Introduction
The actin system comprises a major part of the cytoskeleton of eukaryotic cells.[1] In non muscle cells there is a roughly equal proportion of polymeric F-actin and monomeric G–actin (globular). The polymerisation of globular actin requires ATP. Among known natural products that disturb the actin system is cytochalasin which blocks the polymerisation of G-actin by binding to the end of the growing F-actin filaments.[2] On the other hand, the cyclic peptide phalloidin[3] prevents depolymerisation of F-actin by binding between actin monomers. Another key component of the cytoskeleton is the tubulin system. In this case the monomer tubulin forms a dynamic equilibrium with the polymer called microtubules. Compounds like taxol or the epothilones that disturb the tubulin system gained clinical relevance as anticancer drugs.[4]
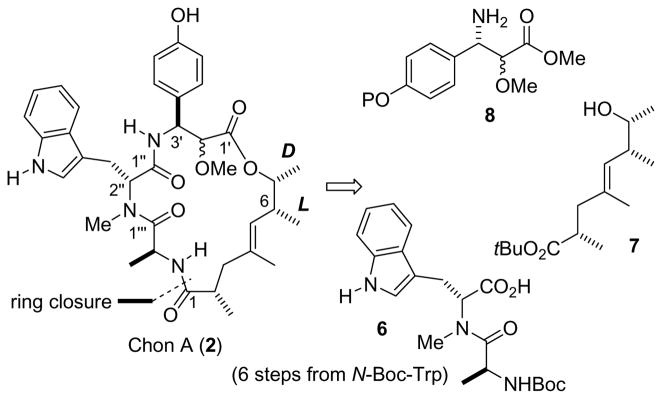
Besides phalloidin, the jaspamide/chondramide family of cyclic depsipeptides[5] does also stabilize F-actin. In this regard, they are interesting lead compounds for drug design. For example, appropriate analogs thereof might be able to bind F-actin in a species dependent manner. Jaspamide (Jasplakinolide, jas) (1) features a 19-membered macrocyclic ring (Figure 1). It was isolated from the marine sponge[6] Jaspis splendens. Related compounds were later found in several other sponges.[7] While showing promising biological properties as an anti-actin agent, this activity is accompanied by significant toxicity. The major difference between the chondramides, which were isolated from the myxobacterium Chondromyces crocatus,[8] and jaspamide is a different polyketide moiety, resulting in an 18-membered ring. Quite recently the configurational assignment for chrondramide C (chon C) was achieved independently by the Waldmann[9] and Kalesse[10] groups. Not surprisingly, the configurations of the three amino acids L-alanine, D-N-methyltryptophan, and L-β-tyrosine turned out to be the same as in jaspamide. However, the proposal[11] for the configuration of the ω-hydroxy acid required revision. We became interested in the synthesis of chondramide A (chon A) since preliminary results showed a higher therapeutic index against the parasite toxoplasma gondii as compared to chon B, chon C and jaspamide. While the L-configuration for the amine-bearing stereocenters in the α-methoxy-β-tyrosine could be assumed, the configuration at the α-carbon remained to be solved. In this paper we describe syntheses of diastereomers of the methoxytyrosine subunit and their incorporation into the chrondramide scaffold leading to the total synthesis and configurational assignment of chondramide A.
Figure 1.
Structures of jasplakinolide and the chondramides. The atom numbering follows the suggestions from the isolation papers.
Results and Discussion
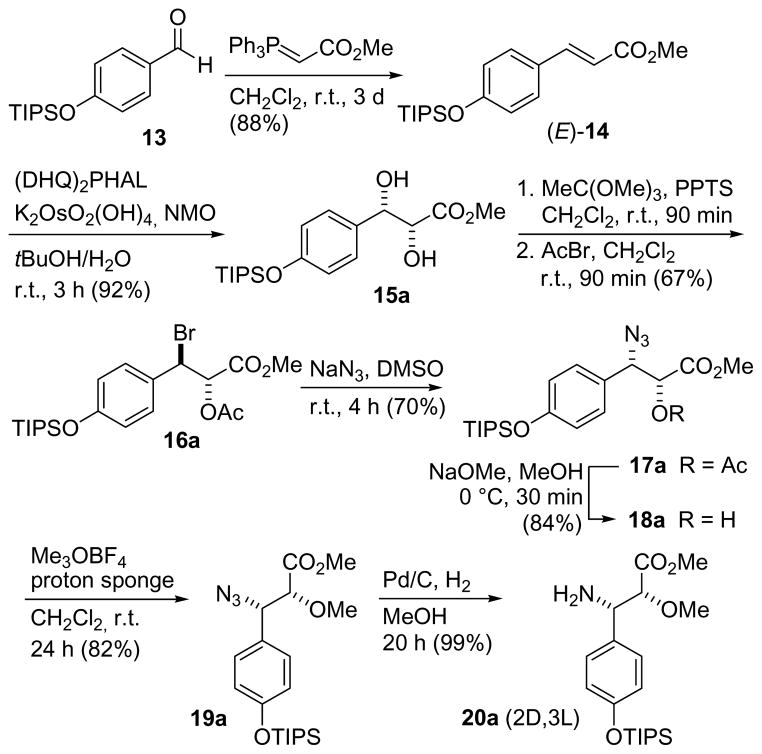
It was planned to close the macrocyclic ring by intramolecular amide formation (Figure 2). The corresponding acyclic ester would originate from the known dipeptide acid 6, a β-tyrosine isomer 8 and the hydroxy ester 7. The dipeptide acid 6 is available in six steps from N-Boc-D-tryptophan.[12]
Figure 2.
Key fragments for the synthesis of chondramide A diastereomers.
For the more challenging hydroxy ester 7 we recently reported a concise route that relies on a Kobayashi vinylogous aldol reaction with acetaldehyde, a Mitsunobu inversion at C7, and an asymmetric allylation to incorporate the last propionate unit (Scheme 1).[13] Overall this 12-step sequence allows for the preparation of ester 7 in gram amounts.
Scheme 1.
Summary of the synthesis of hydroxy ester 7.
