. Although originally identified according to four stemness-related gene sets, nuclear reprogramming can be tailored depending on the parental cell type. Here, we tested two of the most common reprogramming strategies, with and without the oncogene c-MYC, to induce fibroblasts into their pluripotent ground state and quantify the ability of clones to consistently differentiate into functional cardiac cells.
In the absence of c-MYC, nuclear reprogramming of ordinary fibroblasts with transgenic expression of three human stemness-related factors, namely OCT3/4, SOX2 and KLF4, generated pluripotent progeny (3F-iPS) in similar fashion to all four factors applied together (4F-iPS). The acquired cardiogenicity innate to each of the strategies was compared head-to-head through formation of de novo cardiac precursors and bona fide cardiac tissue according to gene expression profiles, sarcomerogenesis, beating activity, and calcium transients [23]. Proficient cardiac lineage was most consistently obtained from 3F-iPS clones compared to inconsistent and poor differentiation of the cardiac lineage observed in 4F-iPS clones. Therefore, a valid approach to optimize the cardiogenic capacity of iPS would be to ensure nuclear reprogramming without overexpression of c-MYC, which is here authenticated as a potent stemness factor that hinders subsequent cardiogenesis within the microenvironment of fibroblasts converted into an embryonic stem cell-like state.
METHODS
Somatic cell source
Mouse embryonic fibroblasts (MEF) were obtained from embryos at 14.5 days post coitum (dpc) [4]. Internal organs and head were removed prior to digestion with 0.25% trypsin-EDTA (Invitrogen, Carlsbad, CA). Cell suspensions were inactivated with equal volume of MEF maintenance medium, Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% FCS, 1% L-glutamine (Invitrogen) and penicillin/streptomycin (Invitrogen). Resulting fibroblasts were plated and grown to confluence for two passages. Transduced MEF were cultured in embryonic stem cell (ES) maintenance medium, Dulbecco’s modified Eagle’s medium (Millipore, Billerica, MA) supplemented with pyruvate (Lonza, Basel, Switzerland) and L-glutamine (Invitrogen), non-essential amino acids (Mediatech, Herndon, VA), 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO), 15% FCS (Invitrogen) and LIF (Millipore).
Nuclear reprogramming
Transfer vectors were generated with pSIN-SEW based vector [4], pSIN-CSGWdlNotI, with full-length human OCT3/4, SOX2, KLF4 and c-MYC cDNAs (Open Biosystems, Huntsville, AL). Human stemness-related factors were driven by a spleen focus-forming virus (SFFV) promoter. HIV vectors were produced by transient transfection of 293T cells using FuGene6 (Roche, Indianapolis, IN) with a weight ratio of 2:1:1 of vector to packaging to VSV-G plasmids. The parental packaging plasmid, pCMVR8.91, was engineered to increase vector transduction efficiency [4]. Transfected cells were washed and grown for 48 h, and supernatants harvested and passed through a 0.45-μm filter. Primary fibroblasts were plated at 105 cells per 24-well plate for 12 h prior to transduction with a combination of infectious supernatants containing four (OCT3/4, SOX2, KLF4 and c-MYC; 4F-iPS) or three (c-MYC-less; 3F-iPS) human stemness genes. Infectious supernatants were replaced with ES maintenance medium after 12 h. Transduced fibroblasts were replated at confluence, with individual iPS clones identified and isolated according to morphology within 3 weeks post-transduction [13,18]. Clonogenic expansion produced reprogrammed cell lines that were maintained in ES maintenance medium. Vector integration was PCR confirmed from genomic DNA (Sigma-Aldrich, XNAT2) using primers for OCT4-R AGCCGCCTTGGGGCACTAGCCC, KLF4-R CGCAAGCCGCACCGGCTCCGCC, SOX2-R AGCCTCGTCGATGAACGGCCGC, c-MYC-R GGGAGAAGGGTGTGACCGCAAC and SFFVprom-F CTCACTCGGCGCGCCAGTCCTC. PCR products were resolved on 1% agarose gel electrophoresis. Both 4F-iPS and 3F-iPS clones were labeled with lacZ using an HIV vector carrying lacZ (pLenti6/UbC/V5-GW/LacZ, Invitrogen) [4]. LacZ vector was concentrated at 105 g using a Beckman L7 ultracentrifuge (SW41 rotor, 25000 rpm, 1.5 h, 4°C) resuspended in 250 μl of serum free medium, and used to label 105 iPS in a 24-well plate. LacZ labeled clonal populations were trypsinized and incubated with Fluorescein di[®-D-galactopyranoside] (FDG) (Sigma-Aldrich, F2756). Cells wereisolated using a FACS Aria SE flow cytometer (BD Biosciences) where forward and side scatter parameters were used to gate viable cell population, and FITC was excited with a 488 nm argon laser and detectedthrough a 530/30 nm bandpass filter.
