Abstract
Topical antimicrobials, or microbicides, are being developed to prevent HIV transmission through local, mucosal delivery of antiviral compounds. While hydrogel vehicles deliver the majority of current microbicide products, intravaginal rings (IVRs) are an alternative microbicide modality in preclinical development. IVRs provide a long-term dosing alternative to hydrogel use, and might provide improved user adherence. IVR efficacy requires sustained delivery of antiviral compounds to the entire vaginal compartment.
A two-dimensional, compartmental vaginal drug transport model was created to evaluate the delivery of drugs from an intravaginal ring. The model utilized MRI-derived ring geometry and location, experimentally defined ring fluxes and vaginal fluid velocities, and biophysically relevant transport theory.
Model outputs indicated the presence of potentially inhibitory concentrations of antiviral compounds along the entire vaginal canal within 24 hours following IVR insertion. Distributions of inhibitory concentrations of antiviral compounds were substantially influenced by vaginal fluid flow and production, while showing little change due to changes in diffusion coefficients or ring fluxes. Additionally, model results were predictive of in vivo concentrations obtained in clinical trials.
Overall, this analysis initiates a mechanistic computational framework, heretofore missing, to understand and evaluate the potential of IVRs for effective delivery of antiviral compounds.
Keywords: HIV/AIDS, transport theory, dapivirine, COMSOL Multiphysics, IVR
INTRODUCTION
Microbicides are topical, anti-viral products designed to block sexual transmission of HIV through directed, local administration of inhibitory compounds. These products are applied to the vaginal or rectal epithelium and aim to prevent transmission of HIV through various mechanisms, including: providing a physical barrier, inhibiting viral entry, blocking viral replication, enhancing natural vaginal defenses, and/or reducing virus-induced immune activation.1-5 Since microbicide products are designed as targeted, local, drug delivery systems, their effectiveness will strongly depend on the abilities of their delivery vehicles to ensure the presence of pharmacologically active compounds in sufficient concentrations around/within vulnerable tissues. Thus, successful development of an effective microbicide requires integrated scientific investigation of both active compounds and their delivery vehicles.
Intravaginal rings (IVRs) are an emerging microbicide delivery modality due to their promise as easy-to-use, longer-term, non-coitally dependent, dosage forms.6-12 Users need only insert a ring once a month, or every few months, to achieve continuous protection.6,8 This dosing schedule could potentially increase user adherence, and therefore microbicide efficacy, compared to coitally-dependent hydrogel-based microbicide delivery vehicles.6,13-15 IVRs have successfully delivered small hydrophobic compounds in contraception and hormone replacement therapy applications,13-16 and development of an IVR for microbicide use has begun with delivery of low-molecular weight, reverse transcriptase (RT) inhibitors of HIV.6-9,11,12 Additionally, the prolonged release and uninterrupted dosing regimen of a ring are ideal for RT inhibitor functionality, since those compounds must attain threshold tissue concentrations prior to HIV exposure to effectively inhibit infection. The analysis here creates a general analytical framework, and illustrates its application with reference to the non-nucleoside RT inhibitor (NNRTI) dapivirine. This is a diarylpyrimidine (DAPY) analogue that has efficacy against a wide range of wild-type and resistant viruses at nanomolar concentrations.12,17 Early clinical results of dapivirine delivery from a silicone elastomer ring are promising: an initial, small clinical trial measured inhibitory concentrations of dapivirine along the entire vaginal canal at 4 hours after ring insertion.12
Compartmental pharmacokinetic modeling of drug delivery by an intravaginal ring could provide enhanced information to assist in understanding and development of effective microbicide delivery. Clinical data on in vivo drug distributions and concentrations from an IVR are limited in temporal and spatial resolution, as every data point requires a patient encounter. Since drug distribution is governed by many different factors, including, ring characteristics, active ingredient(s), the host environment, and multiple transport processes, there can be significant spatial as well as temporal variability in drug concentration within the vagina and surrounding tissues. Therefore, compartmental mathematical analyses could provide greater details into the spatio-temporal distribution of an IVR delivered microbicide. The analysis here introduces a two-dimensional, finite element, pharmacokinetic compartmental model of microbicide delivery by an IVR, using in vitro ring release data, MRI-derived vaginal geometry, and mathematical first principles of drug transport processes. This framework can be applicable to a diverse set of conditions, helping to contribute to optimized IVR design and development.
