Abstract
Background and purpose
Few detailed studies about the correlations among the expanded prevalence, elevated function of Treg cells in tumor microenvironment of hepatocellular carcinoma (HCC), and different clinical tumor stage were reported. The purpose of the present study was to examine the presence and functions of CD4+CD25high regulatory T cell (Treg cell) in tumor microenvironment from early and late stages and reveal the potential underlying mechanisms that may be responsible.
Method
The prevalence of Treg in peripheral blood and fresh tissue samples from 31 patients with HCC after radical hepatectomy and 9 controls was detected. CD127 was selected as a Treg cell maker to test the cell populations and compared its expressions with ICOS. The expressions of FOXP3 mRNA were analyzed. The migration, proliferation, and suppression functions of Treg cell were observed. IFN-γ., IL-10, TGF-ß, CCL-17, CCL-22, and SDF-1 in cell supernatant were detected. Among all of the tests, the relations among the different TNM tumor stages, populations, and functions of Treg cells were evaluated.
Results
The prevalence of Treg cell was significantly higher in the peripheral blood and in tumor tissue compared with those in normal donors. Increased numbers of Treg cell were showed in peripheral blood as well as in tumor tissue. High levels of IL-10 and TGF-ß, but little IFN-γ, were detected in the tumor microenvironment. Treg cells potently suppressed the functions and proliferation of CD4+CD25− T cells. High levels of SDF-1 were detected in malignant biopsies compared with those in benign regions, significantly increased in stage III. Plasma from the same patient was able to chemoattract Treg cell but that was lesser extent than those in tumor supernatant. Also, supernatant in advanced stage tumors exhibited powerful chemoattractic activity. SDF-1 played an important role in the recruited functions of Treg cell into tumor microenvironment of early and advanced stages. The expressions of Foxp3 mRNA increased in different TNM stages. The increased prevalence and expanded function of Treg cells in the tumor microenvironment of HCC were correlated with the cancer stage.
Conclusion
The increase in frequency of Treg cells might play a role in modulation of the immune response against HCC in different TNM stages. The substance secreted in tumor microenvironment recruited CD4+CD25+ Treg cells to tumor sites to contribute to the prosperity and growth of the tumors. The performance of Treg cells in different TNM stages of tumor microenvironment might be acted as the route to evaluate the immunotherapy-based methods, promote therapy effect, and consequently to increase the survival rate in HCC.
Keywords: Hepatocellular carcinoma, Regulatory T cell, Tumor microenvironment
Introduction
HCC is the fifth most common solid tumor in the world (Parkin et al. 2001), and the highest incidence of HCC is seen in China (Hao et al. 2003). It is a critical disease due to late presentation with large tumors, and lack of medical expertise and facilities. Immunotherapy has emerged as an alternative therapeutic approach in recent years; however, significant objective clinical response rates of the immunotherapy are low (Takayama et al. 2000), because the most tumor-associated antigens (Ags) are self-Ags and are expressed either during tumor development or in normal adult tissue. Homeostatic Tregs mediate immune tolerance to self-antigens by suppressing auto reactive immune cells simultaneously (Asano et al. 1996; Dieckmann et al. 2001). It may also suppress the immune response against cancer in homeostasis (Nishikawa et al. 2005; Piccirillo and Thornton 2004; Sakaguchi 2000). CD4+CD25high Treg cell prevents anti-tumor immunity by the suppression of self-reactive T cell and may also suppress the immune response against cancer cells (Maloy and Powrie 2001; O’Garra and Vieira 2004). Recently, accumulating evidence suggested that the amount of Treg cells increased in the peripheral blood and tumor microenvironment in different types of cancers including pancreas, breast, liver, and prostate cancers. Many chemokines secreted from the tumor cell and microenvironment had been reported to have strong chemotactic effect on Treg cells. For example, CCL22 produced by the macrophages in ovarian carcinoma could mediate Treg trafficking to the tumor site (Curiel et al. 2004). Miller et al. (2006) demonstrated that some PC cell lines, the ascites fluid from patients with malignant prostate cancer, and prostate tumor biopsies in culture contain or secrete CCL22 and can chemoattract Treg cells in an in vitro migration assay. Linhua Zou et al. (2004) showed the recruitment of Tregs into bone marrow through CXCL12. And the cytokines in the tumor microenvironment that may attract the Treg cells still need to extensive study. It has been suggested that the excessive presence of Treg cells can explain the poor clinical efficacy of immunotherapeutic effects in human tumors (Curiel et al. 2004; Liyanage et al. 2002; Miller et al. 2006; Ormandy et al. 2005), and the presence of increased numbers of Tregs may predict for reduced survival rate in late ovarian cancer (Curiel et al. 2004). Targeting on Treg cells therefore provides an attractive therapeutic strategy to support anti-tumor therapy (Greten and Jaffee 1999). But the correlations among the elevated prevalence and function of Treg cell in tumor microenvironment of HCC and TNM stage, and the cellular mechanisms that may be responsible for this phenomenon are still largely unknown.
In this study, we were able to gain access to blood and fresh HCC tissue samples to examine the presence and functions of CD4+CD25high T cells in tumor site of HCC at different TNM stages and test the secretion levels of cytokines in the tumor microenvironment to find the potential candidates for the chemotactic response of CD4+CD25high T cells in tumor site of patients with HCC.