Pluripotent validation and chimeric embryo formation
To determine expression of pluripotent markers, 4F-iPS and 3F-iPS were stained with anti-SSEA1 antibody (MAB4301; dilution 1:50; Millipore) along with secondary goat anti-mouse IgG Alexa Fluor 568 (A11031; 1:250). Nuclei were labeled with 4,6′-diamidino-2-phenylindole (DAPI; Invitrogen). Immunostaining of derivatives was performed using monoclonal mouse anti-alpha-actinin (Sigma A7811) 1:200, rabbit anti-Mef2c (proteintech 10056-1-AP) 1:50 and monoclonal mouse anti-myosin light chain 2v (MLC2v, Synaptic Systems 311011) 1:250. Secondary antibodies, namely goat anti-mouse IgG Alexa Fluor 568 (A11031) and goat anti-rabbit IgG Alexa Fluor 488 (A11008), were used at a 1:250 dilution rate. Alkaline phosphatase (AP) was revealed using a fast red violet and napthol AS-BI phosphate-based detection kit (Millipore, SCR004). For lacZ staining, samples were fixed with 0.25% gluteraldehyde for 15 min at room temperature prior to β-galactosidase staining. Transduced cells were differentiated into three-layer embryoid bodies using the hanging-drop method in differentiation medium supplemented with 20% FCS without LIF [24,25]. Briefly, 25 μl drops from a 25,000 cell/ml suspension were cultured on the lid of a plate for 48 h. Embryoid bodies were flushed and kept in suspension for 2 days to allow spontaneous differentiation for a total of 5 days. For further differentiation, embryoid bodies were transferred into cell culture plates coated with 0.1% gelatin. Cells were maintained in differentiation medium that was changed every 2–3 days. Beating areas were monitored daily. Finally, in vivo contribution of transduced cells within an embryonic development environment was assessed through diploid aggregation with pre-implantation morula [26]. CD1 females at 3 weeks of age were superovulated using intraperitoneal injection of pregnant mare serum gonadotropin and human chorionic gonadotrophin, followed by pairing with adult CD1 males for timed pregnancy. Embryos at 2.5 dpc were harvested, washed in EmbryoMax M2 medium (Millipore) and denuded from zona pelucida to produce morula competent for stem cell integration. After washing through M2 and EmbryoMax KSOM (Millipore) solutions, embryos were plated as pairs in microwells to facilitate aggregation. LacZ-labelled cells cultured for at least two passages after thawing were partially digested using trypsin 0.25%-EDTA (Invitrogen) and preplated for 45 min to allow attachment of feeders to the plate. Floating clumps (8–15 cells) were individually picked, washed in M2 medium and KSOM medium before being placed adjacent to the pair of embryos in microwells. The aggregation complex was incubated in a table-top incubator (Thermofisher, Waltham, MA) with continuous flow of a humidified gas mixture (5% CO2/5% O2/90% N2) for 24 h until cavitation of the blastocysts [26]. CD-1 females in estrus were identified and paired with vasectomized studs two days prior to aggregation to produce pseudopregnant mice. Surrogate mothers were anesthetized (2–3% inhaled isoflurane), uterus dissected through a minimal flank incision, and blastocyst-stage chimeric aggregates containing reprogrammed cells were transferred into the distal portion of the uterus [26]. Pregnancy was supported by pseudopregnant females until day 9.5 dpc, when embryos were harvested and analyzed for engraftment and distribution of lacZ-labelled progenitors. Pictures were taken in a Zeiss stereo Discovery V20 microscope using a ProgRes C3 camera. Embryos were fixed with 0.25% gluteraldehyde for 15 min at room temperature prior to standard β-galactosidase staining.
Cardiac lineage characterization
Expression of pluripotency, gastrulation and cardiogenesis markers was detected by RT-PCR [27,28]. Total RNA was extracted with a combination of gDNA Eliminator and RNeasy columns (Qiagen, Valencia, CA). cDNA was prepared from total RNA samples using Superscript III First Strand (Invitrogen). Mouse Gapdh (4352932E; Applied Biosystems, Foster City, CA) was used as control. Analyzed genes included Sox2 (Mm00488369_s1), Pou5f1 (Mm00658129_gH), Fgf4 (Mm00438917_m1), Lhx1 (Mm00521776_m1), Gsc (Mm00650681_g1), Sox17 (Mm00488363_m1), Sox7 (Mm00776876_m1), Cxcr4 (Mm01292123_m1), Kdr (Mm00440099_m1), Mesp1 (Mm00801883_g1), Mesp2 (Mm00655937_m1), Nkx2.5 (Mm00657783_m1), Tbx5 (Mm00803521_m1), Mef2c (Mm01340839_m1) and Myocardin (Mm00455051_m1; Applied Biosystems). To assess intracellular calcium dynamics, cells were loaded with the fluorescent probe Fluo 4-AM (Invitrogen), imaged with a Zeiss LSM live 5 laser confocal microscope, and analyzed using LSM software [18, 29].
