Abstract
Sulfonamide antimicrobials such as sulfamethoxazole (SMX) have been associated with drug hypersensitivity reactions, particularly in patients with AIDS. A reactive oxidative metabolite, sulfamethoxazole-nitroso (SMX-NO), forms drug-tissue adducts that elicit a T cell response. Antioxidants such as ascorbic acid (AA) and glutathione (GSH) reduce SMX-NO to the less reactive hydroxylamine metabolite (SMX-HA), which is further reduced to the non-immunogenic parent compound by cytochrome b5 (b5) and its reductase (b5R). We hypothesized that deficiencies in AA and GSH would enhance drug-tissue adduct formation and immunogenicity towards SMX-NO, and that these antioxidant deficiencies might also impair the activity of the b5/b5R pathway. We tested these hypotheses in guinea pigs fed either a normal or AA-restricted diet, followed by buthionine sulfoximine treatment (250 mg/kg SC daily, or vehicle); and SMX-NO (1 mg/kg IP 4 days per week, or vehicle), for 2 weeks. Guinea pigs did not show any biochemical or histopathologic evidence of SMX-NO related toxicity. Combined AA and GSH deficiency in this model did not significantly increase tissue drug-adduct formation, or splenocyte proliferation in response to SMX-NO. However, combined antioxidant deficiency was associated with decreased mRNA and protein expression of cytochrome b5, as well as significant decreases in SMX-HA reduction in SMX-NO treated pigs. These results suggest that SMX-HA detoxification may be down-regulated in combined AA and GSH deficiency. This mechanism could contribute to the higher risk of SMX hypersensitivity in AIDS patients with antioxidant depletion.
Keywords: Guinea pig, antioxidants, drug hypersensitivity, NADH hydroxylamine reductase
Introduction
Sulfamethoxazole (SMX) is the antimicrobial of choice for the prevention and treatment of Pneumocystic carinii (now jiroveci) pneumonia in immuno-compromised patients, particularly in those with AIDS (DiRienzo et al. 2002; Furrer et al. 1999; Goldie et al. 2002; Grimwade and Swingler 2003; Rabaud et al. 2001). However, the use of SMX and other potentiated sulfonamides is limited by delayed-type drug hypersensitivity reactions, which include fever, skin eruptions, and, less commonly, multi-organ toxicity (Carr et al. 1994; Gordin et al. 1984; Walmsley et al. 1998). The reported incidence of sulfonamide hypersensitivity reactions in AIDS patients is 25-55% (Gordin et al. 1984; Medina et al. 1990; Walmsley et al. 1998), which is much higher than that reported for HIV-negative patients given sulfonamide antimicrobials (3%) (Jick 1982; Koch-Weser et al. 1971).
The pathogenesis of sulfonamide hypersensitivity, and the reasons for its high incidence in the setting of HIV infection, are not fully understood. An oxidative metabolite of SMX, SMX-nitroso (SMX-NO; Figure 1), can haptenize lymphocytes and keratinocytes (Naisbitt et al. 2002; Naisbitt et al. 1999; Reilly et al. 2000), and lead to the generation of anti-SMX antibodies and drug-specific T cell clones (Farrell et al. 2003; Gill et al. 1997; Naisbitt et al. 2001). Therefore, SMX-NO is thought to be a proximate immunogen in sulfonamide hypersensitivity reactions.
Fig 1.
Biotransformation of sulfamethoxazole (SMX) in humans. The parent drug is predominantly cleared by N-acetylation, leading to a non-immunogenic product. SMX is alternatively oxidized to SMX-HA, which spontaneously generates the nitroso metabolite (SMX-NO). SMX-NO forms adducts with tissue proteins that can lead to T cell activation. Ascorbate, and the thiols glutathione or cysteine, can non-enzymatically reduce SMX-NO to the hydroxylamine. SMX-HA is further detoxified by cytochrome b5 and its reductase (Kurian et al. 2004).
SMX-NO can be reduced non-enzymatically to SMX-hydroxylamine (SMX-HA) by antioxidants such as glutathione (GSH), cysteine, and ascorbate (AA) (Cribb et al. 1991; Naisbitt et al. 1999; Trepanier et al. 2004). SMX-HA is further reduced enzymatically by cytochrome b5 (b5) and its reductase (b5R) to the parent sulfonamide (Kurian et al. 2004), which is non-immunogenic. Therefore, these antioxidants and the b5/b5R pathway represent detoxification routes in the setting of SMX hypersensitivity. In addition, antioxidant deficiencies may also directly affect cytochrome b5 expression (Degkwitz and Kim 1973; Degkwitz et al. 1973). Patients with HIV infection have low thiol and ascorbate levels, and a decreased capacity to reduce SMX metabolites back to the parent drug (Naisbitt et al. 2000; Trepanier et al. 2004; Walmsley et al. 1997). We therefore hypothesized that combined ascorbate and glutathione deficiency might lead to enhanced drug adducts and T cell responses to SMX-NO, and might also influence the expression of the b5/b5R pathway.
The objectives of this study, therefore, were to evaluate the effects of combined AA and GSH deficiency on the development of drug-tissue adducts and the degree of drug-specific T cell proliferation in guinea pigs administered SMX-NO, and to determine whether antioxidant depletion impairs SMX-HA reduction via the b5/ b5R pathway.
Methods
Animals
The guinea pig was chosen as an animal model because, like humans, this species cannot synthesize ascorbate in vivo, and becomes ascorbate-deficient with dietary restriction. Two month old male Hartley guinea pigs weighing at least 0.5 kg were purchased from Charles River labs (Wilmington, Mass). Animals were housed individually in standard plastic cages with sterilized corn cob bedding. The study protocol was approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Madison.
Animal experiments and dosing
A vitamin C-deficient guinea pig diet (2 ppm ascorbate; Harlan Teklad, Madison, WI) and water were fed ad libitum for the entire period of study. Eighteen guinea pigs were randomly allocated to 4 different treatments (Figure 2). The ascorbate replete groups were supplemented with oral AA (Sigma Aldrich, St. Louis, MO) in a sucrose solution, at 75 mg/kg/day (equivalent to approximately 1200-2000 ppm of AA in diet), for the 4 weeks of the study. The AA restricted groups received oral AA in the same sucrose solution at 7.5 mg/kg/day (equivalent to approximately 120-200 ppm of AA in diet) over the same 4 weeks. This level of supplementation was designed to lead to ascorbate deficiency without overt scurvy (Council 1995).
Fig 2.