Our initial model outputs the spatio-temporal dependence of local microbicide concentration within the vaginal mucosa and luminal fluids, as well as within the IVR. It demonstrates the significant role of small amounts of vaginal fluid in drug delivery from IVRs. Model results also provided estimates of the magnitude, onset, and maintenance of threshold levels of microbicide concentrations predicted for efficacy against HIV. These results demonstrate the time-dependence of critical details of microbicide concentration profiles within the lumen and surrounding tissues.
MATERIALS AND METHODS
Analysis was performed using COMSOL Multiphysics (COMSOL, Inc., Burlington, MA), a finite element analysis and solver software package.
Ring Parameters and Mesh Geometry
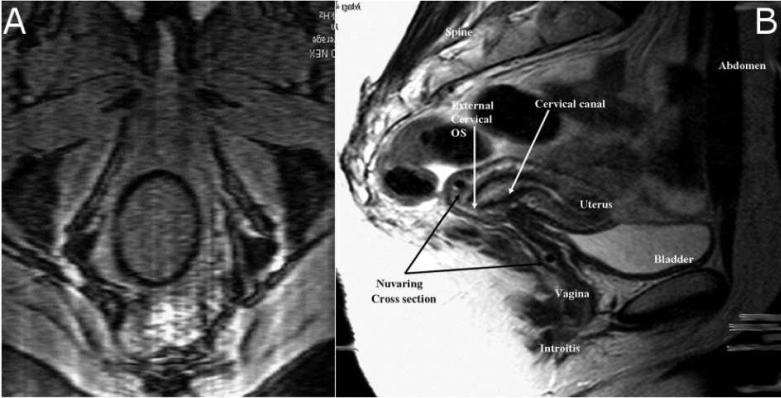
IVRs are being created with a range of dimensions (diameter of the ring ‘loop,’ and the smaller diameter of the ring material cross-section). We chose to analyze the NuvaRing®, a commercially available, FDA approved, IVR indicated for contraception.18 Data are published for both the dimensions and the vaginal position of this device, the latter based on MRI images. The NuvaRing® is a flexible, transparent, colorless, contraceptive ring, made of ethylene vinyl acetate copolymers and magnesium stearate; it measures 54mm in diameter with a 4mm cross-section.19 Sagittal MRI images (Figure 1a) of the NuvaRing in vivo show that the most superior portion of the ring rests within the fornix behind the cervical os.18 Axial MRI images (Figure 1b) reveal that the vaginal tissues compress the NuvaRing into a 57mm × 50mm oval.18 These images strongly suggest that the entire ring is in contact with vaginal tissues, and therefore ring diameter can be used to approximate the width of the vaginal canal at about 50mm. While the width of the human vagina is variable along its length20, we assumed constant average width in our analysis.
Figure 1.
Axial (a) and Sagittal (b) MRI images of NuvaRing® placement within the vagina. Reprinted from18, with permission from Elsevier.