RESULTS
iPS produced with and without c-MYC
Early passage fibroblasts (MEFs) were transduced with a combination of either 4 genes (SOX2, OCT4, KLF4 and c-MYC) or 3 genes (SOX2, OCT4 and KLF4) using a lentivirus-based approach for ectopic expression of human stemness factors. Flat and fusiform ordinary fibroblasts were converted into compact clusters of embryonic stem cell-like morphology after two weeks of 4 factor and after three-four weeks of 3 factor based transduction (Figure 1A). Isolated individual clones were expanded and genotyped for stable integration of vector-derived transgenes to confirm the presumed identity of bioengineered iPS clones (4F-iPS and 3F-iPS). Clones isolated and genotyped contained the expected 4F or 3F transgenic profile that was absent from the untransduced parental source (Figure 1B). Three independent isolated clones for each 4F-iPS and 3F-iPS were randomly selected for in vitro characterization of growth and differentiation potential. At day 0, all six undifferentiated iPS clones were indistinguishable as isolated clusters exhibited a condensed morphology in contrast to the flat untransduced neighboring fibroblasts (Figure 2A). This appearance was maintained for at least 5 passages from the original isolated colony. No gross morphological differences were observed among 4F-iPS and 3F-iPS grown under undifferentiation conditions. Allowing each clone to undergo spontaneous differentiation within embryoid bodies (EB) generated similar growth kinetics leading to equivalent three-dimentional aggregates. In fact, each iPS clone was capable of forming spheroids with regular and defined edges that grew to within 300–400 μm in diameter after 5 days of culture (Figure 2B). Thus, homogeneous morphology was observed in both 4F-iPS and 3F-iPS clones independent of c-MYC transduction.
Figure 1. iPS production with and without c-MYC.
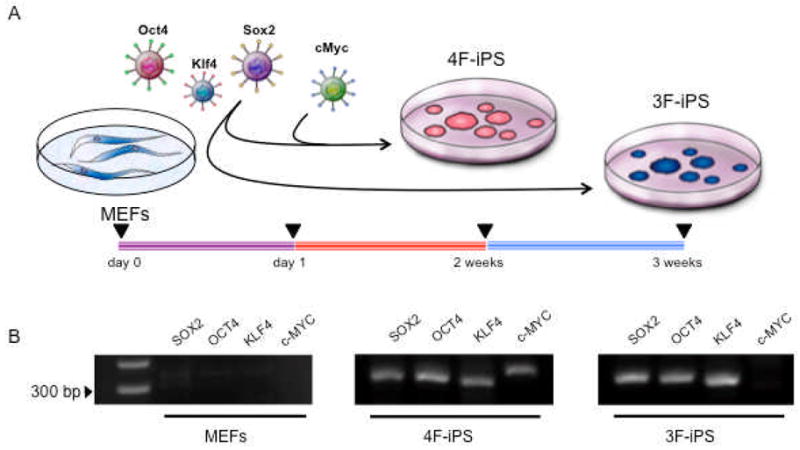
A, Mouse embryonic fibroblasts were transduced one day after plating with 4 genes (SOX2, OCT4, KLF4 and c-MYC) or 3 genes (SOX2, OCT4 and KLF4) using HIV-derived lentiviruses containing human genes. Round and compact embryonic-stem-cell-like colonies were observed after 2 weeks in 4 factor-induced (4F-iPS) cultures and after 3 weeks in 3 factor-induced (3F-iPS) clones. B, Genomic integration of viral constructs was detected in transduced progeny, with c-MYC absent from the 3 factor-induced lines. Parental fibroblasts demonstrate no viral sequence integration and are shown as a negative control.
Figure 2. 4F-iPS and 3F-iPS show similar growth kinetics and embryoid body morphology.

A, Distinct clones of 4F-iPS (left) and 3F-iPS (right) were kept in the undifferentiated state on feeders, and showed indistinguishable morphology consisting of compact colonies. B, Hanging drops were made with multiple clones of 4F-iPS (left) and 3F-iPS (right). Embryoid bodies from each of the clones demonstrated equivalent size and shape within 5 days of differentiation. Bar=500 μm.
Pluripotent capacity of c-MYC-dependent and independent reprogramming strategies
Surrogate pluripotent markers and chimeric embryogenesis validated the pluripotent capacity of the two reprogramming strategies. Clones from both 4F-iPS and 3F-iPS groups induced robust expression of alkaline phosphatase (AP) and embryonic SSEA-1 antigen, initial markers of induced stemness (Figure 3A). Applying a more stringent criterion, diploid aggregation was used to confirm functional pluripotency as only genuine pluripotent stem cells are able to assimilate within a developing embryo at the morula stage [13,18,26,30]. With non-coerced diploid aggregation, 8–15 lacZ-labeled 4F-iPS and 3F-iPS cells were allowed to integrate with two denuded wild type morulae and form a chimeric blastocysts within 24 h of coincubation (Figure 3B). Chimeric blastocysts were then transplanted into surrogate mothers and both 4F-iPS and 3F-iPS clones demonstrated normal embryogenesis within embryos containing high contribution of iPS-derived tissues (Figure 3C). Moreover, chimeric embryos contained lacZ-expressing iPS progeny throughout the cardiac structures such as inflow tract, outflow tract, and both ventricles (Figure 3C, insets) revealing the ability of 4F-iPS and 3F-iPS to generate in vivo cardiac tissue. Thus, functional pluirpotency and innate cardiogenic potential in the context of embryogenesis was confirmed for iPS reprogrammed with and without c-MYC.