Materials and methods
Patients and normal donors
Peripheral blood and fresh tissue samples of benign and malignant portions of 31 patients with HCC after radical hepatectomy were obtained. Blood samples were also obtained from 9 normal healthy volunteers. HCC TNM stage was identified following the standard of UICC-TNM classification (2002). Of all the 31 patients, 14 carried TNM stage III. None of these patients had received any hormonal, immunosuppressive, or radiation therapy before hepatectomy. The study was approved by the local ethics committee and China Anti-Cancer Association. The nature of the study was explained fully to the patients, and informed consent was obtained from all. The patient characteristics were listed in Table 1.
Table 1.
Clinical characteristics of patients with HCC
| Characteristic | Number |
|---|---|
| Mean age (years) | 62 (46–71) |
| Sex | |
| Male | 22 |
| Female | 9 |
| Child-Turcotte-Pugh classification | |
| A | 27 |
| B | 4 |
| Alpha_-fetoprotein level | |
| ≥1000 ng/mL | 18 |
| ≤1000 ng/mL | 13 |
| Tumor number | |
| Single | 14 |
| Multiple | 17 |
| Hepatitis status | |
| Hepatitis B | 26 |
| Hepatitis C | 5 |
| TNM stage | |
| Stage I | 6 |
| Stage II | 11 |
| Stage III | 14 |
| Total no. | 31 |
Blood and liver sample preparation
Blood samples were collected in sterile heparinized vials. Peripheral blood mononuclear cells (PBMC) (HCC and normal) were isolated by centrifugation on Ficoll-Paque (Pharmacia). The T cells were harvested and washed in PBS without calcium and magnesium (PBS, pH 7.2), and resuspended in EMBM for further analysis. The tumor tissue was kept on ice immediately after operation. The absence of tumor cells along the parenchyma transaction line was confirmed histologically. Tissue samples (benign and malignant tissue) were cut into small 23-mm pieces and placed in PBS, and then incubated in 1 ml of EMBM medium containing 10% human serum albumin (Pharmacia) in a 96-well plate to obtain single cell suspensions. After 48 h, tumor infiltrate lymphocytes (TILs) that outgrown from the tissue were collected for further analysis.
Cell lines
The human hepatic cancer cell line HepG2 was maintained in RPMI 1640 medium supplemented with 10% FCS, 2 mm/l-glutamine, and 25 μg/ml gentamicin.
Cell isolation and sorting
PBMCs were further separated using the regulatory T-cell CD4+CD25+ bead selection kit. (Miltenyi Biotec) according to the manufacturer’s instructions. Enriched cells were >90% pure as determined by flow cytometry. For sorting of the cells, CD4+ T cells were purified using the CD4+ T cell isolation kit and sorted into CD4+CD25+ and CD4+CD25− cells using the cell sorting system (Becton–Dickinson, Heidelberg, Germany). The purity of the cells after sorting was 98–99% as determined by flow cytometry.
FACS analysis
To determine the frequency and phenotype of Treg cells, Four-color flow cytometry was performed on a FACS Calibur (BD Biosciences) with the following markers: CD3-FITC, CD4-PerCP, CD25-allophycocyanin, or ICOS-PE with corresponding isotype-matched controls (BD Biosciences). CD127 staining was conducted according to the manufacturer’s protocol (BD Bioscience). To determine the percentage of Tregs, lymphocytes were gated by plotting forward vs side scatter followed by gating on CD3+CD4+ T cells, and these cells were then analyzed for the expression of CD25. The expressions of ICOS and CD127 inside the CD4+CD25+ T cells gate were analyzed.
ELISA
The concentrations of IFN-γ, TGF-β, IL-10, SDF-1 CCL-17, and CCL-22 in culture supernatant of benign and malignant tissue were measured by using ELISA kits (BioSource International, Camarillo, CA).
Real-time PCR
The expressions of Foxp3mRNA were analyzed by real-time PCR. Total RNA was isolated from dissected tissues using the RNeasy Mini kit (Qiagen, Germany). RNA was isolated following the protocol. RNA samples were quantified using an ® Ultrospec™ 1100 Pro UV–Visible Spectrophotometer (Amersham Biosciences) and diluted to the same concentration. Reverse transcription was done on 1 μg of total RNA using the Reverse Transcription System (Invitrogen Life Technologies, Karlsruhe, Germany). Transcript levels of Foxp3 and GAPDH were quantified using real-time quantitative PCR for the following primers: Foxp3 forward 5′-CAG CAC ATT CCC AGA GTT CCT C3′ and reverse 5′-GCG TGT GAA CCA GTG GTA GAT C3′ and GAPDH forward 5′-TGTTGCCATCA ATGA- CCCC-3′ and reverse 5′-ACTCCACGA CGT AC TCAGCG-3′. Target cDNA was quantified using the ΔΔ(ct) method. Results were shown as relative levels of Foxp3 mRNA.
Immunosuppression and proliferation assay in vitro
The suppressive function was assessed. CD4+CD25+ and CD4+CD25− T cells were isolated from the peripheral blood of patients using the bead selection kit as described above. To determine the suppressive and proliferation ability of Treg cell, cocultures of CD4+CD25− T cells with increasing concentrations of CD4+CD25+ Treg cells were incubated with anti-CD3- and anti-CD28-coated 96-well plates. After 48 h of coculture, the concentrations of IFN-γ were measured. Proliferation was measured after 72 h by 3H incorporation. [3H]thymidine (Amersham, Freiburg, Germany) was added to the cultures, and cell proliferation was measured by incorporation of radiolabeled thymidine for 16–18 h. Incorporated radioactivity was measured using a scintillation counter (Wallac, Turku, Finland).
