Abstract
Purpose
The aim of the present study was to investigate the prognostic value of tumor-infiltrated lymphocytes (TILs), especially the prognostic value of Foxp3+ regulatory T cells (Tregs), CD8+ CTLs and Tregs/CD8+ ratios in gastric cancer patients after R0 resection.
Patients and methods
From 133 patients, CD4+, CD8+ and Foxp3+ TILs were assessed by immunohistochemistry in tissue microarrays and N1 regional lymph nodes sections containing gastric cancer. The prognostic effects of low- or high-density TIL subsets were evaluated by Cox regression and Kaplan–Meier analysis using median values as cutoff, while the effects of Foxp3+/CD8+ ratios were evaluated using the value determined by ROC cure analysis as cutoff.
Results
It was found that CD4+ and CD8+ TILs were not associated with overall survival (OS). In the tumor sites, higher Foxp3+ Tregs/CD8+ ratio was an independent factor for worse OS (multivariate analysis HR = 2.827, P = 0.037). The 1-year, 2-year and 3-year OS rates were 90, 77.5 and 70% for the group with intratumoral high Tregs/CD8+ ratio, compared with 100, 94.3 and 90.5% for the group with intratumoral low ratio. At the same time, the presence of intratumoral high Foxp3+ Tregs was also associated with worse OS (log rank test, P = 0.025); however, it was not an independent predictor and correlated with intratumoral Foxp3+ Tregs/CD8+ ratio (χ 2 test, P < 0.001). Although the infiltration of Foxp3+ Tregs in N1 regional lymph nodes was associated with lymph node metastasis (P = 0.028), it was not associated with prognosis (P = 0.458).
Conclusions
Intratumoral high Foxp3+ Tregs/CD8+ ratio was an independent predictor for the prognosis of gastric cancer. It can be inferred that a combination of deletion of Tregs and stimulation of CD8+ effector T cells may be an effective immunotherapy to prolong survival after surgery.
Keywords: Regulatory T cells, Gastric cancer, Prognosis, Lymph nodes
Introduction
Tumor infiltration of lymphocytes (TILs) are considered to be manifestations of host immune reactions against cancers. It has been demonstrated that the majority of TILs in solid tumors are the CD3+ T-cell phenotype. It can be stratified further into CD4+ helper cells (including the TH1 and TH2 subtypes based on their cytokine profile), CD4+ Tregs and CD8+ cytotoxic effector cells (CTLs). CD4+ Tregs and CTLs have different effect on the incidence and prognosis of the tumor.
CD4+ CD25+ regulatory T cells (Tregs) are believed to regulate T-cell immunity and to be the main obstacle in immunotherapy. A body of evidence suggests that Tregs within the tumor microenvironments might play a significant role in the suppression of local antitumor immune responses (Zou 2006). Tregs express various surface antigens, including CD25, CD103, OX-40, CTLA-4 and the glucocorticoid-induced tumor necrosis factor receptor, none of which seems to be entirely specific to Tregs (Piccirillo and Thornton 2004). Foxp3, forkhead/winged helix transcription factor, which is crucial for the development of Tregs, is the most reliable marker for Tregs and it is possible to define Tregs more strictly as CD4+ CD25+ regulatory T cells (Hori and Sakaguchi 2004). Therefore, in recent studies, Foxp3+ is used to identify Tregs. It was reported that higher Foxp3 expression is associated with a higher risk of recurrence and poor overall survival of patients with some solid neoplasms (Gao et al. 2007; Amy et al. 2008; Petersen et al. 2006; Curiel et al. 2004; Griffiths et al. 2007; Bates et al. 2006; Hiraoka et al. 2006; Fu et al. 2007).
However, discrepancies remain among different cancer types. It was found that tumor-infiltrating Tregs had no prognostic influence on anal squamous cell carcinoma (Grabenbauer et al. 2006). In head as well as neck squamous cell carcinoma, Tregs correlated positively with regional control (Badoual et al. 2006). Specifically, the balance between cytotoxic and regulatory T cells is believed to be of greater significance. The ratio of CD8+ CTLs and Tregs in ovarian cancer (Sato et al. 2005), as well as the concurrent low Tregs density and high CTLs density in Hodgkin’s lymphoma (Alvaro et al. 2005), has been reported to be more valuable than the single TILs subtypes. A new case in point is that peritumoral Tregs, but not intratumoral Tregs, are independent prognostic factors for clear cell renal cell carcinoma (Li et al. 2008). It was also reported that Foxp3+ Treg density in different areas had different impacts on survival in colorectal cancer. High Foxp3+ Treg density in normal mucosa was associated with worse prognosis, while a high density of Foxp3+ Treg in tumor tissue was associated with improved survival (Salama et al. 2008).
Gastric cancer is the fourth most common cancer in the world (Kamangar et al. 2006) and the overall survival of patients remains poor despite improved diagnosis and treatment strategies, including resection, which is of first priority. Recent studies have found that CD4(+) CD25high T cells in the regional lymph nodes in patients with gastric cancer were significantly higher in comparison to those in the control lymph nodes (Kawaida et al. 2005) and that Tregs were associated with the development and outcome of gastric cancer (Mizukami et al. 2008; Giuseppe et al. 2008). However, whether Foxp3+ Tregs are unfavorable predictors for gastric cancer is still controversial and the prognostic value of Tregs in different areas of gastric cancer has not been studied either. In addition, tumor-infiltrating CD8+ T lymphocytes are believed to be front fighters against cancers and have prognostic role in many cancer types (Grabenbauer et al. 2006; Sato et al. 2005; Nakano et al. 2001). So far as we know, there is still no information on the prognostic value of CD8+ TILs in gastric cancer.