Summary of the different treatment groups used in the present experiments. The following treatment groups were compared for different end points: to establish a model of antioxidant depletion, group 1 vs. 2, and group 3 vs. 4 (ANOVA with Bonferroni's post hoc test); to compare drug adducts and T cell proliferation, group 3 vs. 4 (unpaired t test); to evaluate clinical toxicity (e.g. serum ALT activity): groups 1, 2, 3, and 4 (ANOVA with Bonferroni's post hoc test); and to evaluate the effect of antioxidant depletion on b5 and b5R expression, groups 1 vs. 2 (unpaired t test).
After 2 weeks of diet run-in, the animals were given saline vehicle or BSO (250 mg/kg/day SC; Dalton Chemicals, Toronto, ON; equivalent to 1.1 mmol/kg) to inhibit GSH synthesis, for the second 2 weeks of the study. This protocol was based on previous studies in mice in which BSO (1 to 3 mmol/kg/day) decreased hepatic GSH concentrations by about 40 to 60% (Yu and Brown 1984), and from our pilot studies in guinea pigs, which showed renal tubular necrosis in some pigs given 400 mg/kg/day of BSO (1.8 mmol/kg/day) for 2 weeks.
In addition, SMX-NO (Dalton Chemicals, Toronto, ON) or 20% DMSO vehicle was given using a pulse protocol shown to be immunogenic in other rodents (Naisbitt et al. 2001), at 1 mg/kg/day IP for 4 days per week, for the second 2 weeks of the study.
Guinea pigs were examined daily and body weights were taken weekly. Blood was collected from pigs under isoflurane anesthesia by mask induction, at baseline, 2 weeks, and 4 weeks, for measurement of erythrocyte GSH and plasma AA concentrations, as well as a complete blood count (CBC) and limited biochemical panel. The animals were euthanized at 4 weeks (Beuthanasia IV, Schering-Plough Animal Health Corp., Union, NJ), 24 h after the last SMX-NO and BSO treatments. Liver, whole blood, peritoneum, and other tissues were collected, flash frozen in liquid nitrogen, and stored at -80°C. Half of each spleen was washed with normal saline and used immediately for T cell proliferation experiments; the reminder was snap frozen for adduct experiments.
Anti-oxidant assays in liver and plasma using HPLC
Heparinized blood (2 ml) was placed on ice immediately after venipuncture, and treated with 200 μl of diluted monobromobimane (Invitrogen, Carlsbad, CA; 37.5 μl of a 180 mM stock in acetonitrile, diluted to 250 μl in PBS just prior to addition) to stabilize glutathione. Plasma and erythrocytes were harvested from chilled whole blood within 1 h of venipuncture, and frozen at -80°C overnight prior to AA and reduced GSH measurements, respectively. Red blood cell (RBC) reduced GSH was measured using HPLC, as previously described (Trepanier et al. 2004). For hepatic GSH content, 0.1 g of liver was homogenized in PBS, pH. 7.4, containing bromobimane (final concentration, 2.45 mM). The homogenate was centrifuged at 3000 g for 15 min at 4°C. The supernatant was processed as for HPLC analysis in erythrocytes.
Plasma AA was measured using our previously validated plasma AA assay (Trepanier et al. 2004), modified to obtain cleaner peak separations and more complete protein precipitation in the guinea pig. An equal volume of 50 mM of perchloric acid (PCA) was added to 200 μl of heparinized plasma to precipitate proteins. The sample was vortexed, incubated on ice for 5 min, and centrifuged at 16,000 g for 10 min at 4°C; 350 μl of the supernatant was then added to 70 μl of 50 mM PCA. Samples were again vortexed and centrifuged to precipitate remaining proteins. The supernatant was filtered first through 0.45 μm, followed by 0.22 μm, Costar nylon spin-X filter tubes (Corning inc., Corning, NY) by centrifugation at 16,000 g for 5 min at 4°C. The supernatant was then used for AA analysis using HPLC, using a refrigerated autosampler unit (Beckman Model 508, Fullerton, CA), a C18 Ultrasphere ODS column (4.6 mm × 25 cm; Beckman Coulter), and ultraviolet detection at 254 nm. Gradient elution was performed with 100% mobile phase A (0.05% triethylamine and 1.0% glacial acetic acid in water), changing to 80% mobile phase A and 20% mobile phase B (acetonitrile) over 5 min, followed by isocratic elution with 20% mobile phase B over 10 min, followed by reequilibration. The flow rate was 0.5 ml/min, yielding a retention time for plasma ascorbate of 6.9 min.
For hepatic AA content, 0.1 g of liver was homogenized in 10 volumes of cold 50 mM PCA. The homogenate was centrifuged at 9,300 g for 10 min at 4°C. The supernatant was filtered using 0.45 μm Costar nylon spin-X filter tubes (Corning inc., Corning, NY) under identical centrifugation conditions. To 250 μl of liver tissue supernatant, 50 μl of 50 mM PCA was added, vortexed and incubated on ice for 10 min, and followed by centrifugation at 16,000 g for 8 min at 4°C. The supernatant was again filtered through 0.22 μm costar nylon spin-X filter tubes (Corning inc., Corning, NY) for 5 min at 16,100 g and the filtrate was used for liver AA analysis. The same HPLC method was used as for plasma AA, except for a gradient to 20% mobile phase B over 20 minutes, followed by reequilibration.
Immunoblots for in-vivo drug- adduct detection
SMX-protein adducts were evaluated in peritoneum, serum, and spleen. Peritoneal and splenic tissue lysates were prepared by homogenization of frozen tissues in cold PBS. Protein concentrations were quantified with the method of Lowry (Lowry et al. 1951), using a commercial kit (BioRad, Herculus, CA). A total of 50 μg of tissue or serum proteins were diluted with Laemmli buffer (without mercaptoethanol; Bio-Rad, Hercules, CA), electrophoresed on 12% SDS-polyacrylamide gels, and transferred to polyvinylidene difluoride (PDVF) membranes for immunoblotting. Immunoblotting for drug-tissue adducts was performed with polyclonal rabbit anti-SMX sera (1:200) (Lavergne et al. 2006) or rabbit pre-immune sera (1:200), with horseradish-peroxidase linked (HRP)-labeled anti-rabbit IgG as the secondary antibody (1:2000; Jackson ImmunoResearch Laboratories, West Grove, PA). Protein signals were visualized with an enhanced chemiluminescence (ECL) immunoblotting reagent (Pierce, Rockford, IL) and the image captured using a digital camera (UVP Inc., Upland, CA); densitometry analysis was done using Image J (version 1.38) software from NIH (Abramoff et al. 2004). Drug-tissue adducts for each guinea pig were quantified by subtracting densitometry readings obtained from rabbit pre-immune sera from those obtained with anti-SMX polyclonal sera. Guinea pigs that were not treated with SMX-NO were used as negative controls.
