Abstract
Nitrogenase catalyzes the nucleotide-dependent conversion of dinitrogen to ammonia at the iron-molybdenum cofactor (FeMoco) center of its molybdenum-iron (MoFe) protein component. Biosynthesis of FeMoco is arguably one of the most complex processes in the field of bioinorganic chemistry, which involves the participation of a number of nif (nitrogen fixing) gene products. One key player in this process, NifEN (encoded by nifE and nifN), is homologous to the MoFe protein with regard to both the primary sequences and the types of the metal centers. Recently, an all-iron precursor has been identified on NifEN, which closely resembles the Fe/S core structure of the mature cofactor. Such a precursor-bound form of NifEN has not only served as an excellent platform for the investigation of FeMoco assembly, but also facilitated the examination of the capacity of NifEN as a catalytic homolog of MoFe protein. This perspective will focus on the recent advances toward elucidating the dual functions of NifEN in nitrogenase assembly and catalysis, and the insights afforded by these advances into the evolution and mechanism of nitrogenase.
Introduction
Nitrogenase provides the biological machinery for nitrogen fixation, an ATP-dependent process in which the inert atmospheric dinitrogen (N2) is converted to ammonia (NH3), a usable form of nitrogen.1–6 The importance of nitrogen fixation can be appreciated from two perspectives. One, the product of the reaction—NH3 (or “fixed” nitrogen)—is essential for the existence of the entire human population. Two, the ambient condition under which the reaction occurs—contrary to the high energy demand of the industrial nitrogen fixation process—makes nitrogenase a desirable target for developing biomimetic strategies of NH3 production. Thus, nitrogenase has remained a focal point of research in the field of bioinorganic chemistry for the past decades.
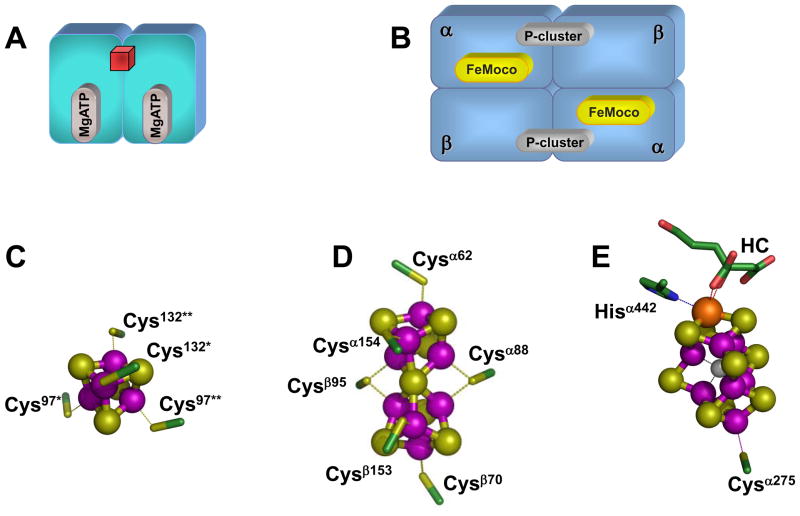
Three homologous nitrogenases [i.e., the molybdenum (Mo)-, vanadium (V)- and iron (Fe) only-nitrogenases] have been identified to date, which are distinguished by the heterometals (i.e., Mo, V and Fe) at their respective cofactor centers.7 Among them, the most extensively studied is the Mo-nitrogenase, which consists of two redox-active metalloproteins: the iron (Fe) protein and the molybdenum-iron (MoFe) protein.1 The α2-dimeric Fe protein contains a [Fe4S4] cluster at the subunit interface and an ATP-binding site within each subunit (Fig. 1(A));8 whereas the α2β2-tetrameric MoFe protein contains two unique clusters per αβ-dimer: the P-cluster ([Fe8S7]), which is bridged between the α-and β-subunits; and the iron-molybdenum cofactor, or FeMoco ([MoFe7S9X-homocitrate], where X = C, N or O), which is located within the α-subunit (Fig. 1(B)).9,10 Both clusters are complex, high-nuclearity metal centers: the P-cluster (in the reduced PN state) can be viewed as a symmetrical double cubane in which two [Fe4S4] subclusters share a μ6-sulfide in between (Fig. 1(D))†; while the FeMoco can be visualized as an asymmetrical double cubane comprising a [MoFe3S3] subcluster (where Mo is further coordinated by an organic homocitrate entity) and a [Fe4S3] subcluster that are bridged by three μ2-sulfides and a μ6-light central atom X (Fig. 1(E)). Nitrogenase catalysis likely involves repeated association and dissociation between the Fe protein and the MoFe protein, during which process electrons are transferred from the [Fe4S4] cluster of Fe protein, through the P-cluster, to the FeMoco of MoFe protein, where substrate reduction eventually occurs.
Fig. 1. Component proteins and metal centers of Mo-nitrogenase.
