Abstract
According to the 2008 American Cancer Society statistics, cancer remains the second leading cause of death in American today. Early detection, innovative surgery, new drugs and increased public education regarding avoidable risk factors, such as smoking, have had significant impact on the incidence and survival rates of many cancers, while overall death rates from all cancers have declined a modest 5% over the past 50 years. Ovarian cancer statistics, however, have not been as encouraging. Despite recent advances in the management of this disease, 5-year survival has not improved, and the search continues for rationally designed new treatments. Müllerian Inhibiting Substance is a strong candidate because it addresses many of the deficiencies of existing treatments. Namely, Müllerian Inhibiting Substance has little demonstrated toxicity, it complements the activity of known anticancer drugs, it is highly specific against cancers expressing its receptor and it inhibits the proliferation of drug-resistant tumors.
Keywords: anti-Müllerian hormone, Müllerian Inhibiting Substance, ovarian cancer
For nearly two centuries, the primary treatment of breast and gynecological cancers has been surgical; physically removing a tumor is an obvious goal. However, complete removal of a tumor is not always possible; therefore, adjuvant measures, such as radiotherapy and/or chemotherapy, are often employed. In addition to these approaches, adjuvant hormonal therapy has played a significant role in the management of these cancers, because the majority of them are responsive to endogenous hormones, if not actually dependent upon them. The rationale for manipulating the hormonal environment, of course, is that by ablating endogenous sources of stimulatory hormones or by administering antihormones and other biologicals that specifically inhibit cancer cell proliferation, there should be a beneficial effect in reducing tumor burden. This adjuvant endocrine approach to the management of cancer has its origins in the mid-to-late 19th Century. The early strategies that were employed included surgical menopause by ovariectomy, an operation first proposed for breast cancer in 1889 by Albert Schinzinger and later accomplished by George Beatson in 1895 (for a brief review of the history of ovariectomy for cancer see [1]). Subsequently, as gonadal and adrenal steroids, as well as pituitary and hypothalamic polypeptides, were isolated and characterized, it became possible to design hormonal antagonists. As a result, ovariectomy could be replaced, when appropriate, by systemic therapy using these compounds. While considerable success has been attained with breast and most reproductive tract tumors, with anti-estrogens and aromatase inhibitors for example, the results with epithelial ovarian cancers has been disappointing. Unfortunately, results with adjuvant chemotherapy have not been much better. With 5-year survival rates for ovarian cancer hovering around 30% [2], ovarian cancer could be considered a candidate disease for novel therapies.
As suggested by the title of this article, such a new, potentially nontoxic, therapy could be Müllerian Inhibiting Substance (MIS), also termed anti-Müllerian hormone (AMH). This gonadal protein hormone regresses Müllerian structures in fetal males, and it has also been shown to block the proliferation of cancer cells of Müllerian (and other) origin in vitro and in vivo in animals. Before a review of the preclinical MIS cancer studies can be presented, it is appropriate to provide some relevant MIS biochemistry as useful background information to understand better MIS as a biological response modifier with therapeutic potential.
Müllerian Inhibiting Substance/anti-Müllerian hormone
Background
Mammalian embryos begin development with the capacity to produce both female and male reproductive tracts. The Müllerian ducts will become the upper third of the vagina, the cervix, uterus, Fallopian tubes and the outer lining of the ovaries. The Wolffian ducts evolve into the seminal vesicles, vasa deferens and epididymides. Early in embryogenesis, both ductal systems grow independently of one another. After the genetic sex of the embryo is declared based upon its chromosomal makeup, the undifferentiated gonads become either ovaries or testes in response to the sex-determining region of the Y chromosome in the case of genetic males [3]. As gonads begin to differentiate, at approximately 10 weeks of gestation in the human, one of the reproductive tract primordia must be destroyed and the other will proliferate and differentiate.
Key insights into how this selection is accomplished at a molecular level were provided by Jost [4]. Before Jost’s observations, it was believed that if embryonic testes were present, the testosterone secreted would promote male development and destroy the female tract. In the presence of ovaries, the male tract would undergo atrophy without testosterone and the female tract would be spared.
Jost performed experiments that proved testosterone alone could not be responsible for eliminating the embryonic female reproductive tract precursors, that is, the Müllerian ducts. He discovered that some other testicular factor, a ‘Müllerian Inhibitor’, as he named it, was responsible for ductal regression [4]. After further study, it was determined that Müllerian duct regression was the result of apoptosis, autophagocytosis, disruption of basement membranes and epithelial mesenchymal transformation of cells followed by migration of cells in the direction of the mesonephros [5–10]. In addition, Jost’s studies showed that Müllerian ducts persisted and developed even in the absence of an ovary; thus, there seemed to be no ovarian contribution to Müllerian duct development in utero.
More than 20 years later, it was determined that MIS was a protein synthesized and secreted by the Sertoli cells of the testes in a number of mammalian species, including rat [11,12], bovine [13–15] and human [16,17]. Subsequently, it has been identified in all species examined, including birds, reptiles and fish [18–19]. Surprisingly MIS expression is not limited to the fetus or to males; it is a sexually dimorphic gene. Transcription of its gene in the testis appears to be regulated by a number of factors, including SF1, SOX9 and WT-1 in utero, and postnatal testosterone suppresses MIS secretion [20–25]. MIS inhibits steroid synthesis in ovaries [26] and represses the expression of ovarian aromatase [27] in fetal [28] and adult testes [29–31]. The converse has been observed for males, at least, where testosterone is reported to reduce MIS expression [32].
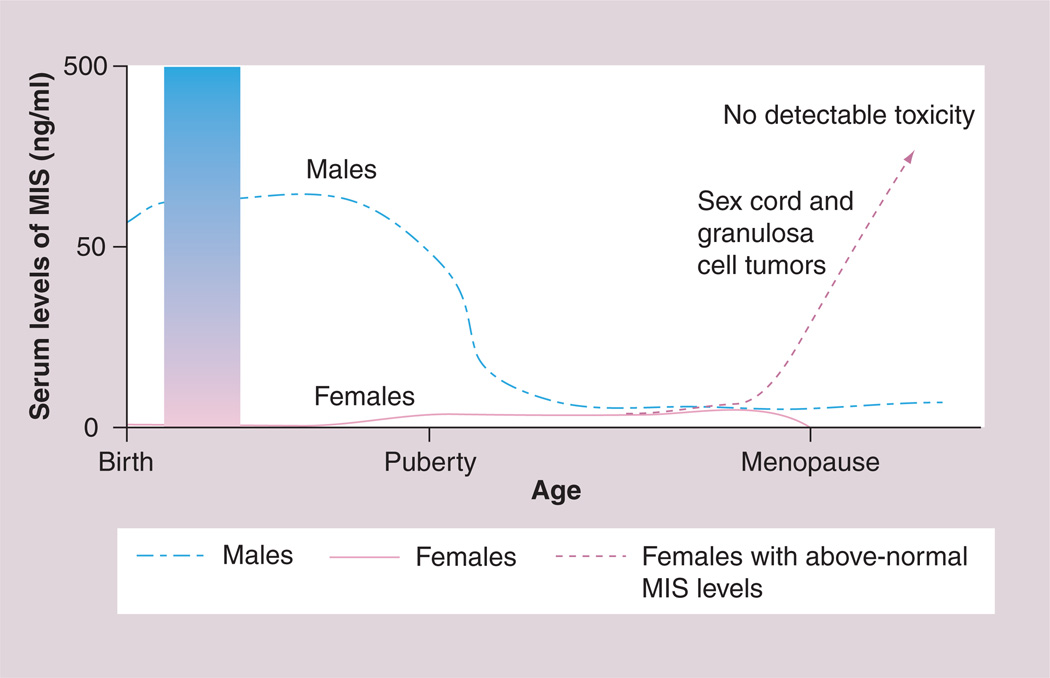
As stated earlier, MIS is only expressed in significant amounts by males during fetal life; however, postnatally, both granulosa cells in females and Sertoli cells in males produce the protein. In males, serum MIS levels remain at their highest until puberty, dropping to basal levels, thereafter. MIS mRNA and protein have been detected in sheep and mouse ovaries shortly after birth [33,34] and in low levels in prepuberal females by ELISA [35]. After puberty, female serum MIS levels reach those of adult males (Figure 1). The detection of MIS in male and female serum, even after the regression and differentiation of the Müllerian duct in the embryo and after birth [36–38], suggests multifunctional roles for MIS.
Figure 1. The pattern of MIS in human serum is sexually dimorphic and varies with age.
MIS in males is high at birth and, after a transient drop at approximately 2 years of age, levels are maintained until puberty. Serum MIS in females becomes measurable in the prepubertal period and is sustained until the menopause, after which it is no longer produced. The shaded bar demonstrates why serum MIS is useful in the perinatal period for evaluating cases of ambiguous genitalia. MIS is absent in genetic females and positive in cases with testicular tissue. The higher the values, the more normal the tissue. Serum testosterone is undetectable in normal males at this time. Rising serum MIS over normal limits in adult females (dotted line) is consistent with granulosa or sex cord tumors. MIS: Müllerian Inhibiting Substance.
The measurement of MIS secretion by both sexes after birth was recognized immediately as potentially useful in a number of clinical circumstances, including ambiguous genitalia and undescended testes and delayed puberty, where serum testosterone is not particularly informative, and in sex cord or granulosa cell tumors. Variations of research assays used to measure MIS in biological fluids [32–34] are now commercially available; thus, serum MIS data in the management of pediatric endocrine, reproductive medicine and gynecologic oncology are becoming more widely used. If this protein is to be used for the treatment of certain cancers (as discussed later), having these reliable and sensitive MIS assays available now is critical for the analysis of human clinical trials.
Serum MIS, for example, is an excellent predictor of the presence of functional testicular tissue in cases of nonpalpable testes [39] and in cases of sexual ambiguity. Normal MIS levels for age predict normal testes, undetectable MIS suggests absent testes, where the cause of ambiguity may be androgen insensitivity, whereas lower than normal MIS is most probably a form of gonadal dysgenesis. Thus, serum MIS data can often be more valuable than hCG stimulation tests in the evaluation of gonadal status.
In adult females, on the other hand, serum MIS is a useful predictor of ovarian reserve [40,41] as well as a marker of response to IVF protocols [42–47], and it appears to be prognostic for pregnancy outcome for infertile couples with advanced female age [48,49]. Abnormally high serum MIS has been detected in patients with polycystic ovary syndrome [50], a finding that has provided a new area of investigation for this complex benign disease. MIS is also elevated in malignant gonadal disease.
Sex cord [51] and granulosa cell tumors [52] secrete MIS, and serum levels predict recurrence and response to therapy as well as tumor volume [53]. These tumors may also express the MIS receptor [54], but the fact that these tumors are unresponsive to the MIS they produce suggests a loss of function downstream from the receptor itself. As a result, these ovarian tumors are not targets for MIS therapy. Serum concentrations of MIS above the upper limit of normal for age is consistent with the presence of either one of these tumors; rising serum MIS reflects tumor growth, and decreases show response to therapy [51,55]. Inhibin B is another marker of these tumors, but MIS appears to be more specific because it is not synthesized elsewhere [56]. In numerous cases, serum MIS rises to thousands-fold the normal range without any significant adverse reactions; therefore, MIS administered to cancer patients as a therapy may be well tolerated. Whether the recombinant protein is toxic will be the topic of detailed toxicology studies to be completed when clinical-grade MIS is available.