Migration assay in vitro
Migration of CD4+CD25+ Treg cells was allowed to proceed in chemotaxis chambers (NeuroProbe). Briefly, lower chambers of plates were filled with 500 μl of cell supernatant (malignant, benign tissue and HepG2), plasma, or migration medium (phenol red-free RPMI 1640 plus 0.1% human serum albumin) with or without chemokines (rhSDF-1α, 0.1–100 ng/mL, R&D system,USA). Anti-CXCR4Ab (a specific inhibition of SDF-1 receptor) (1–500 μg/mL, MAB171, R&D system, USA) was added to the cell supernatant of malignant tissue (stages I–III HCC) in dose depends to observe the inhibition of CD4+CD25+ Treg cell migration in different tumor stages. A 5-μm pore polyvinylpyrrolidine-free polycarbonate filter (NeuroProbe) was then placed over the plate, 5 × 104 Treg cells in 100 μl were added to the top chamber, and migration was allowed to proceed at 37°C. After 4 h, the migrated cells were counted microscopically (×400) in five different fields per filter.
Statistical analysis
All data are mean ± SEM. Statistical analysis was performed using ANOVA, q-test, and x 2 test. All analyses were performed using SPSS13.0 software.
Results
Increased population of CD4+CD 25high Treg cells
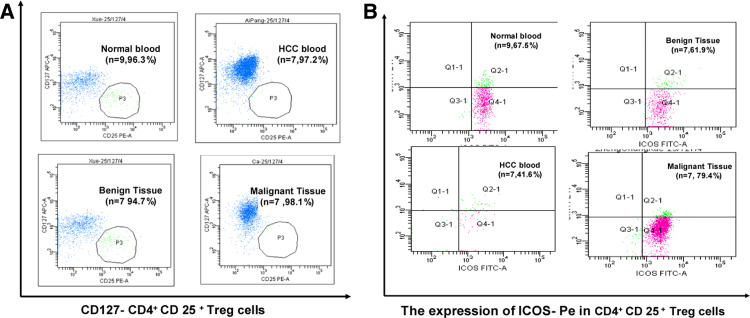
The population of CD4+CD25high Treg cells in total tumor-infiltrating lymphocyte (TIL) was identified by labeling cell with CD4 and CD25, two cell surface markers that can be used as the stringent gating criteria (Fig. 1a). Individual frequencies of CD4+CD25high Treg cells as well as the cumulative data for all the patients in different tumor stages and healthy donors were represented as scatter plots and histograms (Fig. 1b, c). Our results showed that although there was significant difference between the prevalence of CD4+CD25high Treg cells in the peripheral blood from normal patients and that from patients with HCC, the percentage of CD4+CD25high Treg cells in benign tissue was significantly lower than that in malignant tissue, and significant increase in the prevalence of CD4+CD25high Treg cells could be detected when the HCC progressed from stages I–II to a stage III. The data revealed that the increased prevalence of CD4+CD25high Treg cells in the TIL of patients with HCC was closely related to the malignant progress of HCC.
Fig. 1.
Increased population of CD4+CD25high Treg cell in the peripheral blood and tumor tissue sample of patients with HCC. a Representative flow cytometry plots of CD4+CD25high T cells (p2 region) in peripheral blood from normal donors and patients with HCC at the benign and malignant stages (n = 31 for HCC, n = 9 for normal). b Histogram demonstrates the statistic results of the percentage of CD4+CD25high Treg cells in peripheral blood and the tissue samples and plotted as means ± s.e (n = 31 for HCC, n = 9 for normal, *p < 0.05). c Scatter plot showing the percentage of CD4+CD25high Treg cells in peripheral blood or tumor tissue from all samples of normal donor and patient with HCC at different TNM stages (n = 9 for normal blood, n = 8 for HCC blood, n = 6 for stage I, n = 11 for stage II, n = 14 for stage III HCC). The bar represents the mean value of each sample group. The percentage of CD4+CD25high Treg cells in peripheral blood of patients with HCC was significantly higher than that in normal donors (*p < 0.05), and increased in malignant tissue samples when compared with the benign tissue samples (*p < 0.05) and also showed significantly increase at stage III when compared with stages I and II (**p < 0.01, *p < 0.05)
Phenotypes of the CD4+CD25high Treg cells
Having revealed the phenomenon that increased population of CD4+CD25high Treg cells in tumor sites at different TNM stage of HCC, We studied both ICOS and CD127 additional cell markers by gating on the CD4+CD25high Treg cell. They were present as follows: surface expression of ICOS was similar to levels of expression between T cells in peripheral blood of normal donors versus in benign tissue of patients with HCC and between T cells from benign vs malignant tissue. The expressions of ICOS in peripheral blood were lower than those in the tissue samples. High levels of expression of CD127 were detected in CD4+CD25high Treg cells in all samples (Fig. 2a, b). Our study revealed that the CD4+CD25high Treg cells in patients with HCC and healthy donors were phenotypically similar to Treg cell described previously in other studies.