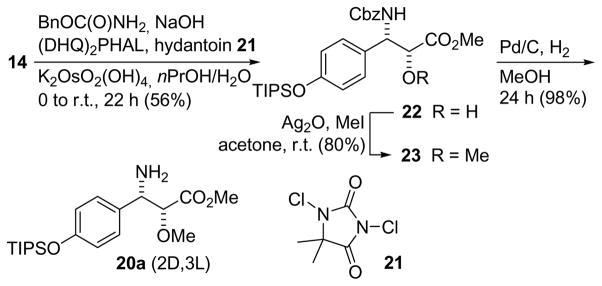
The syn-isomer (2D,3L) of 3-amino-2-methoxyester 8 is similar to the taxol side chain.[14] Accordingly, strategies that came to use in the synthesis of this side chain, a 3-phenylisoserine, or related compounds were considered.[15] For example, asymmetric nitroaldol reactions,[16] the Staudinger reaction leading to β-lactam intermediates,[17] the Sharpless asymmetric aminohydroxylation,[18] or asymmetric aldol reactions of glycolates to imines are known to produce these types of structures.[19] And finally, chemoselective substitution reactions on 2,3-dihydroxy-3-arylpropanoates would provide the syn diastereomer of 8.[20] We opted for the latter due to its operational simplicity (Scheme 2). Thus, starting from 4-(triisopropylsilyloxy)benzaldehyde[21] (13) a Wittig reaction with (methoxycarbonylmethylene)triphenylphosphorane[22] provided cinnamate (E)-14 in good yield. A subsequent asymmetric dihydroxylation[23] in presence of the ligand (DHQ)2PHAL (ADmix-α), furnished an excellent yield of diol 15a. As has been described by Sharpless et al.[20] the benzylic alcohol can be substituted by bromide through reaction of the diol with trimethyl orthoacetate, and treatment of the intermediate mixed orthoester with acetyl bromide. This way, a 67% yield of acetoxy bromo ester 16a could be secured after chromatographic separation of the wrong regioisomer. Substitution of the bromide on 16a with sodium azide in DMSO at room temperature resulted in azide 17a (70% yield). Proof of the regiochemistry came from a HMBC spectrum of 17a. Thus, 3′-H [4.96 (d, J = 5.6 Hz), chon A numbering] showed cross peaks to C-4′ and C-5′. After saponification of the acetate, the 2-hydroxy group of 18a was methylated using Meerwein’s salt (Me3OBF4) [24] and proton sponge in dichloromethane. A final hydrogenation of azide 19a in presence of Pd/C in methanol led to the desired syn-configured amino ester 20a. The optical purity of 15a and the propanoate derivatives derived from it could be estimated at the stage of the tripeptide ester 24a to be yy% by integration the corresponding 2′-H protons.
Scheme 2.
Synthesis of the (2D,3L)-diastereomer 20a via the anti-acetoxy bromo ester 16a.
An alternative route to the (2D,3L)-β-tyrosine 20a was based on an aminohydroxylation reaction[25, 26, 27] of cinnamate 14 using benzylcarbamate, 1,3-dichloro-5,5-dimethylhydantoin[28] and (DHQ)2PHAL in a propanol/water mixture (Scheme 3). This produced the Cbz proptected 2-hydroxy-3-amino acid ester 22. Reductive cleavage of the Cbz protecting group (H2, Pd/C, MeOH) gave as well β-tyrosine 20a. In this case the etherification was performed with MeI and Ag2O in acetone. The optical purity of 20a was estimated at the stage of the tripeptide ester 24a to be 98% by integration the corresponding 2′-H protons.
Scheme 3.
Synthesis of the (2D,3L)-3-amino-2-methoxy acid 20a via an aminohydroxylation reaction.
Construction of the corresponding cyclodepsipeptide started with dipeptide acid 6 (Scheme 4). This acid was condensed with β-tyrosine derivative 20a using TBTU in presence of additional hydroxybenzotriazole (HOBt) yielding tripeptide ester 24a. The subsequent saponification of the ester group turned out to be crucial since cleavage of the TIPS ether or epimerization alpha to the carboxyl group had to be avoided. We therefore turned to the highly chemoselective trimethylstannol which produced tripeptide acid 25a within 5 h at 80 °C.[29] The crude carboxylic acid could then be esterified with hydroxyester 7 under Yamaguchi conditions.[30] This way the Chon A precursor 26a could be secured in 64% yield. The macrocyclization required cleavage of the N-Boc group and the tert-butylester. This was done with TFA in dichloromethane. It seemed that the N-Boc group cleaved quite easy, whereas the ester cleavage necessitated longer reaction times. After concentration of the reaction mixture, macrolactam formation on the residue was carried out in DMF (0.001 M) in presence of TBTU, HOBt and Hünig’s base.[31, 32] After work-up the crude cyclic depsipeptide was treated with TBAF in THF to cleave the phenolic triisopropylsilyl ether. Careful inspection of the NMR data showed that macrocycle 2a did not correspond to natural chondramide A. The macrocycles show characteristic NMR signatures for the four methyl doublets. In 2a (CD3OD) they appear at δ = 0.81, 0.91, 0.92, 1.09 (2-CH3).
Scheme 4.
Synthesis of diastereomer 2a which does not correspond to chondramide A from the three building blocks 6, 20a, and 7.
Therefore, we surmised the (2L,3L)-3-amino-2-methoxy-tyrosine to be the isomer present in chondramide A. Based on the experience with the asymmetric dihydroxylation, we hoped to reach diastereomer 20b from a Z-configured cinnamate. Accordingly, a Horner-Wittig reaction of aldehyde 13 with the Ando phosphonate[33] (NaH, THF) was performed resulting in a 27/73 E/Z-mixture of enoates 14 from which (Z)-14 could be separated by flash chromatography (Scheme 5). Using (Z)-14 a sequence of dihydroxylation [(DHQ)2PHAL] as ligand, orthoester formation [(MeO)3CMe], and reaction of the intermediate orthoester with acetyl bromide was expected to give acetoxy bromoester 16b. The obtained material was converted in four steps to the presumed amino ester 20b. This building block was then utilized for the synthesis of presumed cyclodepsipeptide 2b (for details see supporting information). Again, the spectral date of the macrocycle 2b (expected) did not match with those of chondramide A.
Scheme 5.
Attempted synthesis of amino ester 20b and its incorporation in the cyclodepsipeptide. The obtained product 2c did not correspond to chondramide A.
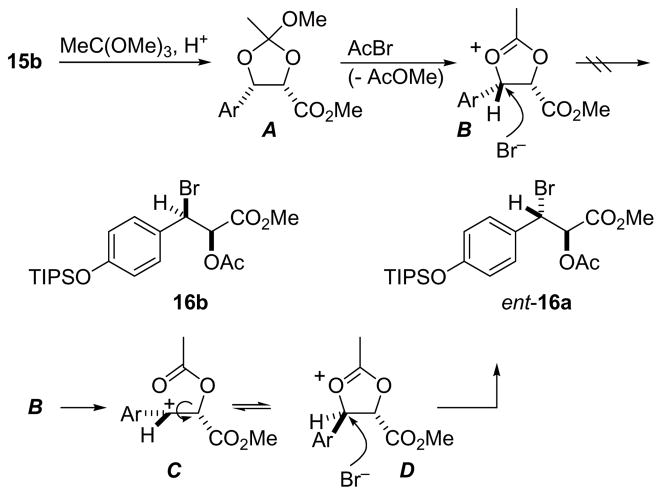
The conclusion from this rather unexpected finding was that either chondramide A contains a β-D-tyrosine derivative or that the substitution reaction on the benzylic position took a different course. The latter turned out to be the case. Thus, it seems that reaction of the presumed orthoester A with acetyl bromide led to isomerization of the cyclic oxonium ion B to the corresponding trans isomer D before reaction with the bromide (Scheme 6). Thus, instead of the syn-isomer, the corresponding trans-acetoxy bromo ester ent-16a was formed. Accordingly, this sequence led to 3-amino-tyrosine ester ent-20a and ultimately to chondramide A analogue 2c.