Although the function of MIS in the fetus is well established, its role after birth is the topic of a number of ongoing studies. MIS blocks meiosis II in the ovary [57], inhibits granulosa cell division and progesterone production [26], and modulates follicular development [58]. The concentrations of MIS in follicular fluid are inversely correlated with the granulosa cell proliferative index [59,42]. Similarly, in males, MIS affects Leydig cell development and blocks the steroidogenic enzyme CYP17 [30,31,60,61] and aromatase [27] transcription. Its spermatogenesis stage-specific expression in Sertoli cells in seminiferous tubules indicates that it must also be one of the factors that control that process [62].
The fact that the MIS receptor is expressed in pituitary and certain neurons suggests even more widespread non-Müllerian roles for the hormone [63,64]. The fact that motor neurons express both the MIS receptor and MIS implies a possible autocrine action of this receptor–ligand pair in some tissues [65]. In addition, the prostate gland [66,67] and ductal epithelium of the mammary gland [68], and some of their cancers [69] are possible targets for MIS (see later). The discovery that MIS receptor expression exists beyond Müllerian-origin structures, particularly in tissues with a sexually dimorphic nature, challenges investigators to examine the place MIS has in the growing list of developmental factors; it seems that the protein could have been MIS-named.
MIS/AMH protein
The MIS protein was purified initially from bovine testes [70–74] and later from recombinant human sources [75,76], using a specific rat embryonic urogenital ridge regression bioassay to monitor progress. Initially, rodent fetal and neonatal testes were selected as a source of MIS for purification, but because of availability and larger size, calf testes were later chosen for this important task.
Conventional biochemical approaches were taken to purify MIS from testicular secretions in vitro [77] or protein extracts [70–77], including dye and carbohydrate affinity, anion- and cation-exchange chromatography, and, once specific antibodies were produced, immunoaffinity chromatography was employed [78,79].
Compositional analyses revealed MIS to be a 140-kDa glycoprotein of approximately 15% carbohydrate by weight. Western analyses under reducing conditions suggested that MIS was actually a disulfide-linked dimer that was partially cleaved into smaller species, perhaps during the biosynthetic and secretion processes. The significance of these findings became more apparent when the MIS gene was cloned [75].
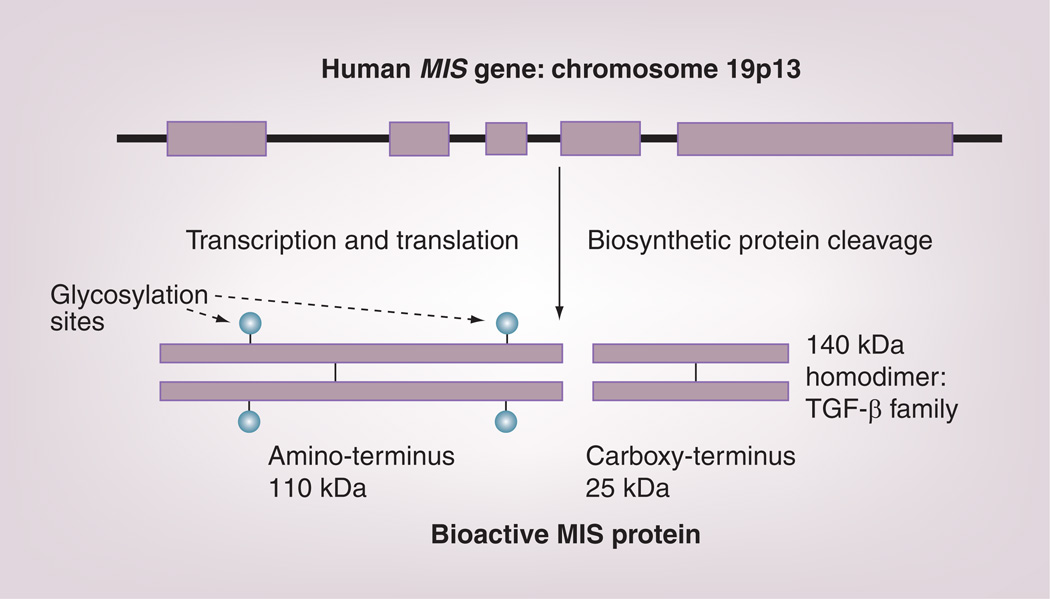
Based on the partial bovine MIS amino acid sequence data, a series of degenerate oligonucleotide primers were designed to facilitate the cloning of the bovine complementary DNA and, later, the human genomic sequence from DNA libraries of bovine testes and human placentae, respectively [75,76]. Sequence analysis of the genes revealed them to be weakly related to the transforming β-family of biological modifiers, with the most striking homology (28%) residing in the carboxy terminal domain. The 2.8-kb human gene contains five exons and four introns and is located on the short arm of chromosome 19 [80]. The deduced protein sequence of this gene contains a 25 amino acid secretion-specific signal peptide and a monomeric protein of 535 amino acids that, upon glycosylation at two putative N-linked sites, has a molecular weight of approximately 70,000 Da (Figure 2). Examination of the primary human MIS sequence showed the presence of a cleavage motif at residue 427, which explains the origins of the major cleavage products of the MIS fragments (12.5 and 55 kDa) appearing on reduced polyacrylamide gels of the purified protein. A weaker motif at residue 229 is also present, but its role in MIS action is unclear. Several studies have clarified the role of MIS carboxy terminal cleavage in the scheme of MIS action. The carboxy terminus results from processing by a biosynthetic protease, most probably a kex-like enzyme such as furin [81,82], into an amino terminal dimer (110 kDa) and a carbohydrate-free carboxy terminal dimer (25 kDa) held together in noncovalent association (Figure 2). It is the carboxy terminal domain that possesses the biological activity [81,83,84]. The amino terminal domain may contribute to proper protein folding and assembly during synthesis and/or it may increase the serum half-life of the carboxy terminus and enhance bioactivity [84]. There is no evidence that the amino terminus interacts directly with either the type I or type II receptors. The carboxy terminal sequence is extremely highly conserved, a fact that explains why MIS from many different species are all active in the rat in vitro bioassay used for MIS purification. A recombinant preparation of MIS carboxy terminus is commercially available for study; however, it is not suited for in vivo work because of its presumed short half-life in serum given the fact that TGF β-1 carboxy terminus, a gene family member with which MIS has structural homology, has a serum half-life of 2–3 min [85].
Figure 2. The human MIS gene has five exons and four introns and encodes a monomer of 70 kDa with two N-linked glycosylation sites (circles).
The monomer forms a homodimer via disulfide bond formation and is activated by biosynthetic kex-like proteases to produce the 25-kDa carboxy terminal dimer, which is the bioactive domain of the molecule. The 110-kDa amino terminus stays associated with the carboxy terminus via noncovalent forces. MIS: Müllerian Inhibiting Substance.
The human gene was transfected into Chinese hamster ovary cells, and the conditioned medium secreted from these cells was then used as a source of recombinant human MIS [86,87], as well as for the purification of the MIS in all of the cancer studies summarized in the following sections. MIS as purified from serum-free conditioned media is proteolytically processed, free of potential contaminants from bovine serum, hamster cells, mouse monoclonal antibodies [87] and significant amounts of endotoxins [69]. The bioactivity of the MIS preparations produced in the laboratory was assessed using the in vitro urogenital ridge Müllerian duct regression assay [12] before the MIS is used in anticancer assays.
At present, no commercial source of bioactive holo-MIS/AMH exists; therefore, the protein must be acquired from academic laboratories. The increased production for large-scale preclinical, pharmacology, and clinical trials will require commercial partnerships.
MIS/AMH receptor
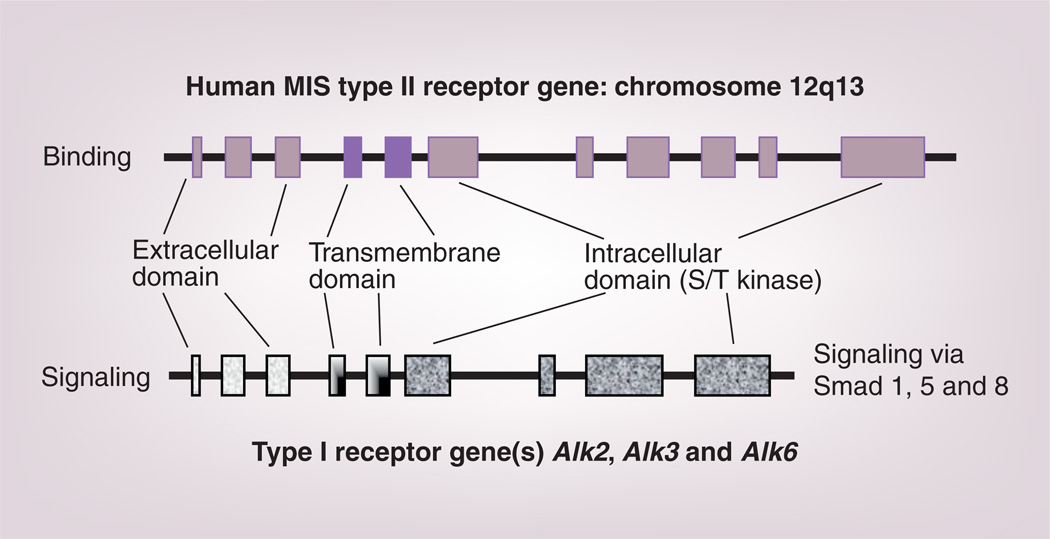
The biological activity of the protein requires interaction with two very similar receptors, termed type I and II. Each is a single membrane spanning, serine-threonine kinase that, after cross-phosphorylation induced by binding to MIS, initiates a series of intracellular cascades leading to the control of cell-cycle-regulating proteins and the altered transcription of a number of genes, depending upon the target tissues. The type II receptor is responsible for ligand binding and the type I heteromer is the signaling receptor (Figure 3). The MIS type II receptor (MISRII) was cloned first by the Themmen laboratory [88] and subsequently by a number of other investigators [89–91]. The human gene, located on chromosome 12q13 [92], has 11 exons and ten introns.
Figure 3. The human MIS type II receptor gene, with its 11 exons, is larger than the nine-exon type I gene.
They share several similar but not identical features, hence the different shadings, including extracellular ligand binding domains, transmembrane spanning regions and intracellular S/T kinase domains. Both types are required for MIS signaling, and mutations in the type II receptor are associated with phenotypic changes in humans.
MIS: Müllerian Inhibiting Substance; S/T: Serine/threonine.
The MISRII is expressed in Müllerian duct mesenchymal cells surrounding the adjacent ductal epithelium, as one might expect since the fetal mesenchyme directs Müllerian duct regression [93], and in fetal and adult gonadal Sertoli and Leydig cells, as well as granulosa cells [88–90], where MIS regulates testosterone [30,60,61], estradiol and progesterone synthesis [26,27], respectively. Functional MISRIIs have also been found in adult rodent uteri [90,94], the human endometrium [94], breast and prostate tissues [67,95] and, unexpectedly, in motor neurons in the mouse brain [65]. It is not yet understood what receptor-mediated actions MIS has on these tissues in normal adults, but MIS inhibits the proliferation of tumor cell lines derived from them (see later), supporting our hypothesis that MIS may be a useful adjuvant agent in the treatment of these diseases.
Josso and colleagues have studied a large number of patients with Persistent Müllerian Duct Syndrome resulting from loss of function mutations in the MISRII or the MIS molecule itself (for a review see [96]). In so doing, they have defined a number of hot spots in the gene, as well as polymorphisms present in the normal sequence. A common defect in the MIS gene is a truncation mutation that produces a protein lacking the carboxy terminal domain and that is, therefore, unable to cause Müllerian duct regression in utero. Heterozygotes of either mutation type, that is, receptor or ligand, have normal phenotypes; affected individuals are either homozygous for a given mutation or compound heterozygotes. Our group has added a newly identified MISRII mutant [97] to the list of the known MIS and MIS receptor mutants that contribute to the Persistent Müllerian Duct Syndrome phenotype [92,96,98].