Fig. 2.
Increased population of in CD4+ CD 25 high Treg cells expressing CD127− and ICOS in tumor tissues of patients with HCC. Representative flow cytometry plots showing the mean percentage of CD4+CD25high Treg cells that co-expressing: a CD127−(p3 regions), b ICOS (Q2-1 regions), in the peripheral blood from normal donors (n = 9) and patients with HCC (n = 7), or from benign (n = 7) and malignant HCC tissues (n = 7). High levels of expression of CD127–(97.6%) were detected in CD4+CD25high Treg cells in all samples. The surface expression of ICOS was similar to levels of expression between Treg cells in peripheral blood of normal donors versus in benign tissue and between Treg cells from benign vs malignant tissue. The expressions of ICOS in peripheral blood were lower than those in the tissue samples
Proliferation and immune suppression test
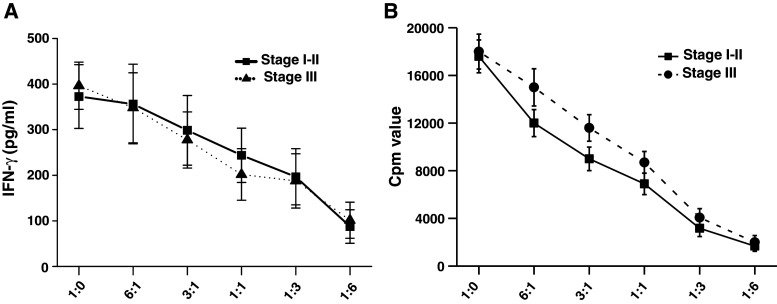
Since the prevalence of CD4+25+ Treg cells increased in the TIL of patients with HCC, we test the CD4+25+ Treg cells’ function on the CD4+25− T cells from the same patient with HCC. CD4+25− T cells were cocultured with CD4+25+ Treg cells in anti-CD3- and anti-CD28-coated 96-well plates at different cell amount ratios of CD4+CD25− T cells/CD4+CD25+ Treg cells. After 48 h of coculture, the proliferation activity and the secretion activity of CD4+CD25− T cells were detected. Our results showed that CD4+25+ Treg cells had strongly suppression inhibitory effects on IFN-γ secretion activity (Fig. 3a) and the proliferation (Fig. 3b) of CD4+CD25− T cells in a dose-dependent manner. This suppressant effect could be observed both in stages I, II, and III but showed no significant difference between the early and advanced stages.
Fig. 3.
Compromised proliferation potential and immunosuppression of CD4+CD25− T cells when cocultured with CD4+CD25+ Treg cells: CD4+CD25− T cells and CD4+CD25+ Treg cells were freshly isolated from blood samples of 14 patients with HCC (n = 8 for stages I–II, n = 6 for stage III) and tested in the following experiments: a CD4+CD25− T cells were cocultured with increasing cell number of CD4+CD25+ Treg cells, which are isolated from same group. The Y axis represents different cell number ratios of CD4+CD25− T cells/CD4+CD25+ Treg cells. The concentration of IFN-γ was tested by ELISA method. Our results showed that with the amount of cocultured CD4+CD25+ Treg cells increased, the concentration of IFN-γ significantly decreased (p < 0.01). This immunosuppression phenomenon was observed in both early stage (stages I–II) and advanced stage (stage III) and showed no difference between these two stages (p > 0.05). b The proliferative capacity of CD4+CD25−T cells was inhibited in the presence of CD4+CD25+ Treg cells in a dose-dependent manner, in which with the cell population ratio of CD4+CD25− T cells/CD4+CD25+Treg cells increased, the suppression of proliferative capacity of CD4+CD25− T cells was aggravated (p < 0.01). There was no significant difference in the proliferation suppression of CD4+CD25+ Treg cells from patients with HCC between stages I, II, and stage III (p > 0.05)
Increased migration of CD4+CD25+ Treg cells in the microenvironment of HCC
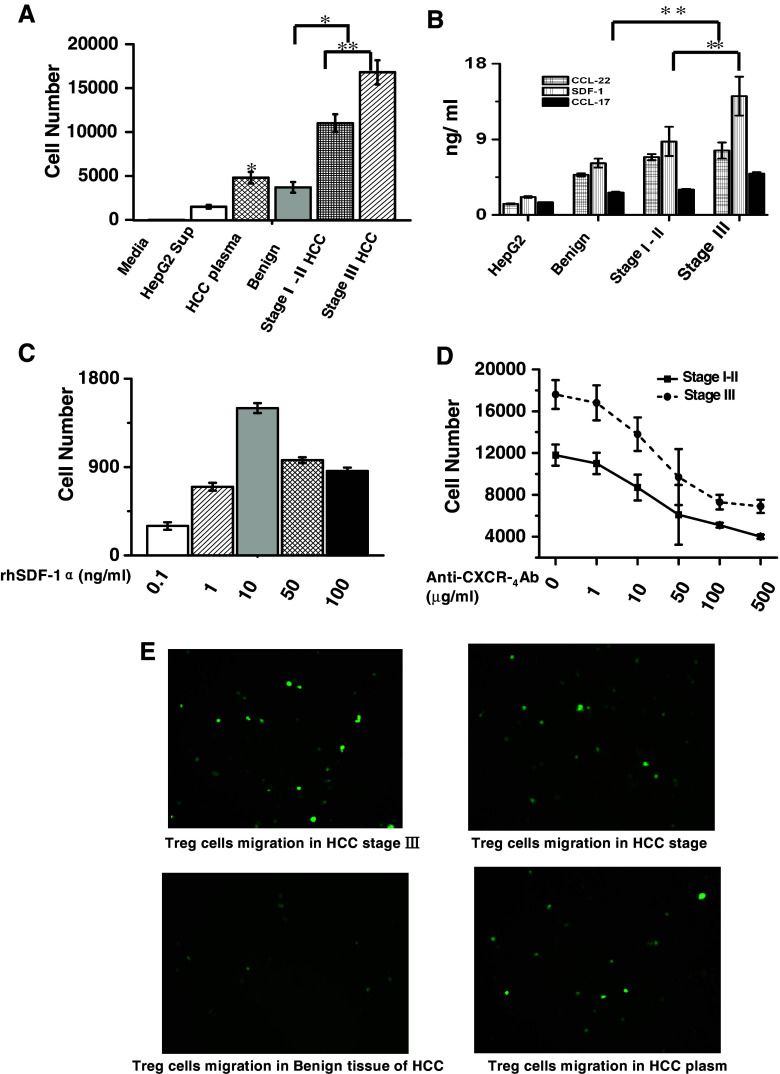
Having identified the immune suppression effect of CD4+CD25+ Treg cells on CD4+25− T cells, we wanted to find the cytokines in the tumor microenvironment that may have chemotactic effect on CD4+CD25+ Treg cells and thus leading to the accumulation of CD4+CD25+ Treg cells in the hepatic tissues of patients with HCC. We separated the hepatic tissue from patients with HCC at benign and malignant stages and cultured the cells in primary cultured medium. The supernatant of the culture medium from different samples was collected separately, and their chemotactic effect on CD4+CD25+ Treg cells was tested, and our results showed that the supernatant of cultured malignant tissue could induce more cell migration than benign supernatant. Malignant tissue culture supernatant obtained in advanced stage (stage III) exhibited powerful chemotactic activity than those in stages I–II (Fig. 4a,e). Plasma from the same patient was also