Scheme 6.
Postulated formation of ent-16a from diol 15b via isomerization of cyclic oxonium ion B to D.
The structure of ent-20a could be proven by performing an aminohydroxylation on enoate (E)-14 with (DHQD)2PHAL as ligand (Scheme 7). In fact, the two compounds showed identical NMR spectra and optical rotation. The optical purity of ent-20a from the aminohydroxylation amounted to 98% whereas the material from the dihydroxylation route (Scheme 5) turned out to be 36% according to analysis of the derived tripeptide.
Scheme 7.
Independent synthesis of 3-amino-2-methoxy-tyrosine derivative ent-20b by aminhydroxylation.
Therefore access to the 2L,3L-diastereomer 20b of 3-amino-2-methoxy tyrosine was sought from a diol precursor by substituting the benzylic hydroxyl function with an ammonia equivalent. Dihydroxylation of E-enoate 14 in presence of (DHQD)2PHAL provided ent-15 in excellent yield (Scheme 8). Initially we tried substitution of the 3-OH with diphenylphosphoryl azide and diethylazodicarboxylate (DEAD). However, under these conditions no product was formed. On the other hand, the Mitsunobu reaction on ester diol ent-15 with hydrazoic acid[34] (HN3) in presence of DEAD/Ph3P gave the desired azide 18b (47% yield) together with the corresponding syn-isomer (8% yield).[35] The two isomers could be separated by chromatography at this stage. The pure anti 2-hydroxy-3-azido ester 18b was carried on to the methylation step (Me3OBF4, proton sponge).[24] Hydrogenation of diastereomer 19b-anti led to 3-amino-2-methoxy-tyrosine derivative 20b. According to the integration of the 2′-H atoms in the 1H NMR spectrum of 24b, the ee of 20b and its precursors is 72%.
Scheme 8.
Synthesis of the anti-diastereomer (2L,3L) 20b via dihydroxylation and substitution of the benzylic hydroxyl group by azide.
As shown in Scheme 9 condensation of 20b with dipeptide acid 6 and the hydroxy ester 7 (three steps) led via tripeptide ester 24b to acyclic chondramide A precursor 26b in good overall yield. Analysis of the 1H NMR spectrum of 24b indicated an ee of 99% for the dihydroxylation reaction (14 to ent-15a). The proven sequence consisting of cleavage of the tert-butyl groups from the carbamate and ester function followed by macrolactam formation and fluoride-induced silyl ether cleavage gave a reasonable yield of cyclodepsipeptide 2b. The spectral data of this synthetic compound proved to be identical to the ones from chondramide A. In 2b (CD3OD) the methyl doublets appear at δ = 0.83, 0.86, 0.92, 1.08 (2-CH3). The optical rotation of synthetic 2b was [α]20D = +7.9 (c 0.80, MeOH), the literature reports [α]20D = +2.1 (c 2.0, MeOH).[8] Furthermore, LC-MS studies at the HZI in Braunschweig confirmed this assignment.
Scheme 9.
Synthesis of chondramide A (2b).
Conclusion
In conclusion we developed a concise route to the cyclodepsipeptide chondramide A (2b). The synthesis established the configuration of the 3-amino-2-methoxy tyrosine to be (2S,3R) or (2L,3L). For the synthesis of the 3-amino-2-methoxy-3-arylpropanoic ester 20b, the diol ent-15 was converted to the corresponding 3-azido-2-hydroxypropanoate 18b via a regioselective Mitsunobu reaction. Extension of the aminoester 20b on the amino function with dipeptide acid 6 and the carboxyl function with the hydroxy ester 7 secured the open chain precursor 26b of chondramide A. Macrolactam formation could be achieved with TBTU in DMF. This strategy should now allow for the preparation of chon A analogues having modifications in all four subunits.
Experimental Section
4-[(Triisopropylsilyl)oxy]benzaldehyde (13)
To a stirred solution of 4-hydroxybenzaldehyde (3.64 g, 29.8 mmol) in CH2Cl2 (60 mL) at 0 °C were added Et3N (4.96 mL, 3.62 mmol) and DMAP (0.364 g, 2.98 mmol). The mixture was stirred for 10 min before TIPSCl (7.00 mL, 32.8 mmol) was added. The mixture was allowed to warm to room temperature. After stirring for 18 h, the reaction mixture was treated with water (20 mL) and the aqueous layer was extracted with CH2Cl2 (2 × 30 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purified by flash chromatography (petroleum ether/EtOAc, 20:1) to afford aldehyde 13 (7.95 g, 96%) as a colorless oil. TLC (petroleum ether/EtOAc, 20:1): Rf = 0.33; 1H NMR (400 MHz, CDCl3): δ [ppm] = 1.10 (d, J = 7.4 Hz, 18H, Si(CH(CH3)2)3), 1.22–1.34 (m, 3H, Si(CH(CH3)2)3), 6.97 (d, J = 8.4 Hz, 2H, Har), 7.77 (d, J = 8.7 Hz, 2H, Har), 9.87 (s, 1H, CHO); 13C NMR (100 MHz, CDCl3): δ [ppm] = 12.7 (Si(CH(CH3)2)3), 17.8 (Si(CH(CH3)2)3), 120.3 (Car), 130.2 (Car), 131.9 (Car), 161.9 (Car), 190.8 (CO).