(A,B) Schematic presentations of the α2-dimeric Fe protein (A), which contains a [Fe4S4] cluster (red cube) at the subunit interface and a MgATP binding site within each subunit; and the α2β2-tetrameric MoFe protein (B), which contains a pair of unique clusters in each αβ-subunit dimer: the P-cluster ([Fe8S7]) at the αβ-subunit interface; and the FeMoco ([MoFe7S9X], where X = C, N, or O) within the α-subunit. (C-E) Structures of the metal centers in Fe protein and MoFe protein. In the Fe protein, the [Fe4S4] cluster is ligated by Cys97 and Cys132 from both subunits (C). In the MoFe protein, the P-cluster is ligated by three Cys residues from the α-subunit (Cysα62, Cysα88 and Cysα154) and three Cys residues from the β-subunit (Cysβ70, Cysβ95 and Cysβ153) (D); and the FeMoco is ligated by only two ligands: Cysα275 and Hisα442. The atoms of the metal centers are colored as follows: Mo, orange; Fe, purple; S, yellow; O, red; C, green; X (C, N or O), light gray. HC stands for homocitrate. These presentations are generated in PYMOL using 1N2C and 1M1N PDB coordinates.10, 32
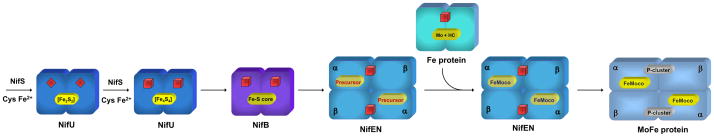
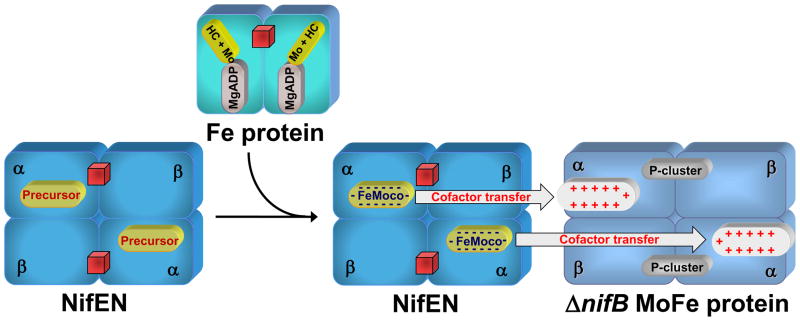
Biosynthesis of the FeMoco is a tremendous chemical feat full of intricate details.11–13 This process occurs independently of the production of the α- and β-polypeptides of MoFe protein. It is likely launched by the concerted action of NifS (encoded by nifS) and NifU (encoded by nifU), through which a protein-bound cysteine persulfide is first formed on NifS and then transferred to NifU for the sequential formation of [Fe2S2] and [Fe4S4] clusters (Fig. 2).14 Subsequently, these small Fe/S building blocks are assembled into a large Fe/S core on NifB (encoded by nifB), a member of the radical S-adenosyl-L-methionine (SAM)-dependent enzyme superfamily (Fig. 2).13 Such a Fe/S core represents a FeMoco precursor that likely contains all Fe and S for the formation of a mature cofactor, and it is further processed on NifEN (encoded by nifE and nifN) before it is delivered to its destined location in the MoFe protein (Fig. 2). In addition to these essential biosynthetic components, the Fe protein (encoded by nifH) also plays a key role in the FeMoco maturation process, and it likely exerts its effect through its interaction with NifEN (Fig. 2).
Fig. 2. Biosynthetic pathway of FeMoco.
NifS and NifU launch the process by synthesizing small Fe/S fragments, such as the [Fe2S2] clusters (red diamonds) and the [Fe4S4] clusters (red cubes). These small Fe/S building blocks are assembled into a large Fe/S core on NifB and further processed on NifEN with the assistance of Fe protein. Upon the completion of assembly on NifEN, the mature FeMoco is subsequently delivered to its target location within the MoFe protein, resulting in the formation of an active holo-MoFe protein. The [Fe4S4] clusters in Fe protein and NifEN are represented by red cubes.
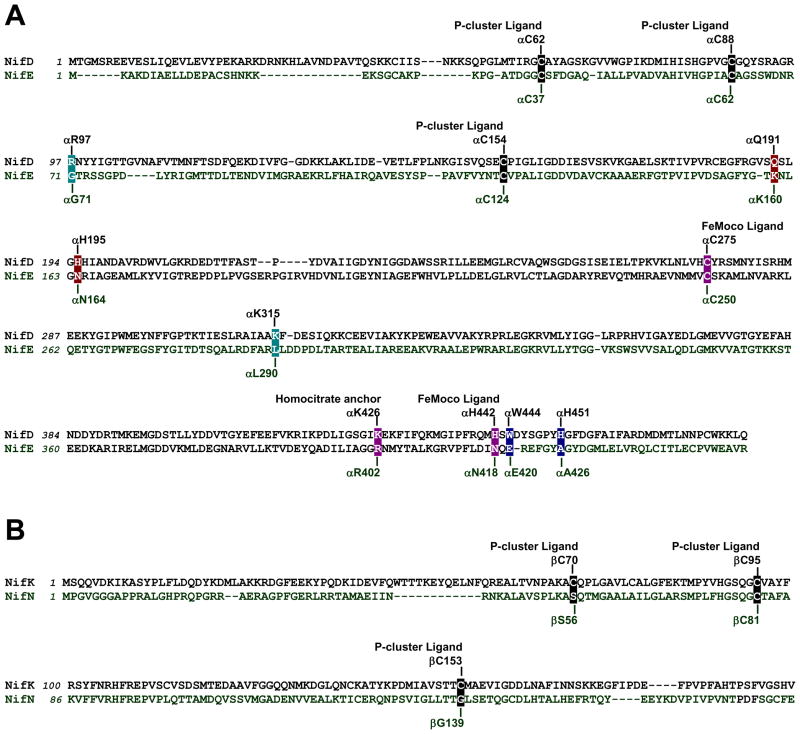
The initial hypothesis of the role of NifEN in FeMoco biosynthesis was based on the considerable degree of similarity between the primary sequences of NifEN and MoFe protein (Fig. 3). Like the MoFe protein, NifEN has the α2β2-tetrameric architecture. Moreover, NifEN contains cluster-binding regions that are homologous to the P-cluster and FeMoco sites in the MoFe protein.15 Consistent with the presence of four Cys ligands that is insufficient for the coordination of a [Fe8S7] structure (Fig. 3), NifEN contains a [Fe4S4] cluster at the “P-cluster site” between the α- and β-subunits.16 The “FeMoco site” of NifEN also bears substantial resemblance to its counterpart in the MoFe protein, as the two ligands that coordinate the two termini of the FeMoco, i.e., Cys at the Fe-end and His at the Mo-end, are either strictly preserved as a Cys residue or analogously replaced by an Asn residue in the α-subunit of NifEN (Fig. 3(A)). Additionally, the Lys residue that coordinates the homocitrate moiety of FeMoco is replaced by a homologous residue, Arg, in the α-subunit of NifEN (Fig. 3(A)). Given the considerable similarity between the cofactor sites in NifEN and MoFe protein, it stands to reason that NifEN is capable of hosting the conversion of a structurally homologous precursor to the mature FeMoco at its cofactor site. Furthermore, should such a FeMoco precursor (or homolog) accumulate on NifEN, it can be speculated that NifEN is capable of reducing some nitrogenase substrates, as it contains clusters that are homologous to both the P-cluster and the FeMoco of MoFe protein. This perspective will focus on the recent advances toward elucidating the dual capacities of NifEN in assembly and catalysis, and the insights afforded by these advances into the evolution and mechanism of nitrogenase.