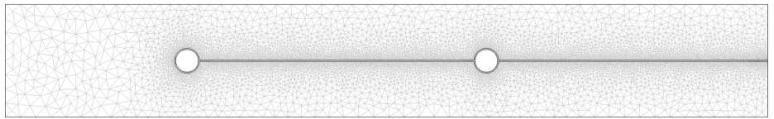
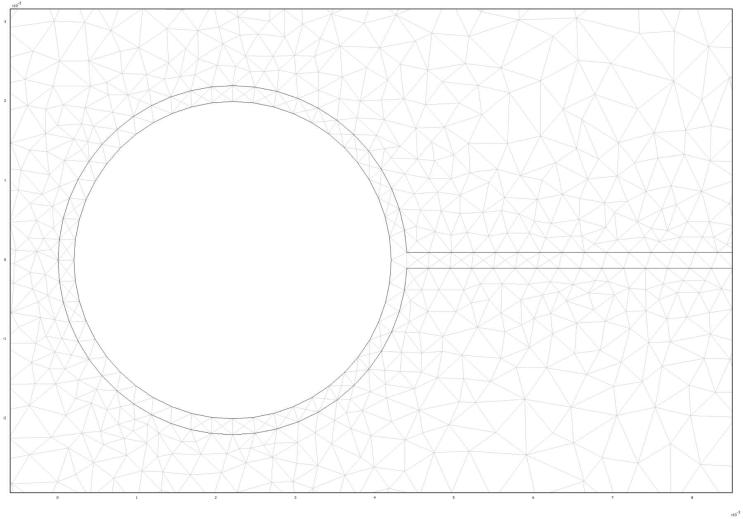
Further analysis of MRI images reveals that the edge of the ring is approximately 47-49mm from the introitus after ambulation, and does not move significantly once in place.18 If we assume that the ring is resting against the posterior wall of the vagina, this estimates vaginal length at about 105mm. That dimension is at the higher range of previous MRI measurements of vaginal length; these found an average length of 63mm, with a range of 41-95mm.20 Such measurements create a theoretical vaginal surface area of approximately 105cm2, which compares well to previous estimates.20 These measurements were used to construct the finite element mesh (Figures 2 & 3) used to solve this problem.
Figure 2.
Finite element mesh used in this computational analysis. The mesh shows a cross-sectional view of an IVR within vaginal tissue. The IVR is represented as the two small circles at the midline of the image; the ring plane is perpendicular to the page. A thin fluid layer runs the length along the midline of the mesh. The remaining area represents vaginal tissue.
Figure 3.
Enlarged region of 2-D mesh showing all three compartments.
The solid cross-section of the ring is shown with the surround layer of vaginal fluid. Again, the plane of the ring extends out of the page. Vaginal tissue surrounds the ring and thin layer of fluid.
Vaginal Fluid Amount and Flow
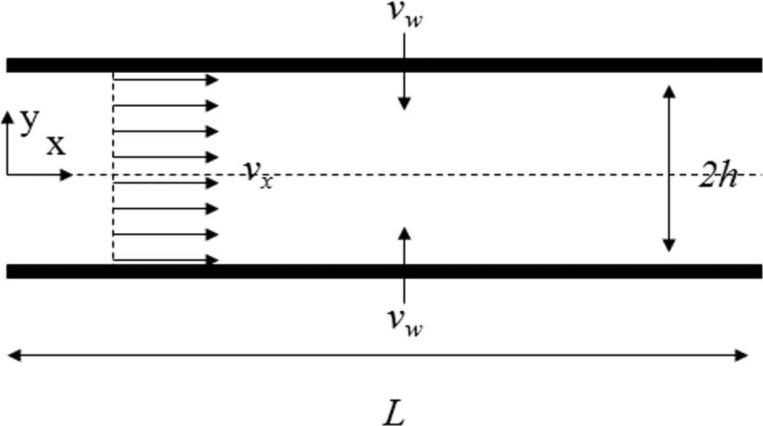
Vaginal tissues exude about 2-8mL of fluid each day. Fluid production is affected by the hormonal changes of the menstrual cycle with more fluid produced at mid-cycle. Approximately 0.5-0.75mL is present at any one time.19,21 This fluid enters the vagina via a transudation process across the vaginal epithelium. As fluid travels along the vaginal canal, increasing fluid volume leads to a greater fluid velocity, due to conservation of mass. Fluid flow in our analysis has been modeled as an axially varying plug flow from the fornix to the introitus. Mathematically, the process can be described as flow in a channel with porous walls. (Figure 4)
Figure 4.