Splenic T-cell proliferation assays for immunogenicity
Spleens were collected on the day of euthanasia (one day after the last dose of SMX-NO or vehicle) for immediate processing. Splenocytes were isolated from individual spleens and equally divided into 3 aliquots of 1 × 106 cells. For the lymphocyte transformation test, splenocyte aliquots were incubated with media only, SMX (1 mM), or SMX-NO (100 μM). Incubations with drug or media were performed for 72 h in 96-well U-bottom cell culture plates at 37°C, 5% CO2, in RPMI-1640 media containing 10% fetal calf serum (Naisbitt et al. 2001). After 72 h, cells were collected, washed, and plated into 96-well plates (2 × 105 cells/well) in triplicate. During the last 16 h, splenocytes were pulsed with [3H] thymidine (0.5 μCi/well) for 16 hours, and T cell proliferation was determined by thymidine uptake (Naisbitt et al. 2001). Briefly, cells were harvested, and incorporated radioactivity was measured in count per minutes (cpm) on a beta counter (PerkinElmer Life Sciences, Cambridge, UK). Proliferative responses were calculated as stimulation indices (SI; cpm in drug-treated cultures/cpm in cultures containing media alone).
Immunoblots for b5 and b5R protein expression
Hepatic microsomal proteins (50 μg) were diluted with Laemmli buffer (without mercaptoethanol), denatured at 99°C for 3 min, electrophoresed on 15% SDS-polyacrylamide gels, and transferred to PDVF membranes for immunoblotting. Membranes were blocked with 5% nonfat dry milk in PBS/0.1% Tween-20, and washed with PBS/0.1% Tween-20. Mouse monoclonal antibody to ß-actin, labeled with HRP (Abcam Inc., Cambridge, MA), was used as a loading control, and was diluted 1:6000 in 1.0% nonfat dry milk and used to probe the membranes for 2 h. After another wash, bands were visualized with the same ECL detection and image capture system as for drug-adduct experiments. The same membrane was re-washed, blocked in 5% nonfat dry milk in PBS/0.1% Tween-20, and washed with PBS/0.1% Tween-20, and re-probed with polyclonal anti-b5 serum (1:10,000) (Kurian et al. 2004) overnight. After washing, HRP-labeled secondary antibody (donkey anti-rabbit IgG; Jackson ImmunoResearch Laboratories, West Grove, PA), diluted 1:10000, was incubated with the membrane for 1 h. Finally, the membrane was washed and the previous antibodies were stripped off using Restore™ PLUS immunoblot stripping buffer (Pierce, Rockford, IL). The membrane was probed with polyclonal anti-b5R (1:10,000) (Kurian et al. 2004), followed by the same secondary antibody system used for b5 imunoblotting. b5 and b5R protein content were expressed in densitometry units normalized to ß-actin.
b5 and b5R mRNA expression
Liver RNA was extracted from control guinea pigs with normal AA intake and no GSH depletion, and from combined (GSH and AA) anti-oxidant deficient guinea pigs, using RNAqueous-4PCR kit (Ambion Inc., Austin, Texas). To remove contaminating DNA, the RNA was DNase-treated using a DNA-Free kit (Ambion, Inc., Austin, Texas). RNA was quantified via optical density readings on an ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE). Because guinea pig b5 and b5R complete gene sequences were not known, primers were first designed to amplify the 405 bp ENSEMBLE predicted guinea pig b5 gene from liver, and the resulting PCR product was sequenced. BLAST analysis of the guinea pig b5 product against the human genome identified homology to human cytochrome b5 type A (microsomal; CYB5A), transcript variant 1 mRNA. For b5R, exon primers specific for the human CYB5R3 gene were used to amplify a 523 bp fragment from guinea pig liver. BLAST analysis of the sequence of the guinea pig product against the human genome matched the human CYB5R3 gene.
Guinea pig primers for real time qPCR were then designed to span b5 and b5R exon-exon junctions using Primer Express (Applied Biosystems, Foster city, CA), with a target annealing temperature of 60°C and product size of less than 150 bp. Both primers sets were designed to detect specifically the microsomal transcripts of each gene (Table 1). From each pig, 2 μg of liver total RNA was reverse transcribed using RETROscript RT (Ambion Inc., Austin, Texas) with oligo dT and random decamer primers to generate cDNA. qPCR reactions were performed in an ABI prism 7500 sequence detection system (Applied Biosystems, Foster City, CA), with 0.5 ng/μl of cDNA and 100nM forward and reverse final primer concentrations, and the Applied Biosystems SYBR Green QPCR Master Mix (Applied Biosystems, Foster city, CA). ß-actin was used as the endogenous control gene. Relative quantification of gene expression was determined after normalizing for ß-actin expression, and computed using the 2-ΔΔCT method (user manual #2, ABI Prism 7700 SDS). In the 2-ΔΔCT analysis, the threshold cycle (CT) from guinea pigs with replete anti-oxidants was used as the calibrator sample. Specific products formed after qPCR were confirmed by a melting curve analysis and by agarose gel analysis of qPCR products.
Table 1.
Primer sequences (5'-3') used in quantitative real-time PCR, and for amplifying full length guinea pig cytochrome b5 and cytochrome b5 reductase cDNAs (microsomal forms).
| Name | Forward primer | Reverse primer | Product size (bp) |
|---|---|---|---|
| Cytochrome b5 (microsomal) | TGTTGGTCATTCTACCGATGC | TGGTCCACCAACTGGAATTAG | 140 bp (spans exons 4 and 6) |
| Cytochrome b5 reductase (microsomal) | CCCAGCTGAGCACGTTG | GCGGGTACTTGATGTCGG | 126 bp (spans exons IM and 2) |
| β-Actin | TTGTTACCAACTGGGACGACATG | GGGTCATCTTCTCACGGTTGG | 116 |
| Cytochrome b5 (microsomal) full length | GGAGGAGCAGGCTGTAAAGTA | TGTAGATGCGATACATCATGG | 380 |
| Cytochrome b5 reductase (microsomal) partial length | CTACCTCTCGGCTCGAATTG | TGAGTTCCTCCAGCTCAGGT | 523 |
Hepatic SMX-HA reduction activities
Microsomes were prepared from livers obtained at euthanasia at the 4 week time point. SMX-HA (500 μM; Dalton Chemical Laboratories, Toronto, Ontario, CA) was incubated with pig liver microsomes (1 mg/ml) in PBS (pH 7.4), with 1 mM ascorbate to prevent further hydroxylamine oxidation. Reactions were started with the addition of 1 mM NADH, and incubated for 15 minutes under conditions approximating linear kinetics; SMX product was measured using HPLC with UV detection (Kurian et al. 2004).
Clinical analyses and histopathology
Blood collected at each time point was used for a CBC and plasma levels of alanine aminotransferese (ALT), alkaline phosphatase, gamma-glutamyl transpeptidase, albumin, blood urea nitrogen, and total bilirubin. At the end of the study, samples of liver, skin, lymph node, bone marrow, spleen, kidney, and lungs were collected from all pigs for histopathology, stained with hematoxylin and eosin, and examined under light microscopy by a board-certified veterinary pathologist (MP), blinded to treatment.