Methyl (2E)-3-{4′-[(triisopropylsilyl)oxy]phenyl}acrylate ((E)-14)
To a stirred solution of aldehyde 13 (2.19 g, 7.87 mmol) in CH2Cl2 (30 mL) was added (carboethoxymethylene)triphenylphosphorane (3.16 g, 9.44 mmol) in one portion. After 3 d the reaction mixture was concentrated in vacuo and the crude product was purified by flash chromatography (petroleum ether/EtOAc, 20:1) to give enoate ((E)-14) (2.32 g, 88%) as a colorless oil. TLC (petroleum ether/EtOAc, 20:1): Rf = 0.33; 1H NMR (400 MHz, CDCl3): δ [ppm] = 1.09 (d, J = 7.4 Hz, 18H, Si(CH(CH3)2)3), 1.17–1.35 (m, 3H, Si(CH(CH3)2)3), 3.78 (s, 3H, OCH3), 6.29 (d, J = 16.0 Hz, 1H, 2-H) 6.86 (d, J = 8.7 Hz, 2H, Har), 7.40 (d, J = 8.7 Hz, 2H, Har), 7.63 (d, J = 16.0 Hz, 1H, 3-H); 13C NMR (100 MHz, CDCl3): δ [ppm] = 12.6 (Si(CH(CH3)2)3), 17.8 (Si(CH(CH3)2)3), 51.5 (OCH3), 115.2 (C-2), 120.3 (Car), 127.4 (Car), 129.7 (Car), 144.6 (C-3), 158.3 (Car), 167.8 (CO); HMRS (ESI): [M+Na]+ calcd for C19H30O3Si 357.18564, found 357.18555.
Methyl (2S,3R)-2,3-dihydroxy-3-{4′-[(triisopropylsilyl)oxy]phenyl}propanoate (ent-15)
To a stirred solution of enoate (E)-14 (899 mg, 2.69 mmol) in tBuOH (2 mL) were added (DHQD)2PHAL (10.0 mg, 0.013 mmol) and NMO [400 mg, 2.96 mmol, 60% in water (270 μL)]. The solution was cooled to 0 °C and K2Os2(OH)4 (2.00 mg, 0.005 mmol) was added. The reaction mixture was allowed to warm to room temperature and after stirring for 5 h, solid Na2SO3 (400 mg), water (2 mL) and EtOAc (5 mL) were added. The layers were separated and the aqueous layer was extracted with EtOAc (2 × 10 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated in vacuo. The crude product was purfied by flash chromatography (petroleum ether/EtOAc, 2:1) to give diol (ent-15) (939 mg, 95%) as a colorless oil. TLC (petroleum ether/EtOAc, 2:1): Rf = 0.25; [α]20D = +1.0 (c 1.00, CH2Cl2); 1H NMR (400 MHz, CDCl3): δ [ppm] = 1.08 (d, J = 7.1 Hz, 18H, Si(CH(CH3)2)3), 1.15–1.32 (m, 3H, Si(CH(CH3)2)3), 2.66 (d, J = 6.4 Hz, 1H, OH), 3.06 (d, J = 6.1 Hz, 1H, OH), 3.76 (s, 3H, OCH3), 4.32 (dd, J = 6.1, 3.3 Hz, 1H, 2-H), 4.90 (dd, J = 6.4, 3.3 Hz, 1H, 3-H), 6.86 (d, J = 8.7 Hz, 2H, Har), 7.24 (d, J = 7.9 Hz, 2H, Har); 13C NMR (100 MHz, CDCl3): δ [ppm] = 12.6 (Si(CH(CH3)2)3), 17.9 (Si(CH(CH3)2)3), 52.7 (OCH3), 74.4 (C-2), 74.8 (C-3), 119.8 (Car), 127.5 (Car), 132.2 (Car), 156.0 (Car), 173.3 (CO); HMRS (ESI): [M+Na]+ calcd for C19H32O5Si 391.19112, found 391.190803.
Generation of a hydrazoic acid (HN3) solution
To a stirred paste of NaN3 (1.56 g, 24 mmol) and water (1.56 mL) was added toluene (8 mL). The mixture was cooled to 0 °C before concentrated sulfuric acid (0.581 mL, 12 mmol) was added dropwise. The organic layer was decanted and dried over Na2SO4. To estimate the concentration, an aliquot (3 mL) of the hydazoic acid solution in toluene was mixed with water (30 mL) and titrated with 0.3 N NaOH solution against phenolphthalein. Typically, concentrations of around 1.6 M were obtained.
Methyl (2S,3S)-3-azido-2-hydroxy-3-{4′-[(triisopropylsilyl)oxy]phenyl}propanoate (18b)
To a stirred solution of diol ent-15 (800 mg, 2.17 mmol) in THF (5 mL) were added PPh3 (683 mg, 2.61 mmol) and HN3 (1.47 mL, 4.34 mmol, ~3M in toluene) at 0 °C, followed by dropwise addition of DEAD (1.29 mL, 2.82 mmol, 40% in toluene) within 4 h. The reaction mixture was allowed to warm to room temperature and after stirring for 14 h, saturated NaHCO3 solution (4 mL) was added, followed by separation of the layers. The aqueous layer was extracted with EtOAc (2 × 15 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (petroleum ether/EtOAc, 9:1) provided azide 18b (399 mg, 47%) as a colorless oil. The syn-diastereomer (66.0 mg, 8%, Rf = 0.16) was also obtained as a colorless oil. TLC (petroleum ether/EtOAc, 9:1): Rf = 0.13; [α]20D = +50.5 (c 1.00, CH2Cl2); 1H NMR (400 MHz, CDCl3): δ [ppm] = 1.08 (d, J = 7.1 Hz, 18H, Si(CH(CH3)2)3), 1.17–1.30 (m, 3H, Si(CH(CH3)2)3), 2.92 (s, 1H, OH), 3.68 (s, 3H, OCH3), 4.49 (d, J = 4.1 Hz, 1H, 2-H), 4.80 (d, J = 4.1 Hz, 1H, 3-H), 6.86 (d, J = 8.7 Hz, 2H, Har), 7.18 (d, J = 8.7 Hz, 2H, Har); 13C NMR (100 MHz, CDCl3): δ [ppm] = 12.9 (Si(CH(CH3)2)3), 18.2 (Si(CH(CH3)2)3), 53.0 (OCH3), 67.1 (C-3), 74.1 (C-2), 120.4 (Car), 126.9 (Car), 129.3 (Car), 157.0 (Car), 172.1 (CO); HMRS (ESI): [M+Na]+ calcd for C19H31N3O4Si 416.19760, found 416.197583.