The purpose of this study was to find greater prognostic significance of various subtypesof TILs (including Treg, CTL, etc.) and their relationship with clinical characteristics of gastric cancer. The results suggest that not only intratumoral Tregs have a role in the promotion of gastric invasiveness, but the other Tregs and CTLs are also predictors of survival.
Patients and methods
Patients and follow-up
In this study, 133 patients with gastric cancer who underwent R0 resections with extended lymph node dissection (D2) between 1999 and 2005 at Zhongshan Hospital (Shanghai, China) were enrolled. The evaluation of resected specimens was performed in accordance with the guidelines of the Japanese Gastric Cancer Association (Japanese Gastric Cancer Association 1998). Each resection referred to the removal of group 1 and 2 lymph nodes (range 7–92, mean 30.3). Stage classification was determined according to the TNM classification for gastric cancer (UICC). Specimens were selected on the basis of the availability of suitable formalin-fixed, paraffin-embedded tissue and complete clinicopathologic as well as follow-up data from the patients. The characteristics of the study subjects are summarized in Table 1. This study was approved by the Zhongshan Hospital Research Ethics Committee and informed consent was obtained from all individuals.
Table 1.
Characteristics of the 133 gastric cancer patients
| Age (years) | |
| Median (minimum–maximum) | 59 (5–80) |
| Gender: number (%) | |
| Male | 89 (66.9%) |
| Female | 44 (33.1%) |
| Primary tumor (T): number (%) | |
| T1 | 30 (22.6%) |
| T2 | 20 (15.0%) |
| T3 | 80 (60.2%) |
| T4 | 3 (2.3%) |
| Regional lymph nodes (N): number (%) | |
| N0 | 52 (39.1%) |
| N1 | 51 (38.3%) |
| N2 | 23 (17.3%) |
| N3 | 7 (5.3%) |
| Distant metastasis (M): number (%) | |
| M0 | 133 (100%) |
| M1 | 0 (0%) |
| Stage grouping (TNM): number (%) | |
| Stage IA, IB | 38 (28.5%) |
| Stage II | 28 (21.1%) |
| Stage IIIA, IIIB | 59 (44.4%) |
| Stage IV | 8 (6.0%) |
| Differentiation: number (%) | |
| I | 5 (3.8%) |
| II | 45 (33.8%) |
| III | 83 (62.4%) |
| Tumor size (cm) | |
| Median (minimum–maximum) | 3.5 (1–11) |
| Death: number (%) | |
| Yes | 38 (28.6%) |
| No | 95 (71.4%) |
None of the patients received chemotherapy or radiation therapy before or after surgery as part of an adjuvant program. Follow-up data were completed on 7 October 2008. A minimum of 3-year follow-up was required. The median follow-up was 43 months (range 36–104 months). Follow-up procedures consisted of interim history, physical examination, tumor markers (CEA, CA199), abdominal ultrasonography or CT and chest X-ray every 6–8 months according to the postoperative time. OS was defined as the interval between surgery and death or between surgery and the last observation for surviving patients. The data were censored at the last follow-up for living patients.
Immunohistochemistry
Tissue microarrays were constructed as previously described (Gao et al. 2007; Amy et al. 2008; Alvaro et al. 2005; Galon et al. 2006; Lee et al. 2006). All cases were histologically reviewed by hematoxylin and eosin staining, and representative areas with small round lymphocyte infiltrate were premarked in the paraffin blocks, away from necrotic and hemorrhagic materials. Duplicate 1-mm diameter cylinders from two different areas, tumor center and nearest noncancerous margin (designated as intratumor and peritumor, respectively), that is, a total of four punches, were included in each case, together with different controls, to insure reproducibility and homogenous staining of the slides (Shanghai Biochip Co., Ltd, Shanghai, China). Furthermore, three N1 regional lymph nodes, which were the most adjacent to the tumor were also obtained from each case to make another tissue section. In short, each patient sample was constructed with four different tissue microarray blocks (Fig. 1b) and three lymph nodes nearest to the tumor. Sections of 4-μm thickness were taken on 3-aminopropyltriethoxysilane-coated slides.
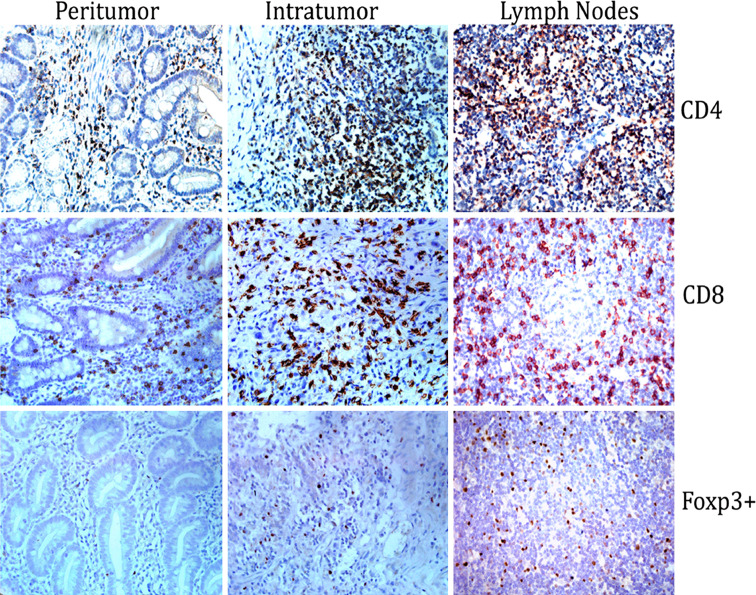
Fig. 1.