Statistical analysis
Data between and among groups were compared by an unpaired t-test or ANOVA with Bonferroni's post hoc test, as appropriate, using data analysis software (GraphPad Software Inc., San Diego, CA). Correlations between antioxidant concentrations and SMX-HA reduction were performed using a Pearson's correlation coefficient. Significance was set at P < 0.05; all data are reported as mean ± SD. A sample size of 4-5 pigs in each group was designed to provide >88% power to detect a doubling of T cell proliferation as quantified by stimulation index, using variability previously reported in mice treated with SMX-NO (Farrell et al. 2003).
Results
Anti-oxidant deficiency model
Guinea pigs were provided with deficient or replete AA supplementation for 4 weeks. At the end of 4 weeks, antioxidant deficient guinea pigs (group 2) had significantly lower hepatic AA concentrations (22.2 ± 6.3 nmoles/gm,) than replete pigs (75.2 ± 18.5 nmoles/gm; group 1; P < 0.001; Figure 3). This difference was also found for pigs given SMX-NO (hepatic AA in deficient pigs (group 4), 18.2 ± 8.6 nmoles/gm; hepatic AA in replete pigs (group 3), 73.4 ± 19.5 nmoles/gm; P < 0.001; Figure 3), with no differences associated with SMX-NO treatment itself. Plasma AA concentrations were 4.6 ± 4.4 μM in the deficient group (group 2) and 9.0 ± 5.4 μM in the replete group (group 1), but this did not reach significance.
Fig 3.
Scatter plot of hepatic ascorbic acid (AA) concentrations in guinea pigs provided replete or deficient levels of AA intake for 4 weeks. For the last two weeks of the study, deficient pigs were also treated with buthionine sulfoximine (BSO; 250 mg/kg/day SC) to inhibit GSH synthesis. Each data point represents one pig at week 4; horizontal lines indicate the group means. * Indicates significantly different from both replete groups (P < 0.001).
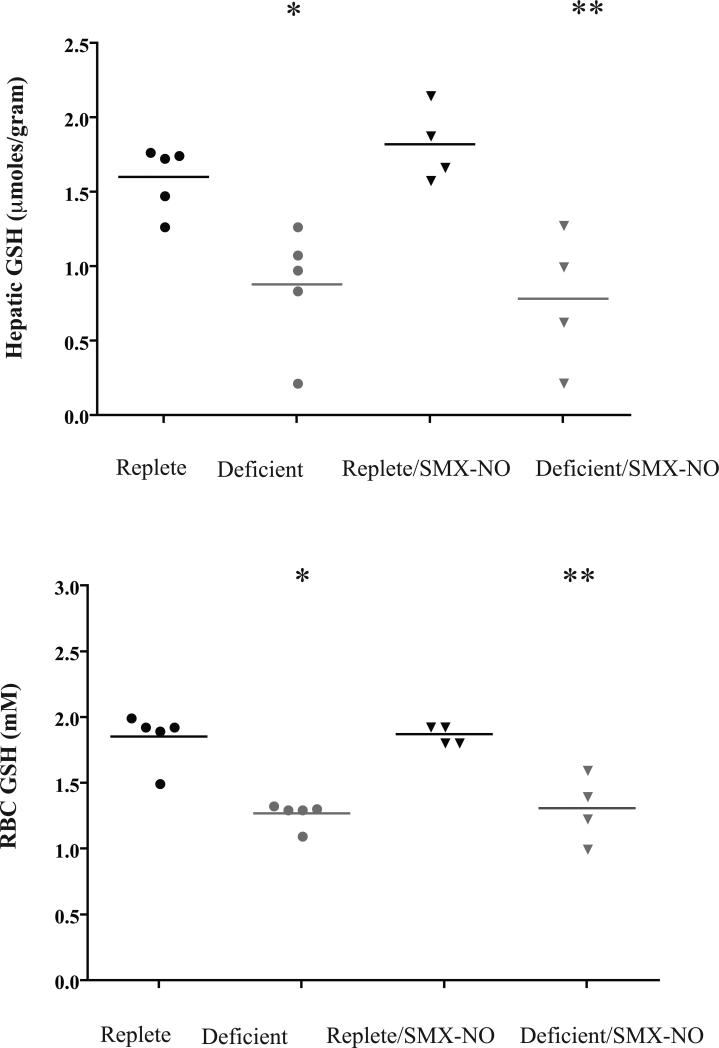
Hepatic GSH concentrations were also significantly lower in the deficient groups, both without and with SMX-NO (Group 2, 0.88 ± 0.40 μmoles/gram; Group 4, 0.78 ± 0.46) compared to respective replete pigs (Group 1, 1.60 ± 0.22 μmoles/gram; Group 3, 1.82 ± 0.25, P = 0.001; Figure 4). Comparable significant differences were also found for erythrocyte GSH between deficient and replete groups (Figure 4).
Fig 4.
Hepatic and red blood cell (RBC) GSH concentrations in guinea pigs depleted of both GSH and AA. Upper panel: Guinea pigs with AA restriction plus BSO treatment (groups 2 and 4) had significantly decreased hepatic GSH concentrations at week 4 compared to replete pigs. * P < 0.05 compared to replete pigs; ** P < 0.01 compared to replete pigs given SMX-NO. Lower panel: Pigs with antioxidant depletion also had significantly decreased RBC GSH concentrations compared to replete pigs. * P < 0.001 compared to replete pigs; ** P < 0.01 compared to replete pigs given SMX-NO.
Clinical toxicity
No histopathologic lesions in the skin, lymph nodes, bone marrow, spleen, kidney, or lungs were associated with SMX-NO administration, compared to respective controls. Antioxidant deficient pigs given SMX-NO had substantial increases in serum ALT activities (693 ± 614 IU/L); however, these were not significantly higher than ALT activities observed in antioxidant deficient pigs without SMX-NO administration (1654 ± 573 IU/L). ALT activities in antioxidant replete pigs were 46 ± 4 IU/L (Group 1) and 46 ± 9 IU/L (Group 3). Increases in ALT were consistent with small foci of hepatic necrosis seen in the livers of some antioxidant deficient pigs. An additional study in 3 pigs restricted in AA intake for 4 weeks (with vehicles only for BSO and SMX-NO; data not shown) suggested that hepatotoxicity was due to BSO administration and/or GSH depletion, and not to AA deficiency alone (ALT 45 ± 9 IU/L, no hepatic necrosis on histopathology).