Figure 3. Pluripotent capacity and embryonic developmental potential in 4F-iPS and 3F-iPS.
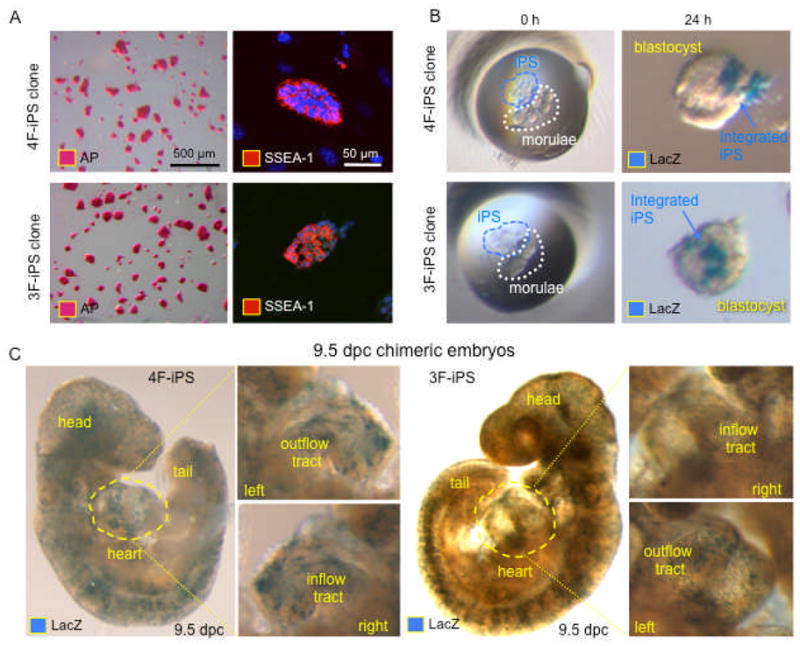
A, 4F-iPS and 3F-iPS stain with similar positive patterns for pluripotency markers alkaline phosphatase (left, AP) and SSEA-1 (right, nuclei revealed with DAPI). B, LacZ-labeled iPS coincubated with diploid embryos (left) revealed the ability of 4F-iPS and 3F-iPS to integrate into host blastocyst (right). C, Integration of both 4F-iPS and 3F-iPS was sustained through embryonic development as shown for 9.5 dpc embryos, containing labeled cells that contributed to most of the tissues including the heart (cardiac inflow and outflow tracts showed in insets).
Removal of c-MYC accelerates cardiogenic gene expression during iPS differentiation
To further characterize the ability of 4F-iPS and 3F-iPS to differentiate into tissue-specific lineages, in vitro models systems were applied independent of the embryonic environment to discriminate innate cardiogenic potential. Gene expression kinetics of 4F-iPS compared to 3F-iPS progeny demonstrated a persistent elevation of pluripotent genes such as Sox2, Oct3/4, and Fgf4 throughout a 12-day differentiation protocol (Figure 4A). Furthermore, spontaneous induction of gastrulation markers was inconsistently upregulated in 4F-iPS compared to the profile of peaking expression levels reliably demonstrated at day 5 in 3F-iPS clones with Lhx1, Gsc, and Sox17 (Figure 4B). Precardiac genes increased expression around day 5 for all tested clones; yet, reproducible induction was augmented in 3F-iPS when compared to 4F-iPS clones (Figure 4C). Cardiac transcription factors were significantly up regulated at day 12 in 3F-iPS, although unreliable for 4F-iPS clones (Figure 4D). Thus, the transition from pluripotent gene expression profiles into bona fide cardiogenic transformation was apparently hampered in iPS clones generated using a c-MYC dependent approach.
Figure 4. Divergent gene expression profiles for 4F-iPS and 3F-iPS during in vitro differentiation.
Differentiation of iPS progenitors was promoted within hanging drops to form embryoid bodies followed by expansion of progeny on gelatinized plates in multi-layer tissues. Cells were sampled from undifferentiated cultures at day 0, floating embryoid bodies at day 5, and differentiating cultures at day 12 for gene expression analysis. A, Pluripotency genes were immediately downregulated in 3F-iPS with initiation of differentiation, whereas expression levels of pluripotent genes was protracted in 4F-iPS. B, Gastrulation markers consistently peaked at day 5 for 3F-iPS clones with inconsistent levels expressed in 4F-iPS. C, Precardiac genes increased at day 5 for all tested clones, However, relative expression was notably higher in 3F-iPS when compared to 4F-iPS clones. D, Upregulation of cardiac transcription factors was observed at day 12 in 3F-iPS with 4F-iPS clones maintaining lower baseline expression levels.