Methyl (2S,3S)-3-azido-2-methoxy-3-{4′-[(triisopropylsilyl)oxy]phenyl}propanoate (19b)
To a stirred solution of alcohol 18b (213 mg, 0.541 mmol) in CH2Cl2 (7 mL) was added Me3OBF4 (280 mg, 1.89 mmol) and proton sponge (580 mg, 2.71 mmol). After stirring for 20 h at room temperature in the dark, water (4 mL) was added and the mixture extracted with CH2Cl2 (2 × 10mL). The combined organic extracts were washed with 1N HCl (5 mL), saturated NaHCO3 solution (5 mL), and saturated NaCl solution (5 mL), dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purfied by flash chromatography (petroleum ether/EtOAc, 9:1) to afford methyl ether 19b (175 mg, 79%) as a colorless oil. TLC (petroleum ether/EtOAc, 9:1): Rf = 0.40; [α]20D = +36.6 (c 1.00, CH2Cl2); 1H NMR (400 MHz, CDCl3): δ [ppm] = 1.08 (d, J = 7.1 Hz, 18H, Si(CH(CH3)2)3), 1.18–1.30 (m, 3H, Si(CH(CH3)2)3), 3.34 (s, 3H, OCH3), 3.71 (s, 3H, OCH3), 3.95 (d, J = 6.6 Hz, 1H, 2-H) 4.68 (d, J = 6.9 Hz, 1H, 3-H), 6.86 (d, J = 8.7 Hz, 2H, Har), 7.22 (d, J = 8.4 Hz, 2H, Har); 13C NMR (100 MHz, CDCl3): δ [ppm] = 12.6 (Si(CH(CH3)2)3), 17.8 (Si(CH(CH3)2)3), 52.2 (OCH3), 59.2 (OCH3), 65.5 (C-3), 83.5 (C-2), 120.1 (Car), 127.5 (Car), 129.3 (Car), 156.5 (Car), 170.3 (CO); HMRS (ESI): [M+Na]+ calcd for C20H33N3O4Si 430.21325, found 430.21319.
Methyl (2S,3S)-3-amino-2-methoxy-3-{4′-[(triisopropylsilyl)oxy]phenyl}propanoate (20b)
To a stirred solution of azide 19b (218 mg, 0.535 mmol) in MeOH (5 mL) was added a catalytic amount of Pd/C. After stirring for 18 h at room temperature the Pd/C was filtered off, and the filtrate concentrated in vacuo. The residue (98 mg, 98%) was used for the next step without further purification. TLC (petroleum ether/EtOAc, 3:7): Rf = 0.23; [α]20D = −6.2 (c 1.00, CH2Cl2); 1H NMR (400 MHz, CDCl3): δ [ppm] = 1.07 (d, J = 7.12 Hz, 18H, Si(CH(CH3)2)3), 1.16–1.30 (m, 3H, Si(CH(CH3)2)3), 2.20 (br s, 2H, NH2), 3.37 (s, 3H, OCH3), 3.59 (s, 3H, OCH3), 4.00 (d, J = 5.3 Hz, 1H, 2-H), 4.26 (d, J = 5.3 Hz, 1H, 3-H), 6.83 (d, J = 8.4 Hz, 2H, Har), 7.17 (d, J = 8.4 Hz, 2H, Har); 13C NMR (100 MHz, CDCl3): δ [ppm] = 12.6 (Si(CH(CH3)2)3), 17.8 (Si(CH(CH3)2)33), 51.6 (OCH3), 57.0 (C-3), 58.9 (OCH3), 85.2 (C-2), 119.7 (Car), 128.0 (Car), 133.7 (Car), 155.5 (Car), 171.2 (CO); HMRS (ESI): [M+H]+ calcd for C20H35NO4Si 382.24081, found 382.24086.
N-(tert-Butoxycarbonyl)-L-alanyl-N-((1S,2S)-2,3-dimethoxy-3-oxo-1-{4′-[(triiso-propylsilyl)oxy]phenyl}propyl)-N-methyl-D-tryptophanamide (24b)
To a stirred solution of amine 20b (151 mg, 0.388 mmol) and acid 6 (148 mg, 0.388 mmol) in DMF (7 mL) was added DIEA (197 μL, 1.16 mmol), HOBT (79.0 mg, 0.582 mmol) and TBTU (182 mg, 0.582 mmol) at 0 °C. After stirring for 2 h at 0 °C, water (4 mL) was added and the mixture extracted with EtOAc (2 × 10mL). The combined organic extracts were washed with 1N HCl solution (5 mL), saturated NaHCO3 solution (5 mL), water (5 mL) and saturated NaCl solution (5 mL), dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purfied by flash chromatography (petroleum ether/EtOAc, 1:1) to give tripeptide 24b (212 mg, 73%) as a colorless foam. TLC (petroleum ether/EtOAc, 1:1): Rf = 0.29; [α]20D = +26.4 (c 1.00, CH2Cl2); 1H NMR (400 MHz, CDCl3): δ [ppm] = 0.90 (d, J = 6.4 Hz, 3H, Ala CH3), 1.06 (d, J = 7.1 Hz, 18H, Si(CH(CH3)2)3), 1.16–1.26 (m, 3H, Si(CH(CH3)2)3), 1.40 (s, 9H, C(CH3)3), 2.94 (s, 3H, OCH3), 3.26 (dd, J = 15.4, 5.2 Hz, 1H, CH2), 3.33–3.39 (m, 1H, CH2), 3.36 (s, 3H, NCH3), 3.53 (s, 3H, OCH3), 4.05 (d, J = 4.8 Hz, 1H, CHOCH3), 4.47–4.55 (m, 1H, Ala CH), 5.37 (dd, J = 8.7, 5.1 Hz, 1H, β-Tyr CH), 5.44 (d, J = 7.4 Hz, 1H, Ala NH), 5.55 (dd, J = 9.7, 6.4 Hz, 1H, Trp CH), 6.75 (d, J = 8.7 Hz, 2H, β-Tyr Har), 6.91 (s, 1H, Trp Har), 7.04–7.11 (m, 4H, β-Tyr NH, β-Tyr Har, Trp Har), 7.14 (t, J = 7.5 Hz, 1H, Trp Har), 7.30 (d, J = 8.1 Hz, 1H, Trp Har), 7.58 (d, J = 7.6 Hz, 1H, Trp Har), 8.33 (br s, 1H, Trp NH); 13C NMR (100 MHz, CDCl3): δ [ppm] = 12.5 (Si(CH(CH3)2)3), 17.8 (Si(CH(CH3)2)3), 18.0 (Ala CH3), 23.3 (CH2), 28.3 (C(CH)3), 30.7 (NCH3), 46.7 (Ala CH), 51.7 (OCH)3, 53.6 (β-Tyr CH), 56.7 (Trp CH), 59.1 (OCH3), 79.5 (C(CH)3), 82.4 (CHOCH3), 110.6 (Trp Car), 111.1 (Trp Car), 118.4 (Trp Car), 119.4 (Trp Car), 119.8 (β-Tyr Car), 122.0 (Trp Car), 122.2 (Trp Car), 127.2 (Trp Car), 128.6 (β-Tyr Car), 129.1 (β-Tyr Car), 136.1 (Trp Car), 155.1 (CO), 155.7 (β-Tyr Car), 169.3 (CO), 170.2 (CO), 174.3 (CO); HMRS (ESI): [M+Na]+ calcd for C40H60N4O8Si 775.40726, found 775.406789.