Drug adducts
Serum, splenic, and peritoneal drug-tissue adducts were detected in all guinea pigs treated with SMX-NO. Serum adducts were observed at 174, 91, and 10 kD; splenic adducts at 67 kD, and peritoneal adducts at 35 kD. There was considerable individual variability in adducts among treated pigs, and no differences in drug-adduct formation could be detected between deficient and replete pigs after treatment with SMX-NO (Figure 5).
Fig 5.
Quantification of SMX-tissue adducts in the (A) serum (175 kD band represented), (B) peritoneum, and (C) spleen of AA and GSH replete (group 3) and deficient (group 4) guinea pigs treated with SMX-NO. Densitometry readings were normalized by subtracting readings obtained with rabbit pre-immune sera. No significant differences in adduct formation could be demonstrated.
Drug-specific T-cell proliferation
Consistent with the lack of difference in drug adduct formation in the spleen, no significant differences in SMX-NO-specific splenocyte proliferation were observed between deficient (SI, 3.6 ± 2.4) and replete (SI, 3.2 ± 2.8; P = 0.12) pigs treated with SMX-NO (Figure 6). In no pigs was the splenocyte response to SMX in vitro increased above vehicle control (SI 1.0 ± 0.34; Figure 6).
Fig 6.
Proliferative response of splenocytes from AA and GSH deficient (group 4) and replete (group 3) guinea pigs treated with SMX-NO. Cells were cultured with SMX-NO (100 μM) in vitro for 72 h; proliferation was measured by incorporation of [3H] thymidine. Data are reported as stimulation index relative to incubation with media alone in vitro. Results represent the mean from four animals in each group, with incubations carried out in triplicate. No response was seen to SMX (1 mM in vitro) over vehicle controls in any pig (last 2 columns on graph).
b5 and b5R expression, and SMX-HA reduction activities
Combined AA and GSH deficiency was associated with decreased immunoreactive hepatic microsomal b5 protein expression (1.4 ± 0.5 densitometry units (normalized to μ-actin), group 2) compared replete guinea pigs (2.6 ± 0.6 for group 1; P = 0.015; Figure 7, upper panel). Apparent decreases in hepatic b5R protein expression were also observed in deficient pigs compared to replete pigs, but this did not reach significance (1.8 ± 0.6 vs. 3.2 ± 1.6; P = 0.10; Figure 7, lower panel). Interestingly, hepatic expression of b5 and b5R immunoreactive proteins was significantly correlated across all treatment groups (r = 0.60, P = 0.009).
Fig 7.
Cytochrome b5 (b5) and b5 reductase (b5R) protein expression in liver microsomes of guinea pigs, measured using immunoblotting. All densitometry readings were normalized to ß-actin as a loading control. (Upper panel): b5 expression was significantly decreased in combined AA and GSH deficient guinea pigs (group 2) compared to anti-oxidant replete guinea pigs (group 1; P = 0.01). (Lower panel): Trend toward reduction in b5R expression in deficient guinea pig liver microsomes (group 2) compared to replete guinea pigs (group 1; P = 0.10).
Expression of the microsomal transcripts of b5 and b5R genes was detected using real time qPCR in guinea pig livers. As found for b5 protein, expression of the microsomal transcript of CYB5A was significantly decreased in deficient pigs (group 2) compared to replete pigs (group 1; P = 0.044 for ΔCT between groups; relative quantity value 0.43). There was no detectable difference in expression of the microsomal transcript of CYB5R3 between deficient and replete groups (P = 0.43 for ΔCT between groups; relative quantity value 0.92).
Hepatic SMX-HA reduction activities were significantly decreased in deficient (2.7 ± 1.1 nmoles/mg/min; group 4) versus replete (5.0 ± 1.6 nmoles/mg/min; group 3) pigs treated with SMX-NO; P = 0.029; Figure 8, upper panel). SMX-HA reduction activities were also weakly but positively correlated with hepatic GSH concentrations across all pigs (r = 0.48, P = 0.04; Figure 8, lower panel).
Fig 8.
SMX-hydroxylamine (SMX-HA) reduction activities in guinea pig liver microsomes, after 4 weeks of differential ascorbate intake and two weeks of treatment with BSO and SMX-NO (or respective vehicles). (Upper panel): Hepatic SMX-HA reduction was significantly decreased in antioxidant deficient versus replete pigs treated with SMX-NO (* P = 0.029 versus replete/SMX-NO group). (Lower panel): Hepatic glutathione concentrations at week 4 were modestly but significantly correlated with reduction of (SMX-HA) to SMX in guinea pig liver microsomes. All 4 groups of guinea pigs are included (n = 18).
Discussion
SMX hypersensitivity is an important clinical problem in humans, particularly in those with AIDS (DiRienzo et al. 2002; Furrer et al. 1999; Goldie et al. 2002; Grimwade and Swingler 2003; Rabaud et al. 2001). SMX is metabolized to a pro-hapten, SMX-HA, in humans and other species (Cribb and Spielberg 1990; Gill et al. 1997). Further auto-oxidation yields SMX-NO (Cribb et al. 1991), which is thought to be a primary immunogen in sulfonamide hypersensitivity. SMX-NO can bind to cell surface proteins, undergo internalization, be presented in association with major histocompatibility complexes, and stimulate metabolite specific T cell clones (Naisbitt et al. 2001; Sanderson et al. 2007).
Antioxidants, such as ascorbate and thiols, reduce SMX-NO non-enzymatically to SMX-HA (Naisbitt et al. 2000; Trepanier et al. 2004). SMX-HA can be further reduced to the parent SMX by the pathway composed of b5 and b5R (Kurian et al. 2004); this may be an important detoxification step, since SMX itself does not form tissue adducts (Naisbitt et al. 2001). Plasma ascorbate and thiol deficiencies in HIV-infected patients lead to decreased capacity for SMX-NO reduction (Naisbitt et al. 2000; Trepanier et al. 2004), and could contribute to increased drug haptenization, drug immunogenicity, and risk of SMX hypersensitivity reactions. In support of this, ascorbate and glutathione have been shown to decrease the formation of SMX-NO adducts in lymphoid cells in vitro (Farrell et al. 2003; Lavergne et al. 2009; Manchanda et al. 2002).
These previous results suggested that antioxidant deficiency could influence the haptenization and immunogenicity of SMX metabolites. Because of the role of both AA and GSH in the clearance of SMX-NO, and given that AA and GSH have interdependent functions (Martensson and Meister 1991), we hypothesized that combined AA and GSH deficiency would lead to increased SMX-NO availability for drug-adduct formation and T cell proliferation. In addition, because ascorbate deficiency had been shown in older studies to decrease cytochrome b5 levels, (Degkwitz et al. 1973) we hypothesized that combined antioxidant deficiency would lead to impaired SMX-HA detoxification via the b5/ b5R pathway.