Fig. 3. Comparison between the primary sequences of NifEN and MoFe protein.
(A) Partial sequence alignment between the α-subunits of MoFe protein (NifD) and NifEN (NifE) of Azotobacter vinelandii. (B) Partial sequence alignment between the β-subunits of MoFe protein (NifK) and NifEN (NifN) of A. vinelandii. Four of the six P-cluster ligands in MoFe protein, Cysα62, Cysα88, Cysα154 and Cysβ95, are preserved as Cysα37, Cysα62, Cysα124 and Cysβ81, respectively, in NifEN (A, B). One of the two FeMoco ligands in MoFe protein, Cysα275, is preserved as Cysα250 in NifEN; whereas the other ligand, Hisα442, is replaced by Asnα418 (A). The homocitrate anchor in MoFe protein, Lysα426, is replaced by a homologous residue, Argα402, in NifEN. Two MoFe protein residues that are important for N2 reduction, Glnα191 and Hisα195, are replaced by Lysα160 and Asnα164, respectively, in NifEN (A). Hisα451 and Trpα444, which could secure FeMoco at the cofactor binding site of MoFe protein, are replaced by Alaα426 and Gluα420, respectively, in NifEN (A). Argα97 and Lysα315, which are part of the positively charged insertion funnel in MoFe protein, are replaced by Glyα71 and Leuα290, respectively, in NifEN (A).
The biosynthetic role of NifEN: a scaffold protein for FeMoco maturation
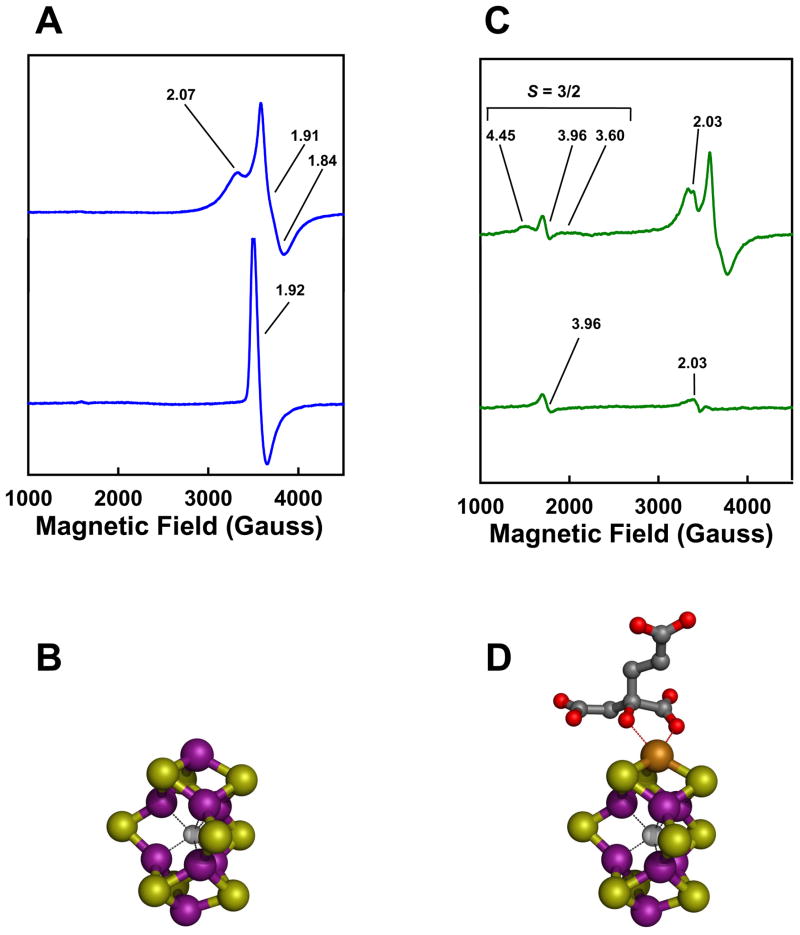
The exact function of NifEN in FeMoco biosynthesis was unveiled following the identification of a FeMoco precursor on NifEN.17 As the proposed biosynthetic flow of Fe and S during the process of FeMoco assembly is NifUS→NifB→NifEN→NifDK (Fig. 2), the absence of the nifDK-encoded MoFe protein (the downstream acceptor of FeMoco) and the nifH-encoded Fe protein (an indispensable factor for FeMoco maturation) should result in the accumulation of a precursor form of FeMoco on NifEN. Indeed, an all-Fe precursor was captured on a NifEN complex in the nifHDK-deficient genetic background, which gives rise to a unique g = 1.93 EPR feature in the IDS-oxidized state (Fig. 4(A), lower trace).17 Furthermore, Fe K-edge XAS/EXAFS analysis reveals that this cluster closely resembles the architecture of the Fe/S core of the mature FeMoco, although it is slightly elongated (Fig. 4(B)).18 This finding is exciting, as it suggests a previously-unsuspected biosynthetic strategy of the cofactor: instead of utilizing a mechanism that involves the coupling of [Fe4S3] and [MoFe3S3] subclusters, the assembly of FeMoco follows a course in which the Fe/S core is fully assembled prior to the attachment of Mo and homocitrate. The occurrence of a finished Fe/S core on NifEN also points to a considerable role of NifB—the biosynthetic component immediately upstream of NifEN (Fig. 2)—in the formation of this distinctive structure and suggests a plausible synthetic route to bridged metalloclusters that is dependent on the radical chemistry at the SAM domain of NifB.11
Fig. 4. Precursor- and FeMoco-bound forms of NifEN.