c-MYC independent nuclear reprogramming favors cardiogenesis in iPS progeny
Divergent gene expression profiles of 4F-iPS and 3F-iPS throughout the 12-day differentiation protocol were validated according to distinct morphology and different beating activities. Specifically, five day old-embryoid bodies from independent 4F-iPS and 3F-iPS clones were plated on gelatinized plates for spontaneous differentiation. After day 8, 4F-iPS clones were morphologically distinct from 3F-iPS clones with 4F-iPS progeny showing areas of detached cells (Figure 5A left). 3F-iPS clones had a consistent growth profile with sustained integrity of the cell layer spreading from the initial embryoid body. In contrast to c-MYC dependent iPS, 3F-iPS remained viable with areas of multi-layer cell growth that continuously expanded to cover the full plate (Figure 5A right). By day 12, extensive cell death was apparent in 4F-iPS cultures (Figure 5B left) together with a slower growth rate while compact, confluent, and continuous masses of 3F-iPS were observed in all studied clones (Figure 5B right). Furthermore, 3F-iPS areas of multi-cellular build-up demonstrated frequent sites of spontaneous beating activity, absent from 4F-iPS clones studied in vitro. During the differentiation time course, 4F-iPS and 3F-iPS were observed daily for beating activity. Within the follow-up period, 4F-iPS clones demonstrated no spontaneous contractility (Figure 6A, n=8). However, robust beating activity was reproducibly documented in 3F-iPS clones with a sustained increasing tendency starting as early as day 7 (Figure 6A, n=4). Individual cardiac cells from 3F-iPS were isolated from the contracting cultures using a density gradient purification strategy [18]. Immunostaining of isolated cardiomyocytes derived from 3F-iPS demonstrated presence of cardiac contractile proteins alpha actinin (Figure 6B right) and myosin light chain-2v (MLC-2v) in combination with cardiac transcription factor Mef2c (Figure 6B left). When loaded with the calcium sensitive Fluo-4AM probe, isolated cardiomyocytes showed fluorescence peaks consistent with calcium transients (Figure 6C). Clumps of cells could be observed to display synchronized calcium pulses, demonstrating cell-to-cell communication within a developing syncytium (Figure 6D) and validating the genuine cardiogenic potential in vitro. Therefore, 3F-iPS progeny were capable of consistent cardiac gene expression, maturation of sarcomeres, and acquisition of rhythmical calcium handling machinery. Thus, c-MYC independent nuclear reprogramming recapitulated the salient features of stem cell derived cardiac tissue that was unreliable in the presence of the protooncogene.
Figure 5. 3F-iPS differentiate into beating areas while 4F-iPS demonstrate inconsistent morphology and viability in vitro.
iPS clones were differentiated into embryoid bodies and plated on day 5 onto gelatinized plates. A, Differences between 4F-iPS and 3F-iPS were noticeable in morphology after a total of 8 days as embryoid bodies. 4F-iPS clones showed areas of round and detached cells (arrows, left). 3F-iPS clones had predictable growth kinetics with sustained integrity of the cell layers that spread from the initial embryoid body towards the periphery of the plate. B, After 12 days, extensive cell loss was apparent in 4F-iPS cultures (arrows, left) while compact and continuous masses of 3F-iPS were observed in all studied clones, including areas of spontaneous beating activity (outlined in red dotted lines). Bar=500 μm.
Figure 6. c-MYC independent nuclear reprogramming favors cardiogenic potential of iPS.
A, Differentiating cultures of 4F-iPS and 3F-iPS were observed daily for appearance of beating activity. Between day 7 and 11 of differentiation, no spontaneous contractility was detected with 4F-iPS clones. In contrast, robust and sustained beating activity was documented in 3F-iPS. B, Immunostaining of isolated cardiomyocytes derived from 3F-iPS demonstrated presence of contractile proteins alpha actinin (left) and myosin light chain-2v (MLC-2v) in combination with cardiac transcription factor Mef2c (right). C, Live cell imaging with calcium sensitive Fluo-4AM demonstrated rhythmical calcium transients within individual isolated cardiomyocytes. D, Calcium imaging of beating syncytium demonstrated coordinated activity between multiple cardiomyocytes and synchronized calcium transients.
Discussion
Nuclear reprogramming reorganizes gene expression profiles in parental cells to reset cell fate, and bioengineer pluripotent stem cells from ordinary somatic tissues [31,32]. In this way, iPS-based cardiogenesis has recently been demonstrated with ectopic infection of fibroblasts with a quartet set containing c-MYC or an equivalent transcription factor, Lin28, along with Oct3/4, Sox2, and Klf4 (4F-iPS) [13–17], or alternatively with the stemness gene triad that did not include c-MYC (3F-iPS) [18]. In the absence of the pro-oncogenic transgene, c-MYC, 3F-iPS clones here required an extended 4-week protocol to generate initial iPS colonies that were reprogrammed with the combination of OCT3/4, SOX2, and KLF4. Although both c-MYC dependent and independent strategies have been previously validated to produce bona fide iPS according to increasing levels of pluripotent stringency, including evidence for cardiac differentiation [13–18], the relative efficiencies for innate cardiogenesis was not previously quantified in a head-to-head comparison between 4F-iPS and 3F-iPS.