We tested this hypothesis in an ascorbate-deficient guinea pig model with concurrent GSH deficiency. The guinea pig was chosen because, like humans, this species cannot synthesize ascorbate in vivo, and becomes ascorbate-deficient with dietary restriction. BSO was chosen to deplete GSH because, unlike diethyl maleate, BSO inhibits GSH synthesis (Griffith and Mulcahy 1999). This may more closely reflect the impaired GSH synthesis thought to occur with HIV infection (Choi et al. 2000).
After 2 weeks of BSO treatment, antioxidant depleted guinea pigs had significantly decreased hepatic and RBC GSH concentrations, with mean reductions of 45% and 31%, respectively. This is comparable to studies in HIV-infected patients, which have shown 21 to 42% lower RBC GSH concentrations compared to healthy controls (Bogden et al. 2000; Jahoor et al. 1999; Lang et al. 2001; Trepanier et al. 2004). We used a BSO dosage (250 mg/kg) that was previously shown to deplete hepatic glutathione in rodents by about 40% (Yu and Brown 1984), and found similar reductions in our study.
After 4 weeks on differential AA intake, hepatic AA concentrations were significantly lower (by more than 70%) in the deficient guinea pigs compared to the replete groups. Although mean plasma AA concentrations were also apparently lower in the deficient group (4.5 μM versus 9.0 μM), this difference was not significant. Our study was likely underpowered for this outcome, given the variability observed in plasma AA among individual pigs.
Deficiency of both AA and GSH, followed by challenge with SMX-NO, did not result in any clinical, biochemical, or histopathologic signs of SMX hypersensitivity. Previous studies in other rodents and in rabbits have also been unsuccessful in creating a systemic model of SMX hypersensitivity (Farrell et al. 2003; Naisbitt et al. 2001). Even in rats depleted of GSH with diethyl maleate and then challenged with either SMX or SMX-HA, signs of sulfonamide hypersensitivity or tissue damage were not observed (Naisbitt et al. 2001).
In our study, drug-proteins adducts were detected by immunoblotting in the peritoneal tissues of SMX-NO treated guinea pigs, which was expected considering the intra-peritoneal route of administration and the reactive nature of SMX-NO. Serum drug-adducts were also observed, indicating that some SMX-NO was absorbed systemically, and in the spleen, from which cells were isolated for SMX-NO-specific T cell proliferation. Drug-tissue adducts that were detected in the spleen were ~67 kD, consistent with the finding of an approximately 70 kD protein adducted by SMX-NO in the spleen and lymph nodes of mice; this protein target was found to be predominantly albumin (Cheng et al. 2008).
We hypothesized that combined AA and GSH deficiency would increase detectable drug-adduct formation due to impaired reduction of SMX-NO and increased availability for drug-tissue binding. However, there were no differences in adducts between deficient and replete groups given SMX-NO. One possibility is that our antioxidant depletion was inadequate; however, we reached blood glutathione concentrations (~ 1.3 mM) that were comparable to those reported in patients with AIDS (1.6 mM) (Bogden et al. 2000). Our SMX antisera was generated against SMX-KLH antigen in rabbits. These antibodies detect the SMX moiety of the drug-protein adduct, and this appears to be independent of the protein adducted (i.e. does not require the presence of KLH, and will react with SMX-human serum albumin conjugate, but not human serum albumin itself). (Lavergne et al. 2006) A recent in vivo rodent study found that the immunogenicity of SMX-NO is independent of the protein that is adducted. (Cheng et al. 2008) Therefore, our antibodies should have been able to detect various types of adducts. It is possible that a more sensitive method for adduct detection, such as flow cytometry (Naisbitt et al. 2001), would have been better able to detect differences between groups.
Splenocytes isolated from our guinea pigs treated with SMX-NO proliferated in response to ex-vivo SMX-NO stimulation, as has been shown previously for other species (Farrell et al. 2003; Naisbitt et al. 2001); however, combined AA and GSH deficiency did not lead to enhanced T cell proliferation. In rats treated with diethyl maleate, GSH depletion was associated with weakly enhanced immunogenicity of SMX-HA in vivo; SMX-NO was not tested under these conditions (Naisbitt et al. 2001). It is possible that larger numbers of animals might have demonstrated a difference in SMX-NO immunogenicity between groups in our study, or that evaluating T cell proliferation from lymph nodes, or at alternative time points after the last SMX-NO dose, would have produced positive results. After our study was near completion, another report showed that SMX-NO-dependent proliferation of lymphocytes from inguinal lymph nodes was higher than that from splenocytes in mice treated by the IP route, and that waiting 4 days after the last dose of SMX-NO lead to enhanced responses (Cheng et al. 2008). In our study, guinea pigs were sacrificed 24 hours after the last dose of SMX-NO.
Hepatic b5 and b5R protein expression were significantly correlated across all treatment groups in our study, which suggests co-regulation of these two proteins. Combined AA and GSH deficiency was associated with a significant reduction in hepatic b5 protein (by more than 40%) and mRNA expression (relative quantity value 0.43). This finding is consistent with older studies in which prolonged AA deficiency in guinea pigs resulted in 40-50% decreases in hepatic b5 protein, as measured by spectrophotometry (Degkwitz and Kim 1973; Degkwitz et al. 1973). Our results suggest down-regulation of b5 expression at the transcriptional or pre-translational level.
We further found that hepatic SMX-HA reduction activity, which is catalyzed by the b5/b5R pathway (Kurian et al. 2004), was significantly decreased in antioxidant deficient versus replete pigs that were treated with SMX-NO. We also found that hepatic SMX-HA reduction activity was modestly but significantly correlated with hepatic GSH depletion, with an approximately 7-fold range in reduction activities across treatment groups. Additional studies are underway in our laboratory to more fully characterize the transcriptional regulation of both b5 and b5R, and its relationship to antioxidant deficiency.
In conclusion, in this model of combined ascorbate and glutathione deficiency, there was no discernible effect on drug-adduct formation or T cell proliferation in response to SMX-NO. However, ascorbate and glutathione depletion was associated with decreased hepatic expression of both b5 mRNA and protein, and decreased SMX-HA reduction activities. Since both GSH and AA deficiencies have been documented in HIV-infected patients (Jahoor et al. 1999; Lang et al. 2001; Trepanier et al. 2004; Walmsley et al. 1997), down regulation of hydroxylamine reduction by the b5/b5R pathway could contribute to the risk of SMX hypersensitivity in these patients.
Acknowledgements
This study was funded by the National Institutes of Health through the National Institute of General Medical Sciences, grant GM61753. The authors would like to thank Alexandra Reynolds and Kendall Wagener for technical assistance.