Several different type I receptors have been identified as interacting with the type II receptor (Alk3, Alk2 and Alk6 [99–102]). How each type I receptor interacts with the type II receptor in human tissues is not yet known, although all are expressed in the human cancer cell lines studied thus far.
MIS/AMH & cancer
The concept that a naturally occurring growth inhibitor such as MIS could be an effective adjuvant treatment for cancer is attractive for a number of reasons. First is the issue of possible reduced toxicity. MIS is a naturally occurring inhibitor that has a mechanism of action that is completely dependent upon target cells expressing its specific receptor. Very few tissues express the MIS receptor; therefore, unlike cytotoxic chemotherapeutic agents, MIS should have limited side effects. It is not possible, of course, to predict the toxicity of a recombinant human protein in a clinical setting. This will be assessed in institutional review board-approved clinical trials using MIS that has been prepared to general manufacturing production specification in a commercial US FDA-approved facility. Second, because the mechanism of action of a naturally occurring biological is different from that of a chemotherapeutic drug, the effects of MIS as an adjuvant could be additive or even synergistic, thereby improving outcomes and perhaps also reducing the doses of toxic agents needed for a favorable response. Finally, MIS may be used as a delivery system for more toxic drugs to receptor-positive tumor cells, limiting exposure to nontarget tissues. Covalently attaching cytotoxic agents to MIS with protease-sensitive linkages would allow the drug to be internalized along with MIS after receptor binding, and normally present cytosolic enzymes would cleave the drug from MIS allowing it to function as usual.
The choice to focus initially on a few of the ovarian cancers for treatment arises from the fact that, compared with other potential MIS targets, they have the worst prognosis and might benefit significantly from this novel biological reagent, thereby offering a new approach to augment the efforts to find other useful strategies to employ. In effect, any tumor expressing functional receptors for MIS is a potential target for the material; however, fortunately, the others, listed later, have considerably better prognoses and will be the subject of MIS trials after experience has been gained with ovarian cancer.
Ovarian cancer
The potential for MIS as an anticancer agent for certain ovarian cancers has been a major focus of our laboratory, The Pediatric Surgical Research Laboratory of the Massachusetts General Hospital (MA, USA), since we hypothesized that any cancer of Müllerian origin, such as certain epithelial ovarian cancers [103], could be a target for MIS treatment three decades ago [104,105]. The idea that MIS could be used to treat ovarian cancer is predicted by the fact that the histology of the embryonic Müllerian ducts is recapitulated in the common ovarian adenocarcinomas that arise from the outer ovarian coelomic epithelium, which, in the embryo, invaginates to form the Müllerian duct [5,6]. Results of more recent experiments, however, identify cervical and endometrial cancers as other potential targets, as well as several non-Müllerian cancers, including breast and prostrate (see later). Thus, the original hypothesis can be extended to include any tumor that expresses a functional MISRII.
Before the hypothesis could be tested, several significant obstacles needed to be overcome. Purification of MIS from animal sources was sufficient for research purposes, but to treat patients, it is optimal to use the human protein. The solution was to clone the human gene sequence, and express and characterize the protein it encodes, ideally in sufficient quantity for human trials. Our laboratory has spent considerable effort optimizing purification protocols for the recombinant human protein secreted from mammalian cells and is exploring a number of alternative sources for enhanced production of MIS. The receptor(s) needed to be identified in order to develop reagents for their detection. Patients’ tumors needed to screened for the presence of receptors, because therapy would only be offered to receptor-positive patients. Preclinical trials in both in vitro and ex vivo systems with cancer cell lines and fresh tumor samples had to be carried out in order to confirm the possibility that the cancers were responsive to MIS. Finally, immunoassays to measure MIS in serum and other biological fluids had to be developed in order to test the pharmacokinetics needed to conduct clinical trials. With the exception of amassing sufficient quantities of clinical-grade recombinant human MIS/AMH for the toxicology, pharmacokinetics and Phase I clinical trials in humans, which have yet to be carried out, all of these intermediate goals have been met.
Epithelial ovarian cancer affects nearly 25,000 Americans each year, and it is the fifth most common malignancy in women, with a 5-year mortality of over 70% and more than 16,000 deaths per year [106]. Although the mortality rate is significantly lower in women with stage Ia or Ib disease, peritoneal seeding and metastatic spread accounts for the fact that fewer than 25% of women are diagnosed early in their disease process. Surgery and cytotoxic drug therapy produces favorable clinical responses in 50–80% of patients; however, unfortunately, the majority will relapse [2].
The earliest experiments began with in vitro studies using human ovarian cancer cell lines followed by a series of ex vivo experiments on these lines; thereafter, the work progressed to the examination of human ovarian cancer cells in ascites collected from patients with recurrent disease [103].
These initial studies began with partially purified bovine MIS, which suppressed the growth of a human ovarian carcinoma cell line in vitro [107] and in nude mice treated systemically with the bovine protein [108]. In addition, a large number of primary tumor cells that had been taken from patients with human ovarian and other reproductive cancers were inhibited in vitro in stem cell assays [109]. Importantly, more than 50% of the stage III ovarian patients from whom abdominal ascites were collected had cells that bound biotinylated recombinant human MIS in flow cytometry studies, expressed the type II receptor by PCR, and were growth inhibited when treated ex vivo [105] with recombinant human MIS. Highly purified human MIS also inhibited other human carcinoma cell lines of Müllerian origin, including OVCAR 3, 5 and 8, IGROV-1 and HOC-1, in vitro [110] and/or in vivo [111]. The observations on fresh surgical specimens and ascitic fluid, however, are perhaps the most significant, as they reflect what is true for patients and not cell lines. It is important to stress that MIS is not a cytotoxic agent [112]; MIS inhibits cancer cell growth in vitro by causing cell cycle arrest.
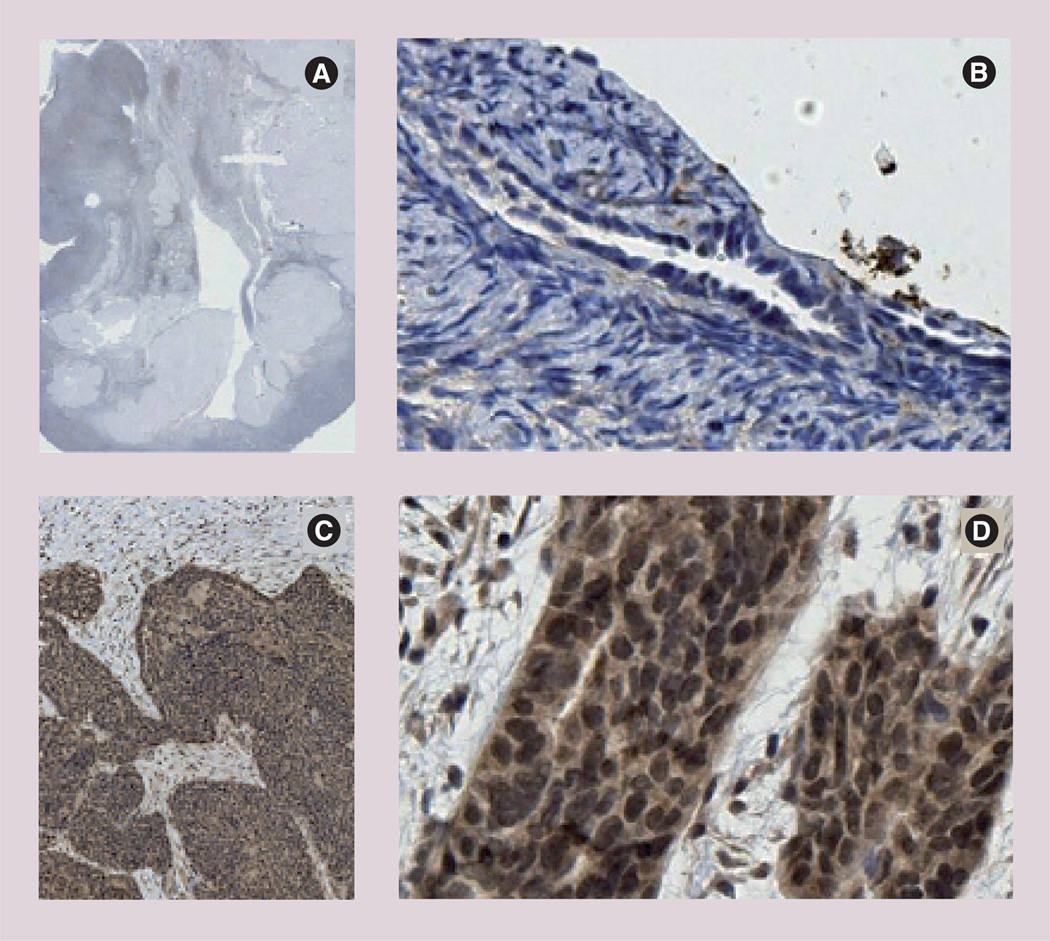
The fact that type II receptor expression is common among random samples of ovarian cancer cases is confirmed by two much more extensive studies using immunohistochemistry and/or real-time PCR that show approximately 70% frequency of receptor expression in over 300 cases in total [113,114]. An example of the immunohistochemical evidence of receptor expression has been presented by Bakkum-Gamez et al. (Figure 4) [113]. This group presents no evidence of the the MISRII in normal tissues. As predicted by the results of earlier studies, a number of other gynecological cancers also produce the type II receptor (Table 1). In contrast to the Bakkum-Gammez et al. study, Song et al. did detect MISRII expression in normal ovarian tissues [114] using in situ hybridization and real-time PCR techniques (Figure 5 & Table 2). Both studies reported similar frequency data for receptor expression in malignant and benign gynecological diseases.
Figure 4. Müllerian Inhibiting Substance type II receptor expression in benign ovarian epithelium and epithelial ovarian cancers.
Immunohistochemical analysis shows no receptor expression in benign postmenopausal ovary and strong staining in serous epithelial ovarian cancer. (A) Postmenopausal ovary and (B) surface epithelium. Serous epithelial ovarian cancers at (C) 1.25× and (D) 20×.
Reprinted with permission from [113].
Müllerian Inhibiting Substance type II receptor expression patterns in malignant and benign gynecologic tissues.
| Tissue | MISIIR expression | p-value |
|---|---|---|
| Ovarian cancer (all EOCs): | 1125 out of 182 (69%) | 0.008†,‡ |
| – Serous | 22 out of 29 (76%) | 0.002†,‡ |
| – Clear cell | 10 out of 25 (40%) | 0.14†,‡ |
| – Endometrioid | 17 out of 31 (55%) | 0.011†,‡ |
| – Mucinous | 3 out of 3 (100%) | 0.018†,‡ |
| Benign ovarian epithelium | 0 out of 5 (0%) | |
| Dysgerminoma | 17 out of 22 (77%) | <0.001§,¶ |
| Benign ovarian stroma | 3 out of 24 (12.5%) | |
| Endometrial cancer (all histologies) | 82 out of 109 (75%) | <0.001§,# |
| Benign endometrium: | 39 out of 139 (28%) | |
| – Proliferative | 11 out of 68 (17%) | <0.001§,†† |
| – Secretory | 16 out of 47 (34%) | <0.001§,†† |
| – Altrophic | 12 out of 24 (50%) | 0.018§,†† |
| Uterine malignant mixed Müllerian tumor | 30 out of 51 (59%) | <0.001§,‡‡ |
| Leiomyosarcoma | 15 out of 29 (52%) | 0.004†,‡‡ |
| Endometrial stromal sarcoma | 4 out of 18(22%) | 0.27†,‡‡ |
| Benign myometrium | 0 out of 11 (0%) | |
Histologic subtype was available for 88 of the 182 EOCs.