Immunohistochemical staining of different tissue microarrays in gastric cancer pathological specimens. Specimens were collected from peritumor, intratumor and tumor-draining lymph nodes followed by immunohistochemical staining for CD4+, CD8+ and Foxp3+. The slides were viewed with light microscopy (magnification ×400). CD4+ and CD8+ staining were noted on the cell surface, whereas Foxp3+ staining was confined to the nucleus
The mouse monoclonal antibodies used were antihuman CD4, CD8 (Nichirei Bioscience Inc, Japan), and Foxp3 (Biolegend, San Diego, CA). Immuno- histochemistry of paraffin sections was carried out using a two-step protocol (Novolink Polymer Detection System, Novocastra, Newcastle, UK) according to the manufacturer’s instructions. Briefly, sections were deparaffinized in xylene and rehydrated in ethanol. After endogenous peroxidase activity blocked with incubation of the slides in 0.3% H2O2, antigen retrieval was done by placing the sections in an electric kitchen pot filled with boiling citrate buffer for 20 min for antigen retrieval. Nonspecific binding sites were blocked with Protein Block (RE7102). After serial incubation with primary antibodies, Post Primary Block (RE7111), and secondary antibodies (RE7112), the sections were developed in 3, 3′-diamiobenzidine solution under a microscope and counterstained with hematoxylin. Negative control slides omitting the primary antibodies were included in all assays.
Evaluation of immunohistochemical variables
We evaluate the immunohistochemistry variables in different ways to count the number of TILs in microarrays and lymph nodes. The number of TILs in microarrays was counted as previously described (Gao et al. 2007) using computerized image analysis system composed of a Hitachi HV-C20A CCD camera (Hitachi, Tokyo, Japan), installed on a Leica DMLA light microscope (Leica Microsystems, Wetzlar, Germany) and attached to a personal computer. Under 400× magnification, there were at least 12 independent and intact computerized microscopic fields for the duplicates of each patient sample. Eight independent microscopic fields (400×), representing the densest lymphocytic infiltrates, were selected for each patient sample to insure representativeness and homogeneity. The numbers of the eight fields were cumulated and then averaged to calculate the final number for one computerized 400× microscopic field (0.0768 mm2/field).
The number of TILs in lymph nodes was analyzed by use of image analysis software (Leica Qwin Image Processing and Analysis Application Software, Wetzlar, Germany). For each slide, all intact HPF (400×) digital images were taken by the aforementioned equipment and then analyzed by the image analysis software to acquire the number of TILs for each field. Finally, average number for one HPF (400×) was calculated to insure the representativeness and homogeneity.
The evaluation of TILs was performed by two independent observers in a blinded fashion. Discrepancies in enumeration, within a range of 5%, were re-evaluated and a consensus decision was made. The ratio of Foxp3+ Tregs/CD8+ T cells was calculated for each specimen.
Statistic analysis
Given that there were no widely accepted standard cutoff points to define the clinical outcome, we selected the median value to be the cutoff for definition of TILs subgroups and determined the most suitable value from the ROC cure analysis to be the cutoff for classification of the ratios. Actuarial overall survival rates were calculated by the Kaplan–Meier method and analyzed by the log-rank test. Univariate and multivariate analyses were based on the Cox proportional hazards regression model. A secondary analysis was performed to assess the relationship among lymphocytic variables and clinicopathologic characteristics. For the comparison of individual variables, paired-sample t tests, ×2 tests and Fisher’s exact tests were carried out as appropriate. Two-tailed P < 0.05 was judged to be significant. All analyses were performed using SPSS 12.0 software (SPSS, Chicago, IL).
Results
Different infiltration of TILs was assessed by immunohistochemistry in tissue microarrays
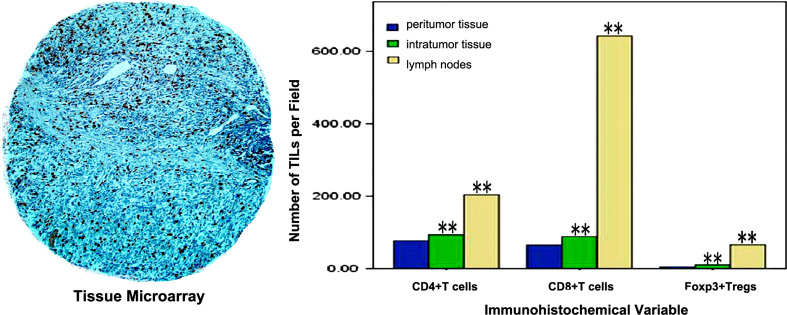
Specimens were collected from peritumor, intratumor and tumor-draining lymph nodes from 133 patients, followed by immunohistochemical staining for analysis of CD4+, CD8+ and Foxp3+ T cells infiltration in tissue microarrays. Using hematoxylin and eosin staining, it was found that immune cell infiltration varied substantially among different patients. Lymphocytes infiltrated gastric cancer and tumor-draining lymph nodes in a diffuse manner or in lymphoid aggregates, with more abundant cells in peritumoral areas, as shown in Fig. 1. Cells were considered positive for CD4+ and CD8+ based on the presence of circular membranous staining, while Foxp3+ was based on distinct nuclear expression. There was significant difference for the frequency of TILs among different tissues (paired-sample t test, P < 0.001), as shown in Fig. 2. The duplicate of spots for each specimen showed a good level of homogeneity for stained cell density.
Fig. 2.
Different frequency of TILs in different tissues. Low power showing core from tissue microarray (magnification ×100). The frequency of intratumoral TILs was significantly higher than in peritumor (paired-sample t test, **P < 0.001); while the frequency of TILs in lymph nodes was significantly higher than in intratumor (paired-sample t test, **P < 0.001)
High Foxp3+ Tregs infiltration were associated with adverse prognosis of gastric cancer
The characteristics of 133 patients are shown in Table 2. At the last follow-up, 38 (28.6%) of all patients had died from the recurrence of gastric cancer. The OS rates were 94.0% for 1 year, 82.0% for 2 years and 78.2% for 3 years, respectively, for the whole study.