Abbreviations
- AA
ascorbic acid
- b5
cytochrome b5
- b5R
cytochrome b5 reductase
- BSO
buthionine sulfoximine
- GSH
glutathione
- RBC
red blood cell
- SMX
sulfamethoxazole
- SMX-HA
sulfamethoxazole hydroxylamine
- SMX-NO
sulfamethoxazole-nitroso
Reference list
- Abramoff MD, Magalhaes PJ, Ram SJ. Image Processing with ImageJ. BIOPHOTONICS INTERNATIONAL. 2004;11:36–43. [Google Scholar]
- Bogden JD, Kemp FW, Han S, Li W, Bruening K, Denny T, Oleske JM, Lloyd J, Baker H, Perez G, Kloser P, Skurnick J, Louria DB. Status of selected nutrients and progression of human immunodeficiency virus type 1 infection. Am J Clin Nutr. 2000;72:809–815. doi: 10.1093/ajcn/72.3.809. [DOI] [PubMed] [Google Scholar]
- Carr A, Gross AS, Hoskins JM, Penny R, Cooper DA. Acetylation phenotype and cutaneous hypersensitivity to trimethoprim-sulphamethoxazole in HIV-infected patients. Aids. 1994;8:333–337. doi: 10.1097/00002030-199403000-00006. [DOI] [PubMed] [Google Scholar]
- Cheng L, Stewart BJ, You Q, Petersen DR, Ware JA, Piccotti JR, Kawabata TT, Ju C. Covalent binding of the nitroso metabolite of sulfamethoxazole is important in induction of drug-specific T-cell responses in vivo. Mol Pharmacol. 2008;73:1769–1775. doi: 10.1124/mol.107.043273. [DOI] [PubMed] [Google Scholar]
- Choi J, Liu RM, Kundu RK, Sangiorgi F, Wu W, Maxson R, Forman HJ. Molecular mechanism of decreased glutathione content in human immunodeficiency virus type 1 Tat-transgenic mice. J Biol Chem. 2000;275:3693–3698. doi: 10.1074/jbc.275.5.3693. [DOI] [PubMed] [Google Scholar]
- Council NR. Nutrient Requirements of Laboratory Animals. National Academies Press; 1995. NUTRIENT REQUIREMENTS OF THE GUINEA PIG. pp. 103–124. [PubMed] [Google Scholar]
- Cribb AE, Miller M, Leeder JS, Hill J, Spielberg SP. Reactions of the nitroso and hydroxylamine metabolites of sulfamethoxazole with reduced glutathione. Implications for idiosyncratic toxicity. Drug Metab Dispos. 1991;19:900–906. [PubMed] [Google Scholar]
- Cribb AE, Spielberg SP. Hepatic microsomal metabolism of sulfamethoxazole to the hydroxylamine. Drug Metab Dispos. 1990;18:784–787. [PubMed] [Google Scholar]
- Degkwitz E, Kim KS. Comparative studies on the influence of L-ascorbate, D-arabino-ascorbate and 5-oxo-D-gluconate on the amounts of cytochromes P-450 and b5 in liver microsomes of guinea pigs. Hoppe Seylers Z Physiol Chem. 1973;354:555–561. doi: 10.1515/bchm2.1973.354.1.555. [DOI] [PubMed] [Google Scholar]
- Degkwitz E, Walsch S, Dubberstein M, Winter J. Influence of L-ascorbate on the contents of cytochromes P-450 and b5 in several organs of guinea pigs. Enzyme. 1973;16:237–245. doi: 10.1159/000459386. [DOI] [PubMed] [Google Scholar]
- DiRienzo AG, van der Horst C, Finkelstein DM, Frame P, Bozzette SA, Tashima KT. Efficacy of Trimethoprim-Sulfamethoxazole for the Prevention of Bacterial Infections in a Randomized Prophylaxis Trial of Patients with Advanced HIV Infection. AIDS Research and Human Retroviruses. 2002;18:89–94. doi: 10.1089/08892220252779629. [DOI] [PubMed] [Google Scholar]
- Farrell J, Naisbitt DJ, Drummond NS, Depta JP, Vilar FJ, Pirmohamed M, Park BK. Characterization of sulfamethoxazole and sulfamethoxazole metabolite-specific T-cell responses in animals and humans. J Pharmacol Exp Ther. 2003;306:229–237. doi: 10.1124/jpet.103.050112. [DOI] [PubMed] [Google Scholar]
- Furrer H, Egger M, Opravil M, Bernasconi E, Hirschel B, Battegay M, Telenti A, Vernazza P, Rickenbach M, Flepp M, Malinverni R. Discontinuation of primary prophylaxis against PCP in HIV1-infected adults treated with combination antiretroviral therapy. Swiss HIV Cohort Study. N Engl J Med. 1999;340:1301–1306. doi: 10.1056/NEJM199904293401701. [DOI] [PubMed] [Google Scholar]
- Gill HJ, Hough SJ, Naisbitt DJ, Maggs JL, Kitteringham NR, Pirmohamed M, Park BK. The relationship between the disposition and immunogenicity of sulfamethoxazole in the rat. J Pharmacol Exp Ther. 1997;282:795–801. [PubMed] [Google Scholar]
- Goldie S, Kaplan J, Losina E, Weinstein M, Paltiel A, Seage G, Craven D, Kimmel A, Zhang H, Cohen C, Freedberg K. Prophylaxis for human immunodeficiency virus-related Pneumocystis carnii pneumonia. Arch Intern Med. 2002;162:921–928. doi: 10.1001/archinte.162.8.921. [DOI] [PubMed] [Google Scholar]
- Gordin FM, Simon GL, Wofsy CB, Mills J. Adverse reactions to trimethoprimsulfamethoxazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1984;100:495–499. doi: 10.7326/0003-4819-100-4-495. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Mulcahy RT. The enzymes of glutathione synthesis: gamma-glutamylcysteine synthetase. Adv Enzymol Relat Areas Mol Biol. 1999;73:209–267. xii. doi: 10.1002/9780470123195.ch7. [DOI] [PubMed] [Google Scholar]
- Grimwade K, Swingler G. Cotrimoxazole prophylaxis for opportunistic infections in adults with HIV. Cochrane Database Syst Rev. 2003;3:CD003108. doi: 10.1002/14651858.CD003108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahoor F, Jackson A, Gazzard B, Philips G, Sharpstone D, Frazer ME, Heird W. Erythrocyte glutathione deficiency in symptom-free HIV infection is associated with decreased synthesis rate. Am J Physiol. 1999;276:E205–211. doi: 10.1152/ajpendo.1999.276.1.E205. [DOI] [PubMed] [Google Scholar]
- Jick H. Adverse reactions to trimethoprim-sulfamethoxazole in hospitalized patients. Rev Infect Dis. 1982;4:426–428. doi: 10.1093/clinids/4.2.426. [DOI] [PubMed] [Google Scholar]