N-(tert-Butoxycarbonyl)-L-alanyl-N-((1S,2S)-3-{[(1′R,2′R,3′E,6′S)-7′-tert-butoxy-1′,2′,4′,6′-tetramethyl-7′-oxo-3′-heptenyl]oxy}-2-methoxy-3-oxo-1-{4″-[(triisopropylsilyl)oxy]phenyl}propyl)-N-methyl-D-tryptophanamide (26b)
To a stirred solution of tripeptide fragment 24b (87.0 mg, 0.116 mmol) in 1,2-dichloroethane (2 mL) was added Me3SnOH (105 mg, 0.580 mmol). After stirring for 5 h at 80 °C, TLC showed complete conversion and the reaction mixture was diluted with KHSO4 (5% in water, 4 mL). The aqueous layer was extracted with EtOAc (2 × 5mL) and the combined organic layers were dried over Na2SO4, filtered, and concentrated in vacuo to afford the crude acid 25b as a colorless foam. This crude product was dissolved in toluene (2.5 mL) and cooled to 0 °C. Et3N (48.0 μL, 0.348 mmol) and 2,4,6-trichlorobenzoyl chloride (20.0 μL, 0.128) were added. After 30 min, hydroxy ester 7 (33.0 mg, 0.128 mmol) in toluene (0.2 mL) and DMAP (57.0 mg, 0.464 mmol) were added and the yellow reaction mixture was stirred for 1 h at 0 °C, allowed to warm to room temperature and stirred again for 1 h. Then, saturated NaHCO3 solution (3 mL) was added and the aqueous layer was extracted with EtOAc (2 × 5 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated in vacuo. Flash chromatography (petroleum ether/EtOAc, 2:1) provided ester 26b (78.0 mg, 69% over two steps) as a colorless foam. TLC (petroleum ether/EtOAc, 2:1): Rf = 0.19; [α]20D = +11.3 (c 1.00, CH2Cl2); 1H NMR (400 MHz, CDCl3): δ [ppm] = 0.81 (d, J = 7.1 Hz, 3H, 2′-CH3), 0.82 (d, J = 6.4 Hz, 3H, 1′-CH3), 0.91 (d, J = 6.6 Hz, 3H, Ala CH3), 1.01 (d, J = 6.9 Hz, 3H, 6′-CH3), 1.05 (d, J = 7.1 Hz, 18H, Si(CH(CH3)2)3), 1.14–1.27 (m, 3H, Si(CH(CH3)2)3), 1.40 (s, 9H, C(CH3)3), 1.41 (s, 9H, C(CH3)3), 1.57 (s, 3H, 4′-CH3), 1.93 (dd, J = 13.7, 7.6 Hz, 1H, CH2), 2.34 (dd, J = 13.6, 6.7 Hz, 1H, CH2), 2.40–2.49 (m, 2H, 6′-H, 2′-H), 2.95 (s, 3H, NCH3), 3.24 (dd, J = 15.5, 9.9 Hz, 1H, Trp CH2), 3.31–3.39 (m, 1H, Trp CH2), 3.40 (s, 3H, OCH3), 4.05 (d, J = 4.3 Hz, 1H, CHOCH3), 4.48–4.59 (m, 2H, Ala CH, 1′-H), 4.86 (d, J = 9.4 Hz, 1H, 3′-H), 5.36 (dd, J = 8.7, 4.3 Hz, 1H, β-Tyr CH), 5.44 (d, J = 7.4 Hz, 1H, Ala NH), 5.52 (dd, J = 9.4, 6.6 Hz, 1H, Trp CH), 6.73 (d, J = 8.7 Hz, 2H, β-Tyr Har), 6.90 (s, 1H, Trp Har), 7.04 (d, J = 8.9 Hz, 1H, β-Tyr NH), 7.07–7.12 (m, 1H, Trp Har), 7.13–7.18 (m, 1H, Trp Har), 7.14 (d, J = 7.9 Hz, 2H, β-Tyr Har), 7.30 (d, J = 7.9 Hz, 1H, Trp Har), 7.58 (d, J = 7.6 Hz, 1H, Trp Har), 8.15 (br s, 1H, Trp NH); 13C NMR (100 MHz, CDCl3): δ [ppm] = 12.6 (Si(CH(CH3)2)3), 16.4 (4′-CH3), 16.6 (6′-CH3), 17.2 (1′-CH3), 17.6 (2′-CH3), 17.9 (Si(CH(CH3)2)3), 18.2 (Ala CH3), 23.4 (Trp CH2), 28.1 (C(CH)3), 28.3 (C(CH)3), 30.8 (NCH3), 37.6 (C-2′), 38.6 (C-6′), 43.4 (C-5′), 46.7 (Ala CH), 53.6 (β-Tyr CH), 56.7 (Trp CH), 59.2 (OCH3), 75.9 (C-1′), 79.5 (C(CH)3), 79.9 (C(CH)3), 81.9 (CHOCH3), 110.9 (Trp Car), 111.1 (Trp Car), 118.6 (Trp Car), 119.5 (Trp Car), 119.6 (β-Tyr Car), 122.1 (Trp Car), 127.3 (Trp Car), 128.0 (C-3′), 129.1 (β-Tyr Car), 129.2 (Trp Car), 133.7 (C-4′), 136.1 (Trp Car), 155.1 (CO), 155.8 (β-Tyr Car), 169.2 (CO), 169.3 (CO), 174.2 (CO), 175.8 (CO); HMRS (ESI): [M+Na]+ calcd for C54H84N4O10Si 999.58489, found 999.584938.