Table 2.
Descriptive statistics of immunohistochemical variables
| Variables | Mean | SE | Median | Range |
|---|---|---|---|---|
| CD4+ TILs | ||||
| Intratumoral | 93.28 | 5.90 | 79.17 | 1.67–400.00 |
| Peritumoral | 76.57 | 6.17 | 54.67 | 0.58–398.33 |
| Tumor-draining LN | 203.72 | 14.89 | 173.61 | 0.02–777.41 |
| CD8+ TILs | ||||
| Intratumoral | 88.31 | 5.43 | 72.67 | 1.3–344.8 |
| Peritumoral | 65.20 | 3.71 | 57.17 | 1.0–197.3 |
| Tumor-draining LN | 642.51 | 267.47 | 621.14 | 0–1524.94 |
| Foxp3+ TILs | ||||
| Intratumoral | 10.59 | 1.14 | 6.33 | 0–87.4 |
| Peritumoral | 4.95 | 0.56 | 2.92 | 0–40.2 |
| Tumor-draining LN | 65.74 | 5.82 | 40.76 | 0–368.71 |
Number of TILs per field (×400) for all variables
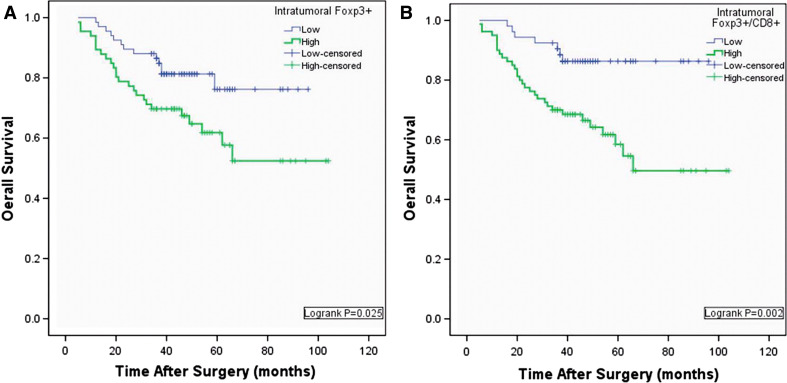
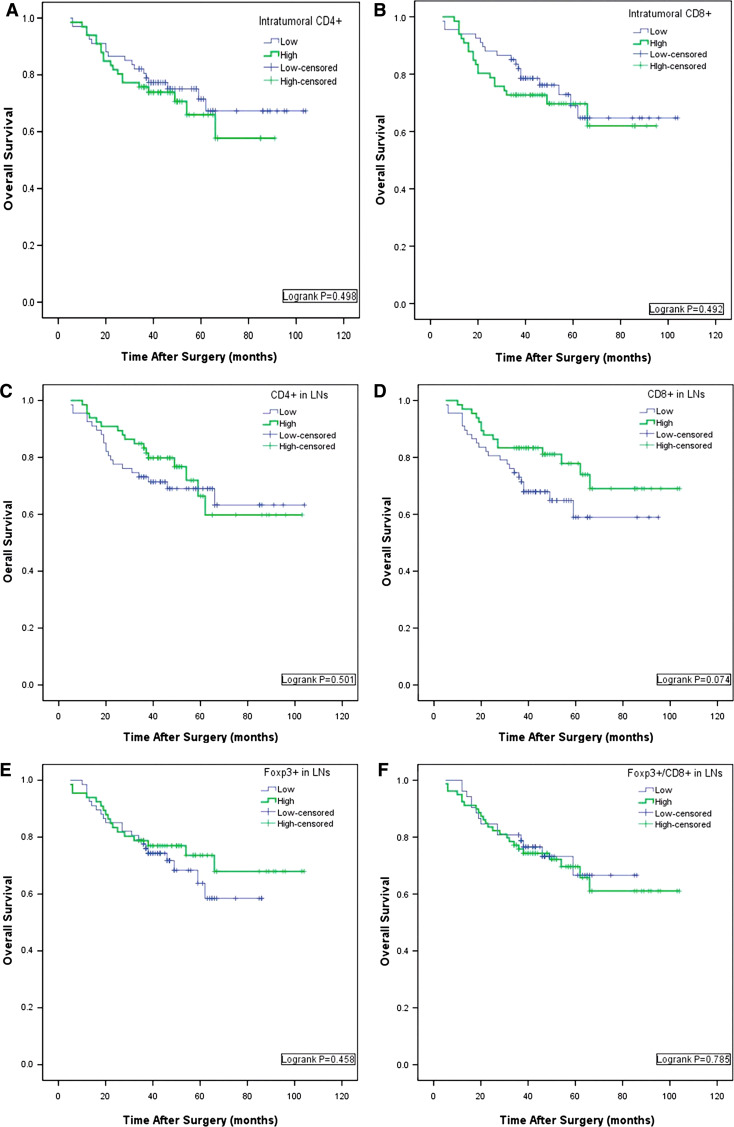
On univariate analysis, age, gender, tumor size and tumor differentiation showed no prognostic significance for OS. TNM staging, lymph node metastasis and the depth of infiltration were associated with OS rates. No lymphocyte types infiltrating the peritumoral area showed any prognostic significance. Neither intratumoral CD4+ TILs nor intratumoral CD8+ TILs were associated with prognosis, whereas intratumoral high Foxp3+ Tregs were associated with adverse prognosis (*P = 0.029, Table 3; Logrank *P = 0.025, Fig. 3a). In the N1 regional lymph nodes, higher numbers of CD8+ TIL were associated with favorable outcome; 1-year and 3-year OS were 83.3 and 81.1%, compared with 82.1 and 67.9% for the group with lower numbers, although this did not reach statistical significance. Neither CD4+ TILs nor Foxp3+ Tregs in lymph nodes were predictors of outcome of gastric cancer after surgery, as shown in Table 3 and Fig. 4 in Appendix.