Fisher’s exact test.
χ2 test.
Compared with benign ovarian epithelium.
Compared with benign ovarian stroma.
Compared with overall benign endometrium.
Compared with endometrial cancer.
Compared with benign myometrium.
EOC: Epithelial ovarian cancer; MISIIR: Müllerian Inhibiting Substance type II receptor.
Data with permission from [113].
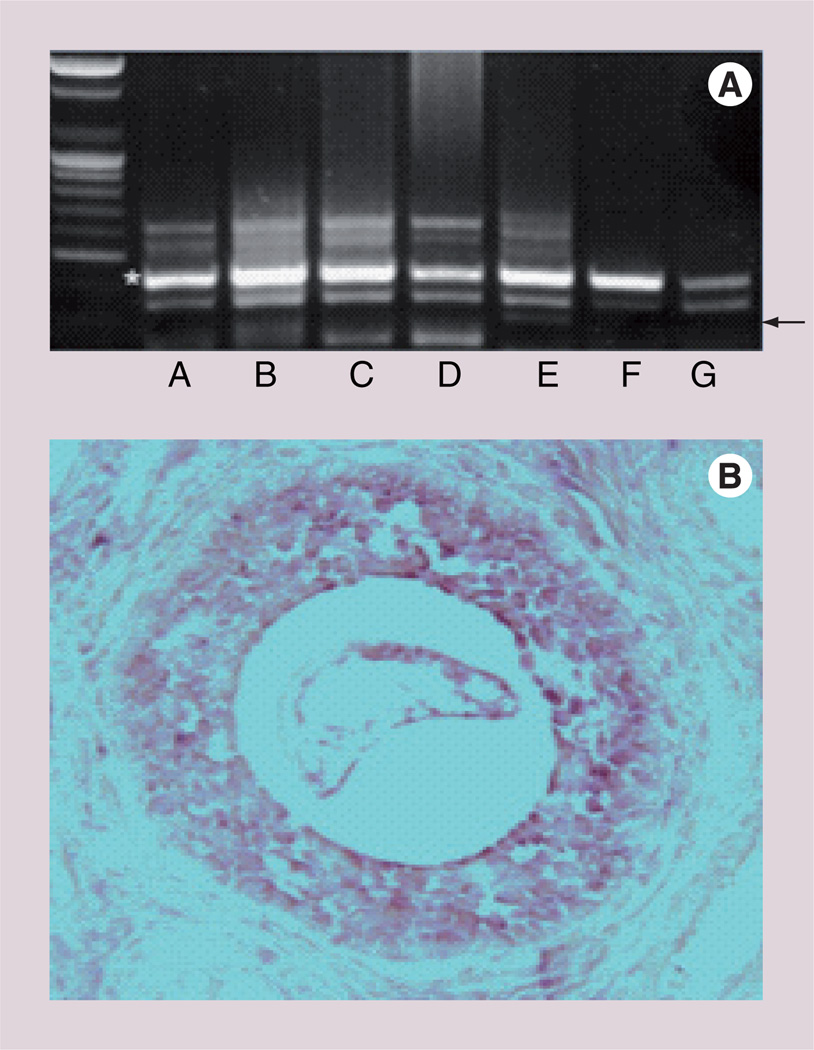
Figure 5. Müllerian Inhibiting Substance type II receptor expression in normal ovarian tissues.
(A) PCR of Müllerian Inhibiting Substance type II receptor (arrow) in human ovary; lane A: secretory phase; lane B: follicular cyst; lane C: luteal cyst; lane D: borderline mucinous cystadenoma; lane E: borderline malignant cystadenoma; lane F: serous adenocarcinoma; lane G: endometrioid adenocarcinoma. (B) Light microscopy of a proliferative phase human ovary. Granulosa cells of the small antral follicle show expression of the Müllerian Inhibiting Substance type II receptor mRNA (400×). Adapted from [114].
Table 2.
Frequency and intensity of Müllerian Inhibiting Substance and Müllerian Inhibiting Substance receptor expression in normal ovary and certain ovarian cancers.
| Expression: tumor (number of cases) |
MIS/AMHR II | MIS/AMHR II mRNA | ||
|---|---|---|---|---|
| Frequency (%) | Expression intensity | Frequency (%) | Expression intensity | |
| Benign (n = 11) | 45.45 | 0.64 ± 0.28 | 45.45 | 0.82 ± 0.31 |
| Borderline (n = 9) | 77.78 | 1.22 ± 0.32 | 55.56 | 100 ± 0.37 |
| Malignancy (n = 40) | 70.00 | 1.22 ± 0.16 | 75.00 | 1.43 ± 0.17 |
| Epithelial (n = 18) | 50.00 | 0.72 ± 0.21† | 55.56 | 0.72 ± 0.19‡ |
| Nonepithelial (n = 22): | 86.36 | 1.64 ± 0.20 | 90.91 | 2.00 ± 0.21‡ |
| – Germ cell (n = 13) | 76.92 | 1.62 ± 0.31 | 84.62 | 1.85 ± 0.31 |
| – Sex-cord stromal (n = 9) | 100 | 1.67 ± 0.24 | 100 | 2.22 ± 0.31 |
Values are the mean ± standard error.
p < 0.05, malignant epithelial vs malignant nonepithelial tumors.
p < 0.001, malignant epithelial vs malignant nonepithelial tumors.
AMHR: Anti-Müllerian hormone receptor; MIS: Müllerian Inhibiting Substance.
Adapted with permission from [114].
Müllerian Inhibiting Substance will most probably be administered to patients as an adjuvant in combination with other drugs. Therefore, studying MIS downstream signaling mechanisms proliferation inhibition is necessary before using MIS in combination with commonly used cytotoxic drugs. It is important to test for synergy or additivity between MIS and any drug to make certain that they do not counteract one another. Studies to date show that MIS downstream signal transduction pathways include type I receptor(s), Smads, cyclin-dependent kinase inhibitors (p16) and cytokine-inducible pathways. MIS also increased the expression of p107 and p130 proteins [115]. An inhibitor of cell cycle progression, p16 functions by binding cyclin/cyclin-dependent kinase complexes, thereby preventing nuclear translocation. p16 is mutated in a number of patients with ovarian cancer and, in fact, it is mutated in the SKOV 3 cell line that does not respond to MIS [105]. p107 and p130 are pocket proteins such as Rb, which, incidentally, is mutated in OVCAR 8 cells. Expression of the downstream protein, E2F1, which is associated with apoptosis, was also enhanced by MIS treatment of OVCAR 8 cells [94].
These results suggest mechanisms different from those of most cytotoxic drugs, namely MIS interrupts cell-signaling pathways that stimulate cell division and arrest the cell cycle. If MIS and cytotoxic drugs can function in combination, it may be able to decrease the dose needed for either agent alone, potentially resulting in decreased toxicity and perhaps improved outcomes. Whether this hypothesis is true will require extensive study in clinical trials when a commercial-grade protein preparation is available.
Preclinical in vitro and in vivo trials for MIS in ovarian cancer were advanced greatly by the development of a new mouse model for the disease [116]. In these animals, the MISRII promoter is used to drive SV40 T antigen expression. Thus, cells expressing transcription factors for the MISRII will also express the Tag oncogene, which inactivates p53 and the pocket proteins, Rb, p107 and p130; as a result, the majority of animals form ovarian cancers [116]. The tumors closely recapitulate the phenotype of the most common human ovarian cancer, serous cystadenocarcinoma.
Cell lines derived from these mouse tumors are known as mouse ovarian carcinoma (MOVCAR) cells, and they are extremely sensitive to MIS for growth inhibition since, by definition, they all express MISRII [117]. MOVCAR cells also grow as explants in immunocompromised mice and they continue to produce MISRII. Tumors appear in a manner proportional to the number of cells injected into the mice; the more cells, the shorter the time to appearance of the tumor.
Intraperitoneal injection of recombinant human MIS into MOVCAR-implanted mice over a period of as long as 11 weeks slowed the time to appearance of the tumors, which were smaller than the tumors seen in vehicle controltreated animals [117]. No apparent toxicity was noted despite the duration of MIS exposure in the mouse being equivalent to approximately 7 years of continuous cancer treatment in humans.
In reality, ovarian cancers arise from any of several genetic alterations, providing multiple options to resist conventional treatment; in fact, drug resistance is common among long-term survivors of this disease. It is rational, therefore, to attack multiple molecular pathways simultaneously in order to increase tumor inhibition. Such a plan may use MIS in combination with one of the approved ovarian cancer drugs. Having extensively studied the downstream signaling pathways of MIS and knowing, at least partially, the mechanism of action of the effective chemotherapeutic agents that are currently in clinical use, it will be possible to make more rational selections of therapies that MIS could be used in combination with.
The results outlined thus far, which show that MIS has the ability to inhibit cell growth with negligible toxicity, raises the possibility that MIS might also be additive or synergistic with commonly used cytotoxic agents. When this hypothesis was tested in vitro with MOVCAR cells, MIS proved to be synergistic with doxorubicin and rapamycin and additive with paclitaxel and cisplatin, and MIS retained its effectiveness against a human ovarian cancer cell line (IGROV-1) that had been made resistant to paclitaxel. Together, these results suggest that MIS can be given in combination with cytotoxic drugs and that it may be a new treatment for drug-resistant disease [118].
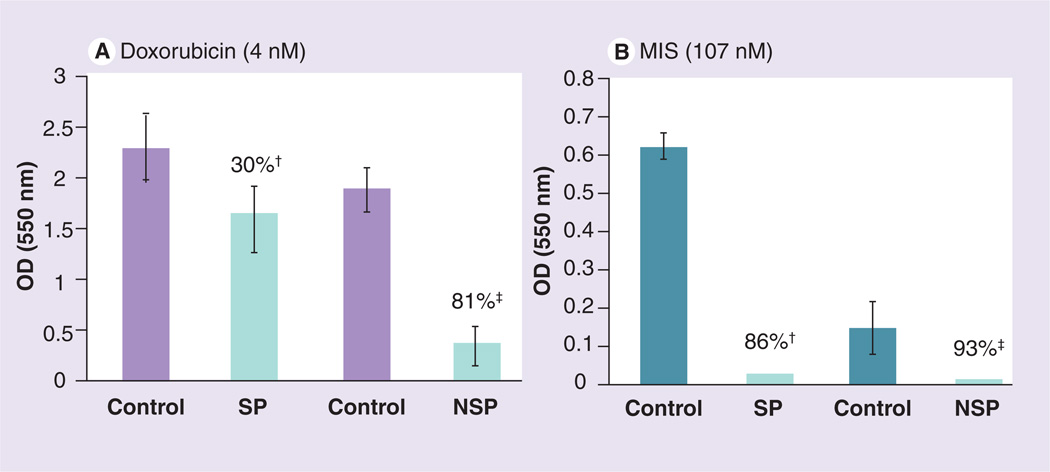
Data in support of this possibility come from studies on tumor-initiating cells, also called side population cells, isolated from MOVCAR cell cultures. These cells exhibit stem-cell-like properties in that they are tumorigenic and are resistant to some of the ovarian cancer drugs [119]. For example, MIS is still able to inhibit these cells from proliferating while they are moderately resistant to doxorubicin (Figure 6).