Fluid flow in a channel with porous walls
The flowing layer of vaginal fluid was assumed to be 0.2mm thick. This value was derived from the volume of fluid within the vagina, vagina surface area, and previous approximations.19,21 The x-component of velocity due to pressure driven flow in a channel is:22
| (Eq. 1) |
We can assume plug flow due to the small channel height and low flow speed. The wall velocity in the y-direction can be expressed as:22
| (Eq. 2) |
if vw is constant, a solution for the pressure gradient can be found:
| (Eq. 3) |
Substituting Eq, (3) into Eq. (2), and assuming vx=0 at x=0, we obtain a solution for the uniaxial velocity of fluid flow along the vagina canal in the presence of percolating fluid from the tissue walls.
| (Eq. 4) |
The wall velocity can be estimated using the approximations of vaginal fluid production and volume.
| (Eq. 5) |
The resulting maximum vaginal fluid velocity is approximately 1 mm/s exiting the introitus. This calculation neglects any reabsorption of fluid as well as any uptake of fluid by the cervix. In the following computations, the calculated fluid speed has been varied to investigate how physiologically relevant changes in fluid flow affect IVR drug delivery.
Ring Flux
Current IVR development has focused on developing rings with constant flux, zero-order release kinetics7,11,12 to provide a consistent dose of drug into the vaginal compartment. Release rate is directly proportional to the amount of drug loaded into the ring, and is affected by ring geometry and/or polymer composition.7 Release rates in vitro for several different dapivirine IVRs range from 50-473μg/day.7,8,11,12 The analysis here emphasizes an IVR that is loaded with 25mg of dapivirine and that releases 50μg/day. Rings with this release rate have been employed previously in a clinical trial,12 providing in vivo data for comparison. However, there are several different IVRs currently under development, each having different dimensions, composition, and/or drug concentration, and therefore a different release rate. Therefore, in order to broaden the interpretation of results obtained here, we considered the general influence of drug release rate on overall drug distribution: we varied the net flux of dapivirine and calculated the subsequent changes to time-dependent tissue concentration distributions of drug.
Drug release from the ring was modeled as a constant flux boundary condition on the ring surface. This is consistent with the zero-order release kinetics demonstrated in IVRs currently in development7,8,11. Concentration profiles within the interior of the ring were not calculated in this analysis. Thus,
The use of a sagittal 2-D model to simulate a 3-D delivery by an IVR will underestimate delivered drug concentrations due to the assumption that all drug present in the 2-D section is delivered solely from the portion of the ring within the same section. In actuality, drug concentrations in vivo will be influenced by the entire 3-D geometry of the ring, which includes portions of the ring that are out of the 2-D plane. This underestimation can be mitigated by employing a set of diffusion-only models (no convection) to adjust the 2-D flux to produce concentration profiles similar to those from a 3-D geometry. Concentration profiles were analyzed for the current 2-D sagittal model of the ring, a 2-D axial model of the ring, and a 3-D diffusion model for an IVR in tissue. Then 2-D sagittal flux values were adjusted to reach maximum concordance with all three models.
A summary of parameters is contained in Table 1.
Table 1.
Parameters used in IVR model
| Parameter | Value |
|---|---|
| IVR dimensions | 57mm × 50mm |
| IVR cross-sectional diameter | 4mm |
| Vaginal length | 105mm |
| Vaginal width | 50mm |
| Vaginal fluid volume | 0.75mL |
| Vaginal fluid output | 6mL/day |
| Fluid flow rate | 9.72×10-4 cm/s |
| Fluid layer thickness | 0.2mm |
| IVR flux | 50μg/day |
| Tissue thickness | 1-3cm |
| Dapivirine MW | 329.4g/mol |
| Dapivirine IC50 (HIV-1 LAI in MT4 cells)12 | 0.33 ng/ml |
| Dapivirine Diffusion Coefficient | 4.86×10-6 cm2/s |
Dapivirine Transport Mechanisms
Drug released into the fluid compartment was transported by both diffusion and convection. The Peclet number (2hvx/D), which characterizes the ratio of transport by convection over transport by diffusion, becomes greater than unity about 25mm from the fornix, and approaches 5 at the introitus, signifying that convective fluid flow drives most of the drug transport along the majority (>75%) of the vaginal canal. However, diffusion remains the primary modality of drug transport transversely into the adjacent tissues. The diffusion coefficient of dapivirine in fluid was approximated using experimentally defined diffusion coefficients of a compound of similar molecular weight, fluorescein.23 As developed above, convection was modeled as an axially varying plug flow field (Eq. 4) from the fornix to the introitus with a velocity equal to the previously calculated flow speed (1.2×10-3 cm/s). Dapivirine is extremely lipophilic, with a logP of 5.29 at pH 9.24 Upon release into the fluid from the ring, it will most likely strongly partition into cellular membranes due to its hydrophobicity. As a result, in this analysis, drug freely transfers between the fluid/tissue boundary due to a unity partition coefficient and a continuity boundary condition. The unity value for the partition coefficient in this analysis may underestimate the kinetics of dapivirine entering tissue. However, this underestimation is minimized due to the relative thinness of the fluid layer with respect to the high concentration gradients driving diffusion. At the edge of the 2-D geometry, there is a zero concentration boundary condition. This reflects immediate entry of dapivirine into the bloodstream.