(A, C) Dithionite-reduced (upper traces) and indigo disulfonate (IDS)-oxidized (lower traces) EPR spectra of precursor-(A) and FeMoco- (C) bound NifEN. The conversion of precursor (A) to FeMoco (C) is accompanied by the appearance of a FeMoco-like, S = 3/2 signal in the dithionite-reduced spectrum (C, upper trace) and the current disappearance of the g = 1.92 feature in the IDS-oxidized spectrum (C, lower trace). The g values are indicated. (B, D) XAS/EXAFS-derived structures of the NifEN-associated precursor (B) and FeMoco (D). Both structures resemble that of the mature cofactor, except for the absence of Mo and homocitrate from the NifEN-associated precursor (B) and an asymmetric coordination of Mo in the NifEN-associated FeMoco (C). The atoms of the metal centers are colored as follows: Mo, orange; Fe, purple; S, yellow; O, red; C, dark gray; X (C, N or O), light gray. These presentations are generated in PYMOL using 1N2C and 1M1N PDB coordinates.10,32
The precursor on NifEN can be further matured, in vitro, by incubation with dithionite, Fe protein, MgATP, molybdate (MoO42−) and homocitrate.19,20 Like the isolated FeMoco, NifEN re-purified after such a treatment can be used as a direct FeMoco source for the reconstitution of the FeMoco-deficient ΔnifB MoFe protein, suggesting that (i) the all-Fe precursor is converted to a fully complemented FeMoco while it is still associated with NifEN; and (ii) upon the completion of assembly, FeMoco is transferred from NifEN to MoFe protein through direct protein-protein interactions.19,20 Maturation of the NifEN-bound precursor to a fully matured FeMoco can be monitored by the disappearance of the precursor-specific, g = 1.92 EPR signal in the IDS-oxidized state (Fig. 4(C), lower trace) and the concurrent appearance of a FeMoco-like, S = 3/2 signal in the dithionite-reduced state of the protein (Fig. 4(C), upper trace).19,20 Furthermore, Fe and Mo K-edge XAS/EXAFS analysis reveals that the NifEN-associated FeMoco is nearly identical in structure to the MoFe protein-associated FeMoco, except for an asymmetric coordination of Mo that likely originates from a different ligand environment at the Mo-end of the cofactor in NifEN (Fig. 4(D)).19,20 Apparently, there is a final “touch-up” of the FeMoco structure through ligand coordination at its target site in MoFe protein. Nevertheless, the fact that the cluster is completely matured prior to its exit from NifEN allows for an accurate account of the biosynthetic events hosted by NifEN.
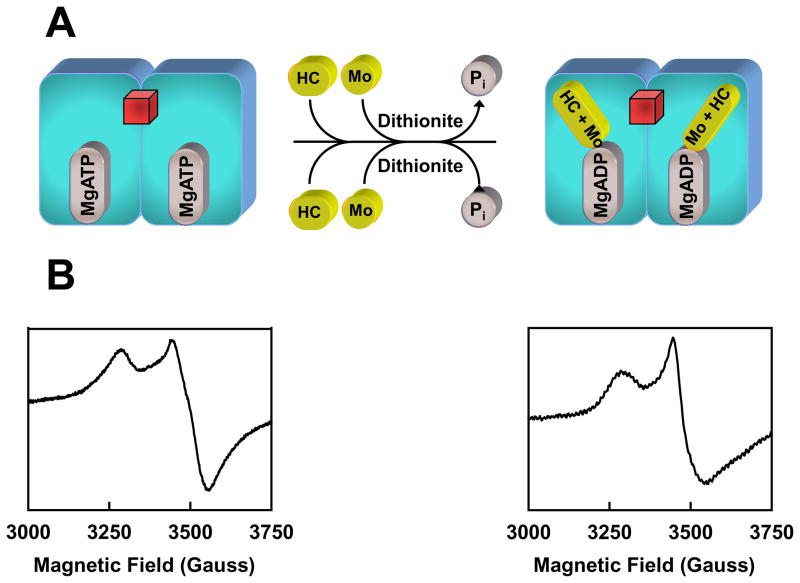
The efficiency of cluster conversion on NifEN relies on the concentrations of reductant (dithionite) and reductase (Fe protein), as the maximum conversion is achieved at a dithionite concentration of 20 mM (ca −440 mV) and a Fe protein/NifEN molar ratio of 4/1.20 Therefore, the incorporation of Mo and homocitrate into the NifEN-associated precursor is a redox-dependent process in which the Fe protein, an ATP-dependent reductase, plays an essential role. Indeed, Fe protein re-purified after incubation with dithionite, MgATP, MoO42−, homocitrate and NifEN is “loaded” with Mo and homocitrate, as it can serve as a direct Mo/homocitrate source for the maturation of NifEN-bound precursor (Fig. 5).21 Furthermore, Mo K-edge XAS of the loaded Fe protein demonstrates a decrease in the number of Mo=O bonds (two or three instead of the four in MoO42−) as well as a decrease in the effective oxidation level of Mo (due to either a change in the formal oxidation state of Mo or a change in the ligation of Mo), suggesting that Mo is reduced upon association with Fe protein.21 Perhaps even more interestingly, the EPR spectrum of the loaded Fe protein assumes a line shape somewhat in between those of the MgADP- and MgATP-bound forms of the Fe protein (Fig. 5(B)).21 This observation coincides with the first crystal structure of the ADP-bound Fe protein, where Mo is bound at a position that corresponds to the γ-phosphate of ATP.8 Given the structural analogy between the phosphate (PO43−) and molybdate (MoO42−), it is plausible that Mo could attach to ADP by replacing the γ-phosphate of ATP. Moreover, a similar nucleotide-dependent mechanism of Mo insertion has been proposed for the biosynthesis of pterin-based cofactors.11 Thus, the association of Mo to the Fe protein-bound ADP could represent the initial attachment of Mo to Fe protein upon ATP hydrolysis (Fig. 5(A)). Currently, the details of the Fe protein-mediated mobilization of Mo and homocitrate have not been clearly defined. Nevertheless, considering the sequence homology between NifEN and MoFe protein, it is likely that the Fe protein may interact with NifEN in a manner that is analogous to its interaction with the MoFe protein, during which process Mo and homocitrate are delivered from the loaded Fe protein to the NifEN-associated precursor.