The similarities of 4F-iPS and 3F-iPS at the pluripotent ground state were here demonstrated within the embryoid body by immunostaining of pluripotent markers, and development of chimeric early stage embryos up to 9.5 dpc. Furthermore, 4F-iPS and 3F-iPS were indistinguishable in vivo with teratoma formation demonstrated for both clones following subcutaneous transplantation into immunodeficient hosts (data not shown) and chimeric embryo formation that demonstrates equivalent abilities to stochastically contribute to all lineages, including cardiac lineage specification within the cardiac fields, as a high-stringency evaluations for pluripotency. However, gene expression profiles following in vitro differentiation began to segregate depending on the presence or absence of c-MYC after 5 days as observed by persistent exposure of pluripotent gene expression. Subsequently, elevated levels of stemness related genes in 4F-iPS were associated with consistently lower levels of gastrulation markers and precardiac mesoderm markers. With the exception of a single clone demonstrating elevated levels of Sox7 at days 0, 5, and 12 of differentiation, all other genes within 3F-iPS clones peaked at day 5 as expected of gastrulation markers [28]. Furthermore, the lack of differentiated cardiac markers in the 4F-iPS compared to the fully cardiogenic 3F-iPS clones highlighted the distinctions after 8 days of in vitro differentiation. Ultimately, the functional cardiac phenotype was reproducible only in the absence of c-MYC nuclear reprogramming as 4F-iPS clones inconsistently differentiated into cardiogenic tissue while all 3F-iPS clones yielded proficient cardiogenic progeny.
Lineage specification of pluripotent stem cells in vitro is a product of intrinsic tissue-specific differentiation and environmental influence [32]. The feasibility of generating iPS progeny independent of c-MYC has recently produced progeny able to differentiate into authentic cardiac tissue [18,33]. In principle, reprogramming pluripotency without the oncogene c-MYC is a favorable strategy for iPS derivation given the reduction in dysregulated gene expression networks and tumorigenic load [34,35]. Indeed, 3F-iPS injected into immunocompetent host did not produce dysregulated tumor growth in long-term follow-up [18]. Moreover, 3F-iPS chimeras also did not demonstrate tumor formation [34,35] and yet maintained a cardiogenic potential from differentiation to functional chimerism [18].
As a pleiotropic transcription factor, c-MYC is a recognized dynamic regulator of the balance between growth and differentiation with ectopic transgene expression modulating lineage-specification [36]. A role for the proto-oncogene in the cardiac lineage was originally revealed in the setting of cardiac hypertrophy, where the c-MYC-dependent fetal gene profile is reactivated with expression of atrial genes and embryonic isoforms of contractile machinery in response to induction of immediate early genes [37]. In fact, promiscuous c-MYC is required for embryonic development with cardiogenic disruption precipitating lethal embryonic dysregulation [38]. This led to the observation that constitutive expression of c-MYC is sufficient to drive cardiac hyperplasia during development [39], and is responsible in part for adaptive compensation and survival benefit under cardiac stress in the adult [40,41]. While overexpression of c-MYC in the context of nuclear reprogramming has generated iPS with cardiogenic capacity [13–17], progeny generated here independent of c-MYC demonstrated particularly robust cardiogenic potential according to gene expression, contractile machinery, and synchronized calcium dynamics when compared to 4F-iPS. This data suggests that the transcription factor is not only dispensable at the time of reprogramming [18], but now establishes the negative impact of c-MYC on efficient cardiogenic output from iPS progeny. Counter productive to cardiogenesis, c-MYC expression binds to a large number of pluripotent genes and primarily activates this class of stemness related genes to induce pluripotency and contribute to self-maintenance of the pluripotent state within embryonic stem cells [42]. Notably, protein synthesis is the most significantly overrepresented biological process within confirmed c-Myc dependent downstream gene targets. In fact, it has been suggested that persistent expression of ectopic c-MYC could prevent proper differentiation of iPS cell lines [43]. Thus, c-MYC dependent nuclear reprogramming may hinder cardiogenesis by reactivation of a potent inductor of pluripotency pathways that are mutually exclusive and potentially contradictory to cardiac differentiation.
In summary, iPS technology promises the next generation of cell-based biologics [9, 23, 31, 44–46]. With the prioritized goal of converting a somatic tissue source for reproducible tissue-specific differentiation, reprogramming strategies initially qualified according to functional pluripotency and reduced tumorigenesis are here validated to produce de novo cardiogenic lineages with efficiency governed by optimal stemness factor induction. Although the present study uses mouse embryonic fibroblasts as a model system, adult somatic cells from multiple tissue sources could further provide variations in the overall efficiencies for iPS bioengineering as indicated recently by the degree of heterogeneity even within individual primary fibroblast cultures [47]. Therefore, continued mapping of the innate characteristics of the somatic source and the cardiogenic potential of the iPS-derived progeny should pave the way to optimize outcome. In this way, reprogramming somatic tissue without transgenic c-MYC expression suggests a reliable platform to ensure proficient iPS-derived differentiation that achieves consistent cardiogenesis.