RESULTS AND DISCUSSION
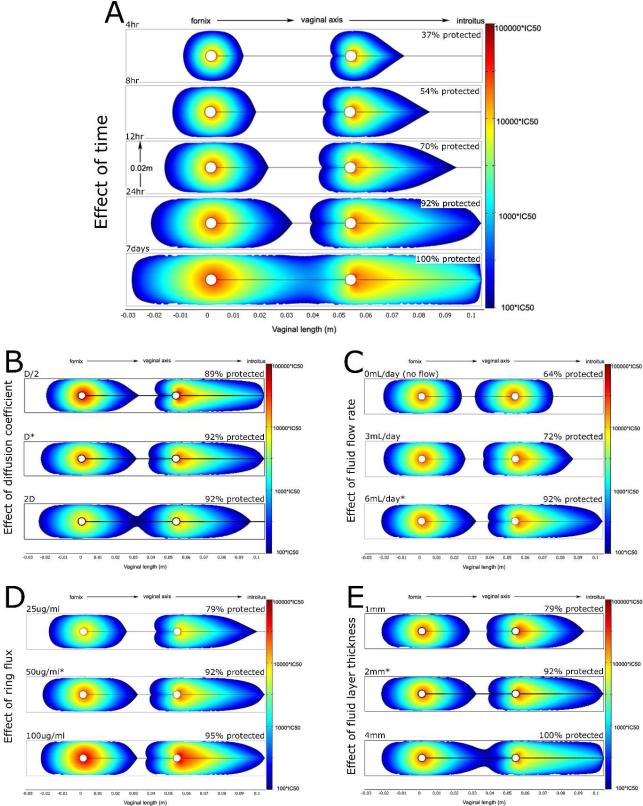
The composite Figure 5 summarizes results. This initial model of IVR drug delivery provides spatio-temporal estimates of local dapivirine concentrations within the vagina. Model results are largely consistent in both magnitude and distribution with clinical data on IVR delivery of dapivirine12, suggesting that this type of computational framework has predictive ability. Furthermore, the model demonstrates that IVRs can effectively distribute antiviral compounds along the vaginal canal and should be pursued as a viable drug delivery modality for microbicides. Additionally, this type of in silico modeling augments and extends in vitro and in vivo information by providing details about the kinetics of drug delivery, and demonstrating how changes to the vaginal environment or the IVR itself affects drug delivery. Specifically, this analysis suggests that an IVR will take approximately 24 hours to distribute its active compound at inhibitory concentrations over >90% of the vaginal epithelium (Figure 5A). While these kinetics do not account for fluid movement during intercourse, and therefore may underestimate the speed and extent of total distribution, 24 hours after insertion may serve as a conservative guideline when designing future clinical trials. For example, it provides guidance for the post insertion period during which protection might not be achieved. We note that while the predicted concentrations of dapivirine closely mirror the measured values within clinical trials, the kinetics are slightly slower than previously measured estimates of IVR delivery: in vivo studies of a dapivirine IVR showed sufficient drug concentrations at three different points along the vaginal axis after only 4hrs.12 Such discrepancies may be due to multiple factors, ranging from sample collection techniques to differences between in vitro measurements (our input parameters) and in vivo behavior. However, the overall message is that consistent protection will not be instantaneous; initial studies should allow extra time prior to viral challenge to allow the true measurement of IVR effectiveness in HIV prevention.
Figure 5.