Figure 6. Mouse ovarian carcinoma (MOVCAR) tumor-initiating cells, also known as SP cells, which can be separated from less tumorigenic NSP cells, respond to MIS in vitro (SP: 86%; NSP: 93%).
SP cells are moderately resistant to doxorubicin (30% inhibition) but are still nearly completely inhibited by MIS.
†p < 0.001.
‡p < 0.0005. MIS: Müllerian Inhibiting Substance; NSP: Non-side population; SP: Side population.
Other Müllerian cancers
The cervix is of Müllerian duct origin; therefore, it is logical to study whether normal cervix and cervical tumors express the MISRII receptor and if its cancers respond to MIS. Three human cervical carcinoma cell lines, CaSki, SiHa and C33A, express the MISRII protein by western analysis and they are all growth inhibited in vitro by MIS [115]. The receptor is detected in normal rat cervix by western blot analysis; however, as expected, it was not found in the non-Müllerian lower vagina or in the small intestine [115]. As was seen with the ovarian cancer cells, MIS specifically upregulated p16, p130 and p107, as well as E2F1 and E2F4 in the C33A cell line, without affecting E2F2, 3 or 5. Since p53 is mutated and Rb is absent in C33A, the MIS affect appears to be independent of both. When p16, p130, p107 or E2F1 were overexpressed in these cells, growth was dramatically inhibited compared with vectors alone [115]. These findings in cervical cancer further broaden the targets for MIS.
In addition to the cervix, Fallopian tubes and the ovarian coelomic epithelial lining, the uterus is also a Müllerian structure. The MISRII mRNA is expressed in the Müllerian ducts of the female rat embryo between days 13 and 19 of development and in the uterine mesenchyme after birth. The receptor protein was also found in a human endometrial cancer cell line, AN3CA [94], as well as in nonpregnant and pregnant adult rat uteri, by western analysis using the MIS receptor antibody developed in our laboratory [94] but not in the small intestine [94]. In the human, MISIIR mRNA is expressed in human myometrium and endometrium of a postmenopausal uterus, and the protein has been observed by western analysis in endometrial tumors.
Two human cell lines, AN3CA and KLE, that express the receptor mRNA and protein were also inhibited by MIS. AN3CA, a cell line derived from a metastasis, showed significant growth inhibition (57–67% inhibition). KLE, a cell line derived from a poorly differentiated endometrial cancer, also demonstrated significant growth inhibition (44.9%) by MIS. Transient transfection of an MIS cDNA construct into these cancer cells produced growth inhibition of an endometrial cancer cell line. These transfection data support the conclusion that the observed effects are due to the MIS molecule rather than a contaminant in the purified protein preparation. Studies of the MIS molecular mechanism in endometrial cancer cells indicate G1 arrest and some apoptosis, as indicated by an increase in Caspase 3 cleavage products. Unlike the ovarian and cervical cancer cell lines, however, AN3CA cells do not express p16 or p21. MIS treatment increases p107 and p130 mRNA at 48 h, while E2F1 initially increases then decreases at 72 h [94].
Non-Müllerian cancers
Müllerian Inhibiting Substance type II receptor is expressed in locations other than Müllerian tissues and the gonads. It is expressed in the normal rat breast in an inversely proportional manner to the state of proliferation of the breast: the more proliferation, the fewer receptors. MISRII is present in virgin breast tissue but not in the lactating breast. The receptor reappears after weaning [68]. MIS enhanced IκB-dependent DNA binding of NF-κB, which resulted in the induction of IEX-1 mRNA [68], an immediate early gene induced by radiation, IFN-γ or TNF-α but not by TGF-β, via an NF-κB pathway [120,121], regardless of the estrogen receptor status of the tumor cells. When female mice aged 5 weeks were treated with MIS, apoptosis was enhanced in the mammary epithelium [68]. Thus, it is likely that breast cancer could be a target for MIS therapy.
Müllerian Inhibiting Substance treatment of cancer may also include the prostate [66,67]. In addition, because MIS suppresses testosterone production [27–31,60], it might indeed exert a dual effect on prostatic cancer, a direct growth inhibition and an indirect effect of lowering testosterone. The MISRII and two candidate MIS type I receptors [97–102] are expressed in a prostatic cancer cell line as well as in multiple human prostate tumors [66]. MIS inhibits the growth of human prostate cancer cell lines, and it induces IRF1, with IFN-γ dramatically enhancing the inhibiting effects of MIS in vitro and in vivo. These findings in breast and prostate tissue significantly broaden the target population and the indications for the clinical uses of MIS. Interestingly, a very recent paper has reported a positive correlation between serum MIS and increased breast cancer risk [122]. The data from this prospective study appear to contradict the idea that MIS may suppress breast cancer tumor growth. Clearly, these new data warrant further investigation; however, the serum MIS data were collected when the subjects were premenopausal and before the breast cancers were detected. It would be very interesting to know what the serum MIS levels were at the time of breast cancer diagnosis. If serum MIS was undetectable, for example, the seeming paradox might be easier to understand.
Conclusion
The existing body of work suggests that MIS, a biological response modifier with very little demonstrated toxicity, may be used to treat any tumor expressing the MISRII. It appears that the type I receptor candidates, with which the type II may dimerize, are ubiquitous. The process of type I receptor selection is not known. There are in vitro and in vivo growth inhibition data for MIS with human and animal cell lines for ovarian, cervical, endometrial, breast and prostate cancers. Potentially, there is a large number of cancer patients who could benefit from MIS therapy. Approximately 25,000 new ovarian cancer patients are diagnosed each year, breast cancer strikes nearly 300,000 women each year, and prostate cancer another 200,000 cases in the USA alone. This larger target population, without even including endometrial and cervical cancer patients, makes development of MIS as an anticancer therapeutic for more than ovarian cancers more compelling. Clearly, ovarian cancer is the prime target because there are the fewest options for therapy of the disease and it carries the most dire of prognoses.
Perhaps the most significant breakthrough in ovarian cancer treatment that accompanies the future use of MIS, however, is not that it should be highly specific and that it is a naturally occurring human protein that should have minimal adverse reactions. Rather, it is the possibility that MIS could treat drug-resistant disease, particularly that arising from tumor-initiating, stem-like cells. As stated earlier, most patients have a significant response to initial surgery and chemotherapy. It is the recurrent, drug-resistant disease that ultimately defeats the best efforts at eradication. MIS offers a new tool that targets not only drug resistance but also the cell type implicated as source of recurrent disease regardless of drug response status.
Clinical trials await the production of sufficient supplies of clinical-grade recombinant human MIS.
Future perspective
In the next 5–10 years, MIS should reach the clinic in two major areas. First, as an adjuvant treatment for ovarian cancer, the protein would augment, most probably additively, the activity of commonly used chemotherapies such as paclitaxel, cisplatin and doxorubicin. The result will be improved clinical control of the cancer and reduced toxicity of the drugs (i.e., less is needed with MIS as an adjuvant). Patients should have a better quality of life and survive longer. In addition, another use for MIS in the clinic will be as a treatment for a particular type of premature puberty in boys known as testotoxicosis, a problem associated with androgen-secreting tumors and low gonadotropins. MIS can directly inhibit the synthesis of testosterone and provide a new treatment strategy, because the usual hormonal therapies to reduce androgen synthesis do not work in these patients. Finally, the future of MIS in the clinic will center on its measurement in serum rather than as a systemic therapy. Serum MIS levels will continue to be useful in the management of granulose/sex-cord tumors. The newer uses will be in monitoring ovarian reserve, and it may have predictive value in IVF protocols.
Executive summary.
-
▪
Müllerian Inhibiting Substance (MIS)/anti-Müllerian hormone is a naturally occurring, highly specific inhibitor of proliferation.
-
▪
MIS’s activity is restricted to cells expressing the MIS receptor.
-
▪
MIS/anti-Müllerian hormone has no measurable toxicity.
-
▪
Drug-resistant tumor cells respond to MIS.
-
▪
Tumor-initiating stem-like cells respond to MIS.
Acknowledgments
This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
David T MacLaughlin, Pediatric Surgical Research Laboratories, Simches Research Building, Massachusetts, General Hospital, 185 Cambridge Street, Boston, MA 02114, USA, Tel.: +1 617 724 1617, Fax: +1 617 726 5057, maclaugh@helix.mgh.harvard.edu.