Table 3.
Univariate analysis of factors associated with overall survival
| Variables | Overall survival | ||
|---|---|---|---|
| HR | 95% CI | P | |
| Age (years) (≤59 vs. >59) | 0.772 | 0.407–1.465 | 0.429 |
| Gender (male vs. female) | 0.581 | 0.306–1.101 | 0.096 |
| Tumor size, cm (≤3.5 vs. >3.5) | 1.303 | 0.687–2.469 | 0.418 |
| Infiltration (T1–T2 vs. T3–T4) | 2.498 | 1.465–4.259 | **0.001 |
| LN staging (N0 vs. N1–N3) | 5.174 | 2.018–13.271 | **0.001 |
| TNM (0–II vs. III–IV) | 6.521 | 2.723–15.616 | **<0.001 |
| Differentiation (I–II vs. III) | 1.512 | 0.761–3.002 | 0.237 |
| Intraltumoral TILs (low vs. high) | |||
| CD4+ | 1.246 | 0.658–2.360 | 0.500 |
| CD8+ | 1.249 | 0.660–2.364 | 0.494 |
| Foxp3+ | 2.114 | 1.080–4.136 | *0.029 |
| Foxp3+/CD8+ | 3.378 | 1.486–7.675 | **0.004 |
| Peritumoral TILs (low vs. high) | |||
| CD4+ | 1.665 | 0.868–3.195 | 0.125 |
| CD8+ | 1.009 | 0.533–1.909 | 0.979 |
| Foxp3+ | 0.803 | 0.424–1.522 | 0.502 |
| Foxp3+/CD8+ | 1.283 | 0.676–2.436 | 0.446 |
| Tumor-draining lymph nodes (low vs. high) | |||
| CD4+ | 0.803 | 0.423–1.525 | 0.503 |
| CD8+ | 0.555 | 0.288–1.071 | 0.079 |
| Foxp3+ | 0.785 | 0.413–1.491 | 0.460 |
| Foxp3+/CD8+ | 0.998 | 0.526–1.890 | 0.994 |
Univariate analysis, Cox proportional hazards regression model
*P < 0.05
**P < 0.01
Fig. 3.
K–M analysis of overall survival for intratumoral Foxp3+ and Foxp3+/CD8. a Low Foxp3+ was associated with prolonged survival (*P = 0.025); b low rate of Foxp3+ and CD8 was also associated with improved survival (**P = 0.002)
Fig. 4.
K–M analysis for the direct correlation between the infiltration of TILs and clinical overall survival (OS) after surgery. a, b The correlation of introtumor infiltrated CD4+, CD8+ T cells with OS. c, d The correlation of infiltrated CD4+, CD8+ T cells in L.N with OS. e, f The correlation of infiltrated Foxp3+ Treg cells with OS
Intratumoral Foxp3+/CD8+ ratio proved to be an independent predictor of gastric cancer
The combined influence of intratumoral Foxp3+/CD8+ ratios was also evaluated. To be more reasonable, we used ROC cure analysis to determine the cutoff (40th percentile) and classify patients into two groups. Significant differences in OS between two groups were found (**P = 0.004, Table 3; Log rank P = 0.002, Fig. 3b).
Clinicopathologic features that proved to have significant prognostic value in univariate analysis were adopted as covariates when multivariate Cox proportional hazards analysis was performed (because TNM staging is a combination of lymph node metastasis and the depth of infiltration, it was not used for the analysis.). TNM staging, lymph node metastasis and the depth of infiltration remained associated with OS. Intratumoral Foxp3+/CD8+ ratio proved to be an independent predictor of gastric cancer (HR = 2.827, *P = 0.037, Table 4), while intratumoral Foxp3+ Tregs were not independent prognostic factors (HR = 1.158, P = 0.720). In another multivariate analysis, in which Foxp3+/CD8+ ratio was not included, intratumoral Foxp3+ seemed to be an independent predictor (HR = 2.039, *P = 0.038, Table 6 in Appendix). A secondary χ 2 test showed that intratumoral Foxp3+ correlated with Foxp3+/CD8+ ratio (**P < 0.001), which is also shown in Table 4.
Table 4.
Multiple analysis of factors associated with overall survival
| Variables | Overall survival | ||
|---|---|---|---|
| HR | 95% CI | P | |
| Infiltration (T1–T2 vs. T3–T4) | 3.183 | 1.212–8.359 | *0.019 |
| LN staging (N0 vs. N1–N3) | 3.591 | 1.363–9.460 | *0.019 |
| Intratumoral Foxp3+/CD8+ (low vs. high) | 2.827 | 1.064–7.513 | *0.037 |
| Intratumoral Foxp3+ (low vs. high) | 1.158 | 0.519–2.584 | 0.720 |
Multivariate analysis, Cox proportional hazards regression model. Variables were adopted for their prognostic significance by univariate analysis
*P < 0.05
Table 6.