Chondramide A (2b)
To a stirred solution of compound 26b (40.0 mg, 0.041 mmol) in CH2Cl2 (1 mL) was added TFA (61.0 μL, 0.820 mmol) at 0 °C. After stirring for 22 h at 0 °C, the solvent was removed in vacuo. For azeotropic removal of TFA the residue was taken up in toluene (3 × 0.5 mL) and concentrated in vacuo each time. The crude product was dissolved in DMF (40 mL) and DIEA (56.0 μL, 0.328 mmol), HOBT (39.0 mg, 0.287 mmol) and TBTU (90.0 mg, 0.287 mmol) were added. The solution was stirred at room temperature for 20 h and then diluted with water (20 mL) and EtOAc (20 mL). The aqueous layer was extracted with EtOAc (3 × 20mL) and the combined organic layers were washed with 5% aqueous KHSO4 solution (20 mL), water (20 mL), saturated NaHCO3 solution (20 mL), water (2×20 mL) and saturated NaCl solution (20 mL). The combined organic extracts were dried over Na2SO4, filtered, and concentrated in vacuo. The crude product was dissolved in THF (0.5 mL) and TBAF·3H2O (26.0 mg, 0.082 mmol) was added at 0 °C. After stirring for 1 h, the solvent was removed in vacuo followed by flash chromatography (petroleum ether/acetone, 3:2) of the residue to give depsipeptide 2b (8.00 mg, 24% over three steps) as a colorless foam. TLC (petroleum ether/acetone, 3:2): Rf = 0.13; [α]20D = +7.9 (c 0.80, MeOH); 1H NMR (400 MHz, MeOD): δ [ppm] = 0.80 (d, J = 7.1 Hz, 3H, Ala CH3), 0.86 (d, J = 6.1 Hz, 3H, 7-CH3), 0.91 (d, J = 6.6 Hz, 3H, 6-CH3), 1.08 (d, J = 6.8 Hz, 3H, 2-CH3), 1.68 (s, 3H, 4-CH3), 2.03 (dd, J = 13.0, 2.6 Hz, 1H, CH2), 2.23 (dd, J = 12.6, 12.6 Hz, 1H, CH2), 2.45–2.57 (m, 1H, 6-H), 2.59–2.72 (m, 1H, 2-H), 3.02 (d, J = 8.1 Hz, 2H, Trp CH2), 3.08 (s, 3H, NCH3), 3.14 (s, 3H, OCH3), 3.85 (d, J = 10.1 Hz, 1H, CHOCH3), 4.47–4.56 (m, 1H, 7-H), 4.73–4.81 (m, 1H, Ala CH), 4.81–4.87 (5-H), 5.03 (d, J = 9.9 Hz, 1H, β-Tyr CH), 5.52 (dd, J = 8.1, 8.1 Hz, 1H, Trp CH), 6.67 (d, J = 8.6 Hz, 2H, β-Tyr Har), 6.83 (s, 1H, Trp Har), 6.95–7.02 (m, 1H, Trp Har), 6.99 (d, J = 8.6 Hz, 2H, β-Tyr Har), 7.02–7.09 (m, 1H, Trp Har), 7.26 (d, J = 8.1 Hz, 1H, Trp Har), 7.57 (d, J = 7.8 Hz, 1H, Trp Har); 13C NMR (100 MHz, MeOD): δ [ppm] = 16.0 (4-CH3), 17.9 (6-CH3), 18.4 (Ala CH3), 18.9 (7-CH3), 19.1 (2-CH3), 26.4 (Trp CH2), 30.9 (NCH3), 38.7 (C-6), 40.2 (C-2), 45.9 (Ala CH), 46.0 (C-3), 55.7 (β-Tyr CH), 56.9 (Trp CH), 58.3 (OCH3), 79.2 (C-7), 83.4 (CHOCH3), 110.1 (Trp Car), 112.2 (Trp Car), 116.1 (β-Tyr Car), 119.4 (Trp Car), 119.6 (Trp Car), 122.3 (Trp Car), 124.4 (Trp Car), 128.5 (Trp Car), 129.1 (C-5), 129.5 (β-Tyr Car), 131.3 (β-Tyr Car), 134.7 (C-4), 137.9 (Trp Car), 157.9 (β-Tyr Car), 171.3 (CO), 173.5 (CO), 174.9 (CO), 176.9 (CO); HMRS (ESI): [M+Na]+ calcd for C36H46N4O7 669.32587, found 669.326358.
Supplementary Material
Table 1. Table Caption.
| Head 1[a] | Head 2 | Head 3[b] | Head 4[c] | Head 5 |
|---|---|---|---|---|
| column 1 | column 2 | column 3 | column 4 | column 5 |
| column 1 | column 2 | column 3 | column 4 | column 5 |
Table Footnote.
…
Acknowledgments
Financial support by the Deutsche Forschungsgemeinschaft, the NIH (grant yy) and the Fonds der Chemischen Industrie is gratefully acknowledged. We thank Graeme Nicholson (Institute of Organic Chemistry) for measuring the HRMS spectra. We also thank Dr. Rolf Jansen, HZI Braunschweig for sending spectra for comparison and for LC-MS measurements.
Footnotes
Supporting information for this article is available on the WWW under http://www.chemeurj.org/ or from the author.
Contributor Information
Dipl.-Chem. Anke Schmauder, Institut für Organische Chemie, Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen (Germany), Fax: (+) (+49) 7071-295137
Prof. David Sibley, Department of Molecular Microbiology, Washington University School of Medicine, 660 S. Euclid Ave., St. Louis, MO 63110-1093, U.S.A.
Prof. Dr. Martin E. Maier, Email: martin.e.maier@uni-tuebingen.de, Institut für Organische Chemie, Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen (Germany), Fax: (+) (+49) 7071-295137
References
- 1.Disanza A, Steffen A, Hertzog M, Frittoli E, Rottner K, Scita G. Cell Mol Life Sci. 2005;62:955–970. doi: 10.1007/s00018-004-4472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a) Kustermans G, Piette J, Legrand-Poels S. Biochem Pharmacol. 2008;76:1310–1322. doi: 10.1016/j.bcp.2008.05.028. [DOI] [PubMed] [Google Scholar]; b) Binder M, Tamm C. Angew Chem. 1973;85:369–381. doi: 10.1002/anie.197303701. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed Engl. 1973;12:370–380. [Google Scholar]
- 3.a) Wieland T. Naturwissenschaften. 1987;74:367–373. doi: 10.1007/BF00405464. [DOI] [PubMed] [Google Scholar]; Cooper JA. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For recent reviews, see: Myles DC. Ann Rep Med Chem. 2002;37:125–132.Altmann KH, Gertsch J. Nat Prod Rep. 2007;24:327–357. doi: 10.1039/b515619j.Altmann KH, Pfeiffer B, Arseniyadis S, Pratt BA, Nicolaou KC. Chem Med Chem. 2007;2:396–423. doi: 10.1002/cmdc.200600206.Nicolaou KC, Chen JS, Dalby SM. Bioorg Med Chem. 2009;17:2290–2303. doi: 10.1016/j.bmc.2008.10.089.
- 5.For review about cyclodepsipeptides, see: Hamada Y, Shioiri T. Chem Rev. 2005;105:4441–4482. doi: 10.1021/cr0406312.