Results from the computational analysis of microbicide delivery by an intravaginal ring. Colored regions denote dapivirine levels >100*IC50; actual concentrations are given by the scale bars on the right. As a quantitative measure, the percentage of vaginal epithelium with inhibitory concentrations of dapivirine is shown above each plot. Unless otherwise stated, all plots show dapivirine concentrations 24 hours post insertion using a IVR with flux = 50μg/day, a fluid thickness of 2mm, and vaginal fluid production rate of 6mL/day. The influence of several factors on dapivirine delivery was evaluated: A) Effect of time. Protection via IVR delivery is not instantaneous. Approximately 24 hours is needed for most of the vulnerable epithelium to be protected. At 4 hours, only about 37% of the tissue is protected, while after 24 hours almost all (92%) tissue is exposed to inhibitory concentrations. B) Effect of diffusion coefficient. The diffusion coefficient of dapivirine was increased and decreased by 2-fold. Changes in rate of diffusion had minimal effect on the range of inhibitory drug concentrations after 24 hours. C) Influence of vaginal fluid production rate. Greater fluid flow rates allow the IVR to protect a greater amount of vaginal epithelium after 24hrs. D) Influence of IVR flux. The fraction of vaginal tissue that is protected by inhibitory concentrations of drug is only slightly influenced by a two-fold increase or decrease of dapivirine flux from the ring. All doses provided significant protection along the entire vaginal canal at 24 hours. E) Effect of fluid layer thickness. Two-fold differences in fluid layer thickness also had minor effects on overall protection.
Additionally, this pharmacokinetic model allowed sensitivity analyses into the effects of changes in diffusion coefficient, fluid flow, ring flux, and fluid layer thickness (Fig 5), summarized as follows.
Diffusion coefficient
IVRs may deliver a range of active compounds with various molecular weights, and therefore different diffusion coefficients. Figure 5B shows the effect of a 2-fold variation in drug diffusion coefficient. Changes in rate of diffusion had minimal effect on the range of inhibitory drug concentrations after 24 hours; assuming their compatibility with IVR materials, differences in active compounds should not affect drug distribution or dictate ring usage patterns.
Fluid flow
Fluid production in the vagina varies widely between women and even within a single woman, due to several physiological factors (cycle phase, age, etc.). Additionally, IVRs have also been associated with increased vaginal fluid production.25 To investigate how changes in fluid flow might affect IVR drug delivery, we predicted active compound concentration distributions for cases of no fluid flow, low fluid flow (3mL/day), or average fluid flow (6mL/day) after 24hours (Fig 5C). Results suggest that fluid flow is the primary mechanism for active compound distribution along the vaginal canal, and therefore has a large effect on distributions of antiviral compounds from an IVR. Convective fluid transport moves drug linearly along the vaginal axis, where it diffuses freely into the adjacent tissues. This critical role of the presence and movement of vaginal fluid is illustrated by our results that drug transport by diffusion alone was not sufficient for adequate protection, even after 7 days (data not shown). This fluid delivery hypothesis is consistent with results of a small IVR clinical trial which detected high concentrations of dapivirine in patients’ vaginal fluids along the entire length of the canal.12 Such factors could influence both optimization of ring design and time to prophylactic onset after ring insertion, since drug distributions will likely be different between women, and even for the same woman at different times in her cycle.
Ring flux
Interestingly, 2-fold changes in ring flux did not have substantial effects on the range of inhibitory concentrations within the vagina. Increased ring fluxes caused higher local concentrations, which might be a concern for local toxicity; however, overall drug distribution remained similar for the two fluxes. While hardly obvious, these findings are in agreement with the findings of a clinical trial that showed that the distribution of dapivirine does not significantly differ between a 25mg ring versus a 200mg ring.12 This suggests that in the absence of toxicity issues, a range of ring fluxes could be sufficient and protective. We also note that these results pertain to altering the surface area of the IVR. Because convection in vaginal fluid is the dominant mechanism of longitudinal drug transport, doubling the ring flux is analogous to doubling the surface area of the IVR. There are a number of different ring shapes and sizes currently under development. Another common ring size has a cross-sectional diameter that is roughly double our modeled ring diameter (8.4mm vs 4mm).12 Since a range of ring fluxes result in similar levels of drug distribution, a range of ring shapes and sizes may also be adequate for microbicide delivery. Final selection could then be based an additional criteria, e.g. acceptability to users.