able to chemoattract these cells but it showed the lesser extent than the tissue cell supernatant (Fig. 4a). The supernatants were then analyzed by ELISA method for the presence of the chemokine CCL22, SDF-1, and CCL17. High levels of SDF-1 were detected in culture supernatants taken from malignant tissue culture medium when compared with those from benign tissue (Fig. 4b). We showed that the migration of CD4+CD25+ Treg cell obviously induced by chemokine SDF-1 in dose depends in vitro (Fig. 4c). To further test SDF-1’s effect on the migration of CD4+CD25+ Treg cells, anti-human-CXCR4Ab was administrated to the culture supernatant, the migration activity of CD4+CD25+ Treg cells from stages I–II and stage III decreased in a dose-dependent manner, but there was no difference between stages I–II and stage III in the migration activity in response to anti-human-CXCR4Ab treatment (Fig. 4d), which indicated that SDF-1 performed this function through activation of CXCR4/CXCL12 signals pathway in HCC.
Fig. 4.
Increased migrations of CD4+CD25+ Treg cells induced by cytokines SDF-1 from the microenvironment of HCC: a The supernatants medium from the cultured HepG2 cells (n =6), benign and malignant region of HCC tissue (n = 11), and the plasma of patients with HCC (n =9) were collected. The tissue culture mediums from both benign and malignant tissues were able to induce the migration of CD4+CD25+ Treg cells. The supernatant from malignant tissues could induce more cell migration than those from benign tissues (*p < 0.05). The supernatant from malignant tissues at advanced stage (stage III, n = 5) exhibited higher chemotactic ability than those at early stage (stages I–II, n = 6, **p < 0.01). Plasma from the same patient also showed chemotactic ability but was weaker than supernatant of malignant tissue (*p < 0.05). b The concentrations of SDF-1, CCL-22, and CCL-17 in culture medium were measured by ELISA. The supernatants of cultured malignant tissue have higher concentrations of SDF-1 than the benign tissue. Its concentration in advanced stage was significant higher than those in early stage with (**p < 0.01). c Induced migration of CD4+CD25+Treg cells in vitro by chemokine SDF-1 showed bell-shaped curve. d The chemotactic phenomenon of CD4+CD25+ Treg cells induced by the supernatant of cultured HCC tissues at different stages was inhibited by the administration of a specific anti-human SDF-1 monoclonal antibody. The cell migrations were inhibited in dose depends manner (p < 0.05), but no significant difference in migration inhibition was observed between the supernatant from stages I–II and stage III (p > 0.05). e The migrated CD4+CD25+Treg cells immunostained with CD25 antibody were showed by using immunofluorescence method (original magnification×200)
Altered secretion of cytokines tumor microenvironment in patients with HCC
Since CD4+CD25+ T cells showed immune suppression effect on the proliferation and secretion of CD4+CD25− T cells, we wanted to explore the underlying mechanism that may contribute to the phenomenon. Previous studies showed that the CD4+CD25+ T cells may perform the immune suppression activity by increasing the secretion of IL-10 and TGF-β and down-regulating the secretion of INF-γ (Dieckmann et al. 2002). We conducted cytokine analysis of the supernatants to investigate which cytokines may be mediating the suppressive effects. It was demonstrated that extremely low levels of IFN-γ and high levels of IL-10 and TGF-β present in supernatants or tissue taken from HCC and in accordance with tumor stages. Cytokines in supernatants from the cultured benign and malignant biopsies were collected and measured after 2 days in culture. The high levels of TGF-β and IL-10 were detected in malignant biopsies. The secretions of TGF-β and IL-10 increased, and the secretion of IFN-γ decreased significantly in malignant compared with those detected in benign supernatant, and also correlated with tumor stage (Fig. 5a, b). Our results suggested that the CD4+CD25+ T cells in the HCC tissues may perform the immune suppression activity by modifying the secretion of cytokines in the tumor microenvironment.