Patricia K Donahoe, Pediatric Surgical Research Laboratories, Simches Research Building, Massachusetts, General Hospital, 185 Cambridge Street, Boston, MA 02114, USA, Tel.: +1 617 724 1617, Fax: +1 617 726 5057, pdonahoe@partners.org.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Love RR, Philips J. Oophorectomy for breast cancer: history revisited. J. Natl Cancer Inst. 2002;94(19):1433–1434. doi: 10.1093/jnci/94.19.1433. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun M. Cancer statistics. J. Clin. Can. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Goodfellow PN, Lovell-Badge R. SRY and sex determination in mammals. Annu. Rev. Genet. 1993;27:71–92. doi: 10.1146/annurev.ge.27.120193.000443. [DOI] [PubMed] [Google Scholar]
- 4.Jost A. Recherches sue la differentiation sexulle de l’embryonde lapen. Arch. Anat. Microsc. Morphol. Exp. 1947;8:379–418. [Google Scholar]
- 5.Price JM, Donahoe PK, Ito Y, Hendren WH., 3rd Programmed cell death in the Müllerian duct induced by Müllerian Inhibiting Substance. Am. J. Anat. 1977;149:353–375. doi: 10.1002/aja.1001490304. [DOI] [PubMed] [Google Scholar]
- 6.Price JM, Donahoe PK, Ito Y. Involution of the female Müllerian duct of the fetal rat in the organ-culture assay for the detection of Müllerian Inhibiting Substance. Am. J. Anat. 1979;156:265–284. doi: 10.1002/aja.1001560207. [DOI] [PubMed] [Google Scholar]
- 7.Trelstad RL, Hayashi A, Hayashi K, Donahoe PK. The epithelial–mesenchymal interface of the male Müllerian duct: loss of basement membrane integrity and ductal regression. Dev. Biol. 1982;92:27–40. doi: 10.1016/0012-1606(82)90147-6. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi A, Donahoe PK, Budzik GP, Trelstad RL. Periductal and matrix glycosamino-glycans in rat Müllerian duct development and regression. Dev. Biol. 1982;92:16–26. doi: 10.1016/0012-1606(82)90146-4. [DOI] [PubMed] [Google Scholar]
- 9.Ikawa H, Trelstad RL, Hutson JM, Manganaro TF, Donahoe PK. Changing patterns of fibronectin, laminin, type IV collagen, and a basement membrane proteoglycan during rat Müllerian duct regression. Dev. Biol. 1984;102:260–263. doi: 10.1016/0012-1606(84)90190-8. [DOI] [PubMed] [Google Scholar]
- 10.Dyche WJ. A comparative study of the differentiation and involution of the Müllerian duct and Wolffian duct in the male and female fetal mouse. J. Morphol. 1979;162:175–209. doi: 10.1002/jmor.1051620203. [DOI] [PubMed] [Google Scholar]
- 11.Picon R. Action du testicule foetal sur le développement in vitro des canaux de Müller chez le rat. Arch. Anat. Microsc. Morph. Exp. 1969;58:1–19. [PubMed] [Google Scholar]
- 12.Donahoe PK, Ito Y, Marfatia S, Hendren WH., 3rd The production of Müllerian Inhibiting Substance by the fetal, neonatal and adult rat. Biol. Reprod. 1976;15:329–334. doi: 10.1095/biolreprod15.3.329. [DOI] [PubMed] [Google Scholar]
- 13.Josso N. In vitro synthesis of Müllerian inhibiting hormone by seminiferous tubules isolated from the calf fetal testis. Endocrinology. 1973;93:829–834. doi: 10.1210/endo-93-4-829. [DOI] [PubMed] [Google Scholar]
- 14.Blanchard MG, Josso N. Source of the anti-Müllerian hormone synthesized by the fetal testis: Müllerian-inhibiting activity of fetal bovine Sertoli cells in tissue culture. Pediatr. Res. 1974;8:968–971. doi: 10.1203/00006450-197412000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Donahoe PK, Ito Y, Price JM, Hendren WH. Müllerian Inhibiting Substance activity in bovine fetal, newborn, and prepubertal testes. Biol. Reprod. 1977;16:238–243. doi: 10.1095/biolreprod16.2.238. [DOI] [PubMed] [Google Scholar]
- 16.Josso N. Permeability of membranes to the Müllerian Inhibiting Substance synthesized by the human fetal testis. J. Clin. Endocrinol. Metab. 1972;34:265–270. doi: 10.1210/jcem-34-2-265. [DOI] [PubMed] [Google Scholar]
- 17.Donahoe PK, Ito Y, Morikawa Y, Hendren WH., 3rd Müllerian Inhibiting Substance in human testes after birth. J. Pediatr. Surg. 1977;12:323–330. doi: 10.1016/0022-3468(77)90008-2. [DOI] [PubMed] [Google Scholar]
- 18.Hutson J, Ikawa H, Donahoe PK. The ontogeny of Müllerian Inhibiting Substance in the gonads of the chicken. J. Pediatr. Surg. 1981;16(6):822–827. doi: 10.1016/s0022-3468(81)80827-5. [DOI] [PubMed] [Google Scholar]
- 19.Austin HB. Extended production of the Müllerian duct regressor in the American alligator. Gen. Comp. Endocrinol. 1994;96(1):122–128. doi: 10.1006/gcen.1994.1164. [DOI] [PubMed] [Google Scholar]
- 20.Shen WH, Moore CCD, Ikeda Y, Parker KL, Ingraham HA. Nuclear receptor steroidogenic factor 1 regulates the Müllerian Inhibiting Substance gene: a link to the sex determination cascade. Cell. 1994;77:651–661. doi: 10.1016/0092-8674(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 21.Giuili G, Shen WH, Ingraham HA. The nuclear receptor SF-1 mediates sexually dimorphic expression of Müllerian Inhibiting Substance, in vivo. Development. 1997;124:1799–1807. doi: 10.1242/dev.124.9.1799. [DOI] [PubMed] [Google Scholar]
- 22.Arango NA, Lovell-Badge R, Behringer RR. Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell. 1999;99:409–419. doi: 10.1016/s0092-8674(00)81527-5. [DOI] [PubMed] [Google Scholar]
- 23.Hossain A, Saunders GF. Role of Wilms tumor 1 (WT1) in the transcriptional regulation of the Müllerian Inhibiting Substance promoter. Biol. Reprod. 2003;69:1808–1814. doi: 10.1095/biolreprod.103.015826. [DOI] [PubMed] [Google Scholar]
- 24.Nachtigal MW, Hirokawa Y, Enyeart-Van Houten DL, Flanagan JN, Hammer GD, Ingraham HA. Wilms’ tumor 1 and DAX-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell. 1998;93:445–454. doi: 10.1016/s0092-8674(00)81172-1. [DOI] [PubMed] [Google Scholar]
- 25.Tremblay JJ, Viger RS. Nuclear receptor Dax-1 represses the transcriptional cooperation between GATA-4 and SF-1 in Sertoli cells. Biol. Reprod. 2001;64:1191–1199. doi: 10.1095/biolreprod64.4.1191. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Seibel MM, MacLaughlin DT, et al. The inhibitory effects of Müllerian Inhibiting Substance on epidermal growth factor induced proliferation and progesterone production of human granulosa-luteal cells. J. Clin. Endocrinol. Metab. 1992;75:911–917. doi: 10.1210/jcem.75.3.1517385. [DOI] [PubMed] [Google Scholar]
- 27.di Clemente N, Ghaffari S, Pepinsky RB, et al. A quantitative and interspecific test for biological activity of anti-Müllerian hormone: the fetal ovary aromatase assay. Development. 1992;114:721–727. doi: 10.1242/dev.114.3.721. [DOI] [PubMed] [Google Scholar]
- 28.Rouiller-Fabre V, Carmona S, Merhi RA, Cate R, Habert R, Vigier B. Effect of anti-Müllerian hormone on Sertoli and Leydig cell functions in fetal and immature rats. Endocrinology. 1998;139:1213–1220. doi: 10.1210/endo.139.3.5785. [DOI] [PubMed] [Google Scholar]
- 29.Racine C, Rey R, Forest MG, et al. Receptors for anti-Müllerian hormone on Leydig cells are responsible for its effects on steroidogenesis and cell differentiation. Proc. Natl Acad. Sci. USA. 1998;95:594–599. doi: 10.1073/pnas.95.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teixeira J, Fynn-Thompson E, Payne A, Donahoe PK. Müllerian Inhibiting Substance regulates androgen synthesis at the transcriptional level. Endocrinology. 1999;140:4732–4738. doi: 10.1210/endo.140.10.7075. [DOI] [PubMed] [Google Scholar]
- 31. Laurich VM, Trbovich AM, O’Neill FH, et al. Müllerian Inhibiting Substance blocks the protein kinase A-induced expression of cytochrome P450 17α-hydroxylase/C17–20 lyase mRNA in a mouse Leydig cell line independent of cAMP responsive element binding protein phosphorylation. Endocrinology. 2002;143:3351–3360. doi: 10.1210/en.2001-211352. ▪▪ Documents the block of androgen synthesis by Müllerian Inhibiting Substance (MIS). This property of MIS may augment prostate cancer treatment and be the basis of treatment for some premature puberty in males.
- 32.Rey R, Lordereau-Richard I, Carel JC, et al. Anti-Müllerian hormone and testosterone serum levels are inversely during normal and precocious pubertal development. J. Clin. Endocrinol. Metab. 1993;77:1220–1226. doi: 10.1210/jcem.77.5.8077315. [DOI] [PubMed] [Google Scholar]
- 33.Bezard J, Vigier B, Tran D, Mauleon P, Josso N. Immunocytochemical study of anti-Müllerian hormone in sheep ovarian follicles during fetal and post-natal development. J. Reprod. Fertil. 1987;80:509–516. doi: 10.1530/jrf.0.0800509. [DOI] [PubMed] [Google Scholar]
- 34.Munsterberg A, Lovell-Badge R. Expression of the mouse anti-Müllerian hormone gene suggests a role in both male and female sexual differentiation. Development. 1991;113:613–624. doi: 10.1242/dev.113.2.613. [DOI] [PubMed] [Google Scholar]
- 35.Lee MM, Donahoe PK, Hasegawa T, et al. Müllerian Inhibiting Substance in humans: normal levels from infancy to adulthood. J. Clin. Endocrinol. Metab. 1996;8:571–576. doi: 10.1210/jcem.81.2.8636269. [DOI] [PubMed] [Google Scholar]
- 36.Hudson PL, Dougas I, Donahoe PK, et al. An immunoassay to detect human Müllerian Inhibiting Substance in males and females during normal development. J. Clin. Endocrinol. Metab. 1990;70:16–22. doi: 10.1210/jcem-70-1-16. [DOI] [PubMed] [Google Scholar]
- 37.Josso N, Legeai L, Forest MG, Chaussain JL, Brauner R. An enzyme linked immunoassay for anti-Müllerian hormone: a new tool for the evaluation of testicular function in infants and children. J. Clin. Endocrinol. Metab. 1990;70:23–27. doi: 10.1210/jcem-70-1-23. [DOI] [PubMed] [Google Scholar]
- 38.Baker ML, Metcalfe SA, Hutson JM. Serum levels of Müllerian Inhibiting Substance in boys from birth to 18, as determined by enzyme immunoassay. J. Clin. Endocrinol. Metab. 1990;70:11–15. doi: 10.1210/jcem-70-1-11. [DOI] [PubMed] [Google Scholar]
- 39.Lee MM, Donahoe PK, Silverman BL, et al. Müllerian Inhibiting Substance in the evaluation of children with nonpalpable gonads. N. Engl. J. Med. 1997;336:1480–1486. doi: 10.1056/NEJM199705223362102. [DOI] [PubMed] [Google Scholar]
- 40.van Rooij IA, Broekmans FJ, te Velde ER, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum. Reprod. 2002;17:3065–3071. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 41.Silberstein T, MacLaughlin DT, Shai I, et al. Müllerian Inhibiting Substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum. Reprod. 2006;21:159–163. doi: 10.1093/humrep/dei270. [DOI] [PubMed] [Google Scholar]
- 42.Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum Müllerian Inhibiting Substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil. Steril. 2002;77:468–471. doi: 10.1016/s0015-0282(01)03201-0. [DOI] [PubMed] [Google Scholar]
- 43.Muttukrishna S, Suharjono H, McGarrigle H, Sathanandan M. Inhibin B and anti-Müllerian hormone: markers of ovarian response in IVF/ICSI patients? Brit. J. Obstet. Gynaecol. 2004;111:1248–1253. doi: 10.1111/j.1471-0528.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 44.Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Barcie P. Serum antiMüllerian hormone/Müllerian Inhibiting Substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil. Steril. 2004;82:1323–1329. doi: 10.1016/j.fertnstert.2004.03.061. [DOI] [PubMed] [Google Scholar]