Multiple analysis of factors associated with overall survival
| Variables | Overall survival | ||
|---|---|---|---|
| HR | 95% CI | P | |
| Infiltration (T1–T2 vs. T3–T4) | 3.041 | 1.162–7.962 | *0.024 |
| LN staging (N0 vs. N1–N3) | 3.946 | 1.509–10.320 | **0.005 |
| Intratumoral Foxp3+ (low vs. high) | 2.039 | 1.039–3.999 | *0.038 |
Multivariate analysis, Cox proportional hazards regression model. Variables were adopted for their prognosis significance by univariate analysis
*P < 0.05
**P < 0.01
There is no obvious direct correlation between the infiltration of TILs and clinicopathologic features
None of the intratumoral lymphocyte types correlated with any clinicopathologic features (Table 5). In N1 regional lymph nodes compared with the metastatic lymph nodes, lymph nodes without metastasis were prone to have more infiltrating Foxp3+ Tregs and CD4+ TILs (*P = 0.028, Table 5; *P = 0.028, Table 7 in Appendix). In addition, lower numbers of CD4+ cells were corrected with III-V TNM staging (*P = 0.012). However, the correlation between CD8+ TILs and clinical characteristics was not found (P > 0.05), as shown in Table 8 in Appendix.
Table 5.
Correlation between intratumoral Foxp3+ TILs and clinical characteristics
| Characteristics | Foxp3 Positive | Foxp3 Positive in tumor-draining LN | ||||
|---|---|---|---|---|---|---|
| Low | High | P | Low | High | P | |
| Age (years) | 0.543 | 0.340 | ||||
| ≤59 | 32 | 35 | 31 | 36 | ||
| >59 | 35 | 31 | 36 | 30 | ||
| Gender | 0.057 | 0.668 | ||||
| Male | 50 | 39 | 46 | 43 | ||
| Female | 17 | 27 | 21 | 23 | ||
| Tumor size (cm) | 0.658 | 0.255 | ||||
| ≤3.5 | 35 | 37 | 33 | 39 | ||
| >3.5 | 32 | 29 | 34 | 27 | ||
| LN staging | 0.945 | *0.028 | ||||
| N0 | 26 | 26 | 20 | 32 | ||
| N1–N3 | 41 | 40 | 47 | 34 | ||
| Tumor infiltration | 0.516 | 0.063 | ||||
| T1, T2 | 27 | 23 | 20 | 30 | ||
| T3, T4 | 40 | 43 | 47 | 36 | ||
| TNM staging | 0.543 | 0.069 | ||||
| 0–II | 35 | 31 | 28 | 38 | ||
| III–IV | 32 | 35 | 39 | 28 | ||
| Tumor differentiation | 0.172 | 0.433 | ||||
| I, II | 29 | 21 | 23 | 27 | ||
| III | 38 | 45 | 44 | 39 | ||
| Foxp3+/CD8+ | **<0.001 | 0.116 | ||||
| Low | 48 | 5 | 31 | 21 | ||
| High | 19 | 61 | 36 | 43 | ||
χ2 tests for all the analysis
*P < 0.05
**P < 0.01
Table 7.
Correlation between CD4+ TILs and clinical characteristics
| Characteristics | Intratumoral CD4+ | CD4+ in Tumor-draining LN | ||||
|---|---|---|---|---|---|---|
| Low | High | P | Low | High | P | |
| Age (years) | 0.794 | 0.340 | ||||
| ≤59 | 33 | 34 | 31 | 36 | ||
| >59 | 34 | 32 | 36 | 30 | ||
| Gender | 0.668 | 0.951 | ||||
| Male | 46 | 43 | 45 | 44 | ||
| Female | 21 | 23 | 22 | 22 | ||
| Tumor size (cm) | 0.100 | 0.547 | ||||
| ≤3.5 | 41 | 31 | 38 | 34 | ||
| >3.5 | 26 | 35 | 29 | 32 | ||
| LN staging | 0.775 | *0.028 | ||||
| N0 | 27 | 25 | 20 | 32 | ||
| N1–N3 | 40 | 41 | 47 | 34 | ||
| Tumor infiltration | 0.314 | 0.433 | ||||
| T1, T2 | 28 | 22 | 23 | 27 | ||
| T3, T4 | 39 | 44 | 44 | 39 | ||
| TNM staging | 0.794 | *0.012 | ||||
| 0–II | 34 | 32 | 26 | 40 | ||
| III–IV | 33 | 34 | 41 | 26 | ||
| Tumor differentiation | 0.516 | 0.671 | ||||
| I, II | 27 | 23 | 24 | 26 | ||
| III | 40 | 43 | 43 | 40 | ||
*P < 0.05
Table 8.
Correlation between CD8+ TILs and clinical characteristics
| Characteristics | Intratumoral CD8 Positive | CD8 positive in tumor-draining LN | ||||
|---|---|---|---|---|---|---|
| Low | High | P | Low | High | P | |
| Age (years) | 0.931 | 0.099 | ||||
| ≤59 | 34 | 33 | 29 | 38 | ||
| >59 | 33 | 33 | 38 | 28 | ||
| Gender | 0.158 | 0.499 | ||||
| Male | 41 | 48 | 43 | 46 | ||
| Female | 26 | 18 | 24 | 20 | ||
| Tumor size (cm) | 0.194 | 0.658 | ||||
| ≤3.5 | 40 | 32 | 35 | 37 | ||
| >3.5 | 27 | 34 | 32 | 29 | ||
| LN staging | 0.435 | 0.435 | ||||
| N0 | 24 | 28 | 24 | 28 | ||
| N1–N3 | 43 | 38 | 43 | 38 | ||
| Tumor infiltration | 0.314 | 0.134 | ||||
| T1, T2 | 28 | 22 | 21 | 29 | ||
| T3, T4 | 39 | 44 | 46 | 37 | ||
| TNM staging | 0.543 | 0.436 | ||||
| 0–II | 35 | 31 | 31 | 35 | ||
| III–IV | 32 | 35 | 36 | 31 | ||
| Tumor differentiation | 0.085 | 0.172 | ||||
| I, II | 30 | 20 | 29 | 21 | ||
| III | 37 | 46 | 38 | 45 | ||
Discussion
The novelty of our study was that Foxp3+ was examined in parallel with CD8+ and CD4+ in intratumoral areas, peritumoral areas and tumor-draining lymph nodes.