Fig. 5. Mobilization of Mo and homocitrate by Fe protein.
(A) In the presence of reductant, Mo and homocitrate (HC) can be “loaded” onto the Fe protein upon ATP hydrolysis. Mo may enter the Fe protein by attaching to the position that corresponds to the γ-phosphate of ATP following the hydrolysis of ATP. Subsequently, the loaded Fe protein can deliver Mo and HC to the NifEN-associated precursor and transform the precursor into a fully matured FeMoco. The [Fe4S4] cluster of the Fe protein is represented by a red cube. (B) EPR spectra of the MgATP-bound Fe protein (left) and the Fe protein loaded with Mo and HC upon ATP hydrolysis (right).
The incorporation of Mo and homocitrate into the precursor signals the completion of the cofactor assembly on NifEN and the transfer of the finished FeMoco from NifEN to its target location within the MoFe protein. The absolute requirement of intermediary FeMoco carrier(s) between NifEN and MoFe protein was ruled out by the earlier report of unaffected nitrogen-fixing ability of the host upon deletions of proposed carrier-encoding genes and the recent observation of direct transfer of cofactor between NifEN and MoFe protein.19 A direct interaction between the two proteins was demonstrated recently by the formation of a complex between the FeMoco-bound NifEN and the FeMoco-deficient ΔnifB MoFe protein (unpublished data). Interestingly, complex formation was not observed between the precursor-bound NifEN and the ΔnifB MoFe protein (unpublished data). Together, these results indicate that NifEN undergoes a conformational rearrangement upon the insertion of Mo and homocitrate, which allows it to dock on the MoFe protein for the subsequent cluster transfer. Based on these observations, a plausible “diffusion” mechanism can be proposed for cluster transfer between the two proteins, which involves the alignment of their respective cofactor sites in close proximity and the subsequent diffusion of FeMoco from its biosynthetic site in NifEN (low-affinity site) to its binding site in MoFe protein (high-affinity site) (Fig. 6).12 Such a mechanism is supported by the analysis of the primary sequences of the two proteins, which reveals that certain residues that either provide covalent ligands to the cofactor or tightly pack the cofactor within the polypeptide matrix of MoFe protein are absent from the NifEN sequence.13 For example, two such residues, Hisα451 and Trpα444, which secure FeMoco at the cofactor binding site of MoFe protein, are replaced by Alaα426 and Gluα420, respectively, in NifEN (Fig. 3(A)). Apart from the ligand effect and steric impact, charge-based interactions could also play an important role in the transfer of FeMoco to the MoFe protein. Crystallographic analysis of the FeMoco-deficient ΔnifB MoFe protein has revealed the presence of a positively charged funnel in the α-subunit that could accommodate the insertion of the negatively charged FeMoco.22 In contrast, some of the positively charged residues in the insertion funnel of MoFe protein, such as Argα97 and Lysα315, are replaced by Glyα71 and Leuα290, respectively, in the NifEN sequence (Fig. 3(A)). This observation points to a significant role of homocitrate in transferring FeMoco from NifEN to MoFe protein, as this organic moiety is responsible for the overall negative charge of the cofactor and, therefore, could facilitate the diffusion of the fully complemented cofactor toward a more positive environment (i.e., the cofactor site in MoFe protein). Consistent with this hypothesis, a recent study showed that a homocitrate-free FeMo cluster could be generated on NifEN; however, unlike the fully complemented FeMoco, this FeMo cluster could not be transferred to the ΔnifB MoFe protein (unpublished data).
Fig. 6. Maturation and incorporation of FeMoco.
Fe protein loaded with Mo and homocitrate (HC) can serve as a direct Mo/HC source for the maturation of NifEN-bound precursor. Incorporation of Mo and HC into the precursor completes the FeMoco maturation on NifEN. Subsequently, the fully matured FeMoco is transferred from NifEN to its target location within the MoFe protein upon direct protein-protein interactions. Charge-based interactions likely play an important role in the transfer of FeMoco between NifEN and MoFe protein, during which process the negatively charged FeMoco is inserted into the MoFe protein via a positively charged insertion funnel. The [Fe4S4] clusters of Fe protein and NifEN are represented by red cubes.