- 6.a) Crews P, Manes LV, Boehler M. Tetrahedron Lett. 1986;27:2797–2800. [Google Scholar]; b) Zabriskie TM, Klocke JA, Ireland CM, Marcus AH, Molinski TF, Faulkner DJ, Xu C, Clardy JC. J Am Chem Soc. 1986;108:3123–3124. [Google Scholar]
- 7.Tanaka C, Tanaka J, Bolland RF, Marriott G, Higa T. Tetrahedron. 2006;62:3536–3542. [Google Scholar]
- 8.Jansen R, Kunze B, Reichenbach H, Höfle G. Liebigs Ann. 1996:285–290. [Google Scholar]
- 9.Waldmann H, Hu T-S, Renner S, Menninger S, Tannert R, Oda T, Arndt H-D. Angew Chem. 2008;120:6573–6577. doi: 10.1002/anie.200801010. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:6473–6477. doi: 10.1002/anie.200801010. erratum: H. Waldmann, T.-S. Hu, S. Renner, S. Menninger, R. Tannert, T. Oda, H.-D. Arndt, Angew. Chem. 2009, 121, 1554; Angew. Chem. Int. Ed. 2009, 48, 1526. [DOI] [PubMed] [Google Scholar]
- 10.Eggert U, Diestel R, Sasse F, Jansen R, Kunze B, Kalesse M. Angew Chem. 2008;120:6578–6582. doi: 10.1002/anie.200801156. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:6478–6482. [Google Scholar]
- 11.Bai R, Covell DG, Liu C, Ghosh AK, Hamel E. J Biol Chem. 2002;277:32165–32171. doi: 10.1074/jbc.M205076200. [DOI] [PubMed] [Google Scholar]
- 12.a) Hirai Y, Yokota K, Momose T. Heterocycles. 1994;39:603–612. [Google Scholar]; b) Marimganti S, Yasmeen S, Fischer D, Maier ME. Chem Eur J. 2005;11:6687–6700. doi: 10.1002/chem.200500319. [DOI] [PubMed] [Google Scholar]
- 13.Schmauder A, Müller S, Maier ME. Tetrahedron. 2008;64:6263–6269. doi: 10.1016/j.tet.2008.04.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.For a classical review, see: Nicolaou KC, Dai WM, Guy RK. Angew Chem. 1994;106:38–69.Angew Chem Int Ed Engl. 1994;33:15–44.
- 15.For a review about β-amino acids, see: Lelais G, Seebach D. Biopolymers. 2004;76:206–243. doi: 10.1002/bip.20088.
- 16.a) Kudyba I, Raczko J, Jurczak J. J Org Chem. 2004;69:2844–2850. doi: 10.1021/jo0358269. [DOI] [PubMed] [Google Scholar]; b) Borah JC, Gogoi S, Boruwa J, Kalita B, Barua NC. Tetrahedron Lett. 2004;45:3689–3691. [Google Scholar]
- 17.a) Farina V, Hauck SI, Walker DG. Synlett. 1992:761–763. [Google Scholar]; b) Huang Y, Calter MA. Tetrahedron Lett. 2007;48:1657–1659. [Google Scholar]
- 18.a) Reddy SHK, Lee S, Datta A, Georg GI. J Org Chem. 2001;66:8211–8214. doi: 10.1021/jo0102516. [DOI] [PubMed] [Google Scholar]; b) Milicevic S, Matovic R, Saicic RN. Tetrahedron Lett. 2004;45:955–957. [Google Scholar]
- 19.Wang Y, He QF, Wang HW, Zhou X, Huang ZY, Qin Y. J Org Chem. 2006;71:1588–1591. doi: 10.1021/jo052298n. [DOI] [PubMed] [Google Scholar]
- 20.a) Wang ZM, Kolb HC, Sharpless KB. J Org Chem. 2002;59:5104–5105. [Google Scholar]; b) Voronkov MV, Gontcharov AV, Wang ZM. Tetrahedron Lett. 2002;44:407–409. [Google Scholar]
- 21.Aitken DJ, Faure S, Roche S. Tetrahedron Lett. 2003;44:8827–8830. [Google Scholar]
- 22.a) Diekmann E, Friedrich K, Lehmann J. Liebigs Ann Chem. 1989:1247–1250. [Google Scholar]; b) Lang RW, Hansen HJ. Org Synth. 1984;62:202–209. [Google Scholar]; Org Synth, Coll. 1990;7:232–236. [Google Scholar]
- 23.Kolb HC, VanNieuwenhze MS, Sharpless KB. Chem Rev. 1994;94:2483–2547. [Google Scholar]
- 24.Diem MJ, Burow DF, Fry JL. J Org Chem. 1977;42:1801–1802. [Google Scholar]
- 25.Li G, Angert HH, Sharpless KB. Angew Chem. 1996;108:2995–2999. [Google Scholar]; Angew Chem Int Ed Engl. 1996;35:2813–2817. [Google Scholar]
- 26.For a review, see: O’Brien P. Angew Chem. 1999;111:339–342.Angew Chem Int Ed. 1999;38:326–329. doi: 10.1002/(SICI)1521-3773(19990201)38:3<326::AID-ANIE326>3.0.CO;2-T.
- 27.see also: Cao B, Park H, Joullie MM. J Am Chem Soc. 2002;124:520–521. doi: 10.1021/ja017277z.Kurosawa W, Kan T, Fukuyama T. J Am Chem Soc. 2003;125:8112–8113. doi: 10.1021/ja036011k.
- 28.a) Barta NS, Sidler DR, Somerville KB, Weissman SA, Larsen RD, Reider PJ. Org Lett. 2000;2:2821–2824. doi: 10.1021/ol006255z. [DOI] [PubMed] [Google Scholar]; b) Bodkin JA, Bacskay GB, McLeod MD. Org Biomol Chem. 2008;6:2544–2553. doi: 10.1039/b803310b. [DOI] [PubMed] [Google Scholar]
- 29.a) Mascaretti OA, Furlán RLE. Aldrichimica Acta. 1997;30:55–68. [Google Scholar]; b) Nicolaou KC, Estrada AA, Zak M, Lee SH, Safina BS. Angew Chem. 2005;117:1402–1406. doi: 10.1002/anie.200462207. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:1378–1382. doi: 10.1002/anie.200462207. [DOI] [PubMed] [Google Scholar]
- 30.Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M. Bull Chem Soc Jpn. 1979;52:1989–1993. [Google Scholar]
- 31.Davies JS. J Pept Sci. 2003;9:471–501. doi: 10.1002/psc.491. [DOI] [PubMed] [Google Scholar]
- 32.Marimganti S, Yasmeen S, Fischer D, Maier ME. Chem Eur J. 2005;11:6687–6700. doi: 10.1002/chem.200500319. [DOI] [PubMed] [Google Scholar]; b) Marimganti S, Wieneke R, Geyer A, Maier ME. Eur J Org Chem. 2007:2779–2790. [Google Scholar]
- 33.a) Ando K. J Org Chem. 1997;62:1934–1939. doi: 10.1021/jo970057c. [DOI] [PubMed] [Google Scholar]; b) Ando K. J Org Chem. 1999;64:8406–8408. doi: 10.1021/jo9907181. [DOI] [PubMed] [Google Scholar]
- 34.For the preparation of a solution of hydrazoic acid, see: Wolff H. The Schmidt Reaction. Org React. 1947;3:307–336. 327.
- 35.Ko SY. J Org Chem. 2002;67:2689–2691. doi: 10.1021/jo015967f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.