Our study confirmed the importance of intratumoral Foxp3+ Tregs as a prognostic factor, which is in agreement with Giuseppe Perrone’s earlier study (Giuseppe et al. 2008). Compared with Giuseppe Perrone’s study, the present study revealed intratumoral Foxp3+/CD8+ ratio, and not intratumoral Tregs, to be an independent prognostic factor. This is the first report demonstrating the prognostic value of intratumoral Foxp3+/CD8+ ratio. Several aspects may contribute to the present results. First, we evaluated not only Tregs, but also the Foxp3+/CD8+ ratio. Our study proved that they were correlative (χ 2 tests: **P = 0.001, Table 5) and maybe that was the reason why the prognostic significance of intratumoral Tregs was apparent but not remarkable. Second, the present study contained stage I–IV disease compared with stage II–III in Giuseppe Perrone’s earlier study. Third, we assessed not only intratumor but also peritumor, and recent studies have shown that Foxp3+ Tregs in different areas had different prognostic significance (Li et al. 2008; Salama et al. 2008). In addition, we used tissue microarray techniques in our study and the cores taken from the sample were smaller than in the previous study, though microarray techniques have been shown to be a powerful tool for evaluating tumors simultaneously with histological and immunohistochemical analyses (Kononen et al. 1998).
Although the mechanisms of immune suppression are still unclear, it has been reported that Tregs can inhibit the function of effector T cells directly by cell-to-cell contact or indirectly via the secretion of immune-suppressive cytokines (Earle et al. 2005; Dieckmann et al. 2002; Longhi et al. 2006). Two major points are suggested to be responsible for the compromised CD8+ CTLs function of tumor-specific killing in more advanced stage: the dysfunction of the cytolytic machineries and an increase in inhibitory molecules, in which both tumor and host may play a part (Chiou et al. 2005; Yu and Fu 2006). In agreement with earlier study (Giuseppe et al. 2008), we did not find any correlation between intratumoral Tregs and clinical characteristics, although correlations between them were found in many other malignancies.
The present study also evaluates the prognostic information on Tregs in N1 tumor-draining lymph nodes for the first time. The result showed that neither Foxp3+ Tregs nor Foxp3+/CD8+ ratio in N1 regional lymph nodes was associated with prognosis in gastric cancer. Though the K–M cure revealed CD8+ TILs in N1, tumor-draining lymph nodes were associated with a favorable prognosis and there was no statistical significance. Beyond our expectation, we found in this study that gastric cancer without lymph node metastasis was prone to have more Tregs and CD4+ cells. It is presumed that a tumor-related factor may induce the recruitment of CD4+ TILs, especially Foxp3+ Tregs, in the tumor-draining lymph nodes adjacent to the tumor in the early stage of cancer. With the progression of cancer, CD4+ TILs expand to distant lymph nodes and peripheral blood, which may be the reason for the higher frequency of Tregs among TILs, lymphocytes derived from tumor-draining regional lymph nodes (LNLs), and peripheral blood lymphocytes (PBLs) in gastric cancer and esophageal cancer patients in comparison to their normal counterparts (Schumacher et al. 2001; Ichihara et al. 2003).
In conclusion, our results demonstrate that although both higher intratumoral Foxp3+ Tregs and higher ratio of intratumoral Foxp3+/CD8+ are associated with adverse OS in gastric cancer, for the first time we found that only intratumoral Foxp3+/CD8+ ratio is an independent prognostic factor for gastric cancer. These results are very important and will provide promising clinical treatments for cancers, at least in three aspects. First, these findings provide a novel independent predictor for the prognosis of gastric cancer. Intratumoral Foxp3+ Tregs/CD8+ ratio can be utilized clinically as a new prognostic factor. Through the evaluation of intratumor Foxp3+/CD8+ ratios in resected specimens after surgery, clinical information may provide a prognosis of the outcome of gastric cancers, such as OS (overall survival). Second, there are no other reports about Foxp3+ Tregs/CD8+ ratio, which is an independent postoperative prognostic factor in other cancers. So, these results may throw lights on the field of research of other cancers. Third, it is more important that the results also suggest that the combination of depletion of Tregs and concomitant stimulation of CD8+ TILs may be an effective strategy for gastric cancer to prolong survival.
However, as reported by Yuan et al., tumor-infiltrating Treg cells with increased Foxp3 expression can mediate immune suppression via COX-2/PGE (Piccirillo and Thornton 2004) production in the gastric cancer microenvironment (Yuan et al. 2009). So, more reliable assays are needed to analyze not only the presence and relevance of intratumorous regulatory T cells, but also the functional analysis of their suppressive capacity on effector T cells after cocultivation. As a result, further studies should be performed to explore the mechanism of the relationship between the tumor microenvironment and infiltrated effector T cells.
Acknowledgments
This work was supported by grants from the National Science Foundation of China (grants 30500280 and 30871312).
Conflict of interest statement
None.
Appendix
See Fig. 4.
Contributor Information
Chunmin Liang, Email: cmliang@fudan.edu.cn.
Yihong Sun, Email: yihongsun@medmail.com.cn.