The catalytic properties of NifEN: a partially defective homolog of MoFe protein
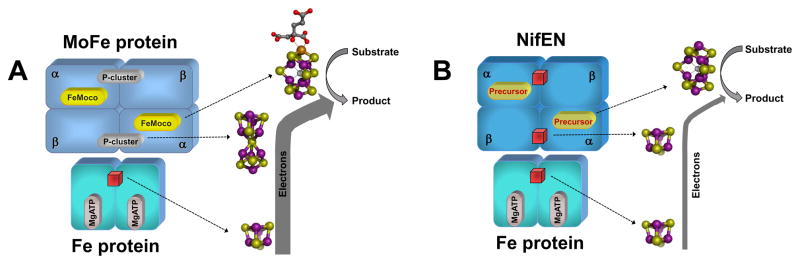
The fact that NifEN contains the cluster equivalents to both the P-cluster and the FeMoco of MoFe protein suggests that NifEN could substitute for the MoFe protein in some of the substrate-reducing reactions of nitrogenase (Fig. 7). Indeed, NifEN is capable of reducing acetylene (C2H2) and azide (N3−), the alternative substrates of the MoFe protein.23 These two NifEN-catalyzed reactions generate C2H4 and NH3 as the respective products, which are identical to those produced by the MoFe protein-catalyzed reactions.23 Like the MoFe protein, NifEN requires the Fe protein as a specific ATP-dependent reductase for substrate reduction, as neither of the two substrates (i.e., C2H2 and N3−) can be reduced by NifEN if ATP is omitted or substituted by ADP or non-hydrolysable ATP analogs, or if the Fe protein is replaced by a variant that is specifically defective in ATP hydrolysis.23 In reactions catalyzed by either NifEN or MoFe protein, C2H2 and N3− are mutually inhibitory for the reduction of each other; whereas CO is a potent inhibitor for the reduction of both substrates.23 These results show a strong parallelism between the catalytic behaviors of NifEN and MoFe protein and suggest that NifEN is a catalytic homolog of the MoFe protein.
Fig. 7. Components of the electron transfer chains in nitrogenase and its homolog.
(A) In nitrogenase (i.e., Fe protein/MoFe protein), electrons likely flow from the [Fe4S4] cluster of the Fe protein to the P-cluster and then the FeMoco of the MoFe protein, where substrate reduction occurs. (B) In nitrogenase homolog (i.e., Fe protein/NifEN), electrons could flow from the [Fe4S4] cluster of the Fe protein to the [Fe4S4] cluster and then the all-Fe FeMoco homolog (or precursor) of NifEN. However, there is a much reduced electron flux through the Fe protein/NifEN system (indicated by a thinner arrow), which is likely due to the limited electron transfer capacity of the [Fe4S4] cluster at the “P- cluster site” of NifEN (B). The [Fe4S4] clusters in Fe protein and NifEN are represented by red cubes. The atoms of the metal centers are colored as follows: Mo, orange; Fe, purple; S, yellow; O, red; C, dark gray; X (C, N or O), light gray. These presentations are generated in PYMOL using 1N2C and 1M1N PDB coordinates.10, 32
On the other hand, NifEN exhibits catalytic features that are distinct from those of the MoFe protein. Except for C2H2 and N3−, NifEN is unable to reduce other substrates of the MoFe protein, such as CN− (requiring four or six electrons for reduction) or N2 (requiring six electrons for reduction).23 Moreover, while the reduction of C2H2 by either NifEN or MoFe protein is a two-electron reaction, the reduction of N3− by NifEN differs from that by MoFe protein in the amount of electrons involved. Contrary to the MoFe protein, which is capable of reducing N3− by two, six or eight electrons to N2+NH3, N2H4+NH3 and NH3, respectively, the NifEN-catalyzed N3−-reduction is “trapped” at the two-electron step, because (i) NifEN is unable to reduce N2; (ii) N2H4 formation is not detected; and (iii) NifEN can not reduce N2H4.23 Thus, the NifEN-catalyzed reactions appear to involve no more than two electrons, which could account for the narrow substrate range of NifEN. Another major discrepancy between the NifEN- and MoFe protein-catalyzed reactions is that the former requires a lower solution potential (as determined by the concentration of dithionite) and a higher excess of reductase (as indicated by the molar excess of Fe protein) than the latter. The maximum C2H2- and N3−-reducing activities of NifEN are achieved at a dithionite concentration of 0.4 mM (ca −490 mV) and a Fe protein/NifEN molar ratio of 70;23 whereas the highest C2H2- and N3−-reducing activities of the MoFe protein are reached at a dithionite concentration of 20 mM (ca −440 mV) and a Fe protein/MoFe protein molar ratio of 30. Even in the presence of a higher reducing power, NifEN is much less active than the MoFe protein in catalysis. Thus, NifEN represents an inefficient MoFe protein homolog that is partially defective in its substrate range.
One account for the catalytic defects of NifEN is the presence of a [Fe4S4]-type cluster—instead of a [Fe8S7] P-cluster—at the αβ-subunit interface of this protein, which has a limited capacity of mediating the electron transfer between the [Fe4S4] center of the Fe protein and the cofactor center of NifEN. As a result, there is an inadequate electron flow through NifEN, which not only makes NifEN ineffective in substrate reduction, but also renders the cofactor center in NifEN in a more oxidized state, to which only select substrates, such as C2H2 and N3−, can bind. Other factors that could account for the deficiency of NifEN in substrate reduction (particularly its inability to catalyze N2 reduction) are the missing constituents from its cofactor, as well as the immediate protein environment surrounding the cofactor. With regard to the former, it has been hypothesized that homocitrate switches from the bi-dentate to the mono-dentate ligation to the Mo atom of FeMoco, which frees up a site for N2 binding.24 Such a mechanism cannot be utilized by NifEN, as its cofactor center does not contain Mo and homocitrate. With regard to the latter, it has been reported that MoFe proteins carrying αQ191K and αH195N mutations cannot reduce N2.25,26 Interestingly, both mutations are precisely “duplicated” in the native sequence of NifEN (Fig. 3(A)), which could explain the inability of NifEN to reduce N2.