Fluid layer thickness
Finally, changes in thickness of the fluid layer also had relatively minor effects on drug distribution after 24hours, suggesting that model results are not especially sensitive to variations in this estimated parameter.
In conclusion, intravaginal rings are emerging as a promising microbicide delivery modality. However, due to the microbicide field's relative inexperience with IVR delivery, little is known about their performance in vivo. To help fill this gap, we have created an MRI-derived, three-compartment, two-dimensional, finite element model of drug delivery by an IVR. We used the model to predict the temporal and spatial distribution of a particular NNRTI within the vaginal compartment. Results mirror and support clinical findings that currently available IVRs can successfully distribute antiviral compounds throughout the vaginal compartment. Additionally, they demonstrate the relative flexibility of IVR design: a wide range of antiviral compounds and ring fluxes should provide sufficient protection against HIV transmission. This model also informs future clinical trials regarding onset kinetics of ring protection, expected differences between female volunteers (e.g. in fluid levels), and the need monitor for local toxicity in tissues adjacent to the ring. Overall, this pharmacokinetic model strongly suggests that IVRs are a viable method for effective microbicide delivery.
ACKNOWLEDGEMENTS
We thank Drs. Fan Yuan and George Truskey for their assistance with relevant transport theory and computational modeling, Dr. Patrick Kiser for information on IVR design, and Dr. Lisa Rohan for her insights into IVR drug delivery. We also acknowledge the support of the NIH, through U19 AI 07728.
REFERENCES
- 1.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458(7241):1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fichorova RN, Tucker LD, Anderson DJ. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. Journal Of Infectious Diseases. 2001;184(4):418–428. doi: 10.1086/322047. [DOI] [PubMed] [Google Scholar]
- 3.Lederman MM, Offord RE, Hartley O. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nature Reviews Immunology. 2006;6(5):371–382. doi: 10.1038/nri1848. [DOI] [PubMed] [Google Scholar]
- 4.Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nature Reviews Immunology. 2005;5(10):783–792. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 5.Stone A. Microbicides: A new approach to preventing HIV and other sexually transmitted infections. Nature Reviews Drug Discovery. 2002;1(12):977–985. doi: 10.1038/nrd959. [DOI] [PubMed] [Google Scholar]
- 6.Klasse PJ, Shattock R, Moore JP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu Rev Med. 2008;59:455–471. doi: 10.1146/annurev.med.59.061206.112737. [DOI] [PubMed] [Google Scholar]
- 7.Gupta KM, Pearce SM, Poursaid AE, Aliyar HA, Tresco PA, Mitchnik MA, Kiser PF. Polyurethane Intravaginal Ring for Controlled Delivery of Dapivirine, a Nonnucleoside Reverse Transcriptase Inhibitor of HIV-1. Journal of Pharmaceutical Sciences. 2008 doi: 10.1002/jps.21331. In Press. [DOI] [PubMed] [Google Scholar]
- 8.Woolfson AD, Malcolm RK, Morrow RJ, Toner CF, McCullagh SD. Intravaginal ring delivery of the reverse transcriptase inhibitor TMC 120 as an HIV microbicide. Int J Pharm. 2006;325(1-2):82–89. doi: 10.1016/j.ijpharm.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 9.Woolfson AD, Malcolm RK, Toner CF, Morrow RJ, Lowry D, Jamil A, McCullagh SD. Potential Use of Vaginal Rings for Prevention of Heterosexual Transmission of HIV: A Controlled-Release Strategy for HIV Microbicides. American Journal of Drug Delivery. 2006;4:7–20. [Google Scholar]