- 45.Penarrubia J, Fabregues F, Manau D, et al. Basal and stimulation day 5 anti-Müllerian hormone serum concentrations as predictors of ovarian response and pregnancy in assisted reproductive technology cycles stimulated with gonadotropin-releasing hormone agonist – gonadotropin treatment. Hum. Reprod. 2005;20:915–922. doi: 10.1093/humrep/deh718. [DOI] [PubMed] [Google Scholar]
- 46.Eldar-Geva T, Ben-Chetrit A, Spitz IM, et al. Dynamic assays of inhibin B, anti-Müllerian hormone and estradiol following FSH stimulation and ovarian ultrasonography as predictors of IVF outcome. Hum. Reprod. 2005;20:3178–3183. doi: 10.1093/humrep/dei203. [DOI] [PubMed] [Google Scholar]
- 47.La Marca A, Broekmans FJ, Volpe A, Fauser BC, Macklon NS the ESHRE Special Interest Group for Reproductive Endocrinology. AMH round table: anti-Mullerian hormone (AMH): what do we still need to know? Hum. Reprod. 2009;24(9):2264–2275. doi: 10.1093/humrep/dep210. [DOI] [PubMed] [Google Scholar]
- 48.Lee TH, Liu CH, Huang CC, Hsieh KC, Lin PM, Lee MS. Impact of female age and male infertility on ovarian reserve markers to predict outcome of assisted reproduction technology cycles. Reprod. Biol. Endocrinol. 2009;7(1):100. doi: 10.1186/1477-7827-7-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.La Marca A, Sighinolfi G, Radi D, et al. Anti-Müllerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum. Reprod. Update. 2010;16(2):113–130. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- 50.Fallat ME, Siow Y, Marra M, Cook C, Carrillo A. Müllerian Inhibiting Substance in follicular fluid and serum: a comparison of patients with tubal factor infertility, polycystic ovary syndrome, and endometriosis. Fertil. Steril. 1997;67:962–965. doi: 10.1016/s0015-0282(97)81417-3. [DOI] [PubMed] [Google Scholar]
- 51.Gustafson ML, Lee MM, Scully RE, et al. Müllerian Inhibiting Substance as a marker for ovarian sex-cord tumor. N. Engl. J. Med. 1992;326:466–471. doi: 10.1056/NEJM199202133260707. [DOI] [PubMed] [Google Scholar]
- 52.Gustafson ML, Lee MM, Asmundson L, MacLaughlin DT, Donahoe PK. Müllerian Inhibiting Substance in the diagnosis and management of intersex and gonadal abnormalities. J. Pediatr. Surg. 1993;28:439–444. doi: 10.1016/0022-3468(93)90245-g. [DOI] [PubMed] [Google Scholar]
- 53.Chang HL, Pahlavan N, Halpern EF, MacLaughlin DT. Serum Müllerian Inhibiting Substance/anti-Müllerian hormone levels in patients with adult granulosa cell tumors directly correlate with aggregate tumor mass as determined by pathology or radiology. Gynecol. Oncol. 2009;114(1):57–60. doi: 10.1016/j.ygyno.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dutertre M, Gouedard L, Xavier F, et al. Ovarian granulosa cell tumors express a functional membrane receptor for anti-Müllerian hormone in transgenic mice. Endocrinology. 2001;142:4040–4046. doi: 10.1210/endo.142.9.8393. [DOI] [PubMed] [Google Scholar]
- 55.La Marca A, Volpe A. The Anti-Müllerian hormone and ovarian cancer. Hum. Reprod. Update. 2007;13:265–273. doi: 10.1093/humupd/dml060. [DOI] [PubMed] [Google Scholar]
- 56.Geerts I, Vergote I, Neven P, Billen J. The role of inhibins B and anti-Müllerian hormone for diagnosis and follow-up of granulosa cell tumors. Int. J. Gynecol. Cancer. 2009;19(5):847–855. doi: 10.1111/IGC.0b013e3181a702d1. [DOI] [PubMed] [Google Scholar]
- 57.Ueno S, Manganaro TF, Donahoe PK. Human recombinant Müllerian Inhibiting Substance inhibition of rat oocyte meiosis is reversed by epidermal growth factor in vitro. Endocrinology. 1988;123:1652–1659. doi: 10.1210/endo-123-3-1652. [DOI] [PubMed] [Google Scholar]
- 58.Durlinger AL, Kramer P, Karel B, et al. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 59.Seifer DB, MacLaughlin DT, Penzias AS, et al. Gonadotropin-releasing hormone agonist-induced differences in granulosa cell cycle kinetics are associated with alterations in follicular fluid Müllerian Inhibiting Substance and androgen content. J. Clin. Endocrinol. Metab. 1993;76:711–714. doi: 10.1210/jcem.76.3.8445031. [DOI] [PubMed] [Google Scholar]
- 60.Trbovich AM, Sluss PM, Laurich VM, et al. Müllerian Inhibiting Substance lowers testosterone in luteinizing hormone-stimulated rodents. Proc. Natl Acad. Sci. USA. 2001;98:3393–3397. doi: 10.1073/pnas.051632298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee MM, Seah CC, Masiakos PT, et al. Müllerian Inhibiting Substance type II receptor expression and function in purified rat Leydig cells. Endocrinology. 1999;140:2819–2827. doi: 10.1210/endo.140.6.6786. [DOI] [PubMed] [Google Scholar]
- 62.Baarends WM, Hoogerbrugge JW, Post M, et al. Anit-Müllerian hormone and anti-Müllerian hormone type II receptor messenger ribonucleic acid expression during postnatal testis development and in the adult testis of the rat. Endocrinology. 1995;136:5614–5622. doi: 10.1210/endo.136.12.7588316. [DOI] [PubMed] [Google Scholar]
- 63.Bedecarrats GY, O’Neill FH, Norwitz ER, Kaiser UB, Teixeira J. Regulation of gonadotropin gene expression by Müllerian Inhibiting Substance. Proc. Natl Acad. Sci. USA. 2003;100:9348–9353. doi: 10.1073/pnas.1633592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lebeurrier N, Launay S, Macrez R, et al. Anti-Müllerian-hormone-dependent regulation of the brain serine-protease inhibitor neuroserpin. J. Cell Sci. 2008;121(Pt 20):3357–3365. doi: 10.1242/jcs.031872. [DOI] [PubMed] [Google Scholar]
- 65.Wang PY, Koishi K, McGeachie AB, et al. Müllerian Inhibiting Substance acts as a motor neuron survival factor in vitro. Proc. Natl Acad. Sci. USA. 2005;102:16421–16425. doi: 10.1073/pnas.0508304102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Segev DL, Hoshiya Y, Hoshiya M, et al. Müllerian Inhibiting Substance regulates NF-kB signaling in the prostate in vitro and in vivo. Proc. Natl Acad. Sci. USA. 2002;99:239–244. doi: 10.1073/pnas.221599298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoshiya Y, Gupta V, Segev DL, et al. Müllerian substance induces NFκB signaling in breast and prostate cancer cells. Mol. Cell. Endocrinol. 2003;211:43–49. doi: 10.1016/j.mce.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 68.Segev DL, Hoshiya Y, Stephen AE, et al. Müllerian Inhibiting Substance regulates NFκB signaling and growth of mammary epithelial cells in vivo. J. Biol. Chem. 2001;276:26799–26806. doi: 10.1074/jbc.M103092200. [DOI] [PubMed] [Google Scholar]
- 69.Hoshiya Y, Gupta V, Kawakubo H, et al. MIS Promotes INF-γ-induced gene expression and apoptosis in breast cancer cells. J. Biol. Chem. 2003;278:51703–51712. doi: 10.1074/jbc.M307626200. [DOI] [PubMed] [Google Scholar]
- 70.Picard JY, Josso N. Anti-Müllerian hormone: estimation of molecular weight by gel filtration. Biomedicine. 1976;25:147–150. [PubMed] [Google Scholar]
- 71.Picard JY, Josso N. Purification of testicular anti-Müllerian hormone allowing direct visualization of the pure glycoprotein and determination of yield and purification factor. Mol. Cell. Endocrinol. 1984;34:23–19. doi: 10.1016/0303-7207(84)90155-2. [DOI] [PubMed] [Google Scholar]
- 72.Swann DA, Donahoe PK, Ito Y, Morikawa Y, Hendren WH. Extraction of Müllerian Inhibiting Substance from newborn calf testis. Dev. Biol. 1979;69:73–84. doi: 10.1016/0012-1606(79)90275-6. [DOI] [PubMed] [Google Scholar]
- 73.Budzik GP, Swann DA, Hayashi A, Donahoe PK. Enhanced purification of Müllerian Inhibiting Substance by lectin affinity chromatography. Cell. 1980;21:909–915. doi: 10.1016/0092-8674(80)90454-7. [DOI] [PubMed] [Google Scholar]
- 74.Budzik GP, Powell SM, Kamagata S, Donahoe PK. Müllerian Inhibiting Substance fractionation by dye affinity chromatography. Cell. 1983;34:307–314. doi: 10.1016/0092-8674(83)90161-7. [DOI] [PubMed] [Google Scholar]
- 75.Cate RL, Mattaliano RJ, Hession C, et al. Isolation of the bovine and human genes for Müllerian Inhibiting Substance and expression of the human gene in animal cells. Cell. 1986;45:685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- 76.Picard JY, Benarous R, Guerrier D, Josso N, Kahn A. Cloning and expression of cDNA for anti-Müllerian hormone. Proc. Natl Acad. Sci. USA. 1986;83:5464–5468. doi: 10.1073/pnas.83.15.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Josso N, Forest MG, Picard JY. Müllerian inhibiting activity of calf fetal testis: relationship to testosterone and protein synthesis. Biol. Reprod. 1975;13:163–167. doi: 10.1095/biolreprod13.2.163. [DOI] [PubMed] [Google Scholar]
- 78.Vigier B, Picard JY, Josso N. A monoclonal antibody against bovine anti-Müllerian hormone. Endocrinology. 1982;110:131–137. doi: 10.1210/endo-110-1-131. [DOI] [PubMed] [Google Scholar]
- 79.Shima H, Donahoe PK, Budzik GP, Kamagata S, Hudson P, Mudgett-Hunter M. Production of monoclonal antibodies for affinity purification of bovine Müllerian Inhibiting Substance activity. Hybridoma. 1984;3:201–214. doi: 10.1089/hyb.1984.3.201. [DOI] [PubMed] [Google Scholar]
- 80.Cohen-Haguenauer O, Pcard JY, Mattei MG, et al. Mapping of the gene for antiMüllerian hormone to the short arm of human chromosome 19. Cytogenet. Cell Genet. 1987;44:2–6. doi: 10.1159/000132332. [DOI] [PubMed] [Google Scholar]
- 81.Pepinsky RB, Sinclair LK, Chow EP, et al. Proteolytic processing of Müllerian Inhibiting Substance produces a transforming growth factor-β-like fragment. J. Biol. Chem. 1988;263:18961–18965. [PubMed] [Google Scholar]
- 82.Nachtigal MW, Ingraham HA. Bioactivation of Müllerian Inhibiting Substance during gonadal development by a kex2/subtilisin-like endoprotease. Proc. Natl Acad. Sci. USA. 1996;93:7711–7716. doi: 10.1073/pnas.93.15.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.MacLaughlin DT, Hudson PL, Graciano AL, et al. Müllerian duct regression and antiproliferative bioactivities of Müllerian Inhibiting Substance reside in its carboxyterminal domain. Endocrinology. 1992;131:291–296. doi: 10.1210/endo.131.1.1612008. [DOI] [PubMed] [Google Scholar]
- 84.Wilson CA, di Clemente N, Ehrenfels C, et al. Müllerian Inhibiting Substance requires its N-terminal domain for maintenance of biological activity, a novel finding within the transforming growth factor-β superfamily: Mol. Endocrinology. 1993;7:247–257. doi: 10.1210/mend.7.2.8469238. [DOI] [PubMed] [Google Scholar]
- 85.Wakefield LM, Winokur TS, Hollands RS, Christopherson K, Levinson AD, Sporn MB. Recombinant latent transforming growth factor β 1 has a longer plasma half-life in rats than active transforming growth factor β 1, and a different tissue distribution. J. Clin. Invest. 1990;86:1976–1984. doi: 10.1172/JCI114932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ragin RC, Donahoe PK, Kenneally MK, Ahmad M, MacLaughlin DT. Human Müllerian Inhibiting Substance: enhanced purification imparts biochemical stability and restores antiproliferative effects. Protein Expr. Purif. 1992;3:236–245. doi: 10.1016/1046-5928(92)90020-w. [DOI] [PubMed] [Google Scholar]