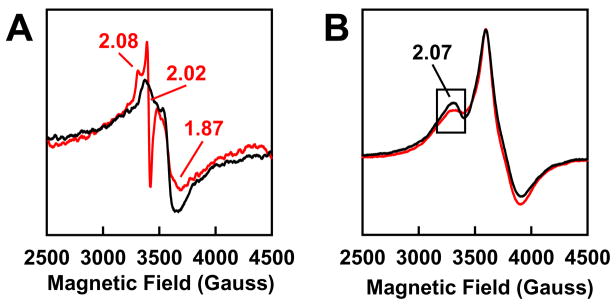
The slow turnover of NifEN has an unexpected advantage in that it permits a direct spectroscopic analysis of enzyme-substrate interactions in NifEN-catalyzed reactions. Upon C2H2 turnover, a new feature appears in the EPR spectrum of NifEN, which reveals its identity at 30 K as an S = 1/2 signal with a sharp inflection at g = 2.02 (Fig. 8(A)).23 Interestingly, an analogous S = 1/2 signal was observed during the turnover of C2H2 by a variant form of the MoFe protein, suggesting that the cofactor centers in NifEN and MoFe protein may undergo similar redox changes upon the turnover of the same substrate.27 In the case of N3−, no new features appear upon turnover; however, the g = 2.07 feature of the NifEN-associated signal becomes smaller, which can be best visualized at 6 K (Fig. 8(B)).23 The appearance of turnover-associated signals suggests that the cofactor in NifEN may have achieved a uniform oxidation state, which facilitates the population of a substrate- or intermediate-bound state of the enzyme during C2H2- or N3−-turnover. This is an important observation, as one of the major hurdles for the mechanistic analysis of nitrogenase is the difficulty in trapping a particular substrate or intermediate at the cofactor center of the wild-type MoFe protein, which is likely due to a highly mixed oxidation state of the FeMoco center that prevents the accumulation of any substrate- or intermediate-bound form of the enzyme. The limited redox versatility of the cofactor center in NifEN, therefore, may prove advantageous in naturally enriching the C2H2- or N3−-bound forms of NifEN and subsequently crystallizing the first intermediate-bound nitrogenase homolog, a feat yet to be accomplished for this complex enzyme system.
Fig. 8. EPR features of NifEN upon substrate turnover.
EPR spectra of NifEN under turnover (red) and non-turnover (black) conditions of C2H2 (A) and N3− (B). Turnover samples contained MgATP (red), which is absent form non-turnover samples (black). Spectra were recorded at 30 K (A) and 6 K (B), respectively. The g values are indicated.
The dual capacities of NifEN: what can we learn from it?
The dual capacities of NifEN in assembly and catalysis point to a possible role of NifEN in nitrogenase evolution. It has been proposed that NifEN and MoFe protein have evolved from the replication and divergence of a common ancestral gene.28 This theory seems to imply a parallel evolution of NifEN and MoFe protein upon branching at the genetic level. The ability of NifEN to reduce some of the MoFe protein substrates, however, suggests that NifEN and MoFe protein may have appeared sequentially during evolution. NifEN could be the predecessor to MoFe protein, acting as a self-assembled, detoxifying enzyme in the primitive earth environment, where toxic substances such as C2H2 and N3− were abundant. It is interesting to note that the maximum substrate-reducing activity of NifEN is achieved when a high reducing power is supplied (see above). This observation would support the theory that NifEN was catalytically active early on in the course of evolution, when the mantle of earth was likely more reduced. Later, NifEN might have gradually evolved into an effective enzyme with a wide range of substrates (i.e., MoFe protein) while in the meantime adjusting its own role toward synthesizing a catalytically more powerful cofactor (i.e., FeMoco).
The striking resemblance of the NifEN-associated all-Fe cluster to the Fe/S core of the mature cofactor is another interesting observation from the evolutionary perspective. Given the structural similarity among the three homologous nitrogenases (i.e., the Mo-, V-, and Fe only-nitrogenases),7 this all-Fe cluster could reasonably serve as a precursor to all three nitrogenase cofactors (i.e., FeMoco, FeVco and FeFeco). However, while the biosynthetic pathways of all three cofactors share the same apparatus at the early stages, they part their ways after the formation of an Fe/S core on NifB (Fig. 2) and continue toward either the formation of FeMoco on NifEN, or the formation of FeVco or FeFeco on VnfEN.13 The appearance of these two homologous yet distinct scaffold proteins (i.e., NifEN and VnfEN) could reflect the conditions of the earth environment (e.g., the availability of certain metals) during the evolution of nitrogenase. More importantly, through these two different scaffold proteins, nature has developed a highly selective strategy to incorporate different heterometals into the various nitrogenase cofactors.
The fact that NifEN is a partially defective homolog of MoFe protein makes it an ideal mutational platform for the (re)construction of a functional MoFe protein equivalent. For example, a P-cluster site can be restored by introducing the missing P-cluster ligands into the NifEN sequence. In addition, a fully complemented FeMoco can be generated by inserting Mo and homocitrate into the all-Fe precursor on NifEN. Finally, the immediate protein environment at the cofactor site of NifEN can be systematically altered to recreate the FeMoco site in MoFe protein. This “add-on” approach (i.e., approach to restore the catalytic features in NifEN) can be combined with a “subtractive” approach (i.e., approach to remove the catalytic features from MoFe protein) and, together, they could provide a comprehensive account of the key features for nitrogenase catalysis. With this knowledge in hand, attempts could be made to generate a “slow” nitrogen-fixing system by partially restoring the catalytic features in NifEN. Such a nitrogenase variant could be used to capture the nitrogenous substrate(s) or intermediate(s) and directly analyze the enzyme-substrate interactions during N2 reduction. While it is too early to predict the outcome of these proposed efforts, the potential of NifEN as a powerful tool in the mechanistic studies of nitrogenase is certainly worth exploring. This NifEN-based approach could complement a MoFe protein-based approach that has been successfully applied to trap a variety of nitrogenase substrates29–31 and serve as an alternative route to a better understanding of the catalytic mechanism of nitrogenase.
Acknowledgments
This work was supported by the National Institutes of Health grant GM67626 (M.W.R.).