Fig. 5.
Altered secretion of TGF-β, IL-10, and TFN-γ in tumor microenvironment with the progress of HCC. The concentrations of TGF-β, IL-10, and IFN-γ in the hepatic tumors tissues or supernatant from cultured hepatic tumors were measured by ELISA and compared among the benign and malignant hepatic tumors at different stages. The statistic results were plotted as means ± s.e. a The secretions of TGF-β and IL-10 were significant higher in both the supernatant and tissues of malignant hepatic tumors than those of benign tumors (n = 31, *p < 0.05). The secretion of IFN-γ was significantly decreased in malignant supernatant and tissues of malignant hepatic tumors. (n = 31, **p < 0.01). b The secretions of TGF-β and IL-10 increased, and the secretion of IFN-γ decreased significantly when the HCC progress into advanced stage. (n = 6 for stage I, n = 11 for stage II, and n = 14 for stage III, **p<0.01, *p<0.05)
Increased level of FOXP3 mRNA expressions with the malignant progress of HCC
Many studies demonstrated that FOXP3 function as the master regulator in the development and function of Treg cells (Fontenot et al. 2003, 2005; Hori et al. 2003; Zhang and Zhao 2007). Animal studies revealed that Tregs that expressing Foxp3 play a critical importance in the transfer of immune tolerance, especially self-tolerance and found that the induction or administration of Foxp3-positive T cells may led to marked reductions in autoimmune disease severity in various animal disease models, including diabetes, asthma, thyroiditis, and renal diseases. (Suri-Payer and Fritzsching 2006).
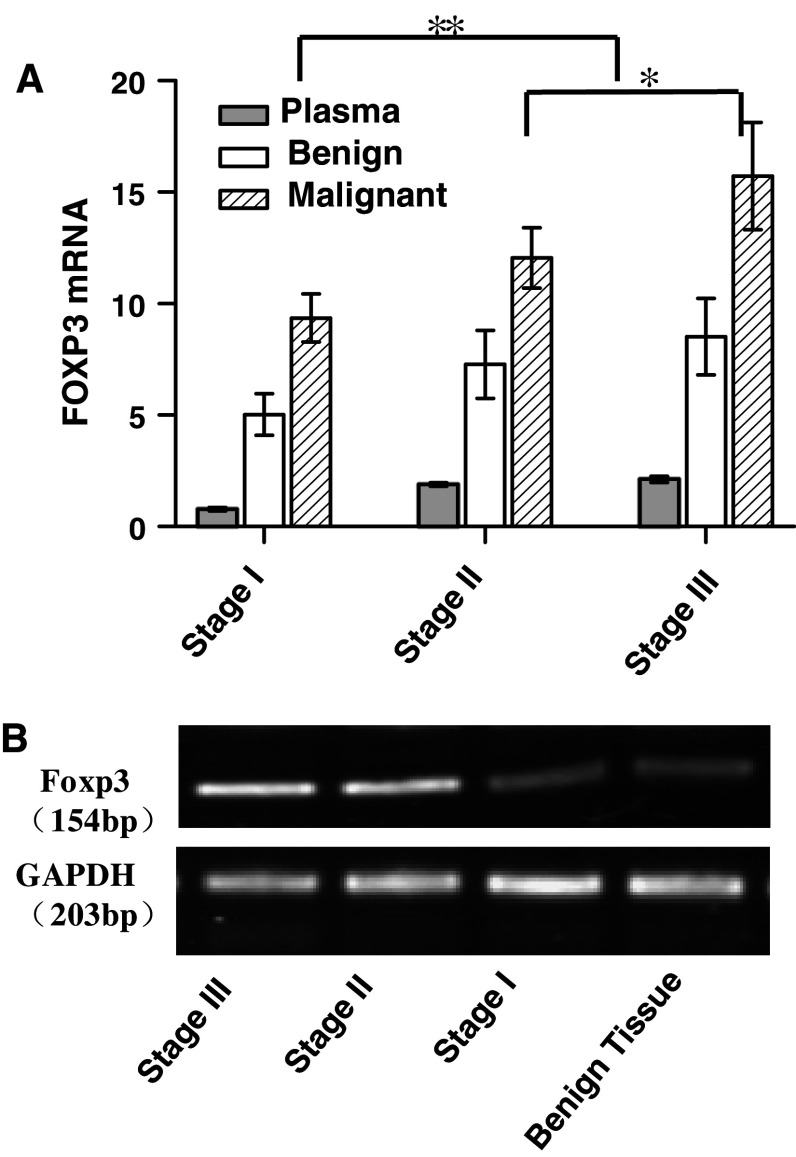
We tested the mRNA expression level of FOXP3 in the hepatic tissues of patients with HCC by real-time RT-PCR. We found that the mRNA expression level of FOXP3 increased in malignant tissue when compared with that in benign tissue. The high levels of FOXP3 mRNA were detected in HCC tissues at stage III than those at stages I–II (Fig. 6a), and the results could be repeated by semi-quantitative RT-PCR as shown in Fig. 6b. Our results indicated that the expression of FOXP3 in HCC tissues may also contribute to the self-tolerance of tumor in the patients.