- 87.Lorenzo HK, Teixeira J, Pahlavan N, Laurich VM, Donahoe PK, MacLaughlin DT. New approaches for high-yield purification of Müllerian Inhibiting Substance improve its bioactivity. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;766:89–98. doi: 10.1016/s0378-4347(01)00436-4. [DOI] [PubMed] [Google Scholar]
- 88.Baarends WM, van Helmond JM, Post M, et al. A novel member of the transmembrane serine /threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the Müllerian duct. Development. 1994;120:189–197. doi: 10.1242/dev.120.1.189. [DOI] [PubMed] [Google Scholar]
- 89.di Clemente N, Wilson C, Faure E, et al. Cloning, expression, and alternative splicing of the receptor for anti-Müllerian hormone. Mol. Endocrinol. 1994;8:1006–1020. doi: 10.1210/mend.8.8.7997230. [DOI] [PubMed] [Google Scholar]
- 90.Teixeira J, He WW, Shah PC, et al. Developmental expression of a candidate Müllerian Inhibiting Substance type II receptor. Endocrinology. 1996;137:160–165. doi: 10.1210/endo.137.1.8536608. [DOI] [PubMed] [Google Scholar]
- 91.Mishina Y, Tizard R, Deng JM, et al. Sequence, genomic organization and chromosomal location of the mouse Müllerian Inhibiting Substance type II receptor gene. Biochem. Res. Commun. 1997;237:741–746. doi: 10.1006/bbrc.1997.7224. [DOI] [PubMed] [Google Scholar]
- 92.Imbeaud S, Faure E, Lamarre I, et al. Insensitivity to anti-Müllerian hormone due to a mutation in the human anti-Müllerian hormone receptor. Nat Genet. 1995;11:382–388. doi: 10.1038/ng1295-382. [DOI] [PubMed] [Google Scholar]
- 93.Tsuji M, Shima H, Yonemura CY, Brody J, Donahoe PK, Cunha GR. Effect of human recombinant Müllerian Inhibiting Substance on isolated epithelial and mesenchymal cells during Müllerian duct regression in the rat. Endocrinology. 1992;131:1481–1488. doi: 10.1210/endo.131.3.1505479. [DOI] [PubMed] [Google Scholar]
- 94.Renaud EJ, MacLaughlin DT, Oliva E, Rueda BR, Donahoe PK. Endometrial cancer is a receptor mediated target for Müllerian Inhibiting Substance. Proc. Natl Acad. Sci. USA. 2005;102:111–116. doi: 10.1073/pnas.0407772101. erratum in: Proc. Natl Acad. Sci. USA 102, 6513 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gupta V, Carey JL, Kawakubo H, et al. Müllerian Inhibiting Substance suppresses tumor growth in the C3(1)T antigen transgenic mouse mammary carcinoma model. Proc. Natl Acad. Sci. USA. 2005;102:3219–3224. doi: 10.1073/pnas.0409709102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Josso N, Belville C, di Clemente N, Picard YP. AMH and AMH receptor defects in persistent Müllerian duct syndrome. Hum. Reprod. Update. 2005;11:351–356. doi: 10.1093/humupd/dmi014. [DOI] [PubMed] [Google Scholar]
- 97.Hoshiya M, Christian B, Cromie W, Zhan Y, MacLaughlin DT, Donahoe PK. Persistent Müllerian duct syndrome with a deletion and a novel splicing mutation in the MIS type II receptor. Birth Def. Res. 2003;67:868–874. doi: 10.1002/bdra.10091. [DOI] [PubMed] [Google Scholar]
- 98.Belville C, van Vlijmen H, Ehrenfels C, et al. Mutations of the anti-Müllerian hormone gene in patients with persistent Müllerian duct syndrome: biosynthesis, secretion and processing of the abnormal proteins and analysis using a three-dimensional model. Mol. Endocrinol. 2004;18:708–721. doi: 10.1210/me.2003-0358. [DOI] [PubMed] [Google Scholar]
- 99.Gouedard L, Chen YG, Thevenet L, et al. Engagement of bone morphogenetic protein type IB receptor and Smad1 signaling by anti-Müllerian hormone and its type II receptor. J. Biol. Chem. 2000;275:27973–27978. doi: 10.1074/jbc.M002704200. [DOI] [PubMed] [Google Scholar]
- 100.Visser JA, Olaso R, Verhoef-Post M, Kramer P, Themmen AP, Inraham HA. The serine/threonine transmembrane receptor ALK2 mediates Müllerian Inhibiting Substance signaling. Mol. Endocrinol. 2001;15:936–945. doi: 10.1210/mend.15.6.0645. [DOI] [PubMed] [Google Scholar]
- 101.Jamin S, Arango NA, Mishina Y, et al. Requirement of Bmpr1a for Müllerian duct regression during male sexual development. Nat. Genet. 2002;32(3):408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- 102.Clarke TR, Hoshiya Y, Yi SE, Liu X, Lyons KM, Donahoe PK. Müllerian Inhibiting Substance signaling uses a BMP-like pathway mediated by ALK2 and induces Smad6 expression. Mol. Endocrinol. 2001;15:946–959. doi: 10.1210/mend.15.6.0664. [DOI] [PubMed] [Google Scholar]
- 103.Scully RE. Ovarian tumors. A review. Am. J. Pathol. 1977;87:686–720. [PMC free article] [PubMed] [Google Scholar]
- 104.Donahoe PK, Swann DA, Hayashi A, Sullivan MD. Müllerian duct regression in the embryo is correlated with cytotoxic activity against a human ovarian cancer. Science. 1979;205:913–915. doi: 10.1126/science.472712. [DOI] [PubMed] [Google Scholar]
- 105. Masiakos PT, MacLaughlin DT, Maheswaran S, et al. Human ovarian cancer, cell lines and primary ascites cells express the human MIS type II receptor, bind, and are responsive to MIS. Clin. Can. Res. 1999;5:488–499. ▪▪ Provides supporting evidence that MIS receptor is expressed in normal human ovaries and human ovarian cancers. This study also shows that recombinant human MIS can inhibit the growth of human ovarian cancer cells in vitro. It also shows evidence that drug-resistant cancer cells are still responsive to MIS and that tumor-initiating cells, which may be the source of metastatic disease, are also MIS targets.
- 106.Berkenblit A, Cannistra SA. Advances in the management of epithelial ovarian cancer. J. Reprod. Med. 2005;50:426–438. [PubMed] [Google Scholar]
- 107.Fuller AF, Jr, Guy SR, Budzik GP, Donahoe PK. Müllerian Inhibiting Substance inhibits colony growth of a human ovarian cancer cell line. J. Clin. Endocrinol. Metab. 1982;54:1051–1055. doi: 10.1210/jcem-54-5-1051. [DOI] [PubMed] [Google Scholar]
- 108.Donahoe PK, Fuller AF, Jr, Scully RE, Guy SR, Budzik GP. Müllerian Inhibiting Substance inhibits growth of a human ovarian cancer in nude mice. Ann Surg. 1981;194:472–480. doi: 10.1097/00000658-198110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fuller AF, Jr, Krane IM, Budzik GP, Donahoe PK. Müllerian Inhibiting Substance reduction of colony growth of human gynecologic cancers in a stem cell assay. Gynecol. Oncol. 1985;22:135–148. doi: 10.1016/0090-8258(85)90019-8. [DOI] [PubMed] [Google Scholar]
- 110.Chin T, Parry RL, Donahoe PK. Human Müllerian Inhibiting Substance inhibits tumor growth in vitro and in vivo. Can. Res. 1991;51:2101–2106. [PubMed] [Google Scholar]
- 111.Stephen AE, Pearsall LA, Christian BP, Donahoe PK, Vacanti JP, MacLaughlin DT. Highly purified Müllerian Inhibiting Substance inhibits ovarian cancer in vivo. Clin. Can. Res. 2002;8:2640–2646. [PubMed] [Google Scholar]
- 112.Wallen JW, Cate RL, Kiefer DM, et al. Minimal antiproliferative effect of recombinant Müllerian Inhibiting Substance on gynecological tumor cell lines and tumor explants. Cancer Res. 1989;49:2005–2011. [PubMed] [Google Scholar]
- 113. Bakkum-Gamez JN, Aletti G, Lewis KA, et al. Müllerian Inhibiting Substance type II receptor (MISIIR): a novel, tissue-specific target expressed by gynecologic cancers. Gynecol. Oncol. 2008;108(1):141–148. doi: 10.1016/j.ygyno.2007.09.010. ▪ Provides supporting evidence that the MIS receptor is expressed in normal human ovaries and human ovarian cancers. This study also shows that recombinant human MIS can inhibit the growth of human ovarian cancer cells in vitro. It also shows evidence that drug-resistant cancer cells are still responsive to MIS and that tumor-initiating cells, which may be the source of metastatic disease, are also MIS targets.
- 114. Song JY, Chen KY, Kim SY, et al. The expression of Müllerian Inhibiting Substance/anti-Müllerian hormone type II Receptor protein and mRNA in benign, borderline and malignant ovarian neoplasia. Int. J. Oncol. 2009;34(6):1583–1591. doi: 10.3892/ijo_00000288. ▪ Provides supporting evidence that MIS receptor is expressed in normal human ovaries and human ovarian cancers. This study also shows that recombinant human MIS can inhibit the growth of human ovarian cancer cells in vitro. It also shows evidence that drug-resistant cancer cells are still responsive to MIS and that tumor-initiating cells, which may be the source of metastatic disease, are also MIS targets.
- 115.Barbie TU, Barbie DA, MacLaughlin DT, Maheswaran S, Donahoe PK. Müllerian Inhibiting Substance inhibits cervical cancer cell growth via a pathway involving p130 and p107. Proc Natl Acad Sci USA. 2003;100:15601–15606. doi: 10.1073/pnas.2636900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Connolly DC, Bao R, Nikitin AY, et al. Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIR promoter develop epithelial ovarian cancer. Can. Res. 2003;63:1389–1397. [PubMed] [Google Scholar]
- 117.Pieretti-Vanmarcke R, Donahoe PK, Szotek P, et al. Recombinant human Müllerian Inhibiting Substance inhibits long term growth of MISRII-directed transgenic mouse ovarian cancers in vivo. Clin. Can. Res. 2006;12(5):1593–1598. doi: 10.1158/1078-0432.CCR-05-2108. [DOI] [PubMed] [Google Scholar]
- 118. Pieretti-Vanmarcke R, Donahoe PK, Pearsall LA, et al. Müllerian Inhibiting Substance enhances subclinical doses of chemotherapeutic agents to inhibit human and mouse ovarian cancer. Proc. Natl Acad. Sci. USA. 2006;103(46):17426–17431. doi: 10.1073/pnas.0607959103. ▪▪ Provides supporting evidence that MIS receptor is expressed in normal human ovaries and human ovarian cancers. This study also shows that recombinant human MIS can inhibit the growth of human ovarian cancer cells in vitro. It also shows evidence that drug-resistant cancer cells are still responsive to MIS and that tumor-initiating cells, which may be the source of metastatic disease, are also MIS targets.
- 119. Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Müllerian Inhibiting Substance responsiveness. Proc. Natl Acad. Sci. USA. 2006;103(30):11154–11159. doi: 10.1073/pnas.0603672103. ▪▪ Provides supporting evidence that MIS receptor is expressed in normal human ovaries and human ovarian cancers. This study also shows that recombinant human MIS can inhibit the growth of human ovarian cancer cells in vitro. It also shows evidence that drug-resistant cancer cells are still responsive to MIS and that tumor-initiating cells, which may be the source of metastatic disease, are also MIS targets.
- 120.Sovak MA, Bellas RE, Kim DW, et al. Aberrant nuclear factor-kB/Rel expression and the pathogenesis of breast cancer. J. Clin. Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Sledge GW. Constitutive activation of NF-κB during progression of breast cancer to hormone-independent growth. Mol. Cell Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dorgan JF, Stanczyk FZ, Egleston BL, et al. Prospective case-control study of serum Müllerian Inhibiting Substance and breast cancer risk. J. Natl Cancer Inst. 2009;101(21):1501–1509. doi: 10.1093/jnci/djp331. [DOI] [PMC free article] [PubMed] [Google Scholar]