Footnotes
The P-cluster can be converted to the POX (or P2+) state by a two-electron oxidation process, during which event one half of this [Fe8S7] cluster is opened up concomitantly with the added coordination from the backbone amide nitrogen of Cysα88 to one Fe atom and the Oγ of Serβ188 to another Fe atom of the cluster, resulting in a loss of symmetry of the double-cubane structure.4
Notes and references
- 1.Burgess BK, Lowe DJ. Chem Rev. 1996;96:2983–3012. doi: 10.1021/cr950055x. [DOI] [PubMed] [Google Scholar]
- 2.Howard JB, Rees DC. Chem Rev. 1996;96:2965–2982. doi: 10.1021/cr9500545. [DOI] [PubMed] [Google Scholar]
- 3.Smith BE. Adv Inorg Chem. 1999;47:159–218. [Google Scholar]
- 4.Rees DC, Tezcan FA, Haynes CA, Walton MY, Andrade SL, Einsle O, Howard JB. Philo Trans R Soc London. 2005;A363:971–984. doi: 10.1098/rsta.2004.1539. [DOI] [PubMed] [Google Scholar]
- 5.Howard JB, Rees DC. Proc Natl Acad Sci USA. 2006;103:17088–17093. doi: 10.1073/pnas.0603978103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters JW, Szilagyi RK. Curr Opin Chem Biol. 2006;10:101–108. doi: 10.1016/j.cbpa.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Eady RR. Chem Rev. 1996;96:3013–3030. doi: 10.1021/cr950057h. [DOI] [PubMed] [Google Scholar]
- 8.Georgiadis MM, Komiya H, Chakrabarti P, Woo D, Kornuc JJ, Rees DC. Science. 1992;257:1653–1659. doi: 10.1126/science.1529353. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Rees DC. Nature. 1992;360:553–560. doi: 10.1038/360553a0. [DOI] [PubMed] [Google Scholar]
- 10.Einsle O, Tezcan FA, Andrade SL, Schmid B, Yoshida M, Howard JB, Rees DC. Science. 2002;297:1696–1700. doi: 10.1126/science.1073877. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz G, Mendel RR, Ribbe MW. Nature. 2009;460:839–847. doi: 10.1038/nature08302. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y, Fay AW, Lee CC, Yoshizawa J, Ribbe MW. Biochemistry. 2008;47:3973–3981. doi: 10.1021/bi7025003. [DOI] [PubMed] [Google Scholar]
- 13.Dos Santos PC, Dean DR, Hu Y, Ribbe MW. Chem Rev. 2004;104:1159–1173. doi: 10.1021/cr020608l. [DOI] [PubMed] [Google Scholar]
- 14.Johnson DC, Dean DR, Smith AD, Johnson MK. Annu Rev Biochem. 2005;74:247–281. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- 15.Roll JT, Shah VK, Dean DR, Roberts GP. J Biol Chem. 1995;270:4432–4437. doi: 10.1074/jbc.270.9.4432. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin PJ, Agar JN, Roll JT, Roberts GP, Johnson MK, Dean DR. Biochemistry. 1998;37:10420–10428. doi: 10.1021/bi980435n. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Fay AW, Ribbe MW. Proc Natl Acad Sci USA. 2005;102:3236–3241. doi: 10.1073/pnas.0409201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corbett MC, Hu Y, Fay AW, Ribbe MW, Hedman B, Hodgson KO. Proc Natl Acad Sci USA. 2006;103:1238–1243. doi: 10.1073/pnas.0507853103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Y, Corbett MC, Fay AW, Webber JA, Hodgson KO, Hedman B, Ribbe MW. Proc Natl Acad Sci USA. 2006;103:17119–17124. doi: 10.1073/pnas.0602647103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshizawa JM, Blank MA, Fay AW, Lee CC, Wiig JA, Hu Y, Hodgson KO, Hedman B, Ribbe MW. J Am Chem Soc. 2009;131:9321–9325. doi: 10.1021/ja9035225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Y, Corbett MC, Fay AW, Webber JA, Hodgson KO, Hedman B, Ribbe MW. Proc Natl Acad Sci USA. 2006;103:17125–17130. doi: 10.1073/pnas.0602651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmid B, Ribbe MW, Einsle O, Yoshida M, Thomas LM, Dean DR, Rees DC, Burgess BK. Science. 2002;296:352–356. doi: 10.1126/science.1070010. [DOI] [PubMed] [Google Scholar]
- 23.Hu Y, Yoshizawa JM, Fay AW, Lee CC, Wiig JA, Ribbe MW. Proc Natl Acad Sci USA. 2009;106:16962–16966. doi: 10.1073/pnas.0907872106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durrant MC, Francis A, Lowe DJ, Newton WE, Fisher K. Biochem J. 2006;397:261–270. doi: 10.1042/BJ20060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomann H, Bernardo M, Newton WE, Dean DR. Proc Natl Acad Sci USA. 1991;88:6620–6623. doi: 10.1073/pnas.88.15.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher K, Dilworth MJ, Newton WE. Biochemistry. 2000;39:15570–15577. doi: 10.1021/bi0017834. [DOI] [PubMed] [Google Scholar]
- 27.Lee HI, Sørlie M, Christiansen J, Yang TC, Shao J, Dean DR, Hales BJ, Hoffman BM. J Am Chem Soc. 2005;127:15880–15890. doi: 10.1021/ja054078x. [DOI] [PubMed] [Google Scholar]
- 28.Fani R, Gallo R, Liò P. J Mol Evol. 2000;51:1–11. doi: 10.1007/s002390010061. [DOI] [PubMed] [Google Scholar]
- 29.Barney BM, Lee HI, Dos Santos PC, Hoffman BM, Dean DR, Seefeldt LC. Dalton Trans. 2006;19:2277–2284. doi: 10.1039/b517633f. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman BM, Dean DR, Seefeldt LC. Acc Chem Res. 2009;42:609–619. doi: 10.1021/ar8002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seefeldt LC, Hoffman BM, Dean DR. Annu Rev Biochem. 2009;78:701–722. doi: 10.1146/annurev.biochem.78.070907.103812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schindelin H, Kisker C, Schlessman JL, Howard JB, Rees DC. Nature. 1997;387:370–376. doi: 10.1038/387370a0. [DOI] [PubMed] [Google Scholar]