Acknowledgments
The authors thank James E. Tarara and the Mayo Clinic Flow Cytometry and Optical Morphology Resource Core for their expertise. This work was supported by National Institutes of Health (R01HL083439, T32HL007111, R56AI074363), Caja Madrid Graduate Program, Marriott Individualized Medicine Program, Marriott Heart Disease Research Program, and Mayo Clinic.
References
- 1.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1(1):39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 4.Nelson TJ, Martinez-Fernandez AJ, Yamada S, Mael AA, Terzic A, Ikeda Y. Induced pluripotent reprogramming from promiscuous human stemness-related factors. Clinical and Translational Science. 2009;2(2):118–126. doi: 10.1111/j.1752-8062.2009.00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 7.Hochedlinger K, Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136(4):509–523. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishikawa S, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nature Reviews of Molecular Cell Biology. 2008;9(9):725–729. doi: 10.1038/nrm2466. [DOI] [PubMed] [Google Scholar]
- 9.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134(5):877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, Jaenisch R. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318(5858):1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 11.Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proceedings of the National Academy of Sciences USA. 2008;105(14):5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu D, Alipio Z, Fink LM, Adcock DM, Yang J, Ward DC, Ma Y. Phenotypic correction of murine hemophilia A using an iPS cell-based therapy. Proceedings of the National Academy of Sciences USA. 2009;106(3):808–813. doi: 10.1073/pnas.0812090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction with human stemness factors induced pluirpotent stem cells. Circulation. 2009;120(5):408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schenke-Layland K, Rhodes KE, Angelis E, Butylkova Y, Heydarkhan-Hagvall S, Gekas C, Zhang R, Goldhaber JI, Mikkola HK, Plath K, MacLellan WR. Reprogrammed mouse fibroblasts differentiate into cells of the cardiovascular and hematopoietic lineages. Stem Cells. 2008;26(6):1537–1546. doi: 10.1634/stemcells.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118(5):507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 16.Narazaki G, Uosaki H, Teranishi M, Okita K, Kim B, Matsuoka S, Yamanaka S, Yamashita JK. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118(5):498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Wilson G, Soerens A, Koonce C, Yu J, Palecek S, Thomson J, Kamp T. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circulation Research. 2009;104(4):e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Fernandez A, Nelson TJ, Yamada S, Reyes S, Alekseev AE, Perez-Terzic C, Ikeda Y, Terzic A. iPS Programmed without c-MYC yield proficient cardiogenesis for functional heart chimerism. Circulation Research. 2009;105(7):648–656. doi: 10.1161/CIRCRESAHA.109.203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321(5893):1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 20.Raya A, Rodríguez-Pizà I, Guenechea G, Vassena R, Navarro S, Barrero MJ, Consiglio A, Castellà M, Río P, Sleep E, González F, Tiscornia G, Garreta E, Aasen T, Veiga A, Verma IM, Surrallés J, Bueren J, Izpisúa Belmonte JC. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature. 2009;460(7251):53–59. doi: 10.1038/nature08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA. Generation of pluripotent stem cells from patients with type 1 diabetes. Proceedings of the National Academy of Sciences USA. 2009;106(37):15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee G, Papapetrou EP, Kim H, Chambers SM, Tomishima MJ, Fasano CA, Ganat YM, Menon J, Shimizu F, Viale A, Tabar V, Sadelain M, Studer L. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009;461(7262):402–406. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chien KR, Domian IJ, Parker KK. Cardiogenesis and the complex biology of regenerative cardiovascular medicine. Science. 2008;322(5907):1494–1497. doi: 10.1126/science.1163267. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Terzic C, Faustino RS, Boorsma BJ, Arrell DK, Niederländer NJ, Behfar A, Terzic A. Stem cells transform into a cardiac phenotype with remodeling of the nuclear transport machinery. Nature Clinical Practice Cardiovascular Medicine. 2007;4(Suppl 1):S68–76. doi: 10.1038/ncpcardio0763. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Terzic C, Behfar A, Méry A, van Deursen JM, Terzic A, Pucéat M. Structural adaptation of the nuclear pore complex in stem cell-derived cardiomyocytes. Circulation Research. 2003;92(4):444–452. doi: 10.1161/01.RES.0000059415.25070.54. [DOI] [PubMed] [Google Scholar]