References
- Alvaro T, Lejeune M, Salvado MT et al (2005) Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res 11:1467–1473 [DOI] [PubMed] [Google Scholar]
- Amy B, Heimberger, Mohamed AG et al (2008) Incidence and prognostic impact of Foxp3+ regulatory T cells in human gliomas. Clin Cancer Res 14:5166–5172 [DOI] [PubMed] [Google Scholar]
- Badoual C, Hans S, Rodriguez J et al (2006) Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res 12:465–472 [DOI] [PubMed] [Google Scholar]
- Bates GJ, Fox SB, Han C et al (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24:5373–5380 [DOI] [PubMed] [Google Scholar]
- Chiou SH, Sheu BC, Chang WC et al (2005) Current concepts of tumor-infiltrating lymphocytes in human malignancies. J Reprod Immunol 67:35–50 [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L et al (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942–949 [DOI] [PubMed] [Google Scholar]
- Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G (2002) Human CD41CD251 regulatory, contact-dependent T cells, induce interleukin 10-producing, contact-independent type 1-like regulatory T cells. J Exp Med 196:247–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle KE, Tang Q, Zhou X, Liu W, Zhu S, Bonyhadi ML, Bluestone JA (2005) In vitro expanded human CD41CD251 regulatory T cells suppress effector T cell proliferation. Clin Immunol 115:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Xu D, Liu Z et al (2007) Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 132:2328–2339 [DOI] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F et al (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964 [DOI] [PubMed] [Google Scholar]
- Gao Q, Qiu SJ, Fan J et al (2007) Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 25:2586–2593 [DOI] [PubMed] [Google Scholar]
- Giuseppe P, Pier AR, Vincenzo C et al (2008) Intratumoural Foxp3+ Regulatory T cells are associated with adverse prognosis in radically resected gastric cancer. Eur J Cancer 44:1875–1882 [DOI] [PubMed] [Google Scholar]
- Grabenbauer GG, Lahmer G, Distel L et al (2006) Tumor-infiltrating cytotoxic T cells but not regulatory T cells predict outcome in anal squamous cell carcinoma. Clin Cancer Res 12:3355–3360 [DOI] [PubMed] [Google Scholar]
- Griffiths RW, Elkord E, Gilham DE et al (2007) Frequency of regulatory T cells in renal cell carcinoma patients and investigation of correlation with survival. Cancer Immunol Immunother 56:1743–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka N, Onozato K, Kosuge T, Hirohashi S (2006) Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 12:5423–5434 [DOI] [PubMed] [Google Scholar]
- Hori S, Sakaguchi S (2004) Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes Infect 6:745–751 [DOI] [PubMed] [Google Scholar]
- Ichihara F, Kono K, Takahashi A, Kawaida H et al (2003) Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patient with gastric and esophageal cancers. Clin Cancer Res 9:4404–4408 [PubMed] [Google Scholar]
- Japanese Gastric Cancer Association (1998) Japanese classification of gastric carcinoma–2nd English edition. Gastric Cancer 1:10–24 [DOI] [PubMed] [Google Scholar]
- Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24(14):2137–2150 [DOI] [PubMed] [Google Scholar]
- Kawaida H, Kono K, Takahashi A et al (2005) Distribution of CD4+ CD25high regulatory T-cells in tumor-draining lymph nodes in patients with gastric cancer. J Surg Res 124:151–157 [DOI] [PubMed] [Google Scholar]
- Kononen J, Bubendorf L, Kallioniemi A et al (1998) Tissue microarrays for high throughput molecular profiling of tumor specimens. Nat Med 4:844–847 [DOI] [PubMed] [Google Scholar]
- Lee AM, Clear AJ, Calaminici M et al (2006) Number of CD4+ cells and location of forkhead box protein P3-positive cells in diagnostic follicular lymphoma tissue microarrays correlates with outcome. J Clin Oncol 24:5052–5059 [DOI] [PubMed] [Google Scholar]
- Li JF, Chu YW, Wang GM et al (2008) The prognostic value of peritumoral regulatory T cells and its correction with intratumoral cyclooxygenase-2 expression in clear cell renal cell carcinoma. BJU Int 103:399–405 [DOI] [PubMed] [Google Scholar]
- Longhi MS, Hussain MJ, Mitry RR et al (2006) Functional study of CD41CD251 regulatory T cells in health and autoimmune hepatitis. J Immunol 176:4484–4491 [DOI] [PubMed] [Google Scholar]
- Mizukami Y, Kono K, Kawaguchi Y et al (2008) Localisation pattern of Foxp3+ regulatory T cell is associated with clinical behavior in gastric cancer. Br J Cancer 98:148–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano O, Sato M, Naito Y et al (2001) Proliferative activity of intratumoral CD8+ T-lymphocytes as a prognostic factor in human renal cell carcinoma: Clinicopathologic demonstration of antitumor immunity. Cancer Res 61:5132–5136 [PubMed] [Google Scholar]
- Petersen RP, Campa MJ, Sperlazza J et al (2006) Tumor-infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer 107:2866–2872 [DOI] [PubMed] [Google Scholar]
- Piccirillo CA, Thornton AM (2004) Cornerstone of peripheral tolerance: naturally occurring CD4+ CD25+ regulatory T cells. Trends Immunol 25:374–380 [DOI] [PubMed] [Google Scholar]
- Salama P, Phillips M, Grieu F et al (2008) Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 27:186–192 [DOI] [PubMed] [Google Scholar]
- Sato E, Olson SH, Ahn J et al (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 102:18538–18543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher K, Haensch W, Roefzaad C et al (2001) Prognostic significance of activated CD8+ T cell infiltrations within esophageal carcinomas. Cancer Res 61:3932–3936 [PubMed] [Google Scholar]
- Yu P, Fu YX (2006) Tumor-infiltrating T lymphocytes: friends or foes? Lab Invest 86:231–245 [DOI] [PubMed] [Google Scholar]
- Yuan XL, Chen L, Li MX et al (2009) Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2- dependent manner. Clin Immunol. 7 Nov 2009 [Epub ahead of print] [DOI] [PubMed]
- Zou W (2006) Regulatory T cells, tumor immunity and immunotherapy. Nat Rev Immunol 6:295–307 [DOI] [PubMed] [Google Scholar]