- Koch-Weser J, Sidel VW, Dexter M, Parish C, Finer DC, Kanarek P. Adverse reactions to sulfosoxazole, sulfamethoxazole, and nitrofurantoin. Manifestations and specific reaction rates during 2,118 courses of therapy. Arch Intern Med. 1971;128:399–404. [PubMed] [Google Scholar]
- Kurian JR, Bajad SU, Miller JL, Chin NA, Trepanier LA. NADH cytochrome b5 reductase and cytochrome b5 catalyze the microsomal reduction of xenobiotic hydroxylamines and amidoximes in humans. J Pharmacol Exp Ther. 2004;311:1171–1178. doi: 10.1124/jpet.104.072389. [DOI] [PubMed] [Google Scholar]
- Lang CA, Huang A, Ramirez JA, Liu MC. Erythrocytic glutathione and plasma cysteine status of human immunodeficient patients. Exp Biol Med (Maywood) 2001;226:866–869. doi: 10.1177/153537020122600910. [DOI] [PubMed] [Google Scholar]
- Lavergne SN, Danhof RS, Volkman EM, Trepanier LA. Association of drug-serum protein adducts and anti-drug antibodies in dogs with sulphonamide hypersensitivity: a naturally occurring model of idiosyncratic drug toxicity. Clin Exp Allergy. 2006;36:907–915. doi: 10.1111/j.1365-2222.2006.02506.x. [DOI] [PubMed] [Google Scholar]
- Lavergne SN, Wang H, Callan HE, Park BK, Naisbitt DJ. “Danger” conditions increase sulfamethoxazole-protein adduct formation in human antigen presenting cells. J Pharmacol Exp Ther Epub ahead of print. 2009 doi: 10.1124/jpet.109.155374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Manchanda T, Hess D, Dale L, Ferguson SG, Rieder MJ. Haptenation of sulfonamide reactive metabolites to cellular proteins. Mol Pharmacol. 2002;62:1011–1026. doi: 10.1124/mol.62.5.1011. [DOI] [PubMed] [Google Scholar]
- Martensson J, Meister A. Glutathione deficiency decreases tissue ascorbate levels in newborn rats: ascorbate spares glutathione and protects. Proc Natl Acad Sci U S A. 1991;88:4656–4660. doi: 10.1073/pnas.88.11.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina I, Mills J, Leoung G, Hopewell PC, Lee B, Modin G, Benowitz N, Wofsy CB. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med. 1990;323:776–782. doi: 10.1056/NEJM199009203231202. [DOI] [PubMed] [Google Scholar]
- Naisbitt DJ, Farrell J, Gordon SF, Maggs JL, Burkhart C, Pichler WJ, Pirmohamed M, Park BK. Covalent binding of the nitroso metabolite of sulfamethoxazole leads to toxicity and major histocompatibility complex-restricted antigen presentation. Mol Pharmacol. 2002;62:628–637. doi: 10.1124/mol.62.3.628. [DOI] [PubMed] [Google Scholar]
- Naisbitt DJ, Gordon SF, Pirmohamed M, Burkhart C, Cribb AE, Pichler WJ, Park BK. Antigenicity and immunogenicity of sulphamethoxazole: demonstration of metabolism-dependent haptenation and T-cell proliferation in vivo. Br J Pharmacol. 2001;133:295–305. doi: 10.1038/sj.bjp.0704074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbitt DJ, Hough SJ, Gill HJ, Pirmohamed M, Kitteringham NR, Park BK. Cellular disposition of sulphamethoxazole and its metabolites: implications for hypersensitivity. Br J Pharmacol. 1999;126:1393–1407. doi: 10.1038/sj.bjp.0702453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbitt DJ, Vilar FJ, Stalford AC, Wilkins EG, Pirmohamed M, Park BK. Plasma cysteine deficiency and decreased reduction of nitrososulfamethoxazole with HIV infection. AIDS Res Hum Retroviruses. 2000;16:1929–1938. doi: 10.1089/088922200750054657. [DOI] [PubMed] [Google Scholar]
- Rabaud C, Charreau I, Izard S, Raffi F, Meiffredy V, Leport C, Guillemin F, Veni P, Aboulker J. Adverse reactions to cotrimoxazole in HIV-infected patients: predictive factors and subsequent HIV disease progression. Scand J Infect Dis. 2001;33:759–764. doi: 10.1080/003655401317074581. group Dt. [DOI] [PubMed] [Google Scholar]
- Reilly TP, Lash LH, Doll MA, Hein DW, Woster PM, Svensson CK. A role for bioactivation and covalent binding within epidermal keratinocytes in sulfonamide-induced cutaneous drug reactions. J Invest Dermatol. 2000;114:1164–1173. doi: 10.1046/j.1523-1747.2000.00985.x. [DOI] [PubMed] [Google Scholar]
- Sanderson JP, Naisbitt DJ, Farrell J, Ashby CA, Tucker MJ, Rieder MJ, Pirmohamed M, Clarke SE, Park BK. Sulfamethoxazole and its metabolite nitroso sulfamethoxazole stimulate dendritic cell costimulatory signaling. J Immunol. 2007;178:5533–5542. doi: 10.4049/jimmunol.178.9.5533. [DOI] [PubMed] [Google Scholar]
- Trepanier LA, Yoder AR, Bajad S, Beckwith MD, Bellehumeur JL, Graziano FM. Plasma ascorbate deficiency is associated with impaired reduction of sulfamethoxazolenitroso in HIV infection. J Acquir Immune Defic Syndr. 2004;36:1041–1050. doi: 10.1097/00126334-200408150-00007. [DOI] [PubMed] [Google Scholar]
- Walmsley SL, Khorasheh S, Singer J, Djurdjev O. A randomized trial of N-acetylcysteine for prevention of trimethoprim-sulfamethoxazole hypersensitivity reactions in Pneumocystis carinii pneumonia prophylaxis (CTN 057). Journal of Acquired Immunodeficiency Syndromes and Human Retrovirology. 1998;19:498–505. doi: 10.1097/00042560-199812150-00009. [DOI] [PubMed] [Google Scholar]
- Walmsley SL, Winn LM, Harrison ML, Uetrecht JP, Wells PG. Oxidative stress and thiol depletion in plasma and peripheral blood lymphocytes from HIV-infected patients: toxicological and pathological implications. Aids. 1997;11:1689–1697. doi: 10.1097/00002030-199714000-00005. [DOI] [PubMed] [Google Scholar]
- Yu NY, Brown JM. Depletion of glutathione in vivo as a method of improving the therapeutic ratio of misonidazole and SR 2508. Int J Radiat Oncol Biol Phys. 1984;10:1265–1269. doi: 10.1016/0360-3016(84)90330-4. [DOI] [PubMed] [Google Scholar]