- 26.Nelson TJ, Martinez-Fernandez A, Terzic A. KCNJ11 knockout morula reengineered by stem cell diploid aggregation. Philosophical Transactions of the Royal Society London B: Biological Sciences. 2009;364(1514):269–276. doi: 10.1098/rstb.2008.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behfar A, Perez-Terzic C, Faustino RS, Arrell DK, Hodgson DM, Yamada S, Puceat M, Niederländer N, Alekseev AE, Zingman LV, Terzic A. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. Journal of Experimental Medicine. 2007;204(2):405–420. doi: 10.1084/jem.20061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson TJ, Chiriac A, Faustino RS, Crespo-Diaz RJ, Behfar A, Terzic A. Lineage specification of Flk-1+ progenitors is associated with divergent Sox7 expression in cardiopoiesis. Differentiation. 2009;77(3):248–255. doi: 10.1016/j.diff.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodgson DM, Behfar A, Zingman LV, Kane GC, Perez-Terzic C, Alekseev AE, Pucéat M, Terzic A. Stable benefit of embryonic stem cell therapy in myocardial infarction. American Journal of Physiology Heart and Circulatory Physiology. 2004;287(2):H471–479. doi: 10.1152/ajpheart.01247.2003. [DOI] [PubMed] [Google Scholar]
- 30.Maherali N, Hochedlinger K. Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;3(6):595–605. doi: 10.1016/j.stem.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Yamanaka S. Pluripotency and nuclear reprogramming. Philosophical Transactions of the Royal Society London B: Biological Science. 2008;363(1500):2079–2087. doi: 10.1098/rstb.2008.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamanaka S. Elite and stochastic models for induced pluripotent stem cell generation. Nature. 2009;460(7251):49–52. doi: 10.1038/nature08180. [DOI] [PubMed] [Google Scholar]
- 33.Kim JB, Sebastiano V, Wu G, Araúzo-Bravo MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D, Meyer J, Hübner K, Bernemann C, Ortmeier C, Zenke M, Fleischmann BK, Zaehres H, Schöler HR. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136(3):411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nature Biotechnology. 2008;26(1):101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 35.Wernig M, Meissner A, Cassady JP, Jaenisch R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell. 2008;2(1):10–12. doi: 10.1016/j.stem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Guo Y, Niu C, Breslin P, Tang M, Zhang S, Wei W, Kini AR, Paner GP, Alkan S, Morris SW, Diaz M, Stiff PJ, Zhang J. c-Myc-mediated control of cell fate in megakaryocyte-erythrocyte progenitors. Blood. 2009;114(10):2097–2106. doi: 10.1182/blood-2009-01-197947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izumo S, Nadal-Ginard B, Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proceedings of the National Academy of Sciences USA. 1988;85(2):339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baudino TA, McKay C, Pendeville-Samain H, Nilsson JA, Maclean KH, White EL, Davis AC, Ihle JN, Cleveland JL. c-Myc is essential for vasculogenesis and angiogenesis during development and tumor progression. Genes and Development. 2002;16(19):2530–2543. doi: 10.1101/gad.1024602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson T, Allard MF, Sreenan CM, Doss LK, Bishop SP, Swain JL. The c-myc proto-oncogene regulates cardiac development in transgenic mice. Molecular Cell Biology. 1990;10(7):3709–3716. doi: 10.1128/mcb.10.7.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao G, Mao S, Baumgarten G, Serrano J, Jordan MC, Roos KP, Fishbein MC, MacLellan WR. Inducible activation of c-Myc in adult myocardium in vivo provokes cardiac myocyte hypertrophy and reactivation of DNA synthesis. Circulation Research. 2001;89(12):1122–1129. doi: 10.1161/hh2401.100742. [DOI] [PubMed] [Google Scholar]
- 41.Zhong W, Mao S, Tobis S, Angelis E, Jordan MC, Roos KP, Fishbein MC, de Alborán IM, MacLellan WR. Hypertrophic growth in cardiac myocytes is mediated by Myc through a Cyclin D2-dependent pathway. EMBO J. 2006;25(16):3869–3879. doi: 10.1038/sj.emboj.7601252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kidder BL, Yang J, Palmer S. Stat3 and c-Myc genome-wide promoter occupancy in embryonic stem cells. PLoS ONE. 2008;3(12):e3932. doi: 10.1371/journal.pone.0003932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brambrink T, Foreman R, Welstead GG, Lengner CJ, Wernig M, Suh H, Jaenisch R. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2(2):151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson TJ, Behfar A, Terzic A. Stem cells: biologics for regeneration. Clinical Pharmacology and Therapeutics. 2008;84(5):620–623. doi: 10.1038/clpt.2008.146. [DOI] [PubMed] [Google Scholar]
- 45.Nelson TJ, Behfar A, Terzic A. Strategies for therapeutic repair: The “R3” regenerative medicine paradigm. Clinical and Translational Science. 2008;1(2):168–171. doi: 10.1111/j.1752-8062.2008.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson TJ, Behfar A, Yamada S, Martinez-Fernandez A, Terzic A. Stem cell platforms for regenerative medicine. Clinical and Translational Science. 2009;2(3):222–227. doi: 10.1111/j.1752-8062.2009.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Byrne JA, Nguyen HN, Reijo Pera RA. Enhanced generation of induced pluripotent stem cells from a subpopulation of human fibroblasts. PLoS ONE. 2009;4(9):e7118. doi: 10.1371/journal.pone.0007118. [DOI] [PMC free article] [PubMed] [Google Scholar]