Fig. 6.
Increased level of Foxp3 mRNA expression in malignant tissues. a Real-time RT-PCR analyses results of Foxp3’s mRNA expression levels for HCC at different stages. GAPDHs were used as sample controls. Folds changes in the mRNA expression levels of Foxp3 were compared among the HCC tissues at different TNM stages and plotted as means ± s.e. Our results showed that higher mRNAs expression level of FOXp3 can be detected in stages II–III than those of benign HCC and HCC at stage I (n = 31, **p< 0.01, *p< 0.05). b The same results could be verified by semi-quantitative RT-PCR
Discussion
In this study, we tested the relationship between the prevalence of CD4+CD25high Treg cells in TILs and the malignant progress in patients with HCC. First, we revealed the elevated prevalence and migration of CD4+CD25high Treg cells increased significantly when HCC progressed from an early stage to an advanced stage. We also explored the changes in various cytokines in the tumor microenvironment. We demonstrated that the secretion of SDF-1 may account for the accumulation of CD4+CD25high Treg cells in the malignant of different HCC stages. We found that the mRNA expression level of FOXP3 strongly increased expressed in malignant tissues when compared with those in benign, and the increased expression is closely correlated with TNM stages.
The increased population of CD4+CD25high Treg cells was showed in tumor sites in different TNM stage of HCC. It had been showed (Ormandy et al.) that the numbers of CD4+CD25high T cells in peripheral blood of patients with hepatocellular carcinoma were increased but did not correlate with stage of HCC. We found that the numbers of CD4+CD25high T cells in of patients with HCC increased in tumor microenvironment as well as those in peripheral blood, and it correlates with TNM stage. Also, FOXP3mRNA strongly expressed in malignant tissues compared with those in benign tissues and it correlated with TNM stages. Our study indicated that investigation into the prevalence and functions of Treg cells in tumor microenvironment is meaningful rather than those in the peripheral blood of patients with HCC.
The Treg cells mainly reside in the CD4+ T cell fraction expressing CD25 at a high level (CD25high). Although CD25 is the typical cell surface marker used to identify Treg cells, its specificity is not limited to Treg cell but also to other types of activated T cell.
In a recent study (Kuniyasu et al. 2000), CD4+CD25+CD127-as the surface marker of Treg cells was characterized in gastric cancer and showed that 88.1–96.1% of CD25+CD127− T cells expressed Foxp3, and above 90% of CD4+CD25+ Treg cells were CD127 negative. We also tested possibility of using CD127− as the surface marker to distinguish CD4+CD25+ Treg cells from activated CD4+ T cells in HCC. We demonstrated that the increased frequency of CD4+CD25+CD127− T cells was 97.6% of CD4+CD25high Treg cells in peripheral blood and TIL, which further confirmed the previous results. ICOS is another phenotype expressed on the surface of Treg cell. In our study, the expression of ICOS in CD4+CD25high Treg cells from HCC tissues was higher than those from blood. It is possible that the CD4+CD25high Treg cells in the tissue are activated or induced by their environment in vivo to express more ICOS in HCC, while the Treg cells in blood are at resting status. We indicated that CD4+CD25+CD127− may be an effective and practical selective biomarker to distinguish CD4+CD25high Treg cells in clinical HCC research.
Tumor microenvironment plays an important role in the recruitment of Tregs. We revealed that CD4+CD25+ Treg cells can inhibit cytokine secretion and proliferation of CD4+CD25− T cells in patient with HCC. This indicated that the CD4+CD25+ Treg cells from patients with HCC had a potential immune suppressive activity. But this immune suppressant function of CD4+CD25+ Treg cells in stages I–II shows equal effects compared with those in advanced stage. One possible reason for this phenomenon is that in our experiment, the HCC biopsies were very small, and the availability of cells was very limited, the functional analysis of the TILs was technically difficult, and the suppressive activity of Treg cells from the peripheral blood cannot represent real performance of that in tumor microenvironment of HCC. Therefore, we conducted cytokine analysis of the supernatants and found that the secretion level of TGF-β, IL-10, and INF-γ in tumor microenvironment of HCC changed correspondingly that changed in microenvironment may synergize with CD4+CD25+ Treg cells to perform the immune suppression effect in vivo, and this immune suppression may allow progressive local growth of tumors. Our finding suggested that the distribution activity and function of CD4+CD25+ Treg cells may be modified by local environmental factors in a tissue- and/or organ-specific manner. Since our study demonstrated that the function of CD4+CD25+ Treg cells was closely correlates with the TNM stage of patients with HCC, it is important to investigate Treg cell function in different tumor stages for guidance of clinical immunotherapeutic protocol.