- 10.Malcolm K, Woolfson D, Russell J, Andrews C. In vitro release of nonoxynol-9 from silicone matrix intravaginal rings. J Control Release. 2003;91(3):355–364. doi: 10.1016/s0168-3659(03)00260-8. [DOI] [PubMed] [Google Scholar]
- 11.Malcolm RK, Woolfson AD, Toner CF, Morrow RJ, McCullagh SD. Long-term, controlled release of the HIV microbicide TMC120 from silicone elastomer vaginal rings. Journal of Antimicrobial Chemotherapy. 2005;56(5):954–956. doi: 10.1093/jac/dki326. [DOI] [PubMed] [Google Scholar]
- 12.Romano J, Variano B, Coplan P, Van Roey J, Douville K, Rosenberg Z, Temmerman M, Verstraelen H, Van Bortel L, Weyers S, Mitchnick M. Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res Hum Retroviruses. 2009;25(5):483–488. doi: 10.1089/aid.2008.0184. [DOI] [PubMed] [Google Scholar]
- 13.Anibal F, Vivian B, Frank A. Pros and Cons of Vaginal Rings for Contraceptive Hormone Delivery. American Journal of Drug Delivery. 2004;2:241–250. [Google Scholar]
- 14.Roumen FJME, Apter D, Mulders TMT, Dieben TOM. Efficacy, tolerability and acceptability of a novel contraceptive vaginal ring releasing etonogestrel and ethinyl oestradiol. Human Reproduction. 2001;16(3):469–475. doi: 10.1093/humrep/16.3.469. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar NN. The combined contraceptive vaginal device (NuvaRing): a comprehensive review. Eur J Contracept Reprod Health Care. 2005;10(2):73–78. doi: 10.1080/13625180500131683. [DOI] [PubMed] [Google Scholar]
- 16.Weisberg E, Fraser IS, Mishell DR, Jr., Lacarra M, Darney P, Jackanicz TM. A comparative study of two contraceptive vaginal rings releasing norethindrone acetate and differing doses of ethinyl estradiol. Contraception. 1999;59(5):305–310. doi: 10.1016/s0010-7824(99)00036-0. [DOI] [PubMed] [Google Scholar]
- 17.Das K, Clark AD, Jr., Lewi PJ, Heeres J, De Jonge MR, Koymans LM, Vinkers HM, Daeyaert F, Ludovici DW, Kukla MJ, De Corte B, Kavash RW, Ho CY, Ye H, Lichtenstein MA, Andries K, Pauwels R, De Bethune MP, Boyer PL, Clark P, Hughes SH, Janssen PA, Arnold E. Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants. J Med Chem. 2004;47(10):2550–2560. doi: 10.1021/jm030558s. [DOI] [PubMed] [Google Scholar]
- 18.Barnhart KT, Timbers K, Pretorius ES, Lin K, Shaunik A. In vivo assessment of NuvaRing(R) placement. Contraception. 2005;72(3):196–199. doi: 10.1016/j.contraception.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Saltzman WM. Drug Delivery: Engineering Principles for Drug Therapy. Oxford University Press; New York: 2001. [Google Scholar]
- 20.Barnhart KT, Izquierdo A, Pretorius ES, Shera DM, Shabbout M, Shaunik A. Baseline dimensions of the human vagina. Human Reproduction. 2006;21(6):1618–1622. doi: 10.1093/humrep/del022. [DOI] [PubMed] [Google Scholar]
- 21.Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59(2):91–95. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 22.Truskey GA, Yuan F, Katz DF. Transport Phenomena in Biological Systems. Pearson Prentice Hall; Upper Saddle River: 2004. [Google Scholar]
- 23.Geonnotti AR, Furlow MJ, Wu T, DeSoto MG, Henderson MH, Kiser PF, Katz DF. Measuring macrodiffusion coefficients in microbicide hydrogels via postphotoactivation scanning. Biomacromolecules. 2008;9(2):748–751. doi: 10.1021/bm701018w. [DOI] [PubMed] [Google Scholar]
- 24.Rohan LC. Feb 8, 2008. Personal communication of unpublished data.
- 25.Roumen FJME. Review of the combined contraceptive vaginal ring, NuvaRing. Therapeutics and Clinical Risk Management. 2008;4(2):441–451. doi: 10.2147/tcrm.s1964. [DOI] [PMC free article] [PubMed] [Google Scholar]