We next explored the potential cytokines in tumor microenvironment and found that SDF-1 may be responsible for the increased recruitment of CD4+CD25high Treg cells to tumor sites of patients with HCC. We found that SDF-1 performed this function through the activation of CXCR4/CXCL12 signals in HCC, since when anti-human-CXCR4Ab was administered, the migration of CD4+CD25+ Treg cells from both early and late TNM stages was inhibited. We showed that although SDF-1 effectively acted on the recruitment of CD4+CD25+ Treg cells, they could not account for the all of the recruitments. This phenomenon could be explained because Treg cells have been reported to express a variety of chemokine receptors depending on their activation status and tissue locality (Iellem et al. 2001). Thus, further studies will be necessary to assess the other possible chemokines secreted in the tumor microenvironment that may be responsible for the increased presence of CD4+CD25+ Treg cells within the different tumor stages. Additionally, the special tumor-associated Ags expressed during tumor development may also contribute to the down-regulated functions of effectors T cell subsets. So, further studies should be performed to investigate the variation and function of tumor-associated Ags in different tumor stages of HCC.
Conclusion
The increase in frequency of Treg cell might play a role in modulation of the immune response against HCC in different TNM stages. The substance secreted in tumor microenvironment of different TNM stages recruited CD4+CD25+ Treg cells to tumor sites to contribute to the prosperity and growth of human tumors. The performance of Treg cells in different TNM stages of tumor microenvironment might be acted as the route to evaluate the immunotherapy-based methods, promote therapy effect, and consequently to increase the survival rate in HCC.
Acknowledgments
This study was funded by: supported by the department of Hepatobiliary Surgery, Cancer Hospital of Tianjin Medical University with the patients collection and providing samples. The authors thank department of Clinical Immunology, Cancer Research Institute of Tianjin Medical University and Department of Pathology, University of Texas M.D. Anderson Cancer Center for their technical support.
Conflict of interest statement
None.
Footnotes
X. Shen and N. Li contributed equally to this work.
References
- Asano M, Toda M, Sakaguchi N, Sakaguchi S (1996) Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med 184:387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942–949 [DOI] [PubMed] [Google Scholar]
- Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G (2001) Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med 193:1303–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G (2002) Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected]. J Exp Med 196:247–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY (2003) Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4:330–336 [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY (2005) Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 22:329–341 [DOI] [PubMed] [Google Scholar]
- Greten TF, Jaffee EM (1999) Cancer vaccines. J Clin Oncol 17:1047–1060 [DOI] [PubMed] [Google Scholar]
- Hao XS, Wang PP, Chen KX, Li Q, He M, Yu SB, Guo ZY, Perruccio A, Rohan T (2003) Twenty-year trends of primary liver cancer incidence rates in an urban Chinese population. Eur J Cancer Prev 12:273–279 [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057–1061 [DOI] [PubMed] [Google Scholar]
- Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D’Ambrosio D (2001) Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med 194:847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuniyasu Y, Takahashi T, Itoh M, Shimizu J, Toda G, Sakaguchi S (2000) Naturally anergic and suppressive CD25(+)CD4(+) T cells as a functionally and phenotypically distinct immunoregulatory T cell subpopulation. Int Immunol 12:1145–1155 [DOI] [PubMed] [Google Scholar]
- Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169:2756–2761 [DOI] [PubMed] [Google Scholar]
- Maloy KJ, Powrie F (2001) Regulatory T cells in the control of immune pathology. Nat Immunol 2:816–822 [DOI] [PubMed] [Google Scholar]
- Miller AM, Lundberg K, Ozenci V, Banham AH, Hellstrom M, Egevad L, Pisa P (2006) CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol 177:7398–7405 [DOI] [PubMed] [Google Scholar]
- Nishikawa H, Kato T, Tawara I, Saito K, Ikeda H, Kuribayashi K, Allen PM, Schreiber RD, Sakaguchi S, Old LJ, Shiku H (2005) Definition of target antigens for naturally occurring CD4(+) CD25(+) regulatory T cells. J Exp Med 201:681–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Garra A, Vieira P (2004) Regulatory T cells and mechanisms of immune system control. Nat Med 10:801–805 [DOI] [PubMed] [Google Scholar]
- Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F (2005) Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res 65:2457–2464 [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P (2001) Estimating the world cancer burden: Globocan 2000. Int J Cancer 94:153–156 [DOI] [PubMed] [Google Scholar]
- Piccirillo CA, Thornton AM (2004) Cornerstone of peripheral tolerance: naturally occurring CD4+CD25+ regulatory T cells. Trends Immunol 25:374–380 [DOI] [PubMed] [Google Scholar]
- Sakaguchi S (2000) Regulatory T cells: key controllers of immunologic self-tolerance. Cell 101:455–458 [DOI] [PubMed] [Google Scholar]
- Suri-Payer E, Fritzsching B (2006) Regulatory T cells in experimental autoimmune disease. Springer Semin Immunopathol 28:3–16 [DOI] [PubMed] [Google Scholar]
- Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, Kakizoe T (2000) Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 356:802–807 [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhao Y (2007) The regulation of Foxp3 expression in regulatory CD4(+)CD25(+)T cells: multiple pathways on the road. J Cell Physiol 211:590–597 [DOI] [PubMed] [Google Scholar]
- Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, Wei S, David O, Curiel TJ, Zou W (2004) Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res 64:8451–8455 [DOI] [PubMed] [